Abstract
Angiogenesis extends pre-existing blood vessels to improve oxygen and nutrient delivery to inflamed or otherwise hypoxic tissues. Mitochondria are integral in this process, controlling cellular metabolism to regulate the proliferation, migration, and survival of endothelial cells which comprise the inner lining of blood vessels. Mitochondrial Complex III senses hypoxic conditions and generates mitochondrial reactive oxygen species (mROS) which stabilize hypoxia-inducible factor (HIF-1α) protein. HIF-1α induces the transcription of the vegfa gene, allowing the translation of vascular endothelial growth factor (VEGF) protein, which interacts with mature and precursor endothelial cells, mobilizing them to form new blood vessels. This cascade can be inhibited at specific points by means of gene knockdown, enzyme treatment, and introduction of naturally occurring small molecules, providing insight into the relationship between mitochondria and angiogenesis. This review focuses on current knowledge of the overall role of mitochondria in controlling angiogenesis and outlines known inhibitors that have been used to elucidate this pathway which may be useful in future research to control angiogenesis in vivo.
Keywords: angiogenesis, mitochondria, endothelial, mROS, HIF-1α, VEGF
Angiogenesis, Endothelial Cells, and Hypoxia Sensation by Mitochondria
Angiogenesis is the process of forming new blood vessels as an extension of pre-existing blood vessels to improve oxygen and nutrient delivery to tissues throughout an organism. Ischemia [1], exercise [2] [3], and inflammation [4] [5] create a hypoxic environment in tissue resulting in low levels of oxygen available for use by the cells. Of primary interest in regards to angiogenesis is the role of mitochondria in either promoting or inhibiting the process of blood vessel formation in response to the varying internal state of the organism. Mitochondrial cascades play a major role in controlling angiogenesis by regulating the proliferation, migration, and survival of endothelial cells, which make up the inner lining of blood vessels [6] [7] [8]. Endothelial cells are highly plastic, and are therefore capable of switching from a resting, quiescent state in established conduit blood vessels, to an active, migratory, proliferative state during the process of angiogenesis [9] [10]. Mitochondria are able to control angiogenesis by sensing fluctuations in oxygen availability in tissue and in turn coordinating changes in energy metabolism and generating reactive oxygen species in order to maintain an internal environment capable of sustaining an organism.
Mitochondrial Complex III and the Generation of Mitochondrial Reactive Oxygen Species
The mitochondrial electron transport chain is composed of four major protein complexes, known as mitochondrial Complex I through IV respectively, which reside on the inner mitochondrial membrane [11]. Mitochondrial Complex III, specifically known as ubiquinolcytochrome c oxidoreductase, is made up of eleven distinct proteins encoded by nuclear and mitochondrial genes [12]. Complex III has three major responsibilities in the process of oxidative phosphorylation: electron transfer, ubisemiquinone radical stabilization, and cellular oxygen sensing [13].
Mitochondrial Complex III catalyzes electron transfer from ubiquinol to cytochrome c. Ubiquinol (QH2), which is the reduced form of ubiquinone (Q), and cytochrome c serve as small electron carriers which ferry electrons from Complex I and II to Complex III and from Complex III to Complex IV, respectively [11]. The electron transfer across Complex III is carried out by the Q cycle [14]. When electrons are transferred from mitochondrial Complexes I and II to ubiquinone, they do so simultaneously in a paired transfer. This newly reduced ubiquinol can then associate with mitochondrial Complex III at the Qo site to begin the transfer of electrons onto Complex III. However, the subsequent transfer of electrons from mitochondrial Complex III to mitochondrial Complex IV via cytochrome c must be conducted sequentially rather than simultaneously, which is the responsibility of the Q cycle [15]. Mitochondrial Complex III contains both high and low potential redox chains [16]. After one electron is transferred from ubiquinol to the high potential redox chain subunit, the Rieske Iron-Sulfur protein, a radical ubisemiquinone intermediate (Q•−) remains until the second electron can be transferred to the low potential redox chain subunit of mitochondrial Complex III, cytochrome b, yielding a newly oxidized ubiquinone.
Stabilization of the ubisemiquinone intermediate is one facet of a larger mechanism controlled by mitochondrial Complex III; generation of mitochondrial reactive oxygen species. The electron transport chain as a whole is responsible for reducing molecular oxygen (O2) to water (H2O), which occurs by means of the final electron transfer from Complex IV to molecular oxygen. The presence of electrophilic molecular oxygen near the Qo site on mitochondrial Complex III allows the possibility of electron transfer from ubisemiquinone to molecular oxygen rather than the low potential redox chain subunit of Complex III, cytochrome b [17]. The probability of this occurring increases in proportion to the amount of time the ubisemiquinone molecule is present [18] [19] [20]. The capture of an electron from ubisemiquinone by molecular oxygen results in the formation of superoxide (O•−2), which, along with other partially reduced oxygen products such as hydrogen peroxide (H2O2) and hydroxyl radicals (•OH), are known as mitochondrial reactive oxygen species (mROS) [21]. Ubisemiquinone stabilization prevents the donation of an electron to molecular oxygen, which inhibits the formation of mROS radicals [18]. These mROS have been shown to contribute to angiogenesis by stabilizing proteins in specific signaling pathways described later [22]. It should be noted that nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase) also produces substantial amounts of reactive oxygen species within endothelial cells and other cell types through the reduction of O2 [23], which can contribute to angiogenesis through similar pathways [22] [24], but this mechanism takes place independently of the mitochondria and is therefore outside the scope of this review. The role of mitochondrial Complex III in cellular oxygen sensing relies on the ubiquinolcytochrome c reductase binding protein (UQCRB) subunit, which is a key player in mitochondria’s role in angiogenesis, and has therefore been the focus of essential research in this discipline.
Control of mROS Generation by Ubiquinol-cytochrome c Reductase Binding Protein
UQCRB is a 13.4-kDa nuclear-encoded subunit of mitochondrial Complex III which plays a role in the maintenance of mitochondrial Complex III while also assisting in the electron transport function of the complex [25]. The vital nature of this subunit in the overall function of mitochondrial Complex III has been proven over the course of several experiments both in vivo and in vitro which look to inhibit UQCRB function and subsequently investigate the downstream effects of this inhibition on mitochondrial function and angiogenesis (Table 1). Terpestacin is a naturally occurring bicyclo sesterterpene molecule which has been isolated from multiple organisms, most notably Embellisia chlamydospora, a fungus. Early work with this molecule proved its general efficacy in inhibiting angiogenesis [26]. It was then shown that terpestacin contributed its inhibiting effects on angiogenesis by binding specifically to the UQCRB subunit of mitochondrial Complex III [27]. Later work in Danio rerio (zebrafish) investigated both terpestacin and gene knockdown of UQCRB with uqcrb-MO (Morpholino oligomer) to investigate downstream effects on angiogenesis. Angiogenesis was dose-dependently inhibited by both factors, accompanied by a substantial decrease in vegfa gene expression [28]. The introduction of human UQCRB-specific siRNA (siUQCRB) to human umbilical vein endothelial cells (HUVECs) decreased the mobilization and invasiveness of HUVECs dose dependently [29], which helps to strengthen the case for UQCRB’s role in the angiogenic cascade as well as the role in angiogenesis of endothelial cell migration and vascular endothelial growth factor (VEGF), which will be described later. mROS generation was also shown to be significantly diminished in cells treated with terpestacin and siUQCRB, implying that the UQCRB subunit also plays a role in mROS production, potentially as a modulator of electron flux through Complex III, which can influence the lifetime of ubisemiquinone, controlling levels of mROS being produced [27]. This inhibition of mROS production decreased the angiogenic proliferation, migration, and survival of endothelial cells [9] [10] [29]. These results indicate that the role of UQCRB in mitochondrial Complex III function and angiogenesis overall involves the production of mROS and VEGF, both of which contribute to downstream factors in the angiogenic pathway of endothelial cells.
Table 1.
Inhibitors of the Angiogenesis Pathway
| Inhibitor | Cell Type | Process Inhibited | Pathway | Reference |
|---|---|---|---|---|
| Stigmatellin | WT cybrids; in vitro | Inhibits HIF-1α stabilization and accumulation | Binding to Qo site inhibits mROS production | [17] |
| Terpestacin in vivo | Murine FM3A breast cancer cells; in vivo | Inhibits tumor angiogenesis | Inhibition of HIF-1α Mediated VEGF expression | [27] |
| Terpestacin in vitro | Human HT1080 fibrosarcoma cells, HUVECs; in vitro | Inhibits VEGF production; inhibits tube formation and angiogenesis by HUVECs | Decreases mROS levels by inhibiting UQCRB which inhibits HIF-1α stabilization and accumulation, inhibiting VEGF production | [27] |
| Uqcrb-MO | Zebrafish embryos | Inhibits VEGF production | Gene knockdown of uqcrb gene leads to decreased expression of vegfa gene | [28] |
| siUQCRB | HUVECs; in vitro | Inhibits endothelial cell invasiveness through inhibition of ERK and Akt pathways | Gene knockdown of uqcrb leads to decreased mROS levels, decreased activation of VEGFR2 | [29] |
| Rotenone and thenoyltrifluoroacetone (TTFA) | Cardiomyocytes; in vitro | Inhibits mROS production | Inhibits formation of ubiquinol by preventing transfer of electrons from Complex I and Complex II | [40] |
| Matairesinol | HUVECs; in vitro and in vivo | Inhibits HUVEC tube formation | Inhibits mROS production which decreases HIF-1α stabilization and VEGF production | [74] |
| GPX1 and catalase | A549 adenocarcinomic human alveolar basal epithelial cells; in vitro | Inhibits HIF-1α stabilization and survival | Breaks down hydrogen peroxide to water which prevents breakdown of prolyl hydroxylase | [76] |
Mitochondrial Metabolism and Intracellular Redox Reactions
Under normoxic conditions, oxidative phosphorylation is the primary metabolic pathway used to recycle ADP to ATP as an energy source for the cell [11]. The electron transport chain on the inner mitochondrial membrane transfers electrons from NADH and FADH2 to a series of specific protein complexes, which then deposit four total electrons onto molecular oxygen, forming water as a final byproduct. This electron transfer powers the transport of protons across the inner mitochondrial membrane into the intermembrane space, creating a mitochondrial membrane potential that contributes to the proton motive force driving the phosphorylation of ADP to ATP [30] [31] [32] [11]. mROS are a natural byproduct of oxygen metabolism, but under normal metabolic conditions, only 0.1% of the total oxygen consumed in the mitochondria is converted to mROS [33].
Hypoxic conditions, or low levels of available oxygen in a tissue, can be caused by ischemia [1], exercise [2] [3], or inflammation [4] [5]. Mitochondria, more specifically the UQCRB subunit of mitochondrial Complex III, play an important role as oxygen sensors in the cell [27]. Sensation of hypoxic conditions increases the generation of mROS which can then be used to regulate downstream hypoxic response pathways [34] [35] [36]. Although it appears to be counterintuitive, due to the decreased availability of oxygen in the cell, studies have shown hypoxic conditions increase mROS production at mitochondrial Complex III [37]. One experiment contributing to the discovery of this phenomenon involved low concentrations of the probe dichlorofluorescein diacetate which is oxidized during the formation of mROS to yield fluorescent dichlorofluorescein (DCF). Under control conditions, the rate of probe oxidation matched the rate of leakage of oxidized probe from the cell, resulting in a stable fluorescence intensity. Hypoxic conditions lead to an increased fluorescence intensity, indicating an overall increase in oxidation within the cell, resulting from the amplified production of mROS [34] [38] [39]. This follows the common redox reaction blueprint, in which the O2 within the mitochondria are being reduced to reactive oxygen species by gaining electrons, and the other entities within the mitochondria, DCF in the case of this experiment, are being oxidized by losing electrons as a result of this reaction. The extent of hypoxia was shown to be proportional with fluorescence intensity, with 1% O2 showing greater fluorescence intensity than 3% and 5% O2 [40], indicating higher levels of oxidation in the mitochondria under increasingly hypoxic conditions, a result of increased mROS production. A review of additional experimentation further confirmed this notion of increased oxidant production under physiological hypoxia in a large range of cell types [37]. Likewise, blocking the ability of mitochondrial Complex I and II to generate ubiquinol, a key step in the electron transport chain during metabolism, was carried out using specific inhibitors [40], and led to attenuation of oxidation in the cell. This confirmed the role of mitochondrial Complex III in mROS production under hypoxic conditions [40], oxidizing ubiquinol to ubisemiquinone, which allows for the reduction of O2 to mROS. mROS can then be used by the mitochondria in hypoxic response signaling, namely, stabilizing the major downstream factor in the angiogenesis pathway, the HIF-1α protein.
mROS Mediated Stabilization of HIF-1α and the VEGF Pathway
Hypoxia inducible factor (HIF-1) is a heterodimer transcription factor protein made up of HIF-1α and HIF-1β domains [41]. Under normoxic conditions, the HIF-1β subunit is stable, while the HIF-1α subunit is easily degradable through hydroxylation by prolyl hydroxylase and subsequent ubiquitination by the von-Hippel-Lindau protein (pVHL)/E3 ubiquitin ligase, allowing for degradation by proteasomes [42] [43] [44] [45] [46]. Under hypoxic conditions, HIF-1α remains viable, accumulating and moving into the nucleus to enable the transcription of genes necessary for a cell and an organism as a whole to respond to hypoxia [11]. One major protein transcriptionally activated by the HIF-1 protein complex is vascular endothelial growth factor (VEGF) [47] [48] [49] [50]. When HIF-1α is in its active state under hypoxic conditions it is able to bind to the 5’ flanking region of the vegfa gene, inducing transcription and leading to translation of the VEGF protein [48]. VEGF, an endothelial cell-specific mitogen, is the major factor affecting angiogenesis [51] [52] [53]. VEGF possesses paracrine function and is released by cells in nearby tissue to bind to vascular endothelial growth factor receptor-2 (VEGFR2), also known as KDR/Flk-1 [54] [55] [56], which has been implicated as the primary mitogenic receptor for VEGF in the angiogenesis pathway [57] [58]. Activation of VEGFR2 leads to phosphorylation of specific downstream signal transduction effectors, including extracellular signal transduction kinase (ERK) [59] [60] and Akt [61]. ERK, a subtype of mitogen-activated protein kinase (MAPK), contributes to angiogenesis by promoting endothelial cell growth and differentiation [62]. Akt inhibits apoptosis to promote endothelial cell survival [63] [64] [65], while also contributing to angiogenesis through the transcription and translation of additional VEGF [66] [67] and promoting the migration of endothelial cells [68] [69]. Increased levels of VEGF in circulation stimulates the mobilization of mature endothelial cells in existing blood vessels as well as the maturation of circulating endothelial precursor cells (CEPs) [70], which both express the VEGFR2 receptor [71] [72], and coordinate to form the inner lining of new blood vessels.
The transcription factor subunit HIF-1α, however, must be stabilized and activated in order to carry out its function. We have discussed how hypoxic conditions lead to increased levels of mROS, which in turn stabilizes the HIF-1α protein [73] and allows transcription of the vegfa gene. Several experiments have implicated the role of hypoxia-induced mROS in the stabilization of HIF-1α by manipulating this pathway through treatment with specific inhibitors (Table 1). For example, treating cells with matairesinol, a natural small molecule originating in Cedrus deodara plants, suppresses hypoxia-induced generation of mROS both in vitro and in vivo, which in turn suppresses HIF-1α and VEGF levels as well as HUVEC tube formation [74]. mROS, as oxidative stressors, carry out this stabilization by contributing to the deactivation of prolyl hydroxylases in the cell, which prevents the hydroxylation and degradation of HIF-1α [75]. To determine which mROS are required for HIF-1α stabilization, copper zinc superoxide dismutase (SOD1) and manganese superoxide dismutase (SOD2), which breakdown superoxide to hydrogen peroxide, or glutathione peroxidase 1 (GPX1) and catalase, which breakdown hydrogen peroxide to water, were introduced to cells in vitro. HIF-1α stabilization was decreased in cells overexpressing GPX1 and catalase, indicating a role for hydrogen peroxide in prolyl hydroxylase deactivation and the accompanying stabilization of HIF-1α [76]. The breakdown of superoxide into hydrogen peroxide by SOD1 and SOD2 contributes to this stabilizing effect of the mROS, but is not sufficient to trigger HIF-1α protein stabilization in the absence of high levels of hydrogen peroxide [76].
Other experiments confirming the role of mROS in HIF-1α stabilization in vitro have employed the knockout of specific mitochondrial components in mutant cell or mitochondrial strains. One study confirmed both the role of the cytochrome b subunit as the second, low potential electron acceptor on mitochondrial Complex III and the role of mROS in HIF-1α stabilization. Cells that were deficient of cytochrome b, while not completing final electron transfer to convert molecular oxygen to water due to this missing link in the electron transport chain, were still able to produce mROS, as evidenced by the stabilization of HIF-1α protein [17]. This supports the ubisemiquinone mechanism of mROS production described earlier, and provides further evidence for the role of mROS in stabilizing HIF-1α. Inhibiting Complex III with a specific inhibitor, stigmatellin, inhibits hypoxic stabilization of HIF-1α in vitro, again confirming the role of this complex in the production of mROS [17]. The binding of terpestacin to the UQCRB subunit of mitochondrial Complex III was also shown to inhibit HIF-1α stabilization by inhibiting mROS production, as discussed. This led to the inhibition of angiogenesis which coincided with decreased expression of VEGF [27], as would be expected without the activated transcription factor for the vegfa gene, the HIF-1 protein complex.
Conclusion
The major link between mitochondria and angiogenesis centers on the production of VEGF in response to oxygen sensation and mROS production by the mitochondria. As the centers of oxidative metabolism, the mitochondria play an integral role in sensing an inflamed or otherwise hypoxic tissue environment, and allowing the transcription of the genes necessary to ensure adequate delivery of nutrients and oxygen to these tissues through the extension of blood vessels by angiogenesis. This angiogenic response ensures the health and survival of both specific tissues and whole organisms. It has been clearly established that mROS are a major signaling molecule generated by the mitochondria to stabilize HIF-1α, leading to the production of VEGF which is released from cells and acts in a paracrine function to activate VEGFR2, inducing downstream pathways. Inhibitors of various steps in this pathway have revealed the overall link between mitochondria and angiogenesis. Future research should be directed toward discovering efficient methods of controlling angiogenesis in vivo by employing these inhibitors, and better understanding mROS signaling, possibly beyond the scope of prolyl hydroxylase deactivation, investigating the possibility of a greater role for these oxidative stressors in the angiogenesis pathway.
Acknowledgements
The authors thank Dave Schumick of The Cleveland Clinic Center for Medical Art and Photography for his illustration work on Figure 1.
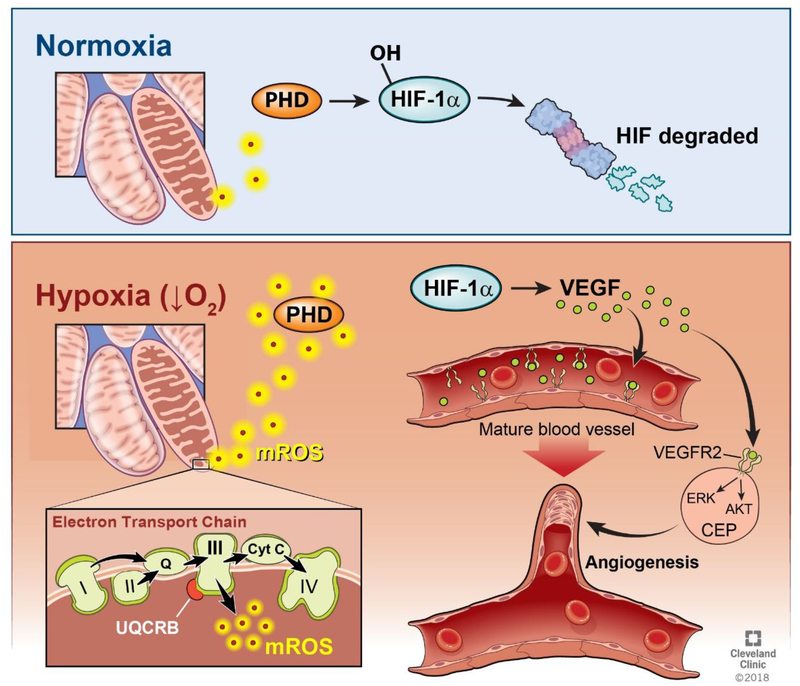
Fig. 1. Summary of the Angiogenesis Pathway.
Ischemia, exercise, and inflammation create a hypoxic tissue environment, resulting in decreased oxygen availability in cells. Under these hypoxic conditions, the electron transport chain produces mROS at mitochondrial Complex III. These mROS exit the mitochondria and deactivate prolyl hydroxylase (PHD). Under normoxic conditions, the levels of mROS being produced are insufficient to deactivate PHD, and PHD therefore hydroxylates the HIF-1α protein, marking it for eventual degradation by proteasomes. When high levels of mROS are produced under hypoxia, HIF-1α is stabilized, allowing the production of the VEGF protein. This VEGF protein attaches to the VEGFR2 receptor on both mature endothelial cells lining blood vessels and circulating endothelial precursors (CEP). This activates the ERK and Akt pathways, causing the maturation and mobilization of endothelial cells, allowing angiogenesis to occur.
Grant support provided by National Institutes of Health grants HL103453, HL081064, HL109250, HL60917, and HL115008, the Alfred Lerner Memorial Chair in Innovative Biomedical Research at the Cleveland Clinic, and in part by the Lerner Research Institute Center of Excellence in Pulmonary Vascular Disease.
Footnotes
Declaration of Interest
The authors declare that they have no conflict of interest.
References
- 1.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451(7181):1008–1012. doi: 10.1038/nature06613 [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson T (2011) Vascular remodelling in human skeletal muscle. Biochem Soc Trans 39 (6):1628–1632. doi: 10.1042/BST20110720 [DOI] [PubMed] [Google Scholar]
- 3.Slopack D, Roudier E, Liu ST, Nwadozi E, Birot O, Haas TL (2014) Forkhead BoxO transcription factors restrain exercise-induced angiogenesis. J Physiol 592 (18):4069–4082. doi: 10.1113/jphysiol.2014.275867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biniecka M, Canavan M, McGarry T, Gao W, McCormick J, Cregan S, Gallagher L, Smith T, Phelan JJ, Ryan J, O’Sullivan J, Ng CT, Veale DJ, Fearon U (2016) Dysregulated bioenergetics: a key regulator of joint inflammation. Ann Rheum Dis 75 (12):2192–2200. doi: 10.1136/annrheumdis-2015-208476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardelean DS, Yin M, Jerkic M, Peter M, Ngan B, Kerbel RS, Foster FS, Letarte M (2014) Anti-VEGF therapy reduces intestinal inflammation in Endoglin heterozygous mice subjected to experimental colitis. Angiogenesis 17 (3):641–659. doi: 10.1007/s10456-014-9421-x [DOI] [PubMed] [Google Scholar]
- 6.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5 (4):434–438. doi: 10.1038/7434 [DOI] [PubMed] [Google Scholar]
- 7.Gale NW, Yancopoulos GD (1999) Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 13 (9):1055–1066 [DOI] [PubMed] [Google Scholar]
- 8.Ferrara N (2000) VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol 11(6):617–624 [DOI] [PubMed] [Google Scholar]
- 9.Ausprunk DH, Folkman J (1977) Migration and proliferation of endothelial cells in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc Res 14 (1):53–65 [DOI] [PubMed] [Google Scholar]
- 10.Folkman J, Klagsbrun M (1987) Angiogenic factors. Science 235 (4787):442–447 [DOI] [PubMed] [Google Scholar]
- 11.Berg JM, Tymoczko JL, Gatto GJ, Stryer L (2015) Biochemistry. Eighth edition edn. W.H. Freeman & Company, a Macmillan Education Imprint, New York [Google Scholar]
- 12.Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK (1998) Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science 281(5373):64–71 [DOI] [PubMed] [Google Scholar]
- 13.Crivellone MD, Wu MA, Tzagoloff A (1988) Assembly of the mitochondrial membrane system. Analysis of structural mutants of the yeast coenzyme QH2-cytochrome c reductase complex. J Biol Chem 263 (28):14323–14333 [PubMed] [Google Scholar]
- 14.Hunte C, Palsdottir H, Trumpower BL (2003) Protonmotive pathways and mechanisms in the cytochrome bc1 complex. FEBS Lett 545 (1):39–46 [DOI] [PubMed] [Google Scholar]
- 15.Saraste M (1999) Oxidative phosphorylation at the fin de siecle. Science 283 (5407):1488–1493 [DOI] [PubMed] [Google Scholar]
- 16.Berry EA, Guergova-Kuras M, Huang LS, Crofts AR (2000) Structure and function of cytochrome bc complexes. Annu Rev Biochem 69:1005–1075. doi: 10.1146/annurev.biochem.69.1.1005 [DOI] [PubMed] [Google Scholar]
- 17.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS (2007) The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol 177 (6):1029–1036. doi: 10.1083/jcb.200609074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turrens JF, Alexandre A, Lehninger AL (1985) Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 237 (2):408–414 [DOI] [PubMed] [Google Scholar]
- 19.Rana M, de Coo I, Diaz F, Smeets H, Moraes CT (2000) An out-of-frame cytochrome b gene deletion from a patient with parkinsonism is associated with impaired complex III assembly and an increase in free radical production. Ann Neurol 48 (5):774–781 [PubMed] [Google Scholar]
- 20.Sun J, Trumpower BL (2003) Superoxide anion generation by the cytochrome bc1 complex. Arch Biochem Biophys 419 (2):198–206 [DOI] [PubMed] [Google Scholar]
- 21.Grivennikova VG, Vinogradov AD (2013) Mitochondrial production of reactive oxygen species. Biochemistry (Mosc) 78 (13):1490–1511. doi: 10.1134/S0006297913130087 [DOI] [PubMed] [Google Scholar]
- 22.Ushio-Fukai M (2007) VEGF signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal 9 (6):731–739. doi: 10.1089/ars.2007.1556 [DOI] [PubMed] [Google Scholar]
- 23.Babior BM (1999) NADPH oxidase: an update. Blood 93 (5):1464–1476 [PubMed] [Google Scholar]
- 24.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH (2007) Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 67 (22):10823–10830. doi: 10.1158/0008-5472.CAN-07-0783 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H, Hosokawa Y, Toda H, Nishikimi M, Ozawa T (1988) Cloning and sequencing of a cDNA for human mitochondrial ubiquinone-binding protein of complex III. Biochem Biophys Res Commun 156 (2):987–994 [DOI] [PubMed] [Google Scholar]
- 26.Jung HJ, Lee HB, Kim CJ, Rho JR, Shin J, Kwon HJ (2003) Anti-angiogenic activity of terpestacin, a bicyclo sesterterpene from Embellisia chlamydospora. J Antibiot (Tokyo) 56 (5):492–496 [DOI] [PubMed] [Google Scholar]
- 27.Jung HJ, Shim JS, Lee J, Song YM, Park KC, Choi SH, Kim ND, Yoon JH, Mungai PT, Schumacker PT, Kwon HJ (2010) Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J Biol Chem 285 (15):11584–11595. doi: 10.1074/jbc.M109.087809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho YS, Jung HJ, Seok SH, Payumo AY, Chen JK, Kwon HJ (2013) Functional inhibition of UQCRB suppresses angiogenesis in zebrafish. Biochem Biophys Res Commun 433 (4):396–400. doi: 10.1016/j.bbrc.2013.02.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HJ, Kim Y, Chang J, Kang SW, Kim JH, Kwon HJ (2013) Mitochondrial UQCRB regulates VEGFR2 signaling in endothelial cells. J Mol Med (Berl) 91(9):1117–1128. doi: 10.1007/s00109-013-1049-6 [DOI] [PubMed] [Google Scholar]
- 30.Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148 [DOI] [PubMed] [Google Scholar]
- 31.Liberman EA, Topaly VP, Tsofina LM, Jasaitis AA, Skulachev VP (1969) Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature 222 (5198):1076–1078 [DOI] [PubMed] [Google Scholar]
- 32.Trumpower BL (1990) The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem 265 (20):11409–11412 [PubMed] [Google Scholar]
- 33.Teshima Y, Takahashi N, Nishio S, Saito S, Kondo H, Fukui A, Aoki K, Yufu K, Nakagawa M, Saikawa T (2014) Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Circ J 78 (2):300–306 [DOI] [PubMed] [Google Scholar]
- 34.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95 (20):11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem 275 (33):25130–25138. doi: 10.1074/jbc.M001914200 [DOI] [PubMed] [Google Scholar]
- 36.Agani FH, Pichiule P, Chavez JC, LaManna JC (2000) The role of mitochondria in the regulation of hypoxia-inducible factor 1 expression during hypoxia. J Biol Chem 275 (46):35863–35867. doi: 10.1074/jbc.M005643200 [DOI] [PubMed] [Google Scholar]
- 37.Guzy RD, Schumacker PT (2006) Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol 91(5):807–819. doi: 10.1113/expphysiol.2006.033506 [DOI] [PubMed] [Google Scholar]
- 38.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT (1998) Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273 (29):18092–18098 [DOI] [PubMed] [Google Scholar]
- 39.Way pa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT (2002) Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res 91(8):719–726 [DOI] [PubMed] [Google Scholar]
- 40.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT (1998) Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273 (19):11619–11624 [DOI] [PubMed] [Google Scholar]
- 41.Wang GL, Jiang BH, Rue EA, Semenza GL (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 92 (12):5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399 (6733):271–275. doi: 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- 43.Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294 (5545):1337–1340. doi: 10.1126/science.1066373 [DOI] [PubMed] [Google Scholar]
- 44.Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107 (1):43–54 [DOI] [PubMed] [Google Scholar]
- 45.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr., (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292 (5516):464–468. doi: 10.1126/science.1059817 [DOI] [PubMed] [Google Scholar]
- 46.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292 (5516):468–472. doi: 10.1126/science.1059796 [DOI] [PubMed] [Google Scholar]
- 47.Wang GL, Semenza GL (1993) Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem 268 (29):21513–21518 [PubMed] [Google Scholar]
- 48.Levy AP, Levy NS, Wegner S, Goldberg MA (1995) Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem 270 (22):13333–13340 [DOI] [PubMed] [Google Scholar]
- 49.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16 (9):4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahluwalia A, Tarnawski AS (2012) Critical role of hypoxia sensor--HIF-1alpha in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr Med Chem 19 (1):90–97 [DOI] [PubMed] [Google Scholar]
- 51.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246 (4935):1306–1309 [DOI] [PubMed] [Google Scholar]
- 52.Levy AP, Tamargo R, Brem H, Nathans D (1989) An endothelial cell growth factor from the mouse neuroblastoma cell line NB41. Growth Factors 2 (1):9–19 [DOI] [PubMed] [Google Scholar]
- 53.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J (1989) Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84 (5):1470–1478. doi: 10.1172/JCI114322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumoto T, Claesson-Welsh L (2001) VEGF receptor signal transduction. Sci STKE 2001 (112):re21. doi: 10.1126/stke.2001.112.re21 [DOI] [PubMed] [Google Scholar]
- 55.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L (2006) VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7 (5):359–371. doi: 10.1038/nrm1911 [DOI] [PubMed] [Google Scholar]
- 56.Holmes K, Roberts OL, Thomas AM, Cross MJ (2007) Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell Signal 19 (10):2003–2012. doi: 10.1016/j.cellsig.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 57.Fong GH, Rossant J, Gertsenstein M, Breitman ML (1995) Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376 (6535):66–70. doi: 10.1038/376066a0 [DOI] [PubMed] [Google Scholar]
- 58.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC (1995) Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376 (6535):62–66. doi: 10.1038/376062a0 [DOI] [PubMed] [Google Scholar]
- 59.Parenti A, Morbidelli L, Cui XL, Douglas JG, Hood JD, Granger HJ, Ledda F, Ziche M (1998) Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem 273 (7):4220–4226 [DOI] [PubMed] [Google Scholar]
- 60.Thakker GD, Hajjar DP, Muller WA, Rosengart TK (1999) The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem 274 (15):10002–10007 [DOI] [PubMed] [Google Scholar]
- 61.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273 (46):30336–30343 [DOI] [PubMed] [Google Scholar]
- 62.Lee KH, Cho HJ, Kim HS, Lee WJ, Lee S, Bang D (2002) Activation of extracellular signal regulated kinase 1/2 in human dermal microvascular endothelial cells stimulated by anti-endothelial cell antibodies in sera of patients with Behcet’s disease. J Dermatol Sci 30 (1):63–72 [DOI] [PubMed] [Google Scholar]
- 63.Basu S, Totty NF, Irwin MS, Sudol M, Downward J (2003) Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14–3–3 and attenuation of p73-mediated apoptosis. Mol Cell 11(1):11–23 [DOI] [PubMed] [Google Scholar]
- 64.Barthwal MK, Sathyanarayana P, Kundu CN, Rana B, Pradeep A, Sharma C, Woodgett JR, Rana A (2003) Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival.J Biol Chem 278 (6):3897–3902. doi: 10.1074/jbc.M211598200 [DOI] [PubMed] [Google Scholar]
- 65.Sugishita Y, Leifer DW, Agani F, Watanabe M, Fisher SA (2004) Hypoxia-responsive signaling regulates the apoptosis-dependent remodeling of the embryonic avian cardiac outflow tract. Dev Biol 273 (2):285–296. doi: 10.1016/j.ydbio.2004.05.036 [DOI] [PubMed] [Google Scholar]
- 66.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL (2001) HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21(12):3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC (2005) Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115 (8):2119–2127. doi: 10.1172/JCI24726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC (2000) Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res 86 (8):892–896 [DOI] [PubMed] [Google Scholar]
- 69.Dimmeler S, Dernbach E, Zeiher AM (2000) Phosphorylation of the endothelial nitric oxide synthase at ser-1177 is required for VEGF-induced endothelial cell migration. FEBS Lett 477 (3):258–262 [DOI] [PubMed] [Google Scholar]
- 70.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275 (5302):964–967 [DOI] [PubMed] [Google Scholar]
- 71.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S (2001) Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med 193 (9):1005–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95 (3):952–958 [PubMed] [Google Scholar]
- 73.Klimova T, Chandel NS (2008) Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ 15 (4):660–666. doi: 10.1038/sj.cdd.4402307 [DOI] [PubMed] [Google Scholar]
- 74.Lee B, Kim KH, Jung HJ, Kwon HJ (2012) Matairesinol inhibits angiogenesis via suppression of mitochondrial reactive oxygen species. Biochem Biophys Res Commun 421 (1):76–80. doi: 10.1016/j.bbrc.2012.03.114 [DOI] [PubMed] [Google Scholar]
- 75.Li YN, Xi MM, Guo Y, Hai CX, Yang WL, Qin XJ (2014) NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1alpha stabilization by inhibiting prolyl hydroxylases activity. Toxicol Lett 224 (2):165–174. doi: 10.1016/j.toxlet.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 76.Brunelle JK, Bell EL, Quesada NM, Vercauteren K, Tiranti V, Zeviani M, Scarpulla RC, Chandel NS (2005) Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab 1(6):409–414. doi: 10.1016/j.cmet.2005.05.002 [DOI] [PubMed] [Google Scholar]