Abstract
Background:
In patients with ST elevation myocardial infarction (STEMI) and concomitant multi-vessel disease (MVD), primary percutaneous coronary intervention (PCI) of the culprit vessel is the preferred reperfusion strategy. However, optimum timing of revascularization for non-culprit artery is unclear. In this Bayesian network meta-analysis (NMA), we compared different PCI-based revascularization strategies in STEMI patients with MVD.
Methods:
11 randomized controlled trials (RCTs) were selected using MEDLINE, EMBASE and CENTRAL (Inception to September 2017). For all outcomes, median estimate of odds ratio from posterior distribution with corresponding 95% credible interval was calculated. The Surface under the Cumulative Ranking Curve (SUCRA) metric was used to estimate the relative ranking probability of each intervention. Sensitivity analysis was conducted by excluding the RCTs in which the staged intervention was performed after two weeks of the index procedure or post discharge.
Results:
In this NMA of 3172 patients, CR-I (instant complete revascularization) was associated with 40% relative risk reduction in all-cause mortality compared with IRA (infarct related artery) [0.60 (0.31–0.89)]. CR-I was superior to CR-S (staged complete revascularization) [0.42 (0.22–0.70)] and IRA [0.50(0.29–0.72)] in reducing the risk of re- infarction. Both CR-I and CR-S significantly reduced the risk of repeat revascularization compared to IRA, whereas the risk of CIN (contrast induced nephropathy) and major bleeding was similar across all interventions. Sensitivity analysis showed, that CR-I was a better strategy compared with CR-S [0.34 (0.12–0.74) ] and IRA (0.60 [0.36–0.97]) in reducing all-cause mortality.
Conclusions:
In this NMA, CR-I was associated with reduction in all-cause mortality and re- infarction compared with IRA.
Keywords: STEMI, Multi-vessel disease, Revascularization
1. Introduction
Approximately 40%−50% of patients with ST-elevation myocardial infarction (STEMI) have been reported to have concomitant multi-vessel disease (MVD), involving at least one additional stenosis in the non-culprit vessel [1,2]. This portends worse prognosis than does single-vessel disease. However, there is uncertainty regarding the appropriate management of non-culprit vessels in such patients. Until recently, clinical practice guidelines recommended against complete revascularization in STEMI patients with MVD, who were otherwise hemodynamically stable unless electrocardiogram localization of the infarction was unclear [3]. A lack of benefit in cardiovascular outcomes with instant complete revascularization was attributed to a higher incidence of complications such as increased risk of major bleeding, CIN requiring renal replacement therapy, stroke, stent thrombosis and fluid overload in the setting of acute STEMI [4–8]. However, most of the earlier trials and meta- analysis were not powered enough to evaluate the effect of complete revascularization on estimates such as all-cause mortality. Moreover, the optimal timing of complete revascularization during the index procedure (CR-1), or as a staged procedure (CR-S) a few weeks later, and its impact on mortality also remains uncertain.
Recently, several randomized controlled trials (RCTs) have shown that complete revascularization (CR) is at least equivalent and likely superior to isolated culprit vessel revascularization in reducing major adverse cardiovascular events (MACE) in patients with STEMI and MVD [9–13]. To update the evidence, we performed a Bayesian network meta-analysis (NMA) to compare the effect of three different revascularization strategies: infarct related artery only revascularization (IRA), complete revascularization during index procedure (CR-I) and staged complete revascularization (CR-S) in patients with stable STEMI and MVD.
2. Methods
The data, analytic methods, and study materials have not been made available to other researchers for purposes of reproducing the results or replicating the procedure.
This NMA was conducted according to the Cochrane Collaboration recommendations [14], Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Protocols (PRISMA) report, and PRISMA extension statement for network meta-analyses [15].
2.1. Inclusion criteria
Eligible studies for this NMA were RCTs that included hemodynamically stable patients with STEMI and MVD undergoing percutaneous coronary intervention (PCI), and compared at least two of the following three revascularization strategies: IRA group (revascularization of only the infarct-related artery determined by the operator), CR-I (complete revascularization of all arteries with significant stenosis by visual estimate or by FFR during the index procedure), and CR-S (a combination approach that include revascularization of the infarct-related artery during the index procedure followed by revascularization of non-culprit arteries as a staged procedure). The staged procedure could have been performed later during the index hospitalization or following discharge. The included studies had to report at least one event in the outcomes of interest (see later text) in adults. There were no restrictions on follow-up duration, co morbidities or sample size. Patients undergoing primary PCI in the setting of cardiogenic shock, chronic total occlusion or left main disease were excluded from the study.
2.2. Study search
We searched PubMed/MEDLINE via OVID (1946 to September 2017), EMBASE via OVID (1980 to September 2017), and Cochrane Central Register of Controlled Trials (inception to September 2017). The search was performed using a combination of the following words and medical subject heading: “percutaneous coronary intervention,” “PCI,” “intervention,” “myocardial infarction,” “STEMI,” “revascularization,” “culprit lesion,” “multivessel,” “multivessel PCI,” “staged PCI,” “complete revascularization,” “infarct artery intervention,” and “randomized controlled trials.” All citations were downloaded into Zotero (Roy Rosen Zweig Center for History and New Media, Research Software, Virginia, USA), and duplicates were manually identified and eliminated by this software. The electronic search was supplemented with a manual review of the references cited in the shortlisted articles. The search was restricted to English language, full-text articles, human participants, and RCTs.
2.3. Data abstraction and quality assessment
Data abstraction on baseline characteristics, events, sample size and follow up duration of each trial was performed by two authors independently (UF and OA) using a structured data collection form. We extracted all the events at longest follow up duration. When possible, data on intention to treat analysis was abstracted. Similar to another NMA, we placed RCTs in the specific revascularization arm based on predominant revascularization strategy if studies had not clearly reported the results according to CR-I or CR-S groups [16]. For instance, in Hamza et al and Compare-Acute, outcomes were reported for CR; however, in both the studies, most of the participants in the CR group underwent CR-I. In Compare-acute, 83.4% of the patients from CR group underwent CR-I, and in Hamza et al, 58% of the patients had CRI from CR group [12,13]. On the same note, in the CvLPRIT trial [10], around 64% of the patients from the CR group underwent CR-I. These studies were included in the CR-I arm based on the pre-dominant revascularization strategy. We also reviewed appendices of the included trials for additional information. Cochrane bias risk assessment tool was used to assess the risk of bias of the included studies (Supplementary Appendix, Table 1)
2.4. Outcomes measures
The primary outcome was all-cause mortality. Secondary outcomes were re-infarction, repeat revascularization [urgent and non-urgent PCI and unplanned coronary artery bypass grafting (CABG)], contrast-induced nephropathy (CIN), and major bleeding events. The definitions of all outcomes were taken as reported in the included trials.
2.5. Statistical analysis
The Bayesian NMA, an extension of a traditional meta-analysis, was conducted using NetMetaXL 1.6.1 (Canadian Agency for Drugs and Technologies in Health, Ottawa, Canada) and WinBUGS 1.4.3 (MRC Biostatistics Unit, Cambridge, UK). The Bayesian method is a sophisticated statistical approach that allows pooling of data related to multiple interventions simultaneously, combining direct and indirect components of the evidence in a single estimate, and enables the comparison of the interventions without a direct connection on the basis of indirect information [17]. Out comes were defined using random effects model. For random effects vague priors, we assumed use the following priors: sd ~ dunif (0,2); where dunif is the density function of the uniform distribution, sd is the vector of standard deviations, and 0 and 2 describe minimum and maximum vector of quantiles, respectively. For informative variance prior, all-cause mortality informative priors were selected based on non-pharmacological intervention with objective outcomes. NetMetaXL uses these selections and bases the informative variance priors on evidence on the extent of heterogeneity noticed in prior meta-analyses, as reported in Turner et al. [18] For all analyses, we assumed vague priors on baseline [dnorm (0,10000)] and basic parameters [dnorm (0,10000)], where function “dnorm” return the value of the probability density function for the normal distribution based on given parameters. Since informative priors, when used properly, can improve modeling efficiency by providing solutions to computational issues, we ultimately applied predictive distributions (informative variance priors) to random effects analyses [18,19]. For all the outcomes, we achieved convergence at 20,000 iterations and autocorrelation was checked and confirmed. The inconsistency was assessed by comparing the deviance residuals and D1C statistics in fitted consistency and inconsistency models [20]. The assessment of between-study heterogeneity variances was interpreted as low (τ = 0.04), moderate (τ = 0.14), and high (τ = 0.40).
Estimates were reported as median estimate of odds ratio from the posterior distribution and reported it with 2.5th to the 97.5th centiles of the distribution (95% credible interval). Markov chain Monte Carlo (MCMC) modeling was used to estimate the relative ranking probability of each intervention [21]. “Rankograms” with surface under the cumulative ranking curve (SUCRA) were reported to provide a comparative hierarchy of efficacy of the interventions [22]. SUCRA is a numerical representation of the probability of effectiveness; briefly, a SUCRA of 90% indicates that the treatment of interest has achieved 90% effectiveness or the safety of that treatment relative to other groups. In all RCTs, the timing of staged intervention was during the index hospitalization or within the first two weeks, except in PRAGUE-13 [23] where staged intervention was planned between day 3–40, in PRIMA [24] around day 27 and in Politi et al [25] staged procedure was performed around day 57. This prompted a sensitivity analysis on major endpoints (all-cause mortality, repeat revascularization, re-infarction and CIN) by excluding these three studies. Publication bias was assessed using Egger’s regression test.
3. Results
The initial search yielded 2419 articles. After excluding studies based on priori inclusion criteria, ultimately 11 RCTs with 3,172 patients were selected in this NMA (Fig. 1). The baseline characteristics of the patients in each study are summarized in Table 1 and the inclusion and exclusion criteria including the endpoint definitions of the included trials are listed in Table 2. In the included studies, the timing of staged intervention was from 72 hours up to 57 days in the CR-S group. The determination of significant stenosis in the non-infarct artery was made by visual estimate during angiography in most of the studies; whereas, FFR was used in others.
Fig. 1.
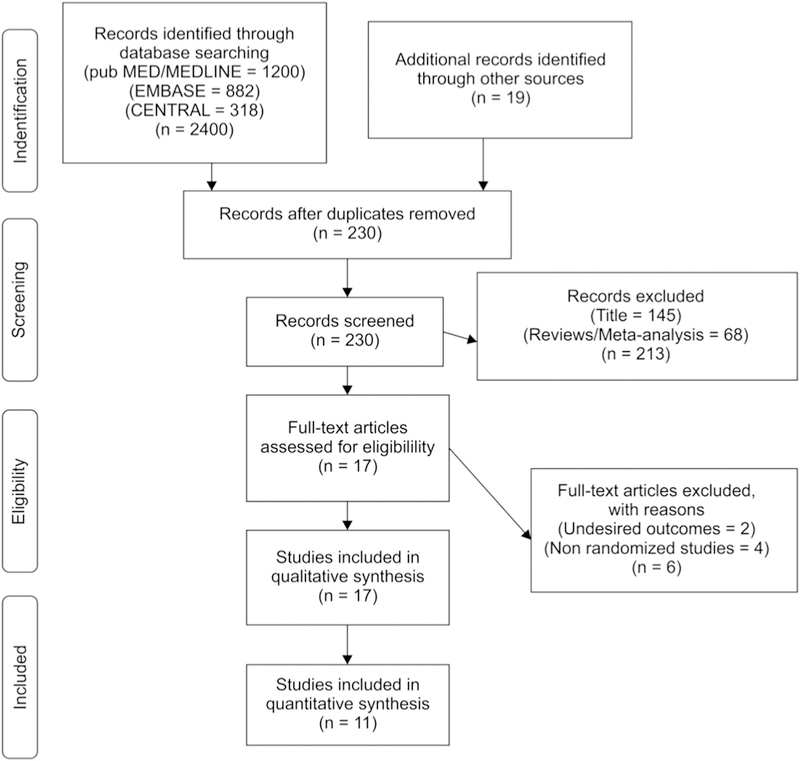
PRISMA flow diagram. Study selection flow diagram, demonstrating search methodology for identification of the eligible studies for the meta-analysis. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analysis.
Table 1.
Baseline patient characteristics.
| Studies | Arms | N = 3172 | Mean age (year) | Men (%) | HTN (%) | DM (%) | Smoking (%) | Prior MI (%) | Follow up (months) |
|---|---|---|---|---|---|---|---|---|---|
| DANAMI-3-PRIMULTI [11] | CR-S | 314 | 64 | 80 | 41 | 9 | 51 | 5 | 27 |
| IRA | 313 | 63 | 81 | 47 | 13 | 48 | 9 | ||
| POLITI et al. [25] | IRA | 84 | 67 | 76 | 60 | 24 | N/A | N/A | 29 |
| CR-S | 65 | 64 | 80 | 65 | 19 | N/A | N/A | ||
| CR-I | 65 | 65 | 77 | 49 | 14 | N/A | N/A | ||
| CvLPRIT [10] | IRA | 146 | 65 | 77 | 36 | 14 | 27 | 3.6 | 12 |
| CR (CR-I 64.6%; CR-S 35.4%) | 150 | 65 | 85 | 37 | 13 | 34 | 4.8 | ||
| PRAMI [9] | CR -I | 234 | 62 | 76 | 40 | 15 | 50 | 8 | 23 |
| IRA | 231 | 62 | 81 | 40 | 21 | 45 | 7 | ||
| HAMZA et al.[12] | IRA | 50 | 52 | 86 | 36 | 100 | 78 | 6 | 6 |
| CR (58% CR-I;42% CR-S) | 50 | 56 | 82 | 26 | 100 | 72 | 10 | ||
| PRAGUE-13 [23] | CR-S | 106 | NA | NA | NA | NA | NA | NA | 38 |
| IRA | 108 | NA | NA | NA | NA | NA | NA | ||
| HELP-AMI [44] | IRA | 17 | 65 | 85 | 59 | 41 | 81 | 12 | |
| CR-I | 52 | 64 | 88 | 37 | 12 | 67 | |||
| GHANI et al. [43] | CR-S | 80 | 62 | 80 | 26 | 6 | 44 | 6 | 36 |
| IRA | 41 | 61 | 81 | 43 | 5 | 48 | 5 | ||
| TARASOV et al. [42] | CR-I | 46 | 59 | 70 | 96 | 26 | 0 | 11 | 6 |
| CR-S | 43 | 59 | 58 | 86 | 21 | 0 | 5 | ||
| PRIMA [24] | CR-I | 48 | 65 | 73 | 52 | 31 | 38 | 29 | 6 |
| CR-S | 44 | 67 | 75 | 48 | 34 | 43 | 23 | ||
| COMPARE ACUTE [13] | CR (83.4% CR-I;16.6%CR-S) | 295 | 62 | 79 | 46 | 15 | 41 | 8 | 12 |
| IRA | 590 | 61 | 76 | 48 | 16 | 49 | 8 | ||
CR-S = complete revascularization staged, CR-I = complete revascularization instant, IRA = infarctrelated artery, CR = complete revascularization, N/A = not available. HTN = hypertension, DM = diabetes mellitus, MI = myocardial infarction.
Table 2.
Inclusion and Exclusion criteria and endpoints of the included trials.
| Study | Year | Inclusion criteria | Exclusion criteria |
Primary endpoint |
Secondary endpoints |
|---|---|---|---|---|---|
| Compare-Acute [13] | 2017 | Patients with STEMI and MVD (>50% stenosis of the N-IRA) | LM disease, CTO, severe valve disease, Killip class III or IV, severe stenosis and complications after IRA treatment. | MACCE (all-cause mortality, nonfatal MI, any revascularization, cerebrovascular events) | NACE (cardiac death, MI, any revascularization, CVA, major bleeding). |
| Hamza et al. [12] | 2016 | Diabetic patients with STEMI and MVD (at least 80% stenosis in N-IRA) | Prior CABG, LM disease and CTO. | Composite of all- cause mortality, recurrent MI, ischemia driven revascularization with PCI or CABG. | Individual component of primary end point, major bleeding and CIN |
| DANAMI 3- PRIMULTI [11] | 2015 | Patients with STEMI, MVD (>50% stenosis in the coronary artery) | Intolerance to contrast, cardiogenic shock, increase bleeding risk, stent thrombosis, indication for CABG | Composite of all- cause mortality, reinfarction, or ischemia driven revascularization of non-IRA. | Components of primary end-point, cardiac death, urgent and non urgent PCI of non-IRA |
| CvLPRIT [10] | 2015 | STEMI or LBBB with MVD (at least one N-IRAwith >70% lesion in one plane or >50% in 2 plane) | Previous Q-wave MI, prior CABG, cardiogenic shock, VSD or severe MR, CKD, thrombosis of previous stent, CTO | MACE (all-cause mortality, MI,HF, ischemia driven PCI OR CABG). | Components of primary endpoints, CV death, stroke, major bleeding, CIN |
| PRAGUE-13 (23) |
2015 | STEMI with successful PCI or IRA, at least 1 stenosis ofN-IRA > 70% with diameter > 2.5 mm, enrolment > 48h following onset of symptoms | Stenosis ofLM, hemodynamically significant valve disease, angina > 1 month prior to STEMI and cardiogenic shock. | All cause mortality, non- fatal MI and stroke. | Hospitalization for unstable angina, revascularization of non-infarct artery, CV mortality, hospitalization for HF, non-fatal MI and all-cause mortality |
| Tarasov et al. [42] | 2014 | STEMI with MVD (>70% stenosis ofat least two or more coronary arteries), target lesion amenable to PCI and target lesion located in native artery | Single lesions, acute heart failure Killip class III-IV, LM stenosis, small vessel <2.5 mm, thrombosis of prior stent | MACE (cardiac or non-cardiac death, re-infarction, repeat coronary revascularization. | Individual components of primary endpoints |
| PRAMI [9] | 2013 | STEMI and MVD (one or more >50% stenosis of the N-IRA) | Cardiogenic shock, prior CABG, LM disease or disease at ostia ofboth circumflex and LAD and CTO. | Composite of death from cardiac cause, nonfatal MI, refractory angina. | Death from non-cardiac cause, repeat revascularization (PC I and CABG) |
| Ghani et al. [43] | 2012 | STEMI with MVD (one or more stenosis in at least 2 major epicardial arteries or the combination of side branch and a main epicardial vessel provided that they supplied different territory | Patients with indication for urgent revascularization, >80 years old, prior CABG, CTO, LM disease, chronic A fib, limited life expectancy. | Ejection fraction at 6 months. | MACE (death, non-fatal re-infarction and additional revascularization). |
| Politi et al. [25] | 2010 | MVD (>70% stenosis of 2 or more epicardial coronary artery or major branches by visual estimate), ST elevation on EKG | Cardiogenic shock, LM disease, CABG, severe valve disease | MACE (CV and Non-CV death, in hospital death, re-infarction, re-hospitalization for ACS and repeat coronary revascularization | Length ofhospitalization and CIN |
| PRIMA [24] | 2004 | STEMI with successful PCI of IRA and at least 1 significant >70% stenosis of the coronary artery other than IRA | LM disease, cardiogenic shock, target lesion in N-IRA not suitable for PCI. | Absolute improvement in the LVEF, recovery time and magnitude of EF increase were assessed. | Safety of single stage PCI. All cause mortality, MI, urgent revascularization, bleeding, unstable angina and CV hospitalization. |
| HELP- AMI [44] | 2004 | STEMI with MVD (IRA and one or more lesion in N-IRA) | Lesion in vein graft, arterial conduits or in segments treated with angioplasty or stent implantation, recent thrombolysis, cardiogenic shock, single vessel disease, LM disease, CTO and side branch >2 mm which required to be covered by the stent. | Rate of repeat revascularization over a period of12 months. | Adverse in hospital events, procedural-cost |
STEMI = ST elevation myocardial infarction, MVD = multi vessel disease, CTO = chronic total occlusion, LM = left main, N-IRA = non infarct related artery, IRA = infarct related artery, MI = myocardial infarction, CABG = coronary artery bypass grafting, CIN = contrast induced nephropathy, CVA = cerebrovascular accident, PCI = percutaneous coronary intervention, VSD = ventricular septal defect, MR = mitral regurgitation, ACS = acute coronary syndrome, HF = heart failure, MACCE = major adverse cardiovascular cerebrovascular events, NACE = net adverse clinical events.
In this NMA, CR-I was associated with 40% relative risk reduction in all-cause mortality compared to IRA [0.60 (0.31–0.89)]. There was 35% decrease in all-cause mortality with CR-I compared to CR-S (0.65 [0.22–1.26)], which did not reach statistical significance (Fig. 2).
Fig. 2.
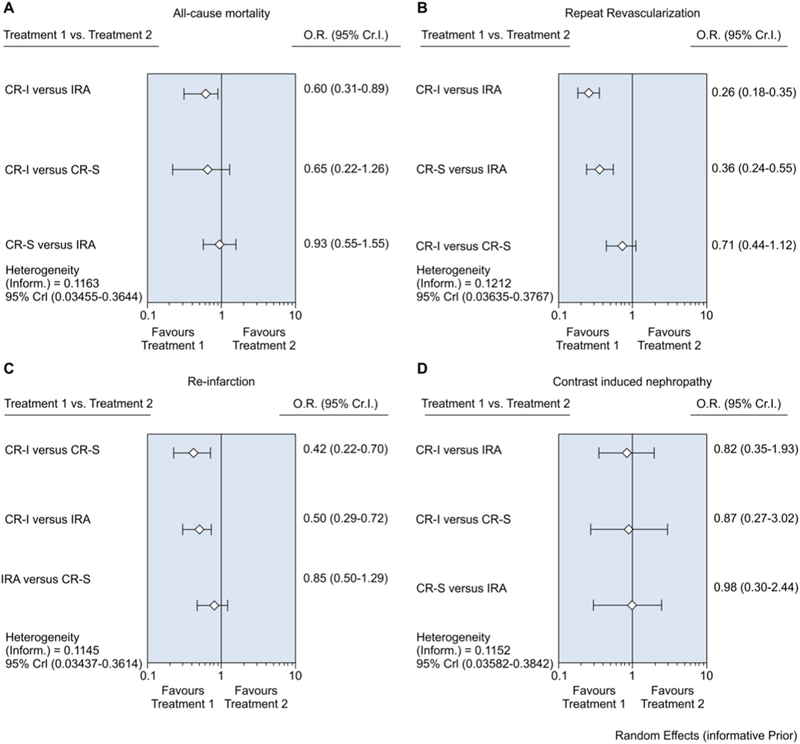
Forest plot for the network meta-analysis comparing infarct-related artery (IRA) revascularization, staged complete revascularization (CR-S), and immediate complete revascularization (CR-I) for all- cause mortality, myocardial infarction (MI), repeat revascularization and contrast induced nephropathy (CIN). The horizontal lines indicate odds ratio with 95% credible interval.
There was no significant difference between CR-S and IRA [0.93 (0.55–1.55)] with regards to all-cause mortality. CR-I was superior to CR-S [0.42 (0.22–0.70)] and IRA [0.50(0.29–0.72)] in reducing the risk of re-infarction. Both CR-I [0.26 (0.18–0.35)] and CR-S [0.36 (0.24–0.55)] significantly reduced the risk of repeat revascularization (urgent and non-urgent PCI and unplanned coronary artery bypass grafting) compared to IRA. The risk of CIN and major bleeding was similar across all the interventions.
In the CR-S group, the timing of staged intervention was heterogeneous across the included trials. Sensitivity analysis was performed after excluding the studies where staged intervention was performed after 2 weeks of the index event or post discharge. The result of sensitivity analysis showed that CR-I was associated with 40% relative risk reduction in all-cause mortality compared with IRA [0.60 (0.36–0.97)] and 66% reduction in mortality when compared with CR-S (0.34 [0.12–0.74]), that was statistically significant. There was no difference in mortality between IRA and CR-S [0.57(0.21–1.19)]. CR-I was associated with reduction in risk of re-infarction when compared with CR-S [0.34(0.15–0.89)] and IRA (0.47 [0.27–0.86]). CR-I was associated with 73% less risk of repeat revascularization compared with IRA, and CR-S was associated with 61% risk reduction in repeat revascularization compared with IRA [0.39(0.22–0.72)] (Fig. 4).
Fig. 4.
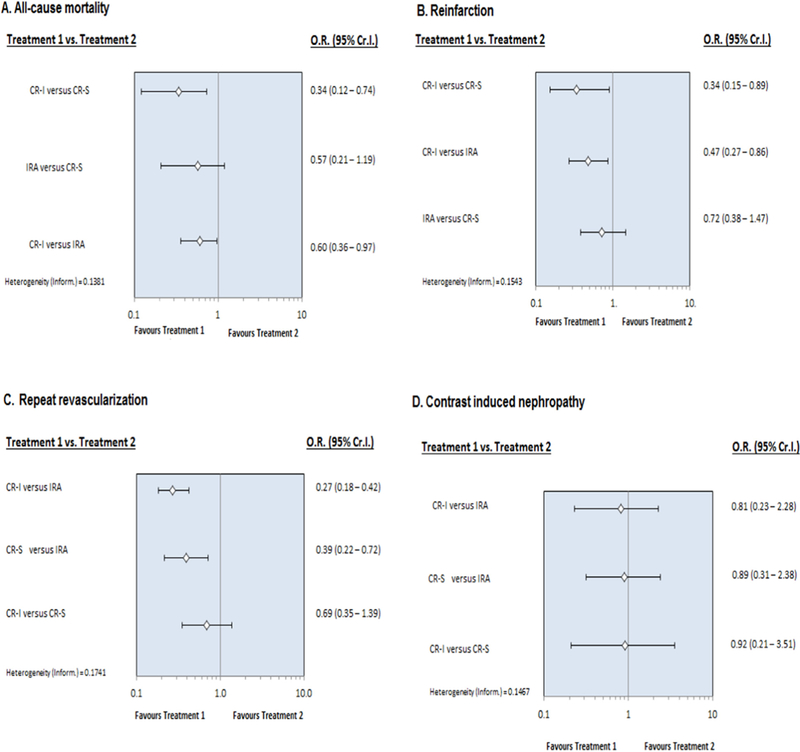
Forest plot for the sensitivity analysis (after excluding the studies where staged intervention was performed >2 weeks after the index procedure or post discharge) comparing infarct-related artery (IRA) revascularization, staged complete revascularization (CR-S), and immediate complete revascularization (CR-I) for all- cause mortality, myocardial infarction (MI), repeat revascularization and contrast induced nephropathy (CIN). The horizontal lines indicate odds ratio with 95% credible interval.
Egger’s regression test could not detect publication bias [(ICR versus SCR: P = 0.92) (SCR versus IRA: P = 0.37) (ICR versus IRA: P = 0.11)].
Probability analysis ranked CR-I as the best intervention for having the lowest risk of all-cause mortality (SUCRA, 94%), re-infarction (SUCRA, 100%), repeat revascularization (SUCRA, 96%) and CIN (SUCRA, 61%). CR-S was ranked as the best intervention to reduce the risk of major bleeding (SUCRA, 92%) (Fig. 3). These findings were consistent in the sensitivity analysis that again ranked CR-I as the most favorable strategy to decrease all-cause mortality, re-infarction, repeat revascularization and CIN (Fig. 5).
Fig. 3.
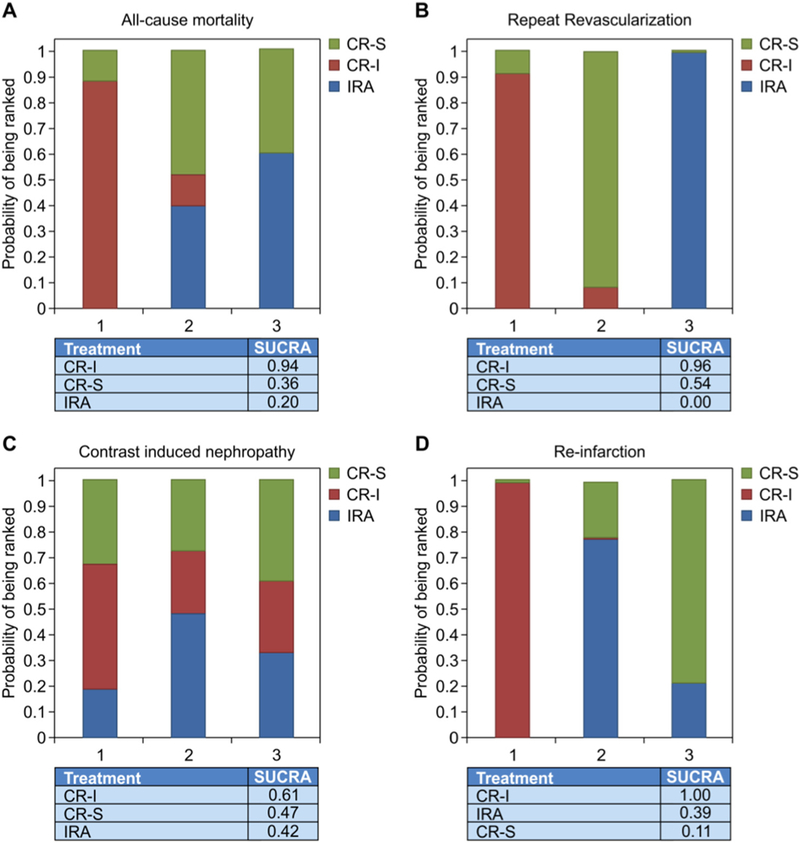
Rankograms for the network meta-analysis comparing infarct-related artery (IRA) revascularization, staged complete revascularization (CR-S), and immediate complete revascularization (CR-I) for all-cause mortality, myocardial infarction (MI), repeat revascularization and contrast induced nephropathy (CIN).
Fig. 5.
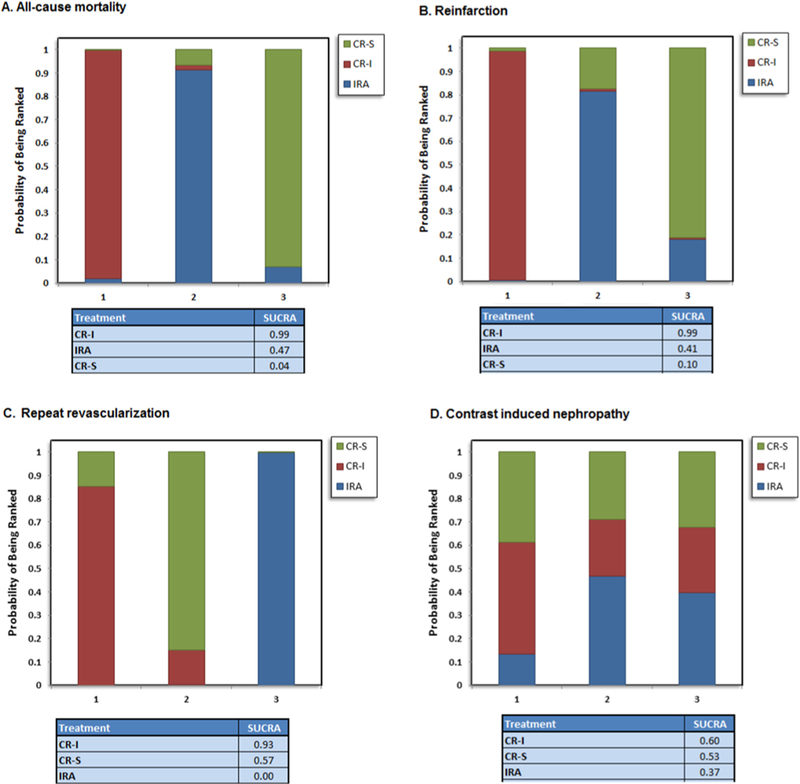
Rankograms for the sensitivity analysis (after excluding the studies where staged intervention was performed >2 weeks) comparing infarct-related artery (IRA) revascularization, staged complete revascularization (CR-S), and immediate complete revascularization (CR-I) for all-cause mortality, myocardial infarction (MI), repeat revascularization and contrast induced nephropathy (CIN).
4. Discussion
In this NMA of 11 RCTs including 3,172 patients with STEMI and MVD, CR-I was associated with significant risk reduction in all-cause mortality compared with IRA. On initial analysis, decrease in all-cause mortality was observed with CR-I when compared with CR-S, however it did not reach statistical significance. Sensitivity analysis was then performed after excluding the studies where staged intervention occurred after 2 weeks of the index event or post discharge, and it showed significant decrease in all-cause mortality with CR-I compared to CR-S and IRA. No difference in mortality was observed between CR-S and IRA A recent NMA by Bangalore et al [16], (11 trials, 3,150 patients) reported that single staged multi-vessel PCI in patients with STEMI was associated with reduction in mortality and myocardial infarction, however no difference in mortality was observed between CR-S and IRA. The mortality benefit seen with single staged multi-vessel PCI in this study is consistent with our findings. These findings demonstrate the benefit of CR-I in decreasing all-cause mortality compared to CR-S and IRA.
Prior meta-analyses have shown significant risk reduction in MACE with complete revascularization, that was primarily driven by decrease in repeat revascularization. However, statistically significant decrease in mortality with complete revascularization, particularly CR-I was not demonstrated [26–32]. This was most likely because most of these studies were either published prior to the new emerging randomized trials or had insufficient sample size, thus limiting the statistical power to determine the estimates. Additionally, most of these analysis included observational studies. A meta-analysis by Elgendy IY [32] and colleagues (10 trials, 2,285 patients) including RCTs only, showed a trend towards decrease in all-cause mortality with CR when compared to IRA intervention however it could not reach statistical significance.
Our study is one of the largest meta-analysis of randomized trials of PCI based revascularization approaches for patients with STEMI and MVD. In our meta-analysis, in addition to the earlier published studies we have incorporated data from the recently published CompareAcute trial that demonstrated reduction in MACE with fractional flow reserve (FFR)-guided complete revascularization compared to selective IRA intervention [13].
With the new emerging data from the recent RCTs, ACC/AHA/SCAI have modified the guidelines for multi-vessel PCI in hemodynamically stable patients with STEMI and MVD from class III to IIb, indicating that PCI of the non-culprit vessels either at the time of the index procedure or as a staged intervention may be considered in selected patients [33]. Also, the updated European Guidelines for STEMI have added a class IIa recommendation for staged PCI of non-culprit lesions in patients with STEMI and MVD prior to the hospital discharge [34]. Our findings and the data from the 2 most recent large meta-analysis [16,35] highlight the benefit of CR-I compared to other revascularization strategies which is a novel finding. Furthermore, with the use of new generation of drug eluting stents, better antiplatelet agents and advances in the PCI based revascularization techniques, the incidence of post procedure complications has significantly reduced [36–41]; making CR-I an effective strategy in acute setting. This novel mortality benefit warrants further validation with RCTs which may impact the current guidelines.
The strength of this meta-analysis lies in the large sample size, inclusion of high-quality RCTs, and application of sophisticated statistical approach. Furthermore, the primary end-point of interest was all-cause mortality which was consistent across the included trials. However, the present findings of decrease in all-cause mortality with CR-I compared to IRA should be interpreted considering certain limitations. First, this study is limited by heterogeneity of the study participants, procedural techniques, variable endpoint definitions, timing of staged intervention and follow-up duration. Second, for few trials that reported outcomes for complete revascularization; these studies were assigned according to the predominant strategy, however each endpoint was measured at the longest available follow-up for each trial. Third, our results demonstrate similar incidence of CIN and bleeding across all groups, however limited number of trials were available that had reported these outcomes.
5. Conclusions
In this NMA of STEMI patients with MVD, we found that a strategy of complete revascularization during the index procedure was superior to a culprit only revascularization in reducing all-cause mortality. Our findings contend that CR-I should be considered as the preferred revascularization strategy in STEMI patients with MVD who are otherwise hemodynamically stable. Further, well-designed RCTs can validate this impression.
Supplementary Material
Acknowledgments
We thank Editage (www.editage.com), for formatting the manuscript.
Sources of Funding
None.
Footnotes
Disclosures
None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.carrev.2018.08.018.
References
- [1].Ibrahim H, Sharma PK, Cohen DJ, Fonarow GC, Kaltenbach LA, Effron MB, et al. Multivessel versus culprit vessel-only percutaneous coronary intervention among patients with acute myocardial infarction: insights from the TRANSLATE-ACS observational study. J Am Heart Assoc 2017;6:1e006343 10.1161/JAHA.117.006343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel diseaseon reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. Eur Heart J 2007;28:1709–16. [DOI] [PubMed] [Google Scholar]
- [3].O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. ACCF/ AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol 2013;2013(61):e78–140. 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- [4].Iqbal MB, Ilsley C, Kabir T, Smith R, Lane R, Mason M, et al. Culprit vessel versus multivessel intervention at the time of primary percutaneous coronary intervention in patients with ST-segment-elevation myocardial infarction and multivessel disease: real-world analysis of 3984 patients in London. Circ Cardiovasc Qual Outcomes 2014;7:936–43. 10.1161/CIRCOUTCOMES.114.001194. [DOI] [PubMed] [Google Scholar]
- [5].Abe D, Sato A, Hoshi T, Takeyasu N, Misaki M, Hayashi M, et al. Initial culprit-only versus initial multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: results from the Ibaraki cardiovascular assessment study registry. Heart Vessels 2014;29:171–7. 10.1007/s00380-013-0342-1. [DOI] [PubMed] [Google Scholar]
- [6].Ornowski R, Mehran R, Dangas G, Nikolsky E, Assali A, Claessen BE, et al. Prognostic impact of staged versus “one -time” multi - vessel percutaneous intervention in acute myocardial infarction: analysis from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol 2011;58:704–11. 10.1016/joacc.2011.02.071. [DOI] [PubMed] [Google Scholar]
- [7].Hannan EL, Samadashvili Z, Walford G, Holmes DR Jr, Jacobs AK, Stamato NJ, et al. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patient with multivessel disease. J Am Coll Cardiol Intv 2010;3:22–31. 10.1016/j.jcin.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [8].Assali AR, Brosh D, Ben-Dor I, Solodky A, Fuchs S, Teplitsky I, et al. The impactof renal insufficiency on patients’ outcomes in emergent angioplasty for acute myocardial infarction. Catheter Cardiovasc Interv 2007;15:395–400. 10.1002/ccd.20939. [DOI] [PubMed] [Google Scholar]
- [9].Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO, et al. Randomized trial of preventive angioplasty in myocardial infarction. N Engl J Med 2013;369: 1115–23. 10.1056/NEJMoa1305520. [DOI] [PubMed] [Google Scholar]
- [10].Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol 2015;65:963–72. 10.1016/jjacc.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Engstrøm T, Kelbsk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, et al. Complete revascularization versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomized controlled trial. Lancet 2015;386:665–71. [DOI] [PubMed] [Google Scholar]
- [12].Hamza M, Mahmoud AN, Elgendy IY. A randomized trial of complete versus culprit-only revascularization during primary percutaneous coronary intervention in diabetic patientswith acute ST elevation myocardial infarction and multi vessel disease. J Interv Cardiol 2016;29:241–7. 10.1111/joic.12293. [DOI] [PubMed] [Google Scholar]
- [13].Smits PC, Abdel-Wahab M, Neumann F-J, Boxma-de Klerk BM, Lunde K, Schotborgh CE, et al. Compare-acute investigators. Fractional flow reserve-guided multivessel angioplasty in myocardial infarction. N Engl J Med 2017;376:1234–44. 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- [14].Cochrane handbook for systematic reviews of interventions version 5.1.0: updated March 2011.
- [15].Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;349 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- [16].Bangalore S, Toklu B, Stone G. Meta-analysis of culprit-only versus multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction and multivessel coronary disease. Am J Cardiol 2018;121(5):529–36. [DOI] [PubMed] [Google Scholar]
- [17].Ades AE, Sculpher M, Sutton A, Abrams K, Cooper N, Welton N, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1–19. [DOI] [PubMed] [Google Scholar]
- [18].Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta analysis, using empirical data from the Cochrane database of systematic reviews. Int J Epidemiol 2012;41:818–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thorlund K, Thabane L, Mills Ej. Modelling heterogeneity variances in multiple treatment comparison meta-analysis - are informative priors the better solution? BMC Med Res Methodol 2013;13 (2-). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Mak 2013;33:641–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Larjo A, Lähdesmäki H. Using multi-step proposal distribution for improved mcmc convergence in Bayesian network structure learning. EURASIP J Bioinform Syst Biol 2015;6 10.1186/s13637-015-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- [23].Hlinomaz O Multivessel coronary disease diagnosed at the time of primary PCI for STEMI: complete revascularization versus conservative strategy (PRAGUE-13 Trial) Paper presented at EuroPCR ; 2015 (May 19, 2015; Paris,France). [Google Scholar]
- [24].Ochala A, Smolka GA, Wojakowski W, Dudek D, Dziewierz A, Krolikowski Z, et al. The function of the left ventricle after complete multivessel one-stage percutaneous coronary intervention in patients with acute myocardial infarction. J Invasive Cardiol 2004;16:699–702. [PubMed] [Google Scholar]
- [25].Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Leuzzi C, et al. A randomised trial of target-vessel versus multi-vessel revascularisation in ST-elevation myocardial infarction: major adverse cardiac events during long-term follow-up. Heart 2010;96: 662–7. 10.1136/hrt.2009.177162. [DOI] [PubMed] [Google Scholar]
- [26].Moreno R, Mehta SR. Nonculprit vessel intervention: let’s COMPLETE the evidence. Rev Esp Cardiol 2017;70:418–20. 10.1016/j.rec.2016.12.029. [DOI] [PubMed] [Google Scholar]
- [27].Bangalore S, Toklu B, Wetterslev J Complete versus culprit-only revascularization for ST-segment elevation myocardial infarction and multivessel disease: a metaanalysis and trial sequential analysis of randomized trials. Circ Cardiovasc Interv 2015;8:e002142 10.1161/CIRCINTERVENTIONS.114.002142. [DOI] [PubMed] [Google Scholar]
- [28].Bangalore S, Kumar S, Poddar KL, Ramasamy S, Rha S-W, Faxon DP. Meta-analysis of multivessel coronary artery revascularization versus culprit-only revascularization in patients with ST-segment elevation myocardial infarction and multivessel disease. Am J Cardiol May 1 2011;107(9):1300–10. [DOI] [PubMed] [Google Scholar]
- [29].Bainey KR, Mehta SR, Lai T, Welsh RC. Complete vs culprit-only revascularization for patients with multivessel disease undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: a systematic review and meta-analysis. Am HeartJ 2014. January;167(1):1–14.e2. [DOI] [PubMed] [Google Scholar]
- [30].Wang C-H, Zhang S- Y, Jin X- F. Complete revascularization versus culprit-only revascularization in ST-segment elevation myocardial infarction and multivessel disease patients undergoing primary percutaneous coronary intervention: a meta-analysis and trial sequential analysis. Int J Cardiol 2017;228:844–52. [DOI] [PubMed] [Google Scholar]
- [31].Gaffar R, Habib B, Filion KB, Reynier P, Eisenberg MJ. Optimal timing of complete revascularization in acute coronary syndrome: a systematic review and meta-analysis. J Am Heart Assoc 2017. April 1;6(4):e005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Elgendy IY, Mahmoud AN, Kumbhani DJ, Bhatt DL, Bavry AA. Complete or culprit-only revascularization for patients with multivessel coronary artery disease under going percutaneous coronary intervention: a pairwise and network meta-analysis of randomized trials. JACC Cardiovasc Interv 2017;10:315–24. 10.1016/j.jcin.2016.11.047. [DOI] [PubMed] [Google Scholar]
- [33].Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol 2015;2016 (67):1235–50. 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- [34].Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation the task force for the management of acute myocardial infarction. Catheter Cardiovasc Interv 2007;15:395–400 (doi: 10). [Google Scholar]
- [35].Pasceri V, Patti G, Pelliccia F, Gaudio C, Speciale G, Mehran R, et al. Complete revascularization during primary percutaneous coronary intervention reduces death and myocardial infarction in patients with multivessel disease: meta-analysis and meta-regression of randomized trials. J Am Coll Cardiol Intv May 2 2018;11(9): 833–43. [DOI] [PubMed] [Google Scholar]
- [36].Vogel B, Mehta SR, Mehran R. Reperfusion strategies in acute myocardial infarctionand multivessel disease. Nat Rev Cardiol 2017;14:665–78. 10.1038/nrcardio.2017.88. [DOI] [PubMed] [Google Scholar]
- [37].Mauri L, Silbaugh T, Garg P, Wolf R, Zelevinsky K, Lovett A, et al. Drug-eluting or bare-metal stents for acute myocardial infarction. N Engl J Med 2008;359: 1330–42. 10.1056/NEJMoa0801485. [DOI] [PubMed] [Google Scholar]
- [38].Kastrati A, Dibra A, Spaulding C, Laarman G, Menichelli M, Valgimigli M, et al. Metaanalysis ofrandomized trials on drug-eluting stents versus bare-metal stents in patients with acute myocardial infarction. Eur Heart J 2007;28:2706–13. 10.1093/eurheartj/ehm402. [DOI] [PubMed] [Google Scholar]
- [39].Jeger R, Jaguszewski M, Nallamothu BN, Luscher TF, Urban P, Pedrazzini GB, et al. Acute multivessel revascularization improves 1-year outcome in ST-elevation myocardial infarction: a nationwide study cohort from the AMIS Plus registry. Int J Cardiol 2014;172:76–81. 10.1016/j.ijcard.2013.12.083. [DOI] [PubMed] [Google Scholar]
- [40].Wiviott SD, Braunwald E, McCabe C, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–15. 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- [41].Wallentin L, Becker R, Budaj A, Cannon C, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009; 361:1045–57. 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- [42].Tarasov RS, Ganyukov VI, Protopopov AV, Barbarash OL, Barbarash LS. Six month results of randomized clinical trial: multivessel stenting versus staged revascularization for ST-elevation myocardial infarction patients with second generation drug eluting stents. Clin Med Res 2014;3:125–9. 10.11648/j.cmr.20140305.12. [DOI] [PubMed] [Google Scholar]
- [43].Ghani A, Dambrink JH, van’t Hof AW, Ottervanger JP, Gosselink AT, Hoorntje JC. Treatment of non-culprit lesions detected during primary PCI: long-term follow-up of a randomised clinical trial. Neth Hear J 2012;20:347–53. 10.1007/s12471-012-0281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Di Mario C, Mara S, Flavio A, et al. Single vs multivessel treatment during primaryangio-plasty: results of the multicentre randomised HEpacoat for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) study. Int J Cardiovasc Interv 2004;6:128–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


