Abstract
-Background:
Multiple BAP-1 inactivated melanocytic tumors (BIMTs) have been associated with a familial cancer-syndrome involving germline mutations in BAP1.
-Objectives:
We sought to describe the clinical and dermoscopic features of BIMTs.
-Methods:
Retrospective, multicenter, case-control study. Participating centers clinical data, dermoscopic images, and histopathological data of biopsy-proven BIMTs. We compared the dermoscopic features between BIMTs and controls.
-Results:
The dataset consisted of 48 BIMTs from 31 patients (22 females, median age=37 years), and 80 controls. Eleven patients had a BAP1 germline mutation. Clinically, most BIMTs presented as pink, dome-shaped papules (n=24). Dermoscopially, we identified 5 patterns: structureless pink-to-tan with irregular eccentric dots/globules (n=14, 29.8%); structureless pink- to-tan with a peripheral vessels (n=10, 21.3%); structureless pink-to-tan (n=7, 14.9%); network with raised, structureless, pink-to-tan areas (n=7, 14.9%); and globular pattern (n=4, 8.5%). The structureless with eccentric dots/globules pattern and network with raised structureless areas pattern were only identified in BIMT and were more common in patients with BAP1 germline mutations (p<0.0001 and p=0.001, respectively)
-Limitations:
Small sample size, retrospective design, absence of germline genetic testing in all patients, inclusion bias towards more atypical-looking BIMTs.
-Conclusion:
Dome-shaped papules with pink-to-tan structureless areas and peripheral irregular dots/globules or network should raise suspicion for BIMT.
Keywords: dermoscopy, bapoma, BAP1 inactivated melanocytic tumors, Wiesner nevus, Spitz tumor, BAP1, dermoscopy, dermatoscopy, Wiesner nevus, BAP1 inactivated melanocytic tumors, atypical spitzoid tumor, melanoma
Introduction
BAP1 inactivated melanocytic tumors (BIMT), also referred as Wiesner nevi or informally bapomas, are melanocytic tumors with unique genetic profiles. While some authors consider these lesions to be variants of Spitz nevi, others think they represent a distinct entity 2 with overlapping clinical and cytologic features.1,2 The occurrence of multiple BIMT has been associated with a familial cancer syndrome involving germline inactivating mutations in the tumor suppressor gene BAP13 BAP1-associated cancer syndrome demonstrates autosomal dominant inheritance, and predisposes to the development of various malignancies, such as mesothelioma, uveal melanoma, renal cell carcinoma, and cutaneous melanoma. Sporadic cases of BIMT without a syndromal association have also been described.3, 4 There are limited reports on the clinical characteristics of BIMT3, 5 and data regarding the dermoscopic appearances of these lesions are sparse.6–9
Herein we sought to describe and correlate the clinical and dermoscopic findings associated with BIMT, features that may raise suspicion for germline BAP1 mutations.
Methods
After Institutional Review Board approval at Memorial Sloan Kettering Cancer Center (MSKCC), we conducted a retrospective multicenter descriptive study via the International Dermoscopy Society (IDS). From August 2016 until November 2017, we promoted the study via the IDS website, dermoscopy conferences, and dermoscopy mailing lists. Physicians from eleven participating centers provided de-identified clinical data using an e-survey (age at diagnosis, lesion size, anatomic site, skin type, reason for excision, personal or family history of cancer, presence or absence of genetic studies in the proband and relatives), clinical and dermoscopic images, and histologic reports and/or scanned slides of biopsy-proven BIMTs. The diagnosis of BIMT was performed by trained dermatopathologists in each participating center, using the diagnostic criteria previously published (biphasic melanocytic proliferation showing one banal- looking melanocytic population showing normal BAP1 expression, together with an area of atypical spitzoid melanocytes showing nuclear loss of BAP13, 10, 11). During the recruitment period we received complete data on 48 BIMTs.
We then created a database form using Access 2013 (Microsoft Corp, Redmond, WA) to collect the data from the e-survey and a checklist of dermoscopic terms based on the 2016 IDS dermoscopic terminology consensus.12 In addition, since the main differential diagnosis of BIMT includes pink-to-tan papules such as intradermal nevi, Spitz nevi or neurofibromas, we included 80 dermoscopic images (~2:1 comparison) of lesions included in its clinical differential diagnosis as controls. Specifically, we included 51 consecutive melanocytic lesions with retained (normal) BAP1 status for which dermoscopic images were available in the MSKCC Dermatology Service’s image database system (Vectra, Canfield, Parsipanny, NJ). Among these lesions, 28 had spitzoid features (16 compound or intradermal nevi with spitzoid features, 6 atypical spitzoid tumors, 1 spitzoid melanoma), and 23 did not have spitzoid features (13 compound nevi, 4 intradermal nevi, 3 junctional nevi, 1 melanoma). We also included 29 non- melanocytic lesions within the clinical differential diagnosis of BIMT (6 neurofibromas, 5 basal cell carcinomas, 4 fibroepitheliomas of Pinkus, 4 dermatofibromas, 3 fibromas, 3 Merkel cell carcinomas, 2 xanthogranulomas, 1 pilomatricoma, and 1 angioma). A board-certified dermatologist (O.Y.) randomized all the images and prepared a slideshow including the BIMTs and the non-BIMT lesions. Two expert dermoscopists (A.A.M., M.A.M.) analyzed the lesions separately and described the dermoscopic features while blinded to the histopathologic diagnosis or clinical data. A third dermatologist (C.N-D.) resolved disagreement in cases of non concordance. Based on the dermoscopic features identified, one of the authors (O.Y.) grouped the BIMTs in different dermoscopic patterns. The dermoscopic structures and patterns encountered in BIMTs were then compared with the features present in controls. We also compared whether these patterns were more commonly found in suspected sporadic vs syndromic BIMTs.
Statistical analysis
Descriptive and relative frequencies were used to describe the distribution of dermoscopic features of the study lesions by each reviewer. Since prevalence estimates for most dermoscopic features were low, prevalence adjusted kappa values were calculated to present agreement between dermoscopic reviewers. A kappa value of 1 indicates perfect agreement, >0.8 indicates excellent agreement, 0.6–0.8 indicates good agreement, 0.4–0.6 indicates fair agreement, and <0.4 indicates poor agreement. Two-sided p values <0.05% were considered statistically significant. A single consensus estimate was created for each characteristic, when agreement was discordant between reviewers. Fisher’s exact test was used to assess the independence of the dermoscopic features between BIMT and non-BIMT lesions. All analyses were performed using Stata v.14.2, Stata Corporation, College Station, TX.
Results
Cohort characteristics
We collected 48 BIMTs from 31 patients (22 females). The average age at diagnosis was 36.9 years (SD=15; range 9–73 years). Nine patients were skin type I, 15 were skin type II, and 7 were skin type III. Eleven patients had a known BAP1 germline mutation and contributed 26 lesions. All patients with known BAP1 germline mutations had multiple BIMTs. One patient had a germline BRCA2 mutation and had a history of breast cancer but no testing for BAP1 was performed. One patient had personal and family history of ocular melanoma and presented with multiple BIMTs, but genetic testing did not reveal evidence of a mutation in BAP1. Three additional patients were suspected to have syndromic BIMTs (cancer history, multiple BIMTs) but genetic results were not available. The remaining 16 patients presented with single BIMTs.
Eight patients had the atypical mole syndrome, 3 of them harboring BAP1 germline mutations. Six patients with the atypical mole syndrome were previously diagnosed with skin cancers (6 melanomas, 2 basal cell carcinomas [BCC]). One patient with a germline BAP1 mutation and the atypical mole syndrome had a renal angiomyolipoma with BAP1 loss. Among the patients without atypical mole syndrome (n=23), 5 had a personal history of melanoma, 3 had a personal history of BCC, and one had occulocutaneous albinism. None of the patients had a personal history of renal cell carcinoma, meningioma, or lung cancer. All patients included in the study did not have local, regional, or distant metastases from BIMT.
Regarding family history, sixteen patients had relatives diagnosed with cancer (9 cutaneous melanomas, 2 mesotheliomas, 2 lung carcinomas, 1 ocular melanoma, 1 renal carcinoma, 1 pancreatic cancer, 1 breast cancer, 1 prostate cancer; 1 testicular cancer, 1 throat cancer, 1 ovarian cancer).
Clinical characteristics of the lesions
Clinically, BIMTs presented as pink, dome-shaped papules in half of all cases (n=24, 50%), followed by brown papules (n=8, 16.6%), pink and brown papules (n=6, 12.5%), red papules (n=4, 8.33%), pink/red to orange papules (n=4, 8.33%), and brown and pink macules (n=2, 4.16%). The average size was 6.85 mm (SD=2.01; range 4–12mm). BIMTs were most frequently located on the head and neck (n=17, 35.41%), followed by the trunk (n=16, 33.33%), upper limbs (n=12, 25%), and lower limbs (n=3, 6.25%). The reasons for biopsy were: exclusion of skin cancer in 32 cases (66.66%), study of a patient with a suspected BAP1-associated cancer syndrome in 9 cases (18.75%), patient concern in 5 cases (10.42%), and irritation of the lesion in 2cases (4.16%).
Dermoscopic characteristics of the lesions
For the dermoscopy analysis, we excluded one case due to poor image quality and ultimately 47 BIMTs were included. Table I summarizes the dermoscopic features present in all the lesions. Dermoscopically, BIMT presented with pink-to-tan structureless areas (n=33, 70.2%), brown irregular dots and globules (clods) (n=19, 40.4%); serpentine vessels (n=18, 38.3%), dotted vessels (n=16, 34%), atypical network (n=6, 12.8%), arborizing vessels (n=3, 6.4%), negative network (n=2, 4.3%), regular globules (n=2, 4.3%), shiny white streaks (n=2, 4.3%) and typical network (n=1, 2.1%). Among these features, irregular globules were significantly more frequent in the BIMTs compared to non-BIMTs (OR 5.23, p=0.002), as well as structureless pink/tan areas (OR 7.6, p<00001). Among BIMTs, the dermoscopic feature with the highest interobserver agreement was irregular globules (k=0.804).
Table I.
Frequencies of the dermoscopic features found in the lesions, interobserver agreement, and comparison of the dermoscopic features and colors identified in BAP1- deficient neoplasms (BIMT) vs controls
| Dermoscopic structures | All lesions (n=127) |
Interobserver agreement, k value |
BIMT (n=47) | Controls (n=80) |
OR [95% CI] | P value |
|---|---|---|---|---|---|---|
| Typical network | 5(3.9%) | 0.505 | 1(2.1%) | 4(5%) | 0.413[0.45−3.8] | 0.651 |
| Atypical network | 15(11.8%) | 0.108 | 6(12.8%) | 9(11.3%) | 1.15 [0.38−3.47] | 0.784 |
| Negative network | 4(3.1%) | 0.485 | 2(4.3%) | 2(2.5%) | 1.73[0.236−12.731] | 0.626 |
| Regular globules | 3(2.4%) | 0.266 | 2(4.3%) | 1(1.3%) | 3.51[0.31−39.8] | 0.554 |
| Irregular globules | 20(15.7%) | 0.804 | 14(29.8%) | 6(7.5%) | 5.23 [1.8−14.8] | 0.002 |
| Regular dots | 2(1.6%) | not significant | 0 | 2(2.5%) | N/A | 0.530 |
| Irregular dots | 20(15.7%) | 0.297 | 12(25.5%) | 8(10%) | 3.086[1.156−8.23] | 0.025 |
| Streaks | 1(0.8%) | not significant | 0 | 1(1%) | N/A | >0.99 |
| Shinny white streaks | 7(5.5%) | 0.788 | 2(4.3%) | 5(6.3%) | 0.667[0.124−3.58] | >0.99 |
| Shinny white blotches and strands | 2(1.6%) | 0.663 | 0 | 2(2.5%) | N/A | 0.530 |
| Blue whitish veil | 3(2.4%) | 0.392 | 0 | 3(3.8%) | N/A | 0.295 |
| Ulceration/erosion | 3(2.4%) | 1 | 0 | 3(3.8%) | N/A | 0.295 |
| Milia-like cysts | 4(3.1%) | 0.324 | 0 | 4(5%) | N/A | 0.296 |
| Comma vessels | 2(1.6%) | not significant | 0 | 2(2.5%) | N/A | 0.530 |
| Dotted vessels | 50(39.4%) | 0.336 | 16(34%) | 34(42.5%) | 0.698[0.33−1.47] | 0.452 |
| Arborizing vessels | 18(14.2%) | 0.367 | 3(6.4%) | 15(18.8%) | 0.295[0.081−1.081] | 0.067 |
| Serpentine vessels | 41(32.3%) | 0.355 | 18(38.3%) | 23(28.8%) | 1.538[0.718−3.295] | 0.327 |
| Glomerular vessels | 1(0.8%) | not significant | 0 | 1(1.3%) | N/A | >0.99 |
| Hairpin vessels | 2(1.6%) | not significant | 0 | 2(2.5%) | N/A | 0.530 |
| Polymorphous vessels | 37(29.1%) | 0.285 | 12(25.5%) | 25(31.3%) | 1.846[0.643−5.3] | 0.548 |
| Structureless pink to tan | 52(40.9%) | 0.346 | 33(70.2%) | 19(23.8%) | 7.6 [3.3−17] | <0.0001 |
| Arrangement of dermoscopic structures | ||||||
| Organized | 44(34.6%) | 0.298 | 18(38.3%) | 26(32.5%) | 1.289[0.608−2.733] | 0.564 |
| Disorganized | 83(65.4%) | 0.298 | 29(61.7%) | 54(67.5%) | 0.776[0.366−1.645] | 0.564 |
| Colors seen on dermoscopy | ||||||
| Brown color | 83(65.4%) | 0.520 | 32(68.1%) | 51(63.8%) | 1.213[0.565−2.605] | 0.701 |
| Black color | 5(3.9%) | 0.478 | 0 | 5(6.3%) | N/A | 0.157 |
| Blue-gray color | 17(13.4%) | 0.339 | 2(4.3%) | 15(18.8%) | 0.2 [0.04−0.88] | 0.029 |
| White color | 16(12.6%) | 0.229 | 2(4.3%) | 14(17.5%) | 0.2 [0.04−0.96] | 0.049 |
| Red color | 5(3.9%) | not significant | 2(4.3%) | 3(3.8%) | 1.141[0.184−7.087] | >0.99 |
| Pink color | 93(73.2%) | 0.315 | 36(76.5%) | 57(71.3%) | 1.321[0.575−3.031] | 0.542 |
Abbreviations: BIMT, BAP1-deficient neoplasm
Considering these dermoscopic findings, we grouped BIMT cases in 5 patterns: structureless pink-to-tan with irregular dots/globules located eccentrically (n=14, 29.8%); structureless pink-to-tan with peripheral vessels (n=10, 21.3%); structureless pink-to-tan (n=7, 14.9%); network with raised, structureless, pink-to-tan areas (n=7, 14.9%); and globular pattern (n=4, 8.5%) (figures 1–3). Five cases (11.6%) did not have a specific pattern. When comparing the presence of these patterns between the BIMTs and controls, the structureless pink-to-tan with irregular dots/globules and the network with raised structureless areas patterns were only identified in BIMTs (p<0.0001) (Table II). Additionally, when comparing lesions on patients with multiple BIMTs associated with BAP1 germline mutations (syndromic BIMTs) vs patients with single BIMTs (suspected sporadic BIMTs), the structureless pink-to-tan with irregular dots/globules pattern was significantly more frequent in cases harboring a BAP1 germline mutation (46.15% vs. 6.25%, OR 12.85, p=0.007) (Table III). Similarly, the pattern showing network and raised structureless areas was only present in syndromic cases. Conversely, a purely globular pattern was not observed in any syndromic case. There was no association for the remaining patterns.
Fig 1.

Schematics showing the five patterns identified in our cohort of BAP1 inactivated melanocytic tumors. A, Structureless pink/tan with atypical eccentric clods B, Structureless pink with radial vessels C, Structureless pink/tan D, Network with raised structureless areas E, Globular
Fig 3.
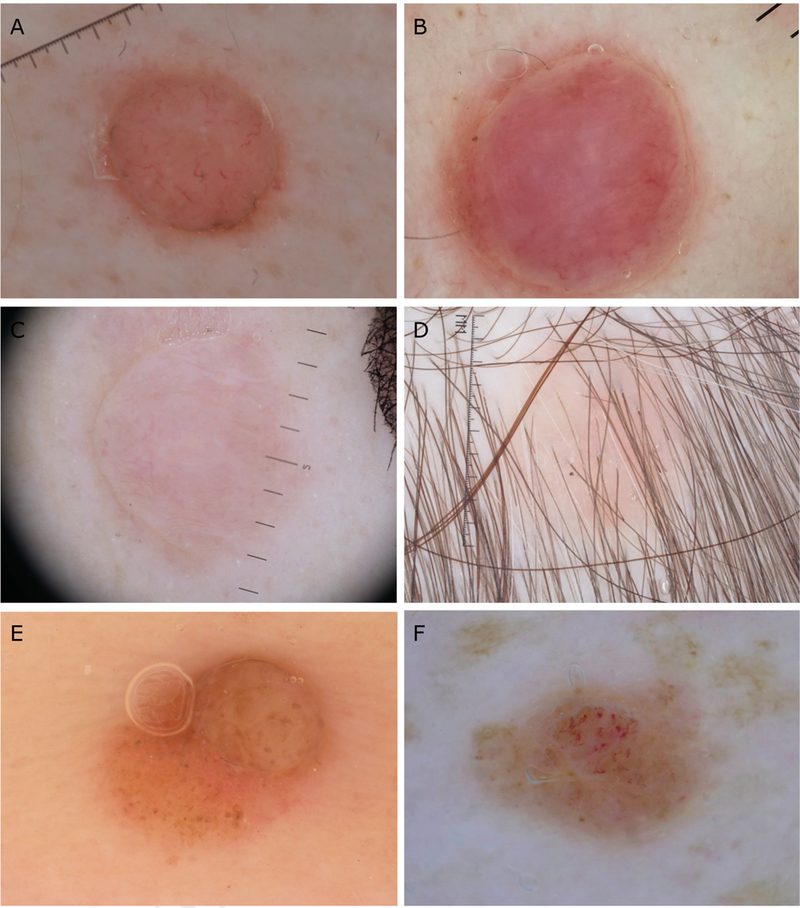
Dermoscopic patterns non-specific for syndromic BAP1 inactivated melanocytic tumors. A-B Dome-shaped papules presenting with structureless pink areas surrounded with radial vessels and a rim of peripheral pigment C-D, Pink papules showing a structureless pink to tan pattern E, Sporadic case presenting as a brown papule with irregular globules on dermoscopy F, Syndromic case with a non-specific pattern presenting with irregular globules and polymorphous vessels.
Table II.
Frequencies and comparison of the dermoscopic patterns identified in BAP1- deficient neoplasms (BIMT) vs non-BIMTs
| Dermoscopic patterns | BIMT (n=47) | Controls (n=80) |
OR [95% CI] | P value |
|---|---|---|---|---|
| Structureless pink/tan with atypical eccentric clods | 14 | 0 | 1.42[1.1−1.7] | <0.0001 |
| Structureless pink/tan | 7 | 7 | 1.11[1.01−1.2] | 0.380 |
| Network with raised structureless | 7 | 0 | 1.17[1.04−1.32] | 0.001 |
| Structureless pink with radial vessels | 10 | 3 | 6.93[1.8−26.7] | 0.004 |
| Globular | 4 | 6 | 1.14[0.3−4.2] | >0.99 |
| Non-specific | 5 | NA | NA | NA |
Abbreviations: BIMT, BAP1-deficient neoplasm
Table III.
Frequencies and comparison of the dermoscopic patterns identified in patients with multiple BIMTs and knownBAP1 germline mutation vs. patients with suspected sporadic cases presenting with single BIMT and no history of a cancer syndrome:
| Dermoscopic patterns | Cases with multiple BIMT, syndromic (n=26) |
Single BIMT, suspected sporadic (n=16) |
OR [95% CI] | P value |
|---|---|---|---|---|
| Structureless pink/tan with a typical eccentric clods | 12 | 1 | 12.85[1.47−112.170] | 0.007 |
| Structureless pink/tan | 4 | 3 | 1.23[0.31−4.8] | >0.99 |
| Network with raised structureless | 6 | 0 | N/A | 0.067 |
| Structureless pink with radial vessels | 4 | 6 | 0.30[0.7−1.31] | 0.142 |
| Globular | 0 | 4 | N/A | 0.016 |
Abbreviations: BIMT, BAP1-deficient neoplasm
Discussion
BIMTs were described by Wiesner et al. in 2011 in two unrelated families both with germline mutations in the tumor suppressor gene called BRCA1-associated protein 1 or BAP1.3 Germline BAP1 mutations are associated with a cancer syndrome that increases the risk for multiple internal and cutaneous neoplasms such as uveal melanoma (28%), pleural and peritoneal mesothelioma (22%), cutaneous melanoma (18%), and renal cell carcinoma (9%)8, 13, 14 Single BIMTs have also been reported to occur sporadically and are not associated with an increased cancer risk. Therefore, since multiple BIMTs are a hallmark of germline BAP1 mutations, the diagnosis of BIMTs is crucial to identify individuals at higher risk to develop multiple cancers.
Clinically, BIMT can be overlooked since they present as non-specific dome-shaped, pink-to-orange papules or papulo-nodules, resembling banal intradermal nevi or fibromas.3, 13 Similarly, the predominant clinical presentation of BIMTs in our study is that of pink-to-tan, sometimes red-to-orange papules. However, we have also shown that BIMT can present as brown, pigmented papules and less frequently as tan macules.
Dermoscopic features of BIMTs have been anecdotally reported and include the presence of pink structureless areas with peripheral linear vessels,6, 7, 9 a multicomponent pattern,9 and pink structureless areas with peripheral pigmented globules.8 In the present study we have identified 3 additional dermoscopic patterns: structureless pink-to-tan, network with raised structureless pink-to-tan areas, and globular patterns. Interestingly, we have identified two patterns which seem characteristic for BIMTs: structureless pink-to-tan with irregular eccentric dots/globules and network with raised structureless pink-to-tan areas. In addition, these two patterns were more frequent in patients with multiple BIMT associated with a known BAP1 germline mutation. On the other hand, the globular pattern seems to be a negative predictor for BAP1 germline mutations as it was not observed in any syndromic case, although only 8.5% of sporadic BIMTs presented with this pattern.
Our results should be interpreted with caution since we only included biopsied BIMTs and there is a chance that some BIMT, especially sporadic lesions, may appear clinically and dermoscopically banal and would thus not warrant a biopsy. In fact, irregular dots and globules, which was the dermoscopic feature more commonly identified in BIMTs, represents a melanoma-specific structure with an OR of 1.7 – 4.8 for melanoma.15 This could explain why the main reason for excision in the BIMTs included in our study (63.6%) was to exclude skin cancer. Interestingly, although irregular dots/globules and structureless pink-to-tan areas were identified in both BIMT and non-BIMTs, its simultaneous presence was only seen in BIMTs (p<0.0001). Nevertheless, we acknowledge that our sample does not include many melanomas, which seems to be an important differential diagnosis from the dermoscopic standpoint. In fact, the melanomas included in our study showed mostly a multicomponent pattern, negative network or polymorphous vessels, which clinically made them easy to suspect as melanomas. Therefore, further studies including a greater number of melanomas, especially melanomas showing atypical dots/globules or structureless areas, are necessary to confirm our results, since if this pattern is confirmed to be unique of BIMT that would be very useful to identify patients at high risk for internal malignancies. Additionally, this pattern may be relatively easy to identify since the identification of irregular dots/globules had excellent interobserver agreement among different observers (k=0.804). Another limitation of our study is that the status of the BAP1 gene was not known in all cases. Therefore, conclusions regarding whether one pattern is more common in syndromic cases vs. sporadic ones should be taken with caution.
Histopathologically, BIMTs are reminiscent of Spitz nevi but lack epidermal hyperplasia, hypergranulosis or Kamino bodies.3, 10, 16 Characteristically, BIMTs present with 2 populations of cells; a more conventional-looking nevus cells population located mostly at the periphery, and a second one with atypical, epitheloid cells with spitzoid characteristics, which typically lacks melanin.3, 10, 11 Immunohistochemially, the atypical spitzoid population presents with nuclear loss of BAP1 and positive staining for VE1 (revealing a BRAF mutation).16, 17 Thus, we believe the structureless areas identified with dermoscopy correspond to the BAP1 inactivated melanocytes, whereas the pigmented clods, network or peripheral pigment correspond to the more conventional-looking melanocytic population (figure 4). Hence, we hypothesize that the different dermoscopic patterns could correspond to different subtypes of BIMTs with a larger or smaller component of either one or the other component of this biphasic proliferation. In other words, BIMT can present with a dermoscopic spectrum which ranges from globular to structureless, depending on the predominant melanocytic population. Thus, one could hypothesize that BIMT undergo a genetic hit that prompts the melanocytes to inactivate BAP1, resulting in increased structureless areas. However, further prospective studies should confirm this hypothesis and evaluate whether these patterns occur de novo or evolve over time.
Fig 4.
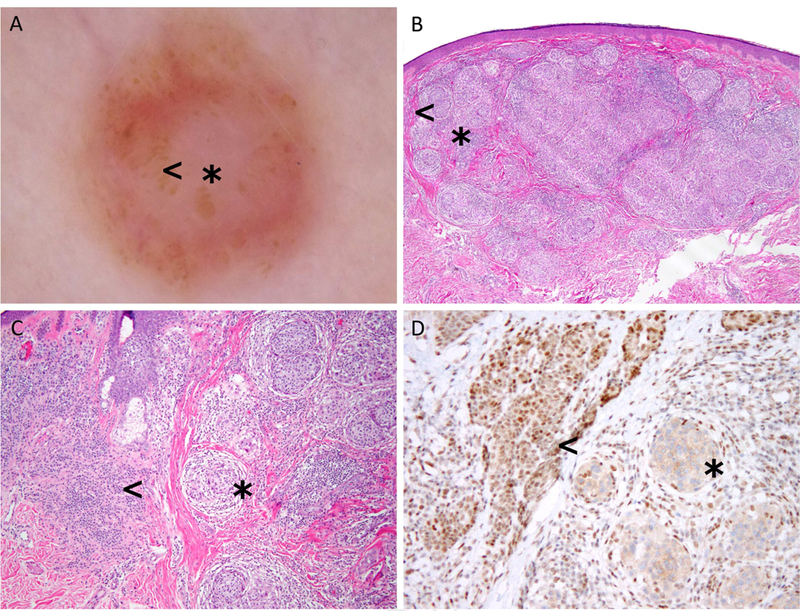
Correlation between dermoscopy and histopathology in a BAP1 inactivated melanocytic tumor. A, Dermoscopy shows a pinkish lesion with central structureless pink to tan areas (asterisk) with atypical dots and globules located mostly at the periphery (arrowhead) B-C, Histologically, this lesion is has a central melanocytic population composed of spitzoid nests located in the dermis (asterisk), surrounded by a more banal-looking melanocytic population located in the periphery (arrowhead) D, Immuhistochemical stains for BAP1 show that spitzoid population (asterisk) corresponds to the BAP1 inactivated population, whereas the pigmented banal melanocytes on the periphery retain BAP1.
Additionally, the relevance of these different patterns and whether they are associated with a better or worse prognosis is unknown. BIMTs are generally indolent,10, 18 although malignant transformation has been described.19 Interestingly, 11 patients with BIMTs had personal history of cutaneous melanoma, and had 9 relatives with cutaneous melanoma. Since some of these cases were diagnosed as spitzoid melanomas, it is possible that some of these lesions were in fact BIMTs removed before 2011 when BIMT was initially described.3 The fact that none of the patients had local or regional recurrence may support this statement. Studies with long-term follow-up are necessary to evaluate the behavior of BIMT and its true malignant potential.
In summary, a subset of BIMT harbors unique dermoscopic features that are not present in lesions in its differential diagnosis. This is important since it may help identify individuals at higher risk for developing multiple malignancies. Specifically, the dermoscopic pattern of pink- to-tan structureless area together with eccentric irregular dots/globules in a young adult should raise suspicion for BIMT associated with BAP1 germline mutations. This could allow the identification of patients at risk for developing multiple cancers and who may benefit from cancer screening. Some authors suggest that total skin examination every 6 months and annual ophthalmological examinations may be beneficial to screen patients with BAP1 germline mutations since they are non-invasive.8 Others also recommend genetic and imaging testing.8, 20. However, there is no consensus guidelines regarding how to screen for malignancies in these patients. Based on our results, if multiple lesions showing one or multiple of the described patterns are identified, it may be worth excising one or two lesions to confirm the diagnosis histopathologically However, we recommend integrating all the available data (clinical, dermoscopic, familial, histologic) together in guiding the management of such patients. Thus, future studies are necessary to generate evidence-based guidelines on how to manage patients harboring BAP1 germline mutations.
Fig 2.
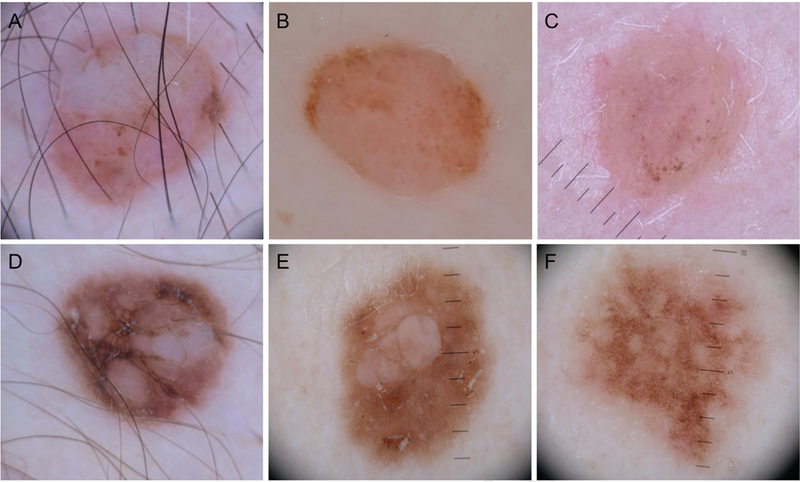
Dermoscopic patterns more frequently identified in suspected syndromic BAP1 inactivated melanocytic tumors. A-C, Structureless pink to tan areas with atypical eccentric clods, which occasionally can coalesce D-F, Network with raised structureless areas, which generally tend to be multiple and sometimes slightly raised.
Capsule summary.
Multiple BAP-1 inactivated melanocytic tumors (BIMTs) have been associated with a familial cancer-syndrome involving germline mutations inBAP1.
We have identified 5 dermoscopic patterns present in BIMT.
Dome-shaped papules with pink-to-tan structureless areas and peripheral irregular dots/globules or network should raise suspicion for BIMT associated withBAP1 germline mutations.
-Acknowledgements
We would like to thank the following collaborators who provided cases for this study: Dr Allan Halpern, Dr Erica Lee, Dr Jennifer DeFazio, Dr Elizabeth A. Quigley, Dr Inés Fernández Canedo, Dr Magand F, Dr Hervet Garat, Dr Perrin P, Dr Martin-Bourret V, Dr Kolonte. We would also like to thank Maria Marino, Iris Zalaudek, and Andreas Blum for their assistance and collaboration in this study.
-IRB: This study was performed under the Memorial Sloan Kettering Cancer Center IRB protocol 16–1194.
-Funding: This research was funded by the NIH/NCI Cancer Center Support Grant P30 CA008748 and Beca Excelencia Fundación Piel Sana.
Abbreviations and acronyms:
- BAP1
BRCA1-Associated Protein 1
- BCC
Basal Cell Carcinoma
- BIMT
BAP1-Deficient Neoplasm
- IDS
International Dermoscopy Society
- MSKCC
Memorial Sloan Kettering Cancer Center
- OR
Odds Ratio
- SD
Standard Deviation
Footnotes
-Conflicts of interest: Pedram Gerami has served as a consultant to DermTech and Castle Biosciences and has received honoraria for this. The other authors do not have conflicts of interest relevant to the current manuscript.
Prior presentation: Partial results of this manuscript have been presented in the 2018 American Academy of Dermatology Meeting (San Diego, 15–20 February 2018) and in the 2018 World Dermoscopy Congress (Thessaloniki, 14–16 June 2018).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vilain RE, McCarthy SW, Thompson JF, Scolyer RA. BAP1-inactivated spitzoid naevi. The American Journal of Surgical Pathology 2015;39:722. [DOI] [PubMed] [Google Scholar]
- 2.Yeh I, Mully TW, Wiesner T, Vemula SS, Mirza SA, Sparatta AJ et al. Ambiguous melanocytic tumors with loss of 3p21. Am J Surg Pathol 2014;38:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nature genetics 2011;43:1018–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiesner T, Murali R, Fried I, Cerroni L, Busam K, Kutzner H et al. A distinct subset of atypical Spitz tumors is characterized by BRAF mutation and loss of BAP1 expression. The American Journal of Surgical Pathology 2012;36:818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busam KJ, Sung J, Wiesner T, von Deimling A, Jungbluth A. Combined BRAF(V600E)- positive melanocytic lesions with large epithelioid cells lacking BAP1 expression and conventional nevomelanocytes. The American Journal of Surgical Pathology 2013;37:193–9. [DOI] [PubMed] [Google Scholar]
- 6.Puig S, Carrera C, Malvehy J. Nevi in patients with Bap1 germ line mutation, red-hair polymorphism, and albinism In: Zalaudek I, Argenziano G and Giacomel J editors. Dermatoscopy of Non-Pigmented Skin Tumors. Boca Raton, FL: CRC Press; 2015. p. 61–2. [Google Scholar]
- 7.Rogers T, Marino ML, Raciti P, Jain M, Busam KJ, Marchetti MA et al. Biologically distinct subsets of naevi: a review. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia 2016. [PMC free article] [PubMed] [Google Scholar]
- 8.Haugh AM, Njauw CN, Bubley JA, Verzi AE, Zhang B, Kudalkar E et al. Genotypic and Phenotypic Features of BAP1 Cancer Syndrome: A Report of 8 New Families and Review of Cases in the Literature. JAMA dermatology 2017;153:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moawad S, Reigneau M, de la Fouchardiere A, Soufir N, Schmutz JL, Granel-Brocard F et al. Clinical, dermoscopic, histological and molecular analysis of BAP1 inactivated melanocytic nevus/tumor in two familial cases of BAP1 syndrome. The British journal of dermatology 2018. [DOI] [PubMed] [Google Scholar]
- 10.Gerami P, Yelamos O, Lee CY, Obregon R, Yazdan P, Sholl LM et al. Multiple Cutaneous Melanomas and Clinically Atypical Moles in a Patient With a Novel Germline BAP1 Mutation. Jama Dermatology 2015;151:1235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busam K, Sung J, Wiesner T, von Deimling A, Jungbluth A. Combined BRAF(V600E)- positive melanocytic lesions with large epithelioid cells lacking BAP1 expression and conventional nevomelanocytes. Am J Surg Pathol 2013;36:193–9. [DOI] [PubMed] [Google Scholar]
- 12.Kittler H, Marghoob AA, Argenziano G, Carrera C, Curiel-Lewandrowski C, Hofmann-Wellenhof R et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. Journal of the American Academy of Dermatology 2016;74:1093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiesner T, Fried I, Ulz P, Stacher E, Popper H, Murali R et al. Toward an Improved Definition of the Tumor Spectrum Associated With BAP1 Germline Mutations. Journal of Clinical Oncology 2012;30:E337–E40. [DOI] [PubMed] [Google Scholar]
- 14.Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G et al. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet 2011;48:856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolner ZJ, Yelamos O, Liopyris K, Rogers T, Marchetti MA, Marghoob AA. Enhancing Skin Cancer Diagnosis with Dermoscopy. Dermatol Clin 2017;35:417–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Llamas-Velasco M, Perez-Gonzalez YC, Requena L, Kutzner H. Histopathologic clues for the diagnosis of Wiesner nevus. Journal of the American Academy of Dermatology 2014;70:549–54. [DOI] [PubMed] [Google Scholar]
- 17.Gerami P, Yelamos O, Lee CY, Obregon R, Yazdan P, Sholl LM et al. Multiple Cutaneous Melanomas and Clinically Atypical Moles in a Patient With a Novel Germline BAP1 Mutation. JAMA Dermatol 2015;151:1235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gammon B, Traczyk TN, Gerami P. Clumped perinuclear BAP1 expression is a frequent finding in sporadic epithelioid Spitz tumors. Journal of cutaneous pathology 2013;40:538– 42. [DOI] [PubMed] [Google Scholar]
- 19.Wiesner T, Fried I, Ulz P, Stacher E, Popper H, Murali R et al. Toward an improved definition of the tumor spectrum associated with BAP1 germline mutations. J Clin Oncol 2012;30:e337–40. [DOI] [PubMed] [Google Scholar]
- 20.Pilarski R, Rai K, Cebulla C, Abdel-Rahman M. BAP1 Tumor Predisposition Syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mefford HC, et al. editors. GeneReviews((R)) Seattle (WA)1993. [Google Scholar]


