Abstract
Decades of research into the molecular and cellular regulation of kidney morphogenesis in rodent models, particularly the mouse, has provided both an atlas of the mammalian kidney and a roadmap for recreating kidney cell types with potential applications for the treatment of kidney disease. With advances in both our capacity to maintain nephron progenitors in culture, reprogram to kidney cell types and direct the differentiation of human pluripotent stem cells to kidney endpoints, renal regeneration via cellular therapy or tissue engineering may be possible. Human kidney models also have potential for disease modelling and drug screening. Such applications will rely upon the accuracy of the model at the cellular level and the capacity for stem-cell derived kidney tissue to recapitulate both normal and diseased kidney tissue. In this review, we will discuss the available cell sources, how well they model the human kidney and how far we are from application either as models or for tissue engineering.
Keywords: pluripotent stem cell, directed differentiation, kidney development, kidney organoid, transdifferentiation, human nephron progenitor
1. Introduction
Chronic kidney disease (CKD) describes the progressive deterioration of kidney function due to a variety of primary and secondary kidney diseases. The prevalence of CKD is rising worldwide and represents a significant burden of morbidity, mortality and healthcare expenditure [1–3]. In 2015, the total Medicare expenditure for all CKD and end-stage kidney disease (ESKD; requiring dialysis or transplantation) in the United States alone exceeded US$98 billion [1]. For many patients CKD progresses to ESKD over years or decades, providing ample opportunity for potential therapeutic intervention. Unfortunately there exists a paucity of disease modifying treatments due to a limited understanding of disease pathobiology for most causes of CKD.
Over the last two decades there has been an interest in augmenting existing treatments for ESKD with novel regenerative medicine approaches. Indeed, a number of approaches have been proposed as options for renal replacement, including adult porcine or embryonic kidney xenotransplantation [4, 5]. Additional approaches envisaged have included bioprinting [6], renal assist devices in which primary cells were seeded on hollow fibre [7, 8], recellularization of decellularized scaffolds [9] and direct cellular therapy, including the use of mesenchymal or bone marrow derived cell types [10]. With the isolation of human embryonic stem cells in 1998 [11] came the prospect of recreating human kidney tissue from this human pluripotent stem cell type. Significant advances in cellular reprogramming, including the capacity to generate induced pluripotent stem cells (iPSC) from any adult somatic cell type [12, 13] and the capacity to enforce cellular transdifferentiation via the overexpression of key pioneer transcription factors [14–16] is beginning to provide additional approaches for the generation of cells for use in renal regeneration. In addition, continuous improvements in our understanding of kidney morphogenesis, and particularly the lineage relationships of the cells within the developing kidney, are also providing approaches for the isolation, maintenance and differentiation of progenitor cell types.
In this article, we will review the current situation with respect to potential cellular sources for renal regeneration, focussing most particularly on the directed differentiation of human pluripotent stem cells (hPSC) to kidney cell types. We will address the key questions of how accurately these recapitulate the human kidney and what challenges remain to using such cell types for disease modelling and regenerative medicine.
2. Embryological origins of the mammalian kidney
In order to recreate, maintain and expand human kidney tissue or individual kidney cell types, a detailed understanding of normal kidney development is required, including specific patterns of gene expression, key cell-cell interactions and critical signalling pathways. Renal regeneration protocols are underpinned by decades of work studying mammalian nephrogenesis predominantly in non-human models (reviewed in [17]). The permanent kidney in mammals, the metanephros, is mesodermal in origin, forming from the cells of the metanephric mesenchyme (MM) and an epithelial side branch of the nephric (Wolffian) duct referred to as the ureteric bud (UB). Both the MM and UB are derivatives of the intermediate mesoderm [18]. The metanephros is connected to the nephric duct via the ureteric bud, which branches from the nephric duct and grows towards the MM in response to the production of glial-derived neurotrophic factor (GDNF) by the MM [19]. Having reached the MM, this UB undergoes extensive rounds of dichotomous branching to form a ureteric epithelial tree. This branching occurs again in response to continued GDNF production by a restricted mesenchymal population, the cap mesenchyme. In both mouse and human, this cap mesenchyme expresses the Six2/SIX2 transcription factor [20]. Lineage tracing studies in mouse have shown that this Six2-expressing cell population gives rise to all cells of the epithelial nephrons [20]. Hence, the cap mesenchyme represents a nephron progenitor cell (NPC) population responsible for all nephron formation. In human, it has recently been confirmed that the cap mesenchyme population has a transcriptional signature similar to mouse, with the notable addition of SIX1 expression, which is not evident in the same cell type in mouse [21]. The triggers to remain as an NPC or to commit to forming a nephron both come, at least in part, from the tips of the branching ureteric tree. More than a decade of research in mouse suggests roles for FGF9, FGF20, non-Smad BMP7 signalling and low canonical Wnt signalling as facilitating NPC maintenance [22–24] while higher levels of canonical Wnt signalling via Wnt9b and subsequently Wnt4 [25, 26], possibly coupled with the initiation of Notch signalling [27, 28], triggers a mesenchyme-to-epithelial transition (MET) initiating nephron commitment. It is this body of knowledge that has assisted in the development of methods for the isolation and culture of NPCs from mouse and human fetal tissue [29–33] as well as approaches for directing the differentiation of pluripotent stem cells to kidney [16, 34].
Following MET of induced cap mesenchyme, the morphological sequence of mammalian nephron development is well established. A pre-tubular aggregate of mesenchyme develops into a renal vesicle, an epithelial structure which becomes polarised, develops a lumen, invades the distal end of the ureteric tip forming a connecting segment and subsequently elongates away from it [35, 36]. Morphological evidence of proximal-distal nephron patterning becomes evident as it elongates it forms into a ‘comma shape’ and an ‘S-shape’ body [37]. The primitive glomerulus and distal tubule juxtapose and the tubular loop between them extends almost clonally into the medulla from an Lgr5+ cell cluster within the medial S-shaped body [38]. Proximal-distal expression patterning has been characterised for many genes in both the pretubular aggregate [39] and the renal vesicle prior to the formation of the connecting segment [36, 40]. A decreasing Wnt signalling gradient, strongest at the ureteric tip and progressively decreasing towards the primitive glomerulus interacts with Notch signalling to pattern the nephron.[41, 42]
Around the branching UB and forming nephrons is the forming vasculature and the renal interstitium, which gives rise to mesangial and perivascular cell types [18]. While less is known about the lineage relationships and origins of these non-epithelial components of the kidney, there is strong evidence that the patterning and morphogenetic events in the kidney are influenced by these cell types. Notably, the loss of expression of the Foxd1+ cortical stroma resulted in what is referred to as the ‘stromaless’ mouse in which the NPC population is greatly expanded and sustained in an undifferentiated state [43]. The recreation of human kidney tissue is also, therefore, likely to require an appropriate stromal population, some of which will also arise from the MM. Again, our understanding of the relevance of murine markers of the interstitial compartment in human kidney development is only just beginning to be investigated. In three landmark publications, the morphology, protein and gene expression of the human trimester 1 and early trimester 2 human kidney have been characterised [21, 44, 45]. What these initial studies show is that while many markers characteristic of cell type or state in developing mouse kidney are present in the developing human kidney, other genes are either not present or mark a potentially subtly different cell population [21, 44, 45]. While incomplete for all cellular components and stages of development at this point in time, these are critical datasets upon which the renal regeneration field must rely.
2.1. Formation of the pronephros and mesonephros
As the embryological origin of the mature adult kidney, the metanephros represents the focus of renal regenerative science. However, the metanephros represents the third pair of excretory organs arising from the IM in a rostrocaudal sequence as the body plan elongates. Both the pronephros and mesonephros form epithelial tubules similar in histological structure to the nephrons that form within the metanephros. Mouse mesonephric tubules undergo patterning and segmentation similar to that seen in metanephric tubule formation [46]. While there is evidence that mesonephric tubules play a role in early filtration and solute transport [47], both the pronephric and mesonephric tubules degenerate as the nephric duct extends along the elongating body plan.
Within an embryo the distinction between mesonephric and metanephric tubules is ultimately made on anatomical grounds. Even in mouse, there are few defining features, other than the absence of a mature loop of Henle, that distinguish mesonephric from metanephric tubules. Whilst the formation of a Six2+ NPC population appears to be unique to the metanephros [20], the repetition of form between tubules arising from the pronephros mesonephros and metanephros provides some challenges for the identification of regenerated human kidney cell types in vitro (elaborated in Section 3.6). Nevertheless, many of the approaches used for isolating, recreating or maintaining human renal cell types draw heavily on our understanding of murine kidney development.
Cellular sources for renal regeneration
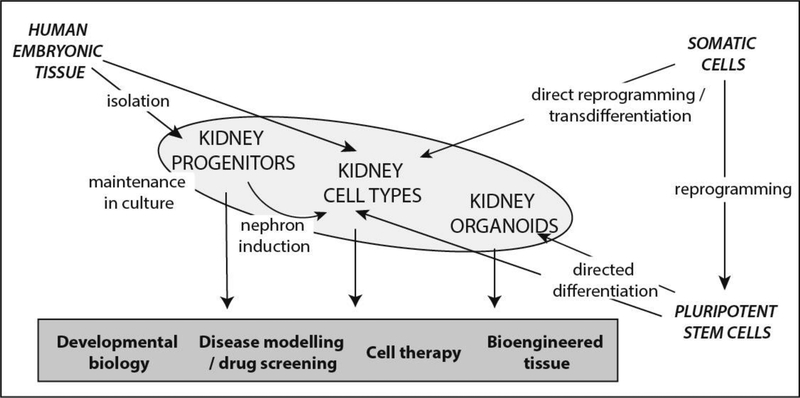
To recreate human kidney tissue or renal cell types for therapy, disease modelling or drug screening, there are three possible sources of kidney cell types; isolated human fetal progenitors, directly reprogrammed cells and human pluripotent stem cells (hPSCs) (Figure 1).
Figure 1:
Summary of the sources of human cells and tissues available for studying kidney development, disease modelling and renal regeneration.
2.2. Isolation and maintenance of nephron progenitor populations
While the nephrons arise from a nephron progenitor population within the developing kidney, this population is terminally differentiated prior to birth in humans [48, 49]. Given the absence of a nephron progenitor population in the postnatal human kidney able to regenerate entire nephrons, there have been a number of attempts to isolate human nephron progenitors from human fetal kidney [50, 51]. This work began with studies into the transplantation of human fetal kidney [52]. Further studies into markers of the progenitor population within the developing human kidney revealed a capacity to selectively enrich for SIX2+ NPCs based on elevated NCAM and lack of CD133 surface expression [32]. By adopting culture in media developed for the maintenance of murine NPC in vitro [29, 30], our group has recently reported robust expansion of human NPC [31]. Single cell profiling of this population, using a restricted biomarker panel approach, showed evidence of mesenchymal uncommitted and committed NPCs, but also revealed that almost immediately upon in vitro culture the NPC fraction formed a substantively heterogeneous population of cellular states.
Having isolated nephron progenitors, the next challenge is their maintenance in culture (Figure 1). This requires not only evidence of maintained NPC marker expression but also evidence of a prolonged capacity to form nephrons when triggered to do so. Despite our extensive understanding in mouse of NPC markers/gene expression and the pathways involved in triggering nephron formation, the in vitro maintenance of this cellular population away from its niche within the developing organ has been a major challenge for the field. A significant breakthrough occurred with the identification of a role for FGF9 and FGF20 in nephron progenitor maintenance in vivo [22]. In addition, while canonical Wnt signalling had been identified as a critical trigger for the transformation of nephron progenitors to nephron epithelia, Karner et al [24] showed that low levels of canonical Wnt signalling were also required for nephron progenitor maintenance. Finally, following a number of studies examining the role of canonical and non-Smad mediated BMP signalling in kidney development [23], Brown et al recently developed media conditions in which they could maintain and expand nephron progenitors isolated from the nephrogenic zone of the developing mouse kidney by facilitating appropriate Wnt, FGF and BMP signalling [29]. At the same time, Tanigawa et al developed similar media that was shown to support isolated NPC [33, 53]. Finally, Li et al [30] reported another media combination able to robustly maintain NPC isolated from either mouse or human developing kidney. While each of these maintenance media contained unique components, there was substantial overlap (Table 1). These advances in protocols for the maintenance of NPC provide approaches for expanding appropriate cell sources for renal regeneration. The provision of a format or scaffold for the therapeutic use of such cells remains to be addressed.
Table 1.
A comparison of media described for the maintenance of isolated nephron progenitors. Adapted from Li et al [30].
| NPSR | NPEM | CDBLY | |||||
|---|---|---|---|---|---|---|---|
| Li et al. 2016 | Brown et al. 2015 | Tanigawa et al. 2016 | |||||
| Culture Period | Until now, more than 17 months and more than 110 passages | Up to 10 passages were shown | Up to around 19 days | ||||
| Culture Condition | Format | 3D | 2D (Matrigel coating) | 2D (iMatrix coating) | |||
| Basal Medium |
DMEM/F12 | APEL | DMEM/F12 | ||||
| Specific Factors | Factor | Final Concentration |
Factor | Final Concentration |
Factor | Final Concentration |
|
| BMP7 | 50ng/ml | BMP7 | 30ng/ml | TGF-α | 10ng/ml | ||
| FGF2 | 200ng/ml | FGF9 | 200ng/ml | FGF2/9 | 50ng/ml | ||
| Heparin | 1ug/ml | Heparin | 1ug/ml | LIF | 5ng/ml | ||
| Y27632 | 10uM | Y27632 | 10uM | Y27632 | 10uM | ||
| CHIR99021 | 1uM | CHIR99021 | 1.25uM | CHIR99021 | 1uM | ||
| LIF | 10ng/ml | LDN193189 | 125nM | BMP7 | 5ng/ml | ||
| BMP4 | 30ng/ml | DAPT | 2.5uM | ||||
| IGF1 | 20ng/ml | ||||||
| IGF2 | 2ng/ml | ||||||
2.3. Direct reprogramming to renal endpoints
Direct reprogramming refers to the direct conversion from one cellular identity to another as a result of the enforced expression of key pioneer transcription factors. This is a second possible approach for the generation of kidney tissue (Figure 1). While the proof of concept was demonstrated by Davis et al [54], where the overexpression of MyoD in a fibroblast was sufficient to reprogram this cell into a myoblast, the ultimate example of direct reprogramming is the generation of induced pluripotent stem cells from any adult somatic cell type via the enforced expression of 4 transcription factors (OCT4, SOC2, KLF4, CMYC [12] or OCT4, LIN28, NANOG, SOX2 [55]). Only a small number of studies have attempted to directly generate kidney cell types using enforced transcription factor expression. Both of these studies have again drawn on the fundamental understanding of normal kidney development in the mouse.
In 2013, Hendry et al performed a lentiviral screen of 15 genes in an attempt to convert an adult human proximal tubule cell line (HK2) into a nephron progenitor state. Six genes were required to generate a cellular state in which there was spontaneous re-expression at the gene and protein level of other nephron progenitor markers and an enhanced capacity for the resulting cells to contribute to the nephron progenitor pool of the developing mouse kidney.
In a second approach to direct reprogramming to kidney, Kaminski et al [34] profiled developing mouse tissues seeking transcription factors with high absolute and relative expression in kidney compared to other tissues. They then selected genes with evolutionary conserved expression in developing kidney and some previous evidence of a role in human or mouse kidney disease. Again using a pooled lentiviral approach, they identified a requirement for 4 genes, EMX2, PAX8, HNF1B, and HNF4A, to transform mouse embryonic fibroblasts to induced renal epithelial cells (iRECs). This transformation resulted in cell types able to integrate into dissociated mouse kidney ex vivo and showed evidence of renal epithelial gene expression and sensitivity to nephrotoxic agents, including gentamycin and cisplatin. However, the segment specification of the resulting cells suggested expression of genes regarded as marking both proximal and distal tubular segments of the nephron. This may therefore represent reprogramming to a more primitive renal epithelial progenitor state or an artificial cell state not present in vivo.
While these represent the only two examples in the literature to date of kidney-related direct reprogramming, as we increase our understanding of the transcriptional profile of the developing human kidney, it is likely that attempts will be made to recreate collecting duct, podocyte and other nephron-specific cell types. The application of such cells for the treatment of renal disease or the regeneration of renal tissue again presumes the identification of an appropriate format for bioengineering or advances in our capacity to deliver and functionally integrate such cells into the endogenous renal parenchyma.
2.4. Differentiation of pluripotent stem cells to kidney
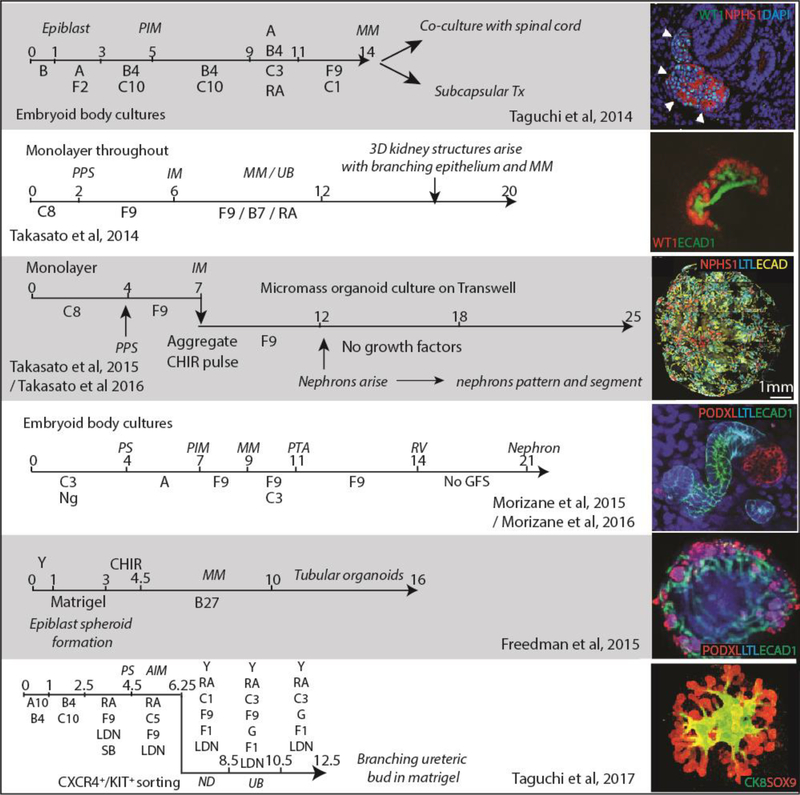
The final source of cells for regenerating human kidney cell types are human pluripotent stem cells (hPSC) (Figure 1). In the ultimate illustration of transdifferentiation, the landmark studies of Takahashi et al [12] and Yu et al [55] demonstrated the capacity to induce the formation of pluripotent stem cells via the introduction of key transcription factors into human fibroblasts. The resulting iPSC displayed the accepted features of pluripotent cells, including a capacity to differentiate towards all three germ layers. While the potential to differentiate such cells to any lineage has been the great promise of the field, this has required the development of protocols specific to each target cell type. While some early studies reported the direct and uniform conversion of human pluripotent stem cells to podocyte [56] and proximal tubule [57], the majority of protocols in the kidney field have applied carefully designed multistep differentiation approaches based upon our existing understanding of kidney development in mouse from the fertilised egg onwards. Successful outcomes have been assessed based upon markers of cell identity defined primarily in mouse. There have now been many protocols described for the directed differentiation of hPSC to kidney tissue [58–68]. While some protocols were developed initially using murine pluripotent stem cells [64], many went directly to hPSC. We will focus here on the most recognised and well characterised protocols, all of which rely upon defined, stepwise differentiation and report the formation of complex multicellular tissues containing cell types and morphological features recognisable as kidney (Figure 2). Many of these protocols involve the formation of 3D structures with multiple cell types. Most describe the formation of segmenting nephrons. All draw on an understanding of embryogenesis (Figure 2). The most recent of these (2017) focusses specifically on the formation of the human ureteric epithelium (Figure 2).
Figure 2. Published protocols for the directed differentiation of human pluripotent stem cells to kidney endpoints.
Stages of differentiation: AIM, anterior intermediate mesoderm; IM, intermediate mesoderm, MM, metanephric mesenchyme; ND, nephric duct; PIM, posterior intermediate mesoderm; PS, primitive streak; PPS, posterior primitive streak; Tx, transplantation; UB, ureteric bud. Compounds added: A, Activin A; A10, activing A 10uM, B4, BMP4; B7, BMP7; C or CHIR, CHIR990201 (GSK3β antagonist / canonical Wnt activator); C1, CHIR 1uM; C3, CHIR 3uM, C5, CHIR 5uM, C8, CHIR 8uM; F1, FGF1; F2, FGF2; F9, FGF9; G, GDNF; LDN, LDN193189 (BMP Type 1 receptor (ALK2/3) inhibitor); Ng, Noggin (BMP inhibitor), RA, retinoic acid; SB, SB431542 (TGFβ Type 1 receptor (ALK5) inhibitor; Y, Y27632 (Rho-kinase inhibitor).
As noted previously, the kidney is mesodermal in origin and hence, from an embryological perspective, kidney development commences with the formation of posterior primitive streak (PPS) which in turn gives rise to the trunk mesoderm. The mediolateral patterning of this mesoderm gives rise to paraxial, intermediate and lateral plate mesoderm, with the coincident expression in mouse of LHX1, OSR1 and PAX2 marking the IM. What is evident when comparing the protocols in Figure 2 is the congruence in signalling pathways / growth factors being applied for these early patterning events, with the activation of BMP/activin A or canonical Wnt signalling predominant. This is not surprising given prior approaches for mesodermal patterning in vitro, as is required for cardiomyocyte or blood differentiation [69, 70]. While the outcomes from these protocols overlap, there is substantial variation in the format of hPSC culture from monolayer culture to embryoid body. However, all protocols involve induction of canonical Wnt signalling and most use FGF9 to pattern to MM. The format used in the Freedman protocol [58] was distinctly different. After an initial expansion of pluripotent cells within Matrigel to form epiblast-like structures comprising pluripotent cells, these cultures were exposed to approximately 1.5 days of CHIR induction followed by culture without added growth factors for 11.5 days allowing for the spontaneous formation of epithelial structures across the monolayer. These were similar to what was previously reported by Takasato et al [66].
The term organoid has now been widely applied to self-organising multicellular tissues generated from hPSC [71]. Our group first shifted from the spontaneous formation of structures across a monolayer [66] to the dissociation and reaggregation of all cells in the culture to form a 3D cellular micromass that was subsequently cultured at an air-media interface [67]. This approach was an homage to the decades of developmental biology studies of the mouse kidney in which the fetal kidney was grown in such a confirmation as an ex vivo tissue. The fact that an embryonic tissue can continue to pattern even after enzymatic dissociation and reaggregation was shown for the embryonic mouse kidney in the 1950s [72] and has been an approach employed by ourselves [16, 73] and others [74] to examine the capacity for exogenous cell types to integrate into the developing kidney. The term kidney organoid is now more widely applied to a number of the published protocols.
2.5. Anterior versus posterior IM derivatives
Cells contributing to the trunk mesoderm do so across time. As the embryo extends, cells patterned as IM include those which are anterior, (early IM), and posterior, (late IM) [64, 75]. The most anterior IM (AIM) gives rise to both the nephric duct (Wolffian duct) and the pronephros whereas the metanephric mesenchyme forms from more posterior IM (PIM). As the collecting duct is a side branch of the nephric duct, this could either be viewed as an AIM structure or an epithelial side branch arising alongside the PIM, depending upon whether one views nephric duct extension as occurring via elongation or rearrangement of IM cells. However, lineage tracing supports the view that the UB arise from the AIM lineage [64]. Hence, there is a clear dichotomy of viewpoint between Taguchi et al [64] and Takasato et al [67] with the former suggesting it is not possible to form both the nephron and the collecting duct elements of the metanephros simultaneously while the latter has chosen an intermediate patterning to achieve both. The tubular structures arising from the protocol of Taguchi et al [64] required induction by co-culture with spinal cord and formed strongly proximalised nephrons. Takasato et al describe the presence of glomerular (NPHS+WT1+), proximal tubule (LTL+ECAD-), distal tubule (ECAD+GATA3-) and collecting duct (GATA3+ECAD+) segments [67]. There is some contention in the field as to whether GATA3 is restricted to the collecting duct or also present in the connecting segment, as is the case for other markers in the mouse [76]. Indeed, our own single cell analysis of E17.5 mouse kidney shows a collecting duct expression pattern for a subset of the distal tubules [77]. Taguchi et al [65] have now reported a method for the targeted generation of AIM for the patterning to collecting duct, describing intervening gene expression marking Wolffian/nephric duct and ureteric bud. This protocol involves a CXCR4+KIT+ enrichment step at day 6.25 in order to enrich for UB-forming cells. The latter can be cultured in Matrigel and shows the formation of a branching epithelium with RET+ tips. Using a protocol developed for mouse, they combined ureteric bud cultures with nephron progenitor cultures and interstitium to recreate a 3D structure with collecting duct and nephrons. This was described as a ‘higher order’ kidney. Other groups have also described protocols for generating collecting duct, including Mae et al [61] and Xia et al [68]. While these vary slightly, Mae et al [61] and Taguchi et al [61] draw upon the published requirement for FGF and RA for Wolffian duct induction and GDNF for RET upregulation and branching. While the approach of Taguchi et al [65] to recombine collecting duct with nephron progenitors and stroma worked for mouse, similar results were not achieved using hPSC. However, the proposed recombination of UB with MM would provide a capacity to more accurately control the placement of each cell type in order to form a more anatomically accurate and hence potentially functionally superior organ model. In contrast, most other methods available allow for stochastic patterning to occur within culture formats that are poor models of the embryo. As a result, while there is evidence in the protocols described here for the formation of structures that mimic aspects of the developing human kidney, there is substantial inherent randomness in the morphology of what arises.
2.6. Cellular diversity and identity within kidney organoids
All protocols currently available for directing differentiation to human kidney show the presence of cellular structures reminiscent of the fetal kidney. Takasato et al [63] was also the first to report the simultaneous patterning of nephrons, collecting duct, endothelium, perivascular and renal stroma within organoids. Several other groups subsequently showed evidence for endothelial cell types [63]. While Takasato et al [61] showed some evidence of invasion of the developing renal corpuscles with endothelial progenitors, as would occur at the capillary loop stage of nephron maturation, this was not a common event. Given the large numbers of protocols published for the generation of kidney cell types from hPSC, there has been little objective characterisation of the individual cell types present within hPSC-derived kidney organoids. This has been compounded by the lack of an accurate point of reference given the paucity of data available on gene expression within the normal human kidney, both adult and fetal. Takasato et al [67] reported the global expression profiling of hPSC-derived kidney organoids in comparison to a panel of human fetal tissues, revealing the highest congruence with trimester 1 human kidney. Of note, profiling of organoids prior to nephron formation showed a closer alignment with gonad, the only other IM-derived tissue available at that time. The complexity of the kidney organoids generated using this method at the level of immunofluorescence would suggest >10 identifiable cell types. However, here as with other protocols, individual cell types have been recognised based upon anticipated protein markers from mouse. What this global profiling does not thoroughly investigate is the presence or prevalence of ‘off-target’ populations or the reproducibility or robustness of the protocol. Organoids are 3D models of embryogenesis and hence may be viewed as ‘targeted’ rather than ‘perfect’ models of development. Single cell transcriptional profiling data from hPSC organoids is just now becoming available [77, 78] and will be critical both to better identify and validate the individual cell types present. This will allow us to not only understand what is being accurately modelled but also unintended end-points. With the recent data from Lindstrom et al characterising the structure and gene expression of normal human kidney [21, 44, 45], there is now a reference point for comparison. In addition, we [79, 80] and others [61, 65] have described a number of hPSC reporter lines which will enable the isolation and transcriptional profiling of individual cell types from within the kidney.
2.7. How well do hPSC-derived kidney models represent the human kidney?
The recent comprehensive studies of histology and gene expression during early (first and second trimester) human kidney development [21, 44, 45] has begun to provide a more secure basis upon which to evaluate and potentially improve protocols for the generation of human kidney tissue from hPSC. While these studies show strong congruence with mouse, they also note some differences between mouse and human both at the level of anatomical structure and gene expression. What is of great promise is that most of the landmarks that have been used to identify kidney cell types formed from hPSC in vitro are conserved. There remain many human kidney cellular components yet to be fully analysed at the transcriptional level and such studies will continue to form the foundation for our understanding of what we hope to regenerate. However, these studies did raise specific questions around the accuracy of what is present in our hPSC-derived kidney models to date.
Lindstrom et al [21, 44, 45] estimated that the transition of a renal vesicle to a capillary loop stage nephron takes in the order of 3 to 10 days. This is consistent with the time taken for a nephron to arise and segment within a kidney organoid generated using the Takasato et al [67] protocol. Here, epithelial elements are evident from around Day 12 and reach capillary loop stage from Day 18. While this timing is consistent with what is observed in the developing human kidney, the human metanephros itself does not begin to arise until week 5 of human gestation. Most protocols for directed differentiation to kidney describe the formation of PPS / IM and MM within 10 days from commencement of differentiation (Figure 2). The human pronephros is reported to arise from 3–4 weeks of gestation (22–28 days) while the mesonephros arises from day 33 (Carnegie stage 14). Hence, the pace at which nephrons are arising in vitro is premature for any of these three excretory structures. Should we anticipate a chronology in vitro reflective of human gestation? There are now protocols for the directed differentiation of hPSC to many tissue types, including those derived from ectoderm (brain, optic cup), endoderm (intestine, stomach, colon, pancreas) and mesoderm (cardiomyocyte, blood). It would be difficult to argue that any of these protocols replicate a gestational clock. There are also a growing number of protocols in which either adult somatic cells (often fibroblasts) or pluripotent stem cells are directed to adopt another lineage via the enforced expression of key pioneer transcription factors. This clearly does not involve an accurate embryological clock. Additionally, it has yet to be established whether in vitro nephron structures follow a similar developmental morphological sequence as that which has been well characterised in vivo, that being polarising renal vesicle, comma shaped body, S shaped body, and so on. What is fair to say is that the pluripotent stem cell field believes the accuracy of what they have generated based on their understanding of the tissue they wish to build, even when that tissue has not yet been comprehensively analysed using human fetal material. The proof will reside in the functional utility of such cells.
As noted above, while arising in a temporal sequence across developmental time in mammals, there are no particular anatomical or histological distinctions between pro-, meso- and metanephric tubules. In our own experience, prolonged culture of hPSC-derived structures results in eventual accumulation of regions of extracellular matrix (ECM), expanded stroma and occasionally the overgrowth of off target cell populations such as cartilage. This mispatterning with prolonged in vitro culture is most likely due to the inappropriate format of the culture itself (that being a tissue mass >7 mm in diameter at an air-media interface without a patent vascular supply). However, are we sure we are not generating mesonephros? As described above, the murine mesonephric tubules appear to form via a similar series of histological changes as those seen in the metanephric tubules. Hence it is not apparent within an hPSC-derived tissue how to distinguish between a pro-, meso- or metanephric tubule. It has previously been proposed in mouse that mesonephric tubules lacked loops of Henle [81] and that glomerular-like structures are only visible in the cranial mesonephric tubules [82, 83], however expression of genes eventually expressed within the metanephric loop of Henle and distal tubules are expressed within mesonephric tubules [46, 84]. Initiation of the MET required to form the mesonephric tubules is also not well understood, although it is thought that a similar Wnt-mediated mechanism is likely to be involved based on the loss of such tubules in Wnt9b mutant mice. There is also evidence of a requirement for FGF8 / Fgfr1/2 signalling which may differ from metanephros [85]. The use of a canonical Wnt signalling trigger in most currently published protocols (either via addition of CHIR [63, 67] or co-culture with spinal cord [64] to initiate MET within their differentiation protocols) makes metanephric and mesonephric protocols indistinguishable.
One solution to this dilemma would be to look for the expression of unique markers. However, there is almost no information about pronephric development in the human and little information, even in mouse, regarding distinguishing markers of pro-, meso- and metanephros. Indeed, nephron patterning at the level of gene expression in mouse mesonephric tubules is very similar to that observed in metanephric nephron morphogenesis [46]. Knockout / transgenic models in mouse showing loss of some or all mesonephric tubules has been reported for many genes also required for metanephric development. For example, loss of Wt1 and Six1 in mouse results in the loss of the caudal mesonephric tubules as well as a loss of metanephric development while a loss of Gata3 or Osr1 results in a loss of all mesonephric tubules as well as defects in nephric duct extension [85]. Conversely, studies have sought markers unique to the metanephric mesenchyme. Early cDNA array profiling of mouse mesonephros versus metanephros performed to identify specific markers of metanephric mesenchyme identified CD24a (EST: BG076069) and Cdh11 as restricted to the metanephric mesenchymal region. Cdh11 expression persisted in the stroma of the developing kidney while Cd24a was evident in the forming nephrons and collecting ducts in mouse [86]. This, however, is not informative for human tissue as Cd24a is a murine specific gene (distinct from CD24 in human tissue).
Taguchi et al [64] performed microarray and QPCR analysis of presumptive mesonephric (E9.5) and metanephric nephron progenitors (E10.5–11.5), showing common expression of Osr1, Pax2 and Six2, despite the fact that Six2 had previously been regarded as specific to metanephros. What they did show was increased expression of Hoxa10, Hoxa11 and Hoxd12 in metanephric progenitors. This concurs with the previous reports of a requirement for Hox11 paralogs for metanephric development [84, 87]. Studies in mouse suggest expression of Hox11 paralogs (Hoxa11/c11/d11) is restricted to the caudal mesonephric and metanephric mesenchyme [84] and, more recently, lineage tracing studies in mouse suggest that this lineage boundary also represents a boundary in which caudal mesonephros / metanephric mesenchyme is Eya1-derived [64]. In mouse models, Brn1 (Pou3f3) is proposed to maintain metanephros-specific expression [84, 88] which might assist in differentiating between meso- and metanephric outcomes in vitro. Lineage tracing studies of Kobayashi et al [20] in mouse suggest that the expression of Brn1, which is conserved in human metanephros, is unique to the MM as a result of regulation by Hoxd11. [87] The co-expression of these genes should therefore indicate a metanephric identity. However, this specification in gene orthologue expression to the metanephric tubule has not been established in human models.
As noted above, there has been limited transcriptional analysis of hPSC-derived kidney tissue to date. Takasato et al [67] reported the presence of HOXD11 protein within patterning mesenchyme based upon antibody staining. While expression of all HOX11 paralogs within these organoids was very low, it increased with time as did expression of HOXA10. CDH11 expression was also present throughout organoid formation while the metanephric-specific tubular genes previously described by Georgas et al [46] (GCNT1 and HALPN1) increased across tubule formation within organoids. A human specific expression of SIX1 in the nephron progenitors of human kidney is a notable difference between the mouse and human developing kidney, although human nephron progenitors also expressed SIX2 and CITED1. In our kidney organoids [67], expression of all these genes is present at the day of aggregation and declines with time. Morizane et al [89] has reviewed the relative prevalence of SIX2+ cells between hPSC kidney differentiation protocols. What is still required is a systematic comparison of protocols at the single cell level. The challenge here is that greater insight into gene expression of these earlier stages of nephric development is not likely to be forthcoming due to a lack of access to such early human material. Human material acquired at spontaneous or elective termination is week 8 of gestation at the earliest, whereas the human pronephros arises at week 4 and the mesonephros at week 5 and regresses by week 8. Indeed, resolving this issue definitively will likely require in vitro lineage tracing within organoids, similar to have been performed in studies of human pancreatic patterning using CRISPR-Cas9 edited iPSC lines [90].
2.8. Lessons from in vivo organoid transplantation
A final yardstick to evaluate the tissue identity of what has been generated in vitro is function.
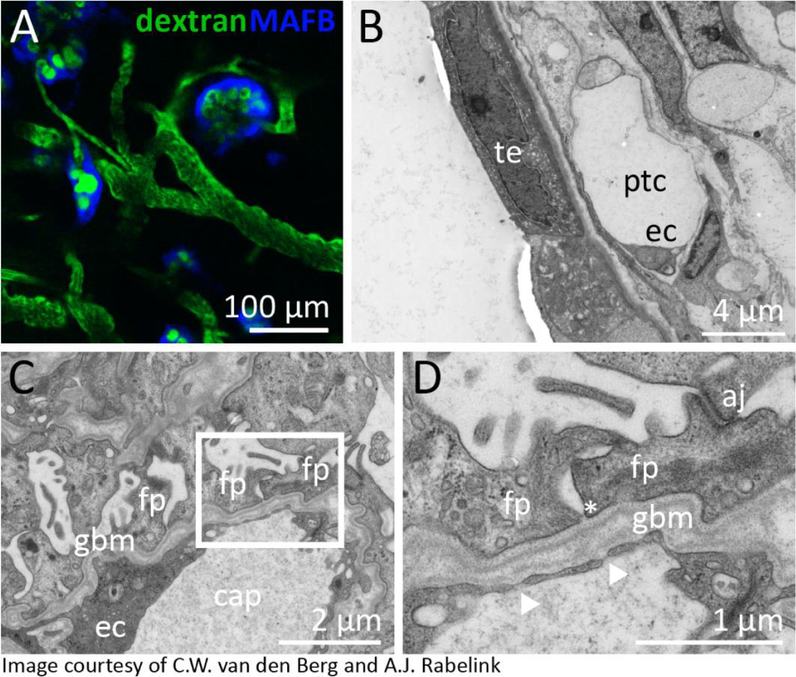
Taguchi et al [64] transplanted hPSC-derived nephrogenic mesenchyme along with spinal cord under the kidney capsule of immune deficient mouse, with the transplanted tissue forming a kidney-like tissue along with vascularized glomeruli. Sharmin et al [91], using the same differentiation protocol, developed an approach for transplanting hPSC-derived nephrons under the renal capsule along with a source of recombinant VEGFA to assist in attracting underlying endothelial sprouting. More recently, van den Berg et al [80] demonstrated that kidney organoids generated using the Takasato protocol [21, 44, 45] were producing endogenous VEGF (Figure 3). Transplantation of these structures under the renal capsule of immunocompromised mice resulted in substantial maturation of all segments of the nephrons. Wide field transmission electron microscopy revealed the formation of glomerular capillaries lined with fenestrated endothelial cells together with evidence for peritubular capillary formation (Figure 3). While prior to transplantation, renal tubules showed a pseudostratified epithelial morphology reminiscent of that observed by Lindstrom et al [44] in early human kidney, 2 weeks of in vivo maturation resulted in resolution of the tubular epithelium into a tight monolayer of polarised cells with ultrastructural features identifiable as characteristic of proximal tubules (mature brush border) and distal tubule / collecting duct (low cuboidal epithelium with evidence of occasional intercalated cells) (Figure 3). Of note, the same ultrastructural evidence of maturation was not achieved with extended in vitro culture. Imaged via a transabdominal window using intravital imaging, it was also possible to visualise murine blood (labelled with FITC-dextran injected into the tail vein) flowing through capillaries within human organoid glomeruli, the latter readily identifiable by virtue of a blue fluorescent protein CRISPR-Cas9 engineered into the MAFB gene locus of the starting iPSC cell line [80]. Using a second reporter line in which mCherry was expressed from the SOX17 locus, it was also possible to see evidence of murine blood flowing through human iPSC-derived endothelial cells, suggesting that at least a portion of the host derived vascular plexus joined with human endothelial progenitors present within the organoids. A second study has transplanted kidney organoids under the skin and also showed evidence of vascularisation from the host [92]. Together these studies would suggest that the presence of a patent vasculature improves the maturation of the hPSC-derived tissue. In summary, while the debate may continue around the specific identity of the cell types present in hPSC-derived kidney tissues, there is growing evidence that these structures do represent a model of kidney with potential utility. It is likely that with time these approaches will undergo continued tweaking and modification, both to improve the reproducibility and identity of the tissue being generated, reduce the formation of ‘off target’ cell types and modify the culture format to better facilitate applications in disease modelling, drug screening of tissue engineering.
Figure 3. Glomerular vascularisation and tubular maturation post in vivo organoid transplantation under the renal capsule.
A. Visualisation of blood flow (FITC-Dextran) through the glomerulus of an hPSC-derived human kidney organoids generated using a CRISPR-Cas9 gene edited iPSC line in which a blue fluorescent protein has been inserted into the MAFB gene locus in order to mark podocytes. B. TEM image of a proximal tubular segment within an hPSC-derived kidney organoid 2 weeks after transplantation under the renal capsule. Ec, endothelial cell; ptc, peritubular capillary; te, tubular epithelium C,D. Low and higher magnification TEM images of a glomerular basement membrane present between podocytes and endothelial cells of the organoid glomerulus. aj, adherens junction; cap, capillary; ec, endothelial cell; fp, foot process; gbm, glomerular basement membrane. Images courtesy of van den Berg et al [80].
3. Disease modelling using hPSC-derived kidney cell types
Chronic kidney disease can take years or even decades to progress to end stage renal disease, providing ample opportunity for potential therapeutic intervention. In spite of this, there exists a paucity of disease modifying treatments. This is in large part due to a limited understanding of molecular pathobiology for most causes of CKD. To date, the use of immortalized cell lines or model animals has underpinned research into kidney disease pathobiology. Mouse models offer a mature and functional mammalian organ within a whole organism providing the opportunity to examine both the renal and non-renal phenotypes. However, as described above, considerable interspecies variation exists in development, anatomy, gene orthologue function and physiology [93, 94]. This variation impedes the translation of findings back to the treatment of human kidney disease in clinical practice. The in vitro study of primary patient-derived cells is thus preferable, but is limited to cell types that are readily cultured from patient biopsies, such as dermal fibroblasts or peripheral blood mononuclear cells. This cannot provide a model where cell-type specific gene expression or phenotype is required and thus the study of kidney disease necessitates a kidney model. Techniques for the isolation of human urine-derived renal epithelial cells (HURECs) have proven useful for the study of ciliopathies [95, 96], however HURECs have a limited capacity for self-renewal and represent a unicellular, two-dimensional model of a complex, multicellular organ.
Compared to these traditional animal and cell-based models of kidney disease, hPSC-derived kidney models, such as kidney organoids, present distinct advantages as a disease modelling platform. Kidney organoids derived from patient iPSC cells allow regeneration of unlimited amounts of patient-specific, multicellular, three-dimensional kidney tissue. As multicellular tissues, kidney organoids also provide the opportunity to interrogate the contribution of different cell types to disease, assisting in the targeting of novel therapies. In addition, compared to two-dimensional culture systems, three-dimensional culture systems demonstrate differences in proliferation, cell-cell and cell-junction interactions, morphology and gene expression that are likely to be highly relevant to the study of human disease [97–99]. Kidney organoids represent multicellular models of the developing organ in question. While this can presumably result in appropriate cell-cell signalling and maintenance of improved identity of individual component cell types, the utility of such structures for disease modelling needs to be evaluated.
Transcriptional profiling of kidney organoids has suggested greatest similarity to trimester 1 human fetal kidney [67]. This would infer that diseases with early- or antenatal-onset phenotypes represent better disease modelling candidates than late-onset diseases.
However, successful disease modelling of age-related diseases such as Parkinson’s and Alzheimer’s diseases in iPSC-derived neuronal models [100], as well as the observation of antenatal phenotypes for classically adult onset diseases such as autosomal dominant polycystic kidney disease (ADPKD) [101, 102] does suggest that modelling of late-onset diseases may be feasible. Indeed, the first use of kidney organoids for inherited renal disease modelling used iPSC in which CRISPR-Cas9 gene editing generated a homozygous PKD1−/− gene knockout in an attempt to model ADPKD. Whilst not patient-derived, these organoids demonstrated epithelial cystic dilatation after extended culture [58] and subsequent manipulation of culture conditions was reported to alter cyst formation metrics [103]. This suggests that the use of hPSC represents a potential model for the investigation of cystogenesis in ADPKD [103] to which patient derived epithelium could be applied.
3.1. Application of hPSC-derived kidney models to genetic renal disease
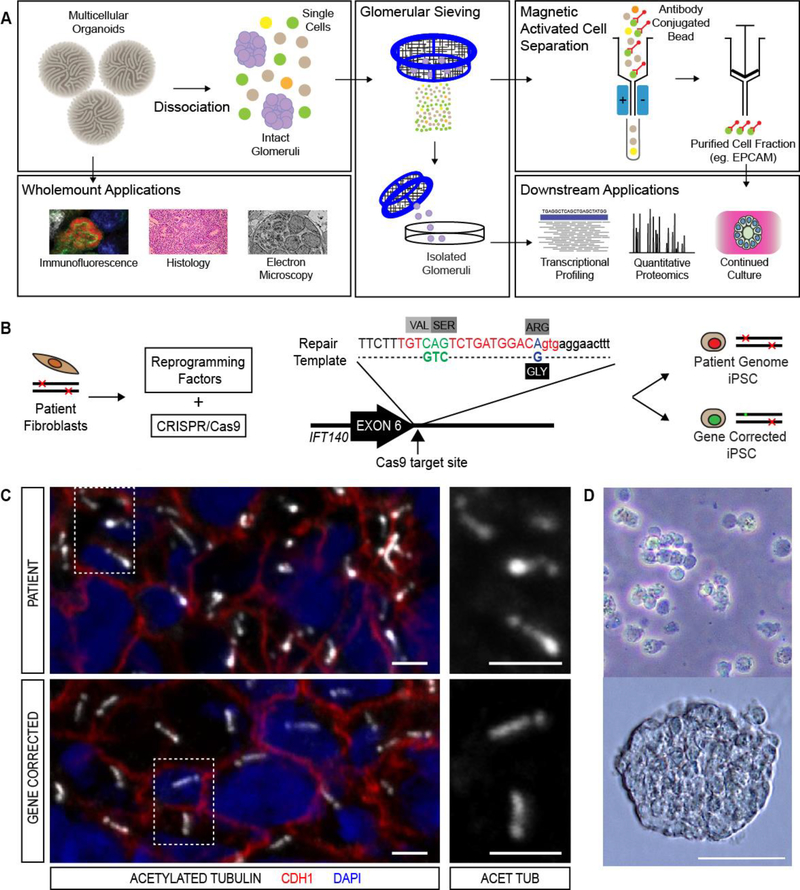
Genetic renal diseases (GRD) currently represent the strongest likely utility for kidney organoid disease modelling. Whilst advances in genomic diagnostics are increasing the overall prevalence of GRD in both adult and paediatric ESKD, many GRDs are rare and thus difficult to study in a clinical context [104, 105]. With the increasing discovery of rare, novel, genomic variants of unknown significance (VUS) by next generation sequencing, kidney organoids represent a ready platform for validation of VUS as being disease-associated. We have demonstrated that the genetic ‘correction’ of a candidate VUS using CRISPR/Cas9 technology can be performed concurrently with reprogramming to generate patient iPSC and gene corrected (or ‘isogenic’) controls from one transfection event [106]. Isogenic clones are currently the gold standard control in the field and provide a comparison whereby any phenotypic variation detected is highly likely to be related to the gene correction event rather than a shift in genomic background between lines. Our own laboratory has utilised this approach to generate iPSC from a patient suffering from the heritable ciliopathic cystic kidney disease, nephronophthisis (NPHP), arising in this patient due to compound heterozygous IFT140 variants. (Figure 4B) As well as generating patient-lines in which point mutations in the IFT140 gene were present in both alleles, CRISPR-Cas9 gene correction was applied to one mutant allele, resulting in a gene-corrected heterozygous (IFT140+/−) control [107]. When compared to gene corrected organoids, patient organoids demonstrated primary ciliary dysmorphology (Figure 4C) and a downregulation of gene expression involved in apico-basal polarity, cell adhesion and dynein motor assembly [107]. Whilst this genotype is not novel, the observation of previously validated cellular and transcriptional phenotypes for the condition within a patient derived organoid demonstrates the fidelity of the disease modelling platform.
Figure 4. Application of hPSC-derived kidney tissues for disease modelling.
A. Outline for the generation of patient-derived and isogenic iPSC lines from a Maizner-Saldino syndrome patient carrying compound heterozygote mutations in the IFT140 gene [107]. The protocol for simultaneous generation of patient and gene corrected lines is described in Howden et al [79]. B. Approach to the characterisation of disease related changes using cells isolated from hPSC-derived kidney organoids [107]. C. Immunofluorescence imaging of renal tubular epithelial cells within iPSC-derived kidney organoids showing the presence of clubbed cilia in patient organoids and wildtype cilia in organoids from gene corrected lines. D. Bright field images of intact glomeruli isolated by sieving dissociated hPSC-derived kidney organoids at low and high power. Figure courtesy of Dr. Lorna Hale.
3.2. Modelling glomerular diseases using hPSC-derived kidney tissue
While modelling of cystic kidney diseases and nephronophthisis bode well for the application of hPSC-derived kidney tissues for modelling heritable kidney diseases that affect tubular epithelium, an accurate model of the glomerulus is more challenging. Congenital nephrotic syndrome frequently arises as a result of mutations in podocyte-specific genes, which encode proteins regulating the podocyte cytoskeleton and the formation of foot processes and the slit diaphragm. There is growing evidence that a subset of steroid sensitive as well as steroid resistant nephrotic syndrome is the result of an as yet unidentified circulating factor. To model this, or any other immune mediated glomerulonephritis, accurately would require a patent circulatory system. Whilst iPSC derived glomeruli can be relatively easily isolated from whole organoids (Figure 4A and D), they lack a functional vascular system.
Glomerulopathies in which the underlying disease occurs as a result of mutations in the extracellular proteins of the glomerular basement membrane (GMB) will also not be feasible to model without the formation of a mature GBM. This requires the presence of the fenestrated endothelial cells of the glomerular capillaries. In many of these disease, the GBM defect arises due to the lack of an appropriate switch in collagens (Alport syndrome) or laminins (Pierson syndrome) that only occur during glomerular maturation and hence may not occur in an in vitro model. As discussed above, several groups have now demonstrated that hPSC-derived kidney tissue can be transplanted in vivo, including under the renal capsule or subcutaneously, resulting in the ingrowth of host-derived vasculature and the formation of patent glomerular capillaries [80, 91]. A requirement for in vivo transplantation for disease modelling makes this a long and slow process that is not tractable to compound screening. Hence, advances are required to facilitate not only an improved glomerular capillary formation in vitro, but the delivery of flow. One approach to this has been the seeding of hPSC-derived podocyte-like cells and primary human glomerular endothelial cells on either side of a laminin coated membrane within a dual channel microfluidic device capable of producing strain on the membrane [108]. In this model, cyclical mechanical strain of the membrane during microfluidic flow increased collagen IV and VEGF production by cultured podocytes as well as interaction between podocyte foot processes and the endothelium [108]. Adriamycin toxicity was modelled by increased permeability of the system to albumin [108] advocating an in vitro platform more amenable to compound screening.
3.3. Applications for hPSC-derived kidney tissue in precision medicine.
One anticipated prospect of regenerated, patient-derived kidney tissue is that of high-content compound screening to inform personalised therapeutic decisions for patients. No such example currently exists and this is likely to be due to deficiencies in the understanding of disease pathobiology and the ensuing difficulty in designing assays that can provide a rapid and clear output. As a prelude to patient-derived high throughput compound screening, forskolin-treated 3D spheroid cultures of PKD1−/− knockout mouse inner medullary collecting duct (IMCD3) cells utilised a series of spheroid metrics to identify compounds that reduced cyst growth [109]. Potential targets included mTOR, IGF-1 receptor and cyclin dependent kinases, all of which have been corroborated in animal models [109]. With the advent of a variety of approaches for generating nephron-like structures, or even specific kidney cell types, coupled with the increasing utility of gene editing to develop reporter-based readouts amenable to automated quantitative imaging, we may soon see high content approaches being applied to iPSC-derived kidney tissue. This may include siRNA screens to examine the effect of pathway modification on disease phenotype and drug repurposing screens to identify potential treatments. Czerniecki et al have recently published a high throughput kidney organoid model using a liquid handling robot which performed both culture and phenotyping of 384 well plates to streamline analysis of ideal culture conditions for new hPSC lines and using a CRISPR edited PKD1 knockout line identified a potentially novel treatment to attenuate cyst growth [110]. Advances in bioprinting techniques and microfluidic chips have regenerated tubular structures capable of receiving flow, examining the epithelial barrier and trans-epithelial transport [97, 111]. Beyond toxicity screening applications described above, it is yet to be seen whether integrating regenerated, patient-derived renal tubular cells into these systems will advance the maturity of in vitro nephrons for downstream disease modelling applications.
3.4. Limitations and challenges to modelling of renal diseases in vitro
Despite the potential application of hPSC-derived kidney tissue for modelling genetic renal disease, current differentiation protocols will remain limited in their ability to model the majority of adult onset kidney disease using patient-derived iPSC. Whilst the addition of supraphysiological glucose levels may be viewed as an approach to mimic diabetic conditions, we doubt that this will be readily modelled using current hPSC-derived structures. However, it may prove possible to create mouse models in which transplanted hPSC-derived tissues can act as avatar tissues in which to provide a more accurate readout of the response of human cells to a systemic pathological challenge [80]. Similarly, while congenital anomalies of the kidney and urinary tract (CAKUT) represent one of the most common group of birth anomalies in man, these represent structural diseases of the entire urogenital tract. Modelling of such structural renal anomalies as duplex ureters, posterior urethral valve, pelviureteric junction obstruction, horseshoe kidney, or even subtle defects such as renal hypodysplasia, is unlikely in vitro. The study of genetic tubular diseases which affect specific segments of the post-glomerular nephron (eg. Bartter Syndrome – ascending loop of Henle; Gordon Syndrome – distal tubule) will require improved maturation of the nephron and the reproducible isolation of specific cell type from the organoid. As a regenerated, embryonic tissue, the removal of any cell type from its microenvironment within the multicellular organoid is likely to immediately affect its identity and endophenotype. Whilst the purification of a cell type from the organoid for immediate lysis and transcriptional or proteomic analysis is viable, the continued culture of these cells in a new environment (for example, a microfluidic chip device) is likely to lead to dedifferentiation affecting transporter expression.
Aside from these disease specific obstacles, one of the most tangible challenges to disease modelling using hPSC-derived protocols is technical variation. This is a caveat to most disease modelling in which multicellular models are generated via the directed differentiation of hPSCs. Unlike the embryo, differentiation of pluripotent cells in vitro does not provide an anatomically precise tissue model every time a differentiation is initiated, even if the same starting cell line is used. Each brain organoid may have several cerebral fields and may or may not include, for example, an eye field. Technical variations with starting cell density or viability, degree of pluripotency, batch variations in growth factors or even culture media will contribute to variations in component cell types and anatomical structures, even if the same iPSC line is used on each occasion. This reality increases the need for appropriate controls during any disease modelling approach. While the use of isogenic lines is optimal, any comparisons between iPSC-derived tissues from distinct clones should be performed repeatedly and preferably simultaneously. With iPSC-derived tissue as complex as most of the available protocols, it may also be preferable to enrich for the specific cell type of interest. In our analysis of the effect of IFT140 mutation, transcriptional profiling and cyst formation assays were performed using EPCAM-enriched tubular epithelium [107]. Even this enrichment represented a mixed epithelial population, and analysis of the transcriptional profiling required adjustment for differential gene expression that was observed within repeated differentiations of the same protocol [112]. It is therefore imperative that an understanding of the inherent variability or robustness of any iPSC differentiation protocol is attained prior to its use for patient disease modelling purposes. Finally, the more selective the purification, the greater the number of organoids required to obtain a reasonable quantity of cell and the greater the discarded fraction of the cultured tissue. Cost effective methods to increase cell yield from differentiation protocols will be required to allow the application of these methods to clinical medicine.
4. Applications of PSC-derived kidney tissues for regenerative medicine
Over the past 20 years, there have been brave pioneers in nephrology preparing the way for alternative approaches to renal replacement. These have included those advocating porcine kidney xenotransplantation [4], fetal xenotransplantation [5], the Charleston Bioengineered Kidney Project [6], recellularistion of decellularised scaffolds [9] and the renal assist device of Professor David Humes [7, 8]. Substantial challenges have arisen with these complex models, which could be attributed to problems with the cell types used as much as with the approach. The regeneration of human kidney cell types using hPSC represents a novel approach with theoretical capacity for the development of autologous or universal renal replacement therapies. Two major approaches to the use of such cells can be envisioned; i) cell therapy, and ii) tissue engineering.
4.1. Cellular therapy
A capacity to generate specific kidney cell types either via direct reprogramming of the directed differentiation of hPSC raises the prospect that delivery of such cells back into a patient may result in functional renal improvement via functional integration. Indeed, given the capacity to generate hPSC from any individual, this may represent a viable source of autologous cell types for therapeutic purposes. As such, this approach should avoid immune rejection. Only a handful of studies have investigated the utility of delivering hPSC-derived cell types into models of renal injury. The direct injection of undifferentiated iPSC into the renal artery during ischemic acute kidney injury (AKI) was reported to reduce damage resulting from oxidative stress [113]. hPSC-derived renal progenitor cells, reportedly expressing OSR1 and SIX2, were shown to reconstitute 3D proximal renal tubule-like structures in vivo when injected under renal capsule of ischemic AKI mice [114]. It was reported that the presence of such structures resulted in the formation of renoprotective factors like Ang-1, VEGF and HGF [114]. However, neither of these studies showed evidence of engraftment of injected cells. Imberti et al reported that intravenous injection of iPSC-derived renal progenitor cells during cisplatin induced AKI robustly engrafted into damaged tubular epithelium and restored renal function and structure [115]. This is the only report to date describing the integration of introduced cells resulting functional regeneration of the injured kidney. Given the discrepancy with respect to the fate of the injected cells, differences in the injury model employed and little standardisation of characterisation of the cell types being delivered, further studies are required.
One major challenge lies in the choice of a route for cell delivery. Intravenous injection of cells in murine injury models is frequently performed via the tail vein. Injection into this site will mean the immediate delivery of the injected cells into the microvasculature of the lungs and potential removal from circulation within the spleen. As a result, it is likely that only a few injected cells will reach the kidney. Arriving via the vasculature represents a further challenge with respect to how such cells can enter the renal parenchyma. Alternatives such as direct renal parenchymal injection is suboptimal due to the risk of excessive bleeding and the long term risk of interstitial fibrosis. Transplantation under the renal capsule does not necessarily facilitate tubular integration, but may provide a better route of access than a vascular route. However, injection into the periphery of the renal cortex can result in cellular integration. This was first shown with the injection of MSC-like cells into the parenchyma of neonatal (day 1) mice [116]. A similar approach was used in recent studies in which fetal murine nephron progenitor cells (NPCs) were injected into neonatal day 1 mouse kidney resulting in the successful engraftment into the tubular epithelium [30]. Injected cells contributed to the formation of chimeric nephrons within the developing kidney suggesting onward differentiation from the nephron progenitor state. Injection of same nephron progenitor cells under the kidney capsule of adult mice during cisplatin induced acute kidney injury (AKI), showed protection in survival and reduced the complication of AKI. Of note, there was no evidence of integration of injected nephron progenitor cells into the injured areas of the kidney [30]. This suggests that the integration of NPCs by adult kidney is completely different compared to the developing neonatal kidney. While this does show the relative utility of NPCs, what remains to be seen is whether a more mature renal cell type will be able to functionally integrate into a postnatal kidney. As protocols for directed differentiation improve or become more targeted towards generating specific cell types, it will be possible to more definitively evaluate the utility of delivering more mature versus progenitor cell types as well as investigating the most amenable temporal or developmental window for successful integration.
4.2. Bioengineering
Tissue engineering refers to the possibility of combining cells and materials to create a tissue substitute that can replace some or all aspects of the function of the original tissue. The source of cells can vary from primary cells harvested from donors to stem cell-derived cell lineages. An ability to generate kidney cell types, from progenitors to specific mature kidney cell types, should allow for the bioengineering of renal replacement tissue. A wide variety of materials have been investigated for their capacity to support cell growth either ex vivo or in vivo. This has included synthetic polymers [117], natural products such as silk [118] and protein hydrogels [119]. Biological scaffolds such as decellularised organs can also be used as the support for cell seeding. As such, material scaffolds can vary in porosity and stiffness and may both support as well as instruct the cells to maintain or adopt a particular identity. Materials have also been evaluated for their capacity to induce an immune response and/or biodegrade over time. Significant advances in bioengineering tissues have been made for tissues such as artificial skin for the treatment of burns [120], cartilage to implant in articular joint disease [121] and engineered functional heart valves to treat cardiac complications [122]. Progress is also being made on bioengineering organs using pluripotent stem cell-derived cell types cultured within artificial biomaterials [123]. However, complex organs like kidney will need very sophisticated engineering techniques to achieve a fully functional and transplantable organ. The major challenge remains the reintroduction of the epithelial and stromal elements of the organ. As the adult human kidney has in excess of 26 distinct cell types, each with a very specific anatomical location within the organ, this poses a major challenge. Either each cell type or their progenitor/s will be required.
5. Major challenges to kidney cellular therapy and bioengineering
While advances in directed differentiation or transdifferentiation will provide a source of cells to drive such developments, there remain considerable challenges. Three key issues include scale, structure and function.
Scale:
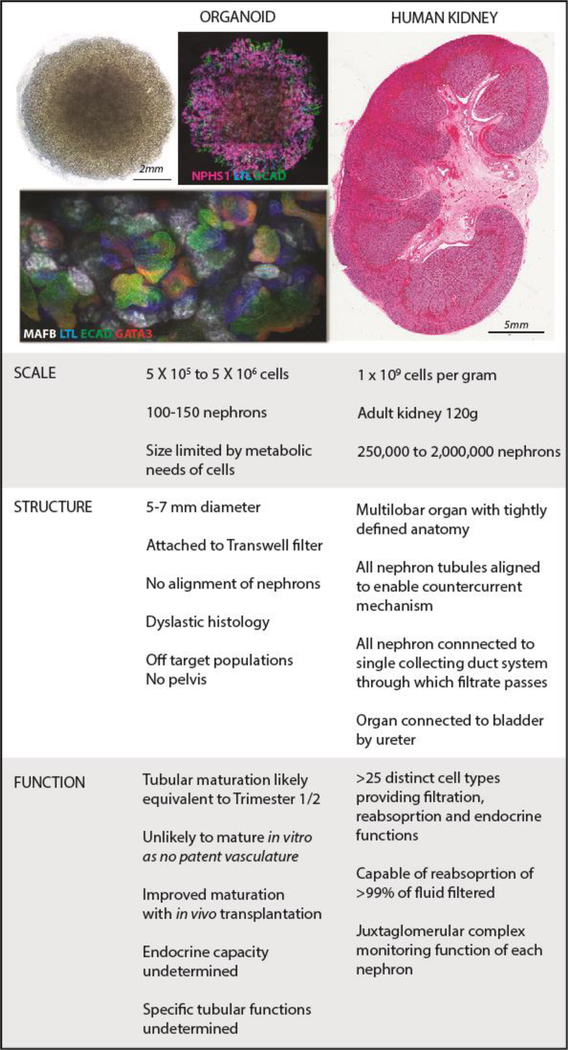
At present in our own directed differentiation protocol an organoid of 500,000 cells (5–7mm in diameter at day 25 of culture) contains 50 to 100 early nephrons (Figure 5). It is also likely to contain a proportion of off target cell populations and, as a multicellular tissue, may vary in relative cellular composition to a much larger extent that an appropriately patterned organ developing in vivo. A healthy adult kidney contains approximately 900,000 to 1 million nephrons [124], and an average gross volume of 134 to 146 cm3. This represents a major scale challenge. Were it even possible to recreate such a structure from a single cell type, the challenge is one of cell manufacturing and with that, quality control. The fact that the adult kidney is composed of >25 distinct cell types suggests that scale up is best done at the stage of pluripotency, with patterning and differentiation to follow. However, the utility of the cells generated for recreating a kidney will rely upon their accurate and reproducible patterning to utilisable kidney endpoints. Hence, the field will need to address how to generate these reliably, reproducibly, and at appropriate scale and cost, using suitable manufacturing processes. An alternative is to develop more controlled approaches for the uniform differentiation of specific kidney cell types. There are a number of reports of specific generation of podocytes or proximal tubular cells [56, 57], but the level of characterisation of these protocols (little or no extensive expression profiling or functional analysis) is such that it is currently insufficient to determine purity or commitment. It would be impractical to recreate an entire tissue one cell type at a time, however such approaches allow for the production of specific cell types for use in cellular therapy or for screening purposes. Any cellular production at scale will need to consider how to evaluate mutation accumulation with cell expansion [125]. This is a major topic of discussion in the iPSC field and a number of groups are now developing approaches for the engineering of suicide switches into the hPSC lines to ensure that any tissue showing oncogenic transformation can be ‘killed’ upon command [126, 127]. Even with these issues addressed, the expansion required to recreate a tissue of the scale of the human kidney may be out of reach technically and economically.
Figure 5. Scale, structure and function represent key challenges to ‘rebuilding a kidney’ using hPSC-derived models.
Images of organoids courtesy of Ms. Pei Xuan Er and Dr. Jessica Vanslambrouck, Murdoch Children’s Research Institute. Image of female human kidney (35 weeks gestation) courtesy of Prof. Mary Jane Black and Dr, Megan Sutherland, Monash University.
Structure:
The second major hurdle will be structure (Figure 5). Currently, the success of isolating, maintaining or generating kidney tissue in vitro is being measured based upon the formation of structures with markers reminiscent of early nephrons within the developing fetal kidney. What is rarely commented upon is the overall histology of such cultures. A scan of the available histological sections suggests that while there are recognisable histological features, the majority of progenitor or hPSC-derived kidney tissue contains nephrons that are poorly patterned and dysplastic [67] and parenchyma that is fibrotic [63] and sometimes contain ectopic tissue types, including cartilage [92]. While the transplantation of organoids in vivo shows a capacity for the nephrons present to draw in blood vessels, some of these tissues contain largely glomeruli and even when there is evidence of a distal tubule / collecting duct-like epithelium, organoids do not necessarily have a unified nephron complement. In addition, unlike the kidney, no human kidney organoid to date has a ureteric tree feeding into a pelvis and in turn connected to a ureter.
One approach to the challenge of ‘structure’ in kidney tissue engineering has been the concept of recellularization of decellularized kidney scaffolds. Such decellularized scaffolds have been generated from a wide variety of tissues, including human lung, heart and kidney [128]. The concept here is that the tissue itself provides an accurate large-scale model of the organ of interest with the remaining ECM components providing both a supportive and an instructive environment into which a source of replacement cells can be provided. Many studies have now demonstrated the feasibility of kidney decellularization in rodent models and using human kidney tissue [9, 129, 130]. Decellularized natural scaffolds can be prepared by continuous rinsing of kidney tissue with sodium dodecyl sulphate (SDS) based solution through the renal artery until all cells are removed. This method has been successfully shown using rat [131, 132], non-human primates [133], pigs [9, 134] and cadaveric human kidneys [9, 135]. Further resin casting analysis of entire decellularized human kidney maintain a well-preserved structure and function of the vasculature, as well as growth factors that are fundamental to achieve a satisfactory recellularization [136]. It is also reported that ECM in the scaffold also instructive to the pluripotent cells to differentiate into appropriate adult cell types. Remuzzi A et al. transplanted decellularized rat kidney scaffolds in vivo. This resulted in no evidence of recullularization by host, but it preserved the scaffold ECM structure [137]. The major challenges lie in how to reintroduce all the required epithelial cells types into the complex narrow channels of the nephrons within the scaffolds. Indeed, to be able to redeliver renal interstitial cells is also a quandary.
Initial studies into arterial recellularization of rat kidney scaffolds with embryonic stem cells and HUVECs showed 95% retention of cells which were well distributed across the vascular network including the glomeruli [9, 131, 138]. Retrograde seeding of embryonic stem cells or neonatal rat kidney cell suspension under negative pressure, through the ureter via renal collecting duct system resulted in a complete organ recellularization, including glomeruli. Embryonic cell cytology was eventually lost over period and formed the well differentiated flattened endothelial cells, mature glomerular podocytes and tubular cells, confirming scaffold guide pluripotent cell fate [9, 131]. Further orthotropic transplantation into rat showed graft survival and also produced urine [9]. Remuzzi et al attempted to recellularize rat kidney scaffolds with mouse embryonic stem cells via renal artery, vein and ureter applying different pressure and flow systems [137]. They concluded that this resulted in very limited and inconsistent cell seeding [137], due in part to the ECM physical barriers acts as a resistant to the cell migration. Clearly there is a long way to go to recreate an organ of this scale in this way. Indeed, the gap remains between hypothesis and reality.
Function:
The final challenge lies in functional maturation. The adult kidney contains more than 20 different cell types, each with distinct functions, many of which are only achieved as a result of the appropriate arrangement of these component cells. For example, a glomerulus will not function appropriately unless podocytes form appropriate foot processes, the endothelial cells fenestrate and, together with the podocytes, generate an appropriate glomerular basement, the capillary tuft is supported by mesangial cells and the renal corpuscle is surrounded by a Bowman’s capsule with appropriately patterned parietal epithelial cells. Proximal tubules need to develop appropriate transporters and microvilli for maximum reabsorption while specialized distal tubule cells are required to create a functional macula densa. The stalks of the ureteric tree must mature to maximally reabsorb water and regulate acid/base balance whilst the medullary cells must form a water impermeable uroplakin layer. Indeed, the perivascular cells, renal fibroblasts, erythropoietin and renin producing cells are required to deliver the endocrine functions of kidney [139]. While many of these cells do arise from a small number of progenitors, much of the functional maturation of these individual cell types and nephron segments does not occur until the postnatal period. By contrast, the kidney structures being generated in vitro from hPSC / progenitor populations are models of the developing organ, not the postnatal structure. It is evident again from recent in vivo transplantation studies [80, 91, 92] that the provision of a patent blood supply facilitates substantial structural maturation. It is likely, although it is not yet proven, that this will facilitate functional maturation. The nature of what is required will depend upon the therapeutic approach being proposed. For cell therapy, integration of a progenitor cell type may be more likely than anticipating a capacity for a quiescent and highly differentiated cell to be able to home, integrate and function. Within bioengineering, the target structure does not need to exactly replicate the original tissue, as long as it achieves the desired functional outcome. That target functional outcome may also only represent a subset of the in vivo tissue functions and hence only require a subset of cell types.
6. Conclusions
In this review, we have provided an update on the use of human pluripotent stem cells for the generation of human kidney cell types. The progress made to date, while a major leap forward, remains preliminary in contrast to the bona fide organ. Indeed, the human adult kidney contains an average of 1 million nephrons, all of which are architecturally aligned to facilitate the countercurrent mechanism essential for concentration of the urinary filtrate. As yet, we have been able to create the fetal cell types and begin to perfuse such structures, but each organoid is effectively dysplastic and very immature. The key challenges include scale, structure and functional maturation, with each representing significant obstacles to renal bioengineering. However, the reproducibility with which fetal kidney cell types can be generated in vitro provides a capacity to re-evaluate previously proposed approaches to renal regeneration. It may be argued that the recreation of an organ as architecturally complex as the kidney in vitro is simply not feasible. Indeed, it is unlikely that this will be possible. However, recreation of a perfect replica of the kidney is not the only solution. At present, only 1 in 4 renal failure patients receives a transplanted organ. All other patients survive on peritoneal or haemodialysis which replaces at best 15% of normal glomerular filtration rate. The provision of a cellular replacement capable of providing better than what is currently afforded by dialysis, even if only transiently, is perhaps a more realistic target. Indeed, this was the objective of the renal assist device in which primary kidney epithelial cells from cadaveric tissues were seeded allow hollow fibres and provided in line with dialysis for the treatment of acute kidney injury [7]. The development of that product reached clinical trial but did not proceed. Revaluating such approaches with currently available cell sources is not without merit. And while therapeutic targets are far off, even with the imperfect kidney tissue that we can generate, we now have a window into human development and disease previously unavailable. The capacity to generate patient-derived kidney cell types for drug efficacy and toxicity screening is a major advance. It is here that stem-cell derived human kidney models are most likely to change clinical practice.
Acknowledgements
MHL is a Senior Principal Research Fellow of the National Health and Medical Research Council of Australia (GNT1136085). TF is an NHMRC Postgraduate Scholar (GNT1114409) and holds an RACP Jacquot NHMRC Excellence Award. Their research is supported by the NHMRC (GNT1098654; GNT1100970) and the National Institutes of Health (DK107344–01). ML holds intellectual property around the generation of kidney organoids from hPSC and consults for Organovo Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.USRDS. Chapter 6: Healthcare Expenditures for Persons with CKD. USRDS Annual Data Report 2017. [cited 2018. [Google Scholar]
- 2.AIHW, Australia’s Health 2014. 2014, Australian Institute of Health and Welfare: Canberra: p. 178. [Google Scholar]
- 3.Caskey F, Steenkamp R, and Thomas K, UK Renal Registry 19th Annual Report: Introduction. Nephron, 2017. 137(suppl 1)(Suppl. 1): p. 1–10. [DOI] [PubMed] [Google Scholar]
- 4.Cowan PJ, Cooper DK, and d’Apice AJ, Kidney xenotransplantation. Kidney Int, 2014. 85(2): p. 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammerman MR, Xenotransplantation of embryonic pig kidney or pancreas to replace the function of mature organs. J Transplant, 2011. 2011: p. 501749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mironov V, Drake C, and Wen X, Research project: Charleston Bioengineered Kidney Project. Biotechnol J, 2006. 1(9): p. 903–5. [DOI] [PubMed] [Google Scholar]
- 7.Humes HD, et al. , Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int, 2004. 66(4): p. 1578–88. [DOI] [PubMed] [Google Scholar]
- 8.Tumlin J, et al. , Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol, 2008. 19(5): p. 1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JJ, et al. , Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med, 2013. 19(5): p. 646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little MH, Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol, 2006. 17(9): p. 2390–401. [DOI] [PubMed] [Google Scholar]
- 11.Thomson JA, et al. , Embryonic stem cell lines derived from human blastocysts. Science, 1998. 282(5391): p. 1145–7. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, et al. , Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 2007. 131(5): p. 861–72. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi K and Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006. 126(4): p. 663–76. [DOI] [PubMed] [Google Scholar]
- 14.Vierbuchen T, et al. , Direct conversion of fibroblasts to functional neurons by defined factors. Nature, 2010. 463(7284): p. 1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, et al. , Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A, 2011. 108(19): p. 7838–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendry CE, et al. , Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol, 2013. 24(9): p. 1424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little MH, Combes AN, and Takasato M, Understanding kidney morphogenesis to guide renal tissue regeneration. Nature Reviews Nephrology, 2016. 12: p. 624. [DOI] [PubMed] [Google Scholar]
- 18.Little MH and McMahon AP, Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol, 2012. 4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costantini F and Kopan R, Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell, 2010. 18(5): p. 698–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi A, et al. , Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell, 2008. 3(2): p. 169–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindstrom NO, et al. , Conserved and Divergent Features of Mesenchymal Progenitor Cell Types within the Cortical Nephrogenic Niche of the Human and Mouse Kidney. J Am Soc Nephrol, 2018. 29(3): p. 806–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barak H, et al. , FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell, 2012. 22(6): p. 1191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blank U, et al. , BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development, 2009. 136(21): p. 3557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karner CM, et al. , Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development, 2011. 138(7): p. 1247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carroll TJ, et al. , Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell, 2005. 9(2): p. 283–92. [DOI] [PubMed] [Google Scholar]
- 26.Park JS, et al. , Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell, 2012. 23(3): p. 637–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung E, et al. , Notch signaling promotes nephrogenesis by downregulating Six2. Development, 2016. 143(21): p. 3907–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung E, Deacon P, and Park JS, Notch is required for the formation of all nephron segments and primes nephron progenitors for differentiation. Development, 2017. 144(24): p. 4530–4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AC, Muthukrishnan SD, and Oxburgh L, A synthetic niche for nephron progenitor cells. Dev Cell, 2015. 34(2): p. 229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Z, et al. , 3D Culture Supports Long-Term Expansion of Mouse and Human Nephrogenic Progenitors. Cell Stem Cell, 2016. 19(4): p. 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pode-Shakked N, et al. , E vidence of In Vitro Preservation of Human Nephrogenesis at the Single-Cell Level. Stem Cell Reports, 2017. 9(1): p. 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pode-Shakked N, et al. , Dissecting Stages of Human Kidney Development and Tumorigenesis with Surface Markers Affords Simple Prospective Purification of Nephron Stem Cells. Sci Rep, 2016. 6: p. 23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanigawa S, et al. , Preferential Propagation of Competent SIX2+ Nephronic Progenitors by LIF/ROCKi Treatment of the Metanephric Mesenchyme. Stem Cell Reports, 2015. 5(3): p. 435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaminski MM, et al. , Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol, 2016. 18(12): p. 1269–1280. [DOI] [PubMed] [Google Scholar]
- 35.Bard JB, et al. , Early nephron formation in the developing mouse kidney. J Anat, 2001. 199(Pt 4): p. 385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Georgas K, et al. , Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol, 2009. 332(2): p. 273–86. [DOI] [PubMed] [Google Scholar]
- 37.Saxen L and Sariola H, Early organogenesis of the kidney. Pediatr Nephrol, 1987. 1(3): p. 385–92. [DOI] [PubMed] [Google Scholar]
- 38.Barker N, et al. , Lgr5(+ve) stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep, 2012. 2(3): p. 540–52. [DOI] [PubMed] [Google Scholar]
- 39.Mugford JW, et al. , High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol, 2009. 333(2): p. 312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunskill EW, et al. , Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell, 2008. 15(5): p. 781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindstrom NO, et al. , Integrated beta-catenin, BMP, PTEN, and Notch signalling patterns the nephron. Elife, 2015. 3: p. e04000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng HT, et al. , Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development, 2007. 134(4): p. 801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das A, et al. , Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol, 2013. 15(9): p. 1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindstrom NO, et al. , Conserved and Divergent Features of Human and Mouse Kidney Organogenesis. J Am Soc Nephrol, 2018. 29(3): p. 785–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindstrom NO, et al. , Conserved and Divergent Molecular and Anatomic Features of Human and Mouse Nephron Patterning. J Am Soc Nephrol, 2018. 29(3): p. 825–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Georgas KM, et al. , Expression of metanephric nephron-patterning genes in differentiating mesonephric tubules. Dev Dyn, 2011. 240(6): p. 1600–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawrence ML, Smith JR, and Davies JA, Functional Transport of Organic Anions and Cations in the Murine Mesonephros. Am J Physiol Renal Physiol, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Hinchliffe SA, et al. , Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest, 1991. 64(6): p. 777–84. [PubMed] [Google Scholar]
- 49.Ryan D, et al. , Development of the Human Fetal Kidney from Mid to Late Gestation in Male and Female Infants. EBioMedicine, 2018. 27: p. 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harari-Steinberg O, et al. , Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med, 2013. 5(10): p. 1556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pleniceanu O, Harari-Steinberg O, and Dekel B, Concise review: Kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells, 2010. 28(9): p. 1649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dekel B, et al. , Human and porcine early kidney precursors as a new source for transplantation. Nat Med, 2003. 9(1): p. 53–60. [DOI] [PubMed] [Google Scholar]
- 53.Tanigawa S, et al. , Selective In Vitro Propagation of Nephron Progenitors Derived from Embryos and Pluripotent Stem Cells. Cell Rep, 2016. 15(4): p. 801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis RL, Weintraub H, and Lassar AB, Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell, 1987. 51(6): p. 987–1000. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, et al. , Induced pluripotent stem cell lines derived from human somatic cells. Science, 2007. 318(5858): p. 1917–20. [DOI] [PubMed] [Google Scholar]
- 56.Song B, et al. , The directed differentiation of human iPS cells into kidney podocytes. PLoS One, 2012. 7(9): p. e46453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narayanan K, et al. , Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int, 2013. 83(4): p. 593–603. [DOI] [PubMed] [Google Scholar]
- 58.Freedman BS, et al. , Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun, 2015. 6: p. 8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang M and Han YM, Differentiation of human pluripotent stem cells into nephron progenitor cells in a serum and feeder free system. PLoS One, 2014. 9(4): p. e94888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lam AQ, et al. , Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol, 2014. 25(6): p. 1211–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mae SI, et al. , Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochem Biophys Res Commun, 2018. 495(1): p. 954–961. [DOI] [PubMed] [Google Scholar]
- 62.Mae SI, et al. , Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun, 2013. 4: p. 1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morizane R, et al. , Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol, 2015. 33(11): p. 1193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taguchi A, et al. , Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell, 2014. 14(1): p. 53–67. [DOI] [PubMed] [Google Scholar]
- 65.Taguchi A and Nishinakamura R, Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell, 2017. 21(6): p. 730–746.e6. [DOI] [PubMed] [Google Scholar]
- 66.Takasato M, et al. , Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol, 2014. 16(1): p. 118–26. [DOI] [PubMed] [Google Scholar]
- 67.Takasato M, et al. , Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature, 2015. 526(7574): p. 564–8. [DOI] [PubMed] [Google Scholar]
- 68.Xia Y, et al. , Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol, 2013. 15(12): p. 1507–15. [DOI] [PubMed] [Google Scholar]
- 69.Giacomelli E, Mummery CL, and Bellin M, Human heart disease: lessons from human pluripotent stem cell-derived cardiomyocytes. Cell Mol Life Sci, 2017. 74(20): p. 3711–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wahlster L and Daley GQ, Progress towards generation of human haematopoietic stem cells. Nat Cell Biol, 2016. 18(11): p. 1111–1117. [DOI] [PubMed] [Google Scholar]
- 71.Ader M and Tanaka EM, Modeling human development in 3D culture. Curr Opin Cell Biol, 2014. 31: p. 23–8. [DOI] [PubMed] [Google Scholar]
- 72.Trinkaus JP and Groves PW, DIFFERENTIATION IN CULTURE OF MIXED AGGREGATES OF DISSOCIATED TISSUE CELLS. Proc Natl Acad Sci U S A, 1955. 41(10): p. 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lusis M, et al. , Isolation of clonogenic, long-term self renewing embryonic renal stem cells. Stem Cell Res, 2010. 5(1): p. 23–39. [DOI] [PubMed] [Google Scholar]
- 74.Unbekandt M and Davies JA, Dissociation of embryonic kidneys followed by reaggregation allows the formation of renal tissues. Kidney Int, 2010. 77(5): p. 407–16. [DOI] [PubMed] [Google Scholar]
- 75.Takasato M and Little MH, The origin of the mammalian kidney: implications for recreating the kidney in vitro. Development, 2015. 142(11): p. 1937–47. [DOI] [PubMed] [Google Scholar]
- 76.Pradervand S, et al. , A comprehensive analysis of gene expression profiles in distal parts of the mouse renal tubule. Pflugers Arch, 2010. 460(6): p. 925–52. [DOI] [PubMed] [Google Scholar]
- 77.Combes AN, et al. , High throughput single cell RNA-seq of developing mouse kidney and human kidney organoids reveal a roadmap for recreating the kidney. bioRxiv, 2017. [Google Scholar]
- 78.H., W., et al. , Comparative analysis of kidney organoid and adult human kidney single cell and single nucleus transcriptomes. bioRxiv, 2017. [Google Scholar]
- 79.Howden SE, Thomson JA, and Little MH, Simultaneous reprogramming and gene editing of human fibroblasts. Nat Protoc, 2018. 13(5): p. 875–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van den Berg CW, et al. , Renal Subcapsular Transplantation of PSC-Derived Kidney Organoids Induces Neo-vasculogenesis and Significant Glomerular and Tubular Maturation In Vivo. Stem Cell Reports, 2018. 10(3): p. 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vize PD, Woolf AS, and Bard JB, The kidney: from normal development to congenital disease. 2003: Academic Press. [Google Scholar]
- 82.Sainio K, et al. , Differential regulation of two sets of mesonephric tubules by WT-1. Development, 1997. 124(7): p. 1293–9. [DOI] [PubMed] [Google Scholar]
- 83.Smith C and Mackay S, Morphological development and fate of the mouse mesonephros. J Anat, 1991. 174: p. 171–84. [PMC free article] [PubMed] [Google Scholar]
- 84.Mugford JW, et al. , Hoxd11 specifies a program of metanephric kidney development within the intermediate mesoderm of the mouse embryo. Dev Biol, 2008. 319(2): p. 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Murashima A, Xu B, and Hinton BT, Understanding normal and abnormal development of the Wolffian/epididymal duct by using transgenic mice. Asian J Androl, 2015. 17(5): p. 749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Challen GA, et al. , Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol, 2004. 15(9): p. 2344–57. [DOI] [PubMed] [Google Scholar]
- 87.Wellik DM, Hawkes PJ, and Capecchi MR, Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev, 2002. 16(11): p. 1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakai S, et al. , Crucial roles of Brn1 in distal tubule formation and function in mouse kidney. Development, 2003. 130(19): p. 4751–9. [DOI] [PubMed] [Google Scholar]
- 89.Morizane R and Bonventre JV, Kidney Organoids: A Translational Journey. Trends Mol Med, 2017. 23(3): p. 246–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi ZD, et al. , Genome Editing in hPSCs Reveals GATA6 Haploinsufficiency and a Genetic Interaction with GATA4 in Human Pancreatic Development. Cell Stem Cell, 2017. 20(5): p. 675–688.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharmin S, et al. , Human Induced Pluripotent Stem Cell-Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation. J Am Soc Nephrol, 2016. 27(6): p. 1778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bantounas I, et al. , Generation of Functioning Nephrons by Implanting Human Pluripotent Stem Cell-Derived Kidney Progenitors. Stem Cell Reports, 2018. 10(3): p. 766–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Perlman RL, Mouse models of human disease: An evolutionary perspective. Evol Med Public Health, 2016. 2016(1): p. 170–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waterston RH, et al. , Initial sequencing and comparative analysis of the mouse genome. Nature, 2002. 420(6915): p. 520–62. [DOI] [PubMed] [Google Scholar]
- 95.Ajzenberg H, et al. , Non-invasive sources of cells with primary cilia from pediatric and adult patients. Cilia, 2015. 4: p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Srivastava S, et al. , A human patient-derived cellular model of Joubert syndrome reveals ciliary defects which can be rescued with targeted therapies. Hum Mol Genet, 2017. 26(23): p. 4657–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Homan KA, et al. , Bioprinting of 3D Convoluted Renal Proximal Tubules on Perfusable Chips. Sci Rep, 2016. 6: p. 34845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sanchez-Romero N, et al. , In vitro systems to study nephropharmacology: 2D versus 3D models. Eur J Pharmacol, 2016. 790: p. 36–45. [DOI] [PubMed] [Google Scholar]
- 99.Pampaloni F, Reynaud EG, and Stelzer EH, The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol, 2007. 8(10): p. 839–45. [DOI] [PubMed] [Google Scholar]
- 100.Srikanth P and Young-Pearse TL, Stem cells on the brain: modeling neurodevelopmental and neurodegenerative diseases using human induced pluripotent stem cells. J Neurogenet, 2014. 28(1–2): p. 5–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grantham JJ, et al. , Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int, 2008. 73(1): p. 108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Piontek K, et al. , A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med, 2007. 13(12): p. 1490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cruz NM, et al. , Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater, 2017. 16(11): p. 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mallett A, et al. , The prevalence and epidemiology of genetic renal disease amongst adults with chronic kidney disease in Australia. Orphanet J Rare Dis, 2014. 9: p. 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fletcher J, et al. , Prevalence of genetic renal disease in children. Pediatr Nephrol, 2013. 28(2): p. 251–6. [DOI] [PubMed] [Google Scholar]
- 106.Howden SE, et al. , Simultaneous Reprogramming and Gene Correction of Patient Fibroblasts. Stem Cell Reports, 2015. 5(6): p. 1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Forbes TA, et al. , Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet, Unpublished results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Musah S, et al. , Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng, 2017. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Booij TH, et al. , High-Throughput Phenotypic Screening of Kinase Inhibitors to Identify Drug Targets for Polycystic Kidney Disease. SLAS Discov, 2017. 22(8): p. 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Czerniecki SM, et al. , High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jansen J, et al. , Human proximal tubule epithelial cells cultured on hollow fibers: living membranes that actively transport organic cations. Sci Rep, 2015. 5: p. 16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Phipson B, et al. , Transcriptional evaluation of the developmental accuracy, reproducibility and robustness of kidney organoids derived from human pluripotent stem cells. Nature Methods Unpublished results. [Google Scholar]
- 113.Lee PY, et al. , Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant, 2012. 21(12): p. 2569–85. [DOI] [PubMed] [Google Scholar]
- 114.Toyohara T, et al. , Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. Stem Cells Transl Med, 2015. 4(9): p. 980–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Imberti B, et al. , Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci Rep, 2015. 5: p. 8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li J, et al. , Collecting duct-derived cells display mesenchymal stem cell properties and retain selective in vitro and in vivo epithelial capacity. J Am Soc Nephrol, 2015. 26(1): p. 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Steffens D, et al. , Update on the main use of biomaterials and techniques associated with tissue engineering. Drug Discov Today, 2018. [DOI] [PubMed] [Google Scholar]
- 118.Altman GH, et al. , Silk-based biomaterials. Biomaterials, 2003. 24(3): p. 401–16. [DOI] [PubMed] [Google Scholar]
- 119.Leijten J, et al. , Spatially and Temporally Controlled Hydrogels for Tissue Engineering. Mater Sci Eng R Rep, 2017. 119: p. 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rennekampff HO, Kiessig V, and Hansbrough JF, Current concepts in the development of cultured skin replacements. J Surg Res, 1996. 62(2): p. 288–95. [DOI] [PubMed] [Google Scholar]
- 121.Oliva A, et al. , Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials, 1996. 17(13): p. 1351–6. [PubMed] [Google Scholar]
- 122.Hockaday LA, et al. , Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication, 2012. 4(3): p. 035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Minuth WW, Sittinger M, and Kloth S, Tissue engineering: generation of differentiated artificial tissues for biomedical applications. Cell Tissue Res, 1998. 291(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 124.Bertram JF, et al. , Human nephron number: implications for health and disease. Pediatr Nephrol, 2011. 26(9): p. 1529–33. [DOI] [PubMed] [Google Scholar]
- 125.Hong SG, Dunbar CE, and Winkler T, Assessing the risks of genotoxicity in the therapeutic development of induced pluripotent stem cells. Mol Ther, 2013. 21(2): p. 272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Itakura G, et al. , Fail-Safe System against Potential Tumorigenicity after Transplantation of iPSC Derivatives. Stem Cell Reports, 2017. 8(3): p. 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yagyu S, et al. , An Inducible Caspase-9 Suicide Gene to Improve the Safety of Therapy Using Human Induced Pluripotent Stem Cells. Mol Ther, 2015. 23(9): p. 1475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gilpin A and Yang Y, Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int, 2017. 2017: p. 9831534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caralt M, et al. , Optimization and critical evaluation of decellularization strategies to develop renal extracellular matrix scaffolds as biological templates for organ engineering and transplantation. Am J Transplant, 2015. 15(1): p. 64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Figliuzzi M, Bonandrini B, and Remuzzi A, Decellularized kidney matrix as functional material for whole organ tissue engineering. J Appl Biomater Funct Mater, 2017. 15(4): p. 0. [DOI] [PubMed] [Google Scholar]
- 131.Ross EA, et al. , Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol, 2009. 20(11): p. 2338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ross EA, et al. , Mouse stem cells seeded into decellularized rat kidney scaffolds endothelialize and remodel basement membranes. Organogenesis, 2012. 8(2): p. 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakayama KH, et al. , Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A, 2010. 16(7): p. 2207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Orlando G, et al. , Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann Surg, 2012. 256(2): p. 363–70. [DOI] [PubMed] [Google Scholar]
- 135.Orlando G, et al. , Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials, 2013. 34(24): p. 5915–25. [DOI] [PubMed] [Google Scholar]
- 136.Peloso A, et al. , Renal Extracellular Matrix Scaffolds From Discarded Kidneys Maintain Glomerular Morphometry and Vascular Resilience and Retains Critical Growth Factors. Transplantation, 2015. 99(9): p. 1807–16. [DOI] [PubMed] [Google Scholar]
- 137.Remuzzi A, et al. , Experimental Evaluation of Kidney Regeneration by Organ Scaffold Recellularization. Sci Rep, 2017. 7: p. 43502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bonandrini B, et al. , Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Eng Part A, 2014. 20(9–10): p. 1486–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zeisberg M and Kalluri R, Physiology of the Renal Interstitium. Clin J Am Soc Nephrol, 2015. 10(10): p. 1831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]