Abstract
Context
Cardiometabolic conditions increase in midlife, but early customized prevention strategies are not established for such women.
Objective
To characterize and identify factors longitudinally related to constellations of cardiometabolic risk components in multiracial/ethnic women in midlife.
Design
We conducted a prospective, longitudinal, multiethnic cohort study of 3003 midlife women undergoing menopausal transition (MT). Metabolic syndrome (MetS) was defined as having at least three of five components: high fasting triglyceride (hTG) level, low high-density lipoprotein cholesterol (lHDL-C) level, high fasting plasma glucose (hGluc) level, large waist circumference (abdominal obesity; Ob), and hypertension (HTN). We described the patterns of constellations and estimated hazard ratios (HRs) for constellations at (i) incident MetS and (ii) recovery from MetS, using multivariable-adjusted Cox regression.
Setting
Seven US sites.
Participants
In all, 1412 non-Hispanic white, 851 black, 272 Japanese, 237 Hispanic, and 231 Chinese women.
Exposures
Race/ethnicity, lifestyle factors, and MT stage.
Main Outcomes Measures
Cardiometabolic constellations, incident MetS, and MetS recovery.
Results
Central obesity was the most frequent component. Having no components was the most frequent (31%) baseline constellation. Physical activity (HR = 1.68; 95% CI: 1.06, 2.68) and lower caloric intake (HR = 0.96; 95% CI: 0.93, 0.99 per 100 cal/d) were associated with recovery from MetS. Ob/hTG/lHDL-C (18%), Ob/HTN/lHDL-C (16%), and Ob/HTN/hGluc (14%) were frequent incident constellations. Physically active women had 26% to 62% lower hazards of incident MetS than inactive women.
Conclusions
Modifiable lifestyle behaviors were related to recovery from MetS and decreased risk of the most frequent MetS constellations in midlife women.
This study characterized longitudinal patterns of cardiometabolic health in multiethnic midlife women. Modifiable lifestyle factors may be targets in midlife to prevent cardiometabolic diseases.
The United States Healthy People 2020 Initiative prioritizes reducing high rates of cardiovascular disease (CVD) and type 2 diabetes (T2D) (1). Women’s CVD rates escalate in midlife toward those of 75-year old men (2). Midlife women have a one-in-three chance of developing metabolic syndrome (MetS) (3, 4), a multifactorial condition that predisposes them to T2D and CVD. MetS is defined as having at least three of five cardiometabolic risk components (5): (i) high fasting triglyceride level (hTG); (ii) low high-density lipoprotein cholesterol (lHDL-C) level; (iii) elevated fasting plasma glucose level; (iv) large waist circumference (abdominal obesity; Ob); and (v) hypertension (HTN). The constellations of these cardiometabolic risk components provide a composite of an individual’s cardiometabolic status and risk of future CVD and T2D.
Certain constellations of these components may confer higher risks of CVD and mortality (6). For example, in the Framingham Heart Study the specific MetS constellations of central obesity/HTN/hyperglycemia and lHDL-C/HTN/high triglycerides conferred twofold to threefold increased risks (6). Black and white women may have different distributions of MetS components and their constellations (7). Having more MetS components may also be related to the severity of coronary heart disease (6).
In the Study of Women’s Health Across the Nation (SWAN), MetS incidence increased as midlife women underwent menopausal transition (MT) (8). Nevertheless, patterns of the cardiometabolic components (constellations) have not been established overall or for racial/ethnic groups. Our objectives were to characterize the longitudinal constellations of cardiometabolic risk components and identify factors associated with progression to or recovery from MetS in women undergoing MT. We leveraged data from SWAN, a longitudinal, prospective, multiracial/ethnic cohort study of premenopausal or early perimenopausal women who underwent MT during follow-up. Midlife women who are identified as having high-risk constellations over time might benefit from early intervention strategies to reduce CVD and T2D.
Methods
Source and study populations
The methods for sampling and recruitment in SWAN were previously described in detail (9). Briefly, in 1996 to 1997, SWAN enrolled 3302 women aged 42 to 52 years who had not taken hormone therapy or oral contraceptives for at least 3 months, were not pregnant or lactating, had not had a hysterectomy or bilateral oophorectomy, and had a menstrual period in the prior 3 months. Each of seven US study sites enrolled non-Hispanic white (NHW) women and one other racial/ethnic group: black (four sites), Hispanics (one site), Japanese (one site), and Chinese (one site). Institutional review boards at all sites approved the study protocols, and all women provided written informed consent.
Our analytic study population consisted of 3003 women at SWAN baseline. In subanalyses, 299 were excluded from this analytic cohort for reasons including unknown MetS status, MT stage, or educational status. Excluded women were more likely to be Hispanic; 16% were excluded because six annual follow-up visits were not completed at the site with Hispanic participants.
Assessment of participant characteristics
Race/ethnicity was self-reported at baseline as non-Hispanic black or African American (black), NHW, Chinese or Chinese American, Japanese or Japanese American, or Hispanic. MT stage was time varying and classified on the basis of self-reported bleeding patterns: premenopausal (a menstrual period in the prior 3 months and no change in menstrual regularity in the prior year); early perimenopausal (menses in the prior 3 months and changes in regularity in the prior year); late perimenopausal (no menses in the prior 3 months but menses in the prior 11 months); postmenopausal (≥12 months of amenorrhea); and unknown due to hormone therapy (hormone therapy use in the previous 12 months). In our analysis, women who underwent a hysterectomy or bilateral oophorectomy were censored at the time of surgery.
At baseline and approximately annual follow-up visits, women completed interviews and self-administered questionnaires, and anthropometric measures and 12-hour fasting blood samples were taken. Time-varying variables included (i) smoking (none/former/active) (10) and total person-hours per week of passive smoke exposure using a validated questionnaire (11); (ii) alcohol intake (<two drinks per week, two to seven drinks per week, and >seven drinks per week); and (iii) physical activity using the Kaiser Physical Activity Survey index (12) (19 questions on level, duration, and intensity of occupational, household, sports and exercise, and daily routine activities). Those who reported exercising for ≥2 hours per week for at least 9 months of the year and who had a moderate increase in their heart rate during exercise were classified as the clinically active group, with those not meeting this level classified as the not clinically active group (13, 14). Baseline dietary caloric intake was assessed using a modified 1995 Block Food Frequency Questionnaire (15, 16) based on the National Health and Nutrition Examination Survey (15, 17, 18).
The five components that define MetS were collected at baseline and at follow-up visits one and three through seven. Serum total cholesterol and triglyceride levels in the collected fasting blood samples were analyzed using enzymatic methods (Hitachi 747 Analyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN), and lHDL-C was isolated using heparin-manganese chloride. Glucose level was measured using a hexokinase-coupled reaction on a Hitachi 747-200 (Roche Molecular Biochemicals Diagnostics, Indianapolis, IN).
MetS constellations and risk components
Women were classified as having MetS as a clustering of cardiometabolic risk factors if they had at least three of the following five components, adapted from component criteria presented by the National Cholesterol Education Program, including medication use specifically for glucose or blood pressure lowering (19): (i) fasting triglyceride level ≥150 mg/dL (hTG); (ii) fasting lHDL-C level <50 mg/dL; (iii) fasting plasma glucose level ≥100 mg/dL or taking antihyperglycemic medication (hGluc); (iv) waist circumference >88 cm or >80 cm for Chinese and Japanese women (Ob); and (v) systolic blood pressure level ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg or taking antihypertensive medication (HTN).
Among participants who did not have MetS at baseline, we defined incident MetS as the first visit at which at least three of the five MetS criteria were present. We categorized the MetS outcome as (i) women with MetS at baseline (prevalent MetS); (ii) women who did not have MetS at baseline and did not develop MetS during follow-up (never MetS); (iii) women who did not have MetS at baseline and developed MetS during follow-up (incident MetS); and (iv) women who had MetS at baseline and then did not have MetS for at least two consecutive follow-up visits (recovery from MetS). In a sensitivity analysis, we also defined incident MetS as two successive visits where at least three of the five components were satisfied. We observed similar results using either definition of incident MetS and therefore present results with only the first definition. We ranked the constellations of MetS components by frequency at the time of incident MetS (incident MetS constellations) and considered each of the three most frequent constellations as outcomes of interest for use in Cox regression models.
Statistical analyses
Descriptive statistics were used to characterize the type of component, number of components, and constellations in women with prevalent MetS, never MetS, and incident MetS by race/ethnicity. In addition, we classified all women by constellation present at baseline and, for each constellation, determined the distribution of constellations at the end of follow-up.
We used Cox regression models to estimate the degree to which participant characteristics (race/ethnicity, MT stage, and modifiable lifestyle factors) were associated with increased or decreased risks of (i) incident MetS; (ii) separately, the three most frequent incident MetS constellations; and (iii) recovery from MetS.
Because data were collected annually, we used interval-censored Cox models to estimate hazard ratios (HRs) and 95% CIs. All models incorporated fixed and time-dependent variables. Censoring and outcome times were assumed to be independent. Censoring occurred at the visit during which a woman had a hysterectomy or bilateral oophorectomy or had unknown MetS status, leaving 10,333 observations available for analysis. For a participant who had missing covariate information, the last visit’s observations were carried forward to address the missing information. MetS components were carried forward if only one visit’s information was missing and the components following the missing visit matched those in the visit before the missingness. We tested the proportional hazards assumption for baseline covariates using tests for interactions between time and each of the baseline covariates; no departures from proportional hazards were found.
Known risk and confounding factors of cardiometabolic conditions were chosen on the basis of existing knowledge. These variables included race/ethnicity, MT stage (time varying), age (independent of MT stage), a priori putative cardiovascular risk factors that are not components of MetS (physical activity, alcohol use, active and passive smoking status, dietary caloric intake, and fiber intake—all time-varying factors), and education level. Because of potential colinearity between study site and race/ethnicity (because some racial/ethnic groups were enrolled at only a single site), we tested models with and without study site. Parameter estimates varied little between the two models; the more parsimonious models without site were used for final analyses.
Fiber intake was not significantly related to MetS, with a very small estimated effect size, and was thus removed from the final model. Categories of smoking and education were each combined to create a more parsimonious model, without appreciable effect on the estimates. We tested for interactions between race/ethnicity and (i) smoking, (ii) alcohol use, (iii) physical activity, and (iv) caloric intake. No significant interactions were found (at α = 0.05). All analyses were conducted on the basis of two-sided hypothesis testing primarily using R (20) and SAS v9.4 (SAS Institute Inc., Cary, NC) statistical software packages.
Results
MetS components at baseline
Among the 3003 women enrolled, 31% had no MetS components at baseline, 25% had one component, and 18% had two components (Table 1). Hispanic women had the highest proportion of women with five components at baseline. Japanese and Chinese women had the highest proportions with no components, whereas black and Hispanic women had higher proportions of women with at least two components. Ob and lHDL-C were the two most prevalent components in each racial/ethnic group except in black women, for whom HTN was more common than lHDL-C.
Table 1.
Frequency Distribution of MetS Components by Race/Ethnicity at Baseline
| All (N = 3003) | Non-Hispanic White (n = 1412) | Black (n = 851) | Japanese (n = 272) | Hispanic (n = 237) | Chinese (n = 231) | |
|---|---|---|---|---|---|---|
| Componenta,b | ||||||
| Abdominal obesity | 40% | 36% | 57% | 20% | 42% | 28% |
| lHDL-C level | 35% | 37% | 37% | 23% | 47% | 23% |
| Hypertension | 30% | 24% | 46% | 19% | 37% | 17% |
| High glucose level | 24% | 19% | 33% | 19% | 33% | 18% |
| High triglyceride level | 19% | 20% | 14% | 20% | 31% | 17% |
| Number of components | ||||||
| 0 | 31% | 35% | 17% | 47% | 19% | 45% |
| 1 | 25% | 25% | 25% | 23% | 24% | 23% |
| 2 | 18% | 16% | 24% | 13% | 21% | 13% |
| 3 | 13% | 10% | 18% | 8% | 14% | 9% |
| 4 | 7% | 7% | 10% | 5% | 10% | 3% |
| 5 | 3% | 3% | 3% | 1% | 6% | 2% |
| NAc | 4% | 3% | 3% | 2% | 5% | 4% |
Column percentages sum to >100% because a participant could have more than one component satisfied.
Abdominal obesity (waist circumference >88 cm or >80 cm for Asians); low fasting lHDL-C level (<50 mg/dL); hypertension (systolic blood pressure level ≥130 mm Hg or diastolic blood pressure level ≥85 mm Hg or taking antihypertensive medication); high glucose level (≥100 mg/dL or taking antihyperglycemic medication); and high triglyceride level (≥150 mg/dL).
Dark to light gray shading per column represents highest to lowest prevalence, respectively, within each racial/ethnic group.
NA refers to nonapplicable women: those with missing information on one or more components.
Women with prevalent MetS
Among 721 women with prevalent MetS, 47% had exactly three components, 31% had four components, and 12% had all five MetS components. Ob/hTG/lHDL-C was the second most frequent constellation overall (12%) and the most frequent in NHW women (18%) and Japanese women (21%). Ob/HTN/hGluc was the most frequent constellation in black women (18%), whereas Ob/hTG/lHDL-C/hGluc was the most frequent constellation in Japanese women (18%).
Baseline characteristics by MetS status
Among the 3003 women, 493 (16%) experienced incident MetS and 1789 (60%) were never MetS (Table 2). Black race/ethnicity was more prevalent among women with prevalent MetS (38%) than among those with incident MetS (32%) or never MetS (24%).
Table 2.
Baseline Characteristics by MetS Status (N = 3003)
|
|
Prevalent MetS at Baseline
|
Never MetS
|
Incident MetS
a |
||||
|---|---|---|---|---|---|---|---|
| Baseline Characteristic | (n = 721) | (n = 1789) |
All (n = 493)
|
Ob/hTG/lHDL-C (n = 85) | Ob/HTN/lHDL-C (n = 74) | Ob/HTN/hGluc (n = 67) | Ob/HTN/hTG (n = 51) |
| Race/ethnicity | |||||||
| Non-Hispanic white | 300 (42%) | 899 (50%) | 213 (43%) | 51 (60%) | 24 (32%) | 17 (25%) | 24 (47%) |
| Black | 271 (38%) | 421 (24%) | 159 (32%) | 12 (14%) | 38 (51%) | 36 (54%) | 14 (27%) |
| Japanese | 39 (5%) | 188 (11%) | 45 (9%) | 7 (8%) | 2 (3%) | 4 (6%) | 6 (12%) |
| Hispanic | 77 (11%) | 124 (7%) | 36 (7%) | 3 (4%) | 8 (11%) | 4 (6%) | 4 (8%) |
| Chinese | 34 (5%) | 157 (9%) | 40 (8%) | 12 (14%) | 2 (3%) | 6 (9%) | 3 (6%) |
| Age, y | 46.1 (2.8) | 45.7 (2.7) | 46.1 (2.6) | 45.6 (2.5) | 45.5 (2.6) | 46.6 (2.5) | 47.3 (2.7) |
| Estimated daily caloric intake, kcal | 1911.7 (754.6) | 1792.2 (673.4) | 1839.8 (745.2) | 1944.5 (740.2) | 1825.4 (826.5) | 1842.9 (679.4) | 1827.9 (731.2) |
| Physical activityb | |||||||
| Not active | 418 (58%) | 696 (39%) | 247 (50%) | 38 (45%) | 38 (51%) | 43 (64%) | 19 (37%) |
| Not clinically active | 244 (34%) | 721 (40%) | 187 (38%) | 39 (46%) | 30 (41%) | 16 (24%) | 18 (35%) |
| Clinically active | 57 (8%) | 370 (21%) | 59 (12%) | 8 (9%) | 6 (8%) | 8 (12%) | 14 (27%) |
| Education | |||||||
| Less than high school | 72 (10%) | 96 (5%) | 39 (8%) | 5 (6%) | 8 (11%) | 9 (13%) | 1 (2%) |
| Completed high school | 415 (58%) | 815 (46%) | 265 (54%) | 42 (49%) | 37 (50%) | 33 (49%) | 29 (57%) |
| Completed college | 234 (32%) | 878 (49%) | 189 (38%) | 38 (45%) | 29 (39%) | 25 (37%) | 21 (41%) |
| Menopausal stage | |||||||
| Early perimenopause | 358 (50%) | 809 (45%) | 223 (45%) | 32 (38%) | 29 (39%) | 32 (48%) | 24 (47%) |
| Premenopause | 363 (50%) | 980 (55%) | 270 (55%) | 53 (62%) | 45 (61%) | 35 (52%) | 27 (53%) |
| Alcohol intake | |||||||
| <2 drinks per wk | 512 (71%) | 985 (55%) | 322 (65%) | 57 (67%) | 53 (72%) | 37 (55%) | 35 (69%) |
| 2–7 drinks per wk | 142 (20%) | 505 (28%) | 107 (22%) | 20 (24%) | 16 (22%) | 20 (30%) | 4 (8%) |
| >7 drinks per wk | 65 (9%) | 285 (16%) | 64 (13%) | 8 (9%) | 5 (7%) | 10 (15%) | 12 (24%) |
| Smoking | |||||||
| Never | 366 (51%) | 1057 (59%) | 284 (58%) | 55 (65%) | 39 (53%) | 44 (66%) | 27 (53%) |
| Former smoker | 186 (26%) | 455 (25%) | 122 (25%) | 18 (21%) | 24 (32%) | 14 (21%) | 17 (33%) |
| Current smoker | 157 (22%) | 263 (15%) | 84 (17%) | 12 (14%) | 10 (14%) | 9 (13%) | 6 (12%) |
| Cholesterol medication | 24 (3%) | 1 (0%) | 7 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (6%) |
| Thyroid medication | 53 (7%) | 84 (5%) | 31 (6%) | 7 (8%) | 5 (7%) | 5 (7%) | 1 (2%) |
| Antihypertensive medication | 216 (30%) | 73 (4%) | 55 (11%) | 1 (1%) | 11 (15%) | 12 (18%) | 12 (24%) |
| MetS components | |||||||
| Number satisfied | 3.6 (0.7) | 0.7 (0.7) | 1.5 (0.7) | 1.4 (0.7) | 1.5 (0.6) | 1.6 (0.6) | 1.3 (0.8) |
| Type satisfied | |||||||
| Ob | 653 (91%) | 313 (17%) | 235 (48%) | 36 (42%) | 49 (66%) | 42 (63%) | 23 (45%) |
| HTN | 462 (64%) | 292 (16%) | 154 (31%) | 5 (6%) | 31 (42%) | 33 (49%) | 29 (57%) |
| lHDL-C | 587 (81%) | 297 (17%) | 177 (36%) | 51 (60%) | 34 (46%) | 5 (7%) | 5 (10%) |
| hTG | 412 (57%) | 80 (4%) | 60 (12%) | 17 (20%) | 0 (0%) | 3 (4%) | 7 (14%) |
| hGluc | 458 (64%) | 157 (9%) | 83 (17%) | 4 (5%) | 0 (0%) | 23 (34%) | 2 (4%) |
Mean (SD) is used for continuous variables and count (%) for categorical variables.
Incident MetS was defined as having at least three of the following components: Ob: waist circumference >88 cm or >80 cm for Asians; HTN: blood pressure level >130/85 mm Hg or taking any blood pressure medication; lHDL-C: <50 mg/dL; hTG: ≥150 mg/dL; hGluc: ≥100 mg/dL or taking antihyperglycemia medication.
The Kaiser Physical Activity Survey sport/exercise index includes a write-in section of sport/exercise with related questions on duration (h/wk) and intensity (none, small, moderate, or large increase in heart rate and breathing). Those who reported exercising for ≥2 h/wk for at least 9 mo of the year and who had a moderate increase in their heart rate during exercise were classified as clinically active; those not meeting this level were classified as not clinically active.
About half of the women in the never MetS and incident MetS groups were NHWs, and one-quarter were blacks (Table 2). Women with never MetS were more likely to be educated, drink more frequently, engage in more physical activity, have lower caloric intake, and be nonsmokers.
Women with incident MetS
Among the 493 women with incident MetS, the three most frequent constellations at the time of incident MetS were Ob/hTG/lHDL-C (18%), Ob/HTN/lHDL-C (16%), and Ob/HTN/hGluc (14%), with Ob present in all three (Table 3). The distribution of race/ethnicity varied among these three constellations, reflecting a higher prevalence of HTN and a lower prevalence of hTG in blacks. NHW women predominated in incident Ob/hTG/lHDL-C and Ob/HTN/hTG constellations, whereas blacks predominated in incident Ob/HTN/lHDL-C and Ob/HTN/hGluc constellations. Ob/hTG/lHDL-C represented the highest proportion of Chinese women (14%) and Ob/HTN/lHDL-C the highest proportion of Hispanic women (11%). In women with incident MetS, Ob was the most frequent (86%) component overall. hTG was most frequent in Japanese women, and HTN was particularly frequent in black (79%) and Hispanic (77%) women and least frequent in Chinese and Japanese women.
Table 3.
Distribution (%) Among SWAN Participants With a MetS Component or Constellation at the Time of Incident MetS by Race/Ethnicity
| All | Non-Hispanic White (n = 213) | Black (n = 159) | Japanese (n = 45) | Chinese (n = 0) | Hispanica (n = 36) | |
|---|---|---|---|---|---|---|
| MetS component | ||||||
| Ob | 86% | 86% | 91% | 70% | 85% | 79% |
| lHDL-C | 66% | 69% | 64% | 58% | 55% | 74% |
| hTG | 65% | 72% | 35% | 80% | 72% | 57% |
| HTN | 60% | 58% | 79% | 58% | 50% | 77% |
| hGluc | 44% | 37% | 50% | 49% | 54% | 47% |
| MetS constellation | ||||||
| Ob/hTG/lHDL-C | 18% | 25% | 8% | 16% | 31% | 9% |
| Ob/HTN/lHDL-C | 16% | 12% | 25% | 5% | 5% | 25% |
| Ob/HTN/hGluc | 14% | 8% | 23% | 9% | 15% | 12% |
| Ob/HTN/hTG | 11% | 12% | 9% | 14% | 8% | 12% |
| HTN/hTG/lHDL-C | 7% | 8% | 5% | 14% | 3% | 6% |
| Ob/lHDL-C/hGluc | 6% | 6% | 8% | 2% | 5% | 6% |
| Ob/hTG/hGluc | 6% | 8% | 2% | 11% | 10% | 0% |
Censored at visit five for Hispanic women and visit seven for all other women.
Constellation patterns (from baseline to last follow-up)
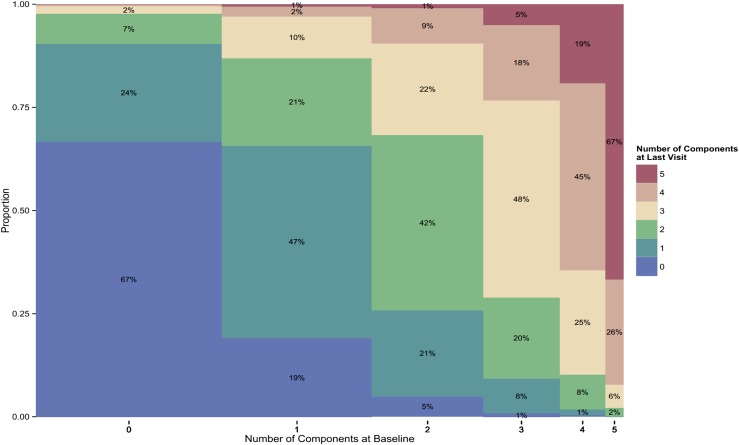
The number of components satisfied at last follow-up increased with the number of components at baseline. In women with MetS at baseline, 74% still had MetS at last follow-up; 23% of women with exactly three components at baseline had four or five at the end of follow-up. In women with zero components, 67% had none and fewer than 3% had MetS at last follow-up (Fig. 1). In women with only one component, 67% ended up with zero or one component, and 13% developed MetS. In women with two components initially, 32% developed MetS, whereas 26% reverted to zero or one component. In women with four components initially, 64% had four or five at last visit.
Figure 1.
Percentage distributions in 2987 SWAN participants of number of MetS components at last follow-up visit (mean, 5.3 y) according to their status at baseline (x-axis). The width of the columns is proportional to the sample size of each constellation at baseline.
Women with hGluc were most likely (66%) to recover to no component at last follow-up, and a greater proportion with only hTG at baseline progressed to MetS. In women who had only Ob at baseline, 30% progressed to having two components, and 15% developed MetS (Fig. 1). Such progression occurred more frequently in black and NHW women and less frequently in Chinese and Japanese women. The majority of women with two components at baseline either maintained these or had fewer components at last follow-up. Women with baseline Ob/hTG/lHDL-C were most likely to progress to more than three components at last follow-up. Of women with hTG/lHDL-C/hGluc, Ob/HTN/hTG, or HTN/lHDL-C/hGluc at baseline, 42% to 47% no longer had MetS at last follow-up.
Factors associated with incident MetS and recovery from incident MetS
Among the 721 women with prevalent MetS at baseline, 119 no longer had MetS on the basis of a net reduction to fewer than three components satisfied by their last follow-up visit (mean follow-up: 5 years) (Table 4). Fifty-one (49%) of these women lost one component, 41 (38%) lost two, 13 (12%) lost three, and one (0.9%) lost four. The most commonly lost component was lHDL-C, and the least commonly lost was Ob. The gain of a component while losing two occurred in 12 women (11%). Only one combination of components was gained/lost in more than one woman; two women lost Ob and hTG and gained hGluc. In multivariable-adjusted modeling, eating fewer kilocalories (HR = 0.96; 95% CI: 0.93, 0.99 per 100 kcal) was associated with resolution of MetS, as was being physically but not clinically physically active (HR = 1.68; 95% CI: 1.06, 2.68).
Table 4.
Multivariable-Adjusted HR Estimates for Recovery From Baseline MetS, Incident MetS, and the Three Most Frequent Constellations at Incident MetS
|
|
|
|
Constellations at Time of Incident MetS
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Recovery From Baseline MetS
|
Incident MetS
|
Ob/hTG/lHDL-C
|
Ob/HTN/lHDL-C
|
Ob/HTN/hGluc
|
|||||
| Characteristicsa | HR (95% CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P | HR (95%CI) | P |
| Baseline age | 1.26 (0.68, 2.35) | 0.63 | 1.04 (1.00, 1.08) | 0.03 | 0.98 (0.89, 1.07) | 0.61 | 0.97 (0.88, 1.07) | 0.58 | 1.11 (1.00, 1.22) | 0.04 |
| Race/ethnicity | ||||||||||
| Non-Hispanic white | Ref | Ref | Ref | Ref | Ref | |||||
| Black | 0.85 (0.51, 1.43) | 0.54 | 1.25 (1.00, 1.57) | 0.05 | 0.31 (0.15, 0.62) | <0.001 | 2.59 (1.48, 4.52) | <0.001 | 4.17 (2.21, 7.86) | <0.001 |
| Chinese | 0.98 (0.40, 2.40) | 0.97 | 0.74 (0.51, 1.06) | 0.1 | 0.72 (0.36, 1.45) | 0.35 | 0.32 (0.07, 1.41) | 0.13 | 1.28 (0.47, 3.5) | 0.63 |
| Hispanic | 0.35 (0.09, 1.34) | 0.13 | 1.61 (1.07, 2.43) | 0.02 | 0.4 (0.11, 1.44) | 0.16 | 2.55 (0.96, 6.81) | 0.06 | 1.65 (0.46, 5.88) | 0.44 |
| Japanese | 1.00 (0.44, 2.28) | 0.99 | 0.73 (0.52, 1.02) | 0.07 | 0.48 (0.22, 1.06) | 0.07 | 0.33 (0.08, 1.42) | 0.14 | 0.98 (0.32, 2.96) | 0.97 |
| Alcohol intake | ||||||||||
| <2 serv per wk | Ref | Ref | Ref | Ref | Ref | |||||
| 2–7 serv per wk | 1.53 (0.80, 2.92) | 0.20 | 0.76 (0.58, 0.98) | 0.04 | 0.55 (0.29, 1.07) | 0.08 | 0.73 (0.36, 1.45) | 0.36 | 0.74 (0.33, 1.66) | 0.46 |
| >7 serv per wk | 2.31 (0.90, 5.92) | 0.08 | 0.94 (0.67, 1.32) | 0.72 | 0.69 (0.29, 1.63) | 0.4 | 0.49 (0.15, 1.61) | 0.24 | 1.79 (0.78, 4.08) | 0.17 |
| Smoking | ||||||||||
| Never | Ref | Ref | Ref | Ref | Ref | |||||
| Former smoker | 1.06 (0.63, 1.77) | 0.84 | 1.09 (0.87, 1.37) | 0.44 | 0.84 (0.48, 1.47) | 0.55 | 1.69 (0.99, 2.9) | 0.05 | 0.74 (0.39, 1.41) | 0.37 |
| Current smoker | 0.51 (0.25, 1.02) | 0.06 | 1.2 (0.92, 1.57) | 0.18 | 0.97 (0.49, 1.92) | 0.93 | 1 (0.48, 2.06) | 1 | 0.62 (0.28, 1.38) | 0.24 |
| Dietary intake (per 100 kcal/d) | 0.96 (0.93, 0.99) | 0.03 | 1.01 (1.00, 1.02) | 0.21 | 1.02 (0.99, 1.06) | 0.12 | 0.99 (0.96, 1.02) | 0.57 | 1 (0.97, 1.03) | 0.94 |
| Menopausal transition stage | ||||||||||
| Pre/early peri | Ref | Ref | Ref | Ref | Ref | |||||
| Late peri/post | 0.51 (0.21, 1.20) | 0.12 | 1.13 (0.89, 1.45) | 0.32 | 0.87 (0.48, 1.6) | 0.67 | 0.74 (0.39, 1.39) | 0.34 | 1.33 (0.67, 2.64) | 0.42 |
| Hormone therapy | 1.53 (0.78, 2.97) | 0.21 | 1.22 (0.89, 1.68) | 0.22 | 0.67 (0.28, 1.59) | 0.36 | 0.63 (0.22, 1.77) | 0.38 | 3.13 (1.56, 6.31) | <0.001 |
| Education | ||||||||||
| Less than HS degree | Ref | Ref | Ref | Ref | Ref | |||||
| HS degree | 0.63 (0.23, 1.75) | 0.37 | 0.73 (0.49, 1.07) | 0.11 | 0.67 (0.24, 1.87) | 0.45 | 0.48 (0.2, 1.17) | 0.1 | 0.47 (0.19, 1.20) | 0.11 |
| College degree | 0.64 (0.22, 1.85) | 0.41 | 0.54 (0.36, 0.81) | <0.001 | 0.54 (0.19, 1.52) | 0.24 | 0.49 (0.19, 1.26) | 0.14 | 0.41 (0.16, 1.11) | 0.08 |
| Physical activity | ||||||||||
| Not active | Ref | Ref | Ref | Ref | Ref | |||||
| Not clinically active | 1.68 (1.06, 2.68) | 0.03 | 0.74 (0.60, 0.90) | <0.001 | 0.77 (0.48, 1.24) | 0.29 | 0.47 (0.27, 0.83) | 0.01 | 0.46 (0.26, 0.82) | 0.01 |
| Clinically active | 0.91 (0.35, 2.37) | 0.85 | 0.39 (0.28, 0.54) | <0.001 | 0.38 (0.17, 0.83) | 0.02 | 0.41 (0.18, 0.95) | 0.04 | 0.38 (0.16, 0.94) | 0.04 |
All characteristics except baseline age, race/ethnicity, and education were time varying.
Abbreviations: HS, high school; peri, perimenopause; post, postmenopause; serv, servings; Ref, reference category.
Hispanic women (HR = 1.61; 95% CI: 1.07, 2.43) and black women (HR = 1.25; 95% CI: 1.00, 1.57) had higher estimated risks of incident MetS (Table 4). Japanese and Chinese women had suggestively decreased risks by 27% (HR = 0.73; 95% CI: 0.52, 1.02) and 26% (HR = 0.74; 95% CI: 0.51, 1.06), respectively, than NHW women. Black women had a fourfold increased risk (HR = 4.17; 95% CI 2.21, 7.86) of developing Ob/HTN/hGluc than NHW women, a 2.5-fold increased risk (HR = 2.59; 95% CI: 1.48, 4.52) of developing Ob/HTN/lHDL-C, and a 69% decreased risk (HR = 0.31; 95% CI: 0.15, 0.62) of developing Ob/hTG/lHDL-C. Physical activity was associated with an ∼60% decreased risk of developing any of the three most frequently occurring MetS constellations except Ob/HTN/hTG. Hormone therapy was associated with a threefold increased risk (HR = 3.13; 95% CI: 1.56, 6.31) of developing Ob/HTN/hGluc. Former smokers had a 1.69-fold risk increase (HR = 1.69; 95% CI: 0.99, 2.90) of developing Ob/HTN/lHDL-C. Higher formal education levels were associated with a decreased risk of Ob/hTG/lHDL-C.
Discussion
This study of midlife women evaluated the racial/ethnic‒specific constellation patterns of cardiometabolic risk components and recovery from MetS. The most frequent constellation was having no MetS components in early midlife women. Physically active women were more likely to recover from MetS and less likely to develop MetS; women with lower daily caloric intake were more likely to recover. Nearly 50% of all the women who developed MetS had one of these constellations: Ob/hTG/lHDL-C (no. 1), Ob/HTN/lHDL-C (no. 2), and Ob/HTN/hGluc (no. 3).
Recovery from MetS occurred in 16.7% of midlife women with MetS at baseline, and lower caloric intake and physical activity were associated with recovery. In Japanese adults older than 20 years, approximately one-third recovered from MetS (median follow-up = 3 years) (21). In adults aged 24 to 39 years in Finland, 30% with MetS recovered 6 years later (22). Interestingly, this recovery from MetS was associated with a more reduced progression of carotid intima-media thickness than was observed in adults with persistent MetS (22).
Midlife women who were physically active had a decreased risk of developing the three most frequent incident MetS constellations. We did not observe a clear association between MT stage and risk of these constellations. We also observed that midlife black and Hispanic women had an increased risk of incident MetS after adjustments for multiple factors. In prior studies, adults had a wide age range or were older postmenopausal women, certain constellations were associated with risks of CVD and mortality (6). These relationships in midlife women remain unclear.
Our results on the distribution of constellations of components differed from those of the Framingham Offspring Study (6). Ob/hTG/lHDL-C was the fifth most frequently occurring constellation, and HTN, not Ob, was the dominant component in that study. Differences in results could be explained in part by differences in racial/ethnic distribution and age range of the samples. Compared with NHW women, black women were more likely to develop constellations of Ob/HTN/lHDL-C and Ob/HTN/hGluc but less likely to develop Ob/hTG/lHDL-C. This is consistent with prior reports that black adults often have high blood pressure (23) and because of distinctive features of lipid metabolism have low triglyceride levels (24).
The current study has limitations. The numbers of women with certain constellations of MetS risk components were small when stratified by race/ethnicity. This limited the statistical power to detect potential associations as significant, especially for black and Asian race/ethnic groups. Models for several outcomes were conducted, leading to the possibility of spurious associations; however, our outcomes of interest were chosen a priori. The results are not directly applicable to other definitions for MetS or for women of other races/ethnicities or ages. SWAN does not have information on medication use for specifically addressing hTG or lHDL-C. Overall, only 1% of women at baseline were taking a lipid-lowering medication (0.9% were taking a statin), whereas such use increased to 12% (nearly all statin) at visit 7. By including lipid-lowering medication use in the definition of lHDL-C, 59 more women were identified with incident MetS at the end of follow-up. When we used a waist circumference threshold of <85 cm instead of <80 cm for Asians, the baseline prevalence of Ob increased by 11% in Chinese and Japanese women, and the baseline prevalence of MetS changed by 1% in Chinese women and 3% in Japanese women. SWAN did not have a sufficient number of women with incident CVD or T2D to evaluate the association between MetS constellations and risk of these outcomes; this warrants future study. Nevertheless, this prospective longitudinal study in midlife women involved detailed MT staging and five race/ethnicities.
In conclusion, midlife women undergoing MT had constellations of cardiometabolic risk components that were related to race/ethnicity. Physical activity was associated with recovery from MetS and a decreased risk of developing MetS, including the three most frequent constellations, during an average follow-up of 5 years. Caloric intake was associated with resolution of MetS; further investigations of the potential role of diet composition across racial/ethnic groups is of interest. An estimated 12% of women who already had MetS early in midlife had all five MetS components. This high-risk subgroup may warrant further attention and tailored preventive strategies. This study supports taking steps to identify tailored race/ethnicity‒specific interventions as well as physical activity and lower dietary caloric intake to facilitate recovery and curb the progression to MetS in midlife women.
Acknowledgments
We thank the study staff at each site and all the women who participated in SWAN.
Financial Support: This publication was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH) through UCSF-CTSI grant no. UL1 RR024131. Supplemental funding from the National Institute on Aging (NIA; 7R21AG040568) is also gratefully acknowledged (to J.S.L.). The SWAN has grant support from the Department of Health and Human Services and NIH through the National Institute on Aging, the National Institute of Nursing Research, and the NIH Office of Research on Women’s Health (grant nos. U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, U01AG017719). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, National Institute of Nursing Research, Office of Research on Women’s Health, or NIH.
Glossary
Abbreviations:
- CVD
cardiovascular disease
- hGluc
high fasting plasma glucose
- HR
hazard ratio
- hTG
high fasting triglyceride
- HTN
hypertension
- lHDL-C
low high-density lipoprotein cholesterol
- MetS
metabolic syndrome
- MT
menopausal transition
- NHW
non-Hispanic white
- NIA
National Institute on Aging
- NIH
National Institutes of Health
- Ob
abdominal obesity
- SWAN
Study of Women’s Health Across the Nation
- T2D
type 2 diabetes
References
- 1. Healthy People 2020 Available at: www.healthypeople.gov/. Accessed 5 March 2019.
- 2. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285(19):2486–2497. [DOI] [PubMed] [Google Scholar]
- 3. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. [DOI] [PubMed] [Google Scholar]
- 4. Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2(3):180–193. [DOI] [PubMed] [Google Scholar]
- 5. American Heart Association Available at: www.heart.org/. Accessed 5 March 2019.
- 6. Franco OH, Massaro JM, Civil J, Cobain MR, O’Malley B, D’Agostino RB Sr. Trajectories of entering the metabolic syndrome: the Framingham Heart Study. Circulation. 2009;120(20):1943–1950. [DOI] [PubMed] [Google Scholar]
- 7. Anuurad E, Chiem A, Pearson TA, Berglund L. Metabolic syndrome components in African-Americans and European-American patients and its relation to coronary artery disease. Am J Cardiol. 2007;100(5):830–834. [DOI] [PubMed] [Google Scholar]
- 8. Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168(14):1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shamsuzzaman ASM, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, Somers VK. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105(21):2462–2464. [DOI] [PubMed] [Google Scholar]
- 10. Ferris BG. Epidemiology standardization project (American Thoracic Society). Am Rev Respir Dis. 1978;118(6 Pt 2):1–120. [PubMed] [Google Scholar]
- 11. Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol. 1989;130(4):696–704. [DOI] [PubMed] [Google Scholar]
- 12. Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the Kaiser physical activity survey in women. Med Sci Sports Exerc. 2000;32(7):1327–1338. [DOI] [PubMed] [Google Scholar]
- 13. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. [DOI] [PubMed] [Google Scholar]
- 14. Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28(3):313–323. [DOI] [PubMed] [Google Scholar]
- 15. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. [DOI] [PubMed] [Google Scholar]
- 16. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. [DOI] [PubMed] [Google Scholar]
- 17. Block G, Norris JC, Mandel RM, DiSogra C. Sources of energy and six nutrients in diets of low-income Hispanic-American women and their children: quantitative data from HHANES, 1982‒1984. J Am Diet Assoc. 1995;95(2):195–208. [DOI] [PubMed] [Google Scholar]
- 18. Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92(6):686–693. [PubMed] [Google Scholar]
- 19. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. [DOI] [PubMed] [Google Scholar]
- 20.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: www.R-project.org/. Accessed 5 March 2019.
- 21. Obokata M, Negishi K, Ohyama Y, Okada H, Imai K, Kurabayashi M. A risk score with additional four independent factors to predict the incidence and recovery from metabolic syndrome: development and validation in large Japanese cohorts. PLoS One. 2015;10(7):e0133884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koskinen J, Magnussen CG, Taittonen L, Räsänen L, Mikkilä V, Laitinen T, Rönnemaa T, Kähönen M, Viikari JS, Raitakari OT, Juonala M. Arterial structure and function after recovery from the metabolic syndrome: the Cardiovascular Risk in Young Finns Study. Circulation. 2010;121(3):392–400. [DOI] [PubMed] [Google Scholar]
- 23. Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988‒1991. Hypertension. 1995;25(3):305–313. [DOI] [PubMed] [Google Scholar]
- 24. Sumner AE, Vega GL, Genovese DJ, Finley KB, Bergman RN, Boston RC. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism. 2005;54(7):902–909. [DOI] [PubMed] [Google Scholar]