Abstract
GATA4 is an essential transcriptional regulator required for gonadal development, differentiation, and function. In the developing testis, proposed GATA4-regulated genes include steroidogenic factor 1 (Nr5a1), SRY-related HMG box 9 (Sox9), and anti-Müllerian hormone (Amh). Although some of these genes have been validated as genuine GATA4 targets, it remains unclear whether GATA4 is a direct regulator of endogenous Amh transcription. We used a CRISPR/Cas9-based approach to specifically inactivate or delete the sole GATA-binding motif of the proximal mouse Amh promoter. AMH mRNA and protein levels were assessed at developmental time points corresponding to elevated AMH levels: fetal and neonate testes in males and adult ovaries in females. In males, loss of GATA binding to the Amh promoter significantly reduced Amh expression. Although the loss of GATA binding did not block the initiation of Amh transcription, AMH mRNA and protein levels failed to upregulate in the developing fetal and neonate testis. Interestingly, adult male mice presented no anatomical anomalies and had no evidence of retained Müllerian duct structures, suggesting that AMH levels, although markedly reduced, were sufficient to masculinize the male embryo. In contrast to males, GATA binding to the Amh promoter was dispensable for Amh expression in the adult ovary. These results provide conclusive evidence that in males, GATA4 is a positive modulator of Amh expression that works in concert with other key transcription factors to ensure that the Amh gene is sufficiently expressed in a correct spatiotemporal manner during fetal and prepubertal testis development.
The anti-Müllerian hormone (AMH) or Müllerian inhibiting substance is a dimeric glycoprotein hormone belonging to the TGFβ superfamily of factors known for their critical roles in growth and development. AMH is present in all vertebrates [reviewed in McLennan and Pankhurst (1)]. In mammals, it exhibits a characteristic sexually dimorphic expression pattern. In males, AMH production is unique to the testis. Expression begins early in development; AMH is the earliest marker of newly differentiated Sertoli cells in the fetal testis (2). This early expression is critical for its best known role in preventing development of the Müllerian ducts into female reproductive structures in genotypically normal males (3). AMH remains high in the fetal testis, and the hormone persists in postnatal life until puberty in humans and around the time of Sertoli cell maturation in rodents (4, 5). Postnatal AMH is thought to continue to have an important role in testis function such as the regulation of Leydig cell differentiation, proliferation, and steroidogenesis (6–11). In humans, Sertoli cell AMH is readily detected in the circulation and has become a useful marker for assessing testicular function in preadolescent boys [reviewed in Edelsztein et al. (12), Josso et al. (13), and Lindhardt et al. (14)].
In the female, AMH is produced by the ovary only after primary follicles appear, which occurs in fetal life in the human (15), and postnatally in rodents (16–20). In both mice and humans, AMH has been shown to be an important inhibitor of primordial follicle growth and/or recruitment and functions to maintain the follicle pool (21, 22). Mice lacking AMH consequently exhibit ovarian deficiency at a premature stage (22, 23). Similarly, AMH is also a useful diagnostic marker of reproductive pathologies in women. An early decrease in AMH levels is linked with diminished ovarian reserve and premature ovarian insufficiency (24, 25). Moreover, elevated AMH is associated with polycystic ovary syndrome and some granulosa cell tumors (26–28).
Given its multiple physiological roles, there has been considerable interest in understanding how AMH production is regulated. Surprisingly, our knowledge of AMH regulation in Sertoli cells remains limited, and we know even less in the ovary. In mouse and human males, high AMH levels produced by the fetal testis begin to decline postnatally. In both species, the decrease in AMH levels correlates with the pubertal rise in testosterone (4, 5). This has led to the widely accepted notion that AMH produced in the postnatal testis is repressed by androgens. In normal mice, it is the high testosterone concentrations present within the testis, and not the serum, that are responsible for AMH downregulation in the prepubertal period (4). In the neonate testis, AMH remains high owing to the absence of the androgen receptor (AR) in Sertoli cells (4, 29). The critical role of AR in mediating AMH downregulation was made evident by mouse models where the receptor is either absent or inactivated (4, 30), or in young human males exhibiting androgen insensitivity syndrome (31). Although the mechanism of androgen repression remains to be fully elucidated, it appears to involve protein–protein interactions between AR and steroidogenic factor 1 [nuclear receptor subfamily 5, group A, member 1 (NR5A1)] directly at the level of the AMH/Amh promoter (29). In contrast to the testis, AMH production in the ovary remains very poorly understood. Recent studies have proposed a role for miRNAs, FSH, and/or specific oocyte-derived growth factors (32–36).
The transcriptional control of the AMH gene has been intensely studied. An initial mouse transgenic study showed that a remarkably short 180-bp fragment upstream of its transcriptional start site was sufficient to confer Amh expression in Sertoli cells (37). A later study, however, revealed that additional upstream sequences were in fact required to confer the correct spatiotemporal expression of the Amh gene (38). The functional region of the human AMH promoter is even longer, with the more distant sequences being necessary for the FSH responsiveness of the gene (39). Both the rodent and human AMH promoters, however, share common regulatory elements that are tightly clustered in their proximal sequences [reviewed in Teixeira et al. (40) and Lasala et al. (41)]. These include binding sites for SRY-related HMG box 9 (SOX9), NR5A1, and a GATA motif known to bind members of the GATA family of transcription factors such as GATA4. Many in vitro studies have shown that synergistic interaction between these and other non–DNA-binding factors such as Wilms tumor 1 (WT1) and friend of GATA 2 contribute to the sex-specific expression of the AMH gene (42–49). Targeted mutagenesis of the SOX9 and NR5A1 sites in mice have validated their relevance to in vivoAmh transcription (50). The in vivo role of the GATA-binding site, however, has not yet been directly assessed. In this study, we report two novel mouse mutants created by CRISPR/Cas9 genome editing that either mutate or delete the Amh GATA promoter element. Our results show that although Amh transcription was initiated normally at the time of Müllerian duct regression, mutation or deletion of the GATA motif significantly impaired the upregulation of Amh expression shortly after gonadal differentiation specifically in the male mouse.
Materials and Methods
Animals
All mouse experiments were carried out in accordance with the Canadian Council of Animal Care guidelines for the care and manipulation of animals used in research. Protocols were approved by the Comité de Protection des Animaux du CHU de Québec (protocol no. 2016043).
Generation and analysis of mouse Amh promoter mutants
The CRISPOR Web tool (www.crispor.tefor.net) was used to scan a 100-bp genomic region of the murine Amh promoter (encompassing the conserved GATA motif) to find a potential sequence for a single-guide RNA (sgRNA) (51). A guide was selected based on its predicted low, overall off-target potential. Plasmid pX330-U6-Chimeric_BBCBh-hSpCas9 (no. 42230) was purchased from Addgene (Cambridge, MA) and used to generate the SpCas9/chimeric sgRNA expression plasmid (52). Two primers were annealed and cloned between the two BbsI sites of the pX330 plasmid (BbsI half sites are underlined and the GATA element is in bold type): forward, 5′-caccgacgcccctatcaacaccaa-3′; reverse, 5′-aaacttggtgttgataggggcgtc-3′. A single-strand oligonucleotide (ssODN) was synthesized as a template for homology-directed repair of the double-strand breaks created by the sgRNA. The ssODN contains the mutated GATA motif of the murine Amh promoter flanked on each side by ∼60-nucleotide-long homology arms (the GATA motif is in bold type and the mutated nucleotides are in uppercase): 5′-ccc acc tgc tgg gca tga aaa gtg cca ggc act gtc ccc caa ggt cac ctt tgg tgt tCCtagg ggc gtc cct ccc aag caa gca atc tgg ctc agc cat aca tat aag cag ggc cac mL-3′. In addition to disrupting the Amh promoter GATA motif, the oligonucleotide was designed to conveniently introduce a restriction site for AvrII (underlined in the sequence above) to allow genotyping of the mutant animals by restriction fragment length polymorphism. The SpCas9/chimeric sgRNA construct was first validated in vitro and then microinjected along with the ssODN into fertilized B6C3F1/J mouse eggs using the microinjection and transgenesis service of the Institut de Recherches Cliniques de Montréal. A total of 53 founder mice were obtained and analyzed. DNA prepared from tail tips collected from the founder mice was used to screen for genomic rearrangements using the AccuStart II mouse genotyping kit (Quantabio, Beverly, MA) and the following primer pair: forward, 5′-ctctgttgaagctgtggtgac-3′; reverse, 5′-taacacagcccccatagtcg-3′. PCR conditions were: initial denaturation for 3 minutes at 95°C followed by 32 cycles of denaturation (30 seconds at 95°C), annealing (30 seconds at 62°C), and extension (30 seconds at 72°C), and a final extension for 3 minutes at 72°C. Amplicons were sequenced and analyzed using the Web tool TIDE (for tracking indels by decomposition) to evaluate the extent of Cas9-mediated rearrangements that occurred (53) and to identify the desired mutated or deleted alleles. Roughly 30% of the founder mice were targeted by the sgRNA. Of these, four founders were successfully repaired by donor ssODN and contained the Amh promoter (pAmh) GATA mutation (GATAmut) that we thereafter named pAmh GATAmut. Interestingly, one founder mouse presented a 40-bp deletion created by nonhomologous end joining. The deletion mutant, which we named pAmh del40, removed both the GATA- and NR5A1-binding elements of the Amh promoter. The pAmh GATAmut and pAmh del40 founder lines were viable and fertile. Both lines were first crossed with C57BL/6J mice (stock no. 000664; The Jackson Laboratory, Bar Harbor, ME) to assess the transmission of the modified alleles and to generate the heterozygous animals carrying the desired mutation or deletion. The lines were backcrossed with C57BL/6J mice for five generations to obtain a pure genetic background.
For both lines, heterozygous mice were crossed to generate homozygous as well as wild-type (WT) control mice (referred to as pAmh WT). Mutant mice were born at the expected Mendelian frequencies with a male/female ratio of 1:1. Testes were dissected from male mice at various developmental ages: embryonic day (E)13.5, E15.5, E18.5, postnatal day (PND)2, and PND90 (adult). Ovaries were collected from adult mice at PND120. Whole reproductive tracts were carefully dissected from adult male mice for anatomical assessment. For neonate male pups at PND2, testes were harvested and prepared for histological analysis or processed for total RNA extraction using TRI Reagent solution (Invitrogen by Thermo Fisher Scientific, Waltham, MA) in accordance with the manufacturer’s instructions. Testes from male E18.5 fetuses were also processed for total RNA extraction, whereas whole E13.5 embryos were prepared for histological analysis as described below.
Quantitative real-time RT-PCR
First-strand cDNA was synthesized from total RNA isolated from tissues using the iScript advanced cDNA synthesis kit for quantitative real-time RT-PCR (qPCR; Bio-Rad Laboratories, Mississauga, ON, Canada). Assessment of gene expression by qPCR was done using a CFX96 plate thermal cycler and SsoAdvanced Universal SYBR Green Supermix from Bio-Rad Laboratories using their standard protocol; PCR primers are listed in Table 1. Primer pairs were optimized beforehand for specificity and efficiency using a temperature gradient to identify the best annealing temperature and by performing a standard curve using a serial dilution of a pool of samples. PCR amplifications were run in duplicate under the following conditions: initial denaturation for 3 minutes at 95°C followed by 40 cycles of denaturation (10 seconds at 95°C), annealing (20 seconds at 62.6°C), and extension (20 seconds at 72°C) with a single acquisition of fluorescence level at the end of each extension step. Differences in mRNA levels between the mouse genotypes was determined using the 2−ΔΔCq method of Livak et al. (54). Ribosomal protein L19 (Rpl19), glyceraldehyde 3-phosphate dehydrogenase (Gapdh) and tubulin α1B (Tuba1b) were used as reference genes.
Table 1.
Oligonucleotide Primers Used for qPCR
| Gene Product | Forward Primer | Reverse Primer |
|---|---|---|
| Amh | 5′-GCAGGCCCTGTTAGTGCTAT-3′ | 5′-GAAAGGCTTGCAGCTGATCG-3′ |
| Gapdh | 5′-GTCGGTGTGAACGGATTTG-3′ | 5′-AAGATGGTGATGGGCTTCC-3′ |
| Rpl19 | 5′-CTGAAGGTCAAAGGGAATGTG-3′ | 5′-GGACAGAGTCTTGATGATCTC-3′ |
| Tuba1b | 5′-CGCCTTCTAACCCGTTGCTA-3′ | 5′-CCTCCCCCAATGGTCTTGTC-3′ |
Abbreviations: Gapdh, glyceraldehyde 3-phosphate dehydrogenase; Rpl19, ribosomal protein L19; Tuba1b, tubulin α1B.
Immunohistochemistry
Tissues were processed for antigen retrieval by deparaffinizing and rehydrating 4-μm sections in graded ethanols and treating them with 10 mM citrate buffer (pH 6.0; 8.2 mM sodium citrate and 0.8 mM citric acid) in a microwave oven for 15 minutes. Endogenous peroxidase activity was inactivated with 0.3% H2O2, and nonspecific binding was blocked by incubation with 0.1% BSA in PBS for 1 hour at room temperature (RT). Sections were incubated overnight at RT with primary antibodies for either goat polyclonal anti-human AMH IgG (55) or goat polyclonal anti-mouse GATA4 IgG (56) diluted 1:100 in blocking solution (PBS containing 0.1% BSA). Absence of primary antibody was used as a negative control. Slides were subsequently washed in PBS and incubated for 1 hour with a biotinylated rabbit anti-goat secondary antibody (57) diluted 1:500 in PBS. After PBS washes, an avidin/biotin complex solution (58) was applied to the sections for 1 hour at RT. A solution of 3-amino-9-ethylcarbazole (Sigma-Aldrich Canada, Oakville, ON, Canada) in 50 mM acetate buffer (pH 5.2) and 0.3% H2O2 was used as chromogen. Sections were counterstained with Gill no. 1 hematoxylin (Ricca Chemical Company, Arlington, TX) and mounted in MOWIOL (EMD Millipore, Gibbstown, NJ). Slides were visualized with a Zeiss Axioscop II epifluorescence microscope (Carl Zeiss Canada, Toronto, ON, Canada) connected to a Spot RT Slider digital camera (Diagnostic Instruments, Sterling Heights, MI). At least three animals per genotype were assessed.
Statistical analysis
Statistical analyses were done using GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA). Significant differences in Amh mRNA levels between the WT and mutant mouse genotypes were identified using the nonparametric Mann–Whitney U test or parametric Student t test where appropriate; P < 0.05 was considered significant.
Results
An intact Amh promoter GATA motif is required for upregulation of testicular but not ovarian Amh expression
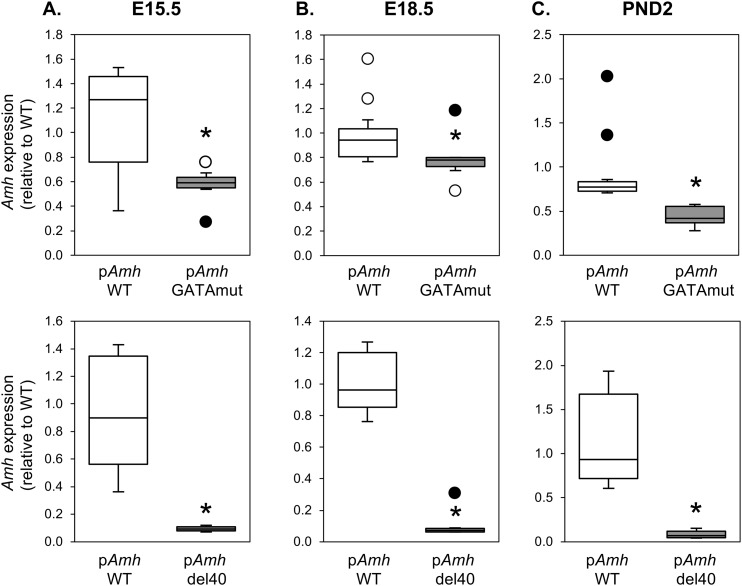
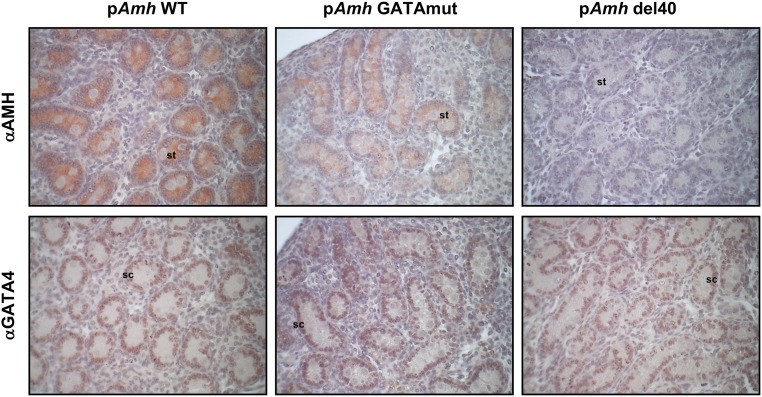
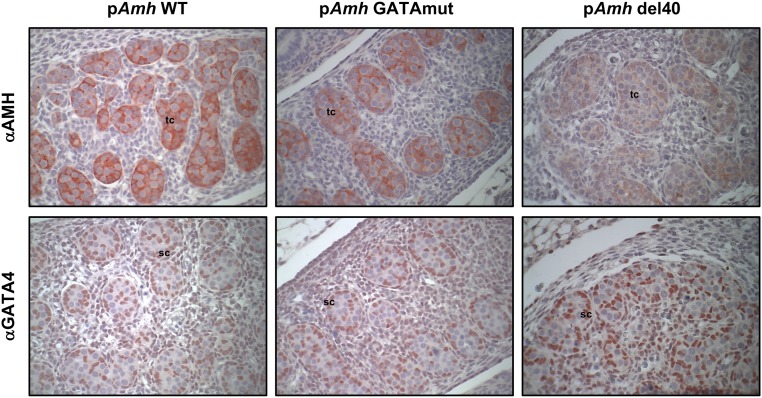
GATA4 has long been proposed to regulate Amh expression. We used CRISPR/Cas9 genome editing to create a two-nucleotide mutation or 40-bp deletion that inactivates and deletes, respectively, the conserved GATA-binding motif in the murine Amh promoter (Fig. 1). We used these novel mouse models to directly assess the requirement of GATA binding for endogenous Amh expression (Fig. 2). Amh mRNA levels in males were assessed at E15.5, E18.5, and PND2, which are three developmental time points when testis Amh expression is at its highest. Homozygous disruption of the GATA-binding motif (pAmh GATAmut) resulted in a significant decrease in Amh mRNA levels when compared with WT (pAmh WT) at all three time points. The maximal decrease (nearly 50%) occurred in the neonate testis at PND2 (Fig. 2C, upper panel). The decrease in Amh mRNA levels was also observed when the Amh promoter GATA-binding motif was deleted in a homozygous context (pAmh del40). The pAmh del40 mutant, however, produced a more dramatic reduction when compared with the two-nucleotide mutation (Fig. 2, lower panels). Amh mRNA levels were reduced by 90% in the fetal testis (Fig. 2A and 2B, lower panels) and were barely detectable by PND2 (Fig. 2C, lower panel). The 40-bp deletion removes not only the GATA-binding motif but also the NR5A1-binding site previously shown to be important for full endogenous Amh expression in the mouse testis (50). The dramatic reduction in Amh expression observed in the pAmh del40 mutant was therefore not unexpected and further reinforces the essential synergistic nature of the two binding sites that was initially reported in vitro (45). We next used immunohistochemistry to determine whether the observed changes in Amh mRNA were also reflected at the level of AMH protein at PND2 (Fig. 3, upper panel). When compared with the pAmh WT mouse, and similar to what we observed for Amh mRNA, the pAmh GATAmut testis showed a noticeable reduction in AMH protein whereas the pAmh del40 testis had very little visible AMH protein in the primitive seminiferous tubules of the neonate testis. In contrast to AMH, no changes were seen in GATA4 protein (Fig. 3, lower panel).
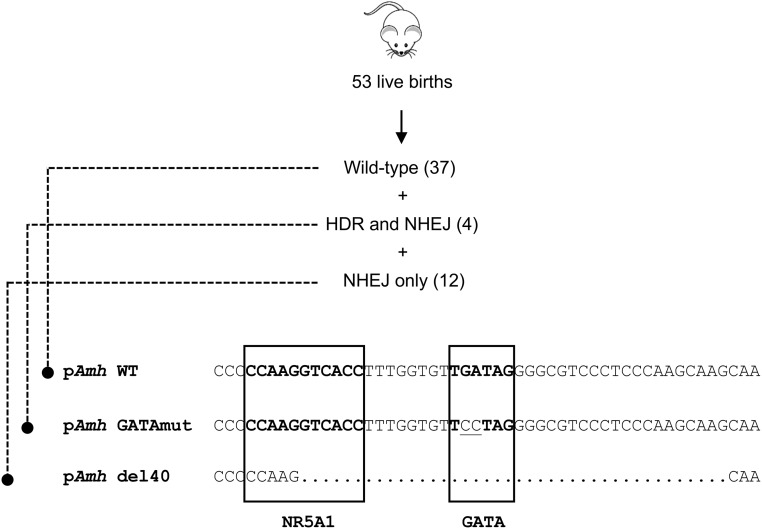
Figure 1.
Generation of mouse Amh promoter mutations. A CRISPR/Cas9 strategy was used to target the conserved GATA-binding motif in the mouse Amh promoter. Of 53 live births obtained, 16 founder mice were found to be cut by the sgRNA. Founder mice presenting suitable modifications were selected for breeding. Four of the 16 founders (7.5% of total births) were repaired by homology-directed repair (HDR) using the ssODN template to incorporate the desired TGATAG to TCCTAG mutation that inactivates the GATA-binding motif in the mouse Amh promoter, termed pAmh GATAmut. Another founder mouse named pAmh del40 was conveniently found to contain a short 40-bp deletion—generated by nonhomologous end-joining repair (NHEJ)—that spanned both the GATA motif and the adjacent NR5A1-binding site. Both mouse lines were first crossed with C57BL/6J mice to assess the transmission of the modified alleles and to isolate the desired point mutation (pAmh GATAmut) or deletion (pAmh del40).
Figure 2.
Comparison of Amh gene expression in pAmh WT, pAmh GATAmut, and pAmh del40 mouse testis. (A–C) Amh expression was assessed by quantitative RT-PCR at (A) E15.5, (B) E18.5, and (C) PND2. Data are reported as the value relative to the age-matched WT (pAmh WT) mouse (n = 4 to 11). Open circles are outliers; filled circles are definitive outliers. *P < 0.05 with regard to differences between the pAmh WT and pAmh GATAmut or pAmh del40 mice.
Figure 3.
Detection of AMH and GATA4 protein in neonate testis sections of pAmh WT, pAmh GATAmut, and pAmh del40 mice. Immunohistochemistry was performed on serial paraffin testis sections obtained from each mouse genotype at PND2 using antibodies specific for either AMH (αAMH) or GATA-binding protein 4 (αGATA4). For each protein and genotype analyzed, the images shown represent results from tissue sections that were processed under identical conditions and on the same day. Original magnification, ×400. sc, Sertoli cell; st, seminiferous tubule.
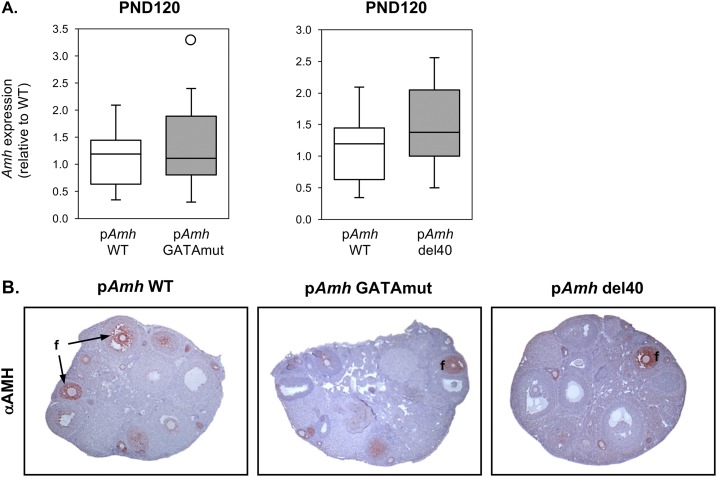
AMH is also found in granulosa cells surrounding growing follicles in the adult ovary. Because granulosa cells also contain members of the GATA and NR5A family (59), a similar dependence on GATA- and NR5A1-binding sites that we observed for the fetal and neonate testis might also apply to ovarian Amh expression. To test this possibility, we compared AMH mRNA and protein in pAmh WT, pAmh GATAmut, and pAmh del40 adult ovaries at PND120 (Fig. 4). In contrast to the testis, Amh expression was unaffected by either the two-nucleotide GATA mutation or 40-bp deletion (Fig. 4A). Similarly, AMH protein was still readily detected in both mouse mutants when compared with WT mice, and the appearance of the ovarian tissue appeared normal with a comparable number of follicles (Fig. 4B).
Figure 4.
AMH gene expression and protein in adult ovary of pAmh WT, pAmh GATAmut, and pAmh del40 mice. (A) Amh expression was assessed by quantitative RT-PCR at PND120. Data are reported as the value relative to the age-matched pAmh WT mouse line (n = 8 to 20). The open circle is the lone outlier. No statistically significant differences were found between the pAmh WT and pAmh GATAmut or pAmh del40 mice (P < 0.05). (B) Immunohistochemistry was performed on serial paraffin ovary sections obtained from each mouse genotype at PND120 using an antibody specific for AMH (αAMH). For each genotype analyzed, the images shown represent results from tissue sections that were processed under identical conditions and on the same day. Original magnification, ×50. f, follicle.
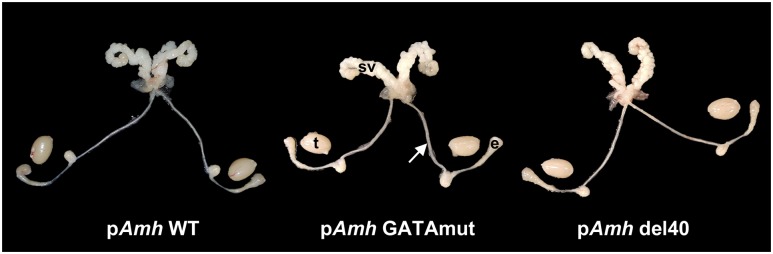
The GATA-binding motif alone or the GATA- and NR5A1-binding sites together in the Amh promoter are not essential for functional Müllerian duct regression
AMH protein is the first functional marker of newly differentiated Sertoli cells in the fetal testis. This early onset of Amh expression and its concomitant protein is essential to initiate regression of the Müllerian ducts and to prevent their development into female reproductive tract structures in the postnatal male. We therefore tested whether sufficient AMH production was initiated in pAmh GATAmut and pAmh del40 males by examining their reproductive tracts at adulthood. As shown in Fig. 5, both mouse mutant lines exhibited normal reproductive tracts that were indistinguishable from the pAmh WT mouse. This result was surprising given the substantial decrease in Amh mRNA and protein that we observed, especially by PND2. This suggested that neither the Amh promoter GATA-binding motif alone, nor the GATA- and NR5A1-binding sites together, are required to initiate Amh transcription at the critical early stage of fetal testis differentiation. To test this possibility, we examined AMH protein in the fetal testis at E13.5 by immunohistochemistry (Fig. 6). When compared with the pAmh WT mouse, AMH was still abundant in the E13.5 pAmh GATAmut testis and, although reduced, was still detectable in the E13.5 pAmh del40 testis (Fig. 6, upper panel). Similar to the PND2 immunohistochemistry data (Fig. 3), changes in AMH protein were not observed with the GATA4 protein, which was used as a labeling control for Sertoli cells (Fig. 6, lower panel).
Figure 5.
Comparative macroscopic anatomy of the reproductive tracts of pAmh WT, pAmh GATAmut, and pAmh del40 adult male mice. Adult males were euthanized at 90 d of age, and their reproductive tracts were dissected and exposed. All three tracts show a normal phenotype with no evidence of retained Müllerian duct derivatives. The arrow points to the vas deferens. e, epididymis; sv, seminal vesicle; t, testis.
Figure 6.
Detection of AMH and GATA4 protein in fetal testis sections of pAmh WT, pAmh GATAmut, and pAmh del40 mice. Immunohistochemistry was performed on serial paraffin testis sections obtained from each mouse genotype at E13.5 using antibodies specific for either AMH (αAMH) or GATA-binding protein 4 (αGATA4). For each protein and genotype analyzed, the images shown represent results from tissue sections that were processed under identical conditions and on the same day. Original magnification, ×400. sc, Sertoli cell; tc, testis cord.
Discussion
The AMH hormone is best known for its role in mammalian sex differentiation by ensuring that the Müllerian ducts do not develop in XY males. The requirement for AMH action is not likely limited to fetal development, as AMH production persists in immature postnatal testis and granulosa cells of developing ovarian follicles. Despite the multiple roles played by AMH, our understanding of how production of this hormone is regulated remains incomplete. The transcriptional regulation of the AMH gene is complex and involves the participation of multiple cis-acting regulatory elements within its upstream promoter sequence. Many of the AMH regulatory elements are shared with the mouse and are located within the first few hundred base pairs upstream of its transcriptional start site (41). These regulatory elements and the factors that bind them have largely been described based on in vitro studies carried out in both homologous and heterologous cell line models. Some, however, have been validated in vivo using targeted mutagenesis approaches in mice (50). This includes the binding sites for both SOX9 and NR5A1 (50). In addition to the aforementioned transcription factors, another factor, namely GATA4, has long been proposed to be a key regulator of AMH transcription (60). In the current study, we have used CRISPR/Cas9 genome editing to target the Amh promoter GATA-binding element. To our knowledge, we provide the first formal in vivo validation that GATA binding to the Amh promoter is essential for maintaining Amh transcription in the male, but not in the female.
GATA4 belongs to only a handful of transcriptional regulators whose function is required prior to and after gonadal differentiation (59, 61–63). In mice, GATA4 is present very early in the developing gonadal primordium (64, 65), where it initiates the first steps of urogenital development in both XY and XX embryos (65). After testis differentiation, GATA4 is abundant in all somatic lineages, and especially the newly differentiated Sertoli cells (59). This early expression pattern, along with the presence of a species-conserved GATA-binding motif, made GATA4 a very interesting candidate regulator to initiate and maintain Amh gene transcription in Sertoli cells (60). Indeed, in vitro characterization studies of either the native or modified Amh promoter have provided strong circumstantial evidence supporting a role for GATA4 in the initiation and/or upregulation of Amh transcription (45, 60, 66). Although ablation of the Amh promoter GATA-binding motif significantly diminished Amh expression in the fetal and neonate testis—in pAmh GATAmut mice and, dramatically so, in pAmh del40 mice—we were surprised that the reduced AMH production was not accompanied by retention of Müllerian duct derivatives in mutant males. We can draw two important conclusions from these observations: (i) GATA4 binding to the Amh promoter is not required to turn on Amh expression in Sertoli cells at the time of testis differentiation, and (ii) very little AMH hormone may be required at this critical early stage to induce Müllerian duct regression. Our results targeting the Amh promoter GATA4-binding element are highly reminiscent of a similar study designed to target the SOX9- and NR5A1-binding sites in the Amh promoter (50). Whereas disruption of SOX9 binding prevented Amh transcriptional initiation and caused Müllerian duct retention in males, a similar blockade of the NR5A1 site significantly reduced Amh transcription but without an effect on Müllerian duct regression (50). Our similar findings with disrupting GATA binding to the Amh promoter therefore reinforces the notion that in mice, AMH is likely produced far in excess of what is needed to initiate Müllerian duct regression.
Although GATA binding to the Amh promoter is not essential for the initiation of Amh transcription in Sertoli cells, it is critical, much like NR5A1 binding, for the upregulation and maintenance of high AMH levels in the later fetal and neonate testis. These results are important not only from the perspective of advancing our basic understanding of Amh transcription, but they also help put to rest the controversy over whether the mechanism of action of GATA binding (presumably GATA4) to the Amh promoter is one of activation or repression. Our present data strongly support GATA4 binding as a positive regulator of Amh transcription. Prior to these findings, some studies, including one characterizing a novel human GATA4 gene mutation affecting AMH expression in men, favored GATA4 as an activator of AMH transcription (44, 45, 60, 66–68). Others, however, favored the opposite view, with GATA4 possibly functioning as a repressor of Amh transcription (69, 70). In one of those studies, GATA1 was proposed to inhibit GATA4 action at the level of the Amh promoter and cause a downregulation in Amh expression prior to puberty (69). The onset of GATA1 protein in the prepubertal testis (being reciprocal with AMH protein) made this a logical speculation. However, the fact that all GATA factors have similar DNA-binding properties (71, 72), as well as similar transcriptional partners in testis cells (59), makes this an unlikely possibility. In a more recent study published in 2015, Amh expression was strongly upregulated and remained high beyond its period of natural decline in mice testis lacking both transcription factors GATA4 and GATA6 (70). Interestingly, GATA1 was also absent in postnatal testis lacking these two factors (70). These observations suggested that GATA factors, although crucial for the regulation of Amh transcription, are important negative regulators of the Amh gene in Sertoli cells (70). However, testes lacking GATA4 and GATA6 were also described as having abnormal steroidogenic function due to the marked loss of 17β-hydroxysteroid dehydrogenase type 3 (HSD17B3), the terminal enzyme required for testosterone synthesis. Although GATA4- and GATA6-deficient testes produced steroids, they did not produce testosterone (70). Because testosterone action acting through the AR in the pubertal period is essential for downregulation of AMH production during this critical developmental window (4, 29), the marked upregulation in Amh expression seen in GATA4- and GATA6-deficient mouse testis is more likely due to the absence of androgen action rather than de-repression caused by the absence of GATA factor binding.
GATA proteins, much like other transcription factors, rarely function alone. Rather, they participate with other ubiquitous or cell-restricted factors to ensure appropriate spatiotemporal gene expression [reviewed in Viger et al. (59)]. Combinatorial interactions between transcription factors play a prominent role in Amh transcription. This includes synergistic interactions between SOX9 and NR5A1, NR5A1 and WT1, GATA4 and WT1, and GATA4 and NR5A1 [reviewed in Lasala et al. (41)]. For the latter, the interaction has been shown to be relevant to activation of both the mouse and human AMH promoters where the GATA- and NR5A1-binding sites are in close proximity to one another (45, 66, 73). Consistent with these findings, we show in the present study that simultaneous deletion of both binding sites in the Amh promoter (pAmh del40 mouse) produced a more severe reduction in AMH mRNA and protein in the fetal and neonate testis when compared with mutation of the GATA site alone (pAmh GATAmut mouse). In contrast to a past report (74), our observations support a genetic interaction between GATA4 and NR5A1, at least on the mouse Amh gene. Surprisingly, this effect was not seen in the adult ovary, which contains both detectable AMH, and at least two GATA proteins (GATA4 and GATA6). Neither mutagenesis of the Amh promoter GATA element (pAmh GATAmut) nor deletion of both the GATA and NR5A1 elements (pAmh del40) resulted in any discernable changes in AMH mRNA or protein. At present, the reason for this male/female difference in Amh regulation by GATA factors is unknown. We can speculate, however, that this difference may reside in the fact that AMH production is differentially regulated in the testis vs the ovary. In Sertoli cells, SOX9 is a critical factor that is absent in females (75). Moreover, androgens via the AR play a critical role in AMH downregulation in the testis but not in the ovary (4, 29). Additionally, in females, NR5A2 rather than NR5A1 might be the more biologically relevant NR5A family member in granulosa cells, as lack of NR5A2 function does not appear to be compensated by NR5A1 (76, 77). Whatever the explanation, this difference in the mode of regulation highlights the complexity of the transcriptional inputs to the AMH gene despite its conserved 5′ upstream regulatory sequence.
Acknowledgments
We thank Dr. Hiroaki Taniguchi for critical reading of the manuscript.
Financial Support: This study was supported by Grant MOP-14796 from the Canadian Institutes of Health Research to R.S.V.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AMH
anti-Müllerian hormone
- AR
androgen receptor
- E
embryonic day
- Gapdh
glyceraldehyde 3-phosphate dehydrogenase
- GATAmut
GATA mutation
- NR5A1
nuclear receptor subfamily 5, group A, member 1
- pAmh
anti-Müllerian hormone promoter
- pAmh del40
anti-Müllerian hormone promoter with 40-bp deletion
- PND
postnatal day
- qPCR
quantitative real-time RT-PCR
- Rpl19
ribosomal protein L19
- RT
room temperature
- sgRNA
single-guide RNA
- SOX9
SRY-related HMG box 9
- ssODN
single-strand oligonucleotide
- Tuba1b
tubulin α1B
- WT
wild-type
- WT1
Wilms tumor 1
References
- 1. McLennan IS, Pankhurst MW. Anti-Müllerian hormone is a gonadal cytokine with two circulating forms and cryptic actions. J Endocrinol. 2015;226(3):R45–R57. [DOI] [PubMed] [Google Scholar]
- 2. Münsterberg A, Lovell-Badge R. Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113(2):613–624. [DOI] [PubMed] [Google Scholar]
- 3. Mullen RD, Behringer RR. Molecular genetics of Müllerian duct formation, regression and differentiation. Sex Dev. 2014;8(5):281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Attar L, Noël K, Dutertre M, Belville C, Forest MG, Burgoyne PS, Josso N, Rey R. Hormonal and cellular regulation of Sertoli cell anti-Müllerian hormone production in the postnatal mouse. J Clin Invest. 1997;100(6):1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rey R, Lordereau-Richard I, Carel JC, Barbet P, Cate RL, Roger M, Chaussain JL, Josso N. Anti-Müllerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. J Clin Endocrinol Metab. 1993;77(5):1220–1226. [DOI] [PubMed] [Google Scholar]
- 6. Racine C, Rey R, Forest MG, Louis F, Ferré A, Huhtaniemi I, Josso N, di Clemente N. Receptors for anti-Müllerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc Natl Acad Sci USA. 1998;95(2):594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rouiller-Fabre V, Carmona S, Merhi RA, Cate R, Habert R, Vigier B. Effect of anti-Mullerian hormone on Sertoli and Leydig cell functions in fetal and immature rats. Endocrinology. 1998;139(3):1213–1220. [DOI] [PubMed] [Google Scholar]
- 8. Salva A, Hardy MP, Wu XF, Sottas CM, MacLaughlin DT, Donahoe PK, Lee MM. Müllerian-inhibiting substance inhibits rat Leydig cell regeneration after ethylene dimethanesulphonate ablation. Biol Reprod. 2004;70(3):600–607. [DOI] [PubMed] [Google Scholar]
- 9. Sriraman V, Niu E, Matias JR, Donahoe PK, MacLaughlin DT, Hardy MP, Lee MM. Müllerian inhibiting substance inhibits testosterone synthesis in adult rats. J Androl. 2001;22(5):750–758. [PubMed] [Google Scholar]
- 10. Wu X, Arumugam R, Baker SP, Lee MM. Pubertal and adult Leydig cell function in Mullerian inhibiting substance-deficient mice. Endocrinology. 2005;146(2):589–595. [DOI] [PubMed] [Google Scholar]
- 11. Teixeira J, Fynn-Thompson E, Payne AH, Donahoe PK. Müllerian-inhibiting substance regulates androgen synthesis at the transcriptional level. Endocrinology. 1999;140(10):4732–4738. [DOI] [PubMed] [Google Scholar]
- 12. Edelsztein NY, Grinspon RP, Schteingart HF, Rey RA. Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int J Pediatr Endocrinol. 2016;2016(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Josso N, Rey RA, Picard JY. Anti-Müllerian hormone: a valuable addition to the toolbox of the pediatric endocrinologist. Int J Endocrinol. 2013;2013:674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindhardt Johansen M, Hagen CP, Johannsen TH, Main KM, Picard JY, Jørgensen A, Rajpert-De Meyts E, Juul A. Anti-Müllerian hormone and its clinical use in pediatrics with special emphasis on disorders of sex development. Int J Endocrinol. 2013;2013:198698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuiri-Hänninen T, Kallio S, Seuri R, Tyrväinen E, Liakka A, Tapanainen J, Sankilampi U, Dunkel L. Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. J Clin Endocrinol Metab. 2011;96(11):3432–3439. [DOI] [PubMed] [Google Scholar]
- 16. Rajpert-De Meyts E, Jørgensen N, Graem N, Müller J, Cate RL, Skakkebaek NE. Expression of anti-Müllerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. J Clin Endocrinol Metab. 1999;84(10):3836–3844. [DOI] [PubMed] [Google Scholar]
- 17. Hirobe S, He WW, Lee MM, Donahoe PK. Mullerian inhibiting substance messenger ribonucleic acid expression in granulosa and Sertoli cells coincides with their mitotic activity. Endocrinology. 1992;131(2):854–862. [DOI] [PubMed] [Google Scholar]
- 18. Ueno S, Takahashi M, Manganaro TF, Ragin RC, Donahoe PK. Cellular localization of Müllerian inhibiting substance in the developing rat ovary. Endocrinology. 1989;124(2):1000–1006. [DOI] [PubMed] [Google Scholar]
- 19. Ueno S, Kuroda T, Maclaughlin DT, Ragin RC, Manganaro TF, Donahoe PK. Mullerian inhibiting substance in the adult rat ovary during various stages of the estrous cycle. Endocrinology. 1989;125(2):1060–1066. [DOI] [PubMed] [Google Scholar]
- 20. Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, Kramer P, Fauser BC, Themmen AP. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. [DOI] [PubMed] [Google Scholar]
- 21. Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223–2227. [DOI] [PubMed] [Google Scholar]
- 22. Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–5796. [DOI] [PubMed] [Google Scholar]
- 23. Visser JA, Durlinger AL, Peters IJ, van den Heuvel ER, Rose UM, Kramer P, de Jong FH, Themmen AP. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinology. 2007;148(5):2301–2308. [DOI] [PubMed] [Google Scholar]
- 24. Pankhurst MW. A putative role for anti-Müllerian hormone (AMH) in optimising ovarian reserve expenditure. J Endocrinol. 2017;233(1):R1–R13. [DOI] [PubMed] [Google Scholar]
- 25. Méduri G, Massin N, Guibourdenche J, Bachelot A, Fiori O, Kuttenn F, Misrahi M, Touraine P. Serum anti-Müllerian hormone expression in women with premature ovarian failure. Hum Reprod. 2007;22(1):117–123. [DOI] [PubMed] [Google Scholar]
- 26. Rey R, Sabourin JC, Venara M, Long WQ, Jaubert F, Zeller WP, Duvillard P, Chemes H, Bidart JM. Anti-Müllerian hormone is a specific marker of Sertoli- and granulosa-cell origin in gonadal tumors. Hum Pathol. 2000;31(10):1202–1208. [DOI] [PubMed] [Google Scholar]
- 27. Rey RA, Lhommé C, Marcillac I, Lahlou N, Duvillard P, Josso N, Bidart JM. Antimüllerian hormone as a serum marker of granulosa cell tumors of the ovary: comparative study with serum α-inhibin and estradiol. Am J Obstet Gynecol. 1996;174(3):958–965. [DOI] [PubMed] [Google Scholar]
- 28. Gustafson ML, Lee MM, Scully RE, Moncure AC, Hirakawa T, Goodman A, Muntz HG, Donahoe PK, MacLaughlin DT, Fuller AF Jr. Müllerian inhibiting substance as a marker for ovarian sex-cord tumor. N Engl J Med. 1992;326(7):466–471. [DOI] [PubMed] [Google Scholar]
- 29. Edelsztein NY, Racine C, di Clemente N, Schteingart HF, Rey RA. Androgens downregulate anti-Müllerian hormone promoter activity in the Sertoli cell through the androgen receptor and intact steroidogenic factor 1 sites. Biol Reprod. 2018;99(6):1303–1312. [DOI] [PubMed] [Google Scholar]
- 30. Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA. 2004;101(18):6876–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rey R, Mebarki F, Forest MG, Mowszowicz I, Cate RL, Morel Y, Chaussain JL, Josso N. Anti-Müllerian hormone in children with androgen insensitivity. J Clin Endocrinol Metab. 1994;79(4):960–964. [DOI] [PubMed] [Google Scholar]
- 32. Convissar S, Armouti M, Fierro MA, Winston NJ, Scoccia H, Zamah AM, Stocco C. Regulation of AMH by oocyte-specific growth factors in human primary cumulus cells. Reproduction. 2017;154(6):745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayes E, Kushnir V, Ma X, Biswas A, Prizant H, Gleicher N, Sen A. Intra-cellular mechanism of anti-Müllerian hormone (AMH) in regulation of follicular development. Mol Cell Endocrinol. 2016;433:56–65. [DOI] [PubMed] [Google Scholar]
- 34. Pierre A, Estienne A, Racine C, Picard JY, Fanchin R, Lahoz B, Alabart JL, Folch J, Jarrier P, Fabre S, Monniaux D, di Clemente N. The bone morphogenetic protein 15 up-regulates the anti-Müllerian hormone receptor expression in granulosa cells. J Clin Endocrinol Metab. 2016;101(6):2602–2611. [DOI] [PubMed] [Google Scholar]
- 35. Roy S, Gandra D, Seger C, Biswas A, Kushnir VA, Gleicher N, Kumar TR, Sen A. Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinology. 2018;159(9):3433–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266(1):201–208. [DOI] [PubMed] [Google Scholar]
- 37. Giuili G, Shen WH, Ingraham HA. The nuclear receptor SF-1 mediates sexually dimorphic expression of Mullerian inhibiting substance, in vivo. Development. 1997;124(9):1799–1807. [DOI] [PubMed] [Google Scholar]
- 38. Beau C, Vivian N, Münsterberg A, Dresser DW, Lovell-Badge R, Guerrier D. In vivo analysis of the regulation of the anti-Müllerian hormone, as a marker of Sertoli cell differentiation during testicular development, reveals a multi-step process. Mol Reprod Dev. 2001;59(3):256–264. [DOI] [PubMed] [Google Scholar]
- 39. Lukas-Croisier C, Lasala C, Nicaud J, Bedecarrás P, Kumar TR, Dutertre M, Matzuk MM, Picard JY, Josso N, Rey R. Follicle-stimulating hormone increases testicular anti-Mullerian hormone (AMH) production through Sertoli cell proliferation and a nonclassical cyclic adenosine 5′-monophosphate-mediated activation of the AMH gene. Mol Endocrinol. 2003;17(4):550–561. [DOI] [PubMed] [Google Scholar]
- 40. Teixeira J, Maheswaran S, Donahoe PK. Müllerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev. 2001;22(5):657–674. [DOI] [PubMed] [Google Scholar]
- 41. Lasala C, Carré-Eusèbe D, Picard JY, Rey R. Subcellular and molecular mechanisms regulating anti-Müllerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol. 2004;23(9):572–585. [DOI] [PubMed] [Google Scholar]
- 42. De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol. 1998;18(11):6653–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell. 1998;93(3):445–454. [DOI] [PubMed] [Google Scholar]
- 44. Miyamoto Y, Taniguchi H, Hamel F, Silversides DW, Viger RS. A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol Biol. 2008;9(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tremblay JJ, Viger RS. Transcription factor GATA-4 enhances Müllerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol. 1999;13(8):1388–1401. [DOI] [PubMed] [Google Scholar]
- 46. Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol. 2011;353(2):229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lasala C, Schteingart HF, Arouche N, Bedecarrás P, Grinspon RP, Picard JY, Josso N, di Clemente N, Rey RA. SOX9 and SF1 are involved in cyclic AMP-mediated upregulation of anti-Mullerian gene expression in the testicular prepubertal Sertoli cell line SMAT1. Am J Physiol Endocrinol Metab. 2011;301(3):E539–E547. [DOI] [PubMed] [Google Scholar]
- 48. Shen WH, Moore CC, Ikeda Y, Parker KL, Ingraham HA. Nuclear receptor steroidogenic factor 1 regulates the Müllerian inhibiting substance gene: a link to the sex determination cascade. Cell. 1994;77(5):651–661. [DOI] [PubMed] [Google Scholar]
- 49. Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK. Endogenous expression of Müllerian inhibiting substance in early postnatal rat Sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc Natl Acad Sci USA. 2000;97(4):1624–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99(4):409–419. [DOI] [PubMed] [Google Scholar]
- 51. Haeussler M, Schönig K, Eckert H, Eschstruth A, Mianné J, Renaud JB, Schneider-Maunoury S, Shkumatava A, Teboul L, Kent J, Joly JS, Concordet JP. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339(6121):819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brinkman EK, Chen T, Amendola M, van Steensel B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014;42(22):e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 55.RRID:AB_649207, https://scicrunch.org/resolver/AB_649207.
- 56.RRID:AB_2108747, https://scicrunch.org/resolver/AB_2108747.
- 57.RRID:AB_2336126, https://scicrunch.org/resolver/AB_2336126.
- 58.RRID:AB_2336827, https://scicrunch.org/resolver/AB_2336827.
- 59. Viger RS, Guittot SM, Anttonen M, Wilson DB, Heikinheimo M. Role of the GATA family of transcription factors in endocrine development, function, and disease. Mol Endocrinol. 2008;22(4):781–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development. 1998;125(14):2665–2675. [DOI] [PubMed] [Google Scholar]
- 61. Viger RS, Silversides DW, Tremblay JJ. New insights into the regulation of mammalian sex determination and male sex differentiation. Vitam Horm. 2005;70:387–413. [DOI] [PubMed] [Google Scholar]
- 62. Tevosian SG. Transgenic mouse models in the study of reproduction: insights into GATA protein function. Reproduction. 2014;148(1):R1–R14. [DOI] [PubMed] [Google Scholar]
- 63. Zaytouni T, Efimenko EE, Tevosian SG. GATA transcription factors in the developing reproductive system. Adv Genet. 2011;76:93–134. [DOI] [PubMed] [Google Scholar]
- 64. Mazaud Guittot S, Tétu A, Legault E, Pilon N, Silversides DW, Viger RS. The proximal Gata4 promoter directs reporter gene expression to Sertoli cells during mouse gonadal development. Biol Reprod. 2007;76(1):85–95. [DOI] [PubMed] [Google Scholar]
- 65. Hu YC, Okumura LM, Page DC. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013;9(7):e1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tremblay JJ, Robert NM, Viger RS. Modulation of endogenous GATA-4 activity reveals its dual contribution to Müllerian inhibiting substance gene transcription in Sertoli cells. Mol Endocrinol. 2001;15(9):1636–1650. [DOI] [PubMed] [Google Scholar]
- 67. Thurisch B, Liang SY, Sarioglu N, Schomburg L, Bungert J, Dame C. Transgenic mice expressing small interfering RNA against Gata4 point to a crucial role of Gata4 in the heart and gonads. J Mol Endocrinol. 2009;43(4):157–169. [DOI] [PubMed] [Google Scholar]
- 68. Lourenço D, Brauner R, Rybczynska M, Nihoul-Fékété C, McElreavey K, Bashamboo A. Loss-of-function mutation in GATA4 causes anomalies of human testicular development. Proc Natl Acad Sci USA. 2011;108(4):1597–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beau C, Rauch M, Joulin V, Jégou B, Guerrier D. GATA-1 is a potential repressor of anti-Müllerian hormone expression during the establishment of puberty in the mouse. Mol Reprod Dev. 2000;56(2):124–138. [DOI] [PubMed] [Google Scholar]
- 70. Padua MB, Jiang T, Morse DA, Fox SC, Hatch HM, Tevosian SG. Combined loss of the GATA4 and GATA6 transcription factors in male mice disrupts testicular development and confers adrenal-like function in the testes. Endocrinology. 2015;156(5):1873–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ko LJ, Engel JD. DNA-binding specificities of the GATA transcription factor family. Mol Cell Biol. 1993;13(7):4011–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Merika M, Orkin SH. DNA-binding specificity of GATA family transcription factors. Mol Cell Biol. 1993;13(7):3999–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tremblay JJ, Viger RS. Nuclear receptor Dax-1 represses the transcriptional cooperation between GATA-4 and SF-1 in Sertoli cells. Biol Reprod. 2001;64(4):1191–1199. [DOI] [PubMed] [Google Scholar]
- 74. Pelusi C, Zhao L, Stallings NR, Parker KL. Combined haploinsufficiency of SF-1 and GATA4 does not reveal a genetic interaction in mouse gonadal development. Sex Dev. 2007;1(3):152–160. [DOI] [PubMed] [Google Scholar]
- 75. Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122(9):2813–2822. [DOI] [PubMed] [Google Scholar]
- 76. Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22(14):1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang C, Large MJ, Duggavathi R, DeMayo FJ, Lydon JP, Schoonjans K, Kovanci E, Murphy BD. Liver receptor homolog-1 is essential for pregnancy. Nat Med. 2013;19(8):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]