Abstract
Epidemiological contact tracing complemented with genotyping of clinical Mycobacterium tuberculosis isolates is important for understanding disease transmission. In Sweden, tuberculosis (TB) is mostly reported in migrant and homeless where epidemiologic contact tracing could pose a problem. This study compared epidemiologic linking with genotyping in a low burden country. Mycobacterium tuberculosis isolates (n = 93) collected at Scania University Hospital in Southern Sweden were analysed with the standard genotyping method mycobacterial interspersed repetitive units-variable number tandem repeats (MIRU-VNTR) and the results were compared with whole genome sequencing (WGS). Using a maximum of twelve single nucleotide polymorphisms (SNPs) as the upper threshold of genomic relatedness noted among hosts, we identified 18 clusters with WGS comprising 52 patients with overall pairwise genetic maximum distances ranging from zero to nine SNPs. MIRU-VNTR and WGS clustered the same isolates, although the distribution differed depending on MIRU-VNTR limitations. Both genotyping techniques identified clusters where epidemiologic linking was insufficient, although WGS had higher correlation with epidemiologic data. To summarize, WGS provided better resolution of transmission than MIRU-VNTR in a setting with low TB incidence. WGS predicted epidemiologic links better which could consolidate and correct the epidemiologically linked cases, avoiding thus false clustering.
Introduction
Tuberculosis (TB) remains a major public health issue in European countries with 323,000 new cases per year1. In 2017, 533 new tuberculosis patients were reported in Sweden, which was a decrease by 6,4% compared to the top year 2015 with influx of asylum seekers from high tuberculosis incidence countries, i.e. Somalia, Eritrea, Afghanistan and Ethiopia2. Sweden is by all means a low incidence country, as the rest of the Nordic countries, with 5.3 tuberculosis cases per 100,000 inhabitants. Most of the infected patients are between 10 to 29 years of age and foreign born, of which this group constituted approximately 86% of the new cases the past years2. To control and prevent disease spreading, epidemiologic tracing with personal communication is the basis, followed by genotyping of the isolated strains. Three genotyping methods were traditionally used before 2016, i.e. restriction fragment length polymorphism (RFLP) and a Mycobacterium tuberculosis-specific multiple locus variable number of tandem repeat (MIRU-VNTR), combined with spoligotyping3–5. In Sweden, MIRU-VNTR was set as the new standard since 2012 by the Public Health Agency of Sweden, to which M. tuberculosis-complex isolates were sent for genotyping. There are some limitations to MIRU-VNTR, as this technique may not distinguish between closely related genotypes and may be suboptimal among immigrants from countries with high incidence of TB, where genetically closely related strains circulate over extended periods of time6,7. Genetic mutations in these strains accumulate, resulting thus often in pairwise SNP distances of 12, but the MIRU-VNTR typing pattern may not change and could wrongly be interpreted as recent transmission in the country of immigration8.
Whole genome sequencing (WGS) is now rapidly becoming the standard for typing M. tuberculosis isolates as this technique provides increased resolution over MIRU-VNTR-based clustering and is considered to be superior in defining the extent and direction of tuberculosis transmission9–14. However, most of the initial studies were from high TB incidence settings, while less is known about this technique in low TB incidence countries. Recently, WGS analysis on outbreak of isoniazid-resistant TB in London showed that this technique may not be useful in identifying the direction of transmission as previously reported15. Other studies from low TB incidence countries showed that WGS is comparable to MIRU-VNTR in native patients, as opposed to foreign-born patients where standard genotyping overestimates recent TB transmission13. In this study, we reanalysed M. tuberculosis strains isolated during the years 2004–2014 with WGS in order to compare the different genotype techniques in a low TB incidence country.
Material and Methods
Study design and population
We sequenced 100 isolates of M. tuberculosis from an archive of 801 frozen cultures obtained between 2004 and 2014 that is held at the Regional Mycobacterial Reference Laboratory for Scania County, Sweden. We selected isolates to estimate genomic diversity within and between hosts. TB is a mandatory notifiable infectious disease to the public health agency of Sweden2 and relevant diagnostic codes, microbiology results and contact tracing were obtained from the Scania county office for infectious disease prevention and control.
Epidemiologic investigation and Traditional genotyping of bacterial strains
M. tuberculosis isolates were cultured at SUS Malmö. 24-loci MIRU-VNTR was performed on 93 laboratory-confirmed, culture-positive isolates at the clinical microbiology laboratory at Sahlgrenska university hospital, Gothenburg, Sweden. Clinical, demographic, and microbiological data were available for each isolate. We obtained epidemiological data from the Scania county office for infectious disease prevention and control. Sweden guidelines for contact tracing recommend screening of household contacts for every new index case, together with at-risk individuals as well as any other pointed contacts in the community if the index case is thought to be infectious.
Bacterial isolates - selection and DNA extraction
Retrospectively, all notified TB cases with M. tuberculosis culture positive samples were identified and stored at −80 C. A total of 100 strains were grown on Lowenstein-Jensen medium for up to 4 weeks in a Biosafety level 3 laboratory. Then approximately one single colony was suspended in 0.5x TE buffer and heat inactivated at 80 C for 20 min before we could sonicate the samples at 35 KHz for 10 min and genomic DNA was extracted using QIAamp DNA Mini Kit (Qiagen) in a Biosafety level 2 laboratory.
Genomic DNA sequencing and library preparation
Sequencing libraries were prepared from 100 ng DNA using the TruSeq Nano DNA sample preparation kit (cat#FC-121,4001/4002 Illumina inc.) according to the manufacturer’s instructions guide (#15041110, HiSeq 2500 system) by SNP&SEQ Technology Platform at Uppsala University. Seven isolates were excluded due to low genomic DNA quantity and quality and the remaining 93 samples were queued for sequencing on 1 lane PE125 and generated 236 M read pair. Raw reads were archived under accession number (to be determined).
Identification, Annotation, and Confirmation of SNPs
Raw reads were trimmed with Trimmomatic [Bolger, A.M., Lohse, M. and Usadel, B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30:15, 2114–2120] to remove adapter sequence and low-quality bases. Only reads at least 36 bases long after trimming were kept. Trimmed reads were mapped to the reference genome CDC1551 (NCBI accession GCA_000008585.1_ASM858v1) using the program BWA mem (http://bio-bwa.sourceforge.net/)16,17, version 0.7.13. The median depth of sequencing was (124.7x), with an average of 99.55% of the reference genome being covered by at least one read after quality control and trimming of reads. The program BWA mem (http://bio-bwa.sourceforge.net/)16,17, version 0.7.13, was used to map the trimmed reads to the reference genome followed by variant calling. Variants were called using freebayes (https://github.com/ekg/freebayes), version 1.0.2. A coverage of at least 8, an alternate allele count of at least two, and an alternate fraction of at least 20% was required to evaluate a position. We only retained positions with phred-scaled quality score of ≥Q20 using vcftools (https://vcftools.github.io/index.html)18. The codon sequences for all single nucleotide polymorphisms (SNPs) positions are extracted using information from annotation with SnpEff19.
The bioinformatics software CLC Genomics Workbench 11.0, Qiagen, was used to study the five largest clusters which comprise more than three patient-isolates. After trimming, the sequences were mapped to the reference genome CDC 1551 (NCBI Reference Sequence: NC_002755.2, assembly accession GCA_000008585.1), followed by a restrict calling to target regions of InDels and structural variants, as well as a Local Realignment. Single nucleotide variants (SNP) were analysed and called for by a Fixed Ploidy Variant Detection step. Fixed ploidy variant parameters- Ploidy: 2, Required variant probability 90%. Variant detection and general filters- Minimum coverage: 30, Minimum count: 10 and Minimum frequency: 20%.
Phylogenetic analyses and strain diversity
Phylogenetic analysis of the single nucleotide polymorphisms (SNPs) was conducted for both the entire set of 93 isolates as well as for four additional genomes/assemblies (CCDC5180 (East Asian lineage 2), EAI/OSDD271 (East African-Indian lineage 3), Haarlem and CTRI-2 (Euro-American lineage 4) that represent the modern M. tuberculosis lineage. These four genomes were aligned to the reference genome CDC1551 using the nucmer program in the MUMmer package, version 3.2320 and SNPs were extracted using the show-snps program in the same package. The resulting lists of SNPs were merged into a vcf-like file and then combined with the variants from the 93 samples using GATK CombineVariants. SNPs located within 12 bp of each other, SNPs in PE/PPE/PGRS genes phage, repeat and transposons were excluded to avoid any concern about errors in the read alignment in those repetitive regions of the genome8. Furthermore, SNPs in additional drug-resistance associated genes21 were also removed to exclude the possibility that homoplasy of drug resistance mutations would significantly decrease the reliability of phylogeny22,23. Ambiguous positions were also excluded and then concatenated alignment was used to generate a midpoint rooted phylogenetic tree in RAxML (version 7.3.4) under GTRCAT substitution model with 100 bootstrap replicates.
The Microbial Genomics Module, CLC, was used to create a Maximum Likelihood (ML) SNP-tree for the five largest clusters, comprising 26 patient isolates. In this analysis the minimum coverage in each sample = 10, minimum coverage percentage of average required = 10, prune distance = 10, minimum z-score = 1,96 and a bootstrap analysis performed with 600 replicates. A MIRU-VNTR phylogenetic tree was performed using BioNumerics v.7.6/Applied Maths (a BioMerieux Company).
Genomic cluster analysis
Genomic clusters were determined independently of the epidemiological data and a cluster was defined if no more than 12 SNPs separated a patient isolate from that of at least one isolate from another patient in the cluster. Twelve SNPs was previously defined as the upper threshold of genomic relatedness noted within hosts and between related hosts10.
Statistical analysis
Prism 6 f for Mac OS X was used for statistical analysis. The Chi-square test was used to analyse differences between patients clustered by WGS and patients that were not; Fisher’s exact test was used for cell counts below five. Significance was accepted at p < 0.05, p < 0.01, or p < 0.001.
Ethical approval
The research was approved by the Medical Ethics Committee at the Lund University, Lund, Sweden (Dnr. 2014/2). The study was approved by the Regional Ethical Review Board (Dnr 2014/2), Lund University, Sweden. All experiments were performed in accordance with relevant guidelines and regulations.
Results
Study selection
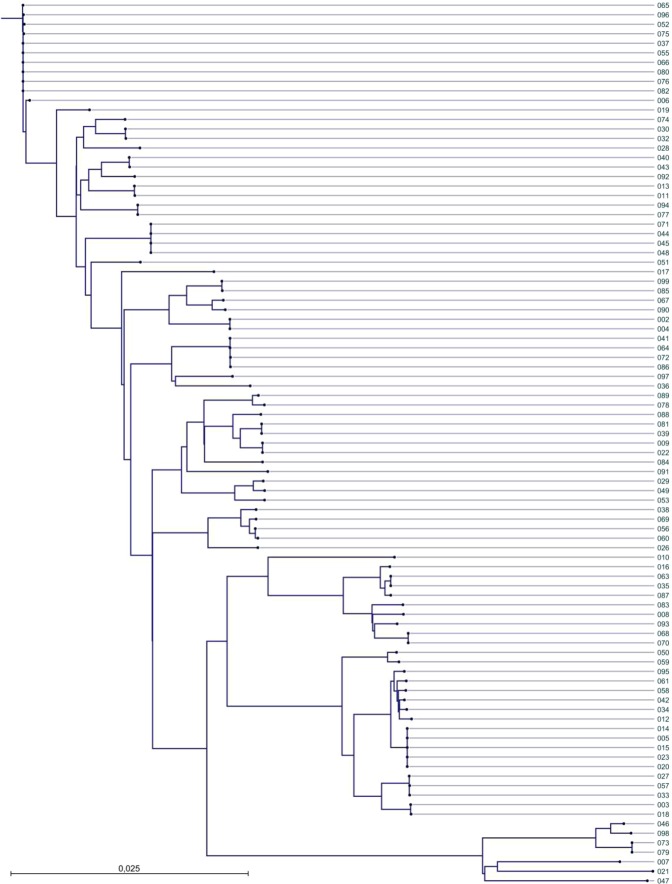
The study population consisted of isolates from 801 eligible TB patients treated between 2004 and 2014 at four infectious disease clinics and one paediatric clinic in Scania County, Sweden. In total, 100 isolates were selected for WGS. The remaining 701 isolates were excluded since they were either culture negative, had no epidemiological or microbiological connection at all, or with an epidemiological connection in small clusters outside Scania County. Of the remaining 100 patient M. tuberculosis isolates, we excluded seven isolates due to insufficient DNA quantity and quality (Fig. 1). The final 93 isolates were selected for WGS due to epidemiological connections in pairs or groups. The selected sample thus contained patient isolates from potential clusters with clinical relevance for contact tracing. The phylogenetic tree revealed that three of the seven main global lineages of M. tuberculosis circulated in Scania county during the time of the sampling (Supplemented Fig. 1) [36,49–51]. The vast majority of isolates belonged to lineage 4 (62%) (Europe, America and Africa) while 30% belonged to lineage 2 (East Asia), with lesser representation from lineage 3 (8%, India and East Africa).
Figure 1.
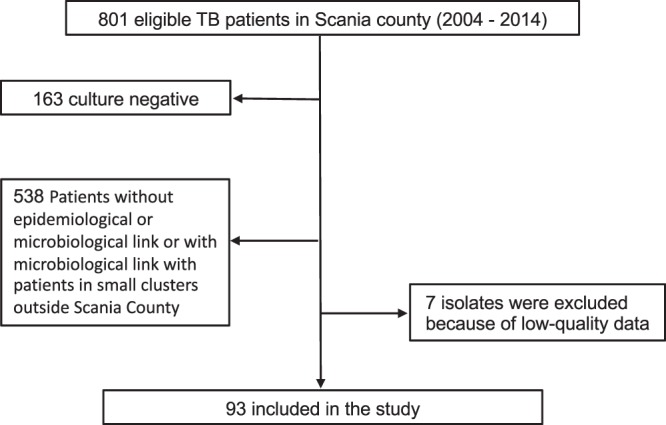
Flow chart of sample collection. Graph show number of isolates/sequences included in the study.
Clinical characteristics
The majority of the patients were adults at the time of the diagnosis (median age 39 years), presenting the disease as pulmonary tuberculosis (76%), extra pulmonary TB (20%) or both simultaneously TB (4%) (Table 1). The age range was 1 to 91 years, of which two patients were infants. None of the patients were positive for the human immunodeficiency virus (HIV). Clinical outcomes were recorded through December 2016, with a minimum of 12 months of follow-up for all patients. The majority of patients had favourable outcomes; 84 patients (91%) were cured or completed the treatment. Five patients (5%) did not complete the treatment, and one patient (1%) had a relapse of pulmonary TB. Three TB related deaths (3% of patients) were recorded during the follow-up period. The mean age of the clustered cases was 40 years (range 1–91) compared to 41 years (range 1–87) of the WGS un-clustered cases. There was no statistical difference between the clustered cases and the un-clustered cases regarding the parameters in Table 1, except in two cases. All homeless patients were found in the clustered group (p = 0.0021), while most newly arrived immigrants were found in the un-clustered group (p = 0.0200). There was no statistical difference in lineage association between clustered and un-clustered patients (lineage 4: 54% vs 69% (p < 0.1373), lineage 2: 34% vs 27% (p = 0.4997) and lineage 3: 12% vs 4% (p = 0.2342)).
Table 1.
Characteristics of 93 Patients with Laboratory-Confirmed Tuberculosis and epidemiologic link in Scania region-Sweden between 2003 and 2014 overall and comparing clustered vs un-clustered.
| Characteristics | All patients (n = 93) | Clustered (n = 52) | Un-clustered (n = 41) |
|---|---|---|---|
| Age–(years) | |||
| Mean (±Standard deviation) | 40.6 (±19.4) | 39.3 (±18.2) | 42.4 (±21.7) |
| Range | 1–91 | 1–91 | 1–87 |
| Gender | |||
| Male | 57 (61) | 32 (62) | 25 (61) |
| Female | 36 (39) | 20 (38) | 16 (39) |
| Place of birth | |||
| Swedish born | 28 (30) | 15 (29) | 13 (32) |
| Foreign born | |||
| Somalia | 17 (18) | 7 (13) | 10 (24) |
| Vietnam | 8 (9) | 4 (8) | 4 (10) |
| Others | 40 (43) | 26 (50) | 14 (34) |
| Tuberculosis Type | |||
| Pulmonary | 71 (76) | 44 (85) | 30 (73) |
| Extra pulmonary | 19 (20) | 9 (17) | 10 (24) |
| Both | 3 (4) | 1 (2) | 2 (5) |
| Smear positive | |||
| Yes | 59 (60) | 36 (69) | 23 (56) |
| No | 34 (37) | 16 (31) | 18 (44) |
| Treatment Outcome | |||
| Completed treatment | 84 (90) | 47 (90) | 37 (90) |
| Incomplete treatment | 5 (5) | 2 (4) | 3 (7) |
| TB-related Death | 3 (3) | 2 (4) | 1 (2) |
| Relapse | 1 (1) | 1 (2) | 0 (0) |
| Risk Factors | |||
| History of TB exposure | 4 (4) | 2 (4) | 2 (5) |
| Immunosuppressive Rx | 5 (5) | 2 (4) | 3 (7) |
| Homelessness | 10 (11) | 10 (19) | 0 (0) |
| Newly arrived Immigrants | 10 (11) | 2 (4) | 8 (20) |
aData are n (%) or mean ± Standard Deviation on WGS analysed patients.
Transmission clusters
WGS and MIRU-VNTR was performed on the 93 M. tuberculosis isolates. MIRU-VNTR typing and pattern analysis resulted in 19 clusters comprising 59 patients. Five clusters comprised more than three patients, while the remaining 14 clusters comprised of less than three patients (Table 2, Fig. 2). These 19 MIRU-VNTR determined clusters comprised of 59 of the 93 patients and corresponded to a clustering proportion of 63%.
Table 2.
Transmission clustering.
| Cluster | Epidemiological link | VNTR | WGS |
|---|---|---|---|
| 1 | 037, 052, 055, 065, 076, 080, 082, 096 and uncertain | 006, 037, 052, 055, 065, 066, 075, 076, 080, 082, 096 | 037, 052, 055, 065, 006, 075, 076, 080, 082, 096 |
| 2 | 044, 045, 048, 071 | 044, 045, 048, 071 | 044, 045, 048, 071 |
| 3 | 041, 064, 072, 086 | 041, 064, 072, 086 | 041, 064, 072, 086 |
| 4 | 027, 033, 057 | 033, 057 | 027, 033, 057 |
| 5 | Uncertain | 005, 012, 014, 015, 020, 034, 049, 061, 095 | 005, 014, 015, 020, 023 |
| 6 | 040, 043 | 040, 043 | 040, 043 |
| 7 | 067, 090 | 067, 090 | No connection |
| 8 | 2,004 | 2,004 | 2,004 |
| 9 | 039, 081 | 039, 081 | 039, 081 |
| 10 | 056, 060 | 056, 060, 069 | 056, 060 |
| 11 | 003, 018 | 003, 018 | 003, 018 |
| 12 | 068, 070 | 068, 070 | 068, 070 |
| 13 | 073, 079 | 073, 079 | 073, 079 |
| 14 | 011, 013 | 011, 013 | 011, 013 |
| 15 | 030, 032 | 030, 032 | 030, 032 |
| 16 | 009, 022 | 009, 022 | 009, 022 |
| 17 | Uncertain | 077, 094 | 077, 094 |
| 18 | Uncertain | 085, 099 | 085, 099 |
| 19 | 035, 063 | 035, 016 | 035, 063 |
| 20 | 007, 010 | No connection | No connection |
| 21 | 036, 046 | No connection | No connection |
*Bold = technique that made the original clustering.
Figure 2.
Phylogenetic tree constructed by pairwise distance (maximum likelihood) of 93 Mycobacterium tuberculosis isolates including CDC1551 as reference genome.
Using a maximum of twelve SNPs as the upper threshold of genomic relatedness noted among hosts10, we identified 18 clusters with WGS comprising 52 patients with an overall pairwise genetic maximum distances ranging from zero to nine SNPs (median 2.5) (Table 2, Fig. 2). Five of the clusters comprised of three patients or more, while the remaining 14 clusters comprised of less than three patients. These 18 WGS transmission clusters corresponded to a clustering proportion of 56%.
Eleven of the clusters involved only foreign-born patients, but none of the clusters involved only Swedish-born patients (Table 1). However, in the largest cluster, which comprised 10 patients, 9 were Swedish-born and one was born in Somalia. The Swedish-born patients belonged to a community of homeless people with alcohol abuse at the time of diagnosis. Interestingly, the Somalian patient did not belong to the homeless community, and in contrast with the native patients, had TB in a lymph node. The time range between the first and last isolate in this cluster was 7 years (Table 1).
Cluster similarities and differences between MIRU-VNTR and WGS
WGS clustered the same isolates as MIRU-VNTR (Table 2). However, there was a major difference in cluster 5 and interesting diversities in clusters 1, 4, 7, 10 and 19. In cluster 5, MIRU-VNTR grouped additional five strains compared to WGS, while WGS included one new strain compared to MIRU-VNTR. Analysis of the phylogenetic tree revealed that the additional strains in cluster 5 added by MIRU-VNTR were 88 SNPs apart for isolate 012, 77 SNPs apart for isolate 034, 76 SNPs for isolate 061 and 72 SNPs for isolate 095. Isolate 049 was calculated to differ 826 SNPs from the rest of WGS cluster 5, hence this strain was probably given the wrong MIRU-VNTR type from the beginning. Similar results were found for the differences in clusters 1, 7, 10 and 19. Interestingly, WGS identified additional strains in clusters 4, 5 and 19 compared with MIRU-VNTR, and one additional strain was identified with MIRU-VNTR compared with WGS analysis in cluster 1. This difference can be explained by the fact that MIRU-VNTR is a 24-locus based molecular typing method, while WGS covers single nucleotide polymorphisms across the entire M. tuberculosis-genome (approximately 4,4 M bases). The SNP-analysis method is therefore much more discriminative and covers regions that might otherwise be missed, hence providing a more accurate picture of real closeness of strains.
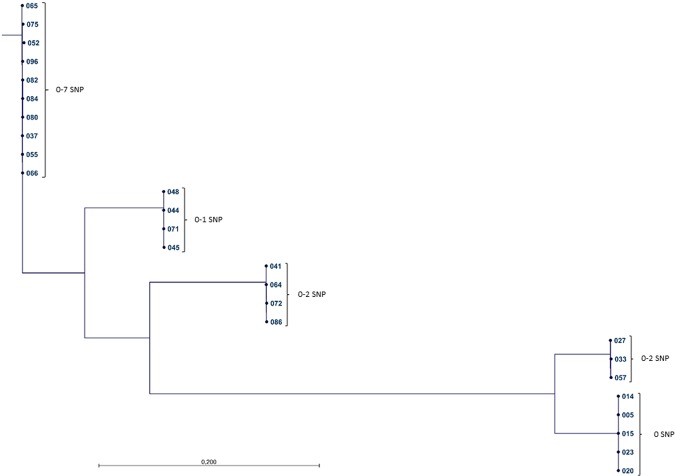
Further analysis of the five largest clusters revealed that all of the M. tuberculosis-isolates were separated by 0–7 SNP, except for patient isolate no. 06, which differed 16–18 SNP in a pairwise comparison. This strain had the same VNTR pattern as the other isolates in the cluster, but no epidemiological contact tracing data were found to support transmission, why this isolate was removed. The isolates of the remaining four clusters differed by only 0–2 SNP in a pairwise comparison in their corresponding groups (Fig. 3).
Figure 3.
ML SNP tree with CLC. A ML SNP-tree of the five largest clusters in the study, comprising of 26 patient isolates was created using the reference genome CDC1551. Within the four smaller clusters the strains differ by 0–2 SNP in a genetic pairwise comparison. In the largest cluster the patient isolates are separated by 0–7 SNP.
Correlation with epidemiologic investigation
Epidemiological contact tracing complemented with M. tuberculosis genotyping is considered to be important for the understanding of transmissions15. Interviews of patients identified 18 possible clusters of which 3 clusters comprised more than three patients (Fig. 2, Table 2). Contact tracing identified 18 clusters comprising 47 patients. Of these, two clusters with two patients in each were removed as they were not supported by MIRU-VNTR and WGS. Interestingly, WGS supported the epidemiological data more accurately and had a higher correlation with epidemiological data than the MIRU-VNTR. Our study revealed that epidemiologic tracing had an 86% sensitivity and 92% specificity using WGS. The sensitivity and specificity of MIRU-VNTR versus WGS were 96% and 80% respectively. The MIRU-VNTR method thus showed more false positive connections while epidemiologic tracing showed more false negative connections.
Geographic and genetic distances within molecular clusters
The 43 patients found in contact tracing represented 16 transmission events that all had ≤12 SNPs genetic distance (range 1–11 SNPs). Of the two epidemiologically linked clusters (010 and 007, 036 and 046), patient strains were separated by more than 12 SNPs and were excluded (Figs 2 and 3). Eight of 59 (13.5%) of the MIRU-VNTR linked patients (006, 012, 034, 049, 061, 095, 069, 016) were separated by more than 12 SNPs and were thus excluded by WGS. The genetic distance for events between patients that could not be epidemiologically linked was up to more than 1300 SNPs; 0–2 SNPs for the seven patients that lacked confirming epidemiological data but that were clustered by WGS (i.e. 066, 075, 005, 014, 015, 020, 023), and approximately 700–1300 SNPs for 36 of the 93 non-epidemiologically linked patients that were not clustered by WGS (007, 008, 010, 012, 016, 017, 019, 021, 026, 027, 028, 029, 033, 036, 038, 042, 046, 047, 050, 051, 057, 058, 059, 069, 074, 078, 083, 084, 087, 088, 089, 091,0 92, 093, 097, 098).
The ability of genomic clustering to identify unclear outbreaks was most evident in the large clusters. In the largest cluster, seven SNPs or fewer separated the 11 patients with a background of homelessness for whom contact tracing had been difficult (Fig. 3). The ability to rule out transmission was particularly evident in the second largest MIRU-VNTR cluster, in which isolates (012, 034, 049, 061 and 095) from recent immigrants from Somalia, shared same pattern with the other patients but were separated with more than 12 SNPs. In this cluster, isolates from five smear negative patients, with no known epidemiological link, could not be genetically linked by ≤12 SNPs, either (Fig. 2).
Discussion
Four hospitals in Scania has Sweden’s third largest uptake area of new TB cases. 93 TB patients whom were previously analysed by traditional genotyping techniques were included in this study. Compared to the standard genotyping and epidemiologic tracing, WGS had an overall high match in identifying cluster transmissions in this patient population. Analysing the two genotyping techniques MIRU-VNTR and WGS, we observed that MIRU-VNTR clustered eight additional patients compared to WGS. This is in good agreement with other studies who state that MIRU-VNTR generally overestimates transmission of M. tuberculosis in countries with low incidence of TB24,25. Our results are also in good agreement with the study of Wampade et al. stating that MIRU-VNTR typing may be suboptimal among immigrants from countries with high incidence of TB, where genetically closely related strains circulate over extended periods of time7. As an example, cluster 5 in our study comprises an immigrant group from Somalia, where both MIRU-VNTR and WGS clustered these patients. However, while WGS clustered five patients, MIRU-VNTR included additional five patients and missed one. The same is true for clusters 10 and 19, with patients from Ethiopia and Vietnam. The diversity in results between the two methods could be explained by the different base of genetical analysis. WGS provides a more accurate picture of the real closeness between strains.
Native cases of TB in Sweden occur especially in subpopulations with alcohol and/or drug abuse, homelessness and poor living conditions as well as individuals of old age. In these populations, endemic strains, responsible for the TB outbreaks, might be frequent, but epidemiologic contact tracing could pose a problem. In contrast, the foreign born are usually identified through health checkups and through contact tracing, but the country of infection could be hard to identify as well as to measure the effect of TB control in Sweden26. In our study, cluster 1 consisted of native homeless patients sampled 2007–2013, where most of the patients had pulmonary TB, compared to an immigrant group from Somalia sampled 2004–2005 comprising cluster 5, with diverse disease locations from joints, pulmonary and pleura. Interestingly, epidemiologic tracing identified the domestic patients, but missed the Somalian patient in cluster 1 and all Somalian patients in cluster 5. This could be due to the diversity in infection location in the Somalian patients making the epidemiologic linking difficult.
Advances in next generation sequencing technology have provided a whole new chapter in informative epidemiology and WGS is now a verified technique for investigation of various aspects of TB22. Currently there is no international standard for the SNP distance cut-off to rule in a possible transmission, and various cut-offs have been applied in studies in different countries, which limits the ability to compare data11,27–30. The upper threshold of 12-SNP distance to identify transmission was proposed by Walker et al. based on studies in two low incidence TB settings, but this limit has been used in other studies as well10,31–33. TB-isolates with a common strain identity should be alike and differ by a few number of SNPs in a pair-wise comparison. A strength in our study is the high coverage between epidemiologic links and WGS. A cut-off of 12 SNPs was also valid in our study in Sweden, a low incidence country. The limit of 12 SNPs is the upper limit and valid only if combined with confirming clinical epidemiological data. Walkers studies furthermore state that isolates separated by five or fewer SNPs are likely a result of recent transmission31. WGS was initially shown to provide increased resolution over MIRU-VNTR-based clustering31, but this technique was recently proven to be insufficient in fully resolving the chains of transmission15. An explanation could be that multiple transmissions can occur with no detectable SNP acquisition, and identical isolate pairs cannot be proven to have resulted from a recent transmission even without supporting epidemiological evidence. International guidelines for clustering cut-offs are however still needed to ensure comparison between different studies.
A limitation of our study is that we determined the sequence of the patient’s dominant genotype, but this approach is recommended as isolating single colonies prior to sequencing is likely lead to overestimation of the SNPs between cases resulting from direct transmission15. Compared to other studies, we analysed four of the modern M. tuberculosis strains in order to reveal geographic distribution13. Different M. tuberculosis strain lineages have also been associated with variable virulence, transmissibility, disease phenotypes and drug resistance profiles34. Interestingly, we found that the vast majority of isolates belonged to lineage 4, suggesting American/European/Middle Eastern origin35,36. Another difference to other studies is that we have used M. tuberculosis CDC1551 as a reference strain, due to its continued passage in humans, in comparison to M. tuberculosis H37Rv that has been passaged in vitro for many years37,38. Also, there is a benefit of using CDC1551 as a reference in WGS for mapping of resistance genes and variant calling, as H37Rv is known for some deletions in its genome39.
To summarize, we conclude that WGS is well suited for identifying transmission clusters in settings with low TB incidence. However, as a future goal an international standardization is needed on all factors to ensure that WGS data are comparable between different studies.
Supplementary information
Acknowledgements
We thank Jenny Pripp and Torbjörn Nilsson at the clinical mycobacteriology laboratory, Malmö, Sweden for their help with the patient material. Support by NBIS (National Bioinformatics Infrastructure Sweden) is gratefully acknowledged. The computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Centre for Advanced Computational Science (3061) under Project SNIC b2016153. This work was supported by the Sten K Johnsons Stiftelse, Kung Oscars II: s jubileumsfond, Alfred Österlund Stiftelse, the Fund of the Hedda and John Forssman Foundation and Heart and Lung Foundation (nr. 20150733).
Author Contributions
N.W. and E.S. collected the material; N.A., E.T.H., J.T., N.W., K.F.-J., B.J. C.W.-O., E.T., P.M., B.K. and G.G. analyzed the data; G.G., N.A. and P.M. designed the study; N.A., E.T.H. and P.M. created the figures; all authors wrote the manuscript. N.A. and E.T.H. contributed equally to the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-39971-z.
References
- 1.2016. Gtr. http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1 (2016).
- 2.Sweden. TPhao. https://www.folkhalsomyndigheten.se/the-public-health-agency-of-sweden/ (2017).
- 3.Supply P, et al. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol. 2000;36:762–771. doi: 10.1046/j.1365-2958.2000.01905.x. [DOI] [PubMed] [Google Scholar]
- 4.Kamerbeek J, et al. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato-Maeda M, Metcalfe JZ, Flores L. Genotyping of Mycobacterium tuberculosis: application in epidemiologic studies. Future Microbiol. 2011;6:203–216. doi: 10.2217/fmb.10.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman Herrador BR, et al. Description of the largest cluster of tuberculosis notified in Norway 1997-2011: is the Norwegian tuberculosis control programme serving its purpose for high risk groups? BMC Public Health. 2015;15:367. doi: 10.1186/s12889-015-1701-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wampande EM, et al. Long-term dominance of Mycobacterium tuberculosis Uganda family in peri-urban Kampala-Uganda is not associated with cavitary disease. BMC Infect Dis. 2013;13:484. doi: 10.1186/1471-2334-13-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenner L, et al. Mycobacterium tuberculosis transmission in a country with low tuberculosis incidence: role of immigration and HIV infection. J Clin Microbiol. 2012;50:388–395. doi: 10.1128/JCM.05392-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardy JL, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364:730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 10.Walker TM, et al. Assessment of Mycobacterium tuberculosis transmission in Oxfordshire, UK, 2007-12, with whole pathogen genome sequences: an observational study. Lancet Respir Med. 2014;2:285–292. doi: 10.1016/S2213-2600(14)70027-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roetzer A, et al. Whole genome sequencing versus traditional genotyping for investigation of a Mycobacterium tuberculosis outbreak: a longitudinal molecular epidemiological study. PLoS Med. 2013;10:e1001387. doi: 10.1371/journal.pmed.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comas I, et al. Whole-genome sequencing of rifampicin-resistant Mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat Genet. 2012;44:106–110. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comas I, et al. Population Genomics of Mycobacterium tuberculosis in Ethiopia Contradicts the Virgin Soil Hypothesis for Human Tuberculosis in Sub-Saharan Africa. Curr Biol. 2015;25(3260-3266):2015/12/22. doi: 10.1016/j.cub.2015.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stucki D, et al. Tracking a tuberculosis outbreak over 21 years: strain-specific single-nucleotide polymorphism typing combined with targeted whole-genome sequencing. J Infect Dis. 2015;211:1306–1316. doi: 10.1093/infdis/jiu601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casali N, et al. Whole Genome Sequence Analysis of a Large Isoniazid-Resistant Tuberculosis Outbreak in London: A Retrospective Observational Study. PLoS Med. 2016;13:e1002137. doi: 10.1371/journal.pmed.1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adane K, et al. Prevalence and drug resistance profile of Mycobacterium tuberculosis isolated from pulmonary tuberculosis patients attending two public hospitals in East Gojjam zone, northwest Ethiopia. BMC Public Health. 2015;15:572. doi: 10.1186/s12889-015-1933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao W, et al. The cyclic peptide ecumicin targeting ClpC1 is active against Mycobacterium tuberculosis in vivo. Antimicrobial agents and chemotherapy. 2015;59:880–889. doi: 10.1128/AAC.04054-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danecek P, et al. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strainw1118; iso-2; iso-3. Fly (Austin) 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz S, et al. Versatile and open software for comparing large genomes. Genome Biol. 2004;5(R12):2004/02/05. doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farhat MR, et al. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nat Genet. 2013;45:1183–1189. doi: 10.1038/ng.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatherell, H. A. et al. Interpreting whole genome sequencing for investigating tuberculosis transmission: a systematic review. Bmc Med; 14. DOI: ARTN 21. 10.1186/s12916-016-0566-x (2016). [DOI] [PMC free article] [PubMed]
- 23.Sandgren A, et al. Tuberculosis drug resistance mutation database. PLoS Med. 2009;6:e2. doi: 10.1371/journal.pmed.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stucki D, et al. Standard Genotyping Overestimates Transmission of Mycobacterium tuberculosis among Immigrants in a Low-Incidence Country. J Clin Microbiol. 2016;54:1862–1870. doi: 10.1128/JCM.00126-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jajou, R. et al. Epidemiological links between tuberculosis cases identified twice as efficiently by whole genome sequencing than conventional molecular typing: A population-based study. PLoS One; 13: e0195413. 2018/04/05, 10.1371/journal.pone.0195413 (2018). [DOI] [PMC free article] [PubMed]
- 26.Control/WHO ECfDPa, and ROfETs and 2017. miE. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/ecdc-tuberculosis-surveillance-monitoring-Europe-2017.pdf (2017).
- 27.Luo T, et al. Whole-genome sequencing to detect recent transmission of Mycobacterium tuberculosis in settings with a high burden of tuberculosis. Tuberculosis (Edinb) 2014;94(434-440):2014/06/04. doi: 10.1016/j.tube.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolayevskyy V, et al. Whole genome sequencing of Mycobacterium tuberculosis for detection of recent transmission and tracing outbreaks: A systematic review. Tuberculosis (Edinb) 2016;98(77-85):2016/05/10. doi: 10.1016/j.tube.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Lee, R. S. et al. Reemergence and amplification of tuberculosis in the Canadian arctic. J Infect Dis; 211: 1905–1914. 2015/01/13, 10.1093/infdis/jiv011 (2015). [DOI] [PubMed]
- 30.Guerra-Assuncao JA, et al. Large-scale whole genome sequencing of M. tuberculosis provides insights into transmission in a high prevalence area. Elife. 2015;4:2015/03/04. doi: 10.7554/eLife.05166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker TM, et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: a retrospective observational study. Lancet Infect Dis. 2013;13:137–146. doi: 10.1016/S1473-3099(12)70277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen KA, et al. Evolution of Extensively Drug-Resistant Tuberculosis over Four Decades: Whole Genome Sequencing and Dating Analysis of Mycobacterium tuberculosis Isolates from KwaZulu-Natal. PLoS Med. 2015;12:e1001880. doi: 10.1371/journal.pmed.1001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryant JM, et al. Whole-genome sequencing to establish relapse or re-infection with Mycobacterium tuberculosis: a retrospective observational study. Lancet Respir Med. 2013;1:786–792. doi: 10.1016/S2213-2600(13)70231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurjav U, et al. Whole Genome Sequencing Demonstrates Limited Transmission within Identified Mycobacterium tuberculosis Clusters in New South Wales, Australia. PLoS One. 2016;11:e0163612. doi: 10.1371/journal.pone.0163612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takiff HE, Feo O. Clinical value of whole-genome sequencing of Mycobacterium tuberculosis. Lancet Infect Dis. 2015;15:1077–1090. doi: 10.1016/S1473-3099(15)00071-7. [DOI] [PubMed] [Google Scholar]
- 36.Merker M, et al. Evolutionary history and global spread of the Mycobacterium tuberculosis Beijing lineage. Nat Genet. 2015;47:242–249. doi: 10.1038/ng.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betts, J. C. et al. Comparison of the proteome of Mycobacterium tuberculosis strain H37Rv with clinical isolate CDC 1551. Microbiology; 146 Pt 12: 3205–3216. 2000/12/02, 10.1099/00221287-146-12-3205 (2000). [DOI] [PubMed]
- 38.Fleischmann RD, et al. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J Bacteriol. 2002;184(5479-5490):2002/09/10. doi: 10.1128/JB.184.19.5479-5490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Toole RF, Gautam SS. Limitations of the Mycobacterium tuberculosis reference genome H37Rv in the detection of virulence-related loci. Genomics. 2017;109(471-474):2017/07/27. doi: 10.1016/j.ygeno.2017.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.