Abstract
Advances in cellular and molecular biology underpin most current therapeutic advances in medicine. Such advances for neurological and neurodegenerative diseases are hindered by the lack of similar specimens. It is becoming increasingly evident that greater access to human brain tissue is necessary to understand both the cellular biology of these diseases and their variation. Research in these areas is vital to the development of viable therapeutic options for these currently untreatable diseases. The development and coordination of human brain specimen collection through brain banks is evolving. This perspective article from the Sydney Brain Bank reviews data concerning the best ways to collect and store material for different research purposes.
Keywords: Brain banking, Brain donation, Neurodegenerative diseases, Human brain tissue processing
Introduction
Neurological disorders are a major public health and socio-economic problem throughout the world. Cellular and molecular research into these disorders is compromised by the lack of adequate animal or in vitro models and still relies heavily on studying post-mortem human brain tissues [1]. Best clinical practice also assumes accurate diagnosis, which for many neurological conditions can only be definitively achieved by neuropathological assessment at post-mortem. Biobanks are therefore essential research tools for medical research and quality control of clinical diagnosis and treatment. With declining post-mortem rates [2] and the need for high-quality fixed and frozen tissue specimens for research, specialized brain banks are essential for the standardized collection, characterization, storage, and distribution of brain and spinal tissue for research purposes and diagnosis. Many of these specialized brain banks operate within networks to enhance collaborations, optimize protocols, and increase the ability to fill requests for tissue for research. Examples of successful brain bank networks include BrainNet Europe, UK Brain Bank Network, The National Alzheimer’s Coordinating Centre, NIH NeuroBioBank, and the Australian Brain Bank Network. Most brain banks operate according to best practice guidelines [3, 4] although differences in recruitment, autopsy procedures, and protocols for tissue disbursement give rise to different brain banking models. This review discusses current operations of the Sydney Brain Bank in the context of best practice for the collection of brain tissue for research into neurodegenerative diseases and ageing.
Donor Recruitment
Establishing effective methods of donor recruitment is essential to all brain banks. There are two time points when recruitment and consent for brain tissue donations can be made: before or after death. After-death recruitment is most effective for programs that require brain tissue from victims of drug abuse and violent or unexpected death. In this instance, brain banks accept and/or request individual donations from those legally responsible at post-mortem, however there are often limited and non-standardized clinical data associated with these tissue donations and a lack of well-characterized longitudinal data for understanding any clinical disease course [5]. This mechanism of recruitment is still the most effective means of collecting tissue from younger (< 75 years) control donors, who are in short supply [6], as most die unexpectedly.
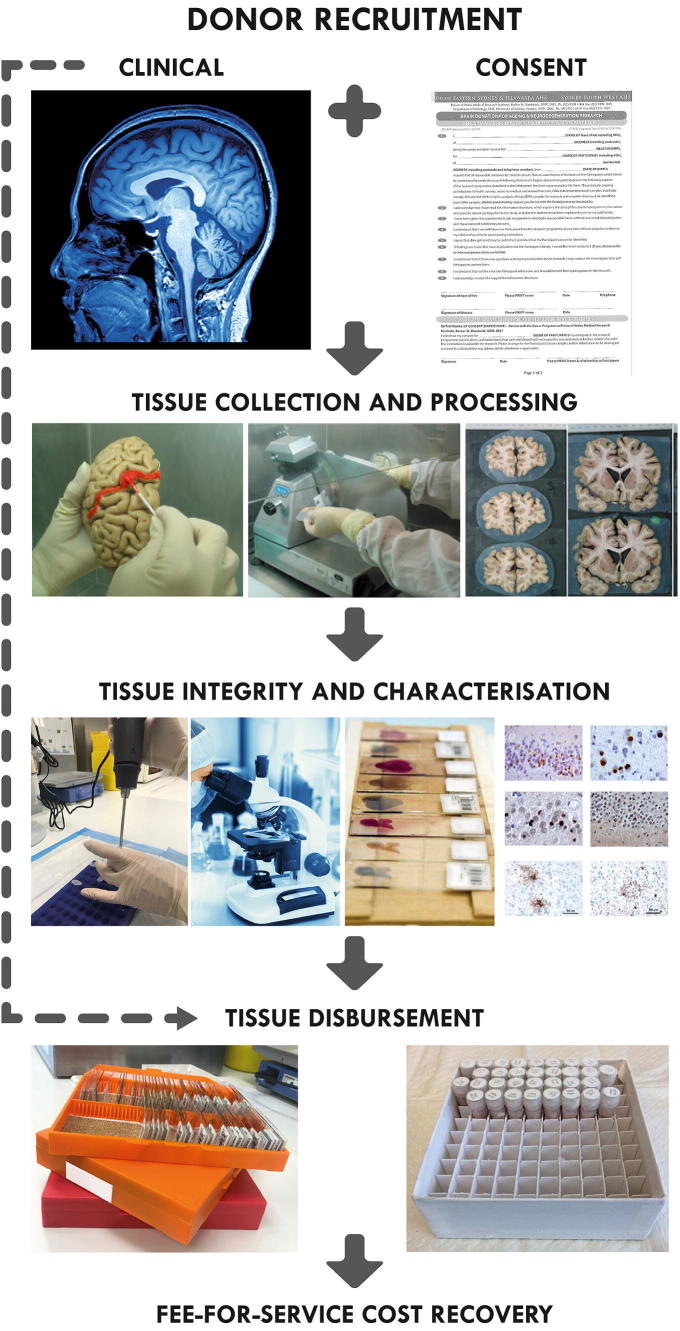
The majority of brain donor recruitment is easier to achieve before death through brain donor programs [7]. In the case of neurodegenerative diseases or collection of tissue from healthy aged individuals, it is agreed that recruitment through longitudinal brain donor programs that collect medical information and clinical records during life is the most valuable type of donors [5]. These programs usually undertake additional detailed assessment of the donors during life, such as neuroimaging, specimen collection, and genetic testing, as well as neuropsychological and cognitive testing (Fig. 1). These programs may be embedded in the brain bank itself or may be managed externally by specialized clinical programs, which are essential for advancing our understanding of the biological basis of disease in the CNS [5]. The neuropathological characterization of these donors has facilitated new clinical diagnostic criteria [8].
Fig. 1.
A schematic representation of Sydney Brain Bank processes for brain donation. Brain donors are recruited through longitudinal clinical research programs undertaking comprehensive clinical assessments. All tissue is characterized according to current research diagnostic criteria and tissue integrity checks are undertaken. Tissue is provided to researchers for projects that have been approved by an independent scientific review committee and minimal fee-for-service cost-recovery is applied.
Tissue Collection
Some brain banks are fully integrated into the local health system and hospital pathology departments (e.g., New York Brain Bank [3]) while others provide their own staff to carry out tissue collection and characterization (e.g., Sydney Brain Bank) or pay a fee for service (e.g., Stanley Foundation Brain Bank [9]). The autopsy may be carried out in a hospital mortuary or a teaching institution. In many circumstances, consent from both the donor and next-of-kin needs to be obtained [7]. In the case of the Sydney Brain Bank, additional approval is required from the host institution where the autopsy is performed to ensure there are no objections to donation from the next-of-kin. This requires careful training and coordination by brain bank staff to ensure all necessary paperwork is in place to enable the autopsy to go ahead.
The body is transferred to the autopsy suite using body transport services (Sydney Brain Bank also uses family-appointed funeral directors). The brain autopsy is performed by a trained individual, who may be a facility attendant (for a fee) or a trained brain bank staff member (e.g., Sydney Brain Bank). In addition to brain donation, the Sydney Brain Bank collects spinal cord tissue from willing donors, as spinal cord pathology has long been recognized as a feature of neurodegenerative disorders such as motor neuron disease where collection is recommended (albeit not essential) for neuropathological diagnosis. Also alpha-synuclein pathology localized in thoracic and sacral spinal cord segments [10] occurs even in asymptomatic aged individuals [11]. Brain banks may therefore wish to consider collection of spinal cords from donors as in our experience individuals are willing to donate both brain and spinal cord tissues. Declining post-mortem rates mean some institutions may have minimal staff and reduced opening hours. Regulatory issues and the location of accessible autopsy suites may also make tissue retrieval within short timeframes difficult. Most brain banks try to provide/use facilities that are close by and convenient for tissue transport back to the biobank, which has important implications for tissue integrity (e.g., Sydney Brain Bank uses nearby university and hospital teaching facilities).
Following tissue retrieval, tissue dissection may occur at the autopsy suite or the tissue may be transported on ice back to the specialized brain bank laboratories for processing. In line with currently recommended practice, tissue is typically prepared so that it may be used for molecular and genetic studies in addition to routine histopathology and stereological methods. In most instances, one hemisphere is fixed for diagnostic evaluation and the other is frozen. In instances where there is obvious asymmetry, the whole brain might be fixed for diagnostic evaluation.
Frozen Tissue Integrity
There are multiple ways in which brain tissue can be frozen for storage. Assessment of freezing between aluminum plates, under ice-cold isopentane or liquid nitrogen revealed advantages and disadvantages of these protocols. The liquid nitrogen vessel provides clear anatomical contours while the aluminum plates prevent cracking and crumbling of the tissue, which are problems associated with immersion in liquid nitrogen or isopentane [12]. A very recent review by The New York Brain Bank of Columbia University evaluated the methods of tissue freezing used by brain banks over the past 35 years, extracting the most valuable aspects in order to optimize the way postmortem brain tissue is frozen [13]. The method they deem most effective includes the use of Teflon-coated aluminum plates pre-cooled to −70°C to −100°C to ‘sandwich’ the samples inside the vessel [13]. This method has the same benefits found in the previous study, including minimal freezing artifacts as well as less stress on the tissue during freezing [12, 13]. The protocol used for freezing the tissue impacts on the ability to provide anatomically accurate tissue samples.
The pH of fresh and frozen postmortem brain tissues has been shown to correlate strongly with RNA integrity [14] and has been adopted by many brain banks, including ours, as an inexpensive way to measure the integrity of fresh or thawed tissue samples [15]. The practice of collecting tissue in the shortest timeframe possible (preferably < 24 h) is universally accepted by brain banks, as extended postmortem intervals have been proposed to adversely affect tissue quality. Cases collected after sudden death are also generally preferred (although rarely available in the setting of a neurodegenerative disease) as hypoxic states resulting from ischemia are often experienced in prolonged agonal states and lead to acidosis.
Despite the overarching belief that these factors contribute to decreased tissue integrity, recent studies have shown that postmortem delay and agonal state do not have significant effects on RNA integrity [13–15]. Indeed, a large study of 556 brains carried out by the Brains for Dementia Research Network Neuropathology Study Group in the UK recently reported no association between postmortem delay or agonal state and either pH or RNA integrity of thawed tissue specimens [15]. Tissue pH levels also remained consistent across various brain regions, thereby negating the need to sample multiple sites for the assessment of tissue integrity [15]. This study refutes the widely held presumption that an extended postmortem delay is detrimental to brain tissue integrity per se. However, extended postmortem delays and postmortem storage temperatures have recently been shown to adversely affect some vulnerable proteins, particularly phospho-tau, and this has important implications for neurodegenerative brain banks [16].
A more important aspect is that samples exposed to even limited freeze-thawing cycles appear to be no longer suitable for biochemical studies [16]. This is most problematic for brain banks as frozen tissue often has to be assessed and sampled from larger frozen blocks to provide requested tissue to researchers. The recent review by The New York Brain Bank of Columbia University also evaluated methods of storing frozen tissues, suggesting the organization of tissue samples by anatomical structure rather than by brain of origin [13]. This method reduces occupation of fridge space, increases efficiency in retrieving samples through a barcoding database, and reduces the frequency of changes in the temperature of the freezers [13]. These preferred methods of brain freezing and storage are consistent with the notion that repeated fluctuations in temperature have a negative impact on tissue integrity.
Tissue Characterization
Previous studies have demonstrated the importance of thorough neuropathological assessment of all tissues collected by brain banks [17]. The importance of standardizing the neuropathological assessment of cases across all brain banks has recently been highlighted in an international survey of brain banking operations. The authors surveyed 24 international brain banks and discovered that different pathological criteria are used to identify control cases, with some brain banks allowing the researcher to indicate the criteria for controls [7]. Given that neurodegenerative disorders are characterized by complex neuropathological features which often display considerable overlap [18], the importance of ensuring brain banks operate according to the most recent neuropathological research diagnostic criteria cannot be overemphasized. Standardization of processes also enables brain banks to facilitate requests through collaboration that they would otherwise be unable to fill due to the availability of tissue [7].
Tissue Disbursement
Many brain banks provide equitable access to researchers wishing to obtain tissue, although some prioritize requests to allow institutional researchers, or researchers with related research projects, priority access to the resource. Some researchers not related to the brain bank or the hosting institution may be refused access to highly sought-after regions such as the substantia nigra or hippocampus. As is the case with the Sydney Brain Bank, applications for tissue are reviewed by an independent panel to confirm the merits of the project and all researchers are required to submit ethical approval for the study in which the tissue will be used. Research projects that have already been peer reviewed and have received grant funding may not be subject to such rigorous review.
Most brain banks supply tissue along with basic demographic information (age, sex, and clinical and pathological diagnosis). Detailed clinical information is also usually available, if requested, but may only be accessible under a collaborative agreement with the recruiting brain donor program/clinician. The provision of tissue is usually given following a tissue transfer agreement being signed between institutions. Such agreements outline intellectual property issues, acknowledgement requirements, and limitations around permitted use. In most cases, recipient scientists are required to complete annual surveys regarding the outcomes of their research using the tissue and/or submit copies of arising publications.
Matching of case and control cohorts represents best practice for research. In the case of human brain tissue research, brain banks can assist by providing control tissue that is appropriately matched to the disease cohort, most commonly for age, sex, and post-mortem delay. This ensures an appropriate supply of control tissue is available, which is one of the most challenging aspects of human brain tissue research. Matching cohorts within banks also minimizes the effect of variations introduced by differences in methodology used for tissue collection, processing, and storage.
Some brain banks such as the Stanley Foundation in the USA supply coded tissue samples. Only once the researchers have finished their study do they send the results to the Stanley Foundation and simultaneously receive the code to facilitate data analysis [9]. The researchers are free to publish their findings but they also agree to share their data with other researchers accessing the same specimens.
Sustainability
The development, implementation, and maintenance of a brain bank is extremely costly. Estimates of the cost of brain banking vary between $10,000 and $30,000 per specimen banked and are predominantly related to personnel costs [19]. Some brain banks are funded by research grants, others through philanthropic or institutional support [20]. As it is a costly exercise, most large brain banks employ user fees to recover some of their costs, although it is estimated that for the most part little more than 5% of actual costs are recovered by this means. Some brain banks provide specimens to researchers free of financial charge but require data to be deposited in a central repository for future use [9].
The issues concerning biobank sustainability have been recently reviewed [20] and highlight the difficulties around biobank sustainability. Brain banking has many additional complexities, particularly with regard to the inability to select the types of cases collected. Indeed, while the characterization of each case contributes to scientific knowledge, not all tissue collected will be requested by researchers, often due to the presence of co-existing or unexpected pathologies, which cannot be identified prior to death. The cost of tissue processing, characterization, and storage remains the same but there is little scope for cost recovery and increased costs associated with tissue storage.
Conclusion
Brain banking is essential for molecular and cellular advances in neurological and neurodegenerative disorders that are becoming increasingly prevalent worldwide. The cost of brain banking is substantial and mechanisms for brain tissue donation remain scarcely known to the public - there is an underdeveloped understanding of the requirement for investment and participation in brain banks in the community. A particular strength of the Sydney Brain Bank lies in the collection of tissue from donors who participate in comprehensive, longitudinal clinical research programs during life. This collaborative relationship between clinical and pathological streams enhances research outcomes and significantly enriches the scientific value of the tissue alone. Access to such well-characterized tissue is crucial for the advancement of knowledge likely to unlock treatment development pathways for neurological and neurodegenerative diseases. Brain Bank networks are essential to ensure the harmonization and standardization of tissue characterization, storage, and processing, and there is a need for universal guidelines for record-keeping of critical variables for optimizing tissue integrity. It is also necessary to develop an agreed-on methodology for tissue integrity checks. This would allow the limited available tissue from multiple brain banks to be utilized in the same studies. Brain banks are the only means of legally obtaining human tissue samples required for research into neurological and neurodegenerative diseases. The development of more strategic regional and worldwide alliances and arrangements for linking up brain banks is now necessary to provide sufficient tissues in a timeframe that will allow for the fastest advances possible for these diseases.
Acknowledgements
We wish to thank the employees of the Sydney Brain Bank, and the institutional funding for the facility from the University of New South Wales and Neuroscience Research Australia.
Conflict of interest
The authors direct and manage the Sydney Brain Bank.
References
- 1.Kretzschmar H. Brain banking: opportunities, challenges and meaning for the future. Nat Rev Neurosci. 2009;10:70–78. doi: 10.1038/nrn2535. [DOI] [PubMed] [Google Scholar]
- 2.Royal College of Pathologists of Australasia Autopsy Working Group P. The decline of the hospital autopsy: a safety and quality issue for healthcare in Australia. Med J Aust 2004, 180: 281–285. [DOI] [PubMed]
- 3.Vonsattel JP, Del Amaya MP, Keller CE. Twenty-first century brain banking. Processing brains for research: the Columbia University methods. Acta Neuropathol 2008, 115: 509–532. [DOI] [PMC free article] [PubMed]
- 4.Klioueva NM, Rademaker MC, Dexter DT, Al-Sarraj S, Seilhean D, Streichenberger N, et al. BrainNet Europe’s Code of Conduct for brain banking. J Neural Transm (Vienna) 2015;122:937–940. doi: 10.1007/s00702-014-1353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Lancet Neurology Brain banking: more effective strategies needed. Lancet Neurol. 2013;12:1035. doi: 10.1016/S1474-4422(13)70249-7. [DOI] [PubMed] [Google Scholar]
- 6.Bell JE, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Budka H, et al. Management of a twenty-first century brain bank: experience in the BrainNet Europe consortium. Acta Neuropathol. 2008;115:497–507. doi: 10.1007/s00401-008-0360-8. [DOI] [PubMed] [Google Scholar]
- 7.Palmer-Aronsten B, Sheedy D, McCrossin T, Kril J. An international survey of brain banking operation and characterization practices. Biopreserv Biobank. 2016;14:464–469. doi: 10.1089/bio.2016.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The stanley foundation brain collection and neuropathology consortium. Schizophr Res. 2000;44:151–155. doi: 10.1016/S0920-9964(99)00192-9. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Mori F, Tanji K, Miki Y, Toyoshima Y, Kakita A, et al. α-Synuclein pathology in the cranial and spinal nerves in Lewy body disease. Neuropathology. 2016;36:262–269. doi: 10.1111/neup.12269. [DOI] [PubMed] [Google Scholar]
- 11.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, et al. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 12.Vonsattel JP, Aizawa H, Ge P, DiFiglia M, McKee AC, MacDonald M, et al. An improved approach to prepare human brains for research. J Neuropathol Exp Neurol. 1995;54:42–56. doi: 10.1097/00005072-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez EPC, Keller CE, Vonsattel JP. The New York Brain Bank of Columbia University: practical highlights of 35 years of experience. Handb Clin Neurol. 2018;150:105–118. doi: 10.1016/B978-0-444-63639-3.00008-6. [DOI] [PubMed] [Google Scholar]
- 14.Ervin JF, Heinzen EL, Cronin KD, Goldstein D, Szymanski MH, Burke JR, et al. Postmortem delay has minimal effect on brain RNA integrity. J Neuropathol Exp Neurol. 2007;66:1093–1099. doi: 10.1097/nen.0b013e31815c196a. [DOI] [PubMed] [Google Scholar]
- 15.Robinson AC, Palmer L, Love S, Hamard M, Esiri M, Ansorge O, et al. Extended post-mortem delay times should not be viewed as a deterrent to the scientific investigation of human brain tissue: a study from the Brains for Dementia Research Network Neuropathology Study Group. UK. Acta Neuropathol. 2016;132:753–755. doi: 10.1007/s00401-016-1617-2. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt A, Bauer M, Heinsen H, Feiden W, Consortium of Brainnet Europe, II, Falkai P, et al. How a neuropsychiatric brain bank should be run: a consensus paper of Brainnet Europe II. J Neural Transm (Vienna) 2007, 114: 527–537. [DOI] [PubMed]
- 17.Nolan M, Troakes C, King A, Bodi I, Al-Sarraj S. Control tissue in brain banking: the importance of thorough neuropathological assessment. J Neural Transm (Vienna) 2015;122:949–956. doi: 10.1007/s00702-015-1376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan RH, Yang Y, Halliday GM. Multiple neuronal pathologies are common in young patients with pathologically proven Frontotemporal lobar degeneration. Neuropathol Appl Neurobiol. 2017 doi: 10.1111/nan.12455. [DOI] [PubMed] [Google Scholar]
- 19.Hulette CM. Brain banking in the United States. J Neuropathol Exp Neurol. 2003;62:715–722. doi: 10.1093/jnen/62.7.715. [DOI] [PubMed] [Google Scholar]
- 20.Watson PH, Nussbeck SY, Carter C, O’Donoghue S, Cheah S, Matzke LA, et al. A framework for biobank sustainability. Biopreserv Biobank. 2014;12:60–68. doi: 10.1089/bio.2013.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]