Abstract
Investigating the pathophysiological mechanisms underlying brain disorders is a priority if novel therapeutic strategies are to be developed. In vivo studies of animal models and in vitro studies of cell lines/primary cell cultures may provide useful tools to study certain aspects of brain disorders. However, discrepancies among these studies or unsuccessful translation from animal/cell studies to human/clinical studies often occur, because these models generally represent only some symptoms of a neuropsychiatric disorder rather than the complete disorder. Human brain slice cultures from postmortem tissue or resected tissue from operations have shown that, in vitro, neurons and glia can stay alive for long periods of time, while their morphological and physiological characteristics, and their ability to respond to experimental manipulations are maintained. Human brain slices can thus provide a close representation of neuronal networks in vivo, be a valuable tool for investigation of the basis of neuropsychiatric disorders, and provide a platform for the evaluation of novel pharmacological treatments of human brain diseases. A brain bank needs to provide the necessary infrastructure to bring together donors, hospitals, and researchers who want to investigate human brain slices in cultures of clinically and neuropathologically well-documented material.
Keywords: Alzheimer’s disease, Brain bank, Brain-derived neurotrophic factor, Depression, Electrical activity, Human brain slice culture, Neuropsychiatric disorders, Organotypic culture, Postmortem human brain tissue, Resected human brain tissue
Introduction
The discovery of the pathophysiological mechanisms underlying human brain disorders and the development of potential treatments based upon new molecular targets present great challenges. Our knowledge on the structure and function of the human brain is far from complete, and we are only beginning to explore strategies to protect our brains against diseases and repair damage caused by disorders. Neuropsychiatric disorders are currently in the top ten of human health burdens. Thus, one of the main efforts in brain research is aimed at obtaining insights into the pathogenesis of neuropsychiatric disorders. Such research is, at present, mainly based upon animal models or upon animal or tumor-derived cell/tissue culture experiments.
Animal models of neuropsychiatric disorders have made a substantial contribution to our understanding of human disorders and are used to evaluate putative novel therapeutics. There are now a great number of transgenic rodent models, non-human primate models, and even invertebrate models for Alzheimer’s disease (AD), mood disorders, and other brain disorders [1–3]. However, due to the unique properties of the human brain, animals do not fully recapitulate the complexity of human neuropsychiatric disorders and only partially mimic symptoms and the underlying processes of pathogenesis. For example, the social defeat rodent model for depression does not mirror the full range of symptoms of patients with major depression (depressed mood, neuro-vegetative symptoms, cognitive symptoms such as guilt, emotional symptoms, and psychomotor agitation or retardation), and it lacks symptoms that are unique to humans and are only revealed through verbal enquiry, such as suicide ideation [4]. The limited number of methods with which we can measure these symptoms objectively in rodents only aggravates the situation. The most commonly-used AD model is the APP/PS1 transgenic mouse, which uses two different amyloid precursor protein (APP) and presenilin 1 (PS1) familial mutations to show limited or incomplete phenotypes. The majority of the AD models have amyloid accumulation, but lack the widespread neurodegeneration, brain atrophy, and intracellular neurofibrillary tangles [5], which are typical features of the AD brain. In addition, in this model, the onset of plaque formation is accompanied by cognitive impairment, which is much earlier than in AD, where cognitive impairment only happens decades after plaque formation [1]. In addition, species differences have been observed in basic properties of neurons. Studies have shown intrinsic differences in electrophysiological characteristics between human and mouse neurons [6, 7]. Neuron size and the anatomical complexity of glial cells differ considerably between humans and animal models [8, 9]. Occasionally, animal models for AD or autism using non-human primates are not practical in terms of time, cost, or availability, although these models may provide essential advantages [10, 11], such as greater genetic similarity to humans and more relevant development of pathology. However, so far there has been no satisfactory animal model for a human brain disease that accommodates face validity (similarity between the behavioral phenotypes in the model and the clinical symptoms of the disorder), construct validity (model and disorder have a similar underlying neuropathogenesis), and predictive validity (amelioration or absence of changes in the behavioral abnormalities in the model as a result of a clinically effective or ineffective treatment) [12].
In vitro animal- or tumor-derived cell/tissue cultures or immortalized cell lines (rather than neurons) offer another tool to study the molecular and cellular mechanisms underlying the pathogenesis of brain disorders, as well as drug efficacy. Traditionally, the dynamic properties of the nervous system have been studied using brain slice cultures from animal models. They comprise processes such as cell migration [13], drug toxicity [14], various disease models including AD [15], and environmental influences on the brain epigenome such as gene (leucine-rich alpha-2-glycoprotein 1) methylation differences caused by lower glucose concentration in preterm births [16]. However, usually there are discrepancies among studies, which are, at least partly, due to species differences [6, 17] and/or technical considerations such as different cell culture medium parameters or cell passages, that complicate their relevance for human brain pathophysiology. There are studies showing that mature neurons can be isolated from the human brain at autopsy, survive in vitro, and maintain their functional properties [18]. However, the local physiological neuronal network functions are interrupted in this model and it is not clear what specific cells are selected by this procedure. Recently, differentiating human embryonic stem cells [19] and induced pluripotent stem cells [20] that develop into neurons or cerebral organoids in three-dimensional culture [21] have been used to mimic human cerebral cortex development and cellular organization in vitro. Although these approaches mark significant progress, challenges regarding cell purity, cell-subtype identity, and the desired maturity of the human neuron-like cells generated need to be addressed. In addition, these approaches can currently neither reconstruct the complicated human neuronal network of the central nervous system, including its regular cortical layering and columnar organization, nor the complex micro-environment, which would be crucial when investigating the physiological characteristics of human brain circuitry or pathophysiological mechanisms leading to the development of neuropsychiatric disorders.
For these reasons, a direct translation from results obtained from animal models or in vitro cell models to human brain disorders is in most cases not possible. Therefore, the development of experimental systems more closely reflecting the human brain in health and disease is needed.
Human Brain Slice Culture
In order to investigate neuropsychiatric disorders, we have explored the potential use of organotypic cultures of postmortem and resected human brain tissue [22–28]. For practical and ethical reasons, the availability, the amount, and the available brain areas of resected brain material obtained at surgery are very limited. Both postmortem and resected tissues can be maintained in vitro for a month and beyond [24, 25] and can be experimentally manipulated [22, 24, 25, 28]. The reactions of postmortem tissue to the processing of slices and transfer to the in vitro conditions are, however, strikingly different from those of resected tissue, due to a reaction to the surgical manipulation of the latter tissue. For a schematic illustration of the difference between the pre-culture histories of postmortem and resected brain tissues, refer to the supporting information Fig. S1 in Verwer et al. [25].
The brain is left in the skull during the postmortem interval and cells that have survived the agonal state and this postmortem interval presumably have reached a kind of resting state when they are finally manipulated and placed in the culture medium. The morphological features of neurons and their histological organization in postmortem tissue slices may remain intact for as long as 50 days in vitro (DIV) as is illustrated in Fig. 1A. Relevant pathological features that are characteristic of a human disease process, such as plaques and tangles in AD, can be assessed in relation to the viability of cells. While the number of viable neurons and the respiratory chain enzyme activity (cytochrome oxidase IV) gradually decrease during the time in vitro [23, 24], viable neurons may still be detected at more distant time points (Fig. 1B–D). Addition of pyruvate, a metabolite that can be directly used by mitochondria, prolongs the activity of cytochrome oxidase [24]. Cells in these slices respond to experimental manipulation [22, 24, 28]. For instance, neurons can be induced to take up recombinant adeno-associated viral (AAV) vectors containing the reporter gene for Escherichia coli β-galactosidase (Lac Z) at DIV 34 and express this enzyme a few days later (Fig. 1E). Viable neurons can express this enzyme even in the vicinity of the pathological hallmarks of AD (Fig. 1F). Thus, neurons that have Alzheimer pathology and experience the agonal state may still possess the machinery to carry out complex physiological processes. Furthermore, when we co-cultured slices with rat embryonic neural stem cells, which were separated by a semi-permeable membrane, we found a higher number of viable neurons than without stem cells (Fig. 1G, H). This suggests that rat embryonic neural stem cells secrete molecules that are beneficial to human neurons in vitro [28]. The presence of morphologically intact synapses [24] and traceable axons [23] suggests that communication between neurons in these slices might be functional. Indeed, using three-dimensional electrode assays (MEA-60, Multi Channel Systems, Reutlingen, Germany) equipped with a grid of 60 spike electrodes with a 200-µm inter-spike distance, spontaneous electrical activity can be recorded in human postmortem cortex slices in culture (Balesar, Verwer et al., unpublished data). Usually, recordings are made at ~2 weeks in vitro, but in a single preparation, activity was detected at a single electrode after 63 DIV. In our experiments, the addition of nerve growth factor, brain-derived neurotrophic factor (BDNF), and neurotrophin-3 appeared helpful to obtain improved activity. In this way, we recorded spontaneous electrical activity in postmortem slices at many electrodes simultaneously or in synchronized waves over groups of electrodes (Fig. 2A), and this was suppressed by the addition of 1 µmol/L tetrodotoxin (TTX) to the medium (Fig. 2B). The TTX effect showed that sodium channels were functionally active in vitro. With some delay, activity resumed after washout of TTX (Fig. 2C). Fig. 2C also illustrates that field potential activity, sparse firing, regular spike firing, and burst firing patterns were produced by neurons in the areas of the electrodes. It should be noted that some electrodes can be inactive for a considerable time and then suddenly start to become active again.
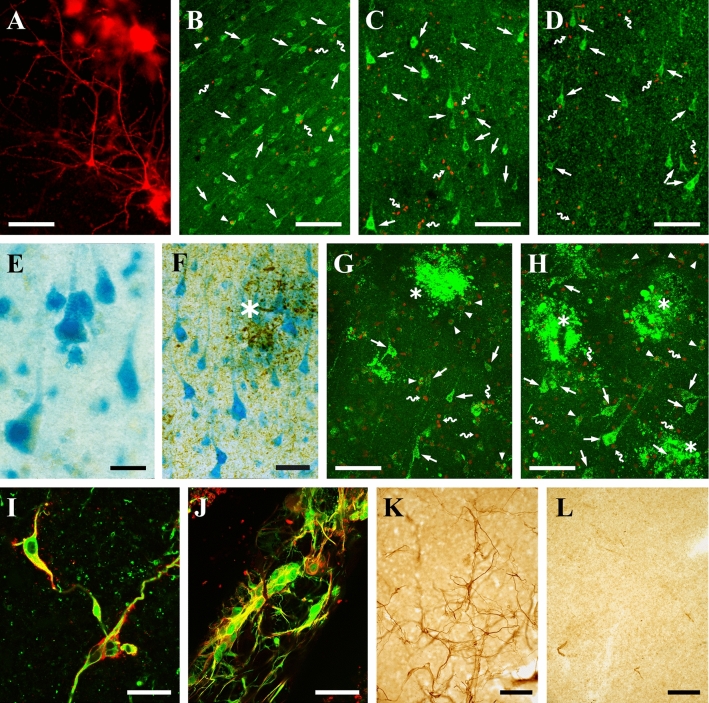
Fig. 1.
Illustrations of human brain tissue in culture. A Two pyramidal neurons stained with DiI (red) in a postmortem slice from a demented, non-AD patient after 50 days in vitro (DIV 50). After this long period in vitro the neuronal morphology is still intact. B–D Viability staining (Live/Dead, Molecular Probes) of postmortem slices from a patient with multiple system atrophy after three different periods in vitro: DIV 1 (B), DIV 17 (C), and DIV 50 (D). E–F Transgene expression of β-galactosidase (blue) in postmortem tissue from a patient with Parkinson’s disease (E) and another with AD (F). The tissue was transfected at DIV 34 and detected at DIV 44 in E. In F, the tissue was transfected at DIV 14 and the enzyme activity was assessed at DIV 24, and tau pathology was detected using immunocytochemistry (stained with AT8; asterisk, neuritic plaques as black deposits,). G–H Viability staining of postmortem tissue from an AD patient at DIV 9. G Untreated slice. H A slice co-cultured with rat embryonic neural stem cells from DIV 0 to DIV 9. Healthy neurons can be surrounded by pathological changes associated with amyloid and neuritic plaques. I–J Resected brain tissue shows a strong injury response involving reactive cells whose origin is not clearly defined because they can express surprising combinations of markers, like the astrocytic marker GFAP (green) and the microglial marker HLA (red) in tissue from an epilepsy patient at DIV 29 (I), and like the astrocytic marker S100β (green) and the early neuronal marker TuJ1 (red) in tissue from an epilepsy patient at DIV 26 (J). K, L It appeared that the emergence of reactive cells (stained for nestin, a reactive cell marker) was prevented by 24-h treatment of the tissue slices with mitomycin C (a cell-division inhibitor) at DIV 0 (Verwer et al., 2015 with permission from Brain Pathology). K Untreated slice at DIV 15. L Slice treated with mitomycin C at DIV 0 and stained at DIV 15. Symbols in (B–D and F–H): arrows indicate viable cells, arrowheads denote viable cells with a permeable (leaky) membrane, snake arrows indicate dead cells without esterase enzyme activity and a leaky membrane, asterisks indicate AD pathology, either in the form of plaques (live/dead) or tau pathology. Scale bars: A–D, G, H, K, and L, 100 µm; F and J, 50 µm; I, 25 µm; E, 20 µm.
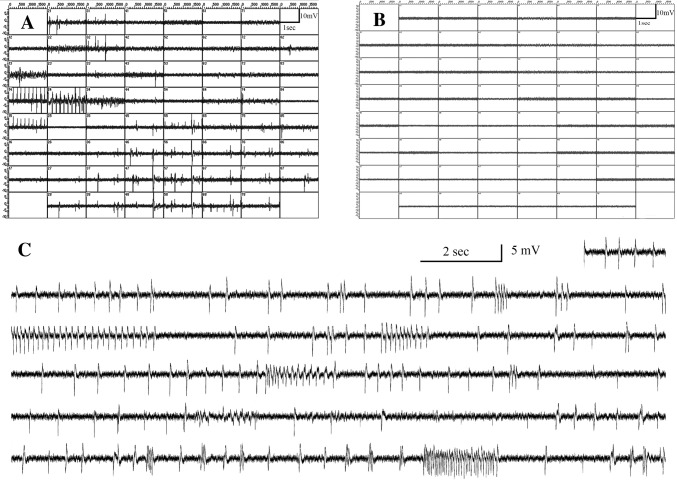
Fig. 2.
Spontaneous electrophysiological activity in postmortem human brain slices after 2 weeks in vitro. A Computer display of a 2-s recording sweep at 60 electrodes simultaneously from an MEA-60 spike-electrode array (Multi Channel Systems, Reutlingen, Germany). The slice was from the visual cortex of a 91-year-old control subject and the recording was made at 14 days in vitro (DIV). Bouts of regular spiking occurred at some electrodes, while more or less synchronous firing occurred at a frequency of about 1 per 2 s at several other electrodes. B A display from the same slice as in panel A after 1 µmol/L TTX was applied and all firing activity stopped. C Trace consisting of 41 sweeps of 2 seconds at one electrode illustrates the patterns of activity that may be observed. This trace was recorded 24 h (DIV 15) after washout of TTX in the same slice as in A and B.
Here, we give, as an example, the application of postmortem human brain slice cultures that were used to study the functional consequences of neurosteroid changes that we found in mood disorders. In postmortem tissue we found a significant decrease in the mRNA level of cytochrome P450 17A1 [CYP17A1, which synthesizes C19 ketosteroids such as dehydroepiandrosterone (DHEA)] in the anterior cingulate cortex, and a significant increase in the mRNA level of hydroxysteroid sulfotransferase 2A (SULT2A1, which catalyzes the sulfate conjugation of DHEA to DHEAS) in the dorsolateral prefrontal cortex from patients with major depressive disorder, suggesting alterations in the levels of DHEA and its sulfate metabolite DHEAS in this neuropsychiatric disorder. In addition, we found decreased mRNA levels of BDNF and its receptor tyrosine-related kinase B (TrkB) in the same cohort. A wide range of basic and clinical studies has revealed that BDNF and TrkB play a critical role in depression and in the mechanism underlying the action of antidepressants [29, 30]. Interestingly, we found a significant positive correlation between the mRNA levels of CYP17A1 and TrkB in control subjects but not in depressed patients. We subsequently tested the effects of DHEA and DHEAS on postmortem human prefrontal cortex slice cultures for five days, in particular on the transcriptional levels of BNDF and TrkB, to gain insight into the possible neurobiological basis of DHEA/DHEAS treatment in the human brain and to test DHEA/DHEAS as a potential drug for depression [22]. Treatment with DHEA did increase the BDNF mRNA level in human brain slices compared to vehicle-treated slices, indicating a close functional relationship between the DHEA/DHEAS and BDNF-TrkB pathways and that steroids such as DHEA may provide promising novel targets for therapeutic strategies in major depressive disorder. Actually, patients with major or minor mild-life depression who were given DHEA showed improvement in their Hamilton depression rating scores in a randomized controlled trial [31], and the positive response of patients taking DHEA was maintained for 8 months, especially among those with increased DHEAS levels [32]. This shows that human brain slice culture may not only be useful for functional studies of putative therapeutic compounds, but may also have predictive value in terms of positive clinical effects in a psychiatric disorder.
In addition, postmortem human cortical brain slices that have been cultured for 1 week in poly I:C- or LPS-conditioned astrocyte medium, show better neuronal survival than slices cultured in a traditional non-conditioned medium, suggesting that astroglial Toll-like receptor-3-mediated production of a variety of neuroprotective factors can maintain the viability of neurons in culture [33]. Furthermore, postmortem human brain slices cut from fresh-frozen cerebellar cortex or the hippocampal formation were incubated for 30 min at 37 °C with vehicle alone or with vehicle plus human recombinant insulin or human recombinant insulin-like growth factor 1 (IGF-1) at near-physiological doses. It was first found that insulin and IGF-1 stimulated signaling pathways via different insulin receptor substrate (IRS) isoforms, IRS-1 and IRS-2, respectively [34]. Insulin resistance associated with IRS-1 dysfunction occurs in the cerebellar cortex and is more marked in the hippocampal formation of AD patients. IGF-1 resistance associated with IRS-2 dysfunction is severe in both the cerebellar cortex and the hippocampal formation of AD cases [34]. It might be of interest to mention that a short-term culture procedure has been used for tracing afferents and efferents and measuring alterations in the axonal transport rate in human postmortem tissue blocks obtained within 8 h after death [35–40]. When neurobiotin and biotinylated dextran amine were used as tracers, transport was observed over distances of 0.5 cm–1.5 cm along axons from injection sites in different brain areas. Dai et al. did not use slices for this purpose but trimmed small tissue blocks for tracer injection. These blocks were incubated in artificial cerebrospinal fluid at room temperature and provided with glucose and 95% O2 + 5% CO2 for < 24 h followed by fixation, sectioning, and staining for microscopy. In this way, the fibers of the human retino-hypothalamic tract terminating in the suprachiasmatic nucleus (SCN) were visualized [35, 36]. In control subjects, a network comprising efferents of the SCN to other hypothalamic areas and axonal transport appeared to be comparable to those found in rats [37, 38], whereas axonal transport appeared to be impaired in the temporal cortex in AD patients [39] or by adding cortisol to the medium [40].
Resected brain tissue consists of normal, unaffected tissue that needs to be surgically removed because it obstructs access to and removal of a brain area afflicted by a pathological process (i.e. epileptic tissue or a tumor). If this tissue is not needed for diagnosis, it can be used for research purposes. At the time of operation, this tissue is perfectly healthy, but its reaction to the interruption of circulation, the mechanical manipulations of the operation, the processing of slices, and the transfer to in vitro conditions is a severe injury response [25, 27]. As a result, neurons degenerate rapidly during the first week in culture and reactive glial cells evolve over the ensuing weeks. Surprisingly, the reactive glial cells are able to co-express markers of different cellular lineages, thus obscuring the lineage (astrocyte, microglia, or early neuron) from which they originated (Fig. 1I, J). The emergence of reactive cells can be prevented by the application of a cell-division inhibitor (Fig. 1K, L). Unfortunately, preventing the occurrence of reactive cells does not reverse the neuronal degeneration. Of course, a treatment that could ameliorate the degeneration of neurons would have immense clinical implications. It should be noted that we have also observed the emergence of some reactive cells in postmortem tissues from a few patients, but this is too infrequent an occurrence to allow a systematic analysis. There are also reports showing that neurons in neocortical slice culture from resected brain tissue obtained at epilepsy surgery maintain typical electrophysiological properties, such as robust action potential generation, normal resting potential, and synaptic connectivity (excitatory and inhibitory postsynaptic potentials) for up to three weeks using human cerebrospinal fluid (hCSF) as the culture medium [41]. In that study, dense populations of NeuN-positive cells with intact somato-dendritic morphology in all cortical layers were much more frequent in hCSF-treated slices than in slices in traditional medium cultured for 18 days. Extracellular population recording has revealed tonic and rhythmic network activity in human brain slices [41]. Furthermore, in hippocampal and temporal lobe slices, characteristic epileptiform activity can be maintained and recorded in vitro [42, 43]. The in vitro spontaneous interictal-like activity recorded in the subiculum of human acute temporal lobe slice cultures appears to resemble intracranial EEG recordings from the same patient with temporal lobe epilepsy [44]. Human temporal cortical slices exposed to amyloid-beta oligomers (AβOs) present robust binding of AβOs and elevated levels of hyperphosphorylated Tau, the second hallmark of AD [45]. In addition, hippocampal slices from patients who underwent surgical removal of epileptic foci have been maintained successfully in culture for up to 25 days, and the cell viability was > 50%. AβOs exposure of slices from three different donors for 24 h altered the expression of 27 genes as revealed by microarray analysis and confirmed by qPCR, notably with down-regulation of the mRNA and protein levels of synaptophysin [46]. This is a presynaptic vesicle membrane protein and the results suggested that the oligomers cause synaptic failure. Thus, adult human brain slice cultures exposed to exogenous AβOs seem to be a good addition to AD mouse models to study the mechanisms or potential targets of novel diagnostic or therapeutic strategies for AD. The tumor- and non-tumor cerebral cortical slices collected intra-operatively from patients undergoing craniotomy for tumor, trauma, arteriovenous malformation, aneurysm, or epilepsy remain intact in culture for a long period of time (~11 days) without any significant change to the tissue cytoarchitecture as shown by immunohistochemical and electron microscopic analyses [47]. Astrocytes with intact somata and processes, neuropil, endothelial cells, basal lamina, and myelinating and non-myelinating axons are well preserved. Also, organelles in these cells are intact, including mitochondria, rough endoplasmic reticulum, Golgi apparatus, microtubules, synapses, and synaptic vesicles. Mitotic bodies in tumor slices are also seen, indicating that the tumor is still proliferating [47]. Human brain tumor slice cultures may provide new avenues for studying cell migration and progression, cellular composition, and potential pharmacological therapies for brain tumors. Acute human brain slices from patients with temporal lobe epilepsy and deep brain tumors exhibit excellent viability and intrinsic and active neuronal membrane properties for at least three days [48]. Importantly, the laminar architecture of the neocortex remains intact, with no overt signs of cell dispersion. Furthermore, widespread enhanced yellow fluorescent protein-labeled neurons can be seen as early as two days after Herpes Simplex Virus type-1 infection, exhibiting membrane properties largely comparable to uninfected neurons over this short timeframe [48]. Neurons in human temporal neocortical and hippocampal tissue slices transduced with a lenti-viral (LV) vector containing the channelrhodopsin-2 (ChR2) gene under the control of the human synapsin promoter effectively express ChR2, respond to light stimulation by generating action potentials, and induce synaptic responses in neighboring neurons cultured in standard medium for up to 2 weeks [49]. That study shows that optogenetics can become a practical tool to explore synaptic connectivity and neuronal networks in human brain tissue cultures. Recent work has explored the optimal conditions for long-term cultures of human brain slices for up to one month [43]. Studies have shown that the regional organization and neuronal physiology and morphology of the dentate gyrus, CA2/3, subiculum, and cortex surgically excised from the human temporal lobe and peri-tumoral or dysplastic cortex maintained for 5 weeks–6 weeks using a new defined culture medium are similar to acute slices, as indicated by immunohistochemistry, whole cell recording, and spontaneous synaptic events [43]. In contrast, some studies [25, 27] have reported the emergence of reactive glial cells and concurrent degeneration of neurons in cultured resected brain tissue. This may be attributed to surgical manipulations and differences in the culture procedure and the medium. Consequently, in these studies no electrical activity was detected in cultured resected tissue (Balesar, Verwer et al., unpublished data). Transgenes such as GCaMP6 (a Ca2+ indicator), carried by AAV or LV vectors and applied during culture preparation, can be stably expressed at 2 weeks–3 weeks, allowing simultaneous electrical and optical recordings of neuronal Ca2+ transients [43]. The findings described above show that, under suitable circumstances, resected human brain slices in culture can show long-term survival and enable experimental approaches such as transgene expression. Under different conditions, other processes such as the injury response may be studied.
Thus, cultures of postmortem and resected human brain tissue can be used to address many different, but important, aspects of human brain functioning.
Human brain slice cultures have some advantages. They partly conserve the original three-dimensional brain architecture, neuronal shape, synaptic connectivity, and micro-environment with glial cells and even blood vessels in very well-controlled culture conditions. This is one of the closest experimental representations to an in vivo human model without using living human subjects. Long-term slice culture allows experiments such as pharmacological intervention, cytoarchitectural evaluation, migration, disease model evaluation, and experiments that might be impossible to do in vivo, such as viral genetic labeling for tracing or the neuromodulation of human neurons by optogenetics. It should also be noted that these tools have some disadvantages. A concern in the use of resected brain tissue from patients with epilepsy or brain tumors is that neurons not involved in the pathological process may have experienced the effects of epileptic seizures for many years or may have been interfered with by drug treatment. In addition, surgical manipulations or culture procedures and the medium used may induce an injury reaction [27]. On the other hand, postmortem brain tissue undergoes biochemical and neuroanatomical changes from the moment of death to tissue collection [50, 51] and during culture. Samples with short postmortem delays are desirable, but the premortem health and previous medical conditions/treatments of patients may be of equal importance. Specific procedures and conditions for the collection and processing of postmortem and surgical materials are important to achieve the optimal viability of human brain slices. Last but not least, well-documented archives of patient data are needed to guarantee the scientific importance of the research efforts.
Brain Donation and Brain Banks
As can be concluded from the examples above, donations of postmortem brain tissue for research are necessary if we are to achieve a better understanding of the physiological functions of the human brain and their alterations in neuropsychiatric disorders. It should be noted that neuropsychiatric disorders are in general the result of an interaction between genetic and epigenetic factors that cause changes in brain development, resulting in vulnerability to environmental stress and functional disorders. Research with donated postmortem human tissue is, therefore, one of the most effective means of studying and gaining an understanding of these complex disease processes. The only way to determine the differences between illness and normality is to experimentally compare the diseased brain with a similar sample from normally-functioning controls without brain disorders. To make such comparisons, scientists need to have both diseased and normal control brain tissues that should be matched for many factors such as age, sex, agonal state, postmortem time, and hour and season of death, and the patients should be extensively documented in terms of clinical history and neuropathology [52]. Controls are thus just as important as cases with neuropsychiatric disorders. The above aspects require well-organized brain banks.
Anyone who wishes to support scientific research by donating his or her brain after death can become a donor. By providing written consent, the donor becomes registered in the donor database of a brain bank. A human brain bank is an organization that stores and provides well-documented high-quality postmortem brain tissue from patients with different brain disorders and well-matched controls to be used by research groups. Information about the disease process and about what treatments the donors and controls receive is needed. In addition, microscopic investigation by a neuropathologist is indispensable to confirm the clinical diagnosis and the possible presence of other neuropsychiatric disorders. One of the major goals of a human brain bank is to facilitate research into the most prevalent neuropsychiatric disorders, but the study of controls can also reveal crucial information about the way a healthy brain functions. Innovative research using human brain tissue may lead to major breakthroughs in our understanding of the human brain in health and disease and may yield novel therapeutic strategies to treat brain disorders.
Brain banks are currently well established in high-income countries and use standardized operational procedures to support collaborative studies across the world [53]. Recently, the China Brain Bank Consortium has been set up, with standardized brain banking procedures with advice from international experts, and a tissue-sharing system is under construction [54]. This will provide an important addition to neuroscience research in China.
Conclusions
To sum up, human brain slice culture is an emerging experimental system that effectively keeps the complex in vivo neuronal network intact, and seems to be a useful strategy to study the cellular and molecular mechanisms underlying the pathophysiology of neuropsychiatric disorders, and to evaluate potential therapeutic treatments for such diseases. The infrastructure needed for the availability of such an endeavor, involving brain banks that provide clinically and neuropathologically well-documented postmortem brain tissue, is being set up in China.
Acknowledgements
We are grateful to the Netherlands Brain Bank (Director Dr. I. Huitinga) at the Netherlands Institute for Neuroscience for providing brain material and patient information, and to Wilma Verweij for secretarial assistance. This review was supported by the National Natural Science Foundation of China (81501172), the China Exchange Programme of the Royal Netherlands Academy of Arts and Sciences (10CDP0037 and 05CD9027), the Shanghai Municipal Commission of Health and Family Planning (20154Y0016), an Innovation Project of the Chinese Academy of Sciences (KSCX2-SW-217), a National Basic Research Development Program of China (2006CB500705), the Internationale Stichting Alzheimer Onderzoek (05501), the Jan Dekkerstichting and dr. Lutgardine Bouwmanstichting, and the Stichting Vrienden van het Herseninstituut.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Drummond E, Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2017;133:155–175. doi: 10.1007/s00401-016-1662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nader MA. Animal models for addiction medicine: From vulnerable phenotypes to addicted individuals. Prog Brain Res. 2016;224:3–24. doi: 10.1016/bs.pbr.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Czeh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 5.Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, et al. APP mouse models for Alzheimer’s disease preclinical studies. EMBO J. 2017;36:2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Testa-Silva G, Verhoog MB, Linaro D, de Kock CP, Baayen JC, Meredith RM, et al. High bandwidth synaptic communication and frequency tracking in human neocortex. PLoS Biol. 2014;12:e1002007. doi: 10.1371/journal.pbio.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhoog MB, Goriounova NA, Obermayer J, Stroeder J, Hjorth JJ, Testa-Silva G, et al. Mechanisms underlying the rules for associative plasticity at adult human neocortical synapses. J Neurosci. 2013;33:17197–17208. doi: 10.1523/JNEUROSCI.3158-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oberheim NA, Wang XH, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Dam D, De Deyn PP. Non human primate models for Alzheimer’s disease-related research and drug discovery. Expert Opin Drug Discov 2017, 12: 187–200. [DOI] [PubMed]
- 11.Bauman MD, Schumann CM. Advances in nonhuman primate models of autism: Integrating neuroscience and behavior. Exp Neurol. 2018;299:252–265. doi: 10.1016/j.expneurol.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willner P. The validity of animal models of depression. Psychopharmacology (Berl) 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- 13.Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods. 2005;37:208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Buendia I, Parada E, Navarro E, Leon R, Negredo P, Egea J, et al. Subthreshold concentrations of melatonin and galantamine improves pathological AD-hallmarks in hippocampal organotypic cultures. Mol Neurobiol. 2016;53:3338–3348. doi: 10.1007/s12035-015-9272-5. [DOI] [PubMed] [Google Scholar]
- 15.Shahani N, Subramaniam S, Wolf T, Tackenberg C, Brandt R. Tau aggregation and progressive neuronal degeneration in the absence of changes in spine density and morphology after targeted expression of Alzheimer’s disease-relevant tau constructs in organotypic hippocampal slices. J Neurosci. 2006;26:6103–6114. doi: 10.1523/JNEUROSCI.4245-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cartier J, Piyasena C, Sparrow SA, Boardman JP, Drake AJ. Alterations in glucose concentrations affect DNA methylation at Lrg1 in an ex vivo rat cortical slice model of preterm brain injury. Eur J Neurosci. 2018;47:380–387. doi: 10.1111/ejn.13825. [DOI] [PubMed] [Google Scholar]
- 17.Molnar G, Rozsa M, Baka J, Holderith N, Barzo P, Nusser Z, et al. Human pyramidal to interneuron synapses are mediated by multi-vesicular release and multiple docked vesicles. Elife 2016, 5. [DOI] [PMC free article] [PubMed]
- 18.Konishi Y, Lindholm K, Yang LB, Li R, Shen Y. Isolation of living neurons from human elderly brains using the immunomagnetic sorting DNA-linker system. Am J Pathol. 2002;161:1567–1576. doi: 10.1016/S0002-9440(10)64435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi XR, Luchetti S, Verwer RWH, Sluiter AA, Mason MRJ, Zhou JN, et al. Alterations in the steroid biosynthetic pathways in the human prefrontal cortex in mood disorders: A post-mortem study. Brain Pathol. 2018;28:536–547. doi: 10.1111/bpa.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verwer RW, Baker RE, Boiten EF, Dubelaar EJ, van Ginkel CJ, Sluiter AA, et al. Post-mortem brain tissue cultures from elderly control subjects and patients with a neurodegenerative disease. Exp Gerontol. 2003;38:167–172. doi: 10.1016/S0531-5565(02)00154-7. [DOI] [PubMed] [Google Scholar]
- 24.Verwer RW, Hermens WT, Dijkhuizen P, ter Brake O, Baker RE, Salehi A, et al. Cells in human postmortem brain tissue slices remain alive for several weeks in culture. FASEB J. 2002;16:54–60. doi: 10.1096/fj.01-0504com. [DOI] [PubMed] [Google Scholar]
- 25.Verwer RW, Sluiter AA, Balesar RA, Baaijen JC, de Witt Hamer PC, Speijer D, et al. Injury response of resected human brain tissue in vitro. Brain Pathol. 2015;25:454–468. doi: 10.1111/bpa.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verwer RW, Sluiter AA, Balesar RA, Baayen JC, Noske DP, Dirven CM, et al. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain. 2007;130:3321–3335. doi: 10.1093/brain/awm264. [DOI] [PubMed] [Google Scholar]
- 27.Verwer RW, Sluiter AA, Balesar RA, Baayen JC, Speijer D, Idema S, et al. Altered loyalties of neuronal markers in cultured slices of resected human brain tissue. Brain Pathol. 2016;26:523–532. doi: 10.1111/bpa.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu L, Sluiter AA, Guo HF, Balesar RA, Swaab DF, Zhou JN, et al. Neural stem cells improve neuronal survival in cultured postmortem brain tissue from aged and Alzheimer patients. J Cell Mol Med. 2008;12:1611–1621. doi: 10.1111/j.1582-4934.2007.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 30.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 31.Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, et al. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999;156:646–649. doi: 10.1176/ajp.156.4.646. [DOI] [PubMed] [Google Scholar]
- 32.Rabkin JG, McElhiney MC, Rabkin R, McGrath PJ, Ferrando SJ. Placebo-controlled trial of dehydroepiandrosterone (DHEA) for treatment of nonmajor depression in patients with HIV/AIDS. Am J Psychiatry. 2006;163:59–66. doi: 10.1176/appi.ajp.163.1.59. [DOI] [PubMed] [Google Scholar]
- 33.Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 34.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai JP, Swaab DF, Buijs RM. Recovery of axonal transport in “dead neurons”. Lancet. 1998;351:499–500. doi: 10.1016/S0140-6736(05)78689-X. [DOI] [PubMed] [Google Scholar]
- 36.Dai JP, Van der Vliet J, Swaab DF, Buijs RM. Human retinohypothalamic tract as revealed by in vitro postmortem tracing. J Comp Neurol. 1998;397:357–370. doi: 10.1002/(SICI)1096-9861(19980803)397:3<357::AID-CNE4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Dai JP, Swaab DF, Van der Vliet J, Buijs RM. Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. J Comp Neurol. 1998;400:87–102. doi: 10.1002/(SICI)1096-9861(19981012)400:1<87::AID-CNE6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 38.Dai JP, Van der Vliet J, Swaab DF, Buijs RM. Postmortem anterograde tracing of intrahypothalamic projections of the human dorsomedial nucleus of the hypothalamus. J Comp Neurol. 1998;401:16–33. doi: 10.1002/(SICI)1096-9861(19981109)401:1<16::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Dai JP, Buijs RM, Kamphorst W, Swaab DF. Impaired axonal transport of cortical neurons in Alzheimer’s disease is associated with neuropathological changes. Brain Res. 2002;948:138–144. doi: 10.1016/S0006-8993(02)03152-9. [DOI] [PubMed] [Google Scholar]
- 40.Dai JP, Buijs R, Swaab D. Glucocorticoid hormone (cortisol) affects axonal transport in human cortex neurons but shows resistance in Alzheimer’s disease. Br J Pharmacol. 2004;143:606–610. doi: 10.1038/sj.bjp.0705995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarz N, Hedrich UBS, Schwarz H, P AH, Dammeier N, Auffenberg E, et al. Human cerebrospinal fluid promotes long-term neuronal viability and network function in human neocortical organotypic brain slice cultures. Sci Rep 2017, 7: 12249. [DOI] [PMC free article] [PubMed]
- 42.Wickham J, Brodjegard NG, Vighagen R, Pinborg LH, Bengzon J, Woldbye DPD, et al. Prolonged life of human acute hippocampal slices from temporal lobe epilepsy surgery. Sci Rep. 2018;8:4158. doi: 10.1038/s41598-018-22554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Duigou C, Savary E, Morin-Brureau M, Gomez-Dominguez D, Sobczyk A, Chali F, et al. Imaging pathological activities of human brain tissue in organotypic culture. J Neurosci Methods. 2018;298:33–44. doi: 10.1016/j.jneumeth.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 45.Mendes ND, Fernandes A, Almeida GM, Santos LE, Selles MC, Lyra ESNM, et al. Free-floating adult human brain-derived slice cultures as a model to study the neuronal impact of Alzheimer’s disease-associated Abeta oligomers. J Neurosci Methods. 2018;307:203–209. doi: 10.1016/j.jneumeth.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 46.Sebollela A, Freitas-Correa L, Oliveira FF, Paula-Lima AC, Saraiva LM, Martins SM, et al. Amyloid-beta oligomers induce differential gene expression in adult human brain slices. J Biol Chem. 2012;287:7436–7445. doi: 10.1074/jbc.M111.298471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaichana KL, Capilla-Gonzalez V, Gonzalez-Perez O, Pradilla G, Han J, Olivi A, et al. Preservation of glial cytoarchitecture from ex vivo human tumor and non-tumor cerebral cortical explants: A human model to study neurological diseases. J Neurosci Methods. 2007;164:261–270. doi: 10.1016/j.jneumeth.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ting JT, Kalmbach B, Chong P, de Frates R, Keene CD, Gwinn RP, et al. A robust ex vivo experimental platform for molecular-genetic dissection of adult human neocortical cell types and circuits. Sci Rep. 2018;8:8407. doi: 10.1038/s41598-018-26803-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson M, Avaliani N, Svensson A, Wickham J, Pinborg LH, Jespersen B, et al. Optogenetic control of human neurons in organotypic brain cultures. Sci Rep 2016, 6. [DOI] [PMC free article] [PubMed]
- 50.Lavenex P, Lavenex PB, Bennett JL, Amaral DG. Postmortem changes in the neuroanatomical characteristics of the primate brain: hippocampal formation. J Comp Neurol. 2009;512:27–51. doi: 10.1002/cne.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fountoulakis M, Hardmeier R, Hoger H, Lubec G. Postmortem changes in the level of brain proteins. Exp Neurol. 2001;167:86–94. doi: 10.1006/exnr.2000.7529. [DOI] [PubMed] [Google Scholar]
- 52.Bao AM, Swaab DF. The art of matching brain tissue from patients and controls for postmortem research. Handb Clin Neurol. 2018;150:197–217. doi: 10.1016/B978-0-444-63639-3.00015-3. [DOI] [PubMed] [Google Scholar]
- 53.Samarasekera N, Salman RA, Huitinga I, Klioueva N, McLean CA, Kretzschmar H, et al. Brain banking for neurological disorders. Lancet Neurol. 2013;12:1096–1105. doi: 10.1016/S1474-4422(13)70202-3. [DOI] [PubMed] [Google Scholar]
- 54.Yan XX, Ma C, Bao AM, Wang XM, Gai WP. Brain banking as a cornerstone of neuroscience in China. Lancet Neurol. 2015;14:136. doi: 10.1016/S1474-4422(14)70259-5. [DOI] [PubMed] [Google Scholar]