Abstract
Epilepsy is a neurological disorder in the brain that is characterized by unprovoked seizures. Epileptic seizures are attributed to abnormal synchronous neuronal activity in the brain. To detect the seizure as early as possible, the identification of specific electroencephalogram (EEG) dynamics is of great importance in investigating the transition of brain activity as the epileptic seizure approaches. In this study, we investigated the transition of brain activity from interictal to preictal states preceding a seizure by combining EEG network and clustering analyses together in different frequency bands. The findings of this study demonstrated the best clustering performance of k-medoids in the beta band; in addition, compared to the interictal state, the preictal state experienced increased synchronization of EEG network connectivity, characterized by relatively higher network properties. These findings can provide helpful insight into the mechanism of epilepsy, which can also be used in the prediction of epileptic seizures and subsequent intervention.
Keywords: Epileptic seizure, EEG network, K-medoids, Preictal state, Synchronization
Introduction
Epilepsy is a neurological disorder characterized by the paroxysmal occurrence of seizures (Burns et al. 2014). Neurologically, an epileptic seizure is attributed to abnormal synchronous neuronal activity in the brain (Fisher et al. 2005, 2014). Although substantial progress has been made in understanding the neurological pathogenesis of epileptic seizures (Hussain 2018; Mateos et al. 2018), patients still bear excruciating pressures. The repeatability of epileptic seizures damages the central neural system in patients, as well as disrupts their behavior and cognition (Blumenfeld 2012; Cheung et al. 2006; Hommet et al. 2006); meanwhile, compared to healthy individuals, patients also experience a greater possibility of suffering from psychosis.
To detect the epileptic seizure as early as possible, the identification of early changes in electroencephalogram (EEG) dynamics has the potential capacity to provide valuable insight (Myers and Kozma 2018; Wang et al. 2011; Zhang et al. 2011). Since related spectrum power and chaoticity are found to dynamically fluctuate before, during, and after a seizure in patients (Blanco et al. 2013; Iasemidis et al. 1990), these EEG dynamics can be used to anticipate epileptic seizures (Mateos et al. 2018) and can also be used to identify different epileptic states, such as interictal and preictal states (Raghu et al. 2017). As previously demonstrated, the transition of epileptic states usually occurs within several minutes to hours before a seizure, which can herald the occurrence of a seizure (Chavez et al. 2003; Le Van Quyen et al. 2001). To date, substantial progress has been made (Blanco et al. 2013; Drury et al. 2003). In contrast to those attempts that failed to detect specific and sustained fluctuations preceding a seizure (Qu and Gotman 1995; Rogowski et al. 1981) by visually inspecting the multiple-channel EEG, studies based on chaotic dynamics have shown a reliable capability of seizure prediction using scalp or intracranial EEG (Drury et al. 2003; Hussain 2018; Mormann et al. 2006; van Drongelen et al. 2003). For example, by estimating the Lyapunov exponent of electrocorticograms before, during, and after a seizure in epileptic patients, Iasemidis and colleagues found the lowest Lyapunov exponent corresponded to the epileptic seizure (Iasemidis et al. 1990).
The human brain is one of the most complex systems and works as a large-scale network. Information is commonly processed between spatially distributed but functionally linked brain areas with coherent temporal dynamics (Dasdemir et al. 2017; Fields and Glazebrook 2017; Li et al. 2016; Sun et al. 2012). Despite the EEG chaoticity, the EEG network seems to be of great importance when investigating the pathogenesis of epilepsy (Epstein et al. 2014; Gao et al. 2017; Myers and Kozma 2018). By using spectral Granger causality in epileptic children, Protopapa and colleagues found the causal connectivity disrupted in the alpha band and highest in the beta band during the Go condition in an experimental task but the opposite for the NoGo condition, which achieved an accuracy of 87.6% when classifying the subjects in the epileptic and control groups (Protopapa et al. 2016). In addition, by using an adaptive directed transfer function, our previous study demonstrated dynamic EEG network architectures originating from the epileptogenic zone in the interictal EEG state (Zhang et al. 2017). Herein, the investigation of the EEG network in epileptic patients can provide helpful insight into the mechanisms of epilepsy and potentially raise a biomarker for the anticipation of epileptic seizures and clinical intervention.
A preictal state is established several minutes to an hour before the epileptic seizure (Bou Assi et al. 2017; Le et al. 2001; Schwartz et al. 2011), and along with signaling the approaching seizure, the brain activity becomes progressively less chaotic. Inspired by that, a smooth transition gradually develops over time toward the onset of the seizure (Chavez et al. 2003). In this study, we first analyzed the dynamic networks of 1-h intervals of EEG signals preceding seizures in different frequency bands. Clustering analysis was then applied to the EEG networks to capture the transition of brain activity from the interictal to preictal state, as well as the dynamics of EEG network connectivity preceding seizures.
Materials and methods
Participants
The protocol was approved by the Medical Ethics Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, and this study was carried out in accordance with the recommendations of Medical Ethics Committee of Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital. Before 24-h EEG monitoring, all patients were required to read the written informed consent and then sign their name on it. Thirteen epileptic patients (7 females, 6 males, age ranged from 8 to 58 years) were diagnosed by doctors at the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital and encouraged to participate in this study. Detailed clinical information of the 13 patients is provided in Table 1. In this study, to eliminate the effects of antiepileptic drugs, all patients were required to not take antiepileptic drugs before 24-h EEG monitoring was initiated.
Table 1.
Detailed clinical information for the 13 patients
| Subject | Gender (female = F; male = M) | Age | Epileptic type (generalized = G; focal = F) | Epileptogenic zone |
|---|---|---|---|---|
| Sub01 | F | 13 | Clonic seizures (F) | – |
| Sub02 | F | 23 | Tonic–clonic seizures (G) | – |
| Sub03 | F | 15 | Bilateral tonic–clonic seizures (F) | Left hippocampal |
| Sub04 | M | 14 | Tonic–clonic seizures (unknown) | Temporal lobe |
| Sub05 | M | 47 | Bilateral tonic–clonic seizures (F) | Right basal ganglia |
| Sub06 | F | 24 | Tonic–clonic seizures (G) | Frontal lobe |
| Sub07 | M | 58 | Tonic–clonic seizures (G) | Bilateral frontal–parietal lobe |
| Sub08 | F | 8 | Tonic–clonic seizures (G) | – |
| Sub09 | F | 8 | Tonic–clonic seizures (G) | – |
| Sub10 | M | 15 | Tonic–clonic seizures (G) | – |
| Sub11 | F | 24 | Tonic–clonic seizures (G) | – |
| Sub12 | M | 25 | Bilateral tonic–clonic seizures (F) | Bilateral parietal–occipital lobe |
| Sub13 | M | 10 | Tonic–clonic seizures (F) | – |
Twenty-four hour EEG recording
The 24-h EEG data sets were collected using the Australia-based Compumedics Grael series of digital video EEG with a sampling rate of 256 Hz. Sixteen Ag/AgCl electrodes (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, and T6) were positioned on the scalp in compliance with the international 10–20 system. Additionally, the electrocardiogram and electromyogram were separately recorded by two extra electrodes. Meanwhile, during the 24-h EEG recording, a single or dual cameras were simultaneously used to monitor the clinical behaviors of the epileptic patients.
EEG data analysis
In this study, EEG data sets with a 1-h duration preceding the seizure were used. Since 29 epileptic seizures were recorded in the 13 patients, 29 1-h EEG data sets were extracted. Meanwhile, to reduce the effect of the last seizure on subsequent analyses, a standard was considered in which two adjacent seizures should be separated by at least 40 min. In this study, we confirmed that between any two adjacent seizures, a time interval of at least 2 h was found for the 29 data sets.
In addition, before constructing the EEG network, all data sets were preprocessed with procedures including averaging reference, bandpass filtering to extract the 1–45 Hz signal offline, and segmenting the data into 10-s epochs. In data segmenting, EEG epochs of 10 s were extracted; thus, each 1-h EEG data set was divided into 360 10-s segments. Afterwards, we excluded those segments with absolute amplitude exceeding 100 μV that were considered to be artifacts (Li et al. 2018). Aiming to guarantee the reliability of our results, if the remaining number of 10-s segments was relatively low (i.e., less than 70), we would have excluded this 1-h EEG data set. In this study, the remaining numbers of 10-s segments were 228.2 ± 83.4.
Afterwards, to investigate the transition of epileptic states preceding a seizure, the EEG networks were constructed by using coherence. Coherence is a commonly used method when analyzing synchrony-defined assemblies at a specific frequency between two signals (Li et al. 2015; Salant et al. 1998; Zhang et al. 2015). Here, considering the two variables, x(t) and y(t), in each segment, let Pxx(f) and Pyy(f) be the autospectral densities of x(t) and y(t) at the frequency f, respectively, and Pxy(f) is the corresponding cross-spectral density; coherence was then formulated as follows:
| 1 |
where Cxy(f) was the frequency-dependent measure of synchrony between x(t) and y(t) at frequency f. Since multiple studies have validated the EEG dynamics of epileptic seizures in different frequency bands (Chavez et al. 2003; Protopapa et al. 2016), we then constructed the related EEG networks in five frequency bands, i.e., delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and low gamma (30–45 Hz) (Dasdemir et al. 2017; Mumtaz et al. 2017). For each specific band, the EEG network was obtained by averaging the Cxy within its frequency range, which led to the final weighted EEG network per 10-s segment. In this coherence definition, the corresponding spectral densities Pxx(f), Pyy(f) and Pxy(f) are essentially calculated from the fast Fourier transform (FFT) instead of the wavelet transform.
Four weighted network properties, i.e., clustering coefficient (CC), characteristic path length (CPL), local efficiency (LE), and global efficiency (GE), corresponding to the weighted network were adopted to quantitatively evaluate the dynamics of EEG network synchronization toward the seizure. In this study, these four weighted network properties were formulated in the following definitions and further calculated by using the brain connectivity toolbox (BCT, http://www.nitrc.org/projects/bct/) (Rubinov and Sporns 2010):
| 2 |
| 3 |
| 4 |
| 5 |
where and denote the connection weight estimated by coherence and shortest weighted path length between nodes i and j, respectively, Ψ denotes the set of all nodes in the network, and n denotes the number of nodes.
Clustering analysis is an unsupervised machine learning method, which does not need prior knowledge of the data sets. Clustering analysis can automatically divide all objects into distinct clusters by inferring the data similarity, which guarantees high similarities within the same cluster but large intercluster differences between distinct clusters. Because no prior information was provided when investigating the transition of epileptic states toward the seizure, the investigation could only depend on the EEG dynamics derived from the time series. Therefore, based on the EEG networks in each band, for each 1-h length of EEG data, four clustering methods, including k-medoids, k-means, fuzzy C-means (FCM), and hierarchical, were used. Since both interictal and preictal states preceding epileptic seizure were reported (Chavez et al. 2003; Drury et al. 2003) in this study, two clusters were considered. To guarantee reliable performance, each method was repeated 1000 times; the data would only be considered clustered data if at least 900 out of 1000 times (i.e., 90%), the clustering divided the 1-h data set into two clusters.
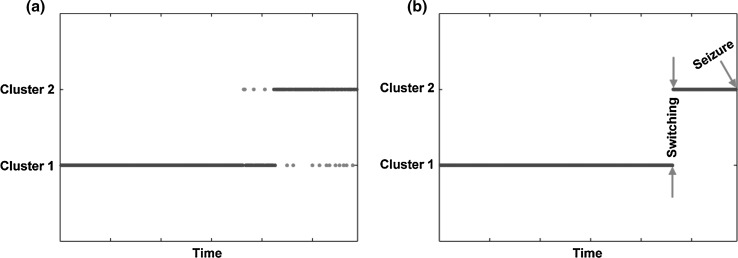
After clustering, there might exist some isolated points (Fig. 1a) that affected further results. In essence, adaptive median filtering adapts to automatically estimate the size of the filtering window based on noise density. Adaptive median filtering can not only suppress noise but also preserve details; thus, it is widely used in noise elimination (Chen and Hong Ren 2001). In this study, to filter out isolated points, we adopted adaptive median filtering following the clustering analysis. Eventually, we investigated the clustering performances of different methods and then compared the differences of weighted network properties between interictal and preictal states.
Fig. 1.
Temporal clustering of EEG networks in the interictal and preictal states. a Clustering before adaptive median filtering and b clustering after adaptive median filtering. On the x-axis, each point over the 1-h interval denotes a brain network constructed for each 10-s EEG segment. On the y-axis, two clusters (i.e., interictal and preictal) are presented. Both blue and red dots denote the EEG networks constructed of different durations; in particular, red dots in subfigure (a) denote the isolated network points. (Color figure online)
Results
As illustrated in Fig. 1, the continuity of EEG networks that belong to either “cluster 1” or “cluster 2” was revealed. Specifically, in the interictal state, the related EEG networks were clustered into “cluster 1”, which lasted for several minutes; however, as the epileptic seizure approached in time, the EEG networks were then clustered into “cluster 2” (i.e., preictal state).
Table 2 quantitatively shows the number of 1-h EEG data sets that were discriminated using the four clustering methods for five frequency bands. Compared to other bands, the beta band consistently displayed a larger number of 1-h data sets that were clustered (k-medoids: 20; k-means: 14; FCM: 18; hierarchical: 16); meanwhile, among four methods, the k-medoids in the beta band had the best performance since 20 out of 29 1-h EEG data sets were clustered into interictal and preictal states.
Table 2.
The number of 1-h EEG data sets that were discriminated into clusters using the four clustering methods in five frequency bands
| k-medoids | k-means | FCM | hierarchical | |
|---|---|---|---|---|
| Delta | 7 | 8 | 5 | 9 |
| Theta | 9 | 9 | 5 | 6 |
| Alpha | 12 | 10 | 12 | 13 |
| Beta | 20 | 14 | 18 | 16 |
| Low gamma | 14 | 10 | 14 | 11 |
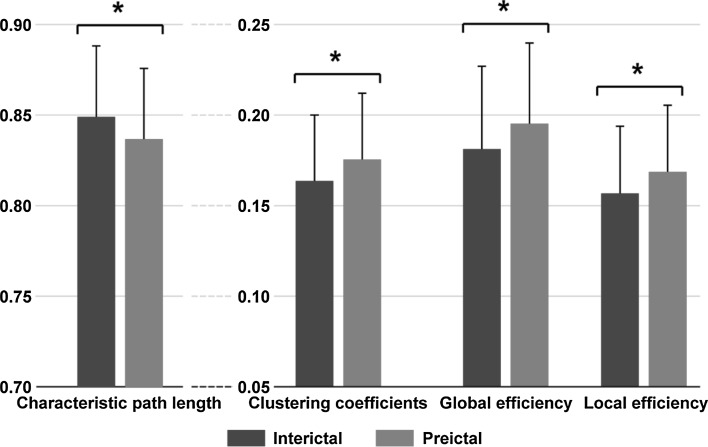
As displayed in Table 2, the k-medoids in the beta band showed the largest number of clustered 1-h EEG data sets. We then investigated the differences in the weighted network properties between the interictal and preictal states only in the beta band and only using k-medoids. The comparison of network properties between the two states displayed in Fig. 3 further demonstrated the significantly shorter (p < 0.05) CPL and higher CC, GE, and LE in the preictal state compared to the interictal state.
Fig. 3.
The weighted network properties between interictal and preictal states. The blue and red solid bars denote the weighted network properties corresponding to interictal and preictal states, respectively, and * denotes p < 0.05. (Color figure online)
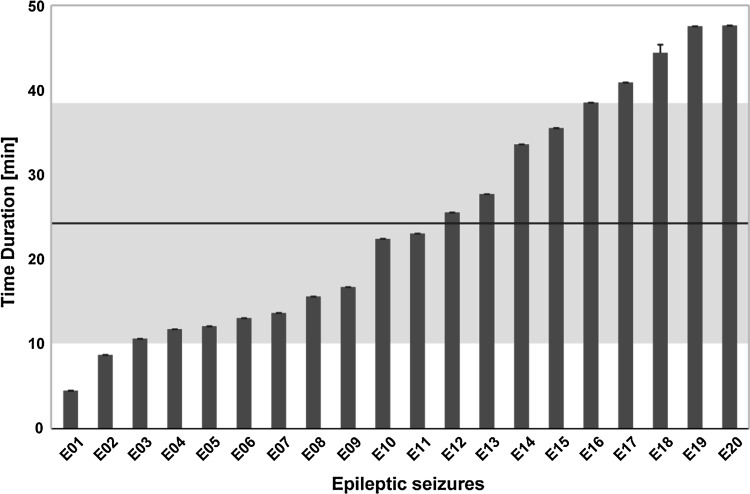
In addition, we calculated the time duration underlying the transition from “cluster 1” to “cluster 2” for each clustered 1-h EEG data set (20 total) in the beta band, which is depicted in Fig. 2. Specifically, durations that varied from 4.52 to 47.80 min were demonstrated; in other words, after a mean of 24.78 ± 14.00 min, the brain would transition from an interictal to a preictal state, which heralded the onset of the epileptic seizure.
Fig. 2.
The various durations before the transition of epileptic states from an interictal to a preictal state for the clustered 1-h EEG data sets (20 in total and shown on the x-axis). The black solid line and gray shadow area denote the mean and standard deviation of the time duration across the 20 data sets, respectively. (Color figure online)
Discussion
Studies that have attempted to detect the preictal state preceding a seizure have provided meaningful findings (Gadhoumi et al. 2016; Le et al. 2001; Lehnertz et al. 2001; Mormann et al. 2007). For example, Blanco and colleagues showed increased entropy in the high band (32–128 Hz) in the moments preceding the attack (Blanco et al. 2013); meanwhile, the spatiotemporal organization of preictal activity was also found several minutes (sometimes longer than 30 min) before a seizure (Chavez et al. 2003). In essence, early changes in EEG dynamics in the time preceding the seizure in different bands helped to herald the epileptic seizure in patients. In this study, we combined EEG network and clustering analyses to capture the transition of brain activity from interictal to preictal states (Fig. 1) and to investigate the corresponding dynamics of EEG network connectivity synchronization.
The relatively high chaoticity and low synchronization were demonstrated within several minutes to hours before seizure; however, as the seizure gradually approached in time, decreased chaoticity and increased synchronization occurred, followed by the epileptic seizure along with the highest synchronization and lowest chaoticity. The brain is one of the most complex systems, and the transition from interictal to preictal states usually occurs from several minutes to hours before the seizure, which can be indexed by the decreased brain network complexity (Lehnertz et al. 2001). For instance, the 6-s EEG signal prior to the seizure showed a high similarity in the frequency range that experienced the largest coherence values with that in epileptic seizure (Salant et al. 1998). Iasemidis and colleagues estimated the Lyapunov exponent of electrocorticograms before, during, and after a seizure, and revealed the lowest Lyapunov exponent in the epileptic seizure (Iasemidis et al. 1990). In this study, we investigated the EEG networks in five bands to uncover the transition of epileptic states from the interictal to preictal state. As shown in Table 2, 20 out of 29 1-h EEG data sets were discriminated into “cluster 1” (interictal) and “cluster 2” (preictal) in the beta band. In addition, the varied durations (4.52–47.80 min) were also demonstrated in Fig. 2, when the brain transitioned from an interictal to a preictal state, which preceded the epileptic seizure.
Theoretically, coherence measures the synchrony-defined neuronal assemblies at a specific frequency between two EEG signals (Salant et al. 1998); the high synchronization of EEG network connectivity corresponds to higher network properties (i.e., high CC, GE, LE, and shorter CPL) (Xu et al. 2013; Zhang et al. 2013). Electrophysiologically, the epileptic seizure occurs in the form of abnormal neuronal activity (Fisher et al. 2005; Staley et al. 2005). By investigating the KIV model, whose architecture was represented by the areas of the limbic system, Myers and Kozma simulated epileptogenesis; when the external weights that join the three networks increased, seizure activity then entrained the entire system (Myers and Kozma 2018). Our previous study also demonstrated the contributions of distributed brain regions to the synchronized activity in the interictal state (Zhang et al. 2017). When the brain transitioned to the preictal state (24.78 ± 14.00 min before the seizure), the results shown in Fig. 3 were consistent with previous studies that investigated the EEG dynamics preceding seizure (Blanco et al. 2013; Salant et al. 1998); indeed, there was an increased synchronization of network connectivity in the beta band since relatively higher CC, GE, LE and shorter CPL were demonstrated in the preictal state than in the interictal state.
In conclusion, these results that were derived from the combination of EEG network and clustering analyses validated the similar tendency that the smooth transition of epileptic states towards the seizure gradually developed over time, which was demonstrated by the increased synchronization of network connectivity. Moreover, the EEG network properties could quantitatively reveal the related EEG dynamics across the transitional process.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (#61522105, #61603344, #81330032, #71601136, and #81771925), the Open Foundation of Henan Key Laboratory of Brain Science and Brain–Computer Interface Technology (No. HNBBL17001), and the Longshan academic talent research supporting program of SWUST (#17LZX692).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fali Li and Yi Liang have contributed equally to this work.
Contributor Information
Liang Yu, Phone: +86-028-83206978, Email: 18981838653@163.com.
Peng Xu, Phone: +86-028-83206978, Email: xupeng@uestc.edu.cn.
References
- Blanco S, Garay A, Coulombie D. Comparison of frequency bands using spectral entropy for epileptic seizure prediction. ISRN Neurol. 2013;2013:287327. doi: 10.1155/2013/287327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Assi E, Nguyen DK, Rihana S, Sawan M. Towards accurate prediction of epileptic seizures: a review. Biomed Signal Process. 2017;34:144–157. doi: 10.1016/j.bspc.2017.02.001. [DOI] [Google Scholar]
- Burns SP, et al. Network dynamics of the brain and influence of the epileptic seizure onset zone. Proc Natl Acad Sci USA. 2014;111:E5321–E5330. doi: 10.1073/pnas.1401752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez M, Van Quyen ML, Navarro V, Baulac M. Spatio-temporal dynamics prior to neocortical seizures: amplitude versus phase couplings. IEEE Trans Biomed Eng. 2003;50:571–583. doi: 10.1109/TBME.2003.810696. [DOI] [PubMed] [Google Scholar]
- Chen T, Hong Ren W. Adaptive impulse detection using center-weighted median filters. IEEE Signal Proc Lett. 2001;8:1–3. doi: 10.1109/97.889633. [DOI] [Google Scholar]
- Cheung MC, Chan AS, Chan YL, Lam JMK, Lam W. Effects of illness duration on memory processing of patients with temporal lobe epilepsy. Epilepsia. 2006;47:1320–1328. doi: 10.1111/j.1528-1167.2006.00556.x. [DOI] [PubMed] [Google Scholar]
- Dasdemir Y, Yildirim E, Yildirim S. Analysis of functional brain connections for positive–negative emotions using phase locking value. Cogn Neurodyn. 2017;11:487–500. doi: 10.1007/s11571-017-9447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury I, Smith B, Li DZ, Savit R. Seizure prediction using scalp electroencephalogram. Exp Neurol. 2003;184:S9–S18. doi: 10.1016/S0014-4886(03)00354-6. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Adhikari BM, Gross R, Willie J, Dhamala M. Application of high-frequency Granger causality to analysis of epileptic seizures and surgical decision making. Epilepsia. 2014;55:2038–2047. doi: 10.1111/epi.12831. [DOI] [PubMed] [Google Scholar]
- Fields C, Glazebrook JF. Disrupted development and imbalanced function in the global neuronal workspace: a positive-feedback mechanism for the emergence of ASD in early infancy. Cogn Neurodyn. 2017;11:1–21. doi: 10.1007/s11571-016-9419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, Boas WVE, Blume W, Elger C, Genton P, Lee P, Engel JJE., Jr Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Fisher RS, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Gadhoumi K, Lina JM, Mormann F, Gotman J. Seizure prediction for therapeutic devices: a review. J Neurosci Methods. 2016;260:270–282. doi: 10.1016/j.jneumeth.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Gao ZK, Cai Q, Yang YX, Dong N, Zhang SS. Visibility graph from adaptive optimal kernel time-frequency representation for classification of epileptiform EEG. Int J Neural Syst. 2017;27:1750005. doi: 10.1142/S0129065717500058. [DOI] [PubMed] [Google Scholar]
- Hommet C, Sauerwein HC, De Toffol B, Lassonde M. Idiopathic epileptic syndromes and cognition. Neurosci Biobehav Rev. 2006;30:85–96. doi: 10.1016/j.neubiorev.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Hussain L. Detecting epileptic seizure with different feature extracting strategies using robust machine learning classification techniques by applying advance parameter optimization approach. Cogn Neurodyn. 2018;12:271–294. doi: 10.1007/s11571-018-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iasemidis LD, Sackellares JC, Zaveri HP, Williams WJ. Phase space topography and the Lyapunov exponent of electrocorticograms in partial seizures. Brain Topogr. 1990;2:187–201. doi: 10.1007/BF01140588. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, et al. Anticipation of epileptic seizures from standard EEG recordings. Lancet. 2001;357:183–188. doi: 10.1016/S0140-6736(00)03591-1. [DOI] [PubMed] [Google Scholar]
- Le VQM, Martinerie J, Navarro V, Baulac M, Varela FJ. Characterizing neurodynamic changes before seizures. J Clin Neurophysiol. 2001;18:191–208. doi: 10.1097/00004691-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Lehnertz K, et al. Nonlinear EEG analysis in epilepsy: its possible use for interictal focus localization, seizure anticipation, and prevention. J Clin Neurophysiol. 2001;18:209–222. doi: 10.1097/00004691-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Li F, et al. Relationships between the resting-state network and the P3: evidence from a scalp EEG study. Sci Rep. 2015;5:15129. doi: 10.1038/srep15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, et al. The time-varying networks in P300: a task-evoked EEG study. IEEE Trans Neural Syst Rehabil Eng. 2016;24:725–733. doi: 10.1109/TNSRE.2016.2523678. [DOI] [PubMed] [Google Scholar]
- Li F, et al. Top-down disconnectivity in schizophrenia during P300 tasks. Front Comput Neurosci. 2018 doi: 10.3389/fncom.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos DM, Guevara Erra R, Wennberg R, Perez Velazquez JL. Measures of entropy and complexity in altered states of consciousness. Cogn Neurodyn. 2018;12:73–84. doi: 10.1007/s11571-017-9459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Elger CE, Lehnertz K. Seizure anticipation: from algorithms to clinical practice. Curr Opin Neurol. 2006;19:187–193. doi: 10.1097/01.wco.0000218237.52593.bc. [DOI] [PubMed] [Google Scholar]
- Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–333. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- Mumtaz W, Vuong P, Xia LK, Malik A, Bin Abd Rashid R. An EEG-based machine learning method to screen alcohol use disorder. Cogn Neurodyn. 2017;11:161–171. doi: 10.1007/s11571-016-9416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MH, Kozma R. Mesoscopic neuron population modeling of normal/epileptic brain dynamics. Cogn Neurodyn. 2018;12:211–223. doi: 10.1007/s11571-017-9468-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopapa F, Siettos CI, Myatchin I, Lagae L. Children with well controlled epilepsy possess different spatio-temporal patterns of causal network connectivity during a visual working memory task. Cogn Neurodyn. 2016;10:99–111. doi: 10.1007/s11571-015-9373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H, Gotman J. A seizure warning system for long-term epilepsy monitoring. Neurology. 1995;45:2250–2254. doi: 10.1212/WNL.45.12.2250. [DOI] [PubMed] [Google Scholar]
- Raghu S, Sriraam N, Kumar GP. Classification of epileptic seizures using wavelet packet log energy and norm entropies with recurrent Elman neural network classifier. Cogn Neurodyn. 2017;11:51–66. doi: 10.1007/s11571-016-9408-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowski Z, Gath I, Bental E. On the prediction of epileptic seizures. Biol Cybern. 1981;42:9–15. doi: 10.1007/BF00335153. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Salant Y, Gath I, Henriksen O. Prediction of epileptic seizures from two-channel EEG. Med Bio Eng Comput. 1998;36:549–556. doi: 10.1007/BF02524422. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Hong SB, Bagshaw AP, Chauvel P, Benar CG. Preictal changes in cerebral haemodynamics: review of findings and insights from intracerebral EEG. Epilepsy Res. 2011;97:252–266. doi: 10.1016/j.eplepsyres.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Sun J, Hong X, Tong S. Phase synchronization analysis of EEG signals: an evaluation based on surrogate tests. IEEE Trans Biomed Eng. 2012;59:2254–2263. doi: 10.1109/TBME.2012.2213597. [DOI] [PubMed] [Google Scholar]
- van Drongelen W, et al. Seizure anticipation in pediatric epilepsy: use of Kolmogorov entropy. Pediatr Neurol. 2003;29:207–213. doi: 10.1016/S0887-8994(03)00145-0. [DOI] [PubMed] [Google Scholar]
- Wang ZG, et al. Altered resting state networks in epileptic patients with generalized tonic-clonic seizures. Brain Res. 2011;1374:134–141. doi: 10.1016/j.brainres.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Xu P, et al. Cortical network properties revealed by SSVEP in anesthetized rats. Sci Rep. 2013;3:2496. doi: 10.1038/srep02496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZQ, et al. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain. 2011;134:2912–2928. doi: 10.1093/brain/awr223. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu P, Huang Y, Cheng K, Yao D. SSVEP response is related to functional brain network topology entrained by the flickering stimulus. PLoS ONE. 2013;8:e72654. doi: 10.1371/journal.pone.0072654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, et al. Efficient resting-state EEG network facilitates motor imagery performance. J Neural Eng. 2015;12:066024. doi: 10.1088/1741-2560/12/6/066024. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Time-varying networks of inter-ictal discharging reveal epileptogenic zone. Front Comput Neurosci. 2017 doi: 10.3389/fncom.2017.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]