Abstract
In this study, the distribution of five Alzheimer’s disease (AD)-related single nucleotide polymorphisms (SNPs) in the Han population was examined in combination with the evaluation of clinical cognition and brain pathological analysis. The associations among SNPs, clinical daily cognitive states, and postmortem neuropathological changes were analyzed in 110 human brains from the Chinese Academy of Medical Sciences/Peking Union Medical College (CAMS/PUMC) Human Brain Bank. APOE ε4 (OR = 4.482, P = 0.004), the RS2305421 GG genotype (adjusted OR = 4.397, P = 0.015), and the RS10498633 GT genotype (adjusted OR = 2.375, P = 0.028) were associated with a higher score on the ABC (Aβ plaque score, Braak NFT stage, and CERAD neuritic plaque score) dementia scale. These results advance our understanding of the pathogenesis of AD, the relationship between pathological diagnosis and clinical diagnosis, and the SNPs in the Han population for future research.
Electronic supplementary material
The online version of this article (10.1007/s12264-019-00343-2) contains supplementary material, which is available to authorized users.
Keywords: Human brain bank, Alzheimer’s disease, APOE ε4, ADAM10, SLC24A4
Background
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by progressive memory loss and cognitive dysfunction. The increasing number of AD patients has imposed huge burdens on individuals, families, and societies [1, 2] and scientists’ exploration of its pathogenesis continues [3]. Since Dr. Alzheimer discovered AD and described its pathological features in 1906, Aβ and tau protein have been the major foci in the exploration of AD mechanisms. Over the years, studies of Aβ have dominated basic research, biomarker development [4], molecular imaging, and drug development. However, with the frequent frustrations of Aβ-targeting drugs [5], it is currently considered that tau protein-related pathological changes are more related than Aβ to clinical symptoms in the dissemination process in the brain and better reflect the severity of AD [6].
Integrating a series of single-nucleotide polymorphisms (SNPs) can help to predict the risk of dementia [7]. APOE ε4 is well-known as the most important AD susceptibility gene. Allen Roses found that the risk of early-onset and late-onset AD in APOE ε4 carriers is significantly higher; carrying one copy of the gene increases the risk by four times, while carrying two copies increases the risk by 12 times [8]. APOE ε4 promotes the accumulation of Aβ in the brain and also interacts closely with tau protein [9].
RS2075650 is located on chromosome 19q13, close to APOE ε4. Since the first AD genome-wide association study (GWAS), it has been found that genes located near the APOE ε4 gene on chromosome 19 also have a close relationship with AD [10]. After further exploration of APOE coding, a small-sample analysis showed that different evolutionary branches can be distinguished by a poly-T morphology repeated in the TOMM40 (translocase of outer mitochondrial membrane 40) intron. Long poly-T is associated with an increased risk of AD and a lower age at onset [11].
The RS2305421 locus is located on human chromosome 15q21.3-q23, and is one of the most common variants in the ADAM10 gene (disintegrin and metalloprotease 10). ADAM10 is an anti-amyloidogenic protease that can significantly mitigate Aβ formation [12]. Kim et al. found that the RS2305421 has a genetic association with AD [13].This correlation has also been confirmed in a GWAS [14].
RS10498633, located on chromosome 14q32.12, encodes the SLC24A4 protein (24 solute carrier family member 4), which is a member of the K+-dependent Na+/Ca2+ exchanger protein family. These exchanger proteins are widely expressed in many tissues of the human body, especially neurons, suggesting that SLC24A4 may play an important role in the nervous system. The SLC24A4 protein is involved in the repair and the development of normal neurons [15], and inhibits the expression of inflammatory mediators in neurons.
Thus, because of their potential relationships with AD, we chose these five SNPs to fulfil the aim of the study which was to determine the distribution of AD-related SNPs in the Han population and analyze the correlation between clinical cognition and brain pathology based on cases from the Chinese Academy of Medical Sciences/Peking Union Medical College (CAMS/PUMC) Human Brain Bank.
Methods
One hundred and ten cases from the CAMS/PUMC Human Brain Bank in Beijing, China were included in this research. The protocols were approved by the Institutional Review Board of the Institute of Basic Medical Sciences of the Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China (Approval Number: 009-2014;031-2017). All ante-mortem written informed consent forms were received from both the potential donor and his/her next-of-kin to guarantee that the donation was completely voluntary and ethically-approved use of the brain tissues in future scientific research was permitted. The brain tissues were collected from 2012 to 2018, all following the international standard human brain banking procedure [16]. The clinical cognitive status was determined using the ECog Insider Questionnaire [17]. For neuropathological analysis, we used the “ABC” dementia score for each case according to the National Institute on Aging and Alzheimer’s Association (NIA-AA) guidelines [18]. Six cases were excluded as they met the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria for vascular dementia [19].
Cognitive Function Assessment
ECog scales including 39 questions aim at assessing ante-mortem everyday function of the brain donor by comparing the participant’s current ability with that of 10 years earlier. There are 6 sub-items in this questionnaire: memory, language, visuospatial functions, planning, organization, and divided attention. The subdivided areas are also associated with different patterns of progression in AD [20].
Ratings were based on a four-point scale: 1, no significant change or better; 2, questionable problems; 3, consistently worse to a small extent; 4, consistently worse to a large extent. An average was calculated for both the global ECog score and the separate score for each ECog domain. The relative items were marked “unknown” if informant could not recall or respond. If more than half of the items were marked “unknown”, the average score was not calculated.
Cognitively normal was defined as ECog ≤ 1.0; mild cognitive impairment as ECog 1.0–2.0; and dementia as ECog > 2.0. ECog 2.0 was set as the dividing point for MCI (mild cognitive impairment) and dementia [21], indicating that at least two cognitive domains were impaired. ECog scales have been demonstrated to be sensitive to early functional damage in both MCI [22] and dementia [18].
Neuropathological Classification
Brain Tissue Preparation and Fixation
Brains were cut into 2 halves. One half was frozen for molecular analysis and the other was fixed in 10% formalin for neuropathological experiments. After two weeks of formalin fixation, the meninges, severity of basilar and carotid artery sclerosis, and cerebral atrophy and infarction were assessed. By cutting along the superior colliculus and mammillary bodies, the midbrain and brainstem together with the cerebellum were separated from the cerebrum. The brainstem and cerebellum were sliced at 0.5 cm along the sagittal axis, and the coronal cerebral sections were cut at 0.3 cm. Based on the NIA-AA guidelines, the following brain regions were sampled for conventional paraffin embedding: superior frontal cortex, primary motor cortex, inferior temporal cortex, hippocampus, anterior cingulate cortex, amygdala, supramarginal cortex, caudate/putamen, midbrain, pons, medulla oblongata, and cerebellar dentate nucleus.
Histopathological Score
In accord with the 2012 NIA-AA guidelines [18], the ABC score was used to incorporate amyloid-β deposits (A), neurofibrillary tangles (NFTs) (B), and neuritic plaques (C). The A score reflects the order of amyloid-β appearance in the brain in a tiered manner. For A and B scoring, the thickness of paraffin-embedded tissue was 5 μm, while for C scoring it was 10 μm.
A and B scores were both based on immunohistochemistry against Aβ (DAKO M0872, mouse monoclonal antibody, diluted 1:200) and p-tau (Thermo MN1020, mouse monoclonal antibody, 1:800). The above primary antibodies were incubated separately overnight at 4 °C, then processed for 30 min with the secondary antibody. The results were visualized with Polink-2 plus (ZSGB-BIO PV-9001, Beijing, China) and 3,3-diaminobenzidine (Boster AR1022, Beijing, China). The C score was determined by modified Bielschowsky stain for neuritic process in senile plaques [23]. The general ABC score was categorized to 4 gradations: none, low, intermediate, and high.
The severity of cerebrovascular changes, such as cerebral amyloid angiopathy and arteriosclerosis, was scored as mild, moderate, or severe according to previous research [24, 25].
SNP Genotyping
Genomic DNA was extracted from frozen prefrontal cortex using tissue DNA extraction kit (GeneOn BioTech GO-BTCD-400, Beijing, China) and detected by PCR-restriction fragment length polymorphism. The genotyping using second-generation sequencing technology was performed by Beijing Zixi Bio Tech Co., Ltd.
Statistical Analysis
We used Spearman analysis to evaluate the correlations among clinical cognitive status, pathological changes, SNPs, and demographic variables (education, gender, age, and post-mortem delay). Age was found to influence both cognition and pathological changes. Therefore, age was set as a control factor in the subsequent analysis of ABC and ECog scores. Since cognitive status (normal, MCI, or dementia) and ABC score (none, low, intermediate, or high) were ordinal variables, OLR (ordinal logistic regression) models were used [26, 27]. First, age, APOE ε4, and three SNPs (RS2075650, RS2305421, and RS10498633) were separately analyzed using a univariate OLR model. Then multivariate OLR analysis was conducted. In multivariate model 1, age and one of the three SNPs were included. In multivariate model 2, age, APOE ε4, and one of the three SNPs were included. In each regression, the estimated parameter of each SNP was divided by the estimated parameter of age to evaluate the influence of the SNP on the progression of cognitive impairment or AD pathology. P < 0.05 was regarded as statistically significant.
Results
Effect of Demographic Variables on Cognition and Pathological Changes
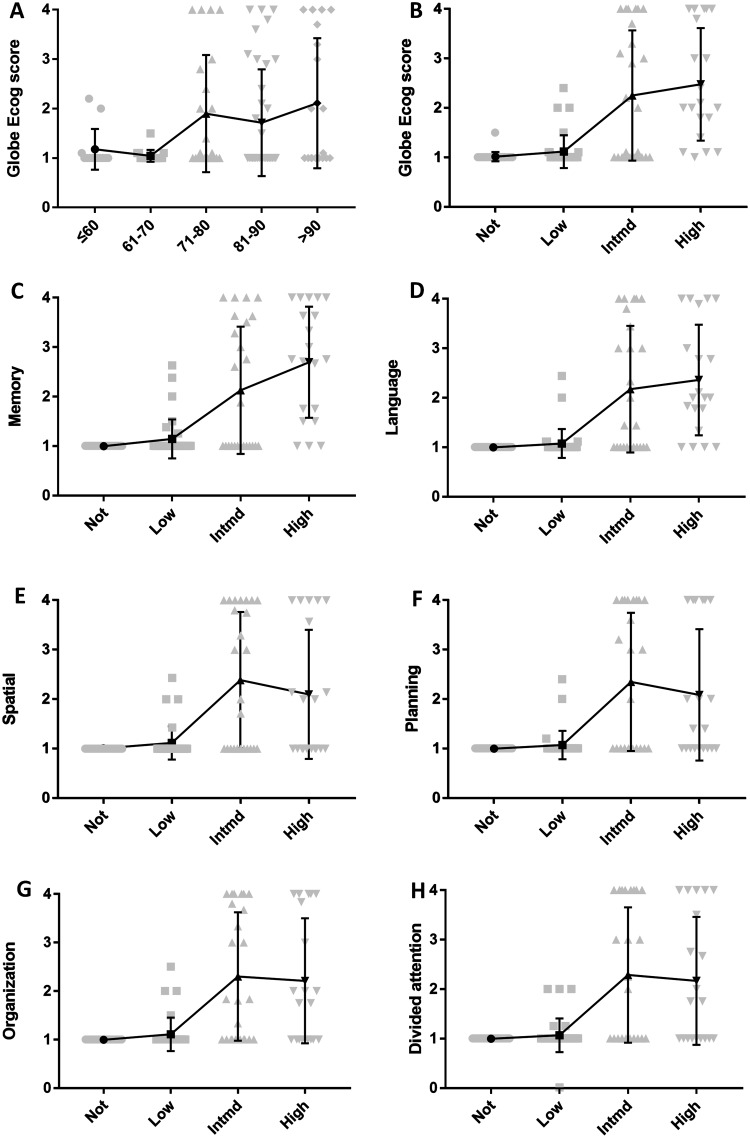
The age at death ranged from 31 years to 102 years, and the average was 78 years. Among the elderly (> 80 years old), 38.5% showed cognitive impairment (Global ECog Score > 2), while this occurred in 10.4% of the younger cases (≤ 80 years old). The ECog score correlated with age in an almost continuous fashion (ANOVA, P = 0.007) (Fig. 1A).
Fig. 1.
Correlations between ECog scores and age or progression of AD pathology. A Correlations between age and Global ECog scores. B Correlations between ECog and ABC scores. C Correlations between memory and ABC scores. D Correlations between language and ABC scores. E Correlations between spatial and ABC scores. F Correlations between planning and ABC scores. G Correlations between organization and ABC scores. H Correlations between divided attention and ABC scores. Intmd, Intermediate.
There was no significant difference in education (Kruskal-Wallis H, P = 0.737), gender (Kruskal-Wallis H, P = 0.267), and post-mortem delay (Kruskal-Wallis H, P = 0.751) between the different general ABC groups (Tables S1 and S2).
Spearman Correlations Among Everyday Cognition Scores, ABC Scores, and Demographic Variables
Spearman correlation analysis was used to further confirm the relationships between ABC scores, ECog scores, and demographic variables. Older participants tended to present with more evident pathological changes and worse cognitive conditions. Neither education nor gender was significantly related to the above concurrent pathologies (Table S3).
The average Global scores for the Intermediate and High groups were 2.0 and 2.1, while those for the None and Low groups were 1.0 and 1.0. As the pathological conditions worsened, cognitive function was more severely impaired (Fig. 1B–H).
Partial correlation analysis was applied to the ECog domains along with A, B, and C. There were extensive connections between them and the B score was more strongly correlated with the ECog score than A and C, as analysis of the B score showed R > 0.4 and P < 0.001 in all domains (Table 1).
Table 1.
Partial correlations between ABC scores, concurrent pathologies and cognitive assessment scales of ECog scores
| Global ECog | Memory | Language | Spatial | Planning | Organization | Divided Attention | |
|---|---|---|---|---|---|---|---|
| General ABC score |
0.490 (< 0.001)*** | 0.534 (< 0.001)*** | 0.484 (< 0.001)*** | 0.393 (< 0.001)*** | 0.379 (< 0.001)*** | 0.417 (< 0.001)*** | 0.417 (< 0.001)*** |
| A score | 0.397 (< 0.001)*** | 0.424 (0.067) |
0.406 (0.001)** | 0.329 (0.001)** | 0.299 (0.002)** | 0.372 (< 0.001)*** |
0.347 (< 0.001)*** |
| B score | 0.465 (< 0.001)*** | 0.465 (< 0.001)*** | 0.465 (< 0.001)*** | 0.410 (< 0.001)*** | 0.410 (< 0.001)*** | 0.418 (< 0.001)*** | 0.405 (< 0.001)*** |
| C score | 0.446 (< 0.001)*** | 0.444 (< 0.001)*** | 0.454 (< 0.001)*** | 0.397 (< 0.001)*** | 0.373 (< 0.001)*** | 0.435 (< 0.001)*** | 0.363 (< 0.001)*** |
| Cerebral amyloid angiopathy | −0.065 (0.516) | −0.045 (0.653) | −0.042 (0.677) | −0.077 (0.442) | −0.084 (0.402) | −0.095 (0.341) |
−0.042 (0.674) |
| Arteriosclerosis | 0.04 (0.693) | 0.004 (0.971) | −0.022 (0.831) | 0.020 (0.844) | 0.021 (0.836) | −0.017 (0.866) | 0.011 (0.913) |
The correlation was adjusted by age. The results are presented as correlation coefficients (P value). *P < 0.05 (2-tailed), **P < 0.01 (2-tailed), ***P < 0.001 (2-tailed)
SNPs
Univariate OLR showed that age and APOE ε4 significantly influenced the cognitive state. Greater age (OR = 1.051, P = 0.004) and APOE ε4 (OR = 5.607, P = 0.002) were associated with the outcome of a worse cognitive state. Thus, age and APOE ε4 were included in the multivariate models as confounding factors.
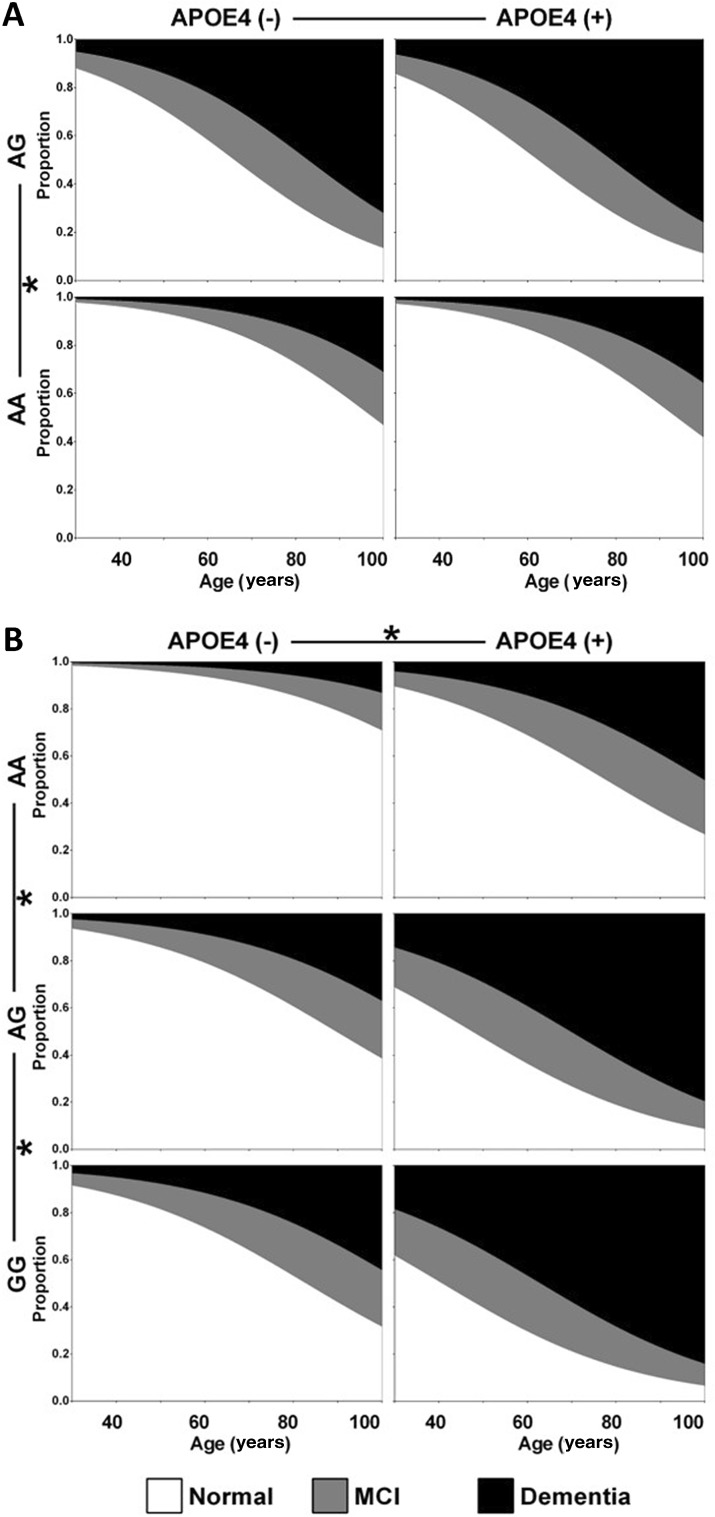
The unadjusted OR for a worse cognitive state in RS2075650 AG carriers was 6.417 (P < 0.001) relative to AA carriers. After adjusting for age, the OR was 6.508 (P < 0.001). The effect of RS2075650 remained significant (OR = 5.697, P = 0.021) after adjusting for age and APOE ε4 (Table 2). According to the regression results, the progression of cognitive impairment began 31.6 years earlier in RS2075650 AG carriers than in AA carriers (Table 3, Fig. 2A). Taking the situation at 80 years of age as an example, there was a probability of 51.4% to have dementia and 21.3% to have MCI for RS2075650 AG carriers with APOE ε4, but for RS2075650 AA carriers without APOE ε4, the probability of having dementia was 13.1% and MCI 14.4%.
Table 2.
Results of ordinal logistic regression for RS2075650, RS2305421, RS10498633, and cognitive state.
| Variables | Cognitive state | Univariate model | Multivariate model 1* | Multivariate model 2* | |||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | MCI | Dementia | Unadjusted OR | P | Adjusted OR | P | Adjusted OR | P | |
| RS2075650 | |||||||||
| AA | 60 | 12 | 11 | Reference | Reference | Reference | |||
| AG | 6 | 3 | 10 | 6.417 (2.370, 17.357) |
< 0.001 | 6.508 (2.340, 18.120) |
< 0.001 | 5.697 (1.303, 24.903) |
0.021 |
| RS2305421 | |||||||||
| AA | 31 | 4 | 4 | Reference | Reference | Reference | |||
| AG | 30 | 9 | 13 | 2.869 (1.122, 7.338) |
0.028 | 2.971 (1.139, 7.752) |
0.026 | 3.869 (1.381, 10.827) |
0.010 |
| GG | 6 | 3 | 4 | 4.267 (1.181, 15.425) |
0.027 | 4.027 (1.090, 14.895) |
0.037 | 5.254 (1.326, 20.822) |
0.018 |
| RS10498633 | |||||||||
| GG | 30 | 4 | 7 | Reference | Reference | Reference | |||
| GT | 33 | 12 | 14 | 1.988 (0.859, 4.604) |
0.109 | 1.728 (0.731, 4.084) |
0.212 | 1.826 (0.736, 4.527) |
0.194 |
*Age and one of the three SNPs were included in multivariate model 1; age, APOE ε4, and one of the three SNPs were included in multivariate model 2.
Table 3.
Parameter estimates of cognitive state for RS2075650 and RS2305421 in model 2.
| Variables | Age | SNP | Change in progression of cognitive impairment (years) | ||
|---|---|---|---|---|---|
| Estimate | P | Estimate | P | ||
| RS2075650 | 0.055 | 0.006 | |||
| AA | Reference | ||||
| AG | 1.740 | 0.021 | 31.6 | ||
| RS2305421 | 0.045 | 0.012 | |||
| AA | Reference | ||||
| AG | 1.353 | 0.010 | 30.1 | ||
| GG | 1.659 | 0.018 | 36.9 | ||
Fig. 2.
Influence of RS2075650 and RS2305421 on the progression of cognitive impairment. A Influence of RS2075650 on the progression of cognitive impairment fitted by ordinal logistic regression in model 2. B Influence of RS2305421 on the progression of cognitive impairment fitted by ordinal logistic regression in model 2.
The unadjusted OR of RS2305421 GG carriers was 4.267 (P = 0.027) and the OR of AG carriers was 2.869 (P = 0.028) relative to AA carriers. After adjusting for age, the OR of GG carriers was 4.027 (P = 0.037) and the OR of AG carriers was 2.971 (P = 0.026). After adjusting for both age and APOE ε4, the OR of GG carriers was 5.254 (P = 0.018) and the OR of AG carriers was 3.869 (P = 0.010) (Table 2). The progression of cognitive impairment began 30.1 years earlier in RS2305421 AG carriers and 36.9 years earlier in GG carriers than in AA carriers (Table 3, Fig. 2B). Taking the situation at 80 years as an example, there was a probability of 68.4% to have dementia and 17.0% to have MCI for RS2305421 GG carriers with APOE ε4, but for RS2305421 AA carriers without APOE ε4, the probability of having dementia was 5.8% and MCI 8.6%.
Besides, RS10498633 showed no evidence of influencing cognitive state before or after adjusting for age and APOE ε4 (Table 2).
Univariate OLR showed that age and APOE ε4 significantly influenced the ABC score. Greater age (OR = 1.070, P < 0.001) and APOE ε4 (OR = 4.482, P = 0.004) were associated with the outcome of a higher ABC score. Thus, age and APOE ε4 were included in the multivariate models as confounding factors.
The unadjusted OR of RS2075650 AG carriers was 3.615 (P = 0.007) relative to AA carriers. After adjusting for age, the OR was 3.340 (P = 0.012). However, the effect of RS2075650 was not significant after adjusting for APOE ε4 (Table 4).
Table 4.
Results of ordinal logistic regression for RS2075650, RS2305421, RS10498633, and ABC score.
| Variables | ABC score# | Univariate model | Multivariate model 1* | Multivariate model 2* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | L | I | H | Unadjusted OR | P | Adjusted OR | P | Adjusted OR | P | |
| RS2075650 | ||||||||||
| AA | 27 | 27 | 21 | 8 | Reference | Reference | Reference | |||
| AG | 2 | 6 | 4 | 7 | 3.615 (1.428, 9.152) |
0.007 | 3.340 (1.306, 8.542) | 0.012 | 2.430 (1.562, 9.217) | 0.192 |
| RS2305421 | ||||||||||
| AA | 13 | 18 | 5 | 3 | Reference | Reference | Reference | |||
| AG | 15 | 13 | 15 | 9 | 2.014 (0.938, 4.323) | 0.073 | 2.136 (0.969, 4.711) | 0.060 | 2.321 (1.041, 5.176) | 0.040 |
| GG | 1 | 4 | 5 | 3 | 4.187 (1.311, 13.383) | 0.016 | 3.896 (1.190, 12.743) | 0.025 | 4.397 (1.326, 14.600) | 0.015 |
| RS10498633 | ||||||||||
| GG | 16 | 14 | 8 | 3 | Reference | Reference | Reference | |||
| GT | 11 | 20 | 16 | 12 | 2.686 (1.275, 5.658) | 0.009 | 2.226 (1.038, 4.773) | 0.040 | 2.375 (1.095, 5.150) | 0.028 |
#ABC score was divided into four grades: N (none), L (low), I (intermediate), H (high).
*Age and one of the three SNPs were included in multivariate model 1; age, APOE ε4 and one of the three SNPs were included in multivariate model 2.
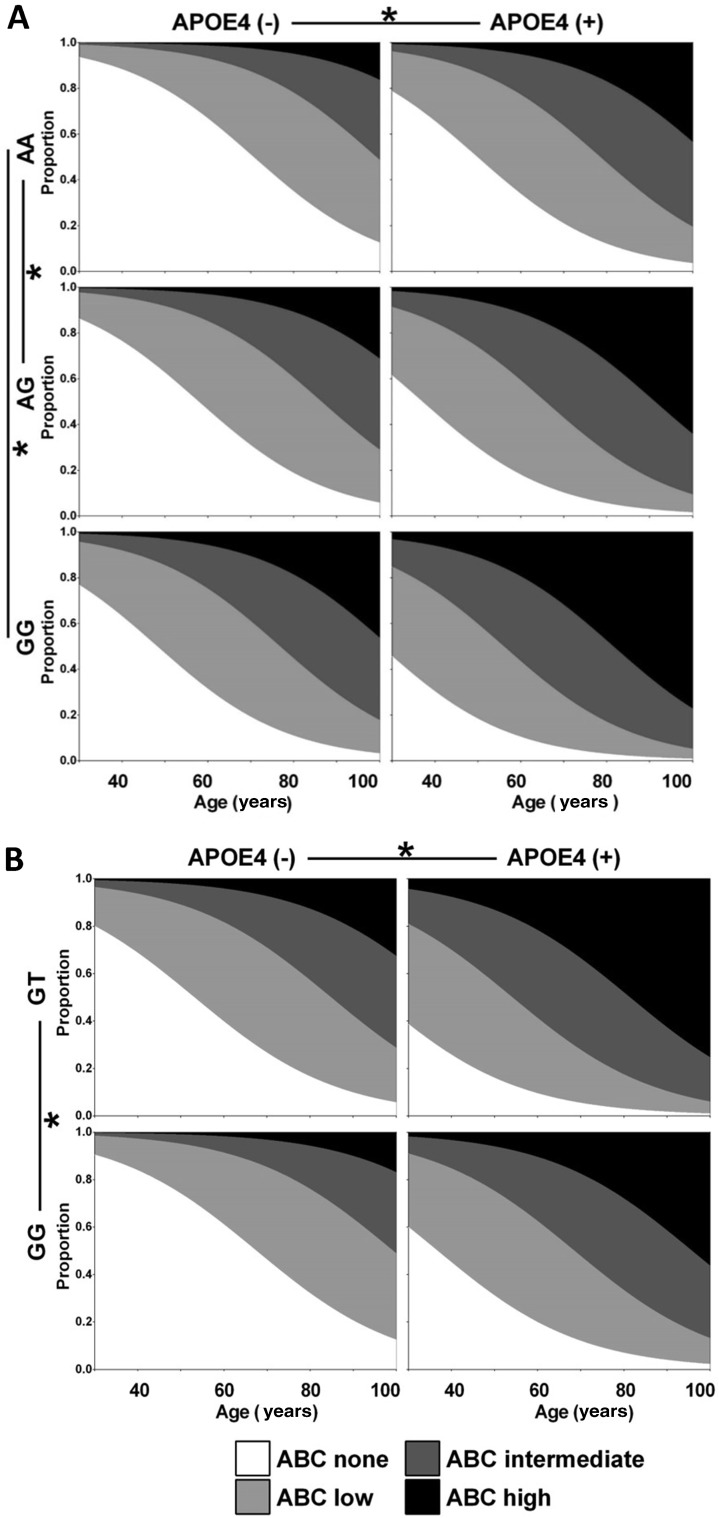
The unadjusted OR of RS2305421 GG carriers was 4.187 (P = 0.016) relative to AA carriers. After adjusting for age, the OR was 3.896 (P = 0.025). After adjusting for both age and APOE ε4, the OR of GG carriers was 4.397 (P = 0.015) and the OR of AG carriers was 2.321 (P = 0.040) (Table 4). The progression of AD pathology began 12.8 years earlier in RS2305421 AG carriers and 22.4 years earlier in GG carriers than in AA carriers (Table 5, Fig. 3A). Taking the situation at 80 years as an example, the probability to have an ABC score of none, low, intermediate, or high was 3.0%, 14.0%, 35.5%, or 47.5% respectively for RS2305421 GG carriers with APOE ε4, but was 34.9%, 43.1%, 17.0%, or 5.0% for RS2305421 AA carriers without APOE ε4.
Table 5.
Parameter estimates of ABC score for RS2305421 and RS10498633 in model 2.
| Variables | Age | SNP | Change in progression of AD pathology (years) | ||
|---|---|---|---|---|---|
| Estimate | P | Estimate | P | ||
| RS2305421 | 0.066 | < 0.001 | |||
| AA | Reference | ||||
| AG | 0.842 | 0.040 | 12.8 | ||
| GG | 1.481 | 0.015 | 22.4 | ||
| RS10498633 | 0.06 | < 0.001 | |||
| GG | Reference | ||||
| GT | 0.865 | 0.028 | 14.4 | ||
Fig. 3.
Influence of RS2305421 and RS10498633 on the progression of AD pathology. A Influence of RS2305421 on the progression of AD pathology fitted by ordinal logistic regression in model 2. B Influence of RS10498633 on the progression of AD pathology fitted by ordinal logistic regression in model 2.
The unadjusted OR of RS10498633 GT carriers was 2.686 (P = 0.009) relative to GG carriers. After adjusting for age, the OR was 2.226 (P = 0.040). After adjusting for both age and APOE ε4, the OR of GT carriers was 2.375 (P = 0.028) (Table 4). The progression of AD pathology began 14.4 years earlier in RS10498633 GT carriers than GG carriers (Table 5, Fig. 3B). Taking the situation at 80 years as an example, the probability to have an ABC score of none, low, intermediate, or high was 3.0%, 14.4%, 34.6%, or 48.0% respectively for RS10498633 GT carriers with APOE ε4, but was 32.0%, 43.9%, 18.2%, or 5.8% for RS10498633 GG carriers without APOE ε4.
Discussion
NFT Stage Widely Correlated with Cognitive Performance
Our results showed that the higher the NFT score, the worse the general cognitive impairment. Both CERAD (Consortium to Establish a Registry for Alzheimer’s Disease) and Thal scoring can be applied as indicators of β-amyloid deposition according to the 2012 NIA-AA guidelines [4]. The A score reveals the distribution of Aβ deposits in separate brain regions, and the C score reflects the density of cortical neuritic plaques [28].
NFTs result from intracellular tau protein accumulation and fibrilization, originally appearing in the entorhinal cortex, then diffusing to the hippocampus and isocortex [29]. Because of hyperphosphorylation, tau loses its normal physiological role in constructing microtubules and maintaining cellular stability [30, 31]. A recent study has also shown that phosphorylation of tau protein is necessary for β-amyloid-induced synapse loss [32]. In recent decades, it has gradually become known that neurofibrillary tau pathology correlates more closely than Aβ with the clinical symptoms of dementia and related diseases such as AD [33, 34, 35].
A meta-analysis has shown that Braak NFT staging is better correlated with cognitive status than the staging of neuritic plaques and diffuse Aβs [36]. In our study, the correlation between Aβ and cognition was not as evident as that for p-tau. A possible reason may be that Aβ is more related to aging than cognitive impairment. A previous study has demonstrated that, along with aging, reduced antioxidant content, mitochondrial dysfunction, and synaptic aging all contribute to the vulnerability to Aβ toxicity [37].
APOE ε4 and AD
We demonstrated that APOE ε4 had a significant negative effect on both cognition and AD pathology, consistent with previous studies [8, 9]. The APOE classification depends on the specific SNPs at the RS429358 and RS7412 loci, which produce low-density lipoproteins, ligands for very-low-density lipoproteins, and chylomicron receptors. As early as 30 years ago, APOE ε4 was shown to be related to AD [38]. The three protein phenotypes of human APOE are APOE ε2, APOE ε3, and APOE ε4. About 15% of the population carry the APOE ε4 gene while 65% of AD patients carry it. Among them, the APOE ε2 and APOE ε3 gene products process lipids better in glial cells, while APOE ε4 is almost incapable of this task, resulting in abnormal accumulation of lipid droplets in glial cells and ineffective isolation of lipid peroxides. With aging, these deficiencies are magnified, and the fundamental functional differences among APOE subtypes make certain individuals more vulnerable to oxidative stress [39]. It is the presence of APOE ε4 that leads to neuronal dysfunction [40].
APOE and tauopathy are closely interlinked. Experiments have found that tau levels are significantly increased in APOE ε4 transgenic mice, and tau proteins have a greater degree of cell body-dendritic redistribution [3]. Epidemiological statistics show that APOE ε4 carriers have elevated blood cholesterol levels, which accelerate the progression of coronary atherosclerosis. There is increasing evidence that these changes damage the blood-brain barrier and promote the deposition of tau protein in the brain [41]. Another experiment showed that higher cerebrospinal fluid (CSF) tau levels are closely associated with more rapid disease progression in AD patients with the APOE ε4 genotype. And when cells are incubated in culture media rich in tau protein or in CSF from AD patients with the APOE ε4 genotype, the apoptotic activity is higher. If, and only if, linked to APOE ε4 is CSF detrimental to astrocyte survival, cortical plasticity, and disease progression [42].
RS2075650 and AD
TOMM40 is the most important of the TOMMs; its abnormal expression directly affects mitochondrial protein import by blocking the nuclear transport of encoded proteins to the mitochondria, so they cannot enter the mitochondria to function properly, eventually leading to mitochondrial dysfunction. Followed by increased production of oxygen free radicals, this genetic abnormality starts a vicious cycle, affecting the normal function of the brain [43, 44].
Ma et al. investigated TOMM40 polymorphism and AD in a Northern Han Chinese population and found that three SNP loci (RS157580, RS2075650, and RS157581) play a role in pathogenesis [45]. However, Bagnoli et al. investigated the polymorphisms of TOMM40 in AD and frontotemporal dementia patients in Italy. By investigating 3 SNPs (RS157580, RS2075650, and RS157581), they found that TOMM40 polymorphism was associated with APOE, and there was no evidence that TOMM40 worked independently from APOE ε4 and became an AD risk factor itself [46]. Bernardi et al. found that in a familial AD population, the age at onset of AD was slightly different under the influence of the TOMM40 (RS10524523) gene, but it was also particularly difficult to exclude the effects of other genetic variants around this SNP [47]. In our study, the effect of RS2075650 on ECog was independent of APOE ε4, but its effect on ABC scores was not significant after adjusting for APOE ε4. Further research is needed to clarify the influence of RS2075650 on the pathogenesis of AD.
RS2305421 and AD
Our data demonstrated that RS2305421 is related to the cognitive impairment and pathogenesis of AD. RS2305421 is considered to be the main component of amyloid precursor protein (APP) α-secretase [48, 49]. Aβ, which is produced by the cleavage of APP by β-secretase and γ-secretase, plays a critical role in AD. α-secretase, on the other hand, converts APP to soluble APPs-α, which hinders the production of Aβ. This phenomenon reported in a northern Asian Han population is consistent with our findings [50]. In order to explore its role in the pathogenesis of AD, further studies on a larger scale involving more ethnic groups are needed.
ABC Score Correlation with RS10498633
In our study, RS10498633 influenced the pathology of AD but not the degree of cognitive impairment. RS10498633 is a mutation in SLC24A4, which is associated with enamel growth, hair color, and skin pigmentation [51–55]. A study of 25,580 AD patients and 48,466 controls confirmed that RS10498633 is significantly associated with AD risk in Caucasians [56]. However, another study showed that RS10498633 may not play a major role in the AD susceptibility of Chinese [57]. In specific case and control groups, allele and gene frequencies in different ethnic groups may vary [58]. Our research provides new evidence for this, but it still needs further investigation to determine whether this mutation is a risk factor or a predictive factor of AD.
Limitations
Our study was cross-sectional, so neither the sequence of exposure and the timing of outcome, nor the causal relationship between exposure and outcome was taken into account. Our statistical data volume was 110, and the number of cases used to study the AD relationship was 104 (6 cases were excluded). Although the amount of data has been greatly extended compared to our previous report [59], it is still relatively small, resulting in some conclusions that are not sufficiently accurate or persuasive. Thus, it should be pointed out that non-significant results do not necessarily mean that an SNP is not related to AD. Since AD is a multifactorial disease, other variables such as marital, mental, and nutritional status, should be added to the collection of clinical data. In addition, long post-mortem intervals in sampling may affect the tissue quality and have an impact on correlation analysis.
Conclusion
The B score, a measure of NFTs, had the strongest correlation with cognitive dysfunction and AD-related pathological changes. The A score, a measure of Aβ plaques, and the C score, a measure of neuritic plaques, also had specific influences. APOE4 was the most important susceptibility gene for AD in our data. RS2305421 and RS10498633 may have correlations with the ABC score, but further evidence is required to confirm or deny this correlation. These results promote the understanding of the pathogenesis of AD, the relationship between the pathological diagnosis and the clinical diagnosis, and the SNPs in a northern Han Chinese population, and provide directions for future research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to extend our appreciation to Ms. Yunli Ling for preparing the mounted paraffin sections. This work was supported by grants from the National Natural Science Foundation of China (81271239, 81771205, and 91632113), the Institute of Basic Medical Sciences/Chinese Academy of Medical Sciences (CAMS) Dean’s Fund (2011RC01), the CAMS Innovation Fund for Medical Sciences (2016-I2M-1004), and the Natural Science Foundation and Major Basic Research Program of Shanghai Municipality, China (16JC1420500 and 16JC1420502).
Contributor Information
Wenying Qiu, Email: qiuwy73@126.com.
Chao Ma, Email: machao@ibms.cams.cn.
References
- 1.Dong MJ, Peng B, Lin XT, Zhao J, Zhou YR, Wang RH. The prevalence of dementia in the People’s Republic of China: a systematic analysis of 1980-2004 studies. Age Ageing. 2007;36:619–624. doi: 10.1093/ageing/afm128. [DOI] [PubMed] [Google Scholar]
- 2.Wang QH, Wang X, Bu XL, Lian Y, Xiang Y, Luo HB, et al. Comorbidity burden of dementia: a hospital-based retrospective study from 2003 to 2012 in seven cities in China. Neurosci Bull. 2017;33:703–710. doi: 10.1007/s12264-017-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang A, Wang C, Song B, Zhang W, Guo Y, Yang R, et al. Attenuation of beta-amyloid toxicity in vitro and in vivo by accelerated aggregation. Neurosci Bull. 2017;33:405–412. doi: 10.1007/s12264-017-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doig AJ, Del Castillo-Frias MP, Berthoumieu O, Tarus B, Nasica-Labouze J, Sterpone F, et al. Why is research on amyloid-beta failing to give new drugs for Alzheimer’s disease? ACS Chem Neurosci. 2017;8:1435–1437. doi: 10.1021/acschemneuro.7b00188. [DOI] [PubMed] [Google Scholar]
- 6.Serrano-Pozo A, Qian J, Muzikansky A, Monsell SE, Montine TJ, Frosch MP, et al. Thal amyloid stages do not significantly impact the correlation between neuropathological change and cognition in the Alzheimer Disease Continuum. J Neuropathol Exp Neurol. 2016;75:516–526. doi: 10.1093/jnen/nlw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desikan RS, Fan CC, Wang Y, Schork AJ, Cabral HJ, Cupples LA, et al. Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score. PLoS Med. 2017;14:e1002258. doi: 10.1371/journal.pmed.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strum JC, Shehee R, Virley D, Richardson J, Mattie M, Selley P, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis. 2007;11:45–51. doi: 10.3233/JAD-2007-11108. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W, et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coon KD, Myers AJ, Craig DW, Webster JA, Pearson JV, Lince DH, et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J Clin Psychiatry. 2007;68:613–618. doi: 10.4088/JCP.v68n0419. [DOI] [PubMed] [Google Scholar]
- 11.Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. Pharmacogenomics J. 2010;10:375–384. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hung AY, Haass C, Nitsch RM, Qiu WQ, Citron M, Wurtman RJ, et al. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem. 1993;268:22959–22962. [PubMed] [Google Scholar]
- 13.Kim M, Suh J, Romano D, Truong MH, Mullin K, Hooli B, et al. Potential late-onset Alzheimer’s disease-associated mutations in the ADAM10 gene attenuate {alpha}-secretase activity. Hum Mol Genet. 2009;18:3987–3996. doi: 10.1093/hmg/ddp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF, et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson M, Duffy DL, Zhu G, Liu JZ, Macgregor S, McRae AF, et al. GWAS findings for human iris patterns: associations with variants in genes that influence normal neuronal pattern development. Am J Hum Genet. 2011;89:334–343. doi: 10.1016/j.ajhg.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samarasekera N, Al-Shahi Salman R, Huitinga I, Klioueva N, McLean CA, Kretzschmar H, et al. Brain banking for neurological disorders. Lancet Neurol. 2013;12:1096–1105. doi: 10.1016/S1474-4422(13)70202-3. [DOI] [PubMed] [Google Scholar]
- 17.Marshall GA, Zoller AS, Kelly KE, Amariglio RE, Locascio JJ, Johnson KA, et al. Everyday cognition scale items that best discriminate between and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res. 2014;11:853–861. doi: 10.2174/1567205011666141001120903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43: 250–260. [DOI] [PubMed]
- 20.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 1995, 16: 271–278; discussion 278–284. [DOI] [PubMed]
- 21.Farias ST, Park LQ, Harvey DJ, Simon C, Reed BR, Carmichael O, et al. Everyday cognition in older adults: associations with neuropsychological performance and structural brain imaging. J Int Neuropsychol Soc. 2013;19:430–441. doi: 10.1017/S1355617712001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, Decarli C. MCI is associated with deficits in everyday functioning. Alzheimer Dis Assoc Disord. 2006;20:217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suenaga T, Hirano A, Llena JF, Yen SH, Dickson DW. Modified Bielschowsky stain and immunohistochemical studies on striatal plaques in Alzheimer’s disease. Acta Neuropathol. 1990;80:280–286. doi: 10.1007/BF00294646. [DOI] [PubMed] [Google Scholar]
- 24.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 25.Yarchoan M, Xie SX, Kling MA, Toledo JB, Wolk DA, Lee EB, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135:3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ananth CV, Kleinbaum DG. Regression models for ordinal responses: a review of methods and applications. Int J Epidemiol. 1997;26:1323–1333. doi: 10.1093/ije/26.6.1323. [DOI] [PubMed] [Google Scholar]
- 27.Suemoto CK, Ferretti-Rebustini RE, Rodriguez RD, Leite RE, Soterio L, Brucki SM, et al. Neuropathological diagnoses and clinical correlates in older adults in Brazil: A cross-sectional study. PLoS Med. 2017;14:e1002267. doi: 10.1371/journal.pmed.1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Abeta, tau, and alpha-synuclein. Science 2015, 349: 1255555. [DOI] [PubMed]
- 30.Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 31.Quinn JP, Corbett NJ, Kellett KAB, Hooper NM. Tau proteolysis in the pathogenesis of tauopathies: neurotoxic fragments and novel biomarkers. J Alzheimers Dis. 2018;63:13–33. doi: 10.3233/JAD-170959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F. The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Abeta oligomers through Tau phosphorylation. Neuron. 2013;78:94–108. doi: 10.1016/j.neuron.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer’s disease. Sci Transl Med 2016, 8: 338ra366. [DOI] [PMC free article] [PubMed]
- 34.Bos I, Verhey FR, Ramakers I, Jacobs HIL, Soininen H, Freund-Levi Y, et al. Cerebrovascular and amyloid pathology in predementia stages: the relationship with neurodegeneration and cognitive decline. Alzheimers Res Ther. 2017;9:101. doi: 10.1186/s13195-017-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y, Huang H, Abner E, Broster LS, Jicha GA, Schmitt FA, et al. Alzheimer’s biomarkers are correlated with brain connectivity in older adults differentially during resting and task states. Front Aging Neurosci. 2016;8:15. doi: 10.3389/fnagi.2016.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quiroz-Baez R, Flores-Dominguez D, Arias C. Synaptic aging is associated with mitochondrial dysfunction, reduced antioxidant contents and increased vulnerability to amyloid-beta toxicity. Curr Alzheimer Res. 2013;10:324–331. doi: 10.2174/1567205011310030012. [DOI] [PubMed] [Google Scholar]
- 38.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, MacKenzie KR, Putluri N, Maletic-Savatic M, Bellen HJ. The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 2017;26(719–737):e716. doi: 10.1016/j.cmet.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Najm R, Xu Q, Jeong DE, Walker D, Balestra ME, et al. Gain of toxic apolipoprotein E4 effects in human iPSC-derived neurons is ameliorated by a small-molecule structure corrector. Nat Med. 2018;24:647–657. doi: 10.1038/s41591-018-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, Jin F, Cao G, Mei R, Wang Y, Long P, et al. ApoE4 may be a promising target for treatment of coronary heart disease and Alzheimer’s disease. Curr Drug Targets. 2018;19:1038–1046. doi: 10.2174/1389450119666180406112050. [DOI] [PubMed] [Google Scholar]
- 42.Koch G, Di Lorenzo F, Loizzo S, Motta C, Travaglione S, Baiula M, et al. CSF tau is associated with impaired cortical plasticity, cognitive decline and astrocyte survival only in APOE4-positive Alzheimer’s disease. Sci Rep. 2017;7:13728. doi: 10.1038/s41598-017-14204-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller BR, Cumsky MG. An unusual mitochondrial import pathway for the precursor to yeast cytochrome c oxidase subunit Va. J Cell Biol. 1991;112:833–841. doi: 10.1083/jcb.112.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen RE, Dunn CD. Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim Biophys Acta. 2002;1592:25–34. doi: 10.1016/S0167-4889(02)00261-6. [DOI] [PubMed] [Google Scholar]
- 45.Ma XY, Yu JT, Wang W, Wang HF, Liu QY, Zhang W, et al. Association of TOMM40 polymorphisms with late-onset Alzheimer’s disease in a Northern Han Chinese population. Neuromolecular Med. 2013;15:279–287. doi: 10.1007/s12017-012-8217-7. [DOI] [PubMed] [Google Scholar]
- 46.Bagnoli S, Piaceri I, Tedde A, Bessi V, Bracco L, Sorbi S, et al. TOMM40 polymorphisms in Italian Alzheimer’s disease and frontotemporal dementia patients. Neurol Sci. 2013;34:995–998. doi: 10.1007/s10072-013-1425-6. [DOI] [PubMed] [Google Scholar]
- 47.Bernardi L, Gallo M, Anfossi M, Conidi ME, Colao R, Puccio G, et al. Role of TOMM40 rs10524523 polymorphism in onset of Alzheimer’s disease caused by the PSEN1 M146L mutation. J Alzheimers Dis. 2013;37:285–289. doi: 10.3233/JAD-130119. [DOI] [PubMed] [Google Scholar]
- 48.Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, et al. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Postina R, Schroeder A, Dewachter I, Bohl J, Schmitt U, Kojro E, et al. A disintegrin-metalloproteinase prevents amyloid plaque formation and hippocampal defects in an Alzheimer disease mouse model. J Clin Invest. 2004;113:1456–1464. doi: 10.1172/JCI20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song JH, Yu JT, Liu M, Yan CZ, Tan L. Genetic association between ADAM10 gene polymorphism and Alzheimer’s disease in a Northern Han Chinese population. Brain Res. 2011;1421:78–81. doi: 10.1016/j.brainres.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Jalloul AH, Rogasevskaia TP, Szerencsei RT, Schnetkamp PP. A functional study of mutations in K+-dependent Na+-Ca2+ exchangers associated with amelogenesis imperfecta and non-syndromic oculocutaneous albinism. J Biol Chem. 2016;291:13113–13123. doi: 10.1074/jbc.M116.728824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Choi M, Richardson AS, Reid BM, Seymen F, Yildirim M, et al. STIM1 and SLC24A4 are critical for enamel maturation. J Dent Res. 2014;93:94S–100S. doi: 10.1177/0022034514527971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parry DA, Poulter JA, Logan CV, Brookes SJ, Jafri H, Ferguson CH, et al. Identification of mutations in SLC24A4, encoding a potassium-dependent sodium/calcium exchanger, as a cause of amelogenesis imperfecta. Am J Hum Genet. 2013;92:307–312. doi: 10.1016/j.ajhg.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bronckers AL, Jalali R, Lytton J. Reduced protein expression of the Na+/Ca2+ +K+ exchanger (SLC24A4) in apical plasma membranes of maturation ameloblasts of fluorotic mice. Calcif Tissue Int. 2017;100:80–86. doi: 10.1007/s00223-016-0197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han J, Kraft P, Nan H, Guo Q, Chen C, Qureshi A, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4:e1000074. doi: 10.1371/journal.pgen.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu G, Zhang L, Feng R, Liao M, Jiang Y, Chen Z, et al. Lack of association between PICALM rs3851179 polymorphism and Alzheimer’s disease in Chinese population and APOEepsilon4-negative subgroup. Neurobiol Aging. 2013;34(1310):e1319. doi: 10.1016/j.neurobiolaging.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Greene CS, Penrod NM, Williams SM, Moore JH. Failure to replicate a genetic association may provide important clues about genetic architecture. PLoS One. 2009;4:e5639. doi: 10.1371/journal.pone.0005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu WY, Yang Q, Zhang W, Wang N, Zhang D, Huang Y, et al. The correlations between postmortem brain pathologies and cognitive dysfunction in aging and Alzheimer’s disease. Curr Alzheimer Res. 2018;15:462–473. doi: 10.2174/1567205014666171106150915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.