Abstract
Interest is growing in the role of infectious agents in the pathogenesis of dementia, but current evidence is limited. We conducted a systematic review and meta-analysis to investigate the effect of any of eight human herpesviruses on development of dementia or mild cognitive impairment (MCI). We searched the Cochrane Library, Embase, Global Health, Medline, PsycINFO, Scopus, Web of Science, clinical trials registers and grey literature sources from inception to December 2017 for observational studies with cohort, case control or self-controlled designs, or randomised controlled trials of interventions against herpesviruses. Pooled effect estimates and 95% confidence intervals (CIs) were generated through random effects meta-analyses across studies with the same design, outcome, and virus type, method and site of measurement. We included 57 studies across various geographic settings. Past infection with herpesviruses, measured by IgG seropositivity, was generally not associated with dementia risk. A single cohort study rated moderate quality showed an association between varicella zoster virus reactivation (ophthalmic zoster) and incident dementia (HR 2.97; 95%CI, 1.89 to 4.66). Recent infection with, or reactivation of, herpes simplex virus type 1 or type 1/2 unspecified, cytomegalovirus and human herpes virus-6 measured by serum IgM, high titre IgG or clinical disease may be associated with dementia or MCI, though results were inconsistent across studies and overall evidence rated very low quality. Longitudinal population studies with robust repeated virus measurements taken sufficiently proximal to dementia onset are needed to establish whether, when and among whom herpesviruses affect dementia risk.
Introduction
Whether chronic viral infections increase the risk of dementia remains controversial: despite decades of research, high-quality population studies are lacking. Nevertheless, only around one third of dementia cases are due to modifiable risk factors1. This, combined with the projected 2.8-fold increase in the global burden of dementia to 131.5 million cases by 20502, and the lack of effective treatments, has added impetus to the search for novel modifiable risk factors.
Infections with a systemic inflammatory component can trigger brain responses through microglial activation and release of pro-inflammatory mediators3. Chronic antigenic stimulation may also contribute to age-associated immune system remodelling and accelerate neurodegeneration in disorders such as dementia4,5. In neuronal and glial cell cultures, herpes simplex virus type 1 (HSV-1) – one of eight herpesviruses that routinely infects humans – induces molecular changes similar to those seen in Alzheimer’s disease (AD), e.g. β-amyloid accumulation6, generation of amyloid precursor protein fragments with neurotoxic potential7, and tau hyperphosphorylation8. It remains unclear, however, whether HSV-1 has similar effects in vivo.
A previous meta-analysis of small case-control studies of varying quality, with heterogeneous methods of herpesvirus detection and dementia diagnosis, suggested tentative associations between HSV-1 and AD (pooled OR 1.38; 95%CI, 1.03–1.84) and Epstein-Barr virus (EBV) and AD (pooled OR 1.55; 95%CI, 1.12–2.13)9. However, interpretation is challenging as data were pooled across studies detecting herpesviruses from different body sites; past infections were not differentiated from recent infections, nor primary infection from virus reactivation. Here, we systematically reviewed literature on infection with, or reactivation of, any of the eight human herpesviruses and risk of developing dementia or mild cognitive impairment (MCI).
Methods
This systematic review and meta-analysis was performed in accordance with PRISMA guidelines10, and registered with the International Prospective Register of Systematic Reviews on 7 January 2017 (Registration no: CRD42017054684).
Search strategy
We searched the Cochrane Library, Embase, Global Health, Medline, PsycINFO, Scopus, Web of Science and grey literature sources for articles in any language reporting associations between any human herpesvirus and dementia or MCI in adults in any setting. Searches were conducted from database inception to 7 March 2017. Upon identifying a key paper published after this date, we repeated searches in 7 December 2017 limited to the years 2016 and 2017 (see data supplement for full search strategy).
Study selection
We included studies of infection with, reactivation of, and where relevant, vaccination against or treatment of, HSV-1, HSV-2, varicella zoster virus (VZV), EBV, cytomegalovirus (CMV) and human herpesviruses types 6, 7 and 8 (HHV-6,-7,-8), defined by clinical or laboratory criteria. Primary outcomes were dementia (all types) and MCI. We included randomised controlled trials (RCTs), cohort, case-control, case crossover and self-controlled case series studies with sufficient data to calculate effect estimates. A detailed protocol is published elsewhere11. Two reviewers (HJF, CWG) scanned all titles and abstracts in parallel to select full-text manuscripts for review. Full-text manuscripts were then reviewed in parallel and reasons for exclusion recorded. A third reviewer (LS) resolved any discrepancies. Where relevant, we emailed authors to obtain missing information.
Data extraction
Two reviewers (HJF, CWG) extracted data according to a standardised data extraction template in parallel for the first three studies, then one reviewer (HJF) extracted data for remaining studies. We included information on author, year, design, setting, study population, and definition and ascertainment of exposures, outcomes and comparators. We also extracted population size, follow up time, number of subjects with the outcome (or exposure for case-control studies), statistical methods, main results and sub-group analyses. Where only raw data were reported, we calculated unadjusted effect estimates and 95% confidence intervals using appropriate online calculators.
Risk of bias and quality assessment
Risk of bias was assessed in line with the Cochrane collaboration approach12,13. For observational studies, we considered biases due to confounding, participant selection, misclassification of variables, missing data and reverse causation. For the intervention study, we included domains relevant to RCTs (selection bias, performance bias, detection bias, attrition bias and reporting bias). Two reviewers assessed risk of bias in parallel for the first three studies, and then one reviewer assessed the remaining studies. We classified each domain as ‘High’, ‘Moderate’, ‘Low’ or ‘Unclear’ risk and, in a sensitivity analysis, removed studies with >one domain rated ‘High risk’. Publication bias was investigated when there were at least ten studies, using a funnel plot and Begg’s test14. Finally, we used Grading of Recommendations, Assessment, Development and Evaluation (GRADE)15, to estimate the quality of cumulative evidence by virus exposure and outcome (see data supplement).
Statistical analysis
Results were presented by exposure (virus type) and outcome (dementia or MCI). Exposures were subdivided into recent infection/reactivation (measured by viral nucleic acid, serum IgM, intrathecal antibody synthesis, high serum IgG titre or a recent history of clinical disease) and past infection (measured by IgG seropositivity). We meta-analysed data if there were at least two studies with the same outcome, design and detailed exposure criteria (virus type, method and site of exposure measurement). We conducted meta-analyses using both fixed and random effects models but presented final results from random effects models only for consistency: random effects models were judged more appropriate when there was at least moderate heterogeneity (I2 > 25%). Sub-group analyses e.g. by age, APOE4 status and underlying cause of dementia were considered where appropriate.
Results
Study characteristics
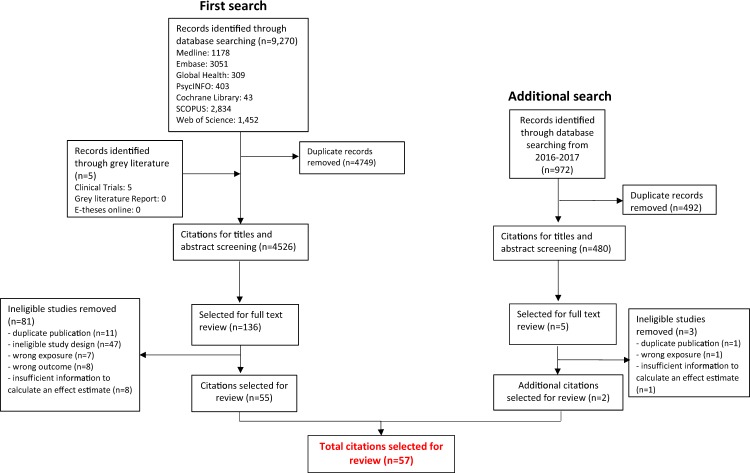
Initial searches retrieved 4,526 citations, of which 136 were selected for full-text review and 55 included. Updated searches retrieved 480 citations, of which five full texts were reviewed and two additional studies included (Fig. 1). The 57 studies (43 case-control, 13 cohort, one RCT) were published between 1974 and 2017 in various geographic settings (UK (n = 14), rest of Europe (n = 22), Far East (n = 8), USA (n = 7) and elsewhere/not reported (n = 6)). For 46 studies, the outcome was dementia alone (AD (n = 35), AIDS-related dementia (n = 5), vascular dementia (n = 2), mixed or unspecified dementia (n = 4)), five studies investigated AD and/or MCI, while the remaining six studies investigated MCI alone (e-table 1). Studies presented 163 estimates for effects of herpesviruses on dementia or MCI (119 main effects; 44 subgroup analyses: e-table 2).
Figure 1.
Flow diagram of study selection.
Effects of individual herpesviruses
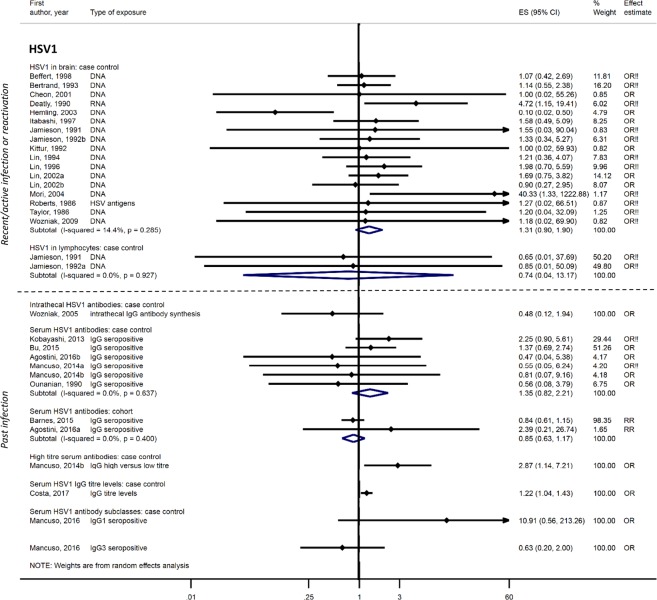
For HSV-1, 32 case-control16–47 and three cohort studies48–50 investigated associations with dementia or MCI. Two studies had no domains at high risk of bias49,50, three had one domain38,43,48 and 30 had ≥two domains at high risk of bias (e-table 3). Seventeen post-mortem case-control studies, all with at least two domains at high risk of bias, showed no evidence of a difference in detection of HSV-1 in the brains of dementia patients compared to controls: pooled OR 1.31; 95%CI, 0.90–1.90, though 14 of 17 studies had <100 participants. There was also no association between HSV1 DNA in lymphocytes (two studies)22,24, intrathecal HSV1 antibody synthesis (one study)36, HSV1 IgG seropositivity (eight studies)25,32,33,38,44,47–49 or IgG subclasses (one study)31 and dementia. One case-control study suggested HSV1 IgG titres were higher in AD patients than controls (adjusted OR 1.22; 95%CI, 1.04–1.43)20 and in a secondary analysis of another case-control study, high-titre serum IgG was associated with dementia: OR 2.87; 95%CI, 1.14–7.2232 (Fig. 2). For MCI, case-control studies showed no association with HSV-1 IgG seropositivity (three studies)25,33,47, HSV-1 IgG subclasses (one study)31, or HSV-1 detection in blood (method unspecified; one study)39 (e-Fig. 1). A population cohort with no domains at high risk of bias also showed no association between rates of cognitive decline over 4 years and HSV-1 baseline antibody titre50.
Figure 2.
Effect of herpes simplex virus type 1 infections on dementia risk.!! No adjustment/matching for age. One study assessing the effect of HSV1 in the brain (Lin, 1998) is not included here as it uses the same data as another study (Lin, 2002a); however, it has been retained for sub-group analyses.
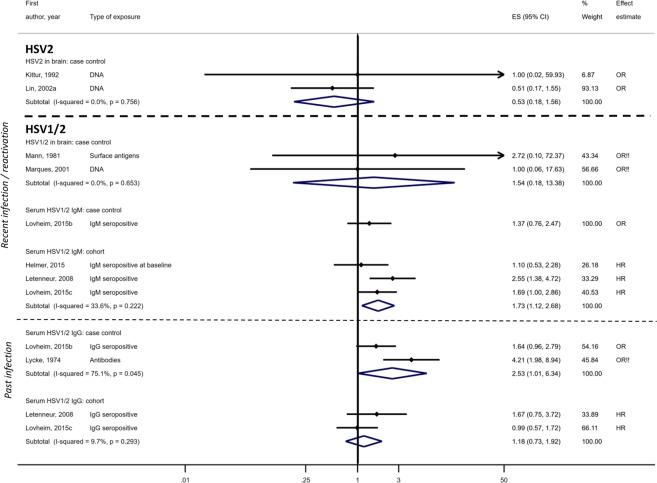
The effect of HSV-1/2 (type unspecified) was assessed in a further five case-control51–55 and three cohort studies56–58. Three had no domains at high risk of bias53,57,58, one had one domain56 and four studies had multiple domains at high risk of bias51,52,54,55. Results were mixed; HSV-1/2 in the brain was not associated with dementia in two small post-mortem case-control studies (pooled OR 1.54; 95%CI, 0.18–13.38) (overall n = 71)51,54. Serum IgM showed an association with dementia in three cohort studies (pooled OR 1·73; 95%CI, 1.12–2.68) with 0–1 domain at high risk of bias56–58 but a case-control study found no effect53. IgG seropositivity to HSV-1/2 was not associated with dementia in two cohort studies (pooled OR 1.18; 95%CI, 0.73–1.92)56,57 but had a marginal association in two case-control studies (pooled OR 2.53; 95%CI, 1.01–6.34)52,53 (Fig. 3). One study found no difference in mean HSV-1/2 serum antibody titres between dementia cases and controls (effect estimates not reported)55.
Figure 3.
Effect of herpes simplex virus type 2 or herpes simplex type unspecified on dementia risk.!! No adjustment/matching for age.
For HSV-2, there were three case-control studies29,39,42, all with ≥two domains at high risk of bias. There was no association between HSV-2 virus detected in the brain and dementia in two studies: pooled OR 0.53; 95%CI, 0.18–1.5629,42, while the third showed an association between HSV-2 (measurement unspecified) in blood and vascular MCI: unadjusted OR 4.29; 95%CI, 2.01-9.1639 (Fig. 3).
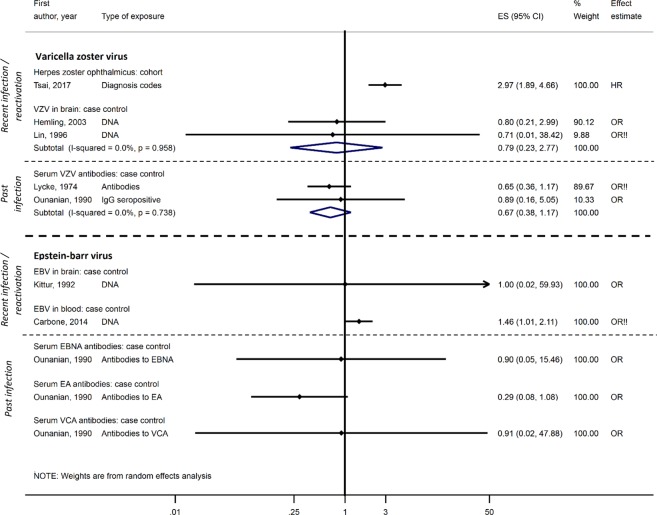
The relationship between VZV and dementia was explored in one cohort study59, with no domains at high risk of bias, and five case-control studies27,40,44,46,52, all with ≥two domains at high risk of bias. The cohort study showed a very strong association between ophthalmic zoster (VZV reactivation) and dementia: adjusted HR 2.97; 95%CI, 1.89-4.6659. Neither VZV DNA detected in post-mortem brain samples in two case-control studies (pooled OR 0.79; 95%CI, 0.23–2.77)27,40 nor VZV IgG serum antibodies from two other case-control studies (pooled OR 0.67; 95%CI, 0.38–1.17) was associated with dementia44,52 (Fig. 4). One additional case-control study reported no association between serum IgG reactivity and AD but did not present estimates46.
Figure 4.
Effect of varicella zoster virus and Epstein-Barr virus on dementia risk.!! No adjustment/matching for age.
For EBV, there were three case-control studies42,44,60, each with ≥two domains at high risk of bias. EBV DNA in the brain was not associated with dementia in one small study of post-mortem brains (n = 10): OR 1.00; 95%CI, 0.02–59.9342, while another study showed a marginal association between EBV DNA in the blood and dementia: unadjusted OR 1.46; 95%CI, 1.01–2.1160. The third study found that serum antibodies to three EBV antigens were not associated with dementia44 (Fig. 4). One additional nested case-control study with one domain at high risk of bias showed an increase in EBV IgG levels among those developing MCI over two year follow-up (p = 0.003)43.
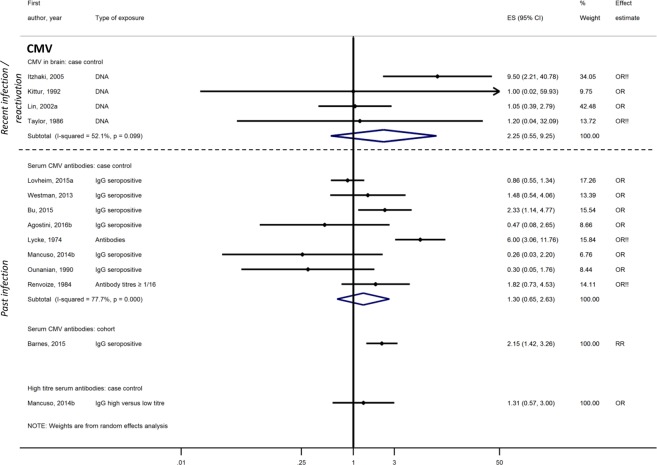
Associations between CMV and dementia or MCI were investigated in 17 case-control29,30,32,38–41,44–47,52,55,60–63 and three cohort studies49,50,64. Four studies had no domains at high risk of bias49,50,63,64, one had one domain38 and 15/20 had ≥two domains at high risk of bias. Neither CMV DNA in the brain (pooled OR from four case-control studies 2.25; 95%CI, 0.55–9.24)29,41,42,45, CMV-specific serum IgG (pooled OR from eight case-control studies 1.30; 95%CI, 0.65–2.63)32,38,44,47,52,61–63, nor high CMV antibody titres (one case-control study)32 were associated with dementia. Three additional case-control studies compared blood CMV IgG titres between dementia cases and controls but did not present effect estimates; two reported no difference46,55 and one found that AD patients had higher mean antibody titres than controls (p = 0.014)60. CMV IgG seropositivity at baseline was associated with AD in one cohort with no domains at high risk of bias: RR 2.15; 95%CI, 1.42–3.2749 (Fig. 5). For MCI, one large Mexican-American population cohort with no domains at high risk of bias reported a decline in MMSE score associated with a one unit increase in serum CMV IgG titre (p < 0.001) and a marked difference between MMSE scores of participants with the highest and lowest antibody titres (p = 0.003)50. In contrast, a smaller Japanese community cohort study, also with no domains at high risk of bias, showed no association between quartiles of CMV titre and cognitive function64. One case-control study found no association between IgG seropositivity and MCI47 while another case-control study reported that CMV in blood (detection method unclear) was associated with MCI in unadjusted analysis: OR 2.17; 95%CI, 1.28–3.6739.
Figure 5.
Effect of cytomegalovirus on dementia risk.!! No adjustment/matching for age.
The relationship between HHV-6 and dementia or MCI was explored in eight case-control studies29,30,36,40,41,46,47,60, all with ≥two domains at high risk of bias and one cohort study, which had one domain at high risk of bias48. HHV-6 DNA detection in the brain was associated with dementia in three case-control studies: pooled OR 2.47; 95%CI, 1.25–4.8629,30,40, and HHV-6 DNA in blood had a marginal association with dementia in data pooled across two further case-control studies: pooled OR 1.22; 95%CI, 1.01–1.4746,60. There was no association between HHV-6 intrathecal antibody synthesis and dementia in two case-control studies36,47, or between HHV-6 IgG seropositivity and dementia in the cohort study48 (e-Fig. 2). One case-control study suggested a negative association between HHV6 seropositivity and MCI: OR 0.32; 95%CI, 0.11–0.9747.
For HHV-8 (and/or Kaposi’s sarcoma (KS)) there were two case-control65,66 and four cohort studies67–70, all carried out among people with HIV. Two studies had no domains at high risk of bias66,70, two had one domain68,69 and two had two domains at high risk of bias65,67. HHV-8 IgG seropositivity was not associated with dementia in either a case-control study: OR 1.22; 95%CI, 0.66-2.2766 or two cohort studies: pooled OR 0.79; 95%CI, 0.37–1.6969,70, all with 0–1 domain at high risk of bias. KS was not associated with dementia in one case-control study65 and had weak evidence of an apparent protective effect against dementia in three cohorts: pooled OR 0.58; 95%CI, 0.33–1.0567,68,70 (e-Fig. 2).
One cohort study, with no domains at high risk of bias, investigated the effect of infection with multiple herpesviruses71. IgG seropositivity to HSV-1, HSV-2 and CMV was associated with an increased risk of cognitive decline compared to infection with 0–1 virus: OR 2.3; 95%CI, 1.1–5.0, however having two herpesviruses compared to 0–1 was not associated with cognitive decline: OR 1.8; 95%CI, 0.9–3.6.
One RCT72 and one cohort study58, each with no domains at high risk of bias, investigated the effect of antiviral treatment for HSV-1 on cognition or dementia. The RCT showed that using valacyclovir for 90 days in patients with HSV encephalitis had no effect on cognitive sequelae at 12 months (adjusted OR 0.81; 95%CI, 0.24–2.77)72. The population cohort study showed that incident dementia rates were similar for people with and without reimbursement claims data for anti-herpetic medication: adjusted HR 0.75, no CIs presented, p = 0.1458.
In sub-group analyses, no differences by age or sex were seen in studies of HSV-1, HSV-1/2, VZV reactivation or HHV-8. Several studies of HSV-1, HSV-1/2, VZV and HHV-6 stratified by APOE4 status, but there were no consistent patterns. Stratifying by dementia subtype gave similar effects to those presented in the main meta-analysis. Finally, removing studies at the highest risk of bias did not result in major differences to findings (e-Figs A1–A3). We did not find evidence of publication bias for studies of HSV-1 DNA in the brain and dementia (p = 0.88) (e-Fig. 4).
Overall, the quality of evidence for VZV reactivation on dementia was judged to be moderate, whilst for every other herpesvirus exposure and dementia or MCI, the quality of evidence was judged to be very low using GRADE criteria, due to serious or very serious risks of bias, inconsistency and imprecision (e-table 4).
Discussion
Our systematic review showed that, while past herpesvirus infection was generally not associated with dementia risk, there were tentative associations between recent infection with, or reactivation of, several herpesviruses and dementia or MCI, though quality of evidence across the 57 included studies was very low. For HSV-1, HSV-1/2, and HSV-2 there was no evidence that DNA in the brain was associated with dementia and little robust evidence that HSV-1 or HSV-1/2 IgG seropositivity was associated with the risk of dementia or MCI. Although some studies suggested that recent infection or reactivation of HSV-1/2, measured by serum IgM or high titre serum IgG, was associated with dementia, results were inconsistent and evidence graded very low quality. Moderate quality evidence from a single study indicated that clinically-diagnosed VZV reactivation (specifically ophthalmic zoster) was associated with increased risk of dementia, while VZV infection or reactivation from brain or serum samples was not associated with dementia. Findings for EBV and CMV were inconsistent and rated very low quality. Although most studies reported no association with dementia, serum IgG CMV seropositivity was associated with dementia in one cohort, and CMV IgG titres with rates of cognitive decline in another large population cohort, but not in a smaller cohort of the very old. Very low quality evidence suggested that HHV-6 recent infection/reactivation, but not past HHV-6 or HHV-8 infection, was associated with dementia risk.
Human herpesviruses establish latency and persist lifelong after initial infection. Our finding that past infection alone was insufficient to raise dementia risk is consistent with current understanding of neurotropic herpesvirus latency, during which viruses such as HSV-1 and VZV show very restricted gene expression and do not produce infective virus particles or induce clinical disease73. Virus reactivation leading to systemic inflammation, glial activation and neuro-inflammation is a putative mechanism to explain our finding that recent infection/reactivation of several herpesviruses may be associated with cognitive decline or dementia74. For the most robust relationship identified in our review – that of ophthalmic zoster with dementia – localised vasculopathy, which occurs when VZV replicates in cerebral arteries75, may contribute to pathogenesis. For other viruses, potential mechanisms are less well-understood. While recent work showing β-amyloid seeding in response to HSV-1 and HHV-6 infection in mouse models and human neuronal cell cultures supports an anti-microbial peptide role for β-amyloid76, the significance for cognitive function in older adults is unknown. A disordered immune response to infections among older people, in part driven by CMV-induced immune system remodelling, has been proposed to promote development of inflammatory disorders such as autoimmune conditions and, potentially, dementia5.
Strengths of our review include the comprehensive search strategy covering seven medical databases as well as clinical trials registers and grey literature for articles in any language, setting or time period. We pre-specified and published our analysis plan11, which included careful consideration of methods of herpesvirus measurement to avoid inappropriate meta-analyses across heterogeneous exposures. Contact with authors and double reviewing throughout identified several instances of duplicate data published in different conference abstracts, which we excluded from meta-analyses. We also conducted a thorough risk of bias assessment for included studies and assessed the quality of evidence overall for each herpesvirus exposure to guide future research. Nevertheless, the strength of evidence is limited by the poor quality of many studies. This included bias associated with difficulty measuring herpesvirus exposures, other than past infection (denoted by serum IgG). Understanding of the nature of sub-clinical herpesvirus reactivation is limited and there is a dearth of standardised methods to assess viral activation. Even markers such as serum IgM, which is known to be associated with recent infection or reactivation, lack sensitivity. It is also unclear whether peripheral virus reactivation correlates with central nervous system reactivation. While some studies detected herpesvirus nucleic acid in post-mortem brains, it is unclear whether this reflects reactivation or peri-mortem changes associated with neurodegeneration. Our outcome definitions were intentionally broad and likely incorporated heterogeneous neuropathologies leading to dementia, although we did not see differences in results stratified by dementia sub-type. Nevertheless, a lack of consistency of dementia definitions and variable reporting of clinical outcomes makes between-study comparisons challenging. In general, our review found a lack of longitudinal studies with repeated measures of exposure, a lack of control for confounding e.g. by age and sex and many small studies that were underpowered to detect an effect.
In contrast to our findings, one previous systematic review and meta-analysis of 35 studies of herpesviruses and dementia reported an association between any herpesvirus in the brain and AD, and specific associations between HSV-1 and EBV with AD9. However, the virus-specific results were pooled across any method of virus detection (PCR, in-situ hybridization, serum IgG, serum IgM) at any site (blood, brain or CSF) and study quality was not assessed systematically. We found no other systematic reviews or meta-analyses of individual herpesviruses and dementia. Two cohort studies from Taiwan using the National Health Insurance Database published after the dates of our search strengthened our findings. One study reported that herpes zoster was associated with a small increase in subsequent dementia risk – adjusted HR 1.11; 95% CI, 1.04–1.1777. The other assessed the association between clinical presentation of new HSV symptoms (either oral or genital) and dementia in patients aged ≥50 years and found an increased risk of incident dementia (adjusted HR 2.56; 95% CI, 2.35–2.80), although the effect was attenuated after removing dementia diagnoses made in the first year after HSV, which were unlikely to be causally linked (adjusted HR 1.65; 95% CI; 1.44–1.87)78. In both studies, antiviral prescriptions after herpesvirus diagnosis were associated with lower dementia rates, although this warrants further investigation.
Our findings that larger longitudinal studies supported a possible association between CMV and dementia or cognitive decline are also consistent with literature highlighting the role of CMV in stimulating ‘inflammaging’ – a state of low-grade chronic inflammation occurring in older individuals which predisposes to a range of adverse health outcomes79. Several studies have investigated the effect of infectious burden on cognition. Cross-sectional analyses show that a greater infectious burden, measured by IgG seropositivity to a range of antigens (including but not limited to herpesviruses), is associated with poorer cognitive performance in young to middle-aged adults80 and with MCI in older adults81 after adjusting for sociodemographic and clinical confounders. One longitudinal study showed a similar association between infectious burden and cognitive decline in the memory domain82.
Our review highlights the complexity of studying the effects of viruses typically acquired in childhood on syndromes such as MCI and dementia, which have long latent periods and are associated with subtle behavioural, nutritional and immune changes that may occur many years before diagnosis. Nevertheless, knowledge of the mechanisms triggering dementia onset remains limited. Herpesviruses are common and a potentially tractable target for prevention with vaccines or treatment: the alpha herpesviruses (HSV-1, HSV-2 and VZV) in particular have rapid replication cycles and are susceptible to antiviral agents such as the synthetic nucleoside analogue acyclovir and related drugs. Although no effect of antiviral therapy on cognitive outcomes was seen in an RCT conducted in a population with herpes simplex encephalitis72, cognitive sequelae may be driven by encephalitis itself rather than any original causative organism83. The effect of antiviral therapy on cognitive outcomes in other HSV infected populations therefore remains unclear. While genetically influenced, herpesvirus acquisition is also socially-patterned, e.g. for CMV, individuals of lower socio-economic status are more likely to become infected earlier and to have higher antibody titres throughout life77, possibly reflecting a greater propensity to reactivation.
Future research should focus on high quality population studies using longitudinal designs to investigate effects of infections on long-term health outcomes such as dementia, ideally using repeated measures taken across the life course. For studies of herpesviruses, methods and timing of measurement are critical; strategies may include active surveillance in population cohort studies for viruses under-reported in electronic health records. For maximum benefit to policymakers and clinicians, future research should seek to understand when and in whom herpesvirus reactivation is associated with greatest risk of adverse health outcomes, to guide targeted intervention strategies.
Supplementary information
Acknowledgements
C.W.G. is supported by a Wellcome Intermediate Clinical Fellowhip (201440_Z_16_Z). B.H.R. and M.N.R. are supported by NIHR University College London Hospitals Biomedical Research Centre.
Author Contributions
C.W.G., H.J.F., J.B., A.C.H., A.M., B.H.R., M.N.R., S.L.T. and L.S. conceptualised the study and contributed to the published protocol. E.W. advised on statistical methods for meta-analysis. C.W.G. and H.J.F. reviewed all titles, abstracts and full texts to select studies for inclusion. C.W.G. and H.J.F. extracted data and assessed risk of bias for the first three studies in parallel. H.J.F. then extracted and meta-analysed data and carried out the remaining quality assessments. C.W.G. wrote the first draft. C.W.G., H.J.F., E.W., J.B., A.C.H., A.M., B.H.R., M.N.R., S.L.T. and L.S. interpreted findings, reviewed further drafts and approved the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-41218-w.
References
- 1.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 2.Prince M, et al. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23. doi: 10.1186/s13195-016-0188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solito E, Sastre M. Microglia Function in Alzheimer’s Disease. Front Pharmacol. 2012;3:14. doi: 10.3389/fphar.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7(2):161–7. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 5.Pereira BI, Akbar AN. Convergence of Innate and Adaptive Immunity during Human Aging. Front Immunol. 2016;7:445. doi: 10.3389/fimmu.2016.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes simplex virus infection causes cellular β-amyloid accumulation and secretase upregulation. Neurosci Lett. 2007;429(2–3):95–100. doi: 10.1016/j.neulet.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 7.De Chiara G, et al. APP Processing Induced by Herpes Simplex Virus Type 1 (HSV-1) Yields Several APP Fragments in Human and Rat Neuronal Cells. Blaho JA, editor. PLoS ONE. 2010;5(11):e13989. doi: 10.1371/journal.pone.0013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alvarez G, Aldudo J, Alonso M, Santana S, Valdivieso F. Herpes simplex virus type 1 induces nuclear accumulation of hyperphosphorylated tau in neuronal cells. J Neurosci Res. 2012;90(5):1020–9. doi: 10.1002/jnr.23003. [DOI] [PubMed] [Google Scholar]
- 9.Steel AJ, Eslick GD. Herpes Viruses Increase the Risk of Alzheimer’s Disease: A Meta-Analysis. J Alzheimers Dis. 2015;47(2):351–64. doi: 10.3233/JAD-140822. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren-Gash C, et al. Association between human herpesvirus infections and dementia or mild cognitive impairment: a systematic review protocol. BMJ Open. 2017;7(6):e016522. doi: 10.1136/bmjopen-2017-016522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JPT, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JA, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 15.Atkins D, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beffert U, Bertrand P, Champagne D, Gauthier S, Poirier J. HSV-1 in brain and risk of Alzheimer’s disease. Lancet. 1998;351(9112):1330–1. doi: 10.1016/S0140-6736(05)79057-7. [DOI] [PubMed] [Google Scholar]
- 17.Bertrand P. Distribution of herpes simplex virus type1 DNA in selected areas of normal and Alzheimer’s disease brains: a PCR study. Neurodegeneration. 1993;2:201–8. [Google Scholar]
- 18.Cheon MS, Bajo M, Gulesserian T, Cairns N, Lubec G. Evidence for the relation of herpes simplex virus type 1 to Down syndrome and Alzheimer’s disease. Electrophoresis. 2001;22(3):445–8. doi: 10.1002/1522-2683(200102)22:3<445::AID-ELPS445>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Deatly AM, Haase AT, Fewster PH, Lewis E, Ball MJ. Human herpes virus infections and Alzheimer’s disease. Neuropathol Appl Neurobiol. 1990;16(3):213–23. doi: 10.1111/j.1365-2990.1990.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 20.Costa AS, et al. Modulation of Immune Responses to Herpes Simplex Virus Type 1 by IFNL3 and IRF7 Polymorphisms: A Study in Alzheimer’s Disease. J Alzheimers Dis. 2017;60(3):1055–63. doi: 10.3233/JAD-170520. [DOI] [PubMed] [Google Scholar]
- 21.Itabashi S, Arai H, Matsui T, Higuchi S, Sasaki H. Herpes simplex virus and risk of Alzheimer’s disease. Lancet. 1997;349(9058):1102. doi: 10.1016/S0140-6736(05)62325-2. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson GA, Maitland NJ, Craske J, Wilcock GK, Itzhaki RF. Detection of herpes simplex virus type 1 DNA sequences in normal and Alzheimer’s disease brain using polymerase chain reaction. Biochem Soc Trans. 1991;19(2):122S. doi: 10.1042/bst019122s. [DOI] [PubMed] [Google Scholar]
- 23.Jamieson GA, Maitland NJ, Wilcock GK, Yates CM, Itzhaki RF. Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J Pathol. 1992;167(4):365–8. doi: 10.1002/path.1711670403. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson G, Maitland N, Itzhaki R. Herpes simplex virus type-1 DNA sequences are present in aged normal and Alzheimer’s disease brain but absent in lymphocytes. Arch Gerontol Geriatr. 1992;3(Suppl):197–202. doi: 10.1016/s0167-4943(05)80019-6. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi N, et al. Increase in the IgG avidity index due to herpes simplex virus type 1 reactivation and its relationship with cognitive function in amnestic mild cognitive impairment and Alzheimer’s disease. Biochem Biophys Res Commun. 2013;430(3):907–11. doi: 10.1016/j.bbrc.2012.12.054. [DOI] [PubMed] [Google Scholar]
- 26.Lin W, Graham J, MacGowan S, Wilcock G, Itzhaki R. Alzheimer’s disease, herpes virus in the brain, apolipoprotein E4 and herpes labialis. Alzheimers Rep. 1998;1(4):274. [Google Scholar]
- 27.Lin WR, Shang D, Itzhaki RF. Neurotropic viruses and Alzheimer disease. Interaction of herpes simplex type 1 virus and apolipoprotein E in the etiology of the disease. Mol Chem Neuropathol. 1996;28(1–3):135–41. doi: 10.1007/BF02815215. [DOI] [PubMed] [Google Scholar]
- 28.Lin W, Jamieson G, Itzhaki R. The possible role of herpes-simplex virus type-1 in the etiology of Alzheimers-disease. Neurobiol Aging. 1994;15:S47. [Google Scholar]
- 29.Lin W-R, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. Herpesviruses in brain and Alzheimer’s disease. J Pathol. 2002;197(3):395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- 30.Lin W-R, Wozniak MA, Wilcock GK, Itzhaki RF. Cytomegalovirus is present in a very high proportion of brains from vascular dementia patients. Neurobiol Dis. 2002;9(1):82–7. doi: 10.1006/nbdi.2001.0465. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso, R. et al. HSV-1 specific IgG subclasses in individuals with Alzheimer’s disease. Alzheimers Dement. 12(7 (supplement)), P1052–1053 (2016).
- 32.Mancuso R, et al. Titers of herpes simplex virus type 1 antibodies positively correlate with grey matter volumes in Alzheimer’s disease. J Alzheimers Dis. 2014;38(4):741–5. doi: 10.3233/JAD-130977. [DOI] [PubMed] [Google Scholar]
- 33.Mancuso R, et al. Relationship between herpes simplex virus-1-specific antibody titers and cortical brain damage in Alzheimer’s disease and amnestic mild cognitive impairment. Front Aging Neurosci. 2014;6:285. doi: 10.3389/fnagi.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori I, et al. Reactivation of HSV-1 in the brain of patients with familial Alzheimer’s disease. J Med Virol. 2004;73(4):605–11. doi: 10.1002/jmv.20133. [DOI] [PubMed] [Google Scholar]
- 35.Roberts GW, et al. Herpes simplex virus: a role in the aetiology of Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 1986;49(2):216. doi: 10.1136/jnnp.49.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wozniak MA, Shipley SJ, Combrinck M, Wilcock GK, Itzhaki RF. Productive herpes simplex virus in brain of elderly normal subjects and Alzheimer’s disease patients. J Med Virol. 2005;75(2):300–6. doi: 10.1002/jmv.20271. [DOI] [PubMed] [Google Scholar]
- 37.Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J Pathol. 2009;217(1):131–8. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 38.Bu X-L, et al. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol. 2015;22(12):1519–25. doi: 10.1111/ene.12477. [DOI] [PubMed] [Google Scholar]
- 39.Deng Y-M, et al. A Case-Control Study: Infectious Burden Increased the Occurrence of Vascular Cognitive Impairment No Dementia. CNS Neurosci Ther. 2016;22(12):1012–4. doi: 10.1111/cns.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemling N, et al. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann Neurol. 2003;54(2):267–71. doi: 10.1002/ana.10662. [DOI] [PubMed] [Google Scholar]
- 41.Itzhaki R, Wozniak M, Wilcock G, Lin W. The role of viruses in dementia: Detection of cytomegalovirus DNA in brain of vascular dementia patients. J Neurol Sci. 2005;229:344–5. [Google Scholar]
- 42.Kittur SD, et al. A molecular hybridization study for the presence of Herpes simplex, cytomegalovirus and Epstein-Barr virus in brain and blood of Alzheimer’s disease patients. Arch Gerontol Geriatr. 1992;15(1):35–41. doi: 10.1016/0167-4943(92)90038-6. [DOI] [PubMed] [Google Scholar]
- 43.Shim S-M, et al. Elevated Epstein-Barr Virus Antibody Level is Associated with Cognitive Decline in the Korean Elderly. J Alzheimers Dis. 2017;55(1):293–301. doi: 10.3233/JAD-160563. [DOI] [PubMed] [Google Scholar]
- 44.Ounanian A, Guilbert B, Renversez JC, Seigneurin JM, Avrameas S. Antibodies to viral antigens, xenoantigens, and autoantigens in Alzheimer’s disease. J Clin Lab Anal. 1990;4(5):367–75. doi: 10.1002/jcla.1860040510. [DOI] [PubMed] [Google Scholar]
- 45.Taylor GR, Crow TJ. Viruses in human brains: a search for cytomegalovirus and herpes virus 1 DNA in necropsy tissue from normal and neuropsychiatric cases. Psychol Med. 1986;16(2):289–95. doi: 10.1017/s0033291700009107. [DOI] [PubMed] [Google Scholar]
- 46.Westman G, et al. Decreased HHV-6 IgG in Alzheimer’s Disease. Front Neurol. 2017;8:40. doi: 10.3389/fneur.2017.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agostini S, et al. Lack of evidence for a role of HHV-6 in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis. 2016;49(1):229–35. doi: 10.3233/JAD-150464. [DOI] [PubMed] [Google Scholar]
- 48.Agostini S, et al. High avidity HSV-1 antibodies correlate with absence of amnestic Mild Cognitive Impairment conversion to Alzheimer’s disease. Brain Behav Immun. 2016;58:254–60. doi: 10.1016/j.bbi.2016.07.153. [DOI] [PubMed] [Google Scholar]
- 49.Barnes LL, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis. 2015;211(2):230–7. doi: 10.1093/infdis/jiu437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aiello AE, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54(7):1046–54. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 51.Marques AR, et al. Lack of association between HSV-1 DNA in the brain, Alzheimer’s disease and apolipoprotein E4. J Neurovirol. 2001;7(1):82–3. doi: 10.1080/135502801300069773. [DOI] [PubMed] [Google Scholar]
- 52.Lycke E, Norrby R, Roos BE. A serological study on mentally ill patients with particular reference to the prevalence of herpes virus infections. Br J Psychiatry J Ment Sci. 1974;124(0):273–9. doi: 10.1192/bjp.124.3.273. [DOI] [PubMed] [Google Scholar]
- 53.Lövheim H, et al. Herpes simplex infection and the risk of Alzheimer’s disease-A nested case-control study. Alzheimers Dement. 2015;11(6):587–92. doi: 10.1016/j.jalz.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 54.Mann DM, Yates PO, Davies JS, Hawkes J. Viruses, Parkinsonism and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1981;44(7):651. doi: 10.1136/jnnp.44.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renvoize EB, Awad IO, Hambling MH. A sero-epidemiological study of conventional infectious agents in Alzheimer’s disease. Age Ageing. 1987;16(5):311–4. doi: 10.1093/ageing/16.5.311. [DOI] [PubMed] [Google Scholar]
- 56.Letenneur L, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer’s disease: a population-based cohort study. PloS One. 2008;3(11):e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lövheim H, Gilthorpe J, Adolfsson R, Nilsson L-G, Elgh F. Reactivated herpes simplex infection increases the risk of Alzheimer’s disease. Alzheimers Dement. 2015;11(6):593–9. doi: 10.1016/j.jalz.2014.04.522. [DOI] [PubMed] [Google Scholar]
- 58.Helmer C, et al. Herpes simplex virus, anti-herpetic medication, and dementia: Results from the Three-city population-based cohort. Alzheimers Dement. 2015;1:P153. [Google Scholar]
- 59.Tsai M-C, et al. Increased risk of dementia following herpes zoster ophthalmicus. PloS One. 2017;12(11):e0188490. doi: 10.1371/journal.pone.0188490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carbone I, et al. Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging. 2014;35(1):122–9. doi: 10.1016/j.neurobiolaging.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 61.Westman G, et al. Decreased proportion of cytomegalovirus specific CD8 T-cells but no signs of general immunosenescence in Alzheimer’s disease. PloS One. 2013;8(10):e77921. doi: 10.1371/journal.pone.0077921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renvoize EB, Hambling MH. Cytomegalovirus infection and Alzheimer’s disease. Age Ageing. 1984;13(4):205–9. doi: 10.1093/ageing/13.4.205. [DOI] [PubMed] [Google Scholar]
- 63.Lövheim H, et al. Cytomegalovirus infection does not increase the risk of alzheimer’s disease. Neurodegener Dis. 2015;15:988. [Google Scholar]
- 64.Kawasaki M, et al. Carotid atherosclerosis, cytomegalovirus infection, and cognitive decline in the very old: a community-based prospective cohort study. Age Dordr Neth. 2016;38(2):29. doi: 10.1007/s11357-016-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dupin N, et al. Relationship between Kaposi’s sarcoma, Kaposi’s sarcoma-associated herpesvirus and AIDS dementia complex. AIDS. 2000;14(3):333–5. doi: 10.1097/00002030-200002180-00023. [DOI] [PubMed] [Google Scholar]
- 66.Polk S, et al. A case-control study of HIV-1-related dementia and co-infection with HHV-8. Neurology. 2002;59(6):950–3. doi: 10.1212/wnl.59.6.950. [DOI] [PubMed] [Google Scholar]
- 67.Dore GJ, Grulich A, Law MG, Brew BJ, Kaldor JM. Kaposi’s sarcoma and protection from AIDS dementia complex. AIDS. 1998;12(17):2354–5. [PubMed] [Google Scholar]
- 68.Mary-Krause M, Billaud E. Impact of Kaposi’s sarcoma in occurrence of opportunistic central nervous system disease. Clinical Epidemiology Group. AIDS. 1999;13(14):1985–7. doi: 10.1097/00002030-199910010-00028. [DOI] [PubMed] [Google Scholar]
- 69.Renwick N, et al. Kaposi’s sarcoma and human herpesvirus 8 infection do not protect HIV-1 infected homosexual men from AIDS dementia complex. AIDS. 2001;15(16):2165–9. doi: 10.1097/00002030-200111090-00012. [DOI] [PubMed] [Google Scholar]
- 70.Rezza G, et al. Does HHV-8 have a protective role on the development of HIV encephalopathy? Italian HIV-Seroconversion Study. Neurology. 1999;53(9):2032–6. doi: 10.1212/wnl.53.9.2032. [DOI] [PubMed] [Google Scholar]
- 71.Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34(9):2126–31. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- 72.Gnann JW, et al. Herpes Simplex Encephalitis: Lack of Clinical Benefit of Long-term Valacyclovir Therapy. Clin Infect Dis. 2015;61(5):683–91. doi: 10.1093/cid/civ369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rovnak J, Kennedy PGE, Badani H, Cohrs RJ. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J Gen Virol. 2015;96(7):1581–602. doi: 10.1099/vir.0.000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bronzuoli MR, Iacomino A, Steardo L, Scuderi C. Targeting neuroinflammation in Alzheimer’s disease. J Inflamm Res. 2016;9:199–208. doi: 10.2147/JIR.S86958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eimer WA, et al. Alzheimer’s disease-associated B-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99:56–63. doi: 10.1016/j.neuron.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen, V. C. et al. Herpes zoster and dementia: a nationwide population-based cohort study. J Clin Psychiatry. 79(1): pii: 16m11312 (2018). [DOI] [PubMed]
- 78.Tzeng NS, et al. Anti-herpetic medications and reduced risk of dementia in patients with herpes simplex infections – a nationawide, population-based cohort study in Taiwan. Neurotherapeutics. 2018;15:417–419. doi: 10.1007/s13311-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aiello AE, Chiu Y-L, Frasca D. How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? GeroScience. 2017;39(3):261–71. doi: 10.1007/s11357-017-9983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gale SD, Erickson LD, Berrett A, Brown BL, Hedges DW. Infectious disease burden and cognitive function in young to middle-aged adults. Brain Behav Immun. 2016;52:161–8. doi: 10.1016/j.bbi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 81.Katan M, et al. Infectious burden and cognitive function: the Northern Manhattan Study. Neurology. 2013;80(13):1209–15. doi: 10.1212/WNL.0b013e3182896e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wright CB, et al. Infectious Burden and Cognitive Decline in the Northern Manhattan Study. J Am Geriatr Soc. 2015;63(8):1540–5. doi: 10.1111/jgs.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Granerod, J. et al. Increased rates of sequelae post-encephalitis in individuals attending primary care practices in the United Kingdom: a population-based retrospective cohort study. J Neurol. 264(2), 407–15 (1Feb 2017). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.