Abstract
Over the past 30 years, the genus Solanum has received considerable attention in chemical and biological studies. Solanum is the largest genus in the family Solanaceae, comprising of about 2000 species distributed in the subtropical and tropical regions of Africa, Australia, and parts of Asia, e.g., China, India and Japan. Many of them are economically significant species. Previous phytochemical investigations on Solanum species led to the identification of steroidal saponins, steroidal alkaloids, terpenes, flavonoids, lignans, sterols, phenolic comopunds, coumarins, amongst other compounds. Many species belonging to this genus present huge range of pharmacological activities such as cytotoxicity to different tumors as breast cancer (4T1 and EMT), colorectal cancer (HCT116, HT29, and SW480), and prostate cancer (DU145) cell lines. The biological activities have been attributed to a number of steroidal saponins, steroidal alkaloids and phenols. This review features 65 phytochemically studied species of Solanum between 1990 and 2018, fetched from SciFinder, Pubmed, ScienceDirect, Wikipedia and Baidu, using “Solanum” and the species’ names as search terms (“all fields”).
Keywords: Solanum, Solanaceae, Phytochemistry, Steroidal saponins and alkaloids, Ethnopharmacology
Introduction
The genus Solanum is considered to be one of the largest and most complex genera among the Angiosperms [1], and the most representative and largest genus of the family Solanaceae [1–4]. It is comprised of about 2000 species distributed across subtropical and tropical regions of Asia [3–9], tropical Africa [10–29], non-arid Africa [30–43], Americas [44–87], Australia [71–74, 81–84] and India [71]. The genus is well represented in Brazil with about 350 species widely distributed from north to south in diverse phytogeographic regions [70, 80]. In Brazil (Ceará, Bahia, Mato Grosso do Sul, Paraná and north-central coast of Santa Catarina State), many Solanum species, usually known as ‘yubeba’, the word that refers to the prickles found on the stems of several of the species, are widely used in traditional medicine [66, 80, 87]. In the northeast of Brazil, 80 Solanum species are distributed throughout the region and used in folk medicine. One of such species is S. capsicoides, commonly known as “Gogoia” [87]. In East Africa, several Solanum species such as S. arundo and S. incanum are known to be poisonous and are reportedly used to induce miscarriages [64].
Solanum genus is rich in economically significant species; the food crops include S. aethiopicum [20, 21], S. anguivi [30, 31] S. lycopersicum, S. melongena, S. muricatum, S. torvum and S. tuberosum [1]. Ornamental species include S. aviculare, S. capsicastrum, S. crispum, S. laciniatum, S. laxum, S. pseudocapsicum, S. rantonnetii, S. seaforthianum and S. wendlandii [1].
A series of pharmacological studies have been carried out to verify and validate the traditional medicinal applications of many plants in this genus. The studied pharmacological activities include analgesic, anthelminthic, antiallergic, anti-anemic, anti-asthmatic, antibacterial, anti- cancer, anti-convulsant, anti-depressant, anti-diabetic, anti-fungal, antihistaminic, antihyperten- sive, anti-inflammatory, anti-leishmanial, antimelanogenetic, anti-molluscicidal, anti-nociceptive, anti-psoriatic, antiplasmodial, antiprotozoa, anti-trypanosomal, antiurolithiatic, antiviral, cardio- vascular, diuretic, hepatoprotective, hypolipidemic, mosquito larvicidal, nephrotoxic, spasmolytic, schistosomicidal and vasorelaxant activities.
In the past, several reviews on Solanum genus have been documented [88–101], however, mostly with singular focus on particular species. The present review is multi faceted, and features 66 medicinal species of Solanum in their geographical distribution, traditional uses, and 670 isolated chemical constituents, including 134 steroidal saponins, 63 steroidal alkaloids, 13 pregnane glycosides, 128 terpenes, 75 flavonoids, 31 lignans, 31 other types of alkaloids, 66 sterols, 52 phenolic compounds, 20 coumarins and coumestans, 4 coumarinolignoids, 23 fatty acids and esters and 30 other compounds. Where applicable, the biological activities of compounds isolated from various species are noted.
Distribution and Ethnopharmacological Uses
Sixty-six species commonly used as important folk medicine, ornamental plants, or wild food sources were selected in this review, and their local names, distribution and ethnopharmacologi- cal uses were summarized in Table 1. Local names are given in different languages with which the inhabitants of a particular region use to identify a specific species. Each species’ natural habitat and/or places of cultivation are mentioned. Traditional as well as modern day applications are presented.
Table 1.
Distribution and ethnopharmalogical uses of Solanum species
| No. | Species | Local names | Distribution | Uses |
|---|---|---|---|---|
| 1 | S. abutiloides | Dwarf tamarillo | Argentina, Bolivia [2, 3] | Ornamental, fruits edible, anti-fungal [2–4] |
| 2 | S. aculeastrum | Goat bitter/poison/gifa/bok-bitter -apple, thola, murulwa, umthuma, itunga, mtuma | Kenya, South Africa, Swaziland [10] | Toothache, ringworm [10], jigger wounds, gonorrhea, anti-molluscicidal [11, 12], anticancer [13–15], antifungal [16], antimicrobial [12, 17], anti-leishmanial [18] |
| 3 | S. aethiopicum | African scarlet/Ethiopian/Chinese scarlet/tomato-fruit eggplant, azoko, garden egg, gilo, golden/love apple, impwa, kumba, losuke, mock/bitter/ruffed tomato, nakasuga, nakati, ngogwe, osun, tokalu, african aubergine, aubergine amère, Ethiopian nightshade, gilo, granadillo, jilo, kumba, meloncillo de olor, meloncillo del campo, pocotillo, quillo, revienta caballo, röd aubergin, shum, silverleaf nightshade, tutía enano | China, India, Japan, Angola, Benin, Botswana,Burkina Faso, Burundi, Cameroon, Cape Verde, Central Africa, Chad, Comoros, Congo DR, Djibouti, Egypt, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Senegal, Sierra Leone, Sudan, Togo, Zambia, Zimbabwe, Australia, Brazil, Italy, France [20, 21] | Fruits/leaves eaten, ornamental [20, 21], anti-ulcer, anticancer [23–26], anti-inflammatory [27] |
| 4 | S. agrarium | Gogóia (Brazil) | Brazil, Guyana, Venezuela [44] | Mycosis, diarrhea, gonorrhea, prostatic, inflammation, abortion [44, 45] |
| 5 | S. americanum | American black/white/small flower/glossy nightshade, maria pretinha (Brazil), quilete (Guatemala), popolo (Hawaii) | Tropical Pacific, Indian Ocean, Hawaii, Indochina, Brazil, Madagascar, Africa | Ripe fruit makes jams, preservative, shoots eaten, antiviral, antimicrobial [46, 47], antidiabetic [48, 49], bladder spasm, joint pains, cooling, cough, gastric ulcer, protozoal infections, vermifuge [49], anticancer [47, 50–52], asthma [53] |
| 6 | S. amygdalifolium | Uruguay, Argentina, Brazil | Decoration [56] | |
| 7 | S. anguivi | Forest bitterberry, African eggplant | Non-arid Africa: Nigeria, Ghana | Leaves/fruits consumed, coughs, dysuria, nasal ulcers, asthma, toothache, cardiac disorder, worm complaints, spinal chord and nervous disorder, fever, diabetes, artherosclerosis carminative, nasal ulcers, asthma, parturition, worm expeller, itching [30–32], hypolipidemic [33, 34], anaemia [31, 32, 35], Huntington’s, Alzheimer, Parkinson, amyotrophic lateral sclerosis [36], antioxidant [33, 37–39], hypotensive [38] |
| 8 | S. arboreum | Costa Rica, Colombia, Trinidad | Anti-leishmanial [60, 61], antimalarial [62] | |
| 9 | S. arundo | Kenya | Abortion [64], hepatoprotective [65] | |
| 10 | S. asperum | Brazil | Anti-molluscicidal [66], antifungal [67] | |
| 11 | S. asterophorum | Jurubeba-de-fogo | Brazil | Liver dysfunctions, antidiarrheal [68], spasmolytic [69] |
| 12 | S. betaceum | English: tree tomato, South America: tamamoro and tomate de árbol, French: arbre à tomates, tomate de La Paz, tomate en arbre. Spanish: tamarillo, tomate de árbol, tomate Serrano | Ecuador, Colombia, Peru, Bolivia, Rwanda, South Africa, India, Nepal China, United States, Chile, Australia, New Zealand, Malaysia, Philippines, Puerto Rico, Bhutan [71–74] | Ripe fruit edible, preservative [71, 72], antioxidant [75] |
| 13 | S. buddleifolium | Unknown | Brazil [79] | Unknown |
| 14 | S. caavurana | Laranjinha do mato, ‘jurubebarana’ or ‘jurubeba-branca’ | Brazil (Ceará, Bahia, Mato Grosso do Sul, Paraná,Santa Catarina States), Paraguay, Argentina | Anemia, liver disorders, digestion [80] |
| 15 | S. capsicoides | Cockroach berry, polohauai’i (Polynesia), devil’s apple | Brazil, Central America, Australia, Brooklyn, New York [81–84] | Ornamental [83], anti-inflammatory [85], anticancer [86], antihypertensive [87] |
| 16 | S. cathayanum | China | Anti-inflammatory, anti-bacterial [102], antitumor, anti-neurodegenerative [102–106] | |
| 17 | S. cernuum | “Panaceia” | Brazil | Gastric ulcers, hepatic injuries, skin disorders, anti-tumor, depurative, diuretic, antihemorrhagic, antiblennorrhoea, cardiac disorders, analgesic, anti-inflammatory, urinary disorders, gastric cancer, gonorrhea [107–112] |
| 18 | S. chrysotrichum | “Sosa” | Mexico | Anti-mycotic, anti-inflammatory [113–120] |
| 19 | S. cornifolium | Latin America | Anti-mycotic [121] | |
| 20 | S. crinitum | “jurubeba” and “fruto-de-lobo” | Brazil, Colombia | Anti-tumor [122, 123] |
| 21 | S. diphyllum | Mexico, Belize, Costa Rica, El Salvador, Guatemala, Honduras, Nicaragua, Florida, Texas, Indonesia, Philippines, West Indies, China, Taiwan, Egypt [124–126] | Anti-tumor [126] | |
| 22 | S. dulcamara | Bittersweet/bitter/European/deadly/blue climbing/woody nightshade, felonwort, violet-bloom, fellen, scarlet/snake berry, mortal, fever twig, staff vine | Northern Africa, North America, Europe, Asia | Skin diseases, cancers, anti-tumors, alterative, anodyne, depurative, mildly diuretic, emetic, expectorant, hepatic, mildly narcotic and purgative [127–131], skin abrasions, inflammation [132] |
| 23 | S. elaeagnifolium | Prairie berry, Silverleaf nightshade, silverleaf/Whitehorse/bull/horse nettle (English); silver-leaf bitter-apple, Satan’s bush (South Africa); trompillo (Spanish); meloncillo del campo quillo-quillo, revienta caballo (Argentina); tomatillo (Chile); trompillo (Honduras)[540] | Mexico, USA, South America, Middle East, Southern Africa, North Africa, Taiwan, Penghu Islands, Brazil, India, Germany, Kenya [539, 540] | Contraceptive, corticosteroid drugs, hepatoprotective, hypoglycemic, hepatotonic, laxative, appetizer, cardiotonic, antispasmodic, antiepileptic, renal pain, analgesic, anti-inflammatory, anticancer, antimolluscicidal [133, 134] |
| 24 | S. erianthum | Aamourette marron (French); big eggplant, black/mullein nightshade, China flowerleaf, flannel bush, tropillo, turkey berry, wild tobacco, jia yan ye shu (Chinese) | Americas, Cuba, Dominican Republic, Haiti, Jamaica, Trinidad, South America | Leukorrhea, abortion, analgesic, vertigo, dysentery, fever, diarrhea, digestive problems, anti-inflammatory, leprosy, sexually-transmitted diseases, malaria, laxative, anti-diuretic, antihepatitis B, anti-tumor [135–139] |
| 25 | S. glabratum | Saudi Arabia, Yemen | Antibacteria, diuretic, scabies, syphilis, cough, hemorrhoids, anticancer [140–144] | |
| 26 | S. glaucophyllum | Brazil, Bolivia, Argentina, Paraguay, Uruguay | Anticancer [145, 146] | |
| 27 | S. guaraniticum | Jurubeba, false-jurubeba | Brazil, Paraguay, Argentina | Anemia, fevers, erysipela, hepatitis, ulcers, uterine tumors, tonic, digestive stimulant, fevers, antioxidants [147–149] |
| 28 | S. incanum | Thorn/bitter/sodom/poison/snake apple, mutongu (Kikuyu), mtunguja mwitu (Kiswahili), ochok (Luo) | Kenya, Uganda,Tanzania, Middle East, India, Australia, Madagascar, Mauritius, Saudi Arabia [150, 151] | Antibacterial [152, 153], antileishmanial [154], anticancer [155] conjunctivitis, inflammations [156] |
| 29 | S. indicum | Poison berry, Indian nightshade, African eggplant, bush tomato, ntunfulu, bhantaki, bari kateri, kateli, kshudra bhantaaki, mahati, mahotika, vartaki, vrihati, kataai kalaan, mullamkatti, papparamulli, barahantaa | India, Sri Lanka, Malaysia, China, Philippine Islands, Africa [157–159] | Diaphoretic, diuretic, expectorant, stimulant, bronchites, itching, bodyaches, asthma, wounds, toothache, narcotic, cutaneous disorders, ringworm, mouthwash [157], anti-inflammatory, respiratory disorders, dropsy, heart diseases, chronic fever, colic, scorpion stings, difficult urination, worm infestation [158], alopecia areata, erectile failure, boost appetite, abdominal pain, distaste, deworming, colitis [159], antitumor [160–163], ascites, edema [164] |
| 30 | S. jabrense | Brazil [165–169] | Anticancer [168], molluscicidal [169] | |
| 31 | S. khasianum | India | Anti-inflammatory, antihelmintic, Anticancer [170–172] | |
| 32 | S. laciniatum | Kangaroo apple | Australia, Tasmania, Wales, New Zealand [173, 174] | Unknown |
| 33 | S. laxum | Potato vine, potato climber, jasmine nightshade, | Australia [175, 176], Uruguay, Argentina [177, 178] | Aphid repellant pesticide [177] |
| 34 | S. ligustrinum | Natri, Tomatillo [541, 542] | Chile | Antipyretic, anti-inflammatory, fever, anti-fungal [179] |
| 35 | S. lycocarpum | Wolf apple, lobeira, fruit-of-wolf, jurubebao (Brazil) fruta-do-lobbo (Portuguese) [543] | Brazil | Anti-inflammatory, antihepatotoxic, hypotensive, antihistamine [180], anticancer [181], antidiabetic [182], antischistosomicidal [183, 184], antileishmanicidal [185], anti-trypanosomal [186] antiprotozoa [187] |
| 36 | S. lycopersicum | Tomatillo (Mexico), tomate (Spanish), tomato (English) | Mexico, South & Central America, Asia, Africa [188] | Antimicrobial [189], antiasthma, antiatherosclerosis [190], antiplatelet [191], anticancer [190, 192] |
| 37 | S. lyratum | Nipplefruit (English), | China South America [193] | Anticancer [88, 89, 194–200], anti-inflammatory [201] |
| 38 | S. melongena | Aubergine, bringal, eggplant, terong, baigan, melongene | India, China, Thailand, Burma, Iran, Egypt, Turkey, East Asia [202, 203] | Antioxidant [90, 91, 204–206], anticancer [206–208], antidiabetic [209], anti-inflammatory, analgesic, sedative, hypnotic, blood circulation [210], antimelanogenesis [211] |
| 39 | S. muricatum | Melon pear, Pepino, Tree melon, sweet cucumber [544–547] | Equador, Colombia, Peru, Chile, Sri Lanka, New Zealand, Western Australia, Spain, Israel, Morocco, Kenya, Hawaii, California [212, 213] | Anti-inflammatory [214], antidiabetic [215], antitumor [212, 213] |
| 40 | S. nienkui | China (Hainan) [216–218] | Unknown | |
| 41 | S. nigrum | Black nightshade, duscle, garden nightshade, Indian nightshade, garden huckleberry, hound’s berry, petty morel, wonder berry, small-fruited black nightshade, or popolo, makoi (Hindi), manathakkali (Tamil) | Eurasia, Americas, Austrasia, South Africa [219–221] | Mouth ulcers, peptic ulcers, dysentery, skin disorders, ringworms, painful periods, cough [219–221], anti-inflammatory, hepatoprotective, diuretic, antipyretic, tuberculosis, cervical carcinoma [220–222], emollient, febrifuge, narcotic, purgative, sedative, analgesic, antispasmodic, vasodilator [222], antihyperlipidemic [131, 223], antimicrobial [224–226], antitumor [92–97, 227–230], anti-molluscicidal [231–233], antinociceptive, antipyretic [230, 234, 235], antiulcerogenic [235], antihistaminic, antiallergic [236, 237], hepatoprotective, anti-inflammatory, antipyretic [98, 236, 237], CNS-depressant action [238] |
| 42 | S. nudum | Caribbean, Haiti, Cuba [239] | Antiplasmodial [240–249] | |
| 43 | S. orbignianum | Brazil [250] | Unknown | |
| 44 | S. paludosum | Brazil | Hypertension, vasorelaxant, antioxidant, antibiotics [251, 252] | |
| 45 | S. paniculatum | Jurubeba, jubeba, juribeba, juripeba, jupela, juripeba, juuna, juvena, jurubebinha, jurubeba-branca, jurubeba-verdadeira | Brazil, Argentina, Paraguay, southern, central, eastern and northern Brazil [253–255] | Anemia, anorexia, bile insufficiency, bladder problems, blood cleansing, bloating, boils, catarrh, congestion, contusions, constipation, convalescence, cystitis, debility, diabetes, digestive sluggishness, dyspepsia, edema, erysipelas, fever, flatulence, gallbladder inflammation, gastric disorders, hangover, headache, heartburn, hepatitis, hives, irritable bowel syndrome, itch, jaundice, liver problems, malaria, menstrual disorders, nausea, skin disorders, spleen inflammation, tumors, ulcers, water retention, wounds [253–255], antiherpes [256], antiulcers [257, 258], antifungal [259, 548], antibacterial [260] |
| 46 | S. pseudocapsicum | Jerusalem/winter cherry, Madeira, | South Africa, Australia, New Zealand, Peru, Ecuador [261–263] | Hepatoprotective [264] |
| 47 | S. rostratum | Buffalobur/spiny nightshade, Colorado bur, Kansas/Mexican/Texas thistle | United States, northern and central Mexico [265–272] | Cardiovascular [273] |
| 48 | S. sarrachoides | Hairy/leafy-fruited nightshade | Columbia [274, 275] | Unknown |
| 49 | S. schimperianum | Somali, Eritrea, Ethiopia, Egypt, Yemen [276] | Antimicrobial [277, 278], antifungal [279] | |
| 50 | S. septemlobum | Qing qi (Chinese) | China (Anhui, Gansu, Hebei, Henan, Jiangsu, Liaoning, Nei Mongol, Shandong, Shanxi, Sichuan, Xinjiang, East Xizang, Zhejiang) [280, 281] | Antipyretic, antidotal [261], anticancer [261, 262] |
| 51 | S. sessiliflorum | Cocona | Peru, Colombia, Venezuela [282–284, 549], Bolivia, Mexico [268] | Antioxidant [550], antimicrobial, hypolipidemic [285] |
| 52 | S. sisymbriifolium | Vila-vila, sticky nighthade, red bufallor bur, fire and ice, litchi tomato, morelle de balbis | Brazil, Argentina, Uruguay, Paraguay [286–288] | Cardiovascular [289], antidiarrheal [290], hypotensive [291, 292], antimicrobial, antioxidative [293], anticonvulsant, CNS depressant [294], antimolluscicidal [295], analgesic [290, 296] |
| 53 | S. spirale | Southern China, India, Bangladesh, Thailand, Laos, Philippines, Australia [551] | Anaesthetic, diuretic and narcotic, antibacterial, anticancer [297–299] | |
| 54 | S. surattense | Cockroach/yellow berry; thorn gourd/eggplant; belladonna; Night-shade, Febrifuge plant (English); Choti kateri/Bhatakataiyya, Rengani (Hindi); | China [300, 301], India [302] | Anti-inflammatory, antibacterial, antitumor, antioxidant, anti-platelet aggregation [303–308], diuretic [308], antiplasmodial [309], anthelmintic, anti-convulsant, antihyperlipide-mic, antiurolithiatic, natriuretic, antiulcer, wound healing, antiasthmatic, hypoglycemic, hepatoprotective [99] |
| 55 | S. torvum | Turkey berry, prickly nightshade, devil’s fig, shoo-shoo bush, wild/pea eggplant (English), aubergine sauvage épineuse, fausse aubergine (French), kantɔsi (Ghana), susumber (Jamaica), berenjena cimarrona (Spanish), kaisurisuri, kausoni, kauvotovotua, soni (Fijian), shui qie (Chinese), bhankatiya, katai (Hindi) [552] | Brazil, Colombia, Caribbean, Central America, Mexico, tropical Africa, Asia, Australia, Hawaii, Guam, American Samoa [310–312] | Antibacterial, anti-platelet aggregation [100, 313], pesticide [314], analgestic [314], anticancer [315–317], antifungal, antimicrobial [318–320], antiulcerogenic [321], antiviral [322], anticonvulsant [323], antihypertensive [324, 325, 553], antinephrotoxicity [326, 327], antioxidants [328–330], anti-inflammatory [331], antidepressant [332, 333], antiplasmodial [334], antidiabetic [335–337], antihelminthic [338] |
| 56 | S. tridynamum | Spanish: mala mujer, sacamanteca, ojo de liebre, berenjena Silvestre | Mexico [339, 340] | Antidiabetic [339–341] |
| 57 | S. trilobatum | Purple fruited pea eggplant, Thai nightshade | India, Myanmar, Thailand, Vietnam, Malaysia [342, 343] | Antifungal, antimitotic, asthma,vomiting, rheumatism, leprosy [342, 343], fever, antioxidant [344], antibacterial [345–347], antidiabetic [348], anticancer [349–355], mosquitocidal [356, 357], anti-inflamatory [358], antinociceptive [359], antihepatitis [360] |
| 58 | S. triste | Venezuela, Trinidad, Martinique, Dominica [361] | Unknown | |
| 59 | S. tuberosum | Potato | Chile, Peru, Bolivia [101, 362, 363] | Antifungal, antimicrobial [364], antioxidants [365, 366], antileishmanial [367, 368], anticancer [369–372], antihypertensive [373] |
| 60 | S. umbelliferum | Bluewitch nightshade | California, Arizona [374–379] | Anticancer [380] |
| 61 | S. uporo | Cannibal’s tomato | Fiji island, Tonga, Samoa, Tuamotus, Hawaii [381–384] | Unknown |
| 62 | S. validinervium | Venezuela [385] | ||
| 63 | S. vestissimum | Toronjo, tumo/coquina melon, lulo fruit | Colombia, Venezuela [386, 387] | |
| 64 | S. villosum | Hairy nightshade, whooly nightshade, red nightshade | Europe, western Asia, northern Africa, North America, Australia, India | Antimolluscicidal [554], mosquito larvicidal [388, 389, 555] |
| 65 | S. violaceum | Ci tian qie (Chinese) | China, India, Myanmar, Thailand, Cambodia, Laos, Vietnam, Malaysia, Indonesia, Philippines | Anticancer, anti-inflammatory, antimicrobial, antioxidant, anthelmintic [390–393] |
| 66 | S. xanthocarpum | Wild eggplant, Kantakari, yellow berried nightshade, huang shui qi (Chinese) | Nepal, Pakistan, Bhutan, Bangladesh, Myanmar, Sri Lanka, China, Iran, Yemen, Thailand, Afghanistan, Saudi Arabia, India | Anthelmintic, anti-inflammatory, anodyne, digestive, carminative, appetizer, stomachic, depurative, sudorific, febrifuge, expectorant, laxative, diuretic, emmenagogue, aphrodisiac, leishmaniasis, immunomodulatory, anti-asthmatic [394–400], antimicrobial [226, 401–405], molluscicidal, hepatoprotective, antidiabetic [406–413] antioxidant, antinociceptive, nephroprotective, mosquitocidal, anti-psoriatic, diuretic, antiurolithiatic [414–429] |
Chemical Constituents and Their Biological Properties
At least 670 compounds, including 134 steroidal saponins (1–134), 63 steroidal alkaloids (135–197), 13 pregnane glycosides (198–210), 128 terpenes (211–338), 72 flavonoids (339–413), 31 lignans (414–444), 31 other types of alkaloids (445–475), 66 sterols (476–541), 52 phenols (542–593), 20 coumarins and coumestans (594–613), 4 coumarinolignoids (614–617), 23 fatty acids and esters (618–640) and 30 other compounds (641–670) were reported from the genus Solanum. Most of them were investigated for various biological activities. The chemical constituents and their biological properties are presented in Table 2, together with their plant sources and parts, alongside the classification of structures.
Table 2.
Phytochemistry, biological properties and classification of Solanum compounds
| No. | Compounds | Plant sources | Parts | Biological properties | References |
|---|---|---|---|---|---|
| Steroidal Saponins | |||||
| 1 | Chlorogenone | S. torvum | Fruit | [430] | |
| 2 | (5α,25S)-Spirostan-3,6-dione | S. torvum | Fruit | [430] | |
| 3 | Solakhasoside | S. khasianum | Fruit | [431] | |
| 4 | Foliumin | S. amygdalifolium | Aerial | [57] | |
| 5 | Foliumin A | S. amygdalifolium | Aerial | [56] | |
| 6 | Neotigogenin | S. paniculatum | Leaf | Cytotoxic | [257] |
| 7 | Diuranthoside A | S. cathayanum | Root | [432] | |
| 8 | Torvoside N | S. torvum | Aerial | Anticancer | [316] |
| 9 | Atroposide E | S. dulcamara | Aerial | [433] | |
| 10 | Degalactotigonin | S. dulcamara | Aerial | [433] | |
| 11 | Trillin | S. paniculatum | Aerial | [258] | |
| 12 | Diosgenin gentiobioside | S. paniculatum | Aerial | [258] | |
| 13 | Diosgenone | S. nudum | Leaf | Hepatoprotective | [242, 247, 249] |
| 14 | (22R, 23S, 25R)-3β,6α, 23-trihydroxy-5α-spirostane 6-O-β-d-xylosyl-(1″”-3″’)-O-[β-d-quinovosyl(1″’-2′)]-O-[α-l-rhamnosyl (1″-3′)] -O-β-d-quinovoside | S. paniculatum | Aerial | [258] | |
| 15 | Nuatigenosido | S. sisymbriifolium | Root | Antihypertensive | [289, 291] |
| 16 | (3β,5α,14β,25R)-3-Hydroxyspirost-8-en-11-one | S. villosum | Leaf | [434] | |
| 17 | (3β,5α,6α,25S)-3-Hydroxyspirostan-6-yl 6-deoxy-3-O-(6-deoxy-α-l-mannosyl) -β-d-glucoside | S. torvum | Whole | [435] | |
| 18 | Torvoside Q | S. torvum | Aerial | [331, 436] | |
| 19 | Dioscin | S. indicum | Fruit | [160] | |
| S. melongena | Fruit | Antimelanogenesis | [211] | ||
| S. rostratum | Aerial | [437] | |||
| 20 | Prosapogenin A | S. indicum | Fruit | [160] | |
| 21 | Diosgenin | S. lycopersicum | Aerial | [438] | |
| S. melongena | Aerial | [439] | |||
| S. nigrum | Fruit | [440] | |||
| S. torvum | Fruit | [430] | |||
| S. tridynamum | Root | [341] | |||
| S. tuberosum | Stem | [441] | |||
| S. violaceum | Aerial | [391, 442] | |||
| 22 | Aspidistrin | S. cathayanum | Root | [432] | |
| 23 | Torvoside M | S. torvum | Aerial | Anticancer | [316] |
| 24 | Protodioscin | S. abutiloides | Root | [7] | |
| S. incanum | Root | [156] | |||
| S. indicum | Fruit | [160, 443] | |||
| S. spirale | Fruit | [444] | |||
| 25 | Methylprotodioscin | S. incanum | Root | [155] | |
| S. indicum | Fruit | [160] | |||
| 26 | Indioside D | S. incanum | Root | [156] | |
| 27 | 26-O-β-d-Glucosyl-22-methoxyfurost-5-ene-3β,26-diol 3-O-α-l-rhamnosyl-(1-2)-β-d-glucoside | S. indicum | Fruit | [160] | |
| S. spirale | Fruit | [444] | |||
| 28 | (3β,22α,25R)-26-(β-d-Glucosyloxy)-22-hydroxyfurost-5-en-3-yl O-β-d-glucosyl-(1-2)-O-β-d-glucosyl-(1-4)–β-d-glucoside | S. cathayanum | Root | [432] | |
| 29 | 25R-Timosaponin H1 | S. cathayanum | Root | [432] | |
| 30 | Torvoside O | S. torvum | Leaf | [445] | |
| 31 | (23S,25R)-spirost-5-en-3,23 diol 3-O-α-l-rhamnosyl-(1-2)-O-α-l-rhamnosyl-1-4)β-d-glucoside | S. glabratum | Aerial | [141] | |
| 32 | 23-β-d-glucosyl (23S,25R)spirost-5-en-3,23 diol 3-O-α-l-rhamnosyl-1-2)O-α-l-rhamnosyl-(1-4)β-d-glucoside | S. glabratum | Aerial | [141] | |
| 33 | (25R)spirost-5-en-3-ol 3-O-α-l-rhamnosyl-1-2)O-β-d-glucosyl-1-3)β-d-galactoside | S. glabratum | Aerial | [141] | |
| 34 | Isonuatigenin-3-O-β-solatriose | S. sisymbriifolium | Root | [446] | |
| 35 | Saponin SC-1 | S. chrysotrichum | Leaf | [118] | |
| 36 | Saponin SC-2 | S. chrysotrichum | Leaf | Antifungal | [113–115, 117] |
| 37 | Saponin SC-3 | S. chrysotrichum | Leaf | Antifungal | [114, 117] |
| 38 | Saponin SC-4 | S. chrysotrichum | Leaf | Antifungal | [114, 117] |
| 39 | Saponin SC-5 | S. chrysotrichum | Leaf | Antifungal | [114, 117] |
| 40 | Saponin SC-6 | S. chrysotrichum | Leaf | Antifungal | [114, 117] |
| S. torvum | Whole | [435] | |||
| 41 | Chlorogenin | S. chrysotrichum | Leaf | [117] | |
| S. tridynamum | Root | [341] | |||
| S. torvum | Fruit | [430] | |||
| 42 | Chrysogenin | S. chrysotrichum | Leaf | [117] | |
| 43 | Laxumin A | S. laxum | Aerial | [178] | |
| 44 | Laxumin B | S. laxum | Aerial | [178] | |
| 45 | Luciamin | S. laxum | Aerial | [177] | |
| 46 | Lyconoside Ia | S. lycocarpum | Fruit | [447] | |
| 47 | Lyconoside Ib | S. lycocarpum | Fruit | [447] | |
| 48 | Lyconoside II | S. lycocarpum | Fruit | [447] | |
| 49 | Lyconoside III | S. lycocarpum | Fruit | [447] | |
| 50 | Lyconoside IV | S. lycocarpum | Fruit | [447] | |
| 51 | 26-O-(β-d-Glucosyl) nuatigenin-3-O-α-l-rhamnosyl-(1-4)-β-d-glucoside | S. surattense | Aerial | [305] | |
| 52 | Aculeatiside A | S. surattense | Aerial | [305] | |
| 53 | (22R, 23S, 25R)-3β,6α,23-trihydroxy-5α-spirostane 6-O-β-d-xylosyl-(1-3) -β-d-quinovoside | S. surattense | Aerial | [305] | |
| 54 | (22R,23S,25S)-3β,6α,23-trihydroxy-5α-spirostane 6-O-β-d-xylosyl-(1-3)-O-β-d-quinovoside | S. surattense | Aerial | [305] | |
| 55 | (22R,23R,25S)-3β,6α,23-trihydroxy-5α-spirostane 6-O-β-d-xylosyl-(1-3)-O-β-d-quinovoside | S. surattense | Aerial | [305] | |
| 56 | Neochlorogenin 6-O-β-d-quinovoside | S. torvum | Aerial | [331, 448] | |
| 57 | Neochlorogenin 6-O-β-d-xylosyl -(1-3)-β-d-quinovoside | S. torvum | Aerial | Anti-inflammatory | [331, 448] |
| 58 | Neochlorogenin 6-O-α-l-rhamnosyl-(1-3)-β-d-quinovoside | S. torvum | Aerial | [448, 449] | |
| 59 | Solagenin 6-O-β-d-quinovoside | S. torvum | Whole | [448–450] | |
| 60 | Solagenin 6-O-α-l-rhamnosyl-(1-3)-β-d-quinovoside | S. torvum | Whole | [448] | |
| 61 | (25S)26-β-d-glucosyloxy)3-oxo-5α-furost-20(22)en-6α-yl-O-β-d-xyloside | S. torvum | Fruit | [451] | |
| 62 | (25S)26-β-d-glucosyloxy)3-oxo-22α-methoxy-5α-furostan-6α-yl-O-β-d-xyloside | S. torvum | Fruit | [451] | |
| 63 | (25S)26-β-d-glucosyloxy)3β-hydroxy-22α-methoxy-5α-furostan-6α-yl-O-α-l-rhamnosyl-1-3)β-d-glucoside | S. torvum | Fruit | [451] | |
| 64 | Torvoside A | S. torvum | Aerial | [313, 449] | |
| 65 | Torvoside B | S. torvum | Root | [449] | |
| 66 | Torvoside E | S. torvum | Root | [449] | |
| 67 | Torvoside F | S. torvum | Root | [449] | |
| 68 | Torvoside H | S. torvum | Fruit | [313] | |
| 69 | (25S)3β-hydroxy-5α-spirostan-6α-yl-O-β-d-xyloside | S. torvum | Fruit | [451] | |
| 70 | (25S)3-oxo-5α-spirostan-6α-yl-O-β-d-xyloside | S. torvum | Fruit | [451] | |
| 71 | (25S)3β-hydroxy-5α-spirostan-6α-yl-O-β-d-glucoside | S. torvum | Fruit | [451] | |
| 72 | (25S)3β,27-dihydroxy-5α-spirostan-6α-yl-O-β-d-glucoside. | S. torvum | Fruit | [451] | |
| 73 | Neochlorogenin | S. tridynamum | Root | [451] | |
| S. torvum | Aerial | [341] | |||
| 74 | Tigogenin | S. americanum | Leaf | [54] | |
| S. torvum | Fruit | [430] | |||
| 75 | Yuccagenin | S. tridynamum | Root | [341] | |
| 76 | Yamogenin | S. violaceum | Aerial | [391] | |
| 77 | Yamogenone | S. violaceum | Aerial | [391] | |
| 78 | Indioside L | S. violaceum | Aerial | [391] | |
| 79 | Indioside M | S. violaceum | Aerial | [391] | |
| 80 | Indioside N | S. violaceum | Aerial | [391] | |
| 81 | Indioside O | S. violaceum | Aerial | [391] | |
| 82 | Indioside G | S. violaceum | Whole | [392] | |
| 83 | Indioside H | S. violaceum | Whole | Anticancer | [392] |
| 84 | Borassoside D | S. violaceum | Whole | [392] | |
| 85 | Borassoside E | S. violaceum | Whole | Anticancer, anti-inflammatory | [392] |
| 86 | Indioside I | S. violaceum | Whole | Anticancer, anti-inflammatory | [392] |
| 87 | Indioside J | S. violaceum | Whole | [392] | |
| 88 | Indioside K | S. violaceum | Whole | [392] | |
| 89 | Yamoscin | S. torvum | Aerial | Anti-inflammatory | [331] |
| S. violaceum | Whole | Anticancer | [392] | ||
| 90 | Zingiberoside A1 | S. violaceum | Whole | [392] | |
| 91 | Solanolactoside A | S. torvum | Aerial | [316] | |
| 92 | Solanolactoside B | S. torvum | Aerial | [316] | |
| 93 | Solanolactoside C | S. torvum | Aerial | [436] | |
| 94 | Solanolide | S. torvum | Aerial | [316] | |
| 95 | Torvoside J | S. surattense | Aerial | Anticonvulsant | [305] |
| S. torvum | Aerial | [323, 331, 452] | |||
| 96 | Torvoside K | S. surattense | Aerial | Anticonvulsant, antifungal | [305] |
| S. torvum | Aerial | [323, 331, 452] | |||
| 97 | Torvoside L | S. surattense | Aerial | Anticonvulsant | [305] |
| S. torvum | Aerial | [323, 331, 435, 452] | |||
| S. paniculatum | Leaf | [260] | |||
| 98 | (22R,23S,25S)-3β,6α,23-trihydroxy-5α-spirostane 6-O-β-d-xylosyl-(1-3)-O-β-d-quinovoside | S. torvum | Aerial | [323, 331] | |
| 99 | (22R,23S,25R)-3β,6α,23-trihydroxy-5α-spirostane 6-O-β-d-xylosyl-(1-3)-O-β-d-quinovoside | S. torvum | Aerial | Anti-inflammatory | [331] |
| 100 | (22R,23R,25S)-3β,6α,23-trihydroxy-5α-spirostane 6-O-β-d-xylosyl-(1-3)-O-β-d-quinovoside | S. torvum | Aerial | Anti-inflammatory | [331] |
| 101 | Gekogenin | S. torvum | Fruit | [430] | |
| 102 | Sisalagenin | S. torvum | Fruit | [430] | |
| 103 | Δ25(27)tigogenin-3-O-β-d-glucoside | S. paniculatum | Leaf | Antiviral | [257] |
| 104 | Soladulcosides A | S. dulcamara | Aerial | [129] | |
| 105 | Soladulcosides B | S. dulcamara | Aerial | [129] | |
| 106 | Abutiloside L | S. abutiloides | Root | [4] | |
| 107 | Abutiloside M | S. abutiloides | Root | [4] | |
| 108 | Abutiloside N | S. abutiloides | Root | [4] | |
| 109 | Abutiloside O | S. abutiloides | Root | [4] | |
| 110 | Torvoside C | S. torvum | Root | [449] | |
| 111 | Torvoside D | S. surattense | Aerial | [305] | |
| S. torvum | Root | [331, 449] | |||
| 112 | Torvoside G | S. torvum | Fruit, Root | [313, 449] | |
| 113 | Torvoside P | S. torvum | Leaf | [445] | |
| 114 | Anguivioside A | S. anguivi | Fruit | [41] | |
| 115 | Anguivioside B | S. anguivi | Fruit | [41] | |
| 116 | Anguivioside C | S. anguivi | Fruit | [41] | |
| 117 | Anguivioside I | S. indicum | Fruit | [443] | |
| 118 | Anguivioside III | S. anguivi | Fruit | [43] | |
| S. indicum | Fruit | [443] | |||
| 119 | Anguivioside XI | S. anguivi | Fruit | [43] | |
| 120 | Anguivioside XV | S. anguivi | Fruit | [43] | |
| 121 | Anguivioside XVI | S. anguivi | Fruit | [43] | |
| 122 | Inunigroside A | S. nigrum | Fruit | [453] | |
| 123 | 25(S)-26-O-β-d-glucosyl-5α-furost-22(20)-en-3β,6α,26-triol 6-O-[α-l-rhamnosyl-(1-3)-O-β-d-quinovoside] | S. torvum | Fruit | Anticancer | [317] |
| 124 | 25(S)-26-O-β-d-glucosyl-5α-furost-22(20)-en-3-one-6α,26-diol 6-O-[α-l-rhamnosyl-(1-3)-O-β-d-quinovoside] | S. torvum | Fruit | Anticancer | [317] |
| 125 | 25(S)-26-O-β-d-glucosyl-5α-furost-22(20)-en-3β,6α,26-triol 6-O-β-d-quinovoside | S. torvum | Fruit | Anticancer | [317] |
| 126 | Paniculonin B | S. torvum | Leaf | [323] | |
| 127 | Smilaxchinoside A | S. rostratum | Aerial | [437] | |
| 128 | 6-O-α-l-rhamnosyl-(1″-3′)-β-d-quinovosyl-(22S,23R,25S)-3β,6α,23-trihydroxy-5α-spirostane | S. paniculatum | Leaf | [260] | |
| 129 | 6-O-β-d-Xylosyl-(1″-3′)-β-d-quinovosyl-(23R,25S)-3β,6α,23-trihydroxy-5α-spirostane | S. paniculatum | Leaf | [260] | |
| 130 | 6-O-β-d-Xylosyl-(1″-3′)-β-d-quinovosyl-(22S,23R,25R)-3β,6α,23-trihydroxy-5α-spirostane | S. paniculatum | Leaf | [260] | |
| 131 | 3-O-α-l-Rhamnosyl-(1″-3′)-β-d-quinovosyl-(22S,23S,25R)-3β,6α,23-trihydroxy-5α-spirostane | S. paniculatum | Leaf | [260] | |
| 132 | 3-O-β-d-Xylosyl-(1″-3′)-β-d-quinovosyl-(22S,23S,25R)-3β,6α,23-trihydroxy-5α-spirostane | S. paniculatum | Leaf | [260] | |
| 133 | 6-O-α-l-Rhamnosyl-(1″-3′)-β-d-quinovosyl-(22S,25S)-1β,3β,6α-trihydroxy-5α-spirostane | S. paniculatum | Leaf | [260] | |
| 134 | 6-O-β-d-Xylosyl-(1″-3′)-β-d-quinovosyl-(22S,25S)-3β,4β,6α-trihydroxy-5α-spirostane | S. paniculatum | Leaf | [260] | |
| Steroidal alkaloids | |||||
| 135 | Demissine | S. tuberosum | Stem | [101] | |
| 136 | Solasodiene | S. torvum | Fruit | [430] | |
| 137 | Solanoside A | S. surattense | Whole | [454] | |
| 138 | Solanoside B | S. surattense | Whole | [454] | |
| 139 | Solamargine | S. abutiloides | Root | [7] | |
| S. aculeastrum | Fruit | [19] | |||
| S. asperum | Root | [66, 67] | |||
| S. buddleifolium | Stem | [79] | |||
| S. americanum | Fruit | [55] | |||
| S. anguivi | Root | [42] | |||
| S. crinitum | Fruit | [122] | |||
| S. erianthum | Leaf | [137, 455] | |||
| S. incanum | Root | [156] | |||
| S. khasianum | Fruit | [456] | |||
| S. lycocarpum | Fruit | Leishmanicidal, antidiabetic, schistosomicidal, trypanocidal | [182, 183, 185, 186, 447, 457] | ||
| S. melongena | Fruit,Root | [206, 439] | |||
| S. nigrum | Whole | [228] | |||
| S. paludosum | Fruit | [253] | |||
| S. sarrachoides | Leaf | Anticancer | [458] | ||
| S. surattense | Aerial | [305] | |||
| S. uporo | Root | Antibacterial, molluscicidal | [384] | ||
| S. xanthocarpum | Fruit | [403, 406] | |||
| 140 | γ-Solamargine | S. nigrum | Whole | [228] | |
| S. umbelliferum | Whole | [380] | |||
| 141 | Khasianine | S. khasianum | Fruit | [456] | |
| S. nigrum | Whole | [228] | |||
| S. surattense | Aerial | Anticancer | [305] | ||
| S. xanthocarpum | Fruit | Antibacterial, molluscicidal | [403, 406, 407] | ||
| 142 | Solasonine | S. americanum | Leaf | [54] | |
| S. amygdalifolium | Aerial | [56] | |||
| S. asperum | Fruit | [66, 67] | |||
| S. crinitum | Aerial | [122, 123, 459] | |||
| S. erianthum | Leaf | [137, 455] | |||
| S. khasianum | Fruit | [456] | |||
| S. lycocarpum | Fruit | Leishmanicidal,antidiabetic, schistosomicidal | [182, 183, 185, 447, 457] | ||
| S. melongena | Fruit,Root | [206, 439] | |||
| S. sarrachoides | Leaf | [458] | |||
| S. sessiliflorum | Fruit | [460] | |||
| S. sisymbriifolium | Fruit | [294] | |||
| 143 | β1-Solasonine | S. nigrum | Whole | [228] | |
| 144 | 12-Hydroxysolasonine | S. lycocarpum | Fruit | [182, 447] | |
| 145 | Solasodine | ||||
| S. americanum | Leaf | [54] | |||
| S. aculeastrum | Fruit | Anticancer | [13] | ||
| S. crinitum | Aerial | [123] | |||
| S. khasianum | Fruit | [172, 456] | |||
| S. laciniatum | Aerial | [461, 462] | |||
| S. lycocarpum | Fruit | [185] | |||
| S. melongena | Fruit | [206] | |||
| S. nigrum | Whole | [163, 440] | |||
| S. sisymbriifolium | Fruit | [294] | |||
| S. surattense | Whole | CNS depressant | [303] | ||
| S. torvum | Whole | Anti-inflammatory | [463] | ||
| S. trilobatum | Whole | [358] | |||
| S. villosum | Whole | [442] | |||
| S. xanthocarpum | Fruit | Antibacterial | [403, 429] | ||
| S. umbelliferum | Whole | [380] | |||
| 146 | N-Hydroxysolasodine | S. paludosum | Root | [464] | |
| 147 | O-Acetylsolasodine | S. umbelliferum | Whole | [380] | |
| 148 | Putuline | S. paludosum | Root | [464] | |
| 149 | Anguivine | S. anguivi | Root | [42] | |
| S. uporo | Root | [384] | |||
| 150 | Isoanguivine | S. uporo | Root | [384] | |
| 151 | Arudonine | S. arundo | Root | [64] | |
| 152 | Solanandaine | S. asperum | Fruit | [66] | |
| 153 | Robeneoside A | S. lycocarpum | Fruit | [182, 447] | |
| 154 | Robeneoside B | S. lycocarpum | Fruit | [182, 447] | |
| 155 | Lobofrutoside | S. lycocarpum | Fruit | [447] | |
| 156 | Solanigroside P | S. nigrum | Whole | [228] | |
| 157 | (22R, 25R)-16β-H-22α-N-Spirosol-3β-ol-5-ene 3-O-α-l-rhamnosyl-(1-2)-[α-l-rhamnosyl-(1-4)]-β-d-glucoside | S. surattense | Aerial | Anticancer | [305] |
| 158 | Solaculine A | S. aculeastrum | Root | [19] | |
| 159 | β-Solamarine | S. aculeastrum | Root | [19] | |
| S. elaeagnifolium | Seed | [465] | |||
| S. incanum | Root | [155] | |||
| 160 | Tomatidenol | S. aculeastrum | Root | [19] | |
| S. palodusum | Root | [464] | |||
| S. lycopersicum | Fruit | [192] | |||
| S. surattense | Aerial | [454] | |||
| 161 | Tomatidine 3-O-β-d-glucoside | S. arboreum | Aerial | [63] | |
| 162 | Dehydrotomatine | S. lycopersicum | Fruit | [192] | |
| 163 | Tomatidine 3-O–O-β-d-xylosyl-1-6)β-d-glucoside] | S. arboreum | Aerial | [63] | |
| 164 | Solaverol A | S. uporo | Root | [384] | |
| 165 | (23S)-23-hydroxyanguivine | S. uporo | Root | [384] | |
| 166 | (23S)-23-hydroxyisoanguivine | S. uporo | Root | [384] | |
| 167 | Tomatidine | S. lycopersicum | Fruit | [192] | |
| S. aculeastrum | Fruit | Anticancer | [13] | ||
| 168 | Tomatine | S. lycopersicum | Fruit | [192, 466] | |
| S. cathayanum | Whole | Neurotoxicity | [106] | ||
| S. sarrachoides | Leaf | [276] | |||
| 169 | 22-Imido-3-[4′-(6″-deoxy-α-l-mannoside)-β-d-glucoside]-5-dehydro spirostane | S. xanthocarpum | Fruit | [407] | |
| 170 | Leptinidine | S. paludosum | Root | [253] | |
| S. orbignianum | Aerial | [250] | |||
| 171 | Leptinine I | S. orbignianum | Aerial | [250] | |
| 172 | Leptinine II | S. orbignianum | Aerial | [250] | |
| 173 | Solanine | S. dulcamara | Stem | [467] | |
| S. indicum | Whole | [162] | |||
| S. tuberosum | Stem | [441] | |||
| S. villosum | Fruit | [468] | |||
| 174 | α-Chaconine | S. tuberosum | Stem | [372, 441] | |
| 175 | β-d-Glucoside, (3β,23β)23-hydroxysolanid-5-en-3-yl | S. orbignianum | Aerial | [250] | |
| 176 | Solanidine | S. villosum | Fruit | [469] | |
| 177 | Solanopubamine | S. schimperianum | Aerial | Antifungal | [279] |
| 178 | Jurubine | S. paniculatum | Fruit | [273, 548] | |
| 179 | Etioline | S. spirale | Root | [470] | |
| 180 | Deacetylveralosine | S. spirale | Root | [470] | |
| S. diphyllum | Root | [126] | |||
| 181 | Solaspiralidine | S. spirale | Root | [470] | |
| 182 | Soladunalinidine | S. arboreum | Aerial | [59] | |
| 183 | 3-epi-Soladunalinidine | S. arboreum | Aerial | [59] | |
| 184 | Caavuranamide | S. caavurana | Fruit | Antibacterial | [80] |
| 185 | 4-Tomatiden-3-one | S. caavurana | Fruit | [80] | |
| 186 | 5-Tomatidan-3-one | S. caavurana | Fruit | [80] | |
| 187 | (22S,25S)-3β-aminospirosol-5-ene | S. arboreum | Aerial | [59] | |
| 188 | (22R,25R)3β-amino-5α-spirosolane | S. triste | Aerial | [362, 471] | |
| 189 | (22R,25R)3β-amino-5-spirosolene | S. triste | Aerial | [362, 471] | |
| 190 | Isojuripidine | S. asterophorum | Aerial | Spasmolytic | [70] |
| 191 | 23,24-2-methyl-tetrahydrofuran)Solanidine | S. cornifolium | Aerial | [472, 473] | |
| 192 | Spiraloside C | S. spirale | Fruit | [474] | |
| 193 | Spiraloside B | S. spirale | Fruit | [474] | |
| 194 | Spiraloside A | S. spirale | Fruit | [474] | |
| 195 | Soladulcine A | S. dulcamara | Aerial | [433] | |
| 196 | Soladulcine B | S. dulcamara | Aerial | [433] | |
| 197 | Esculeoside A | S. lycopersicum | Fruit | [475] | |
| Pregnane glycosides | |||||
| 198 | Solanigroside A | S. nigrum | Whole | [476] | |
| 199 | Solanigroside B | S. nigrum | Whole | [476] | |
| 200 | 5α-Pregn-16-en-3β -ol-20-one lycotetraoside | S. nigrum | Whole | [476] | |
| 201 | (5α)-3-Hydroxypregn-16-en-20-one | S. lyratum | Whole | [194] | |
| 202 | Hypoglaucin H | S. nigrum | Whole | [476] | |
| S. rostratum | Aerial | [437] | |||
| 203 | 16-Dehydropregnolone | S. lyratum | Whole | Anticancer | [194] |
| 204 | 16-dehydropregnenolone 3-O-α-l-rhamnosyl-1-2)β-d-glucosiduronic acid | S. lyratum | Whole | [194] | |
| 205 | Torvpregnanoside A | S. torvum | Aerial | [317, 331] | |
| 206 | 5α-pregn-16-en-3,20-dione-6α-ol-6-O-[α-l-rhamnosyl-(1-3)-β-d-quinovoside] | S. torvum | Fruit | Anticancer | [317] |
| 207 | Torvpregnanoside B | S. torvum | Aerial | [331] | |
| 208 | Ganaxolone | S. torvum | Aerial | [323] | |
| 209 | Allopregnanolone | S. torvum | Aerial | [323] | |
| 210 | Pregnanolone | S. torvum | Aerial | [323] | |
| Triterpenes | |||||
| 211 | Betulinic acid | S. buddleifolium | Stem | [79] | |
| 212 | Lupeol | S. cathayanum | Aerial | [472, 473, 477] | |
| S. schimperianum | Aerial | [278] | |||
| S. spirale | Leaf | Anticancer | [297] | ||
| 213 | Cycloeucalenone | S. cernuum | Leaf | Anticancer | [107] |
| 214 | 24-oxo-31-norcycloartanone | S. cernuum | Leaf | Anticancer | [107] |
| 215 | Friedelin | S. lycopersicum | Seed | [478] | |
| 216 | Ursolic acid | S. lyratum | Whole | [197] | |
| S. torvum | Aerial | [463] | |||
| S. xanthocarpum | Root | [427] | |||
| 217 | 2α,3β-Dihydroxyursolic acid | S. torvum | Aerial | [463] | |
| 218 | Daturaolone | S. arundo | Whole | [65] | |
| 219 | Carbenoxolone | S. cernuum | Leaf | [109] | |
| 220 | β-Amyrin | S. melongena | Aerial | [439] | |
| 221 | Oleanolic acid | S. torvum | Aerial | [463] | |
| S. xanthocarpum | Root | [427] | |||
| 222 | 2α-Hydroxyoleanolic acid | S. torvum | Aerial | [463] | |
| 223 | 3β-Acetoxy-11α,12α-epoxyoleanan-13ß,28-olide | S. torvum | Aerial | [463] | |
| 224 | Solanoglycosydane I | S. torvum | Fruit | [314] | |
| Diterpenes | |||||
| 225 | Phytol | S. pseudocapsicum | Leaf | [263] | |
| S. villosum | Leaf | [434, 479] | |||
| 226 | Kaur-16-ene | S. aculeastrum | Leaf | [11] | |
| 227 | Solanerioside A | S. erianthum | Leaf | [138] | |
| 228 | Tricalysioside U | S. violaceum | Whole | [392] | |
| Sesquiterpenes | |||||
| 229 | Roseoside | S. erianthum | Leaf | [138] | |
| 230 | (6E,10E)-5,12-Dihydroxy-ß-nerolidol 5-O-β-d-glucoside | S. erianthum | Leaf | [138] | |
| 231 | Amarantholidoside IV | S. erianthum | Leaf | [138] | |
| 232 | 3β-Hydroxysolavetivone | S. abutiloides | Root | Antifungal | [3] |
| S. aethiopicum | Root | [29] | |||
| 233 | Solavetivone | S. abutiloides | Root | Antifungal | [3] |
| S. aethiopicum | Root | [29] | |||
| S. indicum | Root | [163] | |||
| S. jabrense | Aerial | [166] | |||
| 234 | 13-Hydroxysolavetivone | S. buddleifolium | Stem | [79] | |
| S. aethiopicum | Root | [29] | |||
| 235 | Lubimin | S. abutiloides | Root | Antifungal | [3] |
| S. aethiopicum | Root | [29] | |||
| 236 | Lubiminoic acid | S. aethiopicum | Root | [29] | |
| 237 | Epilubimin | S. aethiopicum | Root | [29] | |
| 238 | Epilubiminoic acid | S. aethiopicum | Root | [29] | |
| 239 | Lubiminol | S. aethiopicum | Root | [29] | |
| 240 | α-Farnesene | S. aculeastrum | Leaf | [11] | |
| 241 | Nerolidol | S. aculeastrum | Leaf | [11] | |
| 242 | 2,7,10-Trimethyldodecane | S. aculeastrum | Leaf | [11] | |
| 243 | Aethione | S. aethiopicum | Root | [29] | |
| 244 | Anhydro-β-rotunol | S. aethiopicum | Root | [29] | |
| 245 | (4S,5R,7S)-4,11-Dihydroxy-guaia-1(2),9(10)-dien | S. erianthum | Stem | [480] | |
| 246 | Caryophyllene | S. erianthum | Fruit | [481] | |
| 247 | Cadina-1(10),4-diene | S. erianthum | Fruit | [481] | |
| 248 | α-Gurjunene | S. erianthum | Fruit | [481] | |
| 249 | Globulol | S. erianthum | Fruit | [481] | |
| 250 | α-Guaiene | S. erianthum | Fruit | [481] | |
| 251 | α-Calacorene | S. erianthum | Fruit | [481] | |
| 252 | 2-naphthalenemethanol | S. erianthum | Fruit | [481] | |
| 253 | Octahydro-2,2-dimethyl-4a,7a-ethano-5H-cyclobut[e]inden-5-ol | S. erianthum | Fruit | [481] | |
| 254 | 4,5-Dehydroisolongifolene | S. erianthum | Fruit | [481] | |
| 255 | α -Caryophyllene | S. erianthum | Fruit | [481] | |
| 256 | Solafuranone | S. indicum | Root | [163] | |
| 257 | Lyratol D | S. lyratum | Whole | Anticancer | [199] |
| S. septemlobum | Whole | [482] | |||
| 258 | Solajiangxin B | S. lyratum | Whole | Anticancer | [198] |
| S. septemlobum | Whole | [482] | |||
| 259 | Septemlobin D | S. septemlobum | Whole | [483] | |
| 260 | Blumenol A | S. lyratum | Whole | Anticancer | [199, 484] |
| 261 | Blumenol C | S. lyratum | Whole | [484] | |
| 262 | Dehydrovomifoliol | S. lyratum | Whole | Anticancer | [199, 484] |
| 263 | Grasshopper ketone | S. lyratum | Whole | [484] | |
| 264 | 6α-Epoxy-7-megastigmen-9-one | S. lyratum | Whole | [484] | |
| 265 | (1′R,2R,5S,10R)2-1′,2′-dihydroxy-1′-methylethyl)6,10-dimethylspiro[4, 5]dec-6-en-8-one | S. lyratum | Whole | [484] | |
| 266 | (1′S,2R,5S,10R)2-1′,2′-dihydroxy-1′-methylethyl)6,10-dimethylspiro[4, 5]dec-6-en-8-one | S. lyratum | Whole | [484] | |
| 267 | 2-1′,2′-dihydroxy-1′-methylethyl)6,10-dimethyl-9-hydroxyspiro[4, 5]dec-6-en-8-one | S. lyratum | Whole | [200, 484] | |
| 268 | Boscialin | S. lyratum | Whole | [484] | |
| 269 | 1β-Hydroxy-1,2-dihydro-α-santonin | S. lyratum | Whole | [193, 484] | |
| 270 | Lyratol A | S. lyratum | Whole | [485] | |
| 271 | Lyratol B | S. lyratum | Whole | [485] | |
| S. septemlobum | Whole | [482] | |||
| 272 | Lyratol C | S. lyratum | Whole | Anticancer | [199] |
| 273 | Lyratol G | S. lyratum | Whole | [196] | |
| 274 | Solajiangxin A | S. lyratum | Whole | Anticancer | [198] |
| 275 | Solajiangxin C | S. lyratum | Whole | Anticancer | [198] |
| 276 | Solajiangxin D | S. lyratum | Whole | Anticancer | [200] |
| S. septemlobum | Whole | [482] | |||
| 277 | Solajiangxin E | S. lyratum | Whole | Anticancer | [200] |
| 278 | Solajiangxin F | S. lyratum | Whole | Anticancer | [197] |
| S. septemlobum | Whole | [482] | |||
| 279 | Solajiangxin G | S. lyratum | Whole | Anticancer | [197] |
| 280 | 2-hydroxysolajiangxin E | S. lyratum | Whole | Anticancer | [200] |
| 281 | Dehydrocarissone | S. lyratum | Stem | [486] | |
| S. septemlobum | Whole | [482] | |||
| 282 | Atractylenolide I | S. lyratum | Stem | [486] | |
| 283 | Ligucyperonol | S. septemlobum | Whole | [482] | |
| 284 | Nardoeudesmol A | S. septemlobum | Whole | [482] | |
| 285 | Solanerianone A | S. septemlobum | Whole | [482] | |
| 286 | Pterocarptriol | S. torvum | Root | [487] | |
| 287 | Selina-3β,4α,11-triol | S. torvum | Root | [487] | |
| 288 | 2-(1′,2′-dihydroxy-1′-methylethyl)-6,10-dimethylspiro[4, 5]dec-6,9-dien-8-one | S. torvum | Root | [487] | |
| 289 | 10β,12,14-Trihydroxy-allo-aromadendrane | S. torvum | Root | [487] | |
| 290 | 10β,13,14-Trihydroxy-allo-aromadendrane | S. torvum | Root | [487] | |
| 291 | 2-(1′,2′-dihydroxy-1′-methylethyl)-6,10-dimethyl-9-hydroxy-spirodec-6-en-8-one | S. torvum | Root | [487] | |
| 292 | 1β,10β,12,14-Tetrahydroxy-allo-aromadendrane | S. torvum | Root | [487] | |
| 293 | 1β,10β,13,14-Tetrahydroxy-allo-aromadendrane | S. torvum | Root | [487] | |
| 294 | Teferidin | S. schimperianum | Aerial | [278] | |
| 295 | Teferin | S. schimperianum | Aerial | [278] | |
| 296 | Ferutinin | S. schimperianum | Aerial | [278] | |
| 297 | Bisabolol | S. sessiliflorum | Fruit | [488] | |
| 298 | 11,12-O-Isopropylidenesolajiangxin F | S. septemlobum | Whole | [483] | |
| 299 | Eudesmane | S. septemlobum | Whole | [281] | |
| 300 | Vitispirane | S. septemlobum | Whole | [281] | |
| 301 | Septemlobin A | S. septemlobum | Whole | Anticancer | [281] |
| 302 | Septemlobin B | S. septemlobum | Whole | Anticancer | [281] |
| 303 | Septemlobin C | S. septemlobum | Whole | Anticancer | [281] |
| 304 | 3β,11-dihydroxy-4,14-oxideenantioeudesmane | S. torvum | Root | [487] | |
| 305 | Aromadendrene oxide | S. erianthum | Fruit | [481] | |
| 306 | Thujopsene | S. betaceum | Fruit | [77] | |
| 307 | α-Cedrene | S. betaceum | Fruit | [77] | |
| 308 | Cedrol | S. betaceum | Fruit | [77] | |
| 309 | α-Hexylcinnamaldehyde | S. betaceum | Fruit | [77] | |
| 310 | β-Cadinene | S. betaceum | Fruit | [77] | |
| Monoterpenes | |||||
| 311 | Decanal | S. aculeastrum | Leaf | [11] | |
| 312 | Decane | S. aculeastrum | Leaf | [11] | |
| 313 | 2,4-Decadienal | S. aculeastrum | Leaf | [11] | |
| 314 | 1,8-Cineole | S. betaceum | Fruit | [77] | |
| 315 | Terpinen-4-ol | S. betaceum | Fruit | [77] | |
| 316 | Linalool | S. vestissimum | Fruit | [489, 490] | |
| 317 | Geraniol | S. vestissimum | Fruit | [490] | |
| 318 | Limonene | S. vestissimum | Fruit | [490] | |
| 319 | β-Cyclocitral | S. aculeastrum | Leaf | [11] | |
| 320 | β-Ionone | S. aculeastrum | Leaf | [11] | |
| S. pseudocapsicum | Leaf | [263] | |||
| S. betaceum | Fruit | [77] | |||
| 321 | 1, 2-Dihydro-1,1,6-trimethyl-naphthalene | S. aculeastrum | Leaf | [11] | |
| 322 | trans-β -Damascenone | S. aculeastrum | Leaf | [11] | |
| 323 | Loliolide | S. erianthum | Leaf | [137] | |
| S. americanum | Aerial | [49] | |||
| S. pseudocapsicum | Leaf | [263] | |||
| 324 | Hotrienol | S. vestissimum | Fruit | [468, 490] | |
| 325 | Neroloxide | S. vestissimum | Fruit | [468] | |
| 326 | 5-Ethynyltetrahydro-α,α,5-trimethyl-2-furanmethanol | S. vestissimum | Fruit | [490] | |
| 327 | Nerol | S. vestissimum | Fruit | [490] | |
| 328 | 8-Hydroxylinalool | S. vestissimum | Fruit | [491] | |
| 329 | (R)-Linalyl β-d-glucoside | S. vestissimum | Fruit | [492] | |
| 330 | (1R,4E)-1-Ethenyl-6-hydroxy-1,5-dimethyl-4-hexen-1-yl β-d-glucoside | S. vestissimum | Fruit | [492] | |
| 331 | (R)-Linalyl β-vicianoside | S. vestissimum | Fruit | [492] | |
| 332 | 6-O-linked β-d-glucoside of (R)E)2,6-dimethyl-3,7-octadiene-2,6-diol | S. vestissimum | Fruit | [468] | |
| 333 | (3E,6R)-2,6-Dimethyl-3,7-octadiene-2,6-diol | S. vestissimum | Fruit | [468] | |
| 334 | p-Cymenene | S. betaceum | Fruit | [77] | |
| 335 | Dihydroactinidiolide | S. erianthum | Leaf | [137] | |
| 336 | Apiole | S. sessiliflorum | Fruit | [488] | |
| 337 | α-Terpinen-7-al | S. betaceum | Fruit | [77] | |
| 338 | 1,3,8-p-Menthatriene | S. betaceum | Fruit | [77] | |
| Flavonoids | |||||
| 339 | Vitecetin | S. agrarium | Aerial | [31] | |
| 340 | Quercetin | S. anguvi | Fruit | Anticancer | [31] |
| S. elaeagnifolium | Seed | [493] | |||
| S. incanum | Aerial | [494] | |||
| S. melongena | Stem | [205] | |||
| S. muricatum | Whole | [215] | |||
| S. nigrum | Leaf | [92–98, 230–238, 495–497] | |||
| S. torvum | Whole | [498] | |||
| 341 | Kaempferol 7-O-rhamnoside | S. asperum | Fruit | [67] | |
| 342 | Rutin | S. anguvi | Fruit | Anticancer | [31] |
| S. melongena | Stem | [499, 500] | |||
| S. muricatum | Fruit | [215] | |||
| S. nigrum | Leaf | [230] | |||
| S. spirales | Aerial | [470] | |||
| 343 | Kaempferol 3-rutinoside-7-rhamnoside | S. asperum | Fruit | [67] | |
| 344 | Afzelin | S. cernuum | Leaf | [109, 112, 501] | |
| 345 | Quercitrin | S. cernuum | Leaf | [109] | |
| S. melongena | Stem | [205] | |||
| 346 | Astragalin | S. cernuum | Leaf | [501] | |
| S. crinitum | Aerial | [459] | |||
| S. incanum | Aerial | [494] | |||
| S. elaeagnifolium | Aerial | [502] | |||
| 347 | Kaempferol 3-O-[α-apiofuranosyl-(1-2)]-α-rhamnoside | S. cernuum | Leaf | [501] | |
| 348 | Kaempferol 3-O-[α-apiofuranosyl-(1-2)]-β-galactoside | S. cernuum | Leaf | [501] | |
| 349 | Tiliroside | S. asperum | Fruit | [67] | |
| S. crinitum | Aerial | [123, 459] | |||
| S. elaeagnifolium | Whole | Anticancer | [503] | ||
| S. cernuum | Leaf | [501] | |||
| 350 | cis-Tiliroside | S. cernuum | Leaf | [501] | |
| S. elaeagnifolium | Aerial | [502] | |||
| 351 | Kaempferol | S. crinitum | Aerial | [459] | |
| S. elaeagnifolium | Whole | [504] | |||
| S. incanum | Aerial | [494] | |||
| S. indicum | Whole | [505] | |||
| S. nigrum | Leaf | [227] | |||
| S. surattense | Whole | [99] | |||
| S. torvum | Whole | [498] | |||
| 352 | Camelliaside C | S. erianthum | Leaf | [137] | |
| 353 | Baimaside | S. incanum | Aerial | [506] | |
| 354 | Narcissin | S. glabratum | Aerial | [141] | |
| 355 | Isorhamnetin 3-glucoside | S. incanum | Aerial | [506] | |
| 356 | Populnin | S. elaeagnifolium | Aerial | [502] | |
| 357 | Quercetin 3-O-robinoside | S. paniculatum | Aerial | [258] | |
| 358 | Kaempferol 3-O-(6″-O-cis-p-coumaroyl)-O-β-galactoside | S. elaeagnifolium | Aerial | [502] | |
| 359 | Myricetin-3-galactoside | S. melongena | Stem | [205] | |
| 360 | Apigenin | S. lyratum | Whole | [507] | |
| S. torvum | Whole | [498] | |||
| 361 | Pelanin | S. tuberosum | Stem | [508] | |
| 362 | Petanin | S. tuberosum | Stem | [508] | |
| 363 | Peonanin | S. tuberosum | Stem | [508] | |
| 364 | Keracyanin | S. betaceum | Fruit | Anticancer | [75, 76] |
| 365 | Pelargonidin 3-rutinoside | S. betaceum | Fruit | Anticancer | [75, 76] |
| 366 | Tulipanin | S. betaceum | Fruit | Anticancer | [75, 76] |
| 367 | Delphinidin 3-O-α-l-rhamnosyl-(1-6)-β-d-glucoside-3′-O-β-d-glucoside | S. betaceum | Fruit | Anticancer | [75, 76] |
| 368 | Cyanidin 3-O-(2″-O-xylosyl)rutinoside | S. betaceum | Fruit | [76] | |
| 369 | Asterin | S. betaceum | Fruit | [76] | |
| 370 | Biochanin A-7-O-β-d-apiofuranosyl-1-5)β-d-apiofuranosyl-1-6)β-d-glucoside | S. crinitum | Fruit | [122] | |
| 371 | 2R,3R-5,7,4′-trihydroxy-dihydroflavon-3-O-α-d-glucosyl-6″-O-β-d-glucoside-6‴-p-hydroxy benzoate | S. elaeagnifolium | Whole | Anticancer | [503] |
| 372 | 6,2′,3″,5″,4‴-Pentahydroxy-3,7″-biflavone | S. dulcamara | Fruit | [130] | |
| 373 | Kaempferol 8-C-β-d-galactoside | S. elaeagnifolium | Aerial | Hepatoprotective | [502] |
| 374 | Kaempferol 8-C-glucoside | S. elaeagnifolium | Aerial | [502] | |
| 375 | Kaempferol 6-C-glucoside | S. elaeagnifolium | Aerial | [502] | |
| 376 | Vitexin | S. elaeagnifolium | Aerial | [502] | |
| 377 | Vicenin II | S. elaeagnifolium | Aerial | [502] | |
| 378 | Quercetin 6-C-β-glucoside | S. elaeagnifolium | Aerial | [502] | |
| 379 | Quercetin 3-O-β-galactoside | S. elaeagnifolium | Aerial | [502] | |
| 380 | Isoquercitrin | S. elaeagnifolium | Aerial | [502–504] | |
| S. incanum | Aerial | [494] | |||
| S. torvum | Root | [338] | |||
| S. melongena | Stem | [205] | |||
| 381 | Quercetin 3-O-β-apiofuranosyl-(1-2)-O-β-galactoside | S. elaeagnifolium | Aerial | [502] | |
| 382 | 5-Hydroxy,7,2′,3′,5′-tetramethoxyflavone | S. glabratum | Whole | [140] | |
| 383 | Combretol | S. glabratum | Whole | [140] | |
| 384 | Baicalin | S. incanum | Aerial | [506] | |
| 385 | Kaempferol 3‐O‐(6‴‐O‐2,5‐dihydroxycinnamoyl)‐β‐D‐glucosyl(1-2) β‐D‐glucoside | S. incanum | Aerial | [506] | |
| 386 | (±)-Naringenin | S. indicum | Whole | [505] | |
| S. nienkui | Whole | [509] | |||
| S. sessiliflorum | Fruit | [510] | |||
| S. surattense | Whole | [99] | |||
| 387 | Manghaslin | S. lycopersicum | Fruit | [511] | |
| 388 | Genkwanin | S. jabrense | Aerial | [167] | |
| S. palodusum | Aerial | [512] | |||
| 389 | Ombuine | S. jabrense | Aerial | [167] | |
| 390 | Rhamnocitrin | S. jabrense | Aerial | [167] | |
| S. palodusum | Aerial | [513] | |||
| 391 | Retusin | S. jabrense | Aerial | [167] | |
| S. palodusum | Aerial | [512] | |||
| S. schimperianum | Aerial | [278] | |||
| S. torvum | Fruit | [322] | |||
| 392 | Pentamethoxyquercetin | S. jabrense | Aerial | [167] | |
| 393 | 3-O-Methylquercetin | S. jabrense | Aerial | [167] | |
| S. palodusum | Aerial | [513] | |||
| 394 | Kumatakenin | S. jabrense | Aerial | [167] | |
| S. palodusum | Aerial | [513] | |||
| 395 | 3′-Hydroxyflindulatin | S. jabrense | Aerial | [167] | |
| S. palodusum | Aerial | [513] | |||
| 396 | 3,7,8-Trimethylherbacetin | S. jabrense | Aerial | [167] | |
| 397 | 3,7,8,3′,4′-Pentamethylgossypetin | S. jabrense | Aerial | [167] | |
| S. palodusum | Aerial | [512, 513] | |||
| 398 | Diosmetin | S. nienkui | Whole | [509] | |
| 399 | Formononetin | S. lyratum | Whole | [514] | |
| 400 | Ononin | S. lyratum | Whole | [514] | |
| 401 | Daidzein | S. lyratum | Whole | [507, 514] | |
| 402 | Genistin | S. lyratum | Whole | [514] | |
| 403 | 5-Hydroxylononin | S. lyratum | Whole | [514] | |
| 404 | 2,7-Dihydroxy-3-(4-hydroxyphenyl)-5-methoxy-4H-1-benzopyran-4-one | S. nienkui | Whole | [509] | |
| 405 | 5-hydroxy-3,7,4′-trimethoxyflavone | S. schimperianum | Aerial | [278] | |
| 406 | Kaempferol-3-O-β-d-glucoside | S. schimperianum | Aerial | [278] | |
| 407 | Luteolin | S. schimperianum | Aerial | [278] | |
| 408 | Tamarixin | S. torvum | Whole | [498] | |
| 409 | Torvanol A | S. torvum | Root | Antidepressant, antiviral | [322, 332] |
| 410 | 5-methoxy-(3,4″-dihydro-3″,4″-diacetoxy)-2″,2′-dimethyl-(7,8:5″,6″)-flavone | S. erianthum | Leaf | [137] | |
| 411 | 5,7,8,4′-tetrahydroxy-3-methoxyflavone-8-O-β-d-xyloside | S. rostratum | Aerial | [515] | |
| 412 | 3-O-Methylquercetin 3-O-β-d-galactoside | S. rostratum | Whole | [516] | |
| 413 | 3-O-Methylquercetin 3-O-β-d-glucoside | S. rostratum | Whole | [516] | |
| Lignans | |||||
| 414 | Isolariciresinol | S. buddleifolium | Stem | [79] | |
| 415 | 5-Methoxyisolariciresinol | S. buddleifolium | Stem | [79] | |
| 416 | Polystachyol | S. buddleifolium | Stem | [79] | |
| 417 | (+)-Lyoniresinol 3-O-d-glucoside | S. buddleifolium | Stem | [79] | |
| 418 | (-)-Lyoniresinol 3-O-d-glucoside | S. buddleifolium | Stem | [79] | |
| 419 | Alangilignoside C | S. buddleifolium | Stem | [79] | |
| 420 | (+)-(7S,8R,7′E)-4-Hydroxy-3,5,5′,9′-tetram ethoxy-4′,7-epoxy-8,3′-neo-lign-7′-en-9-ol | S. erianthum | Stem | [480] | |
| 421 | (-)-(7R,8S,7′E)-4-Hydroxy-3,5,5′,9′-tetramethoxy-4′,7-epoxy-8,3′-neo-lign-7′-en-9-ol | S. erianthum | Stem | [480] | |
| 422 | Liriodendrin | S. lyratum | Whole | [517] | |
| 423 | Syringaresinol | S. lyratum | Whole | [517] | |
| S. nigrum | Whole | [496] | |||
| S. surattense | Whole | [518] | |||
| 424 | Melongenamide A | S. melongena | Root | [210] | |
| 425 | Cannabisin D | S. melongena | Root | Anti-inflammatory | [210] |
| 426 | Melongenamide B | S. melongena | Root | Anti-inflammatory | [210] |
| 427 | Grossamide | S. melongena | Root | Anti-inflammatory | [210] |
| 428 | Melongenamide C | S. melongena | Root | Anti-inflammatory | [210] |
| 429 | Cannabisin F | S. melongena | Root | Anti-inflammatory | [210] |
| 430 | Melongenamide D | S. melongena | Root | Anti-inflammatory | [210] |
| 431 | Cannabisin G | S. melongena | Root | Anti-inflammatory | [210] |
| 432 | 1,2-dihydro-6,8-dimethoxy-7-hydroxy-1-(3,5-dimethoxy-4-hydroxyphenyl)-N1,N2-bis-[2-(4-hydroxyphenyl)ethyl]-2,3-naphthalene dicarboxamide | S. melongena | Root | [210] | |
| 433 | Sisymbrifolin | S. sisymbriifolium | Fruit | [519] | |
| 434 | Grossamide K | S. melongena | Root | [210] | |
| 435 | Pinoresinol | S. nigrum | Whole | [496] | |
| 436 | Pinoresinol 4-O-β-d-glucoside | S. nigrum | Whole | [520] | |
| 437 | Medioresinol | S. nigrum | Whole | [496] | |
| S. torvum | Stem | [436] | |||
| 438 | Syringaresinol-4′-O-β-d-glucoside | S. nigrum | Whole | [520] | |
| 439 | Glycosmisic acid | S. surattense | Whole | [518] | |
| 440 | Simulanol | S. surattense | Whole | [518] | |
| 441 | Balanophonin | S. surattense | Whole | [518] | |
| 442 | Ficusal | S. melongena | Root | [209] | |
| 443 | Tribulusamide A | S. surattense | Whole | [518] | |
| 444 | Clemastanin B | S. torvum | Fruit | [521] | |
| Other alkaloids | |||||
| 445 | Xylogranatinine | S. cathayanum | Stem | [477] | |
| 446 | Cernumidine | S. cernuum | Leaf | [109, 111, 112] | |
| 447 | Isocernumidine | S. cernuum | Leaf | [111] | |
| 448 | Cernidine | S. cernuum | Leaf | [501] | |
| 449 | Ethyl orotate | S. cathayanum | Stem | [103, 477] | |
| 450 | 3-Indolecarboxylic acid | S. americanum | Aerial | [49] | |
| 451 | L-Valyl-l-isoleucyl-l-leucine | S. asperum | Fruit | [67] | |
| 452 | 2-Methyltetrahydro-β-carboline | S. jabrense | Aerial | [166] | |
| 453 | Proline | S. asperum | Fruit | [67] | |
| 454 | Acetamide | S. schimperianum | Aerial | [277] | |
| 455 | Stearamide | S. schimperianum | Aerial | [277] | |
| 456 | (6E, 9E)N,N-dimethyloctadeca-6,9-dienamide | S. schimperianum | Aerial | [277] | |
| 457 | (2E)-3-(4-Hydroxyphenyl)-N-[(2S)-2-(4-hydroxyphenyl)-2-methoxyethyl]-2-propenamide | S. torvum | Aerial | [450] | |
| 458 | 4-Coumaroyltyramine | S. buddleifolium | Stem | [79] | |
| S. cathayanum | Stem | [522] | |||
| S. indicum | Root | [163] | |||
| S. melongena | Root | [209] | |||
| S. surattense | Whole | [518] | |||
| S. torvum | Aerial | [338] | |||
| S. lyratum | Whole | [507] | |||
| 459 | N-trans-Feruloyltyramine | S. buddleifolium | Stem | [79] | |
| S. cathayanum | Stem | [522] | |||
| S. indicum | Root | [163] | |||
| S. melongena | Root | Antidiabetic | [209] | ||
| S. lyratum | Whole | [507] | |||
| 460 | N-trans-Feruloylmethoxytyramine | S. buddleifolium | Stem | [79] | |
| S. cathayanum | Stem | [522] | |||
| 461 | N-trans-Caffeoyltyramine | S. buddleifolium | Stem | [79] | |
| 462 | N-trans-Feruloyldopamine | S. buddleifolium | Stem | [79] | |
| 463 | N-trans-Feruloyloctopamine | S. cathayanum | Stem | [522] | |
| S. septemlobum | Aerial | [523] | |||
| 464 | N-trans-p-coumaroyloctopamine | S. americanum | Aerial | Antidiabetic | [49] |
| S. torvum | Aerial | [524] | |||
| 465 | N-trans-p-feruloyloctopamine | S. americanum | Aerial | Antidiabetic | [49] |
| 466 | N-trans-p-coumaroyltyramine | S. americanum | Aerial | Antidiabetic | [49] |
| S. melongena | Root | ||||
| 467 | N-trans-p-feruloytyramine | S. americanum | Aerial | Antidiabetic | [49] |
| S. torvum | Aerial | [524] | |||
| 468 | N-cis-p-Coumaroyltyramine | S. melongena | Root | [209] | |
| 469 | Caffeoylputrescine | S. melongena | Stem | [205] | |
| 470 | 3-(3,4-Dihydroxyphenyl)-N-[3-[[4-[[3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl] amino]butyl]amino]propyl]-2-propenamide | S. melongena | Stem | [205] | |
| 471 | Aurantiamide acetate | S. torvum | Aerial | [524] | |
| 472 | N1,N4,N8-Tris(dihydrocaffeoyl) spermidine | S. sessiliflorum | Fruit | [525] | |
| 473 | N-(4-Aminobutyl)-N-[3-[[3-(3,4-dihydroxyphenyl)-1-oxopropyl] amino]propyl]-3,4-dihydroxybenzenepropanamide | S. sessiliflorum | Fruit | [525] | |
| 474 | N-(3-Aminopropyl)-N-[4-[[3-(3,4-dihydroxyphenyl)-1-oxopropyl] amino]butyl]-3,4-dihydroxybenzenepropanamide | S. sessiliflorum | Fruit | [525] | |
| 475 | Soya-cerebroside I | S. torvum | Root | [435] | |
| Sterols | |||||
| 476 | Cilistol G | S. capsicoides | Leaf | [85] | |
| 477 | Capsisteroid A | S. capsicoides | Leaf | [85] | |
| 478 | Capsisteroid B | S. capsicoides | Leaf | [85] | |
| 479 | Capsisteroid C | S. capsicoides | Leaf | [85] | |
| 480 | Capsisteroid D | S. capsicoides | Leaf | [85] | |
| 481 | Capsisteroid E | S. capsicoides | Leaf | [85] | |
| 482 | Capsisteroid F | S. capsicoides | Leaf | [85] | |
| 483 | β-Sitosterol | S. cathayanum | Stem | [477, 522] | |
| S. anguvi | Fruit | [34] | |||
| S. cornifolium | Aerial | [472, 473] | |||
| S. dulcamara | Fruit | [130] | |||
| S. elaeagnifolium | Whole | [134, 504] | |||
| S. indicum | Whole | [160] | |||
| S. lycopersicum | Seed | [478] | |||
| S. melongena | Aerial | [206, 439] | |||
| S. schimperianum | Aerial | [278] | |||
| S. surattense | Aerial | [518] | |||
| S. torvum | Root | [526] | |||
| S. trilobatum | Whole | [356] | |||
| S. xanthocarpum | Fruit | [398] | |||
| 484 | Daucosterol | S. cathayanum | Stem | [522] | |
| S. chrysotrichum | Leaf | [120] | |||
| S. elaeagnifolium | Whole | [504] | |||
| S. glabratum | Whole | [140] | |||
| S. ligustrinum | Aerial | [179] | |||
| S. septemlobum | Aerial | [523] | |||
| S. torvum | Root | [526] | |||
| S. violaceum | Whole | [392] | |||
| 485 | Campesterol | S. elaeagnifolium | Seed | [134] | |
| S. melongena | Root | [439] | |||
| 486 | Cholesterol | S. lycopersicum | Seed | [478] | |
| S. sessiliflorum | Fruit | [285] | |||
| 487 | γ-Sitosterol | S. lycopersicum | Seed | [478] | |
| 488 | 7-Oxositosterol | S. violaceum | Aerial | [391] | |
| 489 | (3β)-7-Hydroxystigmast-5-en-3-yl β-d-glucoside | S. violaceum | Whole | [392] | |
| 490 | Stigmasterol | S. cornifolium | Aerial | [472, 473] | |
| S. dulcamara | Fruit | [130] | |||
| S. elaeagnifolium | Whole | [134, 504] | |||
| S. lycopersicum | Seed | [478] | |||
| S. melongena | Aerial | [439] | |||
| S. septemlobum | Aerial | [523] | |||
| S. surattense | Aerial | [527] | |||
| S. xanthocarpum | Fruit | [398] | |||
| 491 | Brassicasterol | S. elaeagnifolium | Seed | [134] | |
| 492 | Poriferasterol monoglucoside | S. glabratum | Whole | [140] | |
| 493 | 7-Oxostigmasterol | S. violaceum | Aerial | [391] | |
| 494 | β-stigmasteryl-3-O-β-d-6-palmityl) glucoside | S. septemlobum | Aerial | [523] | |
| 495 | Clerosterol | S. elaeagnifolium | Seed | [134] | |
| 496 | 7-Sitoster-3β-ol | S. elaeagnifolium | Seed | [134] | |
| 497 | (3β,5α)Cholest-7-en-3-ol | S. lycopersicum | Seed | [478] | |
| 498 | Stigmasta-5,24(28)-dien-3-ol | S. elaeagnifolium | Seed | [134] | |
| S. torvum | Leaf | [318] | |||
| 499 | Avenasterol | S. elaeagnifolium | Seed | [134] | |
| 500 | 5,24-Stigmastadienol | S. elaeagnifolium | Seed | [134] | |
| 501 | γ-Tocopherol | S. lycopersicum | Seed | [478] | |
| S. villosum | Leaf | [479] | |||
| 502 | Ergosterol | S. lycopersicum | Seed | [478] | |
| 503 | Lanosterol | S. lycopersicum | Seed | [478] | |
| 504 | Peroxyergosterol | S. lyratum | Stem | [486] | |
| S. violaceum | Aerial | [391] | |||
| 505 | 9,11-Dehydroergosterol peroxide | S. lyratum | Stem | [486] | |
| S. violaceum | Aerial | [391] | |||
| 506 | Nigralanostenone | S. nigrum | Leaf | [528] | |
| 507 | Tumacone A | S. nudum | Leaf | [242, 247] | |
| 508 | Tumacone B | S. nudum | Leaf | [242, 247] | |
| 509 | Tumacoside A | S. nudum | Leaf | Antiplasmodial | [242, 247] |
| 510 | Tumacoside B | S. nudum | Leaf | Antiplasmodial | [242, 247] |
| 511 | SN-1 | S. nudum | Aerial | Antiplasmodial | [245] |
| 512 | SN-2 | S. nudum | Aerial | Antiplasmodial | [245] |
| 513 | SN-3 | S. nudum | Aerial | Antiplasmodial | [245] |
| 514 | SN-4 | S. nudum | Aerial | Antiplasmodial | [245] |
| 515 | SN-5 | S. nudum | Aerial | Antiplasmodial | [245] |
| 516 | 9α,11α-epidioxyergosta-6,22-dien-3β-ol | S. septemlobum | Aerial | [523] | |
| 517 | Carpesterol | S. capsicoides | Seed | Anticancer, antifungal | [86] |
| S. sisymbriifolium | Fruit | [519] | |||
| 518 | Carpesterol methyl ether | S. xanthocarpum | Fruit | Antifungal | [401] |
| 519 | Carpesterol ethyl ether | S. xanthocarpum | Fruit | Antifungal | [401] |
| 520 | Stigmast-7-en-6-one, 3-β-d-glucosyloxy)22-hydroxy-4-methyl-(3β,4α,5α,22R) | S. xanthocarpum | Fruit | Antifungal | [401] |
| 521 | Stigmast-7-en-6-one, 3-β-d-glucosyloxy)22-methoxy-4-methyl-(3β,4α,5α,22R) | S. xanthocarpum | Fruit | Antifungal | [401] |
| 522 | Toptriol | S. glaucophyllum | Leaf | [529] | |
| 523 | Cholecalciferol | S. glaucophyllum | Leaf | [530] | |
| 524 | β-d-Glucoside, (1α,3β,5Z,7E)-3,25-dihydroxy-9,10-secocholesta -5,7,10(19) –trien -1-yl | S. glaucophyllum | Leaf | [530] | |
| 525 | Dehydrocholesterol | ||||
| 526 | 3,4-Dihydro-3,5,8-trimethyl-3-(4,8,12-trimethyltridecyl)-2H-1-benzopyran-7-yl acetate | S. villosum | Leaf | [479] | |
| 527 | Tumaquenone | S. nudum | Aerial | [247] | |
| 528 | Abutiloside A | S. abutiloides | Root | [5, 7–9] | |
| 529 | Abutiloside B | S. abutiloides | Root | [5] | |
| 530 | Abutiloside H | S. abutiloides | Root | [5] | |
| 531 | Abutiloside I | S. abutiloides | Root | [5] | |
| 532 | Abutiloside J | S. abutiloides | Root | [5] | |
| 533 | Abutiloside K | S. abutiloides | Root | [5] | |
| 534 | Abutiloside C | S. abutiloides | Root | [7, 8] | |
| 535 | Abutiloside D | S. abutiloides | Root | [6] | |
| 536 | Abutiloside E | S. abutiloides | Root | [6] | |
| 537 | Abutiloside F | S. abutiloides | Root | [6] | |
| 538 | Abutiloside G | S. abutiloides | Root | [6] | |
| 539 | Aethioside A | S. aethiopicum | Stem | [28] | |
| 540 | Aethioside B | S. aethiopicum | Stem | [28] | |
| 541 | Aethioside C | S. aethiopicum | Stem | [28] | |
| Phenolic compounds | |||||
| 542 | 4-Caffeoylquinic acid | S. melongena | Stem,Leaf | [205, 531] | |
| S. lyratum | Whole | [517] | |||
| 543 | 5-Caffeoylquinic acid | S. melongena | Stem | [205] | |
| S. sessiliflorum | Fruit | [525] | |||
| 544 | (1R,3R,4S,5R)-3-(Acetyloxy)-5-[[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl]oxy] -1,4-dihydroxycyclohexanecarboxylic acid | S. melongena | Stem | [205] | |
| 545 | (1S,3R,4R,5R)-3-(Acetyloxy)-4-[[(2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl]oxy] -1,5-dihydroxycyclohexanecarboxylic acid | S. melongena | Stem | [205] | |
| 546 | Chlorogenic acid | S. anguvi | Fruit | Anticancer | [31] |
| S. guaraniticum | Leaf | [146] | |||
| S. incanum | Aerial | [494] | |||
| S. lycocarpum | Fruit | [532] | |||
| S. lyratum | Whole | [517] | |||
| S. melongena | Stem,Leaf | [205, 531] | |||
| S. surattense | Whole | [99] | |||
| 547 | Neochlorogenic acid | S. lyratum | Whole | [517] | |
| 548 | Rosmarinic acid | S. betaceum | Fruit | [78] | |
| S. guaraniticum | Leaf | [146] | |||
| 549 | 3,5-Dicaffeoylquinic acid | S. melongena | Stem | [91] | |
| 550 | (Z)-Neochlorogenic acid | S. melongena | Stem | [91] | |
| 551 | Gallic acid | S. anguvi | Fruit | Anticancer | [31] |
| S. cernuum | Leaf | [112] | |||
| S. spirale | Aerial | [299] | |||
| S. surattense | Whole | [99] | |||
| 552 | 4-hydroxybenzoic acid | S. crinitum | Fruit | [122] | |
| S. americanum | Aerial | [49] | |||
| 553 | Protocatechuic acid | S. lyratum | Whole | [514] | |
| S. spirale | Leaf | [297] | |||
| S. nigrum | Whole | [520] | |||
| 554 | Vanillic acid | S. lyratum | Whole | [514] | |
| S. sessiliflorum | Fruit | [510] | |||
| S. nigrum | Whole | [520] | |||
| S. vestissimum | Fruit | [491] | |||
| 555 | Caffeic acid | S. anguvi | Fruit | Anticancer | [31] |
| S. guaraniticum | Leaf | [146] | |||
| S. incanum | Aerial | [506] | |||
| S. lycocarpum | Fruit | [532] | |||
| S. lyratum | Whole | [194] | |||
| S. melongena | Stem | [205] | |||
| S. muricatum | Whole | [215] | |||
| S. surattense | Whole | [99, 518] | |||
| S. xanthocarpum | Root | [427] | |||
| 556 | P-Coumaric acid | S. americanum | Aerial | [49] | |
| 557 | Isoferulic acid | S. cernuum | Leaf | [109, 112] | |
| 558 | 2,4,6-Trimethoxyphenol | S. torvum | Stem | [533] | |
| 559 | Propionylsyringol | S. torvum | Stem | [533] | |
| 560 | Resveratrol | S. americanum | Fruit | [45] | |
| 561 | cis-p-Coumaric acid ethyl ester | S. crinitum | Fruit | [122] | |
| 562 | cis-p-Coumaric acid | S. crinitum | Fruit | [122] | |
| 563 | trans-p-Coumaric acid ethyl ester | S. crinitum | Fruit | [122] | |
| 564 | trans-p-Coumaric acid | S. crinitum | Fruit | [122] | |
| S. incanum | Aerial | [506] | |||
| 565 | Erythro-1,2-bis-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | S. lyratum | Whole | [517] | |
| 566 | Threo-1,2-bis-(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | S. lyratum | Whole | [517] | |
| 567 | Evofolin B | S. surattense | Whole | [518] | |
| 568 | Ethyl caffeate | S. nienkui | Whole | [509] | |
| 569 | Methyl salicylate | S. nienkui | Whole | [509] | |
| S. aculeastrum | Leaf | [11] | |||
| 570 | p-Hydroxybenzoic acid | S. nigrum | Whole | [520] | |
| 571 | Vanillin | S. nienkui | Whole | [509] | |
| 572 | Protocatechuic aldehyde | S. nienkui | Whole | [509] | |
| 573 | 3,5-Diethoxyphenol | S. nigrum | Leaf | [528] | |
| 574 | Quinic acid | S. sessiliflorum | Fruit | [525] | |
| 575 | Phenol | S. sessiliflorum | Fruit | [525] | |
| 576 | Salicylic acid | S. torvum | Aerial | [524] | |
| 577 | Violaxanthin | S. sessiliflorum | Fruit | [525] | |
| 578 | Lutein | S. sessiliflorum | Fruit | [525] | |
| 579 | α-Carotene | S. sessiliflorum | Fruit | [525] | |
| 580 | Kryptoxanthin | S. sessiliflorum | Fruit | [525] | |
| 581 | Luteoxanthin | S. sessiliflorum | Fruit | [525] | |
| 582 | 15-cis-β-Carotene | S. sessiliflorum | Fruit | [525] | |
| 583 | Foliaxanthin | S. sessiliflorum | Fruit | [525] | |
| 584 | Physoxanthin | S. sessiliflorum | Fruit | [525] | |
| 585 | Coniferol | S. surattense | Whole | [518] | |
| 586 | 1,2-Bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol | S. surattense | Whole | [518] | |
| 587 | Threo-1-(4-Hydroxy-3-methoxyphenyl)-2-[4-[(E)-3-hydroxy-1-propenyl]-2-methoxy phenoxy]-1,3-propanediol | S. surattense | Whole | [518] | |
| 588 | Tyrosol C | S. validinervium | Aerial | [534] | |
| 589 | (E)-Coniferaldehyde | S. melongena | Root | [209] | |
| 590 | trans-Cinnamic acid | S. spirale | Leaf | Antibacterial | [297] |
| 591 | Methyl caffeate | S. torvum | Fruit | Antibacterial,antidiabetic | [315, 320, 335–337] |
| 592 | (E)-2,3-dihydroxycyclopentyl-3-(3′,4′-dihydroxyphenyl)acrylate | S. torvum | Fruit | Antihypertensive | [521] |
| 593 | Eugenol | S. torvum | Stem | [533] | |
| Coumarins and coumestans | |||||
| 594 | Scopolin | S. cathayanum | Stem | Anticancer | [104, 105] |
| S. lyratum | Whole | [194] | |||
| S. septemlobum | Aerial | [523] | |||
| 595 | Scopoletin | S. glabratum | Whole | [140] | |
| S. indicum | Seed | [535] | |||
| S. ligustrinum | Aerial | [179] | |||
| 596 | Coumarin | S. incanum | Leaf | [494] | |
| S. surattense | Whole | [99] | |||
| S. vestissimum | Fruit | [491] | |||
| 597 | Fraxetin | S. indicum | Seed | [536] | |
| 598 | Isofraxidin | S. indicum | Seed | [536] | |
| 599 | Umbelliferone | S. lycopersicum | Aerial | [438] | |
| 600 | 7-hydroxy-6,8-dimethoxy-3-(4′-hydroxy-3′-methoxyphenyl)-coumarin | S. indicum | Seed | [536] | |
| 601 | Cleosandrin | S. indicum | Seed | [535] | |
| 602 | 4,4′-Biisofraxidin | S. indicum | Seed | [535] | |
| 603 | Arteminorin A | S. indicum | Seed | [535] | |
| 604 | Indicumin E | S. indicum | Seed | [536] | |
| 605 | Bergaptin | S. lycopersicum | Aerial | [438] | |
| 606 | Aesculetin | S. lycopersicum | Aerial | [438] | |
| S. validinervium | Aerial | [534, 537] | |||
| 607 | 6, 7-Dimethoxycoumarin | S. melongena | Root | [209] | |
| 608 | Escopoletin | S. nigrum | Whole | [520] | |
| 609 | Isoscopoletin | S. validinervium | Aerial | [534, 537] | |
| 610 | 1′-O-7-esculetin-4′-O-1″-ethylenglycol-β-d-glucose | S. validinervium | Aerial | [534] | |
| 611 | Coumestrol | S. lyratum | Whole | Anti-inflammatory | [88] |
| 612 | 9-hydroxy-2′,2′-dimethyl[5′,6′:2,3]-coumestan | S. lyratum | Whole | Anti-inflammatory | [88] |
| 613 | Solalyratin A | S. lyratum | Whole | Anti-inflammatory | [88] |
| Coumarinolignoids | |||||
| 614 | Indicumine A | S. indicum | Seed | Anti-HBV | [535] |
| 615 | Indicumine B | S. indicum | Seed | Anti-HBV | [535] |
| 616 | Indicumine C | S. indicum | Seed | [535] | |
| 617 | Indicumine D | S. indicum | Seed | [535] | |
| Fatty acids and esters | |||||
| 618 | Hexadecanoic acid | S. aculeastrum | Leaf | [11] | |
| S. vestissimum | Fruit | [490] | |||
| S. villosum | Leaf | [434, 479] | |||
| 619 | Octadecanoic acid, | S. aculeastrum | Leaf | [11] | |
| S. erianthum | Leaf | [137] | |||
| 620 | Linoleic acid | S. aculeastrum | Leaf | [11] | |
| S. glabratum | Whole | [140] | |||
| 621 | Lignoceric acid | S. cathayanum | Stem | [477] | |
| 622 | Corchorifatty acid B | S. americanum | Aerial | [49] | |
| 623 | Linolenic acid | S. erianthum | Leaf | [137] | |
| S. glabratum | Whole | [140] | |||
| 624 | 9(Z),11(E)-Octadecadienoic acid | S. erianthum | Leaf | [137] | |
| 625 | 13S-Hydroxy-9(Z),11(E)-octadecadienoic acid | S. erianthum | Leaf | [137] | |
| 626 | 9S-Hydroxy-10(E),12(Z),15(Z)-octadecatrienoic acid | S. erianthum | Leaf | [137] | |
| 627 | Decosahexaenoic acid | S. glabratum | Whole | [140] | |
| 628 | Decosapentaenoic acid | S. glabratum | Whole | [140] | |
| 629 | Oleic acid | S. glabratum | Whole | [140] | |
| 630 | Eicosapentaenoic acid | S. glabratum | Whole | [140] | |
| 631 | Lauric acid | S. glabratum | Whole | [140] | |
| 632 | Palmitoleic acid | S. glabratum | Whole | [140] | |
| 633 | Arachidonic acid | S. glabratum | Whole | [140] | |
| S. trilobatum | Whole | [356] | |||
| 634 | Myristic acid | S. glabratum | Whole | [140] | |
| 635 | Gamma-linolenic acid | S. glabratum | Whole | [140] | |
| 636 | 9-Oxo-(10E, 12Z)-octadecadienoic acid | S. melongena | Calyx | [91] | |
| 637 | (10Z,12E)-9-Oxo-10,12-octadecadienoic acid | S. melongena | Calyx | [91] | |
| 638 | Eicosanoic acid | S. torvum | Root | [526] | |
| 639 | Octacosanoic acid | S. torvum | Root | [526] | |
| 640 | 4-(3,5-Di-Tert-Butyl-4-Hydroxy Phenyl) butyl Acrylate | S. villosum | Leaf | [479] | |
| Others | |||||
| 641 | Puerariafuran | S. lyratum | Whole | Anti-inflammatory | [88] |
| 642 | 1,2-Benzenedicarboxylic acid | S. aculeastrum | Leaf | [11] | |
| 643 | 1, 4-Dimethyl-benzene | S. aculeastrum | Leaf | [11] | |
| 644 | n-Nonane | S. aculeastrum | Leaf | [11] | |
| 645 | n-Octanol | S. aculeastrum | Leaf | [11] | |
| 646 | Methyl hexadecanoate | S. aculeastrum | Leaf | [11] | |
| 647 | Dodecane | S. aculeastrum | Leaf | [11] | |
| 648 | Undecanal | S. aculeastrum | Leaf | [11] | |
| 649 | Nonanal | S. aculeastrum | Leaf | [11] | |
| 650 | Eicosane | S. aculeastrum | Leaf | [11] | |
| S. betaceum | Fruit | [77] | |||
| 651 | Methyl-9,12-octadecadienoate | S. aculeastrum | Leaf | [11] | |
| 652 | Hexadecane | S. aculeastrum | Leaf | [11] | |
| 653 | 9,17-Octadecadienal | S. aculeastrum | Leaf | [11] | |
| 654 | Hexanal | S. betaceum | Fruit | [78] | |
| 655 | Ethyl butanoate | S. betaceum | Fruit | [78] | |
| 656 | 4-Hydroxy-4-methyl-2-pentanone | S. betaceum | Fruit | [78] | |
| 657 | 2,3-Butanediol | S. betaceum | Fruit | [78] | |
| 658 | cis-3-Hexen-1-ol | S. betaceum | Fruit | [78] | |
| 659 | 3(Z)-Hexenal | S. betaceum | Fruit | [78] | |
| 660 | Ethyl-α-d-arabinofuranoside | S. lyratum | Whole | [514] | |
| 661 | Solalyratin B | S. lyratum | Whole | Anti-inflammatory | [88] |
| 662 | 1-{1-[2-(2 hydroxypropoxy) propoxy] propan-2-yloxy} propan-2-ol | S. schimperianum | Aerial | [277] | |
| 663 | 5-Hydroxymethyl furfural | S. torvum | Stem | [533] | |
| 664 | Solanesol | S. tuberosum | Leaf | [538] | |
| 665 | 3-Hydroxymethyl-7-methoxywutaifuranol | S. cathayanum | Whole | [102] | |
| 666 | Phenylmethyl 2-O-β-d-xylosyl-β-d-glucoside | S. incanum | Aerial | [506] | |
| 667 | Zizybeoside I | S. lycopersicum | Fruit | [511] | |
| 668 | Methyl salicylate 2-O-β-d-glucosyl-(1-2)-[O-β-d-xylosyl-(1-6)]-O-β-d-glucoside | S. lycopersicum | Fruit | [511] | |
| 669 | Phenethyl alcohol 8-O-β-d-glucosyl-(1-2)-[O-α-l-arabinosyl-(1-6)]-O-β-d-glucoside | S. lycopersicum | Fruit | [511] | |
| 670 | Benzyl alcohol 7-O-β-d-glucosyl-(1-2)-[O-α-l-arabinosyl-(1-6)]-β-d-glucoside | S. lycopersicum | Fruit | [511] | |
Steroidal Saponins
Steroidal saponins are prominent characteristic components in Solanum species, from which 134 compounds, 1–134, have been obtained (Fig. 1). Among all the studied species, S. torvum was the one studied mostly, resulting in the isolation of 32 saponins including chlorogenone (1), (5α,25S)-spirostan-3,6-dione (2), diosgenone (13), 56–72, neochlorogenin (73), solanolactosides A–C (91–93), torvosides J–L (95–97) and 98–102 from the leaves, fruits, aerial parts and the whole plant [323, 325, 430, 435, 436, 448, 449, 451, 452, 463].
Fig. 1.




Steroidal saponins 1–134 from Solanum
Included herein are spirostane saponins, SC1–SC6 (35–40), isolated from the leaves of S. chrysotrichum [113–115, 117], and lyconosides Ia (46), Ib (47), II (48), III (49), and IV (50) reported from the fruits of S. lycocarpum. Indiosides G (82) and H (83) with an iso-type F ring were isolated from the methanolic extract of the whole plant of S. violaceum, together with indioside I (86), and two unusual furostanol saponins with a deformed F ring, indiosides J (87) and K (88) [391, 392]. In addition, four steroidal sapogenins, indiosides L–O (78–81) were also obtained from this plant [391]. Indioside L (78) is a rare spirostanoid possessing a 1,4-dien-3-one moiety in ring A. Compounds 80 and 81 represent rare examples of spirostane with the 3β,7α-diol-5,6-ene moiety compared to the normal 3β,7β-diol-5,6-ene derivatives [391].
Two C-22 steroidal lactone saponins, namely solanolactosides A, B (91, 92) and two spirostanol glycosides, torvosides M, N (23, 8) were isolated from ethanol extract of aerial parts of S. torvum. Compounds 91 and 92 possess the aglycon of solanolide (94), while 23 and 8 have the aglycons of yamogenin (76) and neochlorogenin (73), resp. The aglycon of 94 is an unusual C-22 steroidal lactone sapogenin [316].
An avenacoside-type saponin (51) was isolated from aerial parts of S. surattense [305]. Two 23-keto-spirostanol glycosides, torvoside Q (18) and paniculonin B (126) were obtained from aerial parts of S. torvum [323, 331]. Torvosides A (64), B (65), F (67) and G (112) displayed a positive reaction with Ehrlich reagent, suggesting these to be furostanol glycosides [449]. Abutilosides L (106), M (107) and N (108), a 22S,25S-epoxy-furost-5-ene type glycosides, and abutiloside O, being a 20,22-seco-type steroidal glycoside, were isolated from the fresh fruits of S. abutiloides [4].
Anguiviosides III (118) and XI (119) are hydroxylated at C-23 and C-26 on the spirostanol and furostanol skeletons, resp. Anguiviosides XV (120) and XVI (121) are based on a 16, 22-dicarbonyl aglycon, with 121 hydroxylated at C-23 and C-26 followed by ring closure. The biogenetic pathway of 16,22-dicarbonyl compounds such as 120 and 121 might be considered via a 17R-hydroxy spirostanol such as pennogenin, 11 or via a 3β,16β,22,26-tetrahydroxycholesterol glycoside such as anguivioside A (114) [43].
Solanum saponins were reported to have various bioactivies, e.g. cytotoxic [257], anticancer [316, 317, 392], hepatoprotective [242, 247], antihypertensive [289, 291], antimelanogenesis [211], antifungal [113, 114, 117], anti-inflammatory [331, 392, 448] anticonvulsant [305] and antiviral [257].
Nuatigenosido (15) from the roots of S. sisymbriifolium presented anti-hypertensive effect in experimental hypertensive rats [291]. Dioscin (19) showed antimelanogenesis effect on α-melanocyte stimulating hormone (α-MSH)induced melanogenesis in B16 murine melanoma cells. It significantly downregulated the expression of tyrosinase, TRP-1, and TRP-2, which led to the reduction of α-MSH-induced melanogenesis in B16 cells [211]. Degraded diosgenone (13) from S. nudum exhibited hepatoprotective effect on the liver of mice infected with Plasmodium berghei; necrosis of hepatocytes in mice infected with malaria decreased 47–65 [249].
Spirostanic saponins SC2-SC6 (36–40) from the leaves of S. chrysotrichum displayed activity against dermatophytes and yeasts. 36 was the most active in indicating fungicidal effect against Candida albicans and non-albicans strains [113, 114, 117].
Indioside H (83), borassoside E (85), indioside I (86) and yamoscin (89) demonstrated cytotoxic activity against six human cancer cell lines (HepG2, Hep3B, A549, Ca9-22, MDA-MB-231, and MCF-7) (IC50 = 1.83–8.04 μg/mL) [392]. Seperately, 85 and 86 presented inflammation inhibitory effects on SAG (IC50 = 0.62 ± 0.03 and 2.84 ± 0.18 μg/mL, resp.). Compound 85 also inhibited elastase release with IC50 values of 111.05 ± 7.37 μg/mL [392], while 89 showed anti-neutrophilic inflammatory activity against SAG with an IC50 value of 3.59 μM [331].
Torvosides N (8) and M (23) revealed significant cytotoxicity against MGC-803, HepG2, A549 and MCF-7 as compared to the positive control, CDDP [316]. Torvosides J-L (95–97), isolated from the leaves of S. torvum, exhibited substantial anticonvulsant activity in zebrafish seizure assays [323], while 96 also showed considerable antifungal activity against Aspergillus flavus and Fusarium verticillioides with MIC ranging from 31.25 to 250 μg/mL [318]. Compounds 99 and 100 inhibited both inflammatory mediators SAG (IC50 = 3.49 and 2.87 μM) and elastase release (IC50 = 2.69 and 0.66 μM) [331], while 123–125 convinced cytotoxicities against melanoma A375 [317].
Steroidal Alkaloids
Sixty-three steroidal alkaloids (135–197), as other principal components in Solanum were reported from this genus (Fig. 2). Compounds 139–156 are derivatives of solasodine (145), one of the main glycoalkaloid constituents in Solanum spp., even as indicated by several numbers of species from which it has been isolated. Solamargine (139) is the major steroidal alkaloid constituent of Solanum plants and literature data showed that it has been revealed in 18 species.
Fig. 2.


Steroidal alkaloids 135–197 from Solanum
Compounds such as 139, solasonine (142), β1-solasonine (143) and solanigroside P (156) with three sugar units and α-l-rhamnose at C-2 or a hydroxyl group on the steroidal backbone may be potential candidates for the treatment of gastric cancer [228].
Featured here are steroidal pseudoalkaloid oligoglycosides, robeneosides A (153) and B (154) and lobofrutoside (155) from the fruits of S. lycocarpum [182, 447], and a rare 16β-H steroidal alkaloid (157) from aerial parts of S. surattense [305]. Also included are leptinine I (171) and II (172), the solanidane alkaloid glycosides, isolated from aerial parts of S. orbignianum [46].
Two rare C-3 amino steroidal alkaloids, 188 and 189, were isolated from aerial parts of S. triste [362, 471]. Three C-27 steroidal glycoalkaloids, spiralosides A (194), B (193), C (192), were obtained from the fruits of S. spirale [474]. Esculeoside A (197), a tomato saponin, is a significant component of ripened tomatoes isolated by Toshihiro et al. [475].
Various bioactivities e.g. antibacterial [80, 384, 403, 406, 407], anticancer [13, 305, 458], antidiabetic [182, 183], antifungal [279], anti-inflammatory [303], CNS depressant [294], leishmanicidal [182, 183], molluscicidal [384, 403, 406, 407], neurotoxicity [106], schistosomicidal [185, 186, 447, 457], spasmolytic [70] and trypanocidal [185, 186, 447, 457] were highlighted as have been exhibited by steroidal alkaloids of Solanum.
Antioxidant activity of 145 and tomatidine (167) from the berries of S. aculeastrum was investigated using DPPH, ABTS and reducing power assays, and the highest inhibition was observed when the two compounds were combined, followed by 145 and 167 [13]. Furthermore, 145 exhibited significant anti-inflammatory activity at doses of 30 mg/kg, with a maximum inhibition of 77.75% in carrageenan-induced rat paw edema, comparing to indomethacin (81.69%). It also showed stronger (46.79effect in xylene induced ear edema in mice [303]. Intraperitoneal injection of 145 (25 mgkg) significantly delayed latency of hind limb tonic extensor phase in the picrotoxin-induced convulsions, and it also potentiated thiopental-provoked sleep in a dose-dependent manner [294]. Moreover, 145 exhibited not only the antibacterial activity against Klebsiella and Staphylococcus spp. at concentration of 1 mg, together with 139 and 141 [403], but also a potent stemness and invasion inhibitory effect on human colorectal cancer HCT116 cells [155]. Colony Spheroid formation assay showed that solasodine dose-dependently prohibited HCT116 cell stemness. CD133, CD44, Nanog, Oct-4 and Sox-2 were inhibited by 145 to reverse stemness and similar mechanism was stimulated in vivo. Transwell and scratch wound assays revealed that 145 impeded HCT116 cell invasion and migration potential strengthened by TGF-β1. Moreover, solasodine attenuated TGF-β1-induced EMT and decreased MMPs while in vivo study showed the same trend. The results of this study implied that 145 may be a novel therapeutic drug for CRC treatment [155].
Burger et al. documented that the crude extract and aqueous fraction containing 139 displayed potent non-selective cytotoxicity (IC50 15.62 μgmL) and noteworthy 9.1-fold P-glycoprotein inhibition at 100 μgmL [15]. Zhang et al. assessed the molecular mechanism underlying the anti-cancer effect of 139 in human cholangiocarcinoma QBC939 cells. The results revealed that 139 inhibited the viability of QBC939 cells in a dose-dependent manner. Furthermore, it significantly induced the apoptosis of QBC939 cells and altered the mitochondrial membrane potential of cells. Quantitative polymerase chain reaction analysis revealed that 139 decreased the mRNA level of B cell lymphoma-2 (Bcl-2) Bcl-extra-large and X-linked inhibitor of apoptosis protein but increased the mRNA level of Bcl-2-associated X protein (Bax) In addition, western blot analysis demonstrated that 139 inhibited the protein expression of Bcl-2 and poly ADP ribose polymerase (PARP) and promoted the protein expression of Bax, cleaved PARP, caspase 3, cleaved caspase 3 and caspase [97].
Compounds 139, 141 and 157 demonstrated cytotoxicity against A549, whereas 139 and 156 showed cytotoxicity against HepG2 cell lines [305]. Compounds 139 and 141 were confirmed as the effective components for Oncomelania snail control. The death rate of Oncomelania snails was 94.2 at a concentration of 2.50 mg/L (139) [406], while 141 exhibited a lethality of 100against O. hupensis [407]. Moreover, 139 and solasonine (142) displayed not only leishmanicidal activity against promastigote forms of Leishmania amazonensis [185], but also antidiabetic activity by inhibiting the serum glucose increase in oral sucrose-loaded rats and suppressing gastric emptying in mice [182]. A synergistic effect was observed for a mixture of the compounds [183]. Compound 139 also expressed stronger trypanocidal activity (IC50 = 15.3 μg/mL), when compared to benznidazol (IC50 = 9.0 μg/mL), the only drug used to treat Chagas’ disease [186].
Tomatine (168) was illustrated to exert significant neuroprotective effect on H2O2-induced SH-SY5Y cells, by enhancing intracellular anti-oxidant enzyme activity and brain-derived neurotrophic factor expression and restraining H2O2-induced oxidative stress [106]. Isojuripidine (190) displayed spasmolytic activity by hindering phasic contractions induced by both histamine and acetylcholinein guinea-pig ileum [69].
Pregnane Glycosides
Compounds 198–210 from Solanum comprise pregnane glycosides (Fig. 3). These compounds coexist in small amounts and could be biosynthesised from steroidal glycosides [194]. Solanigrosides A (198), B (199), 200 and hypoglaucin H (202) were isolated from S. nigrum [476]. Aerial parts of S. torvum gave the highest number of pregnane glycosides, torvpregnanosides A (205) and B (207), ganaxolone (208), allopregnanolone (209) and pregnanolone (210). The whole plant of S. lyratum afforded compounds 203 and 204 [194].
Fig. 3.

Pregnane glycosides 198–210 from Solanum
Pregnane glycosides have reportedly demonstrated anticancer properties [194, 317]. Compound 203 exhibited substantial cytotoxic activity against A375-S2, HeLa, SGC-7901, and Bel-7402 cell lines, with IC50 values of 13.1 to 49.8 μg/mL [194]. Compound 206 indicated cytotoxicity against human melanoma A375 (IC50 = 39.66 μM) [317].
Triterpenes
Fourteen triterpenes (211–224) were identified in Solanum spp. (Figure 4), with lupeol (212) from S. cathayanum [472, 473, 477], S. schimperianum [278], S. spirale [297] and ursolic acid (216) from S. lyratum [197], S. torvum [463] and S. xanthocarpum [427], as the major ones. Six triterpenes 216–217 and 221–224 were reported from the aerial parts of S. torvum [314, 463]. Two cycloartane triterpenoids, cycloeucalenone (213) and 24-oxo-31-norcycloartanone (214) are the main constituents of S. cernuum leaves [107]. Daturaolone (218) was isolated for the first time from S. arundo [65].
Fig. 4.

Triterpenoids 211–224 from Solanum
Solanum triterpenes have indicated to possess anticancer properties. For instance, 213 presented significant activity against KB-Oral cavity cancer (IC50 = 26.73 μgmL) [297], while 213 exhibited selective activity against lung tumor cell line (NCIH460). The anti-nociceptive activity observed for 213 and 214 was found to be related to the inhibition of different mediators involved in inflammation and nociceptive process. Both compounds decreased cyclooxygenase 2 (COX-2) protein expression, although only 214 reached a significant response (P < 0.05 vs control) [107].
Diterpenes
Four diterpenes, e.g., phytol (225) from S. pseudocapsicum [263], kaur-16-ene (226) from S. aculeastrum [11], solanerioside A (227) from S. erianthum [138], and tricalysioside U (228) from S. violaceum [392] were reported from Solanum spp. (Figure 5). Solanerioside A (227) was the first example of a diterpenoid glucoside featuring a 14, 15-dinor-cyclophytane scaffold [138].
Fig. 5.

Diterpenes 225–228 from Solanum
Sesquiterpenes
Sesquiterpenes, 229–310, have been characterized from Solanum spp. (Figure 6). Majority of these compounds, 260–282, were from S. lyratum [196, 197, 199, 200, 484–486] and S. septemlobum [281, 482, 483]. Likewise, 283–285 and 298–303 were reported from S. septemlobum [281, 482, 483]. Compounds 229–231 and 245–255 were isolated from the leaves and fruits of S. erianthum [138, 481], while 286–293 were from the roots of S. torvum [487]. Compounds 236–239 were isolated from the roots of S. aethiopicum [29], while 240–242 were obtained from the leaves of S. aculeastrum [11]. The fruits of S. betaceum yielded compounds 306–310 [77].
Fig. 6.


Sesquiterpenes 229–310 from Solanum
The bioactivities notedly displayed by sesquiterpenes include anticancer [197–200, 281, 484] and antifungal [3]. 3-β-Hydroxysolavetivone (232), solavetivone (233) and lubimin (235) from the roots of S. abutiloides exhibited anti-fungal activities against Fusarium oxysporum f. sp. Melongenae [3]. The eudesmane-type, solajiangxin D (276), and vetispirane-type, solajiangxin E (277) from S. lyratum demonstrated crucial cytotoxicities (ED50 = 2.1–3.7 μg/mL) against three human cancer lines (P-388, HONE-1, and HT-29) [200]. Solajiangxin B (258), A (274) and C (275) from the whole plant of S. lyratum [198] and Septemlobin D (259), and 11,12-O-isopropylidene solajiangxin F (298) [483] also showed significant cytotoxicities (ED50 = 1.9–3.7, and 3.0–7.3 μM, resp.) against these three cancer cell lines. Lyratol D (257), blumenol A (260), dehydrovomifoliol (262) and lyratol C (272) from the whole plant of S. lyratum displayed critical cytotoxic activities against HONE-1 nasopharyngeal, KB oral epidermoid carcinoma, and HT29 colorectal carcinoma cells (IC50 = 3.7–8.1 μM) [199].
Eudesmane-related sesquiterpenes, septemlobins A (301) and B (302) and vetispirane-type, septemlobin C (303) exhibited significant cytotoxicities against three cancer cell lines (P-388, HONE-1, and HT-29) (IC50 = 3.8–7.5 mΜ) [281].
Monoterpenes
Twenty-eight monoterpenes (311–338) have been characterized from Solanum spp. (Fig. 7), with β-Ionone (320) reported from S. aculeastrum [11], S. pseudocapsicum [263] and S. betaceum [77], and loliolide (323) obtained from S. erianthum [137], S. americanum [49] and S. pseudocapsicum [263], as dominant monoterpenes. Majority of the compounds, 316-318 and 324–333 [468, 489–492], were obtained from the fruits of S. vestissimum. Hotrienol (324), with very sweet and flowery flavor is a well-known constituent of the leaf oil of Cinnamomum camphora. It has also been found in a large number of other natural tissues, such as tea, grapes, wines passion fruit, elderberry flowers, Achillea ligustica and papaya fruit [468]. Seven monoterpenes, 311–313 and 319–322 were reported from the leaves of S. aculeastrum [11], and glycosides 329–332 were the aroma precursors in S. vestissimum fruit peelings [468, 492].
Fig. 7.

Monoterpenes 311–338 from Solanum
Flavonoids
Seventy-two flavonoids 339-413 have been identified in the genus Solanum (Fig. 8), with quercetin (340) and kaempferol (351) as the primary flavonoids. Several glycosylated flavonoids, e.g., afzelin (344), astragalin (346), kaempferol 3-O-[apiofuranosyl-(1 → 2)]- α-rhamnoside (347) and -β-galactoside (348) from S. cernuum [501], and camelliaside C (352) from S. erianthum [137] were obtained. Five kaempferol derivatives 373–377 were reported from S. elaeagnifolium [502]. Moreover, three anthocyanins 361–363 were isolated from the red and purple tubers of S. tuberosum [508], while five anthocyanin rutinosides 364–368 were reported from the fruits of S. betaceum [75, 76]. Anthocyanins are the largest group of water-soluble pigments in the plant kingdom. They are responsible for most red and blue colours in fruits, vegetables, and have been used in the food industry as pigments, owing to their bright attractive colours, high water solubility and associated health benefits [76]. In addition, diverse flavonoids, such as 388–397 from S. jabrense [167] and S. palodusum [513] and 399–403 from S. lyratum [514] were reported.
Fig. 8.


Flavonoids 339–413 from Solanum
Flavonoids of Solanum have displayed various biactivities e.g., anticancer [31, 75, 76, 503], anti-depressant and antiviral [322, 332] and hepatoprotective [502] characteristics. Compound 373 exhibited significant hepatoprotective and curative effects against histopathological and histochemical damage induced by paracetamol in liver [502], while 349 and 371 displayed cytotoxicity against breast MCF7 and liver HPG2 cancer cell lines [503].
Compound 340 and rutin (342) indicated potent and concentration-dependent free radical-scavenging activity [45]. They also inhibited peroxidation of cerebral and hepatic lipids subjected to iron oxidative assault. Compound 340 induced in vitro antiproliferative and apoptotic activities on Jurkat cells (IC50 = 11.77 ± 2.4 mg/mL) [23], while 364-367 showed antioxidant activities [75]. Torvanol A (409) from the roots of S. torvum exhibited antidepressant, anxiolytic and adaptogenic effects [316], as well as anti-HSV-1 activity (IC50 = 9.6 μgmL) [322].
Lignans
Lignans, widely distributed in the plant kingdom, are a family of secondary metabolites produced by oxidative dimerization of two phenylpropanoid units. Although their molecular scaffold consists only of two phenylpropane (C6–C3) units, lignans exhibit an enormous structural diversity originating from various linkage patterns of these phenylpropane units. As the C-8–C-3′/C-7–O–C-4′ linked lignans containing two chiral centers (C-7 and C-8) comprise the core of 2, 3-dihydrobenzo[b]furan [480].
Lignans are rare in the genus Solanum [79], with only 31 compounds (414–444) having been isolated (Fig. 9). Compounds 414–419 were obtained from the stems of S. buddleifolium [79], while 424–432, 434 and 442 were isolated from the roots of S. melongena [208–210]. Several neo-lignans, sisymbrifolin (433) from the fruits of S. sisimbriifolium [519], ficusal (442) from the roots of S. melongena [209], glycosmisic acid (439), simulanol (440) and balanophonin (443) from the whole plant of S. surattense [518] were identified. A pair of new C-8–C-3′/C-7–O–C-4′ linked neolignan enantiomers, 420 and 421, were isolated from the stems of S. erianthum [480]. Lignanamides 424–432 and 434 were obtained from the roots of S. melongena [210].
Fig. 9.

Lignans 414–444 from Solanum
Among lignans from the genus Solanum, only lignanamides (425–432) were reported with bioactivities. They displayed anti-inflammatory activities by inhibition of nitric oxide production in lipopoly-saccharide-induced RAW 264.7 macrophages (IC50 = 16.2 to 58.5 μM) [210].
Other Alkaloids
The alkaloids have a natural (2-aminopyrrolidin-1-yl) carboxamidine alkaloidal base acylated with isoferulic (3-hydroxy-4-methoxycinnamic) acid with Z and E configurations, resp. [111]. Thirty-one alkaloids 445–475 have been isolated from Solanum spp. (Fig. 10), comprising types of cyclic guanidine alkaloids, e.g., cernumidine (446) and isocernumidine (447) from the leaves of S. cernuum [109, 111, 112]. Bioactive long chain amides, 454–456, exhibiting antimicrobial activity against Escherichia coli and Candida albicans were isolated from aerial parts of S. schimperianum [277]. Compounds 472–474 were obtained from S. sessiliflorum [525].
Fig. 10.

Other alkaloids 445–475 from Solanum
Antidiabetic activity was illustrated by Solanum alkaloids [49, 209]. Four amides, N-trans-p-coumaroyl -octopamine (464) and -tyramine (466), and N-trans-p-feruloyl -octopamine (465) and -tyramine (467) exhibited antidiabetic properties by enhancing α-glucosidase inhibitory activity in a study involving dual high-resolution α-glucosidaseradical scavenging inhibition profiling [35]. Moreover, 459, 466 and 468 demonstrated possession of inhibitory activity against α-glucosidase (IC50 = 500.6, 5.3 and 46.3 μM, resp.) [209].
Sterols
Sixty-six sterols (476–541) were obtained from the genus Solanum (Fig. 11), with β-sitosterol (483), daucosterol (484) and stigmasterol (485) as the main sterol constituents. Clistol G (476) and capsisteroids A-F (477–482) were obtained from the leaves of S. capsicoides [85], tumacones A (507) and B (508) and tumacosides A (509) and B (510) were from the leaves of S. nudum [242–247], carpesterol (517) was isolated from the seeds of S. capsicoides [86], and its derivatives (518–521) were reported from the fruits of S. xanthocarpum [401]. From the seeds of S. elaeagnifolium, 491, 495, 496 and 498 were yielded [134]. Additionally, two 26-aminochole- stane-type glycosides, abutilosides A (528) and B (529), and five 26-hydroxycholestane-type glycosides, abutilosides C-G (534–538), were isolated from the fresh roots of S. abutiloides [5–9]. These compounds are important intermediates in the biogenesis of steroidal alkaloids [5].
Fig. 11.


Sterols 476–541 from Solanum
Sterols in Solanum have indicated possession of anticancer [86], antifungal [401], and antiplasmodial [242, 245, 247] features. For instance, 509 and 510 displayed in vitro antimalarial activity against P. falciparum chloroquine-resistant FCB-1 strain (IC50 = 27 and 16 μM) [247]. Compounds 511–515 from aerial parts of S. nudum demonstrated antiplasmodial activity on hepatic trophozoites of P. vivax. All the steroids reduced the number of hepatic P. vivax trophozoites. Among them, 506 and 512 reduced the number of hepatic trophozoites by 47and 39resp. [245]. Compound 517 produced antiproliferative activity in glioma (U251), breast (MCF-7), kidney (786-0), ovary (OVCAR-03), and K562 cell lineages [86]. In addition, 505–509 displayed antifungal activity by inhibiting radial growth of A. niger and T. viride [401].
Phenolic Compounds
Fifty-two phenolic compounds (542–593) were recorded from Solanum (Fig. 12). The fruits of S. crinitum have yielded 552, 561–564 [122]. Aerial parts of S. torvum indicated a great wealth of phenolic compunds, e.g. 558–559, 576, 591–593 [315, 320, 335–337, 521, 524, 533]. The highest numbers of phenols, 542–546, 549–540, 552, 555 and 589 were reported from stems of S. melongena [205] while 574–575 and 577–584 were mentioned from the fruits S. sessiliflorum [525].
Fig. 12.

Phenolic compounds 542–593 from Solanum
Phenolic compounds in Solanum have displayed antibacterial [297, 320, 335–337, 524], anticancer [31], anti- diabetic [297, 320, 335–337, 524] and antihypertensive [521] activities. Chlorogenic acid (546) (21.90 ± 0.02 mgg), gallic acid (551) (17.54 ± 0.04 mgg) and caffeic acid (555) (16.64 ± 0.01 mgg) have indicated potent and concentration-dependent DPPH radical-scavenging activity (IC50 = 275.03 ± 7.8 μg/mL) [31], and 551 and 555 reportedly have great potentials as natural source of antidiabetic and antioxidant drug [336]. trans-Cinnamic acid (590) showed antibacterial activities (MIC = 250 μg/mL) against Staphylococcus aureus [297], and antimycobacterial activities (inhibition zone = 0–22 mm) against Proteus vulgaris, Klebsiella pneumoniae (ESBL-), M. tuberculosis (H37Rv) and M. tuberculosis (Rifampin) [320]. Methyl caffeate (591) not only significantly reduced the cell proliferation, but also increased formation of fragmented DNA and apoptotic body in MCF-7 cells. In this study, Bcl-2, Bax, Bid, p53, caspase-3, PARP and cytochrome c release were detected by western blot analyses [474]. The effects of oral administration of 591 (10, 20 and 40 mgkg) in streptozotocin induced diabetic rats, including body weight, fasting blood glucose, plasma insulin, hemoglobin, glycated hemoglobin, total protein, hepatic glycogen and carbohydrate metabolism enzymes have been studied for 28 days. At 40 mgkg, the compound significantly prevented the increase in blood glucose level after glucose administration at 60 min in comparison to the hyperglycemic control group. It also produced remarkable reductions in blood glucose and increased body weight in streptozotocin induced diabetic rats [335]. Takahashi et al. further established that 591 has a most favorable structure for both sucrase and maltase inhibition against sucrose and that its moderate inhibitory action against alpha-glucosidase provides a prospect for antidiabetic usage of S. torvum fruit [337].
Coumarins and Coumestans
Seventeen coumarins 594–610 and three coumastans 611–613 were isolated from Solanum spp. (Fig. 13). The seeds of S. indicum yielded the highest number of coumarins 597–598 and 600–604 [535, 536], while coumestans 611–613 were from the whole plant of S. lyratum [88]. Scopolin (594), scopoletin (595) and coumarin (596) are the main coumarins in Solanum. Compounds 611–613 showed in vitro anti-inflammatory activities with IC50 values in the range of 6.3–9.1 μM [88].
Fig. 13.
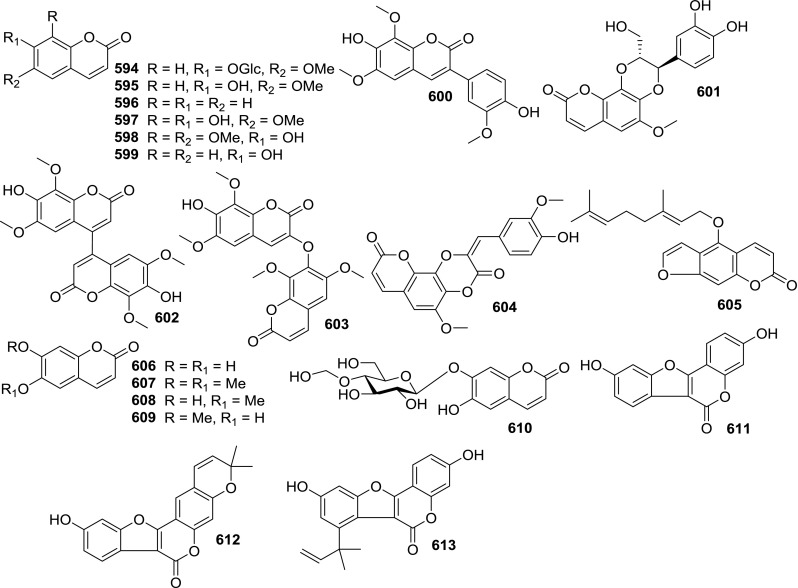
Coumarins and coumestans 594–613 from Solanum
Coumarinolignoids
Four coumarinolignoids known as indicumines A–D (614–617) were obtained from the seeds of S. indicum [535] (Fig. 14). Coumarinolignoids, including cleomiscosins, aquillochins and malloapelins, are unique and rare in nature. Coumarinolignoids of the cleomiscosins type bearing cleomiscosins A–D, 8-epi-cleomiscosin A, and malloapeli A functionalities have been identified in a few genera, including Cleome viscosa, Mallotus apelta, and Rhododendron collettianum. The compounds with such functionalities, especially cleomiscosins A–C and 8-epi-cleomiscosin A, which contributed to biological activities, have been reported with hepatoprotective and tyrosinase inhibition activities [535].
Fig. 14.

Coumarinolignoids 614–617 from Solanum
Fatty Acids and Esters
Nine saturated (618–619, 621, 627–628, 631, 634, 638–639) and 13 unsaturated (620, 622–626, 629, 630, 632, 633, 635–637, 640) fatty acids were reported from Solanum (Fig. 15). The whole plant of S. glabratum has yielded the highest number of fatty acid and esters (627–635) in Solanum spp. [140]. Hexadecanoic acid (618), notably the major fatty acid component in Solanum, was isolated from aerial parts of S. aculeastrum [11] S. vestissimum [489] and S. villosum [434, 479].
Fig. 15.

Fatty acids and esters 618–640 from Solanum
Others
Thirty other kinds of compounds (641–670) were also obtained from Solanum spp. (Fig. 16). Most of them, 642–653, were from the leaves of S. aculeastrum [11] and 654–659 were yielded from the fruits of S. betaceum [78]. An aldehyde puerariafuran (641) and a cyclic eight-membered α,β-unsataturated ketone, solalyratin B (661) were isolated from the whole plant of S. lyratum [88]. Compounds 641 and 661 showed in vitro anti-inflammatory activities, with IC50 values in the range 6.3–9.1 μM [88]. Also presented here are two furans, ethyl-α-D-arabinofuranoside (660) from the whole plant of S. lyratum and 5-hydroxymethyl furfural (663) from the stems of S. torvum [533]. Five aromatic glycosides (666–670) were also isolated from the aerial part of S. incanum [494] and the fruit of S. lycopersicum [511].
Fig. 16.

Other compounds 641–670 from Solanum
Conclusion and Future Prospects
From 1990 to 2017, phytochemical studies on the 65 Solanum species have yielded at least 670 compounds (134 steroidal saponins, 63 steroidal alkaloids, 13 pregnane glycosides, 128 terpenes, 75 flavonoids, 31 lignans, 31 alkaloids, 66 steroids, 52 phenolic compounds, 20 coumarins and coumestans, 4 coumarinolignoids, 23 fatty acids and esters, and 30 other types of compounds).
Pharmacological studies on Solanum genus have focused on antioxidants and anticancer activities. A total of 17 species (fruits of S. aculeastrum, S. americanum, S. muricatum, S. sessiliflorum and S. spirale, seeds of S. capsicoides, the stems of S. cathayanum and S. tuberosum, the roots of S. diphyllum, aerial parts of S. surattense and S. torvum and the whole plant parts of S. aethiopicum, S. nigrum, S. anguivi, S. septemlobum, S. violaceum and S. xanthocarpum) have been explored for anticancer activities and have exhibited significant results.
S. xanthocarpum has outstandingly demonstrated the most diverse pharmacological activities e.g. antioxidants and antitumor, anti-fungal, anti-bacterial, antileishmanial, mosquito larvicidal, molluscicidal, antidiabetic, asthmatic,hepatoprotective, diuretic, nephrotoxicity, antinociceptive, anti-psoriatic, and antiurolithiatic.
Steroidal alkaloids have been presented as being largely responsible for various pharmacological activities of Solanum species, e.g. antibacterial (139, 141 and 145), anticonvulsant and CNS depressant (145), antidiabetic (139, 142 and 144), anti-fungal (145 and 174), anti-inflammatory (145), antileishmanial (139 and 142), molluscicidal (139 and 141), nephrotoxicity (168), antioxidants and antitumor (139, 141, 145, 158, 168 and 180), antiprotozoa (139 and 142), schistosomicidal (139 and 142), spasmolytic (190) and anti-trypanosomal (139).
The genus Solanum seems to possess great potential, yet majority of the species remain unknown or scantily studied for the chemical constituents. It would be very necessary for the phytochemistry researchers to explore and investigate more of its species. The vast pharmacological activities envinced by many compounds from Solanum genus should attract the attention of the pharmacological community to determine their exact target sites, structure–activity relationships and other medicinal applications.
Abbreviations
- ABTS
2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)
- CC50
Cytotoxic concentration of the extracts to cause death to 50% of host’s viable cells
- CDDP
cis-Diamminedichloroplatinum
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- EC50
Half maximal effective concentration
- GABA
Neurotransmitter gamma-aminobutyric acid
- HBV
Hepatitis B Virus
- HSV-1
Herpes simplex virus type 1
- IC50
Minimum inhibition concentration for inhibiting 50% of the pathogen
- LD50
Dose required to kill half the members of a tested population after test duration
- MIC
Minimum inhibitory concentration
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- SAG
Superoxide anion generation
Compliance with Ethical Standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.https://en.wikipedia.org/wiki/Solanum. Accessed 22 Aug 2017
- 2.https://en.wikipedia.org/wiki/Solanum_abutiloides. Accessed 22 Aug 2017
- 3.Yokose T, Katamoto K, Park S, Matsuura H, Yoshihara T. Biosci. Biotechnol. Biochem. 2004;68:2640–2642. doi: 10.1271/bbb.68.2640. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimitsu H, Nishida M, Nohara T. Phytochemistry. 2003;64:1361–1366. doi: 10.1016/j.phytochem.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimitsu H, Nishida M, Yoshida M, Nohara T. Chem. Pharm. Bull. 2002;50:284–286. doi: 10.1248/cpb.50.284. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimitsu H, Nishida M, Nohara T. Chem. Pharm. Bull. 2000;48:556–558. doi: 10.1248/cpb.48.556. [DOI] [PubMed] [Google Scholar]
- 7.Tian RH, Ohmura E, Matsui M, Nohara T. Phytochemistry. 1997;44:723–726. doi: 10.1016/s0031-9422(96)00592-4. [DOI] [PubMed] [Google Scholar]
- 8.Tian RH, Ohmura E, Yoshimitsu H, Nohara T, Matsui M. Chem. Pharm. Bull. 1996;44:1119–1121. doi: 10.1248/cpb.44.1119. [DOI] [PubMed] [Google Scholar]
- 9.Ohmura E, Nakamura T, Tian RH, Yahara S, Yoshimitsu H, Nohara T. Tetrahedron Lett. 1995;36:8443–8444. [Google Scholar]
- 10.http://pza.sanbi.org/solanum-aculeastrum. Accessed 22 Aug 2017
- 11.Koduru S, Asekun OT, Grierson DS, Afolayan AJ, Ess J. Oil-Bear. Plants. 2006;9:65–69. [Google Scholar]
- 12.Wanyonyi AW, Chhabra SC, Mkoji G, Njue W, Tarus PK. Fitoterapia. 2003;74:298–301. doi: 10.1016/s0367-326x(03)00030-3. [DOI] [PubMed] [Google Scholar]
- 13.Koduru S, Jimoh FO, Grierson DS, Afolayan AJ. J. Pharm. Toxicol. 2007;2:160–167. [Google Scholar]
- 14.Koduru S, Grierson DS, Van de Venter M, Afolayan AJ. Pharm. Biol. 2007;45:613–618. [Google Scholar]
- 15.Burger T, Steenkamp V, Cordier W, Mokoka T, Fouche G, Steenkamp P. BMC Comp. Alt. Med. 2018;18:137. doi: 10.1186/s12906-018-2208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Njoki LM, Okoth SA, Wachira PM. Int. J. Microbiol. 2017;2017:5273893. doi: 10.1155/2017/5273893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francisca KK, Jacob M, Omosa LK, Nganga J, Maina N. J. Ethnopharmacol. 2016;192:524–534. doi: 10.1016/j.jep.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laban LT, Mutiso JM, Kiige SG, Ngedzo MM. Iran. J. Basic. Med. Sci. 2015;18:64–71. [PMC free article] [PubMed] [Google Scholar]
- 19.Wanyonyi AW, Chhabra SC, Mkoji G, Eilert U, Njue WM. Phytochemistry. 2002;59:79–84. doi: 10.1016/s0031-9422(01)00424-1. [DOI] [PubMed] [Google Scholar]
- 20.https://en.wikipedia.org/wiki/Solanum_aethiopicum. Accessed 22 Aug 2017
- 21.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+aethiopicum. Accessed 22 Aug 2017
- 22.Chioma A, Obiora A, Chukwuemeka U. J. Trop. Med. 2011;4:163–166. doi: 10.1016/S1995-7645(11)60061-8. [DOI] [PubMed] [Google Scholar]
- 23.Kouassi KC, Mamyrbekova BJA. Res. J. Rec. Sci. 2015;4:81–87. [Google Scholar]
- 24.Anosike CA, Ogbodo NE, Ezugwu AL, Uroko RI, Ani CC, Abonyi O. Am.-Eur J. Toxicol. Sci. 2015;7:104–109. [Google Scholar]
- 25.Adetutu A, Olorunnisola OS, Oyewo EB. Can. J. Pure Appl. Sci. 2013;7:2357–2362. [Google Scholar]
- 26.Nwanna EE, Ibukun EO, Oboh G. Adv. Food Sci. 2013;35:30–36. [Google Scholar]
- 27.Anosike CA, Obidoa O, Ezeanyika LUS. Asian Pac. J. Trop. Med. 2012;5:62–66. doi: 10.1016/S1995-7645(11)60247-2. [DOI] [PubMed] [Google Scholar]
- 28.Tagawa C, Okawa M, Ikeda T, Yoshida T, Nohara T. Tetrahedron Lett. 2003;44:4839–4841. [Google Scholar]
- 29.Nagaoka T, Goto K, Watanabe A, Sakata Y, Yoshihara T. J. Biosci. 2001;56:707–713. doi: 10.1515/znc-2001-9-1007. [DOI] [PubMed] [Google Scholar]
- 30.https://en.wikipedia.org/wiki/Solanum_anguivi. Accessed 22 Aug 2017
- 31.Elekofehinti OO, Kamdem JP, Bolingon AA, Athayde ML, Lopes SR. Asian Pac. J. Trop. Biomed. 2013;3:757–766. doi: 10.1016/S2221-1691(13)60152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalavi C, Patil S. Int. J. Pharm. Sci. Rev. Res. 2016;30:112–120. [Google Scholar]
- 33.Elekofehinti OO, Kamdem JP, Kade IJ, Rocha JBT. S. Afr. J. Bot. 2013;88:56–61. [Google Scholar]
- 34.Elekofehinti OO, Adanlawo IG, Saliu JA, Sodehinde SA. Pharm. Lett. 2012;4:811–814. [Google Scholar]
- 35.Elekofehinti OO, Adanlawo IG, Fakoya A. Asian J. Pharm. Health Sci. 2012;2:416–419. [Google Scholar]
- 36.O.O. Elekofehinti, J.P. Kamdem, D.F. Meinerz, I.J. Kade, I. Joseph, Arch. Pharmacal Res. (2015) [DOI] [PubMed]
- 37.Elekofehinti OO, Kamdem JP, Kade IJ, Adanlawo IG. Asian J. Pharm. Clin. Res. 2013;6:252–257. [Google Scholar]
- 38.Gandhiappan J, Rengasamy R. Pharm. Lett. 2012;4:875–880. [Google Scholar]
- 39.Elekofehinti OO, Adanlawo IG, Saliu JA, Sodehinde SA. Curr. Res. J. Biol. Sci. 2012;4:530–533. [Google Scholar]
- 40.Adanlawo IG, Akanji MA. Rec. Prog. Med. Plants. 2008;19:1–7. [Google Scholar]
- 41.Zhu XH, Ikeda T, Nohara T. Chem. Pharm. Bull. 2000;48:568–570. doi: 10.1248/cpb.48.568. [DOI] [PubMed] [Google Scholar]
- 42.Ripperger H, Himmelreich U. Phytochemistry. 1994;37:1725–1727. doi: 10.1016/s0031-9422(00)89600-4. [DOI] [PubMed] [Google Scholar]
- 43.Honbu T, Ikeda T, Zhu XH, Yoshihara O, Okawa M, Nafady AM, Nohara T. J. Nat. Prod. 2002;65:1918–1920. doi: 10.1021/np020254t. [DOI] [PubMed] [Google Scholar]
- 44.http://eol.org/pages/5695130/data. Accessed 22 Aug 2017
- 45.Correia AC, Macedo CL, Monteiro FS, Alves de Oliveira G. J. Med. Plants Res. 2013;7:2293–2299. [Google Scholar]
- 46.http://www.fireflyforest.com/flowers/2364/solanum-americanum-american-black-nightshade/. Accessed 22 Aug 2017
- 47.https://en.wikipedia.org/wiki/Solanum_americanum. Accessed 22 Aug 2017
- 48.Kadima JN, Kasali FM, Bavhure B, Mahano AO, Bwironde FM. Int. J. Pharmacol. Pharm. 2016;5:196–206. [Google Scholar]
- 49.Silva EL, Almeida LRC, Borges RM, Staerk D. Fitoterapia. 2017;118:42–48. doi: 10.1016/j.fitote.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Vagula JM, Bertozzi J, Castro JC, Celestino OC. Nat. Prod. Res. 2016;30:2230–2234. doi: 10.1080/14786419.2016.1149704. [DOI] [PubMed] [Google Scholar]
- 51.Fidrianny I, Rizkiya A, Ruslan K. J. Chem. Pharm. Res. 2015;7:666–672. [Google Scholar]
- 52.L.U. Colmenares, J. Lai, Determination of Phenolic Content, Antioxidant Activity and Mineral Content of Popolo (Solanum americanum) Leaves. Abstracts, 64th Northwest Regional Meeting of the American Chemical Society, (Tacoma, WA, United States, 2009)
- 53.Hope BE, Massey DG, Fournier MG. Hawaii Med. J. 1993;52:160–166. [PubMed] [Google Scholar]
- 54.Maritza VA, Clara NL. Afinidad. 1999;56:393–396. [Google Scholar]
- 55.Al Chami L, Mendez R, Chataing B, O’Callaghan J, Usubillaga A. Phytother. Res. 2003;56:254–258. doi: 10.1002/ptr.1122. [DOI] [PubMed] [Google Scholar]
- 56.Vazquez A, Ferreira F, Moyna P, Kenne L. Phytochem. Anal. 1999;10:194–197. [Google Scholar]
- 57.Ferreira F, Vazquez A, Moyna P, Kenne L. Phytochemistry. 1994;36:1473–1478. doi: 10.1016/s0031-9422(00)89745-9. [DOI] [PubMed] [Google Scholar]
- 58.http://www.boldsystems.org/index.php/Taxbrowser_Taxonpage?taxid=148709. Accessed 22 Aug 2017
- 59.Maxwell A, Pingal R, Reynolds WF, McLean S. Phytochemistry. 1996;43:913–915. [Google Scholar]
- 60.Londono F, Cardona W, Alzate F, Cardona F, Velez ID. J. Med. Plants Res. 2016;10:100–107. [Google Scholar]
- 61.Chinchilla CM, Valerio CI, Sanchez PR, Bagnarello MV, Rodriguez CD. Rev. Biol. Trop. 2014;62:1229–1240. [Google Scholar]
- 62.Chinchilla M, Valerio I, Sanchez R, Mora V, Bagnarello V, Martinez L. Rev. Biol. Trop. 2012;60:881–891. doi: 10.15517/rbt.v60i2.4024. [DOI] [PubMed] [Google Scholar]
- 63.Maxwell A, Pingal R, Reynolds WF, McLean MS. Stewart Phytochem. 1996;42:543–545. [Google Scholar]
- 64.Fukuhara K, Shimizu K, Kubo I. Arudonine Phytochem. 2004;65:1283–1286. doi: 10.1016/j.phytochem.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 65.Grace MH, Saleh MM. Pharm. 1996;51:593–595. [PubMed] [Google Scholar]
- 66.Silva TMS, Camara CA, Freire KRL, da Silva TG. J. Braz. Chem. Soc. 2008;19:1048–1052. [Google Scholar]
- 67.Pinto FCL, Uchoa DEA, Silveira ER, Pessoa ODL, Espindola LS. Quim. Nova. 2011;34:284–288. [Google Scholar]
- 68.Silva PCB, Clementino NJ, da Silva ADS, da Melo S, Karoline S, Tania MS. Rev. Bras. Farmacogn. 2012;22:131–136. [Google Scholar]
- 69.Oliveira RCM, Lima JT, Ribeiro LAA, Silva JLV, Monteiro FS. J. Biosci. 2006;61:799–805. doi: 10.1515/znc-2006-11-1205. [DOI] [PubMed] [Google Scholar]
- 70.Silva TMS, Costa RA, Oliveira EJ, Barbosa FJM. J. Braz. Chem. Soc. 2005;16:1467–1471. [Google Scholar]
- 71.http://www.hear.org/pier/species/solanum_betaceum.htm. Accessed 23 Aug 2017
- 72.https://en.wikipedia.org/wiki/Tamarillo. Accessed 23 Aug 2017
- 73.http://eol.org/pages/486416/overview. Accessed 23 August 2017
- 74.http://www.iucnredlist.org/details/34636/0. Accessed 23 Aug 2017
- 75.Hurtado NH, Morales AL, Gonzalez-Miret ML, Escudero-Gilete ML, Heredia F. Food Chem. 2009;117:88–93. [Google Scholar]
- 76.Osorio C, Hurtado N, Dawid C, Hofmann T, Heredia M, Francisco J, Morales AL. Food Chem. 2012;132:1915–1921. [Google Scholar]
- 77.Durant AA, Rodriguez C, Santana AI, Herrero C, Rodriguez JC. Rec. Nat. Prod. 2013;7:15–26. [Google Scholar]
- 78.G.J. Maria, P.L. Juliana, O. Coralia, G. Alirio, M. Diana, Mol. 21, (2016)
- 79.Pinto FDCL, Torres MDCM, Silveira ER. Quim. Nova. 2013;36:1111–1115. [Google Scholar]
- 80.Vaz NP, Costa EV, Santos EL, Mikich SB, Marques FA, Braga RA. J. Braz. Chem. Soc. 2012;23:361–366. [Google Scholar]
- 81.https://en.wikipedia.org/wiki/Solanum_capsicoides. Accessed 24 Aug 2017
- 82.http://florida.plantatlas.usf.edu/Plant.aspx?id=609. Accessed 24 Aug 2017
- 83.http://www.flora.sa.gov.au/efsa/lucid/Solanaceae/Solanum%20species/key/Australian%20Solanum%20species/Media/Html/Solanum_capsicoides.htm. Accessed 24 Aug 2017
- 84.http://davesgarden.com/guides/pf/go/38381/. Accessed 24 Aug 2017
- 85.Chen BW, Chen YY, Lin YC. RSC Adv. 2015;5:88841–88847. [Google Scholar]
- 86.Petreanu M, Guimaraes AAA, Broering MF. Nau.-Sch Arch. Pharm. 2016;389:1123–1131. doi: 10.1007/s00210-016-1275-x. [DOI] [PubMed] [Google Scholar]
- 87.Simoes LO, Conceicao FG, Ribeiro TS, Jesus AM, Silva DF. Phytomedicine. 2016;23:498–508. doi: 10.1016/j.phymed.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 88.He J, Ma BZ, Tian XF, Wei FL, Zhao T. Chin. Pharm. Mag. 2014;25:3713–3718. [Google Scholar]
- 89.Xu S, Wang L, Liu H. Shizhen Chin. Med. J. 2006;17:523–524. [Google Scholar]
- 90.Nino GM, Urias OV, Muy MDR, Heredia JB. S. Afr. J. Bot. 2017;111:161–169. [Google Scholar]
- 91.Sun J, Gu YF, Li MM, Su XQ, Li H, Li J, Tu PF. Chin. Herb. Med. 2013;44:2615–2622. [Google Scholar]
- 92.Cao TF, Zhou L, Huang XH, Wang L, Wang DK, Wu Q, Hou WY. Chin. Vet. Med. Mag. 2014;33:31–33. [Google Scholar]
- 93.He J, Zhou CD, Ma BZ, Liu F, Liu X, Zhao T. Chin. Pharm. 2015;26:4433–4436. [Google Scholar]
- 94.Huang H, Zhou J. Food Ind. Tech. 2009;30:315–318. [Google Scholar]
- 95.An L, Tang JT, Liu XM, Gao NN. Chin. J. of Chin. Mat. Med. 2006;31(1225–1226):1260. [PubMed] [Google Scholar]
- 96.Butt G, Romero MA, Tahir F, Farooqi AA. J. of Cell. Biochem. 2018;119:9640–9644. doi: 10.1002/jcb.27258. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X, Yan Z, Xu T, An Z, Chen W, Zhu F. Onc. Lett. 2018;15:6329–6335. doi: 10.3892/ol.2018.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Potawale SE, Sinha SD, Shroff KK, Dhalawat HJ, Boraste SS, Gandhi SP, Tondare AD. Pharmacol. Onl. 2008;3:140–163. [Google Scholar]
- 99.Aftab K, Noreen R, Bukhari SA, Malik A, Sahar A. Oxid. Commun. 2016;39:3079–3089. [Google Scholar]
- 100.Yousaf Z, Wang Y, Baydoun E. J. App. Pharm. Sci. 2013;3:152–160. [Google Scholar]
- 101.Lachman J, Hamouz K, Orsak M, Pivec V. Rost. Vyr. 2001;47:181–191. [Google Scholar]
- 102.Zhou Y, Deng ZS, Cheng F, Dong WJ, Zou K. Chem. Nat. Comp. 2016;52:920–921. [Google Scholar]
- 103.Cheng F, Li X, Wang JZ. Chin. Chem. Lett. 2008;19:68–70. [Google Scholar]
- 104.Yushan K, Huang S, Xie M, Xue Y, Zou J. Med. Plant. 2015;6:1–3. [Google Scholar]
- 105.Kong YS, Huang SL, Xie MX, Xue YH, Zou K, Liu SP. Nat. Prod. Res. Dev. 2014;26:943–946. [Google Scholar]
- 106.Huang SL, He HB, Zou K, Bai CH, Xue YH, Wang JZ, Chen JF. J. Pharm. Pharmacol. 2014;66:844–854. doi: 10.1111/jphp.12205. [DOI] [PubMed] [Google Scholar]
- 107.Lopes LC, de Carvalho JE, Kakimore M, Vendramini CDB. Inflammopharmacology. 2014;22:179–185. doi: 10.1007/s10787-013-0182-8. [DOI] [PubMed] [Google Scholar]
- 108.Grando R, Antonio MA, Araujo CEP, Soares C, Medeiros MA. J. Biosci. 2008;63:507–514. doi: 10.1515/znc-2008-7-807. [DOI] [PubMed] [Google Scholar]
- 109.Mariza AM, Lemos M, Kamila AC, Rodenburg D, McChesney JD. J. Ethnopharmacol. 2015;172:421–429. doi: 10.1016/j.jep.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 110.Damasceno JL, Oliveira PF, Miranda MA, Leandro LF. Biomed. Pharmacother. 2016;83:1111–1115. doi: 10.1016/j.biopha.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 111.Lopes LC, Roman B, Medeiros MA, Mukhopadhyay A. Tetrahedron Lett. 2011;52:6392–6395. [Google Scholar]
- 112.Damasceno JL, de Oliveira PF, Miranda MA, Lima M, Denise C. Biol. Pharm. Bull. 2016;39:920–926. doi: 10.1248/bpb.b15-00638. [DOI] [PubMed] [Google Scholar]
- 113.Herrera A, Jimenez FE, Zamilpa A, Martinez RMA. Planta Med. 2009;75:466–471. doi: 10.1055/s-0029-1185318. [DOI] [PubMed] [Google Scholar]
- 114.Armando HA, Enrique JF, Maria VPA. Planta Med. 2004;70:483–488. [Google Scholar]
- 115.Armando HA, Lopez VEO, Rodriguez TAV, Zamilpa A, Martinez RMA. Afr. J. Trad. Comp. Altern. Med. 2013;10:410–417. [Google Scholar]
- 116.Armando HA, Artemio RS, Maria MR, Eugenia MC, Tortoriello J. Planta Med. 2003;69:390–395. [Google Scholar]
- 117.Zamilpa A, Tortoriello J, Navarro V, Delgado G, Alvarez L. J. Nat. Prod. 2002;65:1815–1819. doi: 10.1021/np020261h. [DOI] [PubMed] [Google Scholar]
- 118.Alvarez L, Perez MDC, Gonzalez JL, Navarro V, Villarreal ML. Planta Med. 2001;67:372–374. doi: 10.1055/s-2001-14332. [DOI] [PubMed] [Google Scholar]
- 119.Lozoya X, Navarro V, Garcia M, Zurita M. J. Ethnopharmacol. 1992;36:127–132. doi: 10.1016/0378-8741(92)90011-f. [DOI] [PubMed] [Google Scholar]
- 120.Alvarez L, Armando HA, Marquina S, Tortoriello J, Zamilpa A, Navarro V. Curr. Top. Steroid Res. 2009;6:89–104. [Google Scholar]
- 121.Gaitaan I, Paz AM, Zacchino SA, Tamayo G, Gimeenez A, Pinzoon R. Pharm. Biol. 2011;49:907–919. doi: 10.3109/13880209.2011.555916. [DOI] [PubMed] [Google Scholar]
- 122.Cornelius MTF, de Carvalho MG, da Silva TMS, Alves CCF. J. Braz. Chem. Soc. 2010;21:2211–2219. [Google Scholar]
- 123.Andressa ES, da Silva TMS, Alves CCF, de Carvalho MG. J. Braz. Chem. Soc. 2002;13:838–842. [Google Scholar]
- 124.https://en.wikipedia.org/wiki/Solanum_diphyllum. Accessed 10 Sept 2017
- 125.https://toptropicals.com/catalog/uid/Solanum_diphyllum.htm. Accessed 10 Sept 2017
- 126.El-Sayed MA, Mohamed AEH, Hassan MK, Hegazy MEF. J. Biosci. 2009;64:644–649. doi: 10.1515/znc-2009-9-1007. [DOI] [PubMed] [Google Scholar]
- 127.https://www.fs.fed.us/database/feis/plants/shrub/soldul/all.html
- 128.https://en.wikipedia.org/wiki/Solanum_dulcamara. Accessed 10 Sept 2017
- 129.Yamashita T, Matsumoto T, Yahara S, Yoshida N, Nohara T. Chem. Pharm. Bull. 1991;39:1626–1628. doi: 10.1248/cpb.39.1626. [DOI] [PubMed] [Google Scholar]
- 130.Sabudak T, Kaya O, Cukurova E. Nat. Prod. Res. 2015;29:308–314. doi: 10.1080/14786419.2014.928878. [DOI] [PubMed] [Google Scholar]
- 131.Mimica-Dukic N, Krstic L, Boza P. Oxid. Commun. 2005;28:536–546. [Google Scholar]
- 132.Tunon H, Olavsdotter HC, Bohlin L. J. Ethnopharmacol. 1995;48:61–76. doi: 10.1016/0378-8741(95)01285-l. [DOI] [PubMed] [Google Scholar]
- 133.https://en.wikipedia.org/wiki/Solanum_elaeagnifolium. Accessed 13 Sept 2017
- 134.Feki H, Koubaa I, Jaber H, Makni J, Damak M. J. Eng. Appl. Sci. 2013;8:708–712. [Google Scholar]
- 135.http://www.cabi.org/isc/datasheet/120139. Accessed 14 Sept 2017
- 136.https://en.wikipedia.org/wiki/Solanum_erianthum. Accessed 14 Sept 2017
- 137.Chou SC, Huang TJ, Lin EH, Huang CH, Chou CH. Nat. Prod. Commun. 2012;7:153–156. [PubMed] [Google Scholar]
- 138.Peng SY, Li H, Yang D, Bai B, Zhu LP, Liu Q. Nat. Prod. Res. 2017;31:810–816. doi: 10.1080/14786419.2016.1247078. [DOI] [PubMed] [Google Scholar]
- 139.Priyadharshini SD, Sujatha V. Int. J. Pharm. Pharm. Sci. 2013;5:652–658. [Google Scholar]
- 140.Monem MA, Azza R. Bull. Fac. Pharm. 2009;47:59–66. [Google Scholar]
- 141.Essam AS, Farag MA, Mahrous EA. Rec. Nat. Prod. 2015;9:94-104/1. [Google Scholar]
- 142.Siddiqui NA, Parvez MA, Al-Rehaily AJ, Al Dosari MS. Saudi Pharm. J. 2017;25:184–195. doi: 10.1016/j.jsps.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mothana RA, Al-Musayeib NM, Al-Ajmi MF, Cos P, Maes L. Evidence-based Comp. Altern. Med. 2014;2014:905639. doi: 10.1155/2014/905639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mothana RAA, Gruenert R, Bednarski PJ, Lindequist U. Pharmazie. 2009;64:260–268. [PubMed] [Google Scholar]
- 145.https://en.wikipedia.org/wiki/Solanum_glaucophyllum. Accessed 14 Sept 2017
- 146.Zanuzzi CN, Nishida F, Portiansky EL, Fontana PA, Gimeno EJ, Barbeito CG. Res. Vet. Sci. 2012;93:336–342. doi: 10.1016/j.rvsc.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zadra M, Piana M, de Brum T, Thiele F, Boligon AA. Molecules. 2012;17:12560–12574. doi: 10.3390/molecules171112560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bonfanti G, Bona KSD, Lucca LD, Jantsch L, Moretto MB. Red. Rep. 2014;19:206–213. doi: 10.1179/1351000214Y.0000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bonfanti G, Bitencourt PR, Karine SB, Priscila SS. Molecules. 2013;18:9179–9194. doi: 10.3390/molecules18089179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.https://en.wikipedia.org/wiki/Solanum_incanum. Accessed 14 Sept 2017
- 151.http://keys.lucidcentral.org/keys/v3/eafrinet/weeds/key/weeds/Media/Html/Solanum_incanum_(Sodom_Apple).htm. Accessed 14 Sept 2017
- 152.Alamri SA, Moustafa MF. Saudi Med. J. 2012;33:272–277. [PubMed] [Google Scholar]
- 153.Taye B, Giday M, Animut A, Seid J. Asian Pac. J. Trop. Biomed. 2011;1:370–375. doi: 10.1016/S2221-1691(11)60082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Al-Sokari SS, Ali NAA, Monzote L, Al-Fatimi M. Biomed. Res. Int. 2015;2015:938747. doi: 10.1155/2015/938747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manase MJ, Mitaine OAC, Pertuit D, Miyamoto T, Tanaka C, Delemasure S. Fitoterapia. 2012;83:1115–1119. doi: 10.1016/j.fitote.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 156.Sundar S, Pillai YJK. Asian J. Pharm. Clin. Res. 2015;8:179–188. [Google Scholar]
- 157.https://easyayurveda.com/2014/06/20/brihati-solanum-indicum-qualities-benefits-dose-side-effect/. Accessed 25 Sept 2017
- 158.http://www.geniusherbs.com/solanum-indicum.html. Accessed 25 Sept 2017
- 159.Zhuang YW, Wu CE, Zhou JY, Zhao ZM, Liu CL, Liu SL. Biochem. Biophy. Res. Commun. 2018;505:485–491. doi: 10.1016/j.bbrc.2018.09.094. [DOI] [PubMed] [Google Scholar]
- 160.Chiang HC, Tseng TH, Wang CJ, Chen CF, Kan WS. Anticancer Res. 1991;11:1911–1917. [PubMed] [Google Scholar]
- 161.Aberoumand A, Deokule SS. J. Food Technol. 2010;8:131–133. [Google Scholar]
- 162.Ma P, Cao TT, Gu GF, Zhao X, Du YG, Zhang Y. Chin. J. Cancer. 2006;25:438–442. [PubMed] [Google Scholar]
- 163.Syu WJ, Don MJ, Lee GH, Sun CM. J. Nat. Prod. 2001;64:1232–1233. doi: 10.1021/np010186v. [DOI] [PubMed] [Google Scholar]
- 164.Huang WH, Hsu CW, Fang JT. Clin. Toxicol. 2008;46:293–296. doi: 10.1080/15563650701385881. [DOI] [PubMed] [Google Scholar]
- 165.https://link.springer.com/article/10.2307/2807835. Accessed 26 Sept 2017
- 166.Silva TMS, Braz-Filho R, de Carvalho MG, Agra MF. Biochem. Syst. Ecol. 2002;30:1083–1085. [Google Scholar]
- 167.Silva TMS, de Carvalho GM, Raimundo BF. Quim. Nova. 2009;32:1119–1128. [Google Scholar]
- 168.Andressa ES, da Silva TMSS, Alves CCF, de Carvalho MG, Echevarria A. Aurea J. Braz. Chem. Soc. 2002;13:838–842. [Google Scholar]
- 169.Silva TMS, Camara CA, Agra MF, de Carvalho MG, Raimundo BF. Fitoterapia. 2006;77:449–452. doi: 10.1016/j.fitote.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 170.Jarald EE, Edwin S, Saini V, Deb L, Gupta VB, Wate SP, Busari KP. Nat. Prod. Res. 2008;22:267–274. doi: 10.1080/14786410701590590. [DOI] [PubMed] [Google Scholar]
- 171.Chand R. Indian Drugs. 1993;30:650. [Google Scholar]
- 172.Rosangkima G, Jagetia GC. J. Pharm. Res. 2015;4:98–103. [Google Scholar]
- 173.http://www.plantoftheweek.org/week351.shtml. Accessed 27 Sept 2017
- 174.http://www.anbg.gov.au/gnp/gnp12/solanum-laciniatum.html. Accessed 27 Sept 2017
- 175.https://en.wikipedia.org/wiki/Solanum_laxum. Accessed 27 Sept 2017
- 176.https://plantsam.com/solanum-laxum/. Accessed 27 Sept 2017
- 177.Soule S, Guntner C, Vazquez A, Argandona V, Moyna P, Ferreira F. Phytochemistry. 2000;55:217–222. [PubMed] [Google Scholar]
- 178.Ferreira F, Soule S, Vazquez A, Moyna P, Kenne L. Phytochemistry. 1996;42:1409–1416. doi: 10.1016/0031-9422(96)00129-x. [DOI] [PubMed] [Google Scholar]
- 179.Delporte C, Backhouse N, Negrete R, Salinas P, Rivas P, Cassels BK. Phytother. Res. 1998;12:118–122. [Google Scholar]
- 180.Munari CC, Oliveira PF, Campos JCL, Martins SPL. J. Nat. Med. 2014;68:236–241. doi: 10.1007/s11418-013-0757-0. [DOI] [PubMed] [Google Scholar]
- 181.Munari CC, de Oliveira PF, Leandro LF, Pimenta LM, Ferreira NH. PLoS ONE. 2014;9:111999. doi: 10.1371/journal.pone.0111999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoshikawa M, Nakamura S, Ozaki K, Kumahara A, Morikawa T, Matsuda H. J. Nat. Prod. 2007;70:210–214. doi: 10.1021/np0680580. [DOI] [PubMed] [Google Scholar]
- 183.Miranda MA, Magalhaes LGM, Tiossi RFJ, Kuehn CC. Parasit. Res. 2012;111:257–262. doi: 10.1007/s00436-012-2827-8. [DOI] [PubMed] [Google Scholar]
- 184.A.M. Mariza, C.C. Kuehn J.F.R. Cardoso, L.G.R. Oliveira, L.G. Magalhaes, Exp. Parasit. 133, 396-402 (2013) [DOI] [PubMed]
- 185.Mariza AM, Tiossi RFJ, da Silva MR, Rodrigues KC, Kuehn CC. Chem. Biodiversity. 2013;10:642–648. doi: 10.1002/cbdv.201200063. [DOI] [PubMed] [Google Scholar]
- 186.Moreira RRD, Martins GZ, Magalhaes NO, Almeida AE, Pietro RCLR. Anais Acad. Bras. Cienc. 2013;85:903–907. doi: 10.1590/S0001-37652013000300006. [DOI] [PubMed] [Google Scholar]
- 187.Martins GZ, Moreira RRD, Planeta CS, Almeida AE, Bastos JK. Pharmacogn. Mag. 2015;11:161–165. doi: 10.4103/0973-1296.157721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.https://en.wikipedia.org/wiki/Tomato. Accessed 03 Oct 2017
- 189.Kralova M, Sanda M, Mackova M, Macek T. Collec. Symp. Series. 2011;13:73–76. [Google Scholar]
- 190.Paoli S, Dias APM, Capriles PVSZ, Costa TEMM. Rev. Bras. Farmacogn. 2008;18:190–196. [Google Scholar]
- 191.Fuentes E, Castro R, Astudillo L, Carrasco G. Evidence-based Comp. Altern. Med. 2012;2012:147031. doi: 10.1155/2012/147031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Friedman M, Levin CE, Lee SU, Kim HJ, Lee IS. J. Agric. Food Chem. 2009;57:5727–5733. doi: 10.1021/jf900364j. [DOI] [PubMed] [Google Scholar]
- 193.http://www.fpcn.net/a/guanmu/20131109/Solanum_lyratum.html. Accessed 07 Oct 2017
- 194.Sun LX, Fu WW, Ren J, Xu L, Bi KS, Wang MW. Arch. Pharm. Res. 2006;29:135–139. doi: 10.1007/BF02974274. [DOI] [PubMed] [Google Scholar]
- 195.Liu SH, Shen XH, Wei XF, Mao XH, Huang T. Immunopharmcol. Immunotoxicol. 2011;33:100–106. doi: 10.3109/08923973.2010.483520. [DOI] [PubMed] [Google Scholar]
- 196.Nie XP, Yao F, Yue XD, Li GS, Dai SJ. Nat. Prod. Res. 2014;28:641–645. doi: 10.1080/14786419.2014.891199. [DOI] [PubMed] [Google Scholar]
- 197.Li GS, Yao F, Zhang L, Yue XD, Dai SJ. Chin. Chem. Lett. 2013;24:1030–1032. [Google Scholar]
- 198.Yao F, Song QL, Zhang L, Li GS, Dai SJ. Fitoterapia. 2013;89:200–204. doi: 10.1016/j.fitote.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 199.Ren Y, Shen L, Zhang DW, Dai SJ. Chem. Pharm. Bull. 2009;57:408–410. doi: 10.1248/cpb.57.408. [DOI] [PubMed] [Google Scholar]
- 200.Yao F, Song QL, Zhang L, Li GS, Dai SJ. Phytochem. Lett. 2013;6:453–456. [Google Scholar]
- 201.Zhang DW, Yang Y, Yao F, Yu QY, Dai SJ. J. Nat. Med. 2012;66:362–366. doi: 10.1007/s11418-011-0581-3. [DOI] [PubMed] [Google Scholar]
- 202.https://en.wikipedia.org/wiki/Eggplant. Accesed 02 Nov 2017
- 203.https://www.thoughtco.com/eggplant-history-solanum-melongena-170820. Accessed 02 Nov 2017
- 204.Atta MAS, Shahid MTK. Oxid. Commun. 2016;39:2249–2259. [Google Scholar]
- 205.Singh AP, Luthria D, Wilson T, Vorsa N, Singh V. Food Chem. 2009;114:955–961. [Google Scholar]
- 206.Zhao A, Sakurai Y, Shibata K, Kikkawa F, Tomoda Y, Mizukami H. Nippon Shokuhin Kagaku Gakkaish. 2014;21:42–47. [Google Scholar]
- 207.Shabana MM, Salama MM, Ezzat SM, Ismail LR. J. Carcinog. Mutagen. 2013;4:1000149/1–1000149/6. [Google Scholar]
- 208.Yoshikawa K, Inagaki K, Terashita T, Shishiyama J, Kuo S, Shankel DM. Mutagen. Res. Gen. Toxicol. 1996;371:65–71. doi: 10.1016/s0165-1218(96)90095-6. [DOI] [PubMed] [Google Scholar]
- 209.Liu X, Luo J, Kong L. Nat. Prod. Commun. 2011;6:851–853. [PubMed] [Google Scholar]
- 210.Sun J, Gu YF, Su XQ, Li MM, Huo HX, Zhang J. Fitoterapia. 2014;98:110–116. doi: 10.1016/j.fitote.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 211.Nishina A, Ebina K, Ukiya M, Fukatsu M, Koketsu M, Ninomiya M. J. Food Sci. 2015;80:H2354–H2359. doi: 10.1111/1750-3841.13068. [DOI] [PubMed] [Google Scholar]
- 212.Ren W, Tang DG. Antican. Res. 1999;19:403–408. [PubMed] [Google Scholar]
- 213.Herraiz FJ, Plazas M, Vilanova S, Prohens J, Villano D, Ferreres F. Int. J. Mol. Sci. 2016;17:394. doi: 10.3390/ijms17030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chang VHC, Chiu TH, Fu SC. J. Sci. Food Agric. 2016;96:192–198. doi: 10.1002/jsfa.7081. [DOI] [PubMed] [Google Scholar]
- 215.Hsu CC, Guo YR, Wang ZH, Yin MC. J. Sci. Food Agric. 2011;91:1517–1522. doi: 10.1002/jsfa.4345. [DOI] [PubMed] [Google Scholar]
- 216.http://www.efloras.org/florataxon.aspx?flora_id=601&taxon_id=200020596. Accessed 07 Nov 2017
- 217.http://www.nhm.ac.uk/natureplus/blogs/china/2010/03/06/the-search-for-solanum-nienkui?fromGateway=true. Accessed 07 Nov 2017
- 218.https://www.wikidata.org/wiki/Q15239976. Accessed 07 Nov 2017
- 219.https://en.wikipedia.org/wiki/Solanum_nigrum. Accessed 08 Nov 2017
- 220.http://naturalhomeremedies.co/Snigrum.html. Accessed 08 Nov 2017
- 221.https://baike.baidu.com/item/%E9%BE%99%E8%91%B5%E8%8A%B1/9378068?fr=aladdin&fromid=11201243&fromtitle=Solanum+nigrum. Accessed 08 Nov 2017
- 222.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+nigrum. Accessed 08 Nov 2017
- 223.Sharma BK, Iyer D, Patil UK. J. Herb. Spic. Med. Plants. 2012;18:257–267. [Google Scholar]
- 224.Sridhar TM, Josthna P, Naidu CV. J. Exp. Sci. 2011;2:24–29. [Google Scholar]
- 225.Matasyoh LG, Murigi HM, Matasyoh JC. Afr. J. Microbiol. Res. 2014;8:3923–3930. [Google Scholar]
- 226.Khan FZ, Saeed MA, Alam M, Chaudhry AR, Ismail M. J. Fac. Pharm. Gazi Uni. 1993;10:105–116. [Google Scholar]
- 227.Yuan HL, Liu XL, Liu YJ. Asian Pac. J. Cancer Prev. 2014;15:10469–10473. doi: 10.7314/apjcp.2014.15.23.10469. [DOI] [PubMed] [Google Scholar]
- 228.Ding X, Zhu F, Yang Y, Li M. Food Chem. 2013;141:1181–1186. doi: 10.1016/j.foodchem.2013.03.062. [DOI] [PubMed] [Google Scholar]
- 229.Prashanth KV, Shashidhara S, Kumar MM, Sridhara BY. Fitoterapia. 2001;72:481–486. doi: 10.1016/s0367-326x(01)00266-0. [DOI] [PubMed] [Google Scholar]
- 230.Huang HC, Syu KY, Lin JK. J. Agric. Food Chem. 2010;58:8699–8708. doi: 10.1021/jf101003v. [DOI] [PubMed] [Google Scholar]
- 231.El-Sherbini GT, Zayed RA, El-Sherbini ET. J. Parasit. Res. 2009;2009:474360. doi: 10.1155/2009/474360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hammami H, Ayadi A. J. Helminth. 2008;82:235–239. doi: 10.1017/S0022149X08982584. [DOI] [PubMed] [Google Scholar]
- 233.Rawani A, Ray AS, Chandra G, Ghosh A, Sakar M. BMC Res. Notes. 2017;10:135. doi: 10.1186/s13104-017-2460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zakaria ZA, Gopalan HK, Zainal H, Mohd PNH. Yakugaku Zasshi. 2006;126:1171–1178. doi: 10.1248/yakushi.126.1171. [DOI] [PubMed] [Google Scholar]
- 235.Jainu M, Devi CSS. J. Ethnopharmacol. 2006;104:156–163. doi: 10.1016/j.jep.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 236.Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW. Chem.-Biol. Int. 2008;171:283–293. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 237.Fang CCHL, Lina WC. J. Ethnopharmacol. 2008;119:117–121. doi: 10.1016/j.jep.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 238.Perez RM, Perez JA, Garcia LM. J. Ethnopharmacol. 1998;62:43–48. doi: 10.1016/s0378-8741(98)00059-2. [DOI] [PubMed] [Google Scholar]
- 239.http://eol.org/pages/5695318/overview. Accessed 10 Nov 2017
- 240.Pabon A, Blair S, Carmona J, Zuleta M, Saez J. Pharm. 2003;58:263–267. [PubMed] [Google Scholar]
- 241.Alvarez G, Pabon A, Carmona J, Blair S. Phytother. Res. 2004;18:845–848. doi: 10.1002/ptr.1534. [DOI] [PubMed] [Google Scholar]
- 242.Lopez ML, Vommaro R, Zalis M, Souza W, Blair S, Segura C. Parasit. Int. 2010;59:217–225. doi: 10.1016/j.parint.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 243.Lopez ML, Blair S, Saez J, Segura C. Memo. Inst. Oswaldo Cruz. 2009;104:683–688. doi: 10.1590/s0074-02762009000500003. [DOI] [PubMed] [Google Scholar]
- 244.Pabon A, Deharo E, Zuluaga L, Maya JD, Saez J, Blair S. Exp. Parasit. 2009;122:273–279. doi: 10.1016/j.exppara.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 245.Londono B, Arango E, Zapata C, Herrera S, Saez J, Blair S, Carmona FJ. Phytother. Res. 2006;20:267–273. doi: 10.1002/ptr.1849. [DOI] [PubMed] [Google Scholar]
- 246.Cardoso OMS, Gomez EJN, Garces LA, Trujillo SB. Rev. Colomb. Biotechnol. 2011;13:186–192. [Google Scholar]
- 247.Saez J, Cardona W, Espinal D, Blair S, Mesa J, Bocar M, Jossang A. Tetrahedron. 1998;54:10771–10778. [Google Scholar]
- 248.Paola GH, Pabon A, Arias C, Blair S. Biomed. 2013;33:78–87. doi: 10.1590/S0120-41572013000100010. [DOI] [PubMed] [Google Scholar]
- 249.Echeverri M, Blair S, Carmona J, Perez P. Am. J. Chin. Med. 2001;29:477–484. doi: 10.1142/S0192415X01000496. [DOI] [PubMed] [Google Scholar]
- 250.Coelho RM, Souza MC, Sarragiotto MH. Phytochemistry. 1998;49:893–897. doi: 10.1016/s0031-9422(98)00220-9. [DOI] [PubMed] [Google Scholar]
- 251.https://commons.wikimedia.org/wiki/Category:Solanum_paludosum. Accessed 14 Nov 2017
- 252.Monteiro FS, Silva ACL, Martins IRR, Correia ACC. J. Ethnopharmacol. 2012;141:895–900. doi: 10.1016/j.jep.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 253.Valverde MLC, Boustie J, Badaoui HE, Muguet B, Henry M. Planta Med. 1993;59:483–484. doi: 10.1055/s-2006-959743. [DOI] [PubMed] [Google Scholar]
- 254.https://en.wikipedia.org/wiki/Solanum_paniculatum. Accessed 14 Nov 2017
- 255.http://rain-tree.com/jurubeba.htm#.WgqWOrVx3IU. Accessed 14 Nov 2017
- 256.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+paniculatum. Accessed 14 Nov 2017
- 257.Valadares YM, Brandao’a GC, Kroon EG, Filho JDS, Oliveira AB, Braga FC. J. Biosci. 2009;64:813–818. doi: 10.1515/znc-2009-11-1210. [DOI] [PubMed] [Google Scholar]
- 258.Vieira GMJ, Rocha QC, Souza RT, Lima CLH, Vilegas W. Food Chem. 2015;186:160–167. [Google Scholar]
- 259.Mesia VS, Santos MT, Souccar C, Lima LMTR, Lapa AJ. Phytomed. Int. J. Phytother. Phytopharm. 2002;9:508–514. doi: 10.1078/09447110260573137. [DOI] [PubMed] [Google Scholar]
- 260.Valerino-Diaz AB, Daylin GT, Zanatta AC, Vilegas W, dos Santos L. J. Agric. Food Chem. 2018;66:8703–8713. doi: 10.1021/acs.jafc.8b01262. [DOI] [PubMed] [Google Scholar]
- 261.https://en.wikipedia.org/wiki/Solanum_pseudocapsicum. Accessed 14 Nov 2017
- 262.https://davesgarden.com/guides/pf/go/54393/. Accessed 14 Nov 2017
- 263.Aliero AA, Asekun OT, Grierson DS, Afolayan AJ. Asian J. Plant Sci. 2006;5:1054–1056. [Google Scholar]
- 264.Vijayan P, Prashanth HC, Vijayaraj P, Dhanaraj SA, Badami S, Suresh B. Pharm. Biol. 2003;41:443–448. [Google Scholar]
- 265.https://en.wikipedia.org/wiki/Solanum_rostratum. Accessed 15 Nov 2018
- 266.https://fireflyforest.net/firefly/2006/07/24/buffalobur-nightshade-a-very-evil-plant/. Accessed 15 Nov 2018
- 267.https://plants.usda.gov/core/profile?symbol=soro. Accessed 15 Nov 2018
- 268.https://www.cabi.org/isc/datasheet/50544. Accessed 15 Nov 2018
- 269.https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/solanum-rostratum. Accessed 15 November 2018
- 270.http://ucjeps.berkeley.edu/eflora/eflora_display.php?tid=44902. Accessed 15 Nov 2018
- 271.https://www.illinoiswildflowers.info/weeds/plants/buffalo_bur.html. Accessed 15 Nov 2018
- 272.https://www.invasiveplantatlas.org/subject.html?sub=6463. Accessed 15 Nov 2018
- 273.Ibarra CA, Rojas A, Mendoza S, Bah M, Gutierrez DM, Hernandez SL, Martinez M. Pharmaceu. Bio. 2010;48:732–739. doi: 10.3109/13880200903271280. [DOI] [PubMed] [Google Scholar]
- 274.https://en.wikipedia.org/wiki/Solanum_sarrachoides
- 275.https://www.apsnet.org/publications/plantdisease/2002/May/Pages/86_5_559.3.asx
- 276.http://plants.jstor.org/compilation/solanum.schimperianum. Accessed 15 Nov 2017
- 277.Hassan WHB, Al-Oqail M, Ahmad MS, Al-Rehaily AJ. Biosci. Biotechnol. Res Asia. 2012;9:593–599. [Google Scholar]
- 278.Hassan WHB, Al-Oqail M, Ahmad MS, Al-Rehaily AJ. Saudi Pharm. J. 2012;20:371–379. doi: 10.1016/j.jsps.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Al-Rehaily AJ, Adnan J, Ahmad MS, Mustafa J, Al-Oqail MM, Khan IA. J. Saudi Chem. Soc. 2013;17:67–76. [Google Scholar]
- 280.http://plants.for9.net/edible-and-medicinal-plants/solanum-septemlobum/. Accessed 15 Nov 2017
- 281.Zhang L, Li GS, Yao F, Yue XD, Dai SJ. Phytochem. Lett. 2015;11:173–176. [Google Scholar]
- 282.https://en.wikipedia.org/wiki/Solanum_sessiliflorum. Accessed 15 Nov 2017
- 283.https://www.hort.purdue.edu/newcrop/morton/cocona.html. Accessed 15 Nov 2017
- 284.http://www.tradewindsfruit.com/content/cocona.htm. Accessed 15 Nov 2017
- 285.Maia JRP, Schwertz MC, Sousa RFS, Rev JPL. Bras. Plantas Med. 2015;17:112–119. [Google Scholar]
- 286.https://en.wikipedia.org/wiki/Solanum_sisymbriifolium. Accessed 15 Nov 2017
- 287.http://www.jeremybartlett.co.uk/2014/10/19/sticky-nightshade-solanum-sisymbriifolium/. Accessed 15 Nov 2017
- 288.http://www.pfaf.org/user/Plant.aspx?LatinName=Solanum+sisymbriifolium. Accessed 15 Nov 2017
- 289.Ibarrola DA, Hellion-Ibarrola MDC, Alvarenga NL. Pharm. Biol. 2006;44:378–381. [Google Scholar]
- 290.Apu AS, Bhuyan SH, Matin M, Hossain F, Khatun F, Taiab Abu A, Matin M. Avicenna J. Phytomed. 2013;3:302–312. [PMC free article] [PubMed] [Google Scholar]
- 291.Ibarrola DA, Ibarrola MCH, Montalbetti Y, Heinichen O, Campuzano MA. Phytomedicine. 2011;18:634–640. doi: 10.1016/j.phymed.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 292.Ibarrola DA, Hellion MCI, Montalbetti Y, Heinichen O, Alvarenga N. J. Ethnopharmacol. 2000;70:301–307. doi: 10.1016/s0378-8741(00)00191-4. [DOI] [PubMed] [Google Scholar]
- 293.Gupta VK, Simlai A, Tiwari M, Bhattacharya K, Roy A. J. Appl. Pharm. Sci. 2014;4:75–80. [Google Scholar]
- 294.Chauhan K, Sheth N, Ranpariya V, Parmar S. Pharm. Biol. 2011;49:194–199. doi: 10.3109/13880209.2010.508499. [DOI] [PubMed] [Google Scholar]
- 295.Bagalwa JJM, Laurence VN, Sayagh C, Bashwira AS. Fitoterapia. 2010;81:767–771. doi: 10.1016/j.fitote.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 296.Siddiqi TO, Ahmad J, Khan SU, Javed K, Khan MSY. Philipp. J. Sci. 1990;119:41–47. [Google Scholar]
- 297.Keawsa-ard S, Natakankitkul S, Liawruangrath S, Teerawutgulrag A. Chiang Mai J. Sci. 2012;39:445–454. [Google Scholar]
- 298.Sukanya KA, Liawruangrath B, Liawruangrath S, Teerawutgulrag A. Nat. Prod. Commun. 2012;7:955–958. [PubMed] [Google Scholar]
- 299.Payum T, Das AK, Shankar R, Tamuly C, Hazarika M. Am. J. PharmTech Res. 2015;5:307–314. [Google Scholar]
- 300.http://www.zhiwutong.com/latin/Solanaceae/Solanum-surattense-Burm-F.htm. Accessed 29 Nov 2017
- 301.http://frps.eflora.cn/frps/Solanum%20surattense. Accessed 29 Nov 2017
- 302.https://easyayurveda.com/2014/04/19/kantakari-solanum-surattense-benefits-dose-usage-side-effects/. Accessed 29 Nov 2017
- 303.Patil SA, Sambrekar SN. Int. J. Res. Pharma. Biomed. Sci. 2012;3:1559–1566. [Google Scholar]
- 304.Ahmed T, Kanwal R, Ayub N, Hassan M. Hum. Ecol. Risk Assess. 2009;15:624–635. [Google Scholar]
- 305.Lu Y, Luo J, Kong L. Phytochemistry. 2011;72:668–673. doi: 10.1016/j.phytochem.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 306.Qasim M, Abideen Z, Adnan MY, Gulzar S, Gul B, Rasheed M. S. Afr. J. Bot. 2017;110:240–250. [Google Scholar]
- 307.Yadav A, Bhardwaj R, Sharma RA. Int. J. Pharm. Pharm. Sci. 2013;5:489–493. [Google Scholar]
- 308.Ahmed MM, Andleeb S, Saqib F, Ch BA, Hussain M, Khatun MN, Rahman H, Comp BMC. Altern. Med. 2016;16:166. doi: 10.1186/s12906-016-1148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ramazani A, Zakeri S, Sardari S, Khodakarim N, Djadidt ND. Malaria J. 2010;9:124. doi: 10.1186/1475-2875-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.https://en.wikipedia.org/wiki/Solanum_torvum. Accessed 6 Dec 2017
- 311.http://fleppc.org/ID_book/solanum%20torvum.pdf. Accessed 6 Dec 2017
- 312.http://tropical.theferns.info/viewtropical.php?id=Solanum+torvum. Accessed 6 Dec 2017
- 313.Maser WH, Yuliana ND, Andarwulan N. J. Liq. Chrom. Rel. Technol. 2015;38:1230–1235. [Google Scholar]
- 314.Paul SB, Choudhury MD, Choudhury R. Asian J. Chem. 2009;21:581–588. [Google Scholar]
- 315.Balachandran C, Emi N, Arun Y, Yamamoto Y, Ahilan B, Sangeetha B. Chem.-Biol. Inter. 2015;242:81–90. doi: 10.1016/j.cbi.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 316.Lu Y, Luo J, Huang X, Kong L. Steroids. 2009;74:95–101. doi: 10.1016/j.steroids.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 317.Li J, Zhang L, Huang C, Guo F, Li Y. Fitoterapia. 2014;93:209–215. doi: 10.1016/j.fitote.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 318.Abhishek RU, Thippeswamy S, Manjunath K, Mohana DC. J. Appl. Microbiol. 2015;119:1624–1636. doi: 10.1111/jam.12956. [DOI] [PubMed] [Google Scholar]
- 319.Chah KF, Muko KN, Oboegbulem SI. Fitoterapia. 2000;71:187–189. doi: 10.1016/s0367-326x(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 320.Balachandran C, Duraipandiyan V, Al-Dhabi NA, Balakrishna K. Indian J. Microbiol. 2012;52:676–681. doi: 10.1007/s12088-012-0313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Nguelefack TB, Feumebo CB, Ateufack G, Watcho P, Tatsimo S. J. Ethnopharmacol. 2008;119:135–140. doi: 10.1016/j.jep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 322.Arthan D, Svasti J, Kittakoop P, Pittayakhachonwut D, Tanticharoen M. Phytochemistry. 2002;59:459–463. doi: 10.1016/s0031-9422(01)00417-4. [DOI] [PubMed] [Google Scholar]
- 323.Challal S, Buenafe OEM, Queiroz EF, Maljevic S, Marcourt L, Bock M, Chem ACS. Neuroscience. 2014;5:993–1004. doi: 10.1021/cn5001342. [DOI] [PubMed] [Google Scholar]
- 324.T.B. Nguelefack, H. Mekhfi, T. Dimo, S. Afkir, M. Nguelefack, P. Elvine, A. Legssyer, A. Ziyyat, J. Comp. Integ. Med. 5, (2008)
- 325.Mohan M, Jaiswal BS, Kasture S. J. Ethnopharmacol. 2009;126:86–89. doi: 10.1016/j.jep.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 326.Mohan M, Kamble S, Gadhi P, Kasture S, Chem F. Toxicology. 2010;48:436–440. doi: 10.1016/j.fct.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 327.Ramamurthy CH, Subastri A, Suyavaran A, Thirunavukkarasu C. Environ. Sci. Pollut. Res. Int. 2016;23:7919–7929. doi: 10.1007/s11356-016-6044-3. [DOI] [PubMed] [Google Scholar]
- 328.Lalmuanthanga C, Lalchhandama C, Lallianchhunga MC, Ali MA. World J. Pharm. Res. 2015;4:1752–1759. [Google Scholar]
- 329.Kusirisin W, Jaikang C, Chaiyasut C, Narongchai P. Med. Chem. 2009;5:583–588. doi: 10.2174/157340609790170443. [DOI] [PubMed] [Google Scholar]
- 330.Ramamurthy CH, Kumar MS, Suyavaran VSA. J. Food Sci. 2012;77:907–913. doi: 10.1111/j.1750-3841.2012.02830.x. [DOI] [PubMed] [Google Scholar]
- 331.Lee CL, Hwang TL, He WJ, Tsai YH, Yen CT, Yen HF, Chen CJ. Phytochemistry. 2013;95:315–321. doi: 10.1016/j.phytochem.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 332.Mohan M, Attarde D, Momin R, Kasture S. Nat. Prod. Res. 2013;27:2140–2143. doi: 10.1080/14786419.2013.778853. [DOI] [PubMed] [Google Scholar]
- 333.Momin R, Mohan M. Nat. Prod. Res. 2012;26:416–422. doi: 10.1080/14786419.2010.495072. [DOI] [PubMed] [Google Scholar]
- 334.Kamaraj C, Kaushik NK, Mohanakrishnan D, Elango G, Bagavan A. Parasit. Res. 2012;111:703–715. doi: 10.1007/s00436-011-2457-6. [DOI] [PubMed] [Google Scholar]
- 335.Gandhi GR, Ignacimuthu S, Paulraj MG, Sasikumar P. Eur. J. Pharmacol. 2011;670:623–631. doi: 10.1016/j.ejphar.2011.09.159. [DOI] [PubMed] [Google Scholar]
- 336.Gandhi GR, Ignacimuthu S, Paulraj MG. Food Chem. Toxicol. 2011;49:2725–2733. doi: 10.1016/j.fct.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 337.Takahashi K, Yoshioka Y, Kato E, Katsuki S, Hosokawa K, Kawabata J. Biosci. Biotechnol. Biochem. 2010;74:741–745. doi: 10.1271/bbb.90789. [DOI] [PubMed] [Google Scholar]
- 338.Kamaraj C, Rahuman AA, Elango G, Bagavan A, Zahir AA. Parasit. Res. 2011;109:37–45. doi: 10.1007/s00436-010-2218-y. [DOI] [PubMed] [Google Scholar]
- 339.http://swbiodiversity.org/seinet/taxa/index.php?taxon=28414. Accessed 19 Dec 2017
- 340.http://www.conabio.gob.mx/malezasdemexico/solanaceae/solanum-tridynamum/fichas/ficha.htm. Accessed 19 Dec 2017
- 341.Brito L, Wendy F, Mejia S, Gaspar M, Francisco CM. Afinidad. 1995;52:49–52. [Google Scholar]
- 342.http://tropical.theferns.info/viewtropical.php?id=Solanum+trilobatum. Accessed 21 Dec 2017
- 343.http://naturalhomeremedies.co/Strilobatum.html. Accessed 21 Dec 2017
- 344.Kanchana A, Panneerselavam C. Int. J. Curr. Res. Rev. 2011;3:37–51. [Google Scholar]
- 345.Thanigaiarassu RR, Kannabiran K, Khanna VG. J. Pharm. Res. 2009;2:273–276. [Google Scholar]
- 346.Kannabiran K, Thanigaiarassu RR, Venkatesan GK. J. Appl. Biol. Sci. 2008;2:109–112. [Google Scholar]
- 347.M. Vanaja, K. Paulkumar, G. Gnanajobitha, S. Rajeshkumar, C. Malarkodi, G. Annadurai, (International Journal of Metals 2014), pp. 1–9 [DOI] [PMC free article] [PubMed]
- 348.Zameer AK, Ahmed SSZ, Ponnusamy P, Senthil KB. Pak. J. Pharm. Sci. 2016;29:1578. [Google Scholar]
- 349.Kanchana MB. Int. J. Pharm. Pharm. Sci. 2011;3:356–364. [Google Scholar]
- 350.Mohanan PV, Devi KS. Cancer Lett. 1996;110:71–76. doi: 10.1016/s0304-3835(96)04463-1. [DOI] [PubMed] [Google Scholar]
- 351.Mohanan PV, Devi KS. Cancer Lett. 1997;112:219–223. doi: 10.1016/s0304-3835(96)04574-0. [DOI] [PubMed] [Google Scholar]
- 352.Shahjahan M, Vani G, Shyamaladevi CS. Chem.-Biol. Int. 2005;156:113–123. doi: 10.1016/j.cbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 353.Jagadeesan P, Prasad DA, Pandikumar P, Ignacimuthu S. Indian J. Nat. Prod. Res. 2011;2:156–163. [Google Scholar]
- 354.Mohanan PV, Devi KS. J. Exp. Clin. Cancer Res. 1998;17:159–164. [PubMed] [Google Scholar]
- 355.Govindarajan P, Chinnachamy C. Pak. J. Pharm. Sci. 2014;27:2101–2107. [PubMed] [Google Scholar]
- 356.Venkatesan PN, Rajendran P, Ekambaram G, Sakthisekaran D. Nat. Prod. Res. 2008;22:1094–1106. doi: 10.1080/14786410802267601. [DOI] [PubMed] [Google Scholar]
- 357.Rajkumar S, Jebanesan A. J. Ins. Sci. 2005;5:15. doi: 10.1093/jis/5.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Pandurangan A, Khosa RL, Hemalatha S. Nat. Prod. Res. 2011;25:1132–1141. doi: 10.1080/14786410903370783. [DOI] [PubMed] [Google Scholar]
- 359.Pandurangan A, Khosa RL, Hemalatha S. J. Asian Nat. Prod. Res. 2010;12:691–695. doi: 10.1080/10286020.2010.497997. [DOI] [PubMed] [Google Scholar]
- 360.Shahjahan M, Sabitha KE, Jainu M, Shyamala DCS. Indian J. Med. Res. 2004;120:194–198. [PubMed] [Google Scholar]
- 361.http://solanaceaesource.org/taxonomy/term/110074/descriptions. Accessed 21 Dec 2017
- 362.Maxwell A, Seepersaud M, Pingal R, Mootoo DR, Reynolds WF. J. Nat. Prod. 1996;59:200–201. doi: 10.1021/np50118a027. [DOI] [PubMed] [Google Scholar]
- 363.https://en.wikipedia.org/wiki/Potato. Accessed 22 Dec 2017
- 364.https://www.hort.purdue.edu/newcrop/duke_energy/Solanum_tuberosum.html. Accessed 22 Dec 2017
- 365.Chandrashekara KD, Dharmesh SM. J. Pharm. Res. 2014;8:1148–1157. [Google Scholar]
- 366.Mohdaly AAA, Sarhan MA, Smetanska I, Mahmoud A. J. Sci. Food Agric. 2010;90:218–226. doi: 10.1002/jsfa.3796. [DOI] [PubMed] [Google Scholar]
- 367.Paik D, Das P, De T, Chakraborti T. Exp. Parasit. 2014;146:11–19. doi: 10.1016/j.exppara.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 368.Paik D, Das P, Pramanik PK, Naskar K, Chakraborti T. Biomed. Pharmacother. 2016;83:1295–1302. doi: 10.1016/j.biopha.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 369.Zuber T, Holm D, Byrne P, Ducreux L, Taylor M, Kaiser M, Stushnoff C. Food Funct. 2015;6:72–83. doi: 10.1039/c4fo00649f. [DOI] [PubMed] [Google Scholar]
- 370.Langner E, Nunes FM, Pozarowski P, Kandefer-Szerszen M. Food Chem. Toxicol. 2013;57:246–255. doi: 10.1016/j.fct.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 371.Reddivari L, Vanamala J, Chintharlapalli S, Safe SH, Miller JC., Jr Carcinogenesis. 2007;28:2227–2235. doi: 10.1093/carcin/bgm117. [DOI] [PubMed] [Google Scholar]
- 372.Friedman M, Lee KR, Kim HJ, Lee IS, Kozukue N. J. Agric. Food Chem. 2005;53:8420. doi: 10.1021/jf050620p. [DOI] [PubMed] [Google Scholar]
- 373.Ojewole JAO, Kamadyaapa DR, Musabayane CT. Cardiovasc. J. S. Afr. 2006;17:166–171. [PubMed] [Google Scholar]
- 374.http://www.calflora.org/cgi-bin/species_query.cgi?where-taxon=Solanum%20umbelliferum. Accessed 23 Dec 2017
- 375.http://calscape.org/Solanum-umbelliferum. Accessed 23 Dec 2017
- 376.https://en.wikipedia.org/wiki/Solanum_umbelliferum. Accessed 23 Dec 2017
- 377.https://www.wildflower.org/plants/result.php?id_plant=soum. Accessed 23 Dec 2017
- 378.http://www.watershednursery.com/nursery/plant-finder/solanum-umbelliferum/. Accessed 23 Dec 2017
- 379.http://www.theodorepayne.org/mediawiki/index.php?title=Solanum_umbelliferum. Accessed 23 Dec 2017
- 380.Kim YC, Che QM, Gunatilaka AAL, Kingston DGI. J. Nat. Prod. 1996;59:283–285. doi: 10.1021/np960125a. [DOI] [PubMed] [Google Scholar]
- 381.http://www.shaman-australis.com/forum/index.php?/topic/33853-cannibals-tomato-solanum-viride-s-uporo-s-anthropophagorum/. Accessed 25 Dec 2017
- 382.http://www.asklepios-seeds.de/gb/solanum-uporo-seeds.html. Accessed 25 Dec 2017
- 383.http://www.tradewindsfruit.com/content/cannibal-tomato.htm. Accessed 25 Dec 2017
- 384.Ripperger H. Phytochemistry. 1997;44:731–734. doi: 10.1016/s0031-9422(96)00575-4. [DOI] [PubMed] [Google Scholar]
- 385.Suarez LEC, Cendales DRM, Clara IOP. Rev. Colomb. Quimica. 2006;35:59–65. [Google Scholar]
- 386.https://en.wikipedia.org/wiki/Solanum_vestissimum. Accessed 25 Dec 2017
- 387.http://tropical.theferns.info/viewtropical.php?id=Solanum+vestissimum. Accessed 25 Dec 2017
- 388.https://en.wikipedia.org/wiki/Solanum_villosum. Accessed 25 Dec 2017
- 389.http://florida.plantatlas.usf.edu/Plant.aspx?id=56. Accessed 25 Dec 2017
- 390.http://tropical.theferns.info/viewtropical.php?id=Solanum+violaceum. Accessed 25 Dec 2017
- 391.Chang FR, Yen CT, El-Shazly M, Yu CY. Bioorg. Med. Chem. Lett. 2013;23:2738–2742. doi: 10.1016/j.bmcl.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 392.Yen CT, Lee CL, Chang FR, Hwang TL, Yen HF, Chen CJ, Chen SL. J. Nat. Prod. 2012;75:636–643. doi: 10.1021/np200877u. [DOI] [PubMed] [Google Scholar]
- 393.Raju GS, Moghal MR, Dewan SMR, Amin MN, Billah M. Avic. J. Phytomed. 2013;3:313–320. [PMC free article] [PubMed] [Google Scholar]
- 394.http://www.homeremediess.com/solanum-xanthocarpum-medicinal-use-and-pictures/. Accessed 28 Dec 2017
- 395.http://www.himalayawellness.com/herbfinder/solanum-xanthocarpum.htm. Accessed 28 Dec 2017
- 396.https://herbpathy.com/Uses-and-Benefits-of-Solanum-Xanthocarpum-Cid1087. Accessed 28 Dec 2017
- 397.Rani D, Dantu PK. Nat. Acad. Sci. Lett. 2015;38:275–279. [Google Scholar]
- 398.Khanam S, Sultana R. Int. J. Pharm. Sci. Res. 2012;3:1057–1060. [Google Scholar]
- 399.Vadnere GP, Gaud RS, Singhai AK. Pharmacol. 2008;1:513–522. [Google Scholar]
- 400.Govindan S, Viswanathan S, Vijayasekaran V, Alagappan R. J. Ethnopharmacol. 1999;66:205–210. doi: 10.1016/s0378-8741(98)00160-3. [DOI] [PubMed] [Google Scholar]
- 401.Singh OM, Subharani K, Singh NI, Devi NB, Nevidita L. Nat. Prod. Res. 2007;21:585–590. doi: 10.1080/14786410701369458. [DOI] [PubMed] [Google Scholar]
- 402.Shiv G, Gaherwal S, Shrivastava CS, Wast N. Eur. J. Exp. Biol. 2015;5:81–89. [Google Scholar]
- 403.Packia LNCJ, Bharath BMS, Anita MA, Raja BJ, Jeeva S. Int. J. Inv. Pharm. Sci. 2013;1:433–437. [Google Scholar]
- 404.Abbas K, Niaz U, Hussain T, Saeed MA, Javaid Z, Idrees A, Rasool S. Acta Poloniae Pharm. 2014;71:415–421. [PubMed] [Google Scholar]
- 405.Kajaria DK, Gangwar M, Kumar D, Kumar SA, Tilak R, Nath G. Asian Pac. J Trop. Biomed. 2012;2:905–909. doi: 10.1016/S2221-1691(12)60251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Li Z, Cheng X, Wang CJ, Li GL, Xia SZ, Wei FH. Chin. J. Parasitol. Parasit. Diseases. 2005;23:206–208. [PubMed] [Google Scholar]
- 407.Li W, Huang X, Qi C, Liu S, Kodama O, Utaka K. China Pest. 2007;46:591–593. [Google Scholar]
- 408.Changbunjong T, Wongwit W, Leemingsawat S, Tongtokit Y, Deesin V. S. Asian J. Trop. Med. Pubic Health. 2010;41:320–325. [PubMed] [Google Scholar]
- 409.Hussain T, Gupta RK, Sweety K, Khan MS, Hussain MD, Sarfaraj AMD. Asian Pac. J Trop. Biomed. 2012;2:454–460. doi: 10.1016/S2221-1691(12)60075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Jalali GB, Ghaffari H, Prakash HS, Kini KR. Pharm. Biol. 2014;52:1060–1068. doi: 10.3109/13880209.2013.877490. [DOI] [PubMed] [Google Scholar]
- 411.Gupta RK, Hussain T, Panigrahi G, Das A, Singh GN, Sweety K. Asian Pac. J Trop. Med. 2011;4:964–968. doi: 10.1016/S1995-7645(11)60227-7. [DOI] [PubMed] [Google Scholar]
- 412.Poongothai K, Ponmurugan P, Ahmed KSZ, Kumar BS. Asian Pac. J Trop. Med. 2011;4:778–785. doi: 10.1016/S1995-7645(11)60193-4. [DOI] [PubMed] [Google Scholar]
- 413.Kar DM, Maharana L, Pattnaik S, Dash GK. J. Ethnopharmacol. 2006;108:251–256. doi: 10.1016/j.jep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 414.Archana C, Jacob J. Asian J. Phytomed Clin. Res. 2015;3:32–36. [Google Scholar]
- 415.Velu P, Iyappan P, Vijayalakshmi A, Indumathi D. Biomed. Pharmacother. 2016;84:430–437. doi: 10.1016/j.biopha.2016.09.060. [DOI] [PubMed] [Google Scholar]
- 416.Kumar S, Pandey AK. BMC Comp. Altern. Med. 2014;14:112. doi: 10.1186/1472-6882-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Kumar S, Sharma UK, Sharma AK, Pandey AK. Cell. Mol. Biol. 2012;58:174–181. [PubMed] [Google Scholar]
- 418.Bhutani KK, Paul AT, Fayad W, Linder S. Phytomedicine. 2010;17:789–793. doi: 10.1016/j.phymed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 419.Rahman MT, Ahmed M, Alimuzzaman M, Shilpi JA. Fitoterapia. 2003;74:119–121. doi: 10.1016/s0367-326x(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 420.Hussain T, Gupta RK, Sweety K, Eswaran B, Vijayakumar M, Rao CV. Asian Pac. J. Trop. Med. 2012;5:686–691. doi: 10.1016/S1995-7645(12)60107-2. [DOI] [PubMed] [Google Scholar]
- 421.Mahesh KP, Murugan K, Kovendan K, Subramaniam J, Amaresan D. Parasit. Res. 2012;110:2541–2550. doi: 10.1007/s00436-011-2797-2. [DOI] [PubMed] [Google Scholar]
- 422.Mahesh KP, Murugan K, Kovendan K, Panneerselvam C. Parasit. Res. 2012;111:609–618. doi: 10.1007/s00436-012-2876-z. [DOI] [PubMed] [Google Scholar]
- 423.Bansal SK, Singh KV, Kumar S. J. Environ. Biol. 2009;30:221–226. [PubMed] [Google Scholar]
- 424.Mohan L, Sharma P, Srivastava CN. J. Environ. Biol. 2005;26:366–401. [PubMed] [Google Scholar]
- 425.Parmar KM, Itankar PR, Joshi A, Prasad SK. J. Ethnopharmacol. 2017;198:158–166. doi: 10.1016/j.jep.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 426.Ranka D, Aswar M, Aswar U, Bodhankar BS. Indian J. Exp. Biol. 2013;51:833–839. [PubMed] [Google Scholar]
- 427.Bhatt B. J. Chem. Pharm. Res. 2011;3:176–181. [Google Scholar]
- 428.Patel PK, Patel MA, Vyas BA, Shah DR, Gandhi TR. J. Ethnopharmacol. 2012;144:160–170. doi: 10.1016/j.jep.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 429.Singh OM, Singh TP. J. Sci. Ind. Res. 2010;69:732–740. [Google Scholar]
- 430.Cuervo AC, Gerald B, Patel AV. Phytochemistry. 1991;30:1339–1341. [Google Scholar]
- 431.Putalun W, Xuan LJ, Tanaka H, Shoyama Y. J. Nat. Prod. 1999;62:181–183. doi: 10.1021/np980301a. [DOI] [PubMed] [Google Scholar]
- 432.Zuo XM, Guo LZ, Cheng F, Guo ZY, Zou K. Nat. Prod. Res. Dev. 2013;25:1205–1208. [Google Scholar]
- 433.Lee YY, Hashimoto F, Yahara S, Nohara T, Yoshida N. Chem. Pharm. Bull. 1994;42:707–709. [Google Scholar]
- 434.Mohy-ud-din A, Khan Z, Ahmad M, Kashmiri MA. Asian J. Chem. 2010;22:2919–2927. [Google Scholar]
- 435.Shu W, Zhang Y, Ye W, Zhou G. J. Jinnan Univ. Nat. Sci. Med. 2011;32:493–497. [Google Scholar]
- 436.Shu W, Wu C, Zhang Y, Ye WC, Zhou G. Nat. Prod. Res. 2013;27:1982–1986. doi: 10.1080/14786419.2013.811406. [DOI] [PubMed] [Google Scholar]
- 437.Hao LJ, Wang S, Zhu JJ, Wang ZM, Wei SH. China J. Chin. Mat. Med. 2014;39:2034–2038. [PubMed] [Google Scholar]
- 438.Al-Sofany RH, Rashwan OA. Bull. Fac. Pharm. 2001;39:99–101. [Google Scholar]
- 439.Ahmed KM. Egyptian J. Pharm. Sci. 1996;37:37–44. [Google Scholar]
- 440.Suthar AC, Mulani RM. Pharmacogn. Mag. 2008;4:112–115. [Google Scholar]
- 441.Hellenas KE, Nyman A, Slanina P, Loof L, Gabrielsson J. J. Chromatogr. 1992;573:69–78. doi: 10.1016/0378-4347(92)80476-7. [DOI] [PubMed] [Google Scholar]
- 442.Keeler RF, Baker DC, Gaffield W. Offic. J. Int. Soc Toxicon. 1990;28:873–884. doi: 10.1016/0041-0101(90)90017-2. [DOI] [PubMed] [Google Scholar]
- 443.Mona EA, Miyashita H, Ikeda T, Lee JH, Yoshimitsu H, Nohara T, Murakami K. Chem. Pharm. Bull. 2009;57:747–748. doi: 10.1248/cpb.57.747. [DOI] [PubMed] [Google Scholar]
- 444.Teng X, Zhang YJ, Yang C. Acta Bot. Yunnan. 2008;30:239–242. [Google Scholar]
- 445.Lu Y, Luo J, Kong L. Mag. Reson. Chem. 2009;47:808–812. doi: 10.1002/mrc.2468. [DOI] [PubMed] [Google Scholar]
- 446.Ferro EA, Alvarenga NL, Ibarrola DA, Hellion IMC, Ravelo AG. Fitoterapia. 2005;76:577–579. doi: 10.1016/j.fitote.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 447.Nakamura S, Hongo M, Sugimoto S, Matsuda H, Yoshikawa M. Phytochemistry. 2008;69:1565–1572. doi: 10.1016/j.phytochem.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 448.Lu YY, Luo JG, Kong LY. Chin. J. Nat. Med. 2011;9:30–32. [Google Scholar]
- 449.Yahara S, Yamashita T, Nozawa N, Nohara T. Phytochemistry. 1996;43:1069–1074. [Google Scholar]
- 450.Shu W, Zhou G, Ye W. Chin. Trad. Herb. Drugs. 2011;42:424–427. [Google Scholar]
- 451.Colmenares AP, Rojas LB, Mitaine OAC, Pouysegu L, Quideau S, Miyamoto T, Tanaka C, Paululat T. Phytochemistry. 2013;86:137–143. doi: 10.1016/j.phytochem.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 452.Iida Y, Yanai Y, Ono M, Ikeda T, Nohara T. Chem. Pharm. Bull. 2005;53:1122–1125. doi: 10.1248/cpb.53.1122. [DOI] [PubMed] [Google Scholar]
- 453.Ohno M, Murakami K, El-Aasr M, Zhou JR, Yokomizo K, Ono M, Nohara T. J. Nat. Med. 2012;66:658–663. doi: 10.1007/s11418-012-0637-z. [DOI] [PubMed] [Google Scholar]
- 454.Nawaz H, Ahmed E, Sharif A, Arshad M, Batool N, Rasool MA, Mukhtar UH. Chem. Nat. Comp. 2014;49:1091–1094. [Google Scholar]
- 455.Nguyen HT, Nguyen VT, Nguyen DT, Chau VM. Tap Chi Duoc Hoc. 2008;48:31–36. [Google Scholar]
- 456.Putalun W, Fukuda N, Tanaka H, Shoyama Y. J. Liq. Chrom. Rel. Technol. 2002;25:2387–2398. [Google Scholar]
- 457.Schwarz A, Pinto E, Haraguchi M, de Oliveira CA, Bernardi MM, Souza SH. Phytother. Res. 2007;21:1025–1028. doi: 10.1002/ptr.2200. [DOI] [PubMed] [Google Scholar]
- 458.Jared JJ, Murungi LK, Torto B. Pest Manag. Sci. 2016;72:828–836. doi: 10.1002/ps.4100. [DOI] [PubMed] [Google Scholar]
- 459.Cornelius MTF, Alves CCF, da Silva TMS, Alves KZ, de Carvalho MG. Rev. Bras. Farmacia. 2004;85:57–59. [Google Scholar]
- 460.Barbosa FJM, Agra MF, Oliveira RA, Paulo MQ, Trolin G. Memo. Inst. Oswaldo Cruz. 1991;86:189–191. doi: 10.1590/s0074-02761991000600043. [DOI] [PubMed] [Google Scholar]
- 461.Manosroi J, Manosroi A, Sripalakit P. Acta Hort. 2005;679:105–111. [Google Scholar]
- 462.Purwanti T. Majalah Farmasi Indon. 1997;8:115–122. [Google Scholar]
- 463.Zhou H, Wang F, Fang Z. China J. Chin. Mat. Med. 2011;36:2096–2098. [PubMed] [Google Scholar]
- 464.Bhattacharyya J, Basilio IJLD, Morais LCSL, Agra MF. Biochem. Syst. Ecol. 2009;37:228–229. [Google Scholar]
- 465.Njeh F, Feki H, Koubaa I, Hamed N, Damak M. Pharm. Biol. 2016;54:726–731. doi: 10.3109/13880209.2015.1073332. [DOI] [PubMed] [Google Scholar]
- 466.Cristea A, Tanasescu M, Adelina PM. Farmacia. 1992;40:25–30. [Google Scholar]
- 467.Butnaru C, Vlase L, Lazar D, Agoroaei L. Farmacia. 2011;59:172–178. [Google Scholar]
- 468.Wintoch H, Morales A, Duque C, Schreier P. Nat. Food Chem. 1993;41:1311–1314. [Google Scholar]
- 469.Sammani A, Shammaa E, Chehna F. Int. J. Pharm. Sci. Rev. Res. 2013;23:23–27. [Google Scholar]
- 470.Ripperger H. Phytochemistry. 1996;43:705–707. [Google Scholar]
- 471.Maxwell A, Seepersaud M, Pingal R, Mootoo DR, Reynolds WF. J. Nat. Prod. 1995;58:625–628. doi: 10.1021/np50118a027. [DOI] [PubMed] [Google Scholar]
- 472.Coy-Barrera CA, Cuca-Suarez LE, Clara IO. Acta Biol. 2005;27:131–134. [Google Scholar]
- 473.Luis ECS, Carlos ACB, Orozco CI. Rev. Colomb. Quimica. 2004;33:7–12. [Google Scholar]
- 474.Li D, Zhao YL, Qin XJ, Liu L, Yang XW, Chen YY, Luo XD. Nat. Prod. Bioprospect. 2016;6:225–231. doi: 10.1007/s13659-016-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Liu JL, Chen SC, Lu FL, Li DP. Guangxi Plant Catal. 2012;32:415–418. [Google Scholar]
- 476.Zhou XL, He XJ, Zhou GX, Ye WC, Yao XS. J. Asian Nat. Prod. Res. 2007;9:517–523. doi: 10.1080/10286020600782488. [DOI] [PubMed] [Google Scholar]
- 477.Xie M, Zhou Y, Zou K, Cheng F, Liu R. J. Chin. Med. Mat. 2008;31:1332–1334. [PubMed] [Google Scholar]
- 478.Luo J, Zhang B, Deng D. J. Chin. Cereals Oils. 2015;30:125–128. [Google Scholar]
- 479.Venkatesh R, Vidya R, Kalaivani K. Int. J. Pharm. Sci. Res. 2014;5:5283–5287. [Google Scholar]
- 480.Li H, Peng SY, Yang DP, Bai B, Zhu LP, Zhao ZM. Chirality. 2016;28:259–263. doi: 10.1002/chir.22571. [DOI] [PubMed] [Google Scholar]
- 481.Mahadev R, Ramakrishnaiah H, Krishna V, Kumar NN. Don. J. Ess. Oil-Bear. Plants. 2012;15:387–391. [Google Scholar]
- 482.Nie X, Zhang L, Yao F, Xiao K, Dai S. China J. Chin. Mat. Med. 2015;40:1514–1517. [PubMed] [Google Scholar]
- 483.Zhang L, Lei H, Lin HQ, Li GS, Yue XD, Dai SJ. Nat. Prod. Res. 2015;29:1889–1893. doi: 10.1080/14786419.2015.1010164. [DOI] [PubMed] [Google Scholar]
- 484.Yue XD, Yao F, Zhang L, Li GS, Dai SJ. China J. Chin. Mat. Med. 2014;39:453–456. doi: 10.1016/j.phytol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 485.Dai SJ, Shen L, Ren Y. Nat. Prod. Res. 2009;23:1196–1200. doi: 10.1080/14786410802396087. [DOI] [PubMed] [Google Scholar]
- 486.Yu SM, Kim HJ, Woo ER, Park H. Arch. Pharm. Res. 1994;17:1–4. [Google Scholar]
- 487.Yuan P, Guo F, Zheng K, Chen K, Jia Q, Li Y. Nat. Prod. Res. 2016;30:1682–1689. doi: 10.1080/14786419.2015.1135142. [DOI] [PubMed] [Google Scholar]
- 488.Marx F, Andrade EHA, Maia JG. Food Res. Technol. 1998;206:364–366. [Google Scholar]
- 489.Suarez M, Duque C, Wintoch H, Schreier P. J. Agric. Food Chem. 1991;39:1643–1645. [Google Scholar]
- 490.Suarez M, Duque C. J. Agric. Food Chem. 1991;39:1498–1500. [Google Scholar]
- 491.Suarez M, Duque C, Bicchi C, Wintoch H, Full G, Schreier P. Flavour Frag. J. 1993;8:215–220. [Google Scholar]
- 492.C. Duque, H. Wintoch, M. Suarez, P. Schreier, Linalool glycosides as aroma precursors in lulo (Solanum vestissimum D.) fruit peelings (Prog. Flavour Precursor. Stud. Proc. Int. Conf. 1993), pp. 279–281
- 493.Feki H, Koubaa I, Damak M. Mediter. J. Chem. 2014;2:639–647. [Google Scholar]
- 494.Lin YL, Wang WY, Kuo YH, Chen CF. J. Chin. Chem. Soc. 2000;47:247–251. [Google Scholar]
- 495.Nirmal SA, Patel AP, Bhawar SB, Pattan SR. J. Ethnopharmacol. 2012;142:91–97. doi: 10.1016/j.jep.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 496.Zhao Y, Liu F, Lou H. Chin. Trad. Herb. Drugs. 2010;33:555–556. [PubMed] [Google Scholar]
- 497.Mohy-Ud-Din A, Khan Z, Ahmad M, Kashmiri MA, Yasmin S, Mazhar H. J. Chilean Chem. Soc. 2009;54:486–490. [Google Scholar]
- 498.Tang WW, Liu XL, Zeng DQ. J. Jiangxi Agric. Univ. 2012;34:483–486. [Google Scholar]
- 499.Ji Y, Sun Y, Hui C. Lishizen Med. Mat. Med. Res. 2009;20:2431–2432. [Google Scholar]
- 500.Lin D, Sun Y, Zhang L, Zeng X. Lishizen Med. Mat. Med. Res. 2009;20:C3–C4. [Google Scholar]
- 501.Ramos AC, Rodrigo RO. Nat. Prod. Res. 2017;31:2405–2412. doi: 10.1080/14786419.2017.1311889. [DOI] [PubMed] [Google Scholar]
- 502.Hawas UW, Soliman GM, Abou LTE, Farrag ARH. J. Biosci. 2013;68:19–28. [PubMed] [Google Scholar]
- 503.Radwan MM, Badawy A, Zayed R, Hassanin H, ElSohly MA. Med. Chem. Res. 2015;24:1326–1330. [Google Scholar]
- 504.Badawy A, Zayed R, Ahmed S, Hassanean H. J. Nat. Prod. 2013;6:156–167. [Google Scholar]
- 505.Wang Y, Li Z, Yang J. J. Yunnan Univ. 1998;20:396–398. [Google Scholar]
- 506.http://www.motherherbs.com/solanum-indicum.html. Accessed 25 Sept 2017
- 507.Ren Y, Shen L, Dai S. China J. Chin. Mat. Med. 2009;34:721–723. [PubMed] [Google Scholar]
- 508.Ishii G, Mori M, Umemura Y, Takigawa S, Tahara S. Nippon Shokuhin Kagaku Kogaku Kaishi. 1996;43:887–895. [Google Scholar]
- 509.Dan W, Chen GW, Han CR, Liu WJ, Yang HZ. Chin. Trad. Herb. Drugs. 2012;43:1068–1070. [Google Scholar]
- 510.Cardona J, Juliana EC, Cuca S, Luis E, Barrera G, Jaime A. Rev. Colomb. Quimica. 2011;40:185–200. [Google Scholar]
- 511.Ono M, Shiono Y, Tanaka T, Masuoka C, Yasuda S, Ikeda T, Nohara T. J. Nat. Med. 2010;64:500–505. doi: 10.1007/s11418-010-0436-3. [DOI] [PubMed] [Google Scholar]
- 512.Siqueira S, Vivyanne FS, Agra MF, Dariva C, Siqueira SJP. J. Sup. Flu. 2011;58:391–397. [Google Scholar]
- 513.Silva STMS, Braz-Filho R, Carvalho MG, Agra MF. Biochem. Syst. Ecol. 2002;30:479–481. [Google Scholar]
- 514.Yin HL, Li J, Li QS, Dong JX. Bull. Acad. Milit. Med. Sci. 2010;34:65–67. [Google Scholar]
- 515.Huang HJ, Wang HM, Cao AC, Zhang CX, Wei SH, Ling TJ. Nat. Prod. Res. 2014;31:1831–1835. doi: 10.1080/14786419.2017.1290621. [DOI] [PubMed] [Google Scholar]
- 516.Shao Q, Chang L, Wei Y, Wei Z. J. Chromatogr. Sci. 2018;56:695–701. doi: 10.1093/chromsci/bmy044. [DOI] [PubMed] [Google Scholar]
- 517.He J, Zhang XJ, Ma BZ, Liu F. Chin. Pharm. J. 2015;50:2035–2038. [Google Scholar]
- 518.Li JH, Yin HL, Dong JX. Acad. Milit. Med. Sci. 2013;37:130–134. [Google Scholar]
- 519.Chakravarty AK, Mukhopadhyay S, Saha S, Pakrashi SC. Phytochemistry. 1996;41:935–939. [Google Scholar]
- 520.Wang L, Wang N, Yao X. Chin. Trad. Herb. Drugs. 2007;30:792–794. [Google Scholar]
- 521.Simaratanamongkol A, Umehara K, Niki H, Noguchi H, Panichayupakaranant P. J. Funct. Foods. 2014;11:557–562. [Google Scholar]
- 522.Zuo X, Deng Z, Guo Z, Zou K, Cheng F, Cent J. China Norm. Univ. 2012;46:322–324. [Google Scholar]
- 523.Xie G, Duan W, Tao B, Li C. Nat. Prod. Res. Dev. 2008;20(627–629):643. [Google Scholar]
- 524.Zhou H, Wang F, He Y, Tang J, Fang Z. West China J. Pharm. Sci. 2011;26:522–524. [Google Scholar]
- 525.Rodrigues E, Mariutti LRB, Mercadante AZ. J. Agric. Food Chem. 2013;61:3022–3029. doi: 10.1021/jf3054214. [DOI] [PubMed] [Google Scholar]
- 526.Wu D, Fang Z. Guangdong Med. Coll. J. 2008;24:139–140. [Google Scholar]
- 527.Sharma AK, Sharma MC, Dobhal MP. Pharm. Lett. 2013;5:355–361. [Google Scholar]
- 528.V. Aeri, Rajkumari, M. Mujeeb, M. Ali, Indian J. Nat. Prod. 21, 40–42 (2005)
- 529.Mathis GA, Toggenburger A, Pokorny R, Autzen S, Ibanez R. J. Steroid Biochem. Mol. Biol. 2014;144:40–43. doi: 10.1016/j.jsbmb.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 530.Bachmann H, Autzen S, Frey U, Wehr U. Brit. Poultry Sci. 2013;54:642–652. doi: 10.1080/00071668.2013.825692. [DOI] [PubMed] [Google Scholar]
- 531.Baek DR, Lee MJ, Baek NI, Seo KH, Lee YH. J. Appl. Biol. Chem. 2016;59:103–106. [Google Scholar]
- 532.Morais MG, Guilherme AFC, Aleixo AA, Lima LARS. Nat. Prod. Res. 2015;29:480–483. doi: 10.1080/14786419.2014.951930. [DOI] [PubMed] [Google Scholar]
- 533.Yuan PL, Wang XP, Chen KX, Li YM, Jia Q. Chin. Patent Med. 2016;38:104–107. [Google Scholar]
- 534.Ricardo MCD, Enrique CSL, Orozco P, Clara I. Actual. Biol. 2005;27:49–52. [Google Scholar]
- 535.Long YH, Li JH, Li J, Li B, Chen L, Dong JX. Fitoterapia. 2013;84:360–365. doi: 10.1016/j.fitote.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 536.Long YH, Hui LJ, Li B, Chen L, Dong JX. J. Asian Nat. Prod. Res. 2014;16:153–157. doi: 10.1080/10286020.2013.841142. [DOI] [PubMed] [Google Scholar]
- 537.Antonio JM, Gracioso JS, Toma W, Lopez LC, Oliveira F, Brito ARMS. J. Ethnopharmacol. 2004;95:83–88. doi: 10.1016/j.jep.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 538.Chen T, Zhang J, Sun X, Xu X, Zhang S. Fine Chem. 2009;26:47–50. [Google Scholar]
- 539.https://species.wikimedia.org/wiki/Solanum_elaeagnifolium. Accessed 13 Sept 2017
- 540.http://www.cabi.org/isc/datasheet/50516. Accessed 13 Sept 2017
- 541.https://garden.org/plants/view/146851/Natri-Solanum-ligustrinum/. Accessed 28 Sept 2017
- 542.https://garden.org/plants/photo/357234/. Accessed 28 Sept 2017
- 543.https://en.wikipedia.org/wiki/Solanum_lycocarpum. Accessed 29 Sept 2017
- 544.https://en.wikipedia.org/wiki/Solanum_muricatum. Accessed 06 Nov 2017
- 545.http://www.pfaf.org/user/plant.aspx?latinname=Solanum+muricatum. Accessed 06 Nov 2017
- 546.https://www.shootgardening.co.uk/plant/solanum-muricatum. Accesed 06 Nov 2017
- 547.https://www.vanmeuwen.com/p/solanum-muricatum-mini-melon/v17830VM. Accessed 06 Nov 2017
- 548.Veira PM, Marinho LPM, Ferri SCS, Lee CC. Anais Acad. Bras. Cienc. 2013;85:553–560. doi: 10.1590/S0001-37652013000200007. [DOI] [PubMed] [Google Scholar]
- 549.http://www.asklepios-seeds.de/gb/solanum-sessiliflorum-seeds.html. Accessed 15 Nov 2017
- 550.D.R.L.H. Mascato, M. M. Passarinho, D.M.L. Galeno, R. P. Carvalho, J. Nutr. Met. 364185/1–364185/8 (2015)
- 551.http://tropical.theferns.info/viewtropical.php?id=Solanum+spirale. Accessed 15 Nov 2017
- 552.http://www.hear.org/pier/species/solanum_torvum.htm. Accessed 6 Dec 2017
- 553.Nguelefack TB, Mekhfi H, Dongmo AB, Dimo T, Watcho P, Zoheir J. J. Ethnopharmacol. 2009;124:592–599. doi: 10.1016/j.jep.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 554.G.T. El-Sherbini, R.A. Zayed, E.T. El-Sherbini, J. Parasit. Res. 2009, (2009) [DOI] [PMC free article] [PubMed]
- 555.Chowdhury N, Ghosh A, Chandra G, Comp BMC. Altern. Med. 2008;8:10. doi: 10.1186/1472-6882-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]


