Abstract
Resistant starch (RS) has been reported to reduce body fat in obese mice. However, this effect has not been demonstrated in humans. In this study, we tested the effects of RS in 19 volunteers with normal body weights. A randomized, double-blinded and crossover design clinical trial was conducted. The study subjects were given either 40 g high amylose RS2 or energy-matched control starch with three identical diets per day throughout the study. The effect of RS was evaluated by monitoring body fat, glucose metabolism, gut hormones, gut microbiota, short-chain fatty acids (SCFAs) and metabolites. The visceral and subcutaneous fat areas were significantly reduced following RS intake. Acetate and early-phase insulin, C-peptide and glucagon-like peptide-1 (GLP-1) secretion were increased, and the low-density lipoprotein cholesterol (LDL-C) and blood urea nitrogen (BUN) levels were decreased after the RS intervention. Based on 16S rRNA sequencing, certain gut microbes were significantly decreased after RS supplementation, whereas the genus Ruminococcaceae_UCG-005 showed an increase in abundance. Other potential signatures of the RS intervention included Akkermansia, Ruminococcus_2, Victivallis, and Comamonas. Moreover, the baseline abundance of the genera Streptococcus, Ruminococcus_torques_group, Eubacterium_hallii_group, and Eubacterium_eligens_group was significantly associated with the hormonal and metabolic effects of RS. These observations suggest that a daily intake of 40 g of RS is effective in modulating body fat, SCFAs, early-phase insulin and GLP-1 secretion and the gut microbiota in normal-weight subjects.
Introduction
Dietary fibre is beneficial for the management of certain chronic diseases. Resistant starch (RS) is a prebiotic dietary fibre that is subject to fermentation by the gut microbiota in the intestine1,2. RS is reported to have beneficial effects on diabetes, obesity, inflammatory bowel disease, intestinal tumours, and cardiovascular diseases. The intake of RS per capita is 14.9 g/d in China3, 3 g/d per capita in Europe4, 3–8 g/d in the United States5, and 3–10 g/d5 worldwide on average, which are too low to have beneficial effects6.
RS has been reported to reduce body fat in obese rodents7–12. However, its effects have not been carefully tested in human subjects, especially in those with normal body weights. The effects of RS on the gut microbiota were reported in obese patients with dysbiosis and in healthy human subjects13,14, although its effects on body fat were not reported in those studies. Studies have shown that RS improves insulin sensitivity and glucose metabolism in overweight and obese subjects or patients with metabolic syndrome or type 2 diabetes, although no effects have been observed on body fat15–19. The current literatures suggest a discrepancy in the effects of RS on body fat between rodents and humans. Although no explanation is available for this discrepancy, differences in dietary components have been considered. Rodent studies have been conducted with controlled diets7–12 but not human studies15–19. The fatty acids in high-fat diets are known to inhibit RS fermentation in the large intestine20,21. Tea polyphenols inhibit fermentation by crosslinking amylose to generate large molecules beyond fermentable size22. Therefore, strict control of dietary components is required for clinical studies of RS.
In this study, we tested the effects of 40 g/d of RS on 19 normal-weight subjects on a controlled diet in a double-blinded and crossover clinical trial. The effects of RS on body fat, glucose, fatty acid metabolism, and the gut microbiota were examined. Our results suggest that RS reduces visceral and subcutaneous fat in normal-weight subjects and induces beneficial changes in gut hormones and the microbiota.
Results
Safety of RS supplementation
To exclude the interference of dietary components in this study, all subjects received a controlled diet with a standardized nutrient composition (Supplementary Table S1). No gastrointestinal adverse events, such as nausea, vomiting, bloating, or constipation were reported. Additionally, no serious adverse events that led to hospitalization or an inability to work were reported during the study period. To further assess the safety of RS supplementation, blood samples were collected for routine clinical chemistry and metabolomics analyses before and after RS and control starch (CS) administration. After 4 weeks of RS administration at 40 g/d, liver function indices, including the aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyl transferase (GGT) levels, and renal function indices, such as the urea nitrogen and creatinine levels, were found to be within the normal ranges (Table 1). Amino acid and bile acid metabolism were also maintained within a normal and stable range (Supplementary Table S2), which confirmed the safety of RS supplementation.
Table 1.
Anthropometric and biochemical assessments before and after 4 weeks’ RS or CS supplementation in normal-weight subjects.
| Variable | CS | RS | p value | ||
|---|---|---|---|---|---|
| 0 week | 4 weeks | 0 week | 4 weeks | ||
| Weight (kg) | 58.73 ± 2.44 | 58.37 ± 2.39 | 58.92 ± 2.39 | 58.78 ± 2.33 | 0.490 |
| BMI (kg/m²) | 21.08 ± 0.41 | 20.95 ± 0.42 | 21.14 ± 0.39 | 21.11 ± 0.37 | 0.423 |
| FM (kg) | 12.23 ± 0.90 | 12.04 ± 0.91 | 12.34 ± 0.88 | 12.33 ± 0.83 | 0.413 |
| FFM (kg) | 44.84 ± 3.33 | 46.39 ± 2.5 | 46.53 ± 2.41 | 46.46 ± 2.44 | 0.948 |
| TBW (kg) | 31.95 ± 1.67 | 32.39 ± 1.68 | 31.87 ± 1.58 | 31.93 ± 1.64 | 0.502 |
| Fat percentage (%) | 21.29 ± 1.72 | 21.12 ± 1.75 | 21.41 ± 1.67 | 21.51 ± 1.65 | 0.826 |
| WC (cm) | 72.08 ± 1.32 | 71.84 ± 1.28 | 72.76 ± 1.47 | 72.09 ± 1.37 | 0.943 |
| HC (cm) | 93.00 ± 1.06 | 92.89 ± 0.97 | 93.57 ± 1.09 | 92.98 ± 1.18 | 0.476 |
| WHR | 0.78 ± 0.01 | 0.77 ± 0.01 | 0.78 ± 0.01 | 0.78 ± 0.01 | 0.560 |
| VFA (cm²) | 26.04 ± 2.52 | 27.05 ± 2.67* | 26.43 ± 2.63 | 21.70 ± 1.78***### | <0.001 |
| SFA (cm²) | 134.18 ± 10.19 | 134.65 ± 10.18 | 135.81 ± 10.57 | 127.33 ± 10.66**# | 0.031 |
| ALT (U/l) | 12.58 ± 1.66 | 11.63 ± 1.30 | 11.79 ± 1.45 | 12.58 ± 1.79 | 0.208 |
| AST (U/l) | 17.74 ± 1.67 | 16.21 ± 0.71 | 16.21 ± 0.85 | 15.95 ± 1.00 | 0.379 |
| GGT (U/l) | 13.32 ± 1.21 | 13.74 ± 1.51 | 14.26 ± 1.37 | 13.68 ± 1.07 | 0.395 |
| TC (mmol/l) | 4.41 ± 0.19 | 4.48 ± 0.16 | 4.38 ± 0.17 | 4.29 ± 0.17# | 0.140 |
| TG (mmol/l) | 0.76 ± 0.08 | 0.80 ± 0.07 | 0.76 ± 0.08 | 0.78 ± 0.06 | 0.873 |
| HDL-C (mmol/l) | 1.40 ± 0.07 | 1.38 ± 0.06 | 1.36 ± 0.07 | 1.31 ± 0.05 | 0.515 |
| LDL-C (mmol/l) | 2.59 ± 0.12 | 2.73 ± 0.12* | 2.62 ± 0.11 | 2.57 ± 0.14# | 0.006 |
| BUN (mmol/l) | 4.11 ± 0.25 | 4.10 ± 0.28 | 4.24 ± 0.25 | 3.66 ± 0.19*# | 0.010 |
| Cr (μmol/l) | 66.05 ± 2.87 | 68.32 ± 2.54 | 67.42 ± 2.59 | 68.84 ± 2.94 | 0.633 |
| UA (μmol/l) | 292.32 ± 12.58 | 286.95 ± 14.63 | 305.58 ± 17.22 | 284.63 ± 13.48* | 0.183 |
Data are presented as mean ± SEM. FM: fat mass; FFM: fat-free mass; TBW: total body water; WC: waist circumference, HC: hip circumference, WHR: Waist hip ratio, VFA: Visceral fat area, SFA: Subcutaneous fat area, TG: triglyceride level; HDL-C: high-density lipoprotein cholesterol. p value was statistical significance between CS effect and RS effect; Significance was determined by generalized estimating equation (GEE) model; *p < 0.05, **p < 0.01, starch 0 week vs. starch 4 weeks, #p < 0.05, ###p < 0.001, RS 4 weeks vs. CS 4 weeks.
RS supplementation reduced visceral subcutaneous and intra-abdominal fat
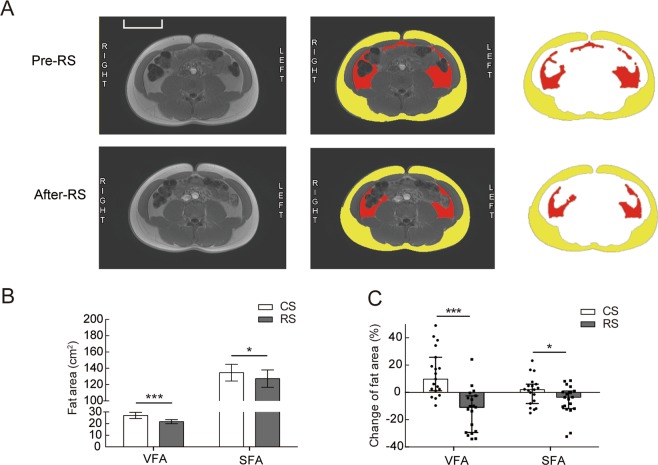
RS intake significantly reduced abdominal adiposity (visceral fat area [VFA] and subcutaneous fat area [SFA]) (Fig. 1A). The VFA showed a significant reduction after RS intake compared to that after CS consumption (27.05 ± 2.67 cm² vs. 21.70 ± 1.78 cm², p < 0.001) (Fig. 1B), whereas no difference was observed in the baseline levels between the two starch intake groups (Table 1). A reduction was also observed in the SFA (Fig. 1B). The SFA and VFA were decreased by −10.88% (−28.96%, −3.30%) and −3.53% (−11.21%, 0.6%), respectively (Fig. 1C). However, no significant change in body weight was observed after RS supplementation (Table 1).
Figure 1.
Body composition before and after 4 weeks of RS or CS supplementation in normal-weight subjects. Nineteen normal-weight subjects were followed up for 4 weeks of dietary supplementation with RS or CS in a crossover study. (A) Representative abdominal MRI of the subjects before and after 4 weeks of RS consumption. Raw (left panel) and marked (middle and right panel) MRI at the navel level; yellow delineates the SFA and red delineates the VFA. (B) The SFA and VFA in the participants after a standardized meal following 4 weeks of consumption of RS or CS (n = 19). Data are presented as the mean ± SEM. (C) Changes in the fat area (%) (SFA and VFA) evaluated by MRI in subjects after 4 weeks of CS or RS intake (n = 19). Data are presented as the median (interquartile range), Significance was determined using the GEE model. Scale bar = 10 cm. *p < 0.05, ***p < 0.001.
We found significant decreases in the low-density lipoprotein cholesterol (LDL-C) (2.73 ± 0.12 vs. 2.57 ± 0.14 mmol/l, p < 0.05) and blood urea nitrogen (BUN) (4.10 ± 0.28 mmol/l vs. 3.66 ± 0.19 mmol/l, p < 0.05) levels in the RS group compared with those of the CS group. Uric acid (UA) was also significantly reduced after RS intake (p < 0.05). No significant changes were found in the ALT, AST, and GGT levels (Table 1). The anthropometric parameters, including the body mass index (BMI), whole body fat mass and percentage, waist circumference, hip circumference, and waist-to-hip ratio, were not significantly changed after RS or CS supplementation. Furthermore, we found that metabolites related to β-oxidation of long-chain fatty acids were reduced in the urine following RS treatment, including carnitine C2:0, carnitine C8:0, and carnitine C8:1 (Supplementary Fig. S1).
RS supplementation promotes secretion of early-phase insulin, gut hormones and SCFAs
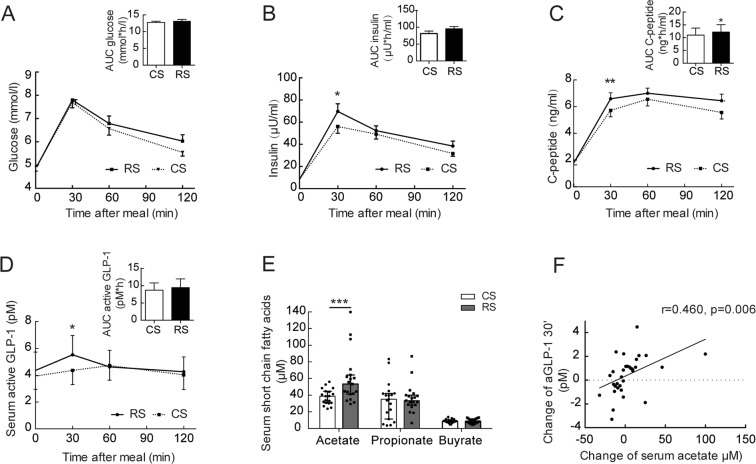
The effect of RS intake on glucose metabolism was examined in two tests: the standard meal tolerance test and the hyperinsulinemic-euglycemic clamp. In the meal tolerance test, the fasting and postprandial glucose levels were not altered by RS intake (Fig. 2A). However, at 30 min after the meal, a significant increase in the insulin (56.98 ± 7.57 μU/ml vs. 69.70 ± 6.90 μU/ml, p < 0.05) and C-peptide (5.72 ± 0.48 ng/ml vs. 6.59 ± 0.45 ng/ml, p < 0.01) levels was seen after RS intake compared to that after CS consumption, and the area under the curve (AUC) for C-peptide was significantly higher after RS intake than after CS consumption (Fig. 2B,C). In contrast, no difference was observed in the baseline levels between the two starch intake groups (Supplementary Table S3). In the hyperinsulinemic-euglycemic clamp test, no significant impact of RS was observed on the glucose infusion rate (Supplementary Table S3). Therefore, the data suggest that early insulin secretion is enhanced by RS supplementation.
Figure 2.
Gut hormones and SCFAs after 4 weeks of RS or CS supplementation in normal-weight subjects. (A) Blood glucose levels and the AUC, (B) blood insulin levels and the AUC, (C) blood C-peptide levels and the AUC, and (D) blood aGLP-1 levels and the AUC after a standardized meal in participants after 4 weeks of RS or CS supplementation. (E) Serum SCFA levels in the participants after 4 weeks of RS or CS supplementation. Data are presented as the mean ± SEM. The aGLP-1 and SCFA data were log transformed before the analysis. Significance was determined with the generalized estimating equation model. *p < 0.05, ***p < 0.001. (F) Relationship between the changes in acetate and the changes in aGLP-1 at 30 min after starch supplementation.
To understand the basis of this early insulin secretion, we examined the serum levels of gut hormones [glucagon-like peptide-1 (GLP-1) and peptide YY (PYY)] and short-chain fatty acids (SCFAs). A significant increase was observed in active GLP-1 (aGLP-1) at 30 min (p < 0.05) in the meal tolerance test after RS intake compared to that after CS consumption (Fig. 2D). The changes in GLP-1 disappeared at 60 and 120 min following the meal tolerance test (Fig. 2D). RS consumption did not significantly affect the PYY level (Supplementary Fig. S2). Furthermore, the serum short-chain fatty acid acetate was significantly increased after RS intake compared to that after CS consumption [53.34 (41.58,63.18) μM vs. 38.79 (30.81,44.38) μM, p < 0.001], whereas the levels of the other SCFAs (propionate, butyrate, isobutyrate, valerate, isovalerate and hexanoate) did not change after RS intake (Fig. 2E, Supplementary Table S2). Furthermore, the change in acetate after starch consumption was positively correlated with the postprandial change in aGLP-1 at 30 min (r = 0.460, p = 0.006, Fig. 2F).
RS intake changes the gut microbiota composition in adults with normal weights
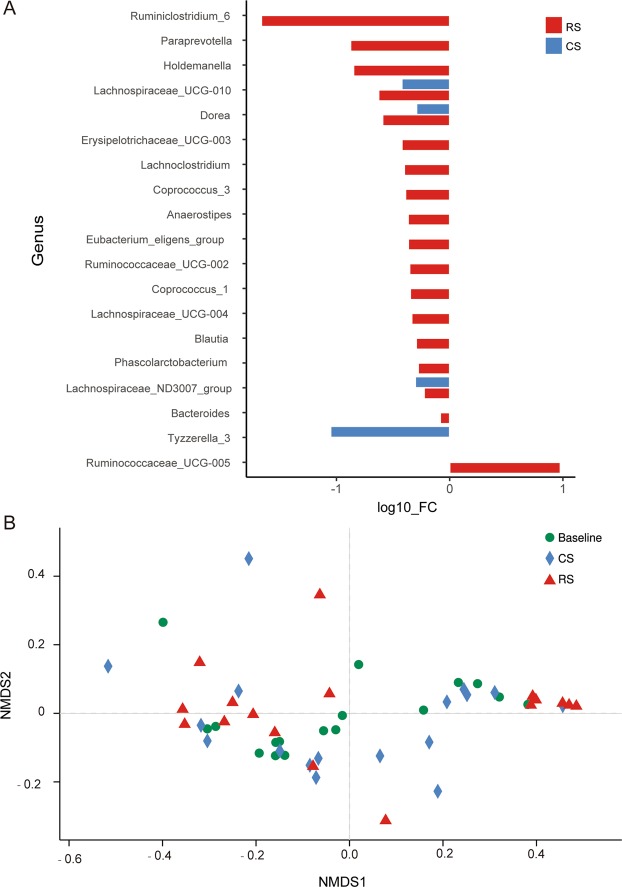
The gut microbiota composition was examined in 17 participants using the 16S rRNA sequencing protocol to understand the mechanism underlying the GLP-1 and PPY alterations in the RS group. A total of 537,298 original sequences were obtained from 51 samples. An operational taxonomic unit (OTU)-based community-level analysis revealed no significant differences in the alpha diversity indices (Shannon, Chao1 and PD_whole_tree) between the values at baseline and after 4 weeks of RS and CS intake (Supplementary Fig. S3). At the phylum level, the composition did not differ among the three groups. Bacteroidetes was the most dominant phylum in all groups, followed by Firmicutes and Proteobacteria (Supplementary Fig. S4). Then, we investigated the effect of RS on the differences in the gut microbiota composition at the genus level. We found that the levels of fifteen bacterial genera were significantly decreased specifically after RS intake relative to the baseline levels (Fig. 3), which included Anaerostipes, Bacteroides, Blautia, Holdemanella, Coprococcus_1, Coprococcus_3, Lachnoclostridium, Lachnospiraceae_UCG-004, Erysipelotrichaceae_UCG-003, Paraprevotella, Phascolarctobacterium, Ruminiclostridium_6, Ruminococcaceae_UCG-002, and Eubacterium_eligens_group. In addition, RS intake significantly increased the abundance of genus Ruminococcaceae_UCG-005. The bacterial genera Lachnospiraceae_UCG-010, Doera and Lachnospiraceae_ND3007_group were decreased in both the RS and CS groups (Fig. 3A). Comparison of the overall communities using non-metric multidimensional scaling (NMDS) with weighted UniFrac distances showed that the samples did not cluster together according to their groups (Fig. 3B). Furthermore, an increasing trend of abundance of Akkermania and Victivallis was observed after RS intake compared to that at baseline (p = 0.059 and p = 0.059, respectively). RS also led to significant abundance changes for a range of bacterial OTUs (Supplementary Figs S5, S6). In addition, metabolites of the gut microbiota, such as N-acetyl-DL-tryptophan and indole lactic acid, were significantly increased in the urine after RS intake (Supplementary Fig. S2).
Figure 3.
The changes in the gut microbial composition after RS supplementation at the genus level. (A) Differences in bacterial abundance at the genus level were analysed before and after 4 weeks of RS or CS intake in normal-weight individuals. Only genera whose abundances were significantly changed are shown (p < 0.05, Wilcoxon signed-rank test, n = 17). The mean abundances of all samples at baseline or after the intervention were used to calculate FC (fold changes) (and then log transformed). If no significance was detected, the fold change was set to 1. (B) Non-metric multidimensional scaling (NMDS) ordination plot based on genus-level microbial beta diversity (UniFrac distances).
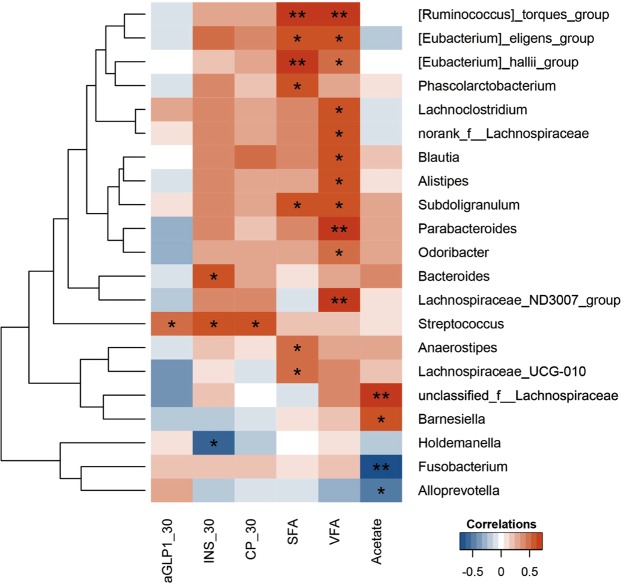
To study the clinical impact of the gut microbiota, we analysed the correlations of the microbiota at baseline and the changes seen in the hormone levels, such as the changes in postprandial aGLP-1, insulin and C-peptide at 30 min, acetate, and the SFA and VFA after RS supplementation. Hormones at 30 min postprandial were elevated by RS and were all correlated with a high baseline abundance of Streptococcus. Additionally, the change in acetate after RS intake was positively correlated with Barmesiella and unclassified_f_Lanchnospiraceae and negatively correlated with Fusobacterium and Alloprevotella. In addition, the decreases in the SFA and VFA after RS supplementation were both correlated with a low baseline abundance of Ruminococcus_torques_group, Eubacterium_hallii_group, and Eubacterium_eligens_group (Fig. 4).
Figure 4.
The correlation heatmap of the baseline gut microbiota with altered host phenotypes and SCFAs. Spearman’s correlations between the abundances of the top 50 most abundant bacterial genera at baseline and the changes in the phenotypes or SCFAs after RS intake were calculated. Blue: negative correlations; red: position correlations. *p < 0.05, **p < 0.01. aGLP1_30: postprandial aGLP-1 at 30 min; INS_30: postprandial insulin at 30 min; CP_30: postprandial C-peptide at 30 min.
Discussion
In this placebo-controlled, double-blinded, and crossover trial, RS was found to reduce abdominal adiposity in normal-weight subjects. Intra-abdominal visceral and abdominal subcutaneous fat were significantly reduced by taking RS at 40 g/d in the 4-week study. RS has been reported to have no effect on body fat in patients with metabolic syndrome or type 2 diabetes mellitus15–19. In those studies, the whole body weight and fat mass were used as indicators of body fat, which might have led to a misinterpretation of data for at least two reasons. First, the fat reduction from RS intake might have been negated by an increase in the intestinal weight, which was not distinguishable from the total body weight. RS is known to increase the tissue weight of the large intestine through a hypertrophic effect on the intestinal wall8,23. Second, diet components were not controlled in these studies, and the dietary impact on the results was not considered. Moreover, interference with the effects of RS by dietary components, such as fatty acids21, tea polyphenols22 and unknown components, may have contributed to the negative results. In the current study, these two issues were addressed using fat-specific analysis and a controlled diet for all subjects. Body fat was measured using a more accurate MRI method that detected changes in the abdominal fat area regardless of alterations in the whole body weight. The fat reduction was observable even in the absence of a reduction in the whole body weight in our study. In addition, we controlled dietary components by providing an identical diet to all subjects. Nonetheless, our nutrient intake data are based on self-reported dietary records, and subjective bias should be considered.
Our data suggest that GLP-1 may play a role in mediating the effects of RS. We observed that the serum GLP-1 level was elevated by RS intake in normal-weight subjects. The elevation in the gut hormone levels provides a mechanism for the effects of RS on abdominal adiposity and early-phase insulin secretion. GLP-1 can increase energy metabolism through its effects on the brain and by promoting insulin secretion through direct stimulation of β cells24,25. Our results are consistent with those observed in rodents for induction of GLP-1 and PYY by RS11,26. The mechanism may be related to the chronic effects of RS on L-cells, which in turn are related to stimulation of GLP-1 secretion by butyrate through G protein-coupled receptor 43 (GPR43)27. RS can also induce L-cell numbers through these chronic effects. The fermentation products of digestion are required for maintenance of mucosal integrity in the colon8,23. Damage to mucosal integrity may lead to a reduction in L-cell numbers. The chronic effects were not examined in these acute studies28–30, which might have contributed to the reduction in GLP-1.
RS was previously found to increase faecal SCFAs, including acetate, propionate and butyrate, in rats8. A significant increase in the serum SCFA acetate level was also found following RS intervention in our study. Moreover, the change in acetate after starch consumption was positively correlated with the postprandial change in aGLP-1 at 30 min. SCFAs in the microbiota act as signalling molecules and nutrients in the colon31. Additionally, SCFAs can be transported from the intestinal lumen into the peripheral blood32, where they affect lipid, glucose and cholesterol metabolism and are taken up by organs33–35. Therefore, our data suggest that RS may induce hormone secretion through stimulation of fermentation and SCFAs.
The gut microbiota was not changed at the phylum level by RS, which was consistent with the findings of a previous report in healthy human subjects13. However, RS intake significantly increased the abundance of Ruminococcaceae_UCG-005, decreased the abundance of fifteen genera, including Bacteroides, Anaerostipes, Blautia, Holdemanella, Coprococcus_1, Coprococcus_3, Lachnoclostridium, Lachnospiraceae_UCG-004, Erysipelotrichaceae_UCG-003, Paraprevotella, Phascolarctobacterium, Ruminiclostridium_6, Ruminococcaceae_UCG-002, and Eubacterium_eligens_group, and increased the trend in the abundance of Akkermania and Victivallis. RS2 intake has been reported to increase the levels of the species Ruminococcus bromii and Eubacterium rectale13. However, we did not observe the same results, possibly because of the limitation in the sensitivity of the 16S sequencing method used in our study.
Bacteroides is a predominant genus within the human intestine and was decreased following RS intake in our study. The effect is similar to that of blackcurrant, dietary α-cyclodextrin and soluble dietary fibre36–38. A decrease in Bacteroides sp. was also observed in overweight or obese children after oligofructose-enriched inulin treatment39, and a similar change was reported in rats after walnut treatment40. Anaerostipes is a genus of anaerobic bacteria from the Lachnospiraceae family, which was also decreased by RS in this study. The decrease in the Anaerostipes levels was also observed in another human study on inulin-type fructan intake41. Blautia, which is another genus of the Lachnospiraceae family, was decreased by RS in this study, which was consistent with observations in healthy Caucasian subjects treated with a walnut-enriched diet42. The reductions in Coprococcus, and Ruminococcus (Lachnospiraceae family) in our study were consistent with those reported in subjects consuming dietary fibre43. Ruminococcaceae_UCG-005, which is a member of family Ruminococcaceae that has been reported to attenuate dietary obesity44, was significantly increased by RS in this study. Akkermansia muciniphila, which is a species of genus Akkermansia, was reported to improve metabolic disorders in dietary obese mice45, and an increasing trend of the Akkermansia genus was also found following RS intake in this study. Therefore, our data suggest that changes in the gut microbiota at the genus level may contribute to the beneficial effects of RS.
We found, for the first time, that the baseline level of genus Streptococcus might mediate the effects of RS on hormones and abdominal fat. Increases in the postprandial levels of active GLP-1, insulin, and C-peptide were associated with a high abundance of genus Streptococcus at baseline in the RS group. This observation was surprising, because an increase in Streptococcus was previously shown to be associated with obesity46. Most species in genus Streptococcus are non-pathogenic, although some are pathogenic. Furthermore, low levels of Ruminococcus_torques_group, Eubacterium_hallii_group, and Eubacterium_eligens_group at baseline were positively associated with reductions in the VFA and SFA in this study, suggesting that baseline levels of Ruminococcus_torques_group are beneficial for the control of body fat. However, a decrease in Ruminococcus_torques_group was reported in a study that evaluated the beneficial effects of the probiotic Akkermansia muciniphila in non obese diabetic mice47. Nonetheless, to obtain further details regarding changes in bacterial genus levels after RS intake, whole-metagenome sequencing, faecal microbiota transplant and a functional study of specific bacteria are warranted in future studies.
In conclusion, RS intake (4 weeks of 40 g/d) significantly reduced the intra-abdominal and subcutaneous fat areas, increased hormone secretion (early-phase insulin, C-peptide, and GLP-1), decreased the levels of several gut bacteria genera, including Anaerostipes, Bacteroides, Blautia, and Holdemanella, and increased that of genus Ruminococcaceae_UCG-005 in normal-weight adults. A higher abundance of the genus Streptococcus promotes an increase in hormones 30 min postprandial by RS, and the lower abundances of Ruminococcus_torques_group, Eubacteriu_hallii_group and Eubacterium_eligen_group promote the effects of RS on abdominal adiposity. The intervention did not lead to any adverse events in the subjects, as indicated by the blood and urine metabolomics. This study provides a basis for the safety and efficacy of RS type 2 supplementation at 40 g/d for the control of abdominal adiposity and glucose metabolism.
Methods
Study subjects and design
Eligible subjects were Chinese individuals between the ages of 18 and 55 years with a BMI < 24 kg/m2 and a waist circumference <85 cm in men and <80 cm in women. The exclusion criteria were as follows: acute or chronic diseases; probiotic supplement or antibiotic use 3 weeks before the study; participation in other clinical trials 4 weeks before the study; medication use that might affect glucose and lipid metabolism; and participation in organized physical activities. The subjects were advised to maintain their current physical activity level throughout the study. Twenty-two subjects with normal weights were screened, and nineteen were finally enrolled between July 2013 and July 2015 from the city of Shanghai, China. The study was conducted in a randomized, double-blind, crossover, and placebo-controlled manner. After enrolment, the subjects were divided randomly into two groups and underwent a 1-week run-in period. A uniform diet was designed and provided by the Department of Nutrition of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital to ensure that all subjects received almost the same food with equal overall macronutrients and caloric intake during the whole process, from the run-in period to the end of the trial. Our uniform diet was designed in line with the Chinese dietary guideline, with 55–60% of the total calories from carbohydrates, ~25% from fat, and 15–20% from protein48. Three consecutive 24-hour dietary records (2 week days and 1 weekend day) at baseline and at the end of each period were required for each subject. The diet information was collected and analysed using a nutrition treatment computing system (NCCW, Qingdao, China). The subjects consumed either HAM-RS2 (Ingredion Inc., Bridgewater, NJ, USA) at 255.4 kcal/day (2.8 kcal/g, 91.2 g, containing 40 g of RS) or matched control starch (Ingredion Inc.) at 255.6 kcal/day (3.55 kcal/g, 72 g, amylopectin, containing 0 g of RS) alternately for four weeks separated by a four-week washout period in the crossover study. The order of starch supplementation was blinded for both the investigator and the participants. An independent researcher performed permuted block randomization and assigned the subjects to the starch supplementations. Computer-generated random numbers were used to assign the subjects in pairs to first receive either RS or CS. The RS and CS were packaged in sealed bags that were identical in appearance, and the subjects and investigators were unaware of the contents of the study starch and the randomization scheme. During the trial, all subjects were provided an identical diet without a change in their exercise habits. Three subjects withdrew from the study before the first intervention, and 19 volunteers, including 10 women and 9 men, completed the study. The data and samples were collected from the subjects through clinical check-ups before and at the end of each intervention.
The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Written informed consent was obtained from all subjects. The trial was registered at the World Health Organization International Clinical Trials Registry Platform with the identification number ChiCTR-TTRCC 13003333 on July 3, 2013.
Anthropometric and biochemical assessments
During the clinical check-ups, the subjects came to the Shanghai Clinical Centre of Diabetes by car or bus in the morning after an overnight (>10 hr) fast. Anthropometric data and venous blood, urine and faecal samples were collected. The waist circumference (WC) was measured at the middle between the lower border of the rib cage and the top of the lateral border of the iliac crest at the end of an expiratory breath. The hip circumference (HC) was measured at the widest part over the greater trochanters by the same researcher. The waist-to-hip ratio (WHR) was calculated. The body composition was measured with the TBF-410 Tanita Body Composition Analyser (Tanita, Tokyo, Japan).
Serum samples were collected and stored at −80 °C prior to the analysis. Fasting plasma glucose (FPG) and postprandial glucose were measured using the standard glucose oxidase method. The blood chemistry parameters included serum AST, ALT, GGT, serum creatinine, blood urea nitrogen (BUN), uric acid (UA), total cholesterol (TC), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), which were measured by enzymatic procedures using an autoanalyser (Hitachi 7600–020 automatic analyser, Tokyo, Japan) according to the manufacturer’s instructions. Fasting and postprandial serum insulin and C-peptide were measured with an electrochemiluminescence immunoassay on a Cobas e411 analyser (Roche Diagnostics GmbH, Mannheim, Germany). Serum active GLP-1 (aGLP-1) and total PYY were measured using quantitative ELISA kits (Millipore, Darmstadt, Germany).
Meal tolerance test (MTT)
To assess glucose metabolism, venous blood samples were taken at serial time points from the subjects following an MTT with instant noodles (1566.6 KJ; 68.4 g carbohydrates and 10.4 g protein). The collection included fasting and postprandial states. Glucose and insulin were examined in the blood as described above.
Hyperinsulinaemic-euglycaemic clamp
Insulin sensitivity was assessed in subjects with a hyperinsulinaemic-euglycaemic clamp as previously described49. The insulin level was maintained at 100 μU/ml by prime-continuous infusion of insulin. The blood glucose concentration was maintained constantly at the basal level by adjusting the glucose infusion rate using the negative feedback principle.
Magnetic resonance imaging
The body fat content was assessed in visceral and subcutaneous adipose tissue areas using a 3.0 T clinical MRI scanner (Archiva, Philips Medical System, Amsterdam, The Netherlands). The standard array coils were used, and the MRI scans were performed by experienced radiologists who were blinded to the intervention groups and laboratory findings. The MRI scans were conducted at the abdominal level between the L4 and L5 vertebrae in the supine position. Segmentation of the images into the VFA and SFA was performed by two trained investigators using the SliceOmatic image analysis software (version 4.2; Tomovision Inc., Montreal, QC, Canada).
Gastrointestinal symptoms
After each treatment in this study, the subjects were given printed sheets to record the incidence and magnitude of GI responses and the details of their bowel movements after consumption of the test starch. Responses to questions on nausea, borborygmi (audible bowel sounds), colic, bloating and flatulence were recorded. The subjects recorded the number of bowel movements and the consistency of their faeces (normal, hard or watery). Information regarding the faecal volume and weight was not gathered.
Metabolomics profiling of human serum and urine samples
Quality control (QC) samples, which were prepared by mixing and blending equal volumes (10 µL) of each sample, were used to estimate a mean profile representing all analytes encountered during the analysis. First, 50 µl of the serum, urine and QC samples were precipitated with 200 µl of methanol in 96-well plates. After vortexing, the filter liquor was obtained via positive pressure and then freeze-dried. The samples were analysed by ultra-performance liquid chromatography (Waters, USA) coupled to a Triple TOF 5600 mass spectrometer (AB SCIEX, USA) system. Pooled QC samples for every ten samples were injected during sample detection to monitor system stability.
Under LC conditions, solvent A was 0.1% (v/v) formic acid/water and solvent B was 0.1% (v/v) formic acid/methanol. The flow rate was set to 0.4 ml/min. For serum sample analysis, each sample was re-dissolved with 50 μl of methanol/water (1:4) solvent. The chromatography column used was a Waters BEH C8 (1.7 μm, 2.1 × 50 mm). The column temperature was 60 °C. The LC gradient was set as follows: 5% B at 0 min and maintained to 0.5 min, 40% B at 2 min, 100% B at 8 min, 100% B at 10 min, 5% B at 10.1 min and maintained for 1.9 min. For urine sample analysis, each sample was re-dissolved with 120 μL of acetonitrile/water (5:95) solvent. The chromatography column was a Waters HSS T3 (1.8 μm, 2.1 × 50 mm). The column temperature was 50 °C. The LC gradient was set as follows: 5% B at 0 min and maintained to 0.5 min, 50% B at 6 min, 90% B at 7 min, 90% B at 8 min, 5% B at 8.1 min and maintained for 1.9 min.
The serum and urine analyses were conducted with the same MS conditions. The mass scanning range was set at 60–1000 m/z for dd-MS2 mode. The sheath gas and curtain gas were set at flow rates of 55 and 35 psi, respectively. The spray voltage was 5.5 kV and the capillary temperature was 550 °C in positive ion mode, whereas the spray voltage was −4.5 kV and the capillary temperature was 450 °C in negative ion mode.
SCFAs in human serum samples
First, 100 µL of serum was mixed with 35 µL of concentrated sulfuric acid diluted with 50% water. Then, 165 µL of ether containing an internal standard was added, mixed thoroughly by vortexing for 1 min, centrifuged at 12,000 rpm for 20 min at 4 °C, and left standing at 4 °C for 30 min. The supernatant ether layer was removed over anhydrous sodium sulfate, which was added to remove traces of water. The resultant supernatant ether layer was transferred to a GC autosampler vial, and short-chain fatty acids were quantitatively determined by gas chromatography (Agilent, USA). The reagent preparation procedure and temperature gradient for the SCFA analysis were adapted from Zheng X. et al.50.
Faecal sample collection and DNA extraction
Fresh faecal samples were collected using a commercial tube with DNA stabilizer (STRATEC Molecular GmbH, Berlin, Germany) before and after each intervention and stored at −80 °C prior to analysis. Stool DNA was extracted using the E.Z.N.A. Soil DNA kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s instructions.
16S rRNA sequencing
The V3 region of the 16S ribosomal RNA (rRNA) gene from each DNA sample was amplified using the bacterial universal forward primer 5′-AGAGTTTGATCCTGGCTCAG-3′ and the reverse primer 5′-TTACCGCGGCTGCTGGCAC-3′. The PCR reaction was conducted under the following conditions: 95 °C for 2 min, 25× (95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s) and 72 °C for 5 min. Roche GS FLX Titanium emPCR kits were used for the PCR amplification, Sequencing was carried out using an Roche Genome Sequencer with the Roche GS FLX + Sequencing Method Manual_XLR70 kit for 2× 300-bp paired-end sequencing.
Bioinformatics analysis
Analysis of the 16S rRNA sequencing data was performed in QIIME51. Sequence data from two subjects were excluded from the analysis because of sample contamination. Adaptors, low-quality reads shorter than 200 bp or those with more than 20% low-quality (score of 20) bases, and low-complexity reads (more than 10 of the same consecutive bases) were filtered. Chimeras were detected and removed using UCHIME52. High-quality sequences were clustered into OTUs at 97% sequence similarity using USEARCH (version 7.1 http://drive5.com/uparse/). Taxonomic assignment of OTUs was performed based on comparison with a database of curated sequences derived from the Silva Database Project (Release 115, http://www.arb-silva.de)53 using RDP Classifiers. The relative abundances of different phyla, genera and OTUs in each sample were calculated and compared before and after the 4-week RS or CS treatments in normal-weight subjects using the Wilcoxon signed-rank test via the SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). A non-metric multidimensional scaling (NMDS) analysis based on the microbiota beta diversity (weighted UniFrac) was performed and visualized in R.
Statistical analysis
All analyses were performed using the SPSS 17.0 software. Sample size calculations were based on equivalence between treatments, which was defined as a difference in the post-treatment visceral fat area of 6 cm2 or more. In our earlier study, the average visceral fat area was 27 ± 5 cm2 in healthy, young, normal-weight adults. We estimated that for a two-sided significance level of 0.05 and 80% power, a minimal sample size of 16 was required. To allow for a 20% drop out rate, at least 22 subjects had to be enrolled54. The general estimating equation (GEE) was used to model the effects of starch treatment and the effects of time, which accounted for the correlation of repeated measurements within subjects. The final results were presented as the mean ± SEM. Data with an abnormal distribution were expressed as medians with interquartile ranges. A two-sided p value less than 0.05 was considered significant.
Supplementary information
Acknowledgements
The RS (HAM-RS2) and CS (matched control starch) supplements were provided free of charge from Ingredion Inc. (Bridgewater, NJ). There was no industrial involvement in the design of the study or the interpretation of the data. This work was supported by the National 973 project of China (2011CB504001), National Natural Science Foundation major international (regional) joint research project (81220108006) and NSFC-NHMRC joint research grant (81561128016) to W.J., Shanghai Pujiang Program (17PJ1407500) and Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ009) to H.L.
Author Contributions
L.Z. carried out the research, analyzed, interpreted the results, and wrote the manuscript. Y.O., P.Y. and G.X. performed untargeted serum and urine metabolomics. H.L. designed the study, analyzed the results and revised the manuscript. L.S. contributed as a clinical nutritionist. Y.N. and G.P. contributed to bioinformatics analysis. G.W., L.Q. and J.Z. contributed to recruited subjects, collected samples and clinical phenotypes. Y.X. performed magnetic resonance imaging. Q.F. reviewed the manuscript. G.X., Y.J. and W.J. designed the study, reviewed and edited the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Zhang and Yang Ouyang contributed equally.
Contributor Information
Huating Li, Email: huarting99@sjtu.edu.cn.
Guowang Xu, Email: xugw@dicp.ac.cn.
Weiping Jia, Email: wpjia@sjtu.edu.cn.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38216-9.
References
- 1.Asp NG, Bjorck I, Holm J, Nyman M, Siljestrom M. Enzyme resistant starch fractions and dietary fibre. Scand J Gastroenterol Suppl. 1987;129:29–32. doi: 10.3109/00365528709095847. [DOI] [PubMed] [Google Scholar]
- 2.Englyst HN, Cummings JH. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am J Clin Nutr. 1985;42:778–787. doi: 10.1093/ajcn/42.5.778. [DOI] [PubMed] [Google Scholar]
- 3.Chen L-Y, et al. Sources and intake of resistant starch in the Chinese diet. Asia Pacific journal of clinical nutrition. 2010;19:274–282. [PubMed] [Google Scholar]
- 4.Birkett A, Jones G, De Silva A, Young G, Muir J. Dietary intake and faecal excretion of carbohydrate by Australians: importance of achieving stool weights greater than 150 g to improve faecal markers relevant to colon cancer risk. European Journal of Clinical Nutrition. 1997;51:625–632. doi: 10.1038/sj.ejcn.1600456. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MM, Douglass JS, Birkett A. Resistant starch intakes in the United States. Journal of the American Dietetic Association. 2008;108:67–78. doi: 10.1016/j.jada.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Penn-Marshall M, Holtzman GI, Barbeau WE. African Americans may have to consume more than 12 grams a day of resistant starch to lower their risk for type 2 diabetes. Journal of medicinal food. 2010;13(4):999–1004. doi: 10.1089/jmf.2009.0195. [DOI] [PubMed] [Google Scholar]
- 7.Harazaki T, Inoue S, Imai C, Mochizuki K, Goda T. Resistant starch improves insulin resistance and reduces adipose tissue weight and CD11c expression in rat OLETF adipose tissue. Nutrition. 2014;30:590–595. doi: 10.1016/j.nut.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Keenan MJ, Raggio A, Williams C, Martin RJ. Dietary-resistant starch improves maternal glycemic control in Goto-Kakizaki rat. Mol Nutr Food Res. 2011;55:1499–1508. doi: 10.1002/mnfr.201000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belobrajdic DP, King RA, Christophersen CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutrition & metabolism. 2012;9:93. doi: 10.1186/1743-7075-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JA, et al. Resistant starch and exercise independently attenuate weight regain on a high fat diet in a rat model of obesity. Nutrition & metabolism. 2011;8:49. doi: 10.1186/1743-7075-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L, et al. Dietary resistant starch increases hypothalamic POMC expression in rats. Obesity (Silver Spring) 2009;17:40–45. doi: 10.1038/oby.2008.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlak DB, Kushner JA, Ludwig DS. Effects of dietary glycaemic index on adiposity, glucose homoeostasis, and plasma lipids in animals. Lancet. 2004;364:778–785. doi: 10.1016/s0140-6736(04)16937-7. [DOI] [PubMed] [Google Scholar]
- 13.Martinez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Upadhyaya B, et al. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Scientific reports. 2016;6:28797. doi: 10.1038/srep28797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabetic medicine: a journal of the British Diabetic Association. 2010;27:391–397. doi: 10.1111/j.1464-5491.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- 16.Bodinham CL, Smith L, Wright J, Frost GS, Robertson MD. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS One. 2012;7:e40834. doi: 10.1371/journal.pone.0040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maki KC, et al. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr. 2012;142:717–723. doi: 10.3945/jn.111.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson MD, et al. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab. 2012;97:3326–3332. doi: 10.1210/jc.2012-1513. [DOI] [PubMed] [Google Scholar]
- 19.Bodinham CL, et al. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocrine connections. 2014;3:75–84. doi: 10.1530/ec-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coate KC, Huggins KW. Consumption of a high glycemic index diet increases abdominal adiposity but does not influence adipose tissue pro-oxidant and antioxidant gene expression in C57BL/6 mice. Nutr Res. 2010;30:141–150. doi: 10.1016/j.nutres.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Charrier JA, et al. High fat diet partially attenuates fermentation responses in rats fed resistant starch from high-amylose maize. Obesity (Silver Spring) 2013;21:2350–2355. doi: 10.1002/oby.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chai Y, Wang M, Zhang G. Interaction between amylose and tea polyphenols modulates the postprandial glycemic response to high-amylose maize starch. J Agric Food Chem. 2013;61:8608–8615. doi: 10.1021/jf402821r. [DOI] [PubMed] [Google Scholar]
- 23.Keenan MJ, et al. High-amylose resistant starch increases hormones and improves structure and function of the gastrointestinal tract: a microarray study. J Nutrigenet Nutrigenomics. 2012;5:26–44. doi: 10.1159/000335319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion. Diabetes. 2003;52:380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald PE, et al. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51:S434–S442. doi: 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 26.Zhou J, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidrine, K. et al. Resistant starch from high amylose maize (HAM-RS2) and Dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity (Silver Spring), 10.1002/oby.20501 (2013). [DOI] [PubMed]
- 28.Bodinham, C. L., Al-Mana, N. M., Smith, L. & Robertson, M. D. Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br J Nutr, 1–5, 10.1017/s0007114513000731 (2013). [DOI] [PubMed]
- 29.Raben A, et al. Resistant starch: the effect on postprandial glycemia, hormonal response, and satiety. Am J Clin Nutr. 1994;60:544–551. doi: 10.1093/ajcn/60.4.544. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Rodriguez, C. E. et al. Postprandial glucose, insulin and gastrointestinal hormones in healthy and diabetic subjects fed a fructose-free and resistant starch type IV-enriched enteral formula. Eur J Nutr, 10.1007/s00394-012-0462-x (2012). [DOI] [PubMed]
- 31.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nature reviews. Endocrinology. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 32.Cummings J, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Z, et al. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes. 2009;58:1509–1517. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fushimi T, et al. Dietary acetic acid reduces serum cholesterol and triacylglycerols in rats fed a cholesterol-rich diet. Br J Nutr. 2006;95:916–924. doi: 10.1079/BJN20061740. [DOI] [PubMed] [Google Scholar]
- 35.Demigné C, et al. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br J Nutr. 1995;74:209–219. doi: 10.1079/BJN19950124. [DOI] [PubMed] [Google Scholar]
- 36.Paturi G, et al. Effects of Blackcurrant and Dietary Fibers on Large Intestinal Health Biomarkers in Rats. Plant Foods Hum Nutr. 2018;73(1):54–60. doi: 10.1007/s11130-018-0652-7. [DOI] [PubMed] [Google Scholar]
- 37.Nihei, N. et al. Dietary α-cyclodextrin modifies gut microbiota and reduces fat accumulation in high-fat-diet-fed obese mice. Biofactors, 10.1002/biof.1429 (2018). [DOI] [PubMed]
- 38.Luo Y, et al. Different Types of Dietary Fibers Trigger Specific Alterations in Composition and Predicted Functions of Colonic Bacterial Communities in BALB/c Mice. Front Microbiol. 2017;8:966. doi: 10.3389/fmicb.2017.00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicolucci A, et al. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology. 2017;153:711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 40.Byerley LO, et al. Changes in the gut microbial communities following addition of walnuts to the diet. The Journal of nutritional biochemistry. 2017;48:94–102. doi: 10.1016/j.jnutbio.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandeputte D, et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66:1968–1974. doi: 10.1136/gutjnl-2016-313271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bamberger C, et al. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients. 2018;10(2):244. doi: 10.3390/nu10020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Healey G, et al. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomised, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr. 2017;119(2):176–189. doi: 10.1017/S0007114517003440. [DOI] [PubMed] [Google Scholar]
- 44.Zhao L, et al. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food & function. 2017;8:4644–4656. doi: 10.1039/C7FO01383C. [DOI] [PubMed] [Google Scholar]
- 45.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langley G, et al. Theimpact of obesity and diabetes on the risk of disease and death due to invasive group A Streptococcus infections in adults. Clinical Infectious Diseases. 2015;62:845–852. doi: 10.1093/cid/civ1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hänninen, A. et al. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut (2017). [DOI] [PubMed]
- 48.Chinese nutrition society. Chinese dietary guidelines summary. 3-13. (People’s Medical Publishing Press, 2017).
- 49.Jia, W. et al. Association of serum retinol-binding protein 4 and visceral adiposity in Chinese subjects with and without type 2 diabetes. The Journal of Clinical Endocrinology & Metabolism92, 3224–3229 (2007). [DOI] [PubMed]
- 50.Zheng X, et al. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics. 2013;9(4):818–827. doi: 10.1007/s11306-013-0500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edgar, R.C. et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics, 10.1093/bioinformatics/btr381 (2011). [DOI] [PMC free article] [PubMed]
- 53.Quast C, et al. The SILVA ribosomal RNA gene database project:improved data processing and web-based tools. Nucl. Acids Res. 2013;41(D1):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kraemer, H. C. & Theimann, S. How many subjects? Statistical power analysis in research. Newbury Park, CA: Sage (1987).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.