Abstract
The genus Agrilus comprises diverse exotic and agriculturally important wood-boring insects that have evolved efficient digestive systems. Agrilus mali Matsumara, an invasive insect, is causing extensive mortality to endangered wild apple trees in Tianshan. In this study, we present an in-depth characterization of the gut microbiota of A. mali based on high-throughput sequencing of the 16S rRNA gene and report the presence of lignocellulose-degrading bacteria. Thirty-nine operational taxonomic units (OTUs) were characterized from the larval gut. OTUs represented 6 phyla, 10 classes, 16 orders, 20 families, and 20 genera. The majority of bacterial OTUs belonged to the order Enterobacteriales which was the most abundant taxa in the larval gut. Cultivable bacteria revealed 9 OTUs that all belonged to Gammaproteobacteria. Subsequently, we examined the breakdown of plant cell-wall compounds by bacterial isolates. Among the isolates, the highest efficiency was observed in Pantoea sp., which was able to synthesize four out of the six enzymes (cellulase, cellobiase, β-xylanase, and β-gluconase) responsible for plant-cell wall degradation. One isolate identified as Pseudomonas orientalis exhibited lignin peroxidase activity. Our study provides the first characterization of the gut microbial diversity of A. mali larvae and shows that some cultivable bacteria play a significant role in the digestive tracts of larvae by providing nutritional needs.
Introduction
Insecta is the largest class among the invertebrates, and species have the ability to feed on different food sources through specialized digestive tracts. The beetles (Coleoptera), with approximately 400,000 species, are the largest order1. The family Buprestidae, also known as the jewel beetle family, has approximately 3000 species, and many of them are invasive exotic insects2,3. Many Buprestidae damage trees, leading to mortality, and are thus considered agriculturally significant pests2. Often, the larvae of Buprestidae parasitize the woody portion of plant tissue, especially the phloem (rarely the xylem), via digestion of plant cell-wall polymers (lignocellulosics). These polymers represent one of the most abundant renewable resources on the planet4,5.
Insects have symbiotic associations with diverse and complex microorganisms, including resident and transient bacteria, fungi, actinomycetes, and archaea6. Over the past decades, there has been an increasing number of works on the gut microbiota of wood-boring beetles because they play a key role in insect physiology and adaptation to the environment and their ecological relevance7. The gut microbiota-host relationship ranges among symbiotic interactions, i.e., from parasitism to mutualism8,9. Microbial symbionts contribute or take part in various physiological processes in insects, including growth, nutrition and vitamin production, development, pathogenesis, immunity, production of components of pheromones, and adaptability to the environment8–10. The gut microbial community is known to be diverse and differs with insect species, different stages and periods of the host life cycle9,11.
In wood-boring beetles, the gut microbiota is prominent in the digestive tract and plays essential roles in compensating for dietary deficiencies and compound detoxification12–14. Moreover, many wood-boring beetles have become significant forest pests that cause extensive mortality of economically important trees. Therefore, exploration of their feeding capabilities is essential for developing pest management programs15–17. The digestive tract and gut microbiota are mainly based on the food source and tissue type. Moreover, certain microbial communities might adapt to the endointestinal lifestyle and have developed mutualistic relationships for host survival. However, little is known about wood-boring larvae feeding behaviour, digestive tracts, gut microbiota, diversity and the symbiotic interactions with insects that develop within the stem phloem and cambium tissues. Furthermore, the potential role of gut microorganisms in lignocellulosic digestion by wood-boring larvae has been thoroughly explored. Therefore, larvae harbour diverse microbial communities, and exploring the role of the gut microbiota helps in understanding insect digestion of plant cell-wall compounds. A symbiotic interaction between gut microbiota and insects likely aids in the digestion of plant cell-wall polymers and provides nutritional supplements for the hosts.
An invasive wood-boring beetle, Agrilus mali Matsumara (Coleoptera: Buprestidae), which is believed to have been introduced from East China in the early 1990s, has caused extensive mortality of wild apple in Tianshan (West China) forests, resulting in severe environmental losses18–20. Beetles of A. mali lay eggs on or in bark crevices of apple trees, and after hatching, neonates immediately bore into the bark and start feeding on the phloem and cambium. The larval stage is the most destructive for the tree because larvae form serpentine galleries throughout the phloem, resulting in disruption of nutrient movement and causing death18–20. The purpose of this study was to characterize the gut microbial communities in depth and to explore the lignocellulolytic activity of cultivable bacteria from fourth-fifth instar A. mali larvae. Characterization of the microbial communities was based on culture-dependent and culture-independent approaches. Six lignocellulolytic assays were used to test cultivable gut bacteria for their ability to degrade plant cell-wall polymers. We found that the A. mali larvae gut includes diverse bacterial species belonging to six phyla, and the most abundant among Proteobacteria species were able to break down plant cell-wall components.
Results
Identification and phylogenetic diversity of bacteria associated with the A. mali larvae gut
The gut microbiota were extracted from the guts of ten A. mali larval insect specimens. High-throughput sequencing of bacterial DNAs of gut microbiota resulted in a total of 96, 206 ± 1319.5 raw reads. After data quality filter processing, the number of quality-controlled reads was 90940 ± 2104.5. The average sequence length of the amplicon was 428 nucleotides. The total number of operational taxonomic units (OTUs) assigned during analysis was 39 bacterial OTUs using 98% similarity in culture-independent methods (Table 1). Analysis revealed that 37 OTUs were found in the gut and 2 OTUs (30 and 35) were detected on the entire sterilized larval body surface.
Table 1.
Bacterial taxa from guts of A. mali larvae identified by 16S rRNA high-throughput sequencing analysis.
| OTU | Phylum | Order | Family | Genus | Predicted species (GenBank #) | Abundance |
|---|---|---|---|---|---|---|
| 38 | Acidobacteria | JF986535* | 2 | |||
| 30 | GU015920* | 3 | ||||
| 9 | Actinobacteria | Propionibacteriales | Propionibacteriaceae | Propionibacterium |
P. acnes CP023676; R. erythropolis MH298510* |
26 |
| 19 | Actinomycetales | Nocardiaceae | Rhodococcus |
R. qingshengii MH064222*; R. degradans KY992558* |
12 | |
| 21 | Bacteroidetes | Bacteroidales | Prevotellaceae | Prevotella | Revotella sp. MG801743 | 14 |
| 14 | Bacteroidales | Rikenellaceae | Rikenella sp. | Rikenella sp. KC417282*** | 39 | |
| 26 | Bacteroidales | Porphyromonoadaceae | Odoribacter | Odoribacter MG052424* | 3 | |
| 11 | Firmicutes | Lactobacillales | Lactobacillaceae | Lactobacillus | L. johnsonii MH393024; | 23 |
| 20 | Lactobacillales | Lactobacillaceae | Lactobacillus | L. intestinalis LC096206* | 12 | |
| 23 | Clostridiales | MG802288 | 10 | |||
| 4 | Clostridiales | AB751303 | 156 | |||
| 31 | Clostridiales | Lachnospiraceae | FJ833589* | 8 | ||
| 37 | Oscillospirales | Ruminococcaceae | AB700360* | 7 | ||
| 15 | Oscillospirales | Ruminococcaceae | KY664658* | 19 | ||
| 25 | Oscillospirales | Ruminococcaceae | Negativibacillus | N. massiliensis NR_147378** | 2 | |
| 39 | Proteobacteria | Burkholderiales | Sutterellaceae | Sutterella | Sutterella sp. KX833879 | 8 |
| 13 | Desulfovibrionales | Desulfovibrionaceae | Bilophila | Bilophila sp. KP055112 | 6 | |
| 12 | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | Desulfovibrio sp. KF760539* | 47 | |
| 3 | Campylobacterales | Campylobacteraceae | Arcobacter | Arcobacter sp. FN397894 | 466 | |
| 10 | Campylobacterales | Helicobacteraceae | Helicobacter |
H. typhlonius NR_041748; H. apri KP120975*; H. mastomyrinus NR_115314*; H. japonicas NR_149210* |
12 | |
| 5 | Pseudomonadales | Moraxellaceae | Acinetobacter |
A. nosocomialis CP029351; A. pittii KY941128; A. calcoaceticus MF149066 |
113 | |
| 24 | Enterobacteriales | Enterobacteriaceae | Erwinia | Erwinia sp. MF525796* | 254 | |
| 22 | Enterobacteriales | Enterobacteriaceae | Erwinia | E. tasmaniensis KF574916* | 16 | |
| 17 | Enterobacteriales | Enterobacteriaceae | Escherichia | E. coli CP008805 | 7 | |
| 1 | Enterobacteriales | Enterobacteriaceae | Pantoea | P. agglomerans MH158658 | 77651 | |
| 18 | Enterobacteriales | Enterobacteriaceae | Pantoea | P. agglomerans KT075213* | 35003 | |
| 8 | Enterobacteriales | Enterobacteriaceae | Pantoea |
P. vagans MH211327*; P. agglomerans MH190052 |
24347 | |
| 32 | Enterobacteriales | Enterobacteriaceae | Pantoea |
P. agglomerans MH165381*; P. vagans MG819435* |
1646 | |
| 2 | Pseudomonadales | Pseudomonadaceae | Pseudomonas |
Ps. congelans LT547855; Ps. syringae KR922152; Ps. cannabina JN167950; Ps. viridiflava HE588020 |
2 | |
| 7 | Pseudomonadales | Pseudomonadaceae | Pseudomonas |
Ps. gessardii MH398505; Ps. reactans MH396741; Ps. synxantha CP011117; Ps. brenneri MF509842; Ps. azotoformans KY939740; Ps. libanensis KY939752; Ps. fluorescens MG977684; Ps. paralactis MG952589 |
101 | |
| 33 | Pseudomonadales | Pseudomonadaceae | Stenotrophomonas |
S. pavanii MF375923; S. maltophilia MH396764 |
2 | |
| 6 | Vibrionales | Vibrionaceae | Vibrio |
V. scophthalmi MG456764*; V. ichthyoenteri KJ817452* |
108 | |
| 28 | Vibrionales | Vibrionaceae | Vibrio |
V. azureus CP018617*; V. ichthyoenteri NR_117888*; V. comitans KT023539*; V. furnissii KR270195*; V. ponticus KF193915 |
13 | |
| 29 | Spirochaetes | Spirochaetales | Spirochaetaceae | Spirochaeta | Spirochaeta sp. DQ340184** | 12 |
| 27 | Spirochaetales | Spirochaetaceae | Spirochaeta sp. DQ340184*** | 23 | ||
| 36 | Unknown | Uncultured bacterium MF259861 | 5 | |||
| 35 | Unknown | Uncultured bacterium MF081102 | 4 | |||
| 34 | Unknown | Uncultured bacterium MF260097 |
*Similarity between 97% < 100%; **similarity between 95% < 97%; less than 95% similarity.
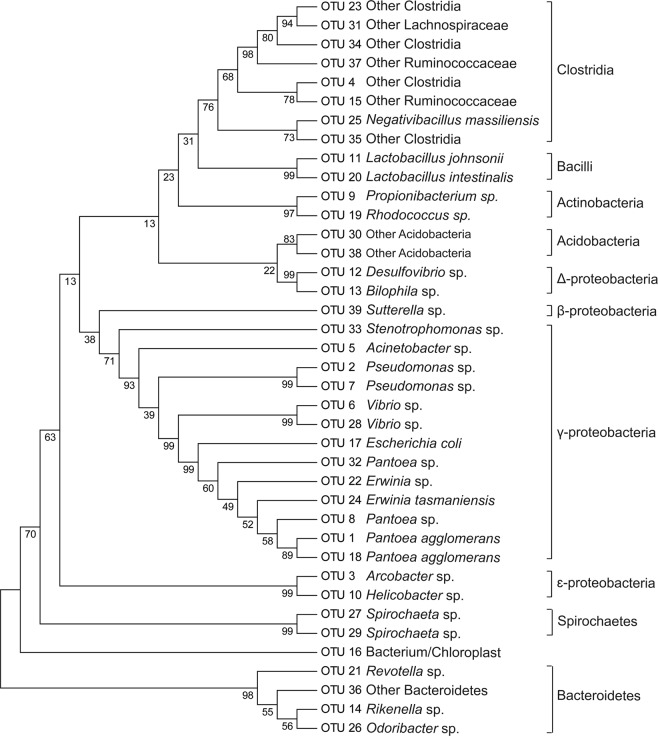
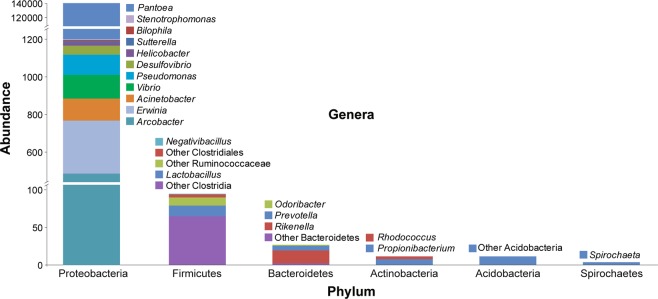
The identification of OTUs was performed by comparison with publicly available sequences in GenBank via BLASTN algorithm search. BLAST analysis revealed that most of the OTUs were distributed into six phyla: Acidobacteria, Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Spirochaetae; ten classes: Acidobacteria, Actinobacteria, Bacteroidetes, Bacilli, Clostridia, Betaproteobacteria, Deltaproteobacteria, Epsilonproteobacteria, Gammaproteobacteria, Spirochaetes; sixteen orders: Acidobacteriales, Propionibacteriales, Actinomycetales, Bacteroidales, Lactobacillales, Burkholderiales, Oscillospirales, Clostridiales, Burkholderiales, Campylobacterales, Pseudomonadales, Enterobacteriales, Pseudomonadales, Xanthomonadales, Vibrionales, and Spirochaetales; twenty families: Acidobacteriaceae, Propionibacteriaceae, Nocardiaceae, Prevotellaceae, Rikenellaceae, Porphyromonadaceae, Lactobacillaceae, other Clostridiales, Lachnospiraceae, Ruminococcaceae, Sutterellaceae, Desulfovibrionaceae, Campylobacteraceae, Helicobacteraceae, Moraxellaceae, Enterobacteriaceae, Pseudomonadaceae, Xanthomonadaceae, Vibrionaceae, and Spirochaetaceae; and twenty genera: other Acidobacteria, Propionibacterium, Rhodococcus, Prevotella, Rikenella, Odoribacter, Lactobacillus, other Clostridiales, other Lachnospiraceae, other Ruminococcaceae, Negativibacillus, Sutterella, Bilophila, Desulfovibrio, Arcobacter, Helicobacter, Acinetobacter, Erwinia, Escherichia, Pantoea, Pseudomonas, Stenotrophomonas, Vibrio, and Spirochaeta. Phylogenetic analysis of the sequenced bacterial species demonstrated that the two largest clades were Proteobacteria and Firmicutes followed by Acidobacteria, Actinobacteria, Bacteroidetes and Spirochaetes (Fig. 1). The Gammaproteobacteria dominated the larval gut libraries (99.3%) and within the class, the most abundant genus was Pantoea (98.8%) (Fig. 2). Within the Firmicutes, the class Clostridia was the most abundant. Bacterial OTUs also contained sequences similar to less-characterized phyla. Two OTUs with low abundance demonstrated close similarity with Acidobacteria, two with Spirochaetes, and one with Bacteroidetes species (Table 1). Four OTUs were highly similar to Pantoea sp. (98.8%). Other genera included Arcobacter (0.33%), two isolates of Erwinia (0.19%), two isolates of Clostridium (0.11%), Lachnospiraceae (0.11%), two isolates of Vibrio (0.09%), two isolates of Pseudomonas (0.07%), Acinetobacter (0.02%), two isolates for Lactobacillus (0.02%), Rikenella (0.02%), Spirochaeta (0.02%), Propionibacterium (0.02%), and other genera with abundances of less than 0.01%. The amount of unidentified bacteria was 0.11%.
Figure 1.
Neighbour-joining tree of partial 16S rRNA sequences retrieved from bacterial community of larvae of A. mali using a culture-independent approach. The evolutionary history was inferred using the UPGMA method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. The evolutionary distances were computed using the Tajima-Nei method and are in units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA7.
Figure 2.
Relative OTU abundance in the A. mali larval gut. The abundance refers to the relative proportion of OTUs containing genera within the distribution of each parent phylum displayed on the x-axis.
Diversity of cultivable bacteria from the A. mali larvae gut
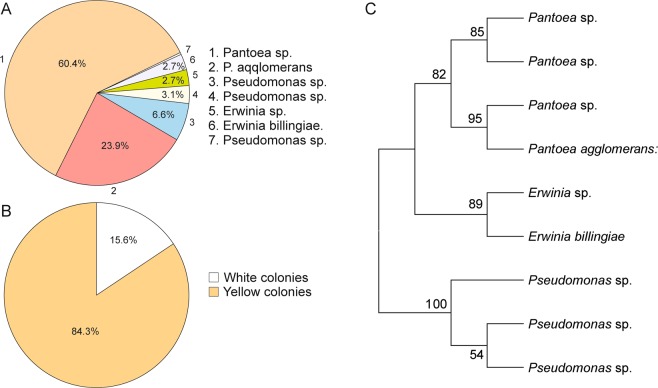
A total of 288 screened bacterial colonies from A. mali larvae were studied. Selection of a colony was randomly based on the morphotype of the colonies. Individual DNA was extracted from each colony, and 16S rRNA sequencing of DNA was performed by traditional Sanger sequencing methods. Sequence analysis of the 16S rRNA gene showed 9 OTUs, identified as various Pantoea, Erwinia and Pseudomonas species, the details of which are shown in Table 2. The identification of sequences by BLAST searches of cultivable bacteria revealed that among the 288 isolates, the most abundant genus was Pantoea (84.3%). Pseudomonas and Erwinia represented 9.7% and 5.5% of the cultivable bacteria, respectively (Fig. 3A). All cultivable bacteria belonged to the class Gammaproteobacteria but showed diversity in colony types (Fig. 3B). A subsequent morphological analysis revealed that 84% of all colonies displayed yellow coloration that corresponded to Pantoea sp. Colonies of Pantoea sp. were smooth, translucent, and convex with entire margins on NA plates with non-pigmented or yellow colonies. Two isolates of Erwinia sp. showed different colony types. Generally, both were circular, smooth and white but different in colony margin with one being entire and the other lobate. One produced a weakly diffusible pink pigment. Pseudomonas sp. showed white colony colour as well either circular or irregular forms.
Table 2.
Bacteria isolated from guts of A. mali larvae identified by 16S rRNA sequence analysis.
| Number of isolates | Genus | Predicted species | GenBank accession | Similarity (%) |
|---|---|---|---|---|
| 8 | Erwinia | E. billingiae | KJ004476 | 100 |
| 8 | Erwinia | E. persicina | MF193907 | 100 |
| E. rhapontici | KF500098 | 100 | ||
| 1 | Pseudomonas | Ps. syringae | MG720019 | 100 |
| Ps. amygdale | CP020351 | 100 | ||
| Ps. cerasi | LT963395 | 100 | ||
| Ps. congelans | JQ320090 | 100 | ||
| Ps. savastanoi | DQ318862 | 100 | ||
| 19 | Pseudomonas | Ps. orientalis | CP018049 | 100 |
| 8 | Pseudomonas | Ps. fluorescens | KT215480 | 99 |
| Ps. synxantha | CP011117 | 99 | ||
| Ps. libanensis | LT629699 | 99 | ||
| Ps. gessardii | MF077145 | 99 | ||
| Ps. azotoformans | LT629702 | 99 | ||
| Ps. chlororaphis | CP011020 | 99 | ||
| 174 | Pantoea | P. agglomerans | MH190052 | 99 |
| P. vagans | KC139414 | 99 | ||
| P. herbicola | U80202 | 99 | ||
| P. ananatis | KC178592 | 99 | ||
| P. conspicua | HF562884 | 99 | ||
| P. brenneri | KX588583 | 99 | ||
| 66 | Pantoea | P. agglomerans | MH158730 | 99 |
| 3 | Pantoea | P. agglomerans | JX077098 | 99 |
| P. vagans | KP099965 | 99 | ||
| P. ananatis | KR361756 | 99 | ||
| 3 | Pantoea | P. agglomerans | MH158730 | 99 |
| P. vagans | CP014129 | 99 |
Figure 3.
Diversity, abundance and clustering analysis of cultivable gut bacteria. Distribution of the cultivable bacterial community of the gut of A. mali larvae by their abundance (A). Coloration of cultivable bacterial isolates (B). Clustering analysis of sequenced cultivable bacteria conducted in MEGA7 (C).
Sequence and clustering analyses demonstrated that the Pantoea genus included four species (Fig. 3C). The most abundant Pantoea species showed close similarity to P. agglomerans, P. vagans, P. herbicola, P. ananatis, P. conspicua, and P. brenneri (60.4%). Other groups showed similarity to P. agglomerans (22.9%); P. agglomerans, P. vagans, and P. ananatis (1%) as well as P. agglomerans and P. vagans (1%). The Pseudomonas genus clustered into three groups. Sequence analysis of the first showed high sequence similarity to Ps. syringae, Ps. amygdale, Ps. cerasi, Ps. congelans and Ps. savastanoi (0.34%); the second group to Ps. orientalis (6.59%); and the third group to Ps. fluorescens, Ps. synxantha, Ps. libanensis, Ps. gessardii, Ps. azotoformans and Ps. chlororaphis (2.77%). Clustering of Erwinia species showed two groups that were highly similar to E. billingiae (2.77%) and to E. persicina and E. rhapontici (2.77%) (Table 2).
Lignocellulolytic activity
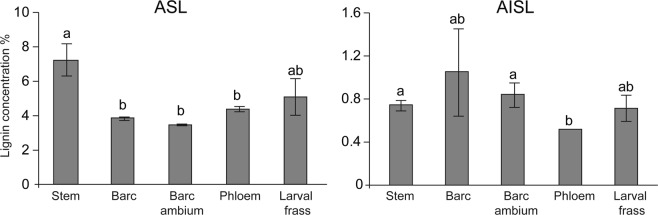
Furthermore, we tested 288 cultivable microbial isolates for their ability to break down plant cell components. Nearly 90% of the bacterial isolates showed lignocellulolytic activity, and cellulose degradation was the most common activity observed among the isolates. In total, 260 isolates showed cellulolytic activity, whereas 242 isolates exhibited xylanase activity, 251 isolates exhibited glucanase activity, 242 isolates exhibited cellobiase activity, and 19 isolates exhibited lignin peroxidase activity (Table 3). The isolates did not show laccase activity. In this context, Pantoea spp. showed higher activity for degrading plant cell wall components than other Proteobacteria. In particular, these species were able to synthesize cellulase, cellobiase, β-xylanase, and β-gluconase enzymes. On the other hand, both identified isolates of Erwinia, namely, Erwinia sp. and E. billingiae actively degraded carboxymethylcellulose. It should be noted that, apart from carboxymethylcellulose degradation ability, E. billingiae also produced glucanase. Representatives of Pantoea, Erwinia and Pseudomonas were not able to degrade Remazol Brilliant Blue molecules except from Pseudomonas orientalis. They also did not demonstrate laccase activity in the ABTS test (Table 3). However, the lignin levels in different apple tree tissues as well as larval frass showed different abundances (Fig. 4). The results demonstrated that ASL accumulated highly in the stem, but AISL did not differ among the tissues. In both AISL and ASL, there was less phloem.
Table 3.
Distribution of enzymatic activities of gut microbial isolates obtained from larvae of A. mali.
| Genus/Species | Colony | CMCa | Xylanase | Glucanase | Cellobiase | RBBRb | LACc |
|---|---|---|---|---|---|---|---|
| Pantoea sp.d | Yellow | 171 | 169 | 171 | 171 | 0 | 0 |
| Pantoea sp. | Yellow | 3 | 3 | 3 | 3 | 0 | 0 |
| Pantoea sp. | Yellow | 3 | 3 | 3 | 3 | 0 | 0 |
| P. aqqlomerans e | Yellow | 66 | 66 | 66 | 66 | 0 | 0 |
| Erwinia sp. | White | 6f | 1 | 8 | 0 | 0 | 0 |
| E. billingiae | White | 8g | 1 | 1 | 0 | 0 | 0 |
| Pseudomonas sp. | White | 0 | 0 | 0 | 0 | 0 | 0 |
| Ps. orientalis | White | 0 | 0 | 0 | 0 | 19 | 0 |
| Pseudomonas sp. | White | 3h | 2 | 0 | 0 | 0 | 0 |
aCellulose activity on CMC agar.
bLignin peroxidase activity in MEA-RBBR.
cLaccase activity on ABTS test.
d80 isolates has less cellulotic, 6 – xylanase, and 8-glucanase activities.
e49 isolates has less cellulotic, 3 – xylanase, and 2-glucanase activities.
f3 isolates has less cellulotic acivity.
g2 isolates has less cellulotic acivity.
h2 isolates has less cellulotic acivity.
Figure 4.
Lignin content in different apple tissues and larval frass. ASL, amino acid soluble lignin; AISL, amino acid insoluble lignin. Values shown are the mean (±SE) of 100 replicates. Different letters show significant differences among months of each stage as determined by one-way ANOVA, followed by a Fisher PLSD post hoc test (P ≤ 0.05).
Discussion
In this study, we explored the diversity of the microbial community that colonizes the larval gut of the invasive wood-borer A. mali to elucidate the digestive process of larvae for plant cell-wall component breakdown. We isolated gut microorganisms using culture-dependent and culture-independent approaches for high-throughput sequencing analysis to determine microbial diversity and to evaluate their lignocellulosic degradation ability. However, the main limitation of this study was the artificial media, which facilitated growth of only a small number of bacterial species present in the larval gut. Phylogenetic analysis demonstrated that the A. mali gut microecosystem is highly diverse with various species abundances.
Overall, our results demonstrated that the gut bacterial community of A. mali larvae is relatively complex. To identify the gut microbiota of A. mali larvae, the variable regions of the 16S rRNA gene were analysed by high-throughput sequencing, and the use of variable regions has already been shown in several studies21–23. In total, we sequenced gut bacterial associates, obtaining 90940 clean reads from culture-independent methods and 288 microorganisms from culture-dependent approaches. We observed a great diversity of bacterial communities representing thirty-nine OTUs belonging to five phyla. Two OTUs were detected in the whole body and 37 OTUs in the larval gut. Two OTUs could be found in the mouth or other parts of the larvae. Approximately 99% of the species found in the larvae gut were β-, ɛ-, Δ-, and γ-proteobacteria and Clostridia. These data are consistent with other reported studies of wood boring beetles17,24,25. In comparison with other wood-boring species whose gut bacterial community was studied17,21,26–29, A. mali larvae had a complex microbiota; however, compared to other wood-boring beetles of Scarabaeidae, Passalidae, Elateridae, Cerambycidae, and Tenebrionidae, the gut microbial diversity of A. mali seems richer in bacterial species27,30. Apparently, the gut diversity of A. mali differed from that reported for another Agrilus species, A. planipennis17. Interestingly, these Agrilus species harbour different bacterial species that belong to almost the same phyla. For example, species belonging to Acidobacteria, Actinobacteria, Firmicutes and Bacteroidetes differed from each other. However, A. mali and A. planipennis commonly share Pseudomonas, Erwinia and Pantoea species.
The bacterial community of A. mali was mainly dominated by Proteobacteria, accounting for 99.7%, with other bacterial classes Firmicutes, Acidobacteria, Actinobacteria, and Bacteroidetes, accounting for less than 1%. To the best of our knowledge, this type of bacterial diversity and large differences in bacterial abundance are reported here for the first time. For example, closely related emerald ash borer A. planipennis larvae harboured 44% Proteobacteria and 38% Firmicutes27. Our study demonstrated that Firmicutes were the second most predominant phylum after Proteobacteria in the A. mali larval gut. In general, our study validates other studies that reported a predominance of Proteobacteria and Firmicutes in wood-boring beetles12,17,21,27, but our results differed in regard to species abundance. Furthermore, recent work by Zhang et al.31 reported bacterial communities of A. mali larvae fed on leaves of different apple (Malus) species under laboratory conditions31. The authors demonstrated that species of γ-proteobacteria accumulated more when larvae were fed M. halliana leaves compared to M. pumila leaves.
High bacterial diversity in the gut of A. mali larvae could indicate that larvae feed on the nutrient-rich cambium and phloem. Colman et al. provided evidence that the diet of the host can affect an organism’s gut microbial community11. This is evidenced by the low gut microbial diversity of the red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae), which feeds on nutrient-poor palm tissues and sap that contains mainly sucrose and glucose32. Sugars affect the complexity of the gut microbiota33, which may account for the low gut bacterial diversity of field sampled larvae21. Conversely, complex substrates such as plant cell-wall lignocellulosics account for the complex bacterial community of the gut11.
Gammaproteobacteria were the most abundant among the identified gut microbial taxa. However, Pantoea had highly dominant species among all identified bacteria (99%). There were four Pantoea sp. identified in the gut, and two of them were identified as two different strains of P. agglomerans. These data were consistent with the sequencing data of cultivable bacteria that also showed four Pantoea species. The results revealed that all four Pantoea species had the ability to break down plant cell-wall polymers. However, this genus did not show lignin-degrading ability. This could be explained by the fact that phloem tissue contains less lignin than xylem tissue. Lourenço et al. reported that lignin composition and structure differed between xylem and phloem34. Our results also indicated that both AISL and ASL levels were significantly lower in phloem and cambium compared to other tissues. Larvae cannot digest the hard lignified xylem. Among the bacterial species, only Ps. orientalis showed the capacity for lignin peroxidase activity, which may help release cellulosics from lignin in the phloem. Interestingly, the lignin content was relatively higher in larval frass compared to the phloem, cambium and bark tissues. This indicates that the frass sample contains less cellulosics due to degradation by the gut bacteria and leads to an increased level of lignin.
The diversity and abundance of the gut bacterial community relies on substrates and their competition with each other. For example, along with Pantoea, species of Erwinia (levels of which were estimated at 0.19%) are also capable of degrading plant cell-wall components. On the other hand, bacterial species could secrete antibacterial compounds when competing for the same substrate. It has been reported that P. agglomerans possesses unique metabolic capabilities to produce antibiotics35–37. These antibiotics could be used for combating fungi and bacterial species present in the gut. Apparently, next-generation sequencing analysis did not detect any fungi in the A. mali gut. The antibiotic herbicolin I, produced by P. agglomerans and P. vagans, has been reported to suppress the pathogen E. amylovora, a pathogen of apple and pear species38.
In conclusion, the results of the current study provide new insight into the diversity of microbial communities and their role in plant cell-wall biopolymer breakdown. This helps to highlight the mechanism of digestion of plant compounds in the larval gut. This work demonstrates that the microbial community of larvae is complex and mainly dominated by γ-proteobacteria. Within the γ-proteobacteria, the Pantoea are the most dominant species in the gut that likely engage in insect-bacteria symbiosis. Moreover, the gut bacterial community might participate in early invasive abilities, leading to host survival in new regions. However, to better understand invasion histories, A. mali samples collected from natural habitats (Eastern China) and invaded regions (Western China) should be compared in regard to gut microbial diversity. Furthermore, the knowledge gained from these studies could be exploited by describing the enzymatic capabilities of gut microorganisms and their roles in host ecophysiology, developing pest management by seeking antagonistic Enterobacteria to inhibit cellulose-degrading bacteria and developing enzyme systems for biotechnological applications.
Materials and Methods
Insect collection and dissection
Agrilus mali insect specimens were collected from April to May 2017 in a wild apple nursery in Mohe Village (43°51N, 82°15W), Gongliu County, Ili-Kazakh District, Xinjiang-Uyghur Autonomous Province, China PR. The first record of A. mali in western China was reported by Ji et al.18; A. mali was later described by DNA barcoding in our recently accepted work20. Insect larvae were collected randomly from infested trees. All larvae were placed inside vented polyethylene containers with woody material (apple twigs) and transported to the laboratory. Insects were surface sterilized in 95% ethanol for 5 s to remove surface microbes39. Larvae were washed five times with sterile Milli-Q water. Surface-sterilized larvae were dissected under aseptic conditions using a scalpel and forceps to extract the digestive tract in 10 mM phosphate-buffered saline (PBS-buffer) under a sterile laminar flow hood17. The larval head and last segment were severed, and the gut was transferred into a 1.5 mL tube with 100 µL PBS buffer (NaCl - 0.137 M, KCl - 0.0027 M, Na2HPO4 - 0.01 M; KH2PO4 – 0.0018 M; pH 7.4) and homogenized using a sterile plastic pestle. Surface sterilized whole larvae were homogenized under aseptic conditions. Homogenates were stored at −20 °C for further culture-independent methods.
Sequencing of non-cultured bacteria
A 16S rRNA gene library from the larvae gut was constructed using ten pooled homogenized guts/whole larvae. Bacterial DNA isolated from the pooled samples was homogenized using the CTAB/SDS method. DNA concentration and purity were monitored on 1% agarose gels. According to the concentration, DNA was diluted to 1 ng/μL using sterile water. Extracted genomic DNA was sent to Novogene China (www.novogene.com) for Illumina generation sequencing. Briefly, amplicons for the 16S rRNA/18SrRNA/ITS genes of distinct regions were amplified using specific primer pairs as follows: for the 16S V4 region, 515F/806R (5′-GTGCCAGCMGCCGCGGTAA-3/5′-GGACTACHVGGGTWTCTAAT-3); for 18S V4 - 528F/706R (5′-GCGGTAATTCCAGCTCCAA-3′/5′-AATCCRAGAATTTCACCTCT-3′); for 18S V9- 1380F/1510R (5′-CCCTGCCHTTTGTACACAC-3′/5′-CCTTCYGCAGGTTCACCTAC-3′) with the barcode. All PCRs were carried out with Phusion® High-Fidelity PCR Master Mix (New England Biolabs). PCRs were carried out in a volume of 30 μL with 15 μL of Master Mix, 0.2 μM of forward and reverse primers, and approximately 10 ng of template DNA. PCR conditions included an initial denaturation for 1 min at 98 °C, 30 cycles of 98 °C for 10 s (denaturation), 50 °C for 30 s (annealing), and at 72 °C for 1 min (elongation), and a final extension at 72 °C for 5 min.
Quantification and quality assessment of PCR products were carried out in the same volume of 1X loading buffer containing SYBR green with PCR products on a 2% agarose electrophoresis gel (Sigma-Aldrich, USA) for visualization. Samples with bright distinct strips between 400–450 bp were chosen for further experiments. Next, PCR products were purified with a Qiagen Gel Extraction Kit (Qiagen, Germany).
Sequencing libraries were generated using the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) following the manufacturer’s recommendations, and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyser 2100 systems. Finally, the library was sequenced on an IlluminaHiSeq2500 platform, and 250 bp paired-end reads were generated.
Isolation of cultivable bacteria
The homogenized larvae tissue was placed into 100 μL PBS solution, and 100 µL aliquots from samples were further serially diluted up to 10−6 and spread on Nutrient Agar (NA) (0.5% peptone, 0.3% beef extract, 1.5% agar, pH 6.8) (Difco, France) for the isolation of bacteria. Incubations were performed at 28 °C for 48 hours. Next, randomly selected single colonies were transferred to fresh NA plates. Selection of colonies was based on colony characteristics such as shape, colony size, colour, margin, elevation, opacity, and consistency.
DNA extraction from cultured bacteria and sequencing
Randomly selected bacterial colonies were incubated in 5 mL Luria-Bertani broth (10 g/L Bacto Tryptone (Oxoid, Canada), 5 g/L Bacto-yeast extract (Oxoid, Canada), 5 g/L NaCl, pH 7.0) on a rotary shaker at 250 rpm at 28 °C overnight. Bacterial suspensions were centrifuged at 10000 rpm for 1 min and treated with proteinase K. Genomic DNA from bacterial isolates was extracted using a TIANamp Bacteria DNA Kit (Tiangen, China) following the manufacturer’s protocol. Extracted DNA was diluted 20-fold and used for polymerase chain reaction (PCR) on a Veriti thermocycler (Applied Biosystems, USA). Forward primer 27 F 5′-AGAGTTTGATCATGGCTCAG-3′ and reverse primer 1492 R 5′-TACGGCTACCTTGTTACGACTT-3′ were used for PCR amplification40. Amplifications were performed in a total volume of 50 µL containing 10 µL of PrimeSTAR HS (Premix) (Takara, Japan) containing an appropriate concentration of dNTPs (0.2 mM) and Taq polymerase (5 U), 1 µL (0.2 µM) of each primer, and 2 µL of diluted DNA. The PCR conditions included 5 min at 95 °C for the initial step followed by 35 cycles at 94 °C for 15 s (denaturation), 55 °C for 30 s (annealing), and 72 °C for 2 min (elongation), with a final extension at 72 °C for 10 min. PCR products were visualized on a 1.5% agarose gel. PCR products were triplicated and sent to the company for further purification and Sanger sequencing at Quintara Bio (China).
Sequence analysis
Paired-end reads were generated based on their unique barcode by removal of barcode and primer sequences. Next, paired-end reads were merged using FLASH V1.2.7 (http://ccb.jhu.edu/software/FLASH)41 for merging paired-end reads for further generating raw tags. Raw tags were filtered under specific filtering conditions to obtain high-quality clean tags42 following QIME V1.7.0 (http://qiime.org/index.html) for the quality-control process. Furthermore, tags were compared with the “Gold” database (http://drive5.com/uchime/uchime_download.html) to remove chimeric sequences43,44 to obtain effective tags. High quality tags were clustered into operational taxonomic units (OTUs) at 97% similarity using UPARSE (v. 7.0.1001)45. Sequences for representative OTUs were classified using the Ribosomal Database Project classifier (RDP)46 in the GreenGene Database47. All raw sequences were submitted to NCBI under Bioproject PRJNA488360 and SRA number SRP072036.
Phylogenetic tree analysis
Sequences obtained with the Sanger method were assembled using SeqMan (DNASTAR Lasergene 7). Sequences of approximately 1400 bp were compared with other 16S RNAs deposited in the nucleotide collection in GenBank using the BLASTN algorithm. Representative OTUs and sequences from the Sanger method were aligned with CLUSTALW. A phylogenetic tree was constructed based on the UPGMA algorithm following the Tajima-Nei model with 1000 bootstrap replicates in MEGA7.
Lignocellulolytic assays
A total of 288 cultivable aerobic bacterial isolates were grown in different media to determine their enzymatic activity. We evaluated the presence of different pathways of lignocellulolytic activity that are likely involved in the degradation of cell-wall components such as lignin, cellulose, β-D-xylan, β-D-cellobiose, and β-D-glucans. To test the lignocellulolytic activity of each pathway, assays were performed in different media supplemented with specific substrates or with direct application onto bacterial colonies. Enzymatic activity was determined by colour change or appearance of halos.
Cellulose hydrolysis was determined using carboxymethylcellulose (CMC, Sigma), which was supplemented into CMC medium (0.94 g/L KH2PO4, 1.9 g/L K2HPO4, 1.6 g/L KCl, 1.43 g/L NaCl, 0.15 g/L NH4Cl, 0.037 g/L MgSO4·7H2O, 0.017 g/L CaCl2, 0.1 g/L yeast extract, 7.5 g/L CMC, and 15 g/L agar, pH 7.0). Individual bacterial isolates were grown on CMC medium as the sole carbon source for 96 hours. After bacterial incubation, bacterial colonies were washed with water. Then, agar plates were stained with 0.5% Congo red solution for 30 min until CMC became dye-bound17. Furthermore, plates were rinsed with 1 M NaCl for 5 min to fix the coloration and then washed with water to clearly observe halos48.
Ligninolytic activity was determined by employing MEA-Remazol Brilliant Blue R (MEA-RBBR) supplemented into agar medium49,50. Bacterial inoculates were incubated on solid media with MEA-RBBR (NA medium, 0.02% wt/vol MEA-RBBR, pH 7.0) at 28 °C for 15 days. The presence of a decolorized area around the colony indicated microbial ligninolytic activity50. For the lignin oxidation assay, bacterial isolates were cultured in NA medium at 28 °C for 16 hours until healthy colonies were visible. Next, to determine lignin oxidation, cool filtered 1 mM 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS, Sigma) was poured over the plates. A substrate colour change to green indicated laccase activity17.
Glucanase, xylanase and cellobiase activities were determined using specific substrates, applying them directly on bacterial colonies27,30. Briefly, substrates 10 mM 4-nitrophenyl β-D-glucopyranoside (Sigma), 4-nitrophenyl β-D-xylopyranoside (Sigma), and 4-nitrophenyl β-D-cellobioside, for β-glucanase, β-xylanase, and β-cellobiase, respectively, were dissolved at 0.6% w/v in 50 mM ammonium acetate buffer, pH 5.0. A drop of these solutions was placed directly on the bacterial colonies, and the plates were incubated at room temperature for 8 hours. The catalytic activity of the microbial enzymes was estimated by the yellow coloration of the substrates, indicating hydrolysis to liberate the p-nitrophenol group (4NP).
Analysis of lignin
Lignin content was determined based on analyses of acid soluble lignin (AISL) (Klason lignin) and insoluble lignin (ASL)51. Briefly, the lignin fractionated into acid insoluble and soluble lignin. The nearest 10 mg of stem, bark, cambium, phloem, and insect frass materials were ground in liquid nitrogen, weighed and dried at 300 °C for 3 hours. Then, 150 µL of 72% sulfuric acid was added and held at room temperature for 1 hour, and samples were vortexed every 10 min. Then, 4200 µL of milli-Q water was added to dilute the acid to a 4% concentration. Samples were autoclaved at 121 °C for 1 hour, and hydrolysates were allowed slowly cool down to room temperature. Samples were centrifuged at high rpm for 1 min, and the supernatant was removed into a new tube for determination of acid soluble lignin by UV-vis (www.thermofisher.com) spectroscopy at 205 nm. The acid soluble52 and insoluble53 lignin contents were calculated using the following equations:
where AISL% = amino acid insoluble lignin, A = weight of lignin and W = oven-dried weight of test specimen, mg.
where ASL% = amino acid soluble lignin, abs = absorbance of sample, V = volume of solution, Coef = known coefficient of plant (here the poplar coefficient = 18.21), and W = amount of starting material.
Statistical analysis
StatView software packages were used to perform Fisher’s PLSD test following an ANOVA (SAS Institute Inc., Cary, NC, USA).
Acknowledgements
We thank the Xinjiang Institute of Ecology and Geography for organizing the expedition to the Tianshan Mountains. The National Key Research Project (2016YFC0501505), Chinese Academy of Sciences ‘Light of West China’ Program (#2016-QNXZ-B-17) and a CAS PIFI fellowship (#2017PB0051) supported this research.
Author Contributions
T.A.B. wrote the manuscript and prepared all tables and figures. B.A.R. contributed to the manuscript writing and corrections. T.A.B. and D.Z. supervised the experiment and manuscript writing. All authors reviewed the manuscript.
Data Availability
Accession Codes: Raw sequence data have been deposited in the Sequence Read Archive of the National Center for Biotechnology Information (SRA, NCBI) under the Bioproject (PRJNA488360) and the SRA (SRP160089).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tohir A. Bozorov, Email: tohirbozorov@yahoo.com
Daoyuan Zhang, Email: zhangdy@ms.xjb.ac.cn.
References
- 1.Bouchard, P. et al. Family-group names in Coleoptera (Insecta). Zookeys, 1–972; 10.3897/zookeys.88.807 (2011). [DOI] [PMC free article] [PubMed]
- 2.Jendek, E. & Poláková, J. Host Plants of World Agrilus (Coleoptera, Buprestidae). A critical review. 706 (Springer, 2014).
- 3.Sydnor TD, Bumgardner M, Todd A. The potential economic impacts of emerald ash borer (Agrilus planipennis) on Ohio, U.S., communities. Arboriculture & Urban Forestry. 2007;33:48–54. [Google Scholar]
- 4.Perez J, Munoz-Dorado J, de la Rubia T, Martinez J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int Microbiol. 2002;5:53–63. doi: 10.1007/s10123-002-0062-3. [DOI] [PubMed] [Google Scholar]
- 5.Beguin P. Molecular biology of cellulose degradation. Annu Rev Microbiol. 1990;44:219–248. doi: 10.1146/annurev.mi.44.100190.001251. [DOI] [PubMed] [Google Scholar]
- 6.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 7.Egert M, Wagner B, Lemke T, Brune A, Friedrich MW. Microbial community structure in midgut and hindgut of the humus-feeding larva of Pachnoda ephippiata (Coleoptera: Scarabaeidae) Appl Environ Microbiol. 2003;69:6659–6668. doi: 10.1128/AEM.69.11.6659-6668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 9.Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon RJ, Vennard CT, Charnley AK. A note: gut bacteria produce components of a locust cohesion pheromone. J Appl Microbiol. 2002;92:759–763. doi: 10.1046/j.1365-2672.2002.01581.x. [DOI] [PubMed] [Google Scholar]
- 11.Colman DR, Toolson EC, Takacs-Vesbach CD. Do diet and taxonomy influence insect gut bacterial communities? Molecular Ecology. 2012;21:5124–5137. doi: 10.1111/j.1365-294X.2012.05752.x. [DOI] [PubMed] [Google Scholar]
- 12.Morales-Jimenez J, Zuniga G, Villa-Tanaca L, Hernandez-Rodriguez C. Bacterial community and nitrogen fixation in the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera: Curculionidae: Scolytinae) Microb Ecol. 2009;58:879–891. doi: 10.1007/s00248-009-9548-2. [DOI] [PubMed] [Google Scholar]
- 13.Geib SM, et al. Microbial community profiling to investigate transmission of bacteria between life stages of the wood-boring beetle, Anoplophora glabripennis. Microb Ecol. 2009;58:199–211. doi: 10.1007/s00248-009-9501-4. [DOI] [PubMed] [Google Scholar]
- 14.Engel P, Moran NA. The gut microbiota of insects - diversity in structure and function. FEMS Microbiol Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- 15.Morales-Jiménez J, Zúñiga G, Ramírez-Saad HC, Hernández-Rodríguez C. Gut-associated bacteria throughout the life cycle of the bark beetle Dendroctonus rhizophagus Thomas and Bright (Curculionidae: Scolytinae) and their cellulolytic activities. Microbial Ecology. 2012;64:268–278. doi: 10.1007/s00248-011-9999-0. [DOI] [PubMed] [Google Scholar]
- 16.Coulson RN. Population dynamics of bark beetles. Annual Review of Entomology. 1979;24:417–447. doi: 10.1146/annurev.en.24.010179.002221. [DOI] [Google Scholar]
- 17.Vasanthakumar A, Handelsman JO, Schloss PD, Bauer LS, Raffa KF. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmaire, across various life stages. Environmental Entomology. 2008;37:1344–1353. doi: 10.1093/ee/37.5.1344. [DOI] [PubMed] [Google Scholar]
- 18.Ji Y, Ji R, Huang RX. Invasive species - Agrilus Mali Matsumura and damage in Xinjiang. Xinjiang Agricultural Sciences. 2004;41:31–33. [Google Scholar]
- 19.Cui XN, Liu DG, Liu AH. Research progress in integrated management of Agrilus mali. Plant Protection. 2015;41:16–23. [Google Scholar]
- 20.Bozorov TA, Luo Z, Li X, Zhang D. Agrilus mali Matsumara (Coleoptera: Buprestidae), a new invasive pest of wild apple in western China: DNA barcoding and life cycle. Ecology and Evolution. 2019;9:1160–1172. doi: 10.1002/ece3.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tagliavia M, Messina E, Manachini B, Cappello S, Quatrini P. The gut microbiota of larvae of Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae) BMC Microbiol. 2014;14:136. doi: 10.1186/1471-2180-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gajardo K, et al. A high-resolution map of the gut microbiota in Atlantic salmon (Salmo salar): A basis for comparative gut microbial research. Sci Rep. 2016;6:30893. doi: 10.1038/srep30893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milani C, et al. Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS One. 2013;8:e68739. doi: 10.1371/journal.pone.0068739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arias-Cordero E, et al. Comparative evaluation of the gut microbiota associated with the below- and above-ground life stages (larvae and beetles) of the forest cockchafer, Melolontha hippocastani. PLoS One. 2012;7:e51557. doi: 10.1371/journal.pone.0051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzi A, et al. Characterization of the bacterial community associated with larvae and adults of Anoplophora chinensis collected in Italy by culture and culture-independent methods. BioMed research international. 2013;2013:420287. doi: 10.1155/2013/420287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delalibera I, Handelsman J, Raffa KF. Contrasts in cellulolytic activities of gut microorganisms between the wood borer, Saperda vestita (Coleoptera: Cerambycidae), and the bark beetles, Ips pini and Dendroctonus frontalis (Coleoptera: Curculionidae) Environmental Entomology. 2005;34:541–547. doi: 10.1603/0046-225X-34.3.541. [DOI] [Google Scholar]
- 27.Rojas-Jimenez K, Hernandez M. Isolation of fungi and bacteria associated with the guts of tropical wood-feeding Coleoptera and determination of their lignocellulolytic activities. Int J Microbiol. 2015;2015:285018. doi: 10.1155/2015/285018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao, G., Gao, J., Hao, C., Dai, L. & Chen, H. Biodiversity and activity of gut fungal communities across the life history of Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae). Int J Mol Sci19, 10.3390/ijms19072010 (2018). [DOI] [PMC free article] [PubMed]
- 29.Briones-Roblero CI, et al. Structure and dynamics of the gut bacterial microbiota of the bark beetle, Dendroctonus rhizophagus (Curculionidae: Scolytinae) across their life stages. PLoS One. 2017;12:e0175470. doi: 10.1371/journal.pone.0175470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vargas-Asensio, G. et al. Uncovering the cultivable microbial diversity of costa rican beetles and its ability to break down plant cell wall components. PLoS One9, e113303; 10.1371/journal.pone.0113303 (2014). [DOI] [PMC free article] [PubMed]
- 31.Zhang ZQ, Jiao S, Li XH, Li ML. Bacterial and fungal gut communities of Agrilus mali at different developmental stages and fed different diets. Sci Rep. 2018;8:15634. doi: 10.1038/s41598-018-34127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagnan, P., Cain, A. H. & Rochat, D. Extraction et identification des composés volatils de la sève de palmier à huile fermentée (vin de palme) attractifs potentiels pour le charancon du palmier. Oléagineux, 135–142 (1992).
- 33.Wong CN, Ng P, Douglas AE. Low-diversity bacterial community in the gut of the fruitfly Drosophila melanogaster. Environ Microbiol. 2011;13:1889–1900. doi: 10.1111/j.1462-2920.2011.02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lourenço A, et al. Lignin composition and structure differs between xylem, phloem and phellem in Quercus suber L. Frontiers in plant science. 2016;7:1612. doi: 10.3389/fpls.2016.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith DD, Kirzinger MW, Stavrinides J. Draft genome sequence of the antibiotic-producing cystic fibrosis isolate Pantoea agglomerans Tx10. Genome Announc. 2013;1:e00904–13. doi: 10.1128/genomeA.00904-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutkiewicz J, Mackiewicz B, Lemieszek MK, Golec M, Milanowski J. Pantoea agglomerans: a mysterious bacterium of evil and good. Part IV. Beneficial effects. Ann Agric Environ Med. 2016;23:206–222. doi: 10.5604/12321966.1203879. [DOI] [PubMed] [Google Scholar]
- 37.Valiente Moro C, Tran FH, Raharimalala FN, Ravelonandro P, Mavingui P. Diversity of culturable bacteria including Pantoea in wild mosquito Aedes albopictus. BMC Microbiol. 2013;13:70. doi: 10.1186/1471-2180-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamber T, et al. Characterization of the biosynthetic operon for the antibacterial peptide herbicolin in Pantoea vagans biocontrol strain C9–1 and incidence in Pantoea species. Appl Environ Microbiol. 2012;78:4412–4419. doi: 10.1128/AEM.07351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demaio J, Pumpuni CB, Kent M, Beier JC. The midgut bacterial flora of wild Aedes triseriatus, Culex pipiens, and Psorophora columbiae mosquitoes. Am J Trop Med Hyg. 1996;54:219–223. doi: 10.4269/ajtmh.1996.54.219. [DOI] [PubMed] [Google Scholar]
- 40.Broderick NA, Raffa KF, Goodman RM, Handelsman J. Census of the bacterial community of the gypsy moth larval midgut by using culturing and culture-independent methods. Appl Environ Microbiol. 2004;70:293–300. doi: 10.1128/AEM.70.1.293-300.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bokulich NA, et al. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat Methods. 2013;10:57–59. doi: 10.1038/nmeth.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas BJ, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics27, 2194–2200, 0.1093/bioinformatics/btr381 (2011). [DOI] [PMC free article] [PubMed]
- 45.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeSantis TZ, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teather RM, Wood PJ. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Applied and Environmental Microbiology. 1982;43:777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vyas, B. & Molitoris, H. Involvement of an extracellular H2O2 dependent ligninolytic activity of the white-rot fungus Pleurotus ostreatus in the decolorization of Remazol Brilliant Blue R. Appl Environ Microbiol, 3919–3927 (1995). [DOI] [PMC free article] [PubMed]
- 50.Machado KMG, Matheus DR, Bononi VLR. Ligninolytic enzymes production and Remazol Brilliant Blue R decolorization by tropical Brazilian basidiomycetes fungi. Brazilian Journal of Microbiology. 2005;36:246–252. doi: 10.1590/S1517-83822005000300008. [DOI] [Google Scholar]
- 51.Yasuda S, Fukushima K, Kakehi A. Formation and chemical structures of acid-soluble lignin I: sulfuric acid treatment time and acid-soluble lignin content of hardwood. Journal of Wood Science. 2001;47:69. doi: 10.1007/BF00776648. [DOI] [Google Scholar]
- 52.Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part II: carbohydrates. J Vis Exp. 2010;37:1837. doi: 10.3791/1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kline MK, Hayes DG, Womac AR, Labbe N. Simplified determination of lignin content in hard and soft woods via UV-spectrophotometric analysis of biomass dissolved in ionic liquids. BioResources. 2010;5:1366–1383. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Accession Codes: Raw sequence data have been deposited in the Sequence Read Archive of the National Center for Biotechnology Information (SRA, NCBI) under the Bioproject (PRJNA488360) and the SRA (SRP160089).