Abstract
Deep brain stimulation (DBS) to the subcallosal cingulate cortex (SCC) is an emerging therapy for treatment resistant depression. Precision targeting of specific white matter fibers is now central to the model of SCC DBS treatment efficacy. A method to confirm SCC DBS target engagement is needed to reduce procedural variance across treatment providers and to optimize DBS parameters for individual patients. We examined the reliability of a novel cortical evoked response that is time‐locked to a 2 Hz DBS pulse and shows the propagation of signal from the DBS target. The evoked response was detected in four individuals as a stereotyped series of components within 150 ms of a 6 V DBS pulse, each showing coherent topography on the head surface. Test–retest reliability across four repeated measures over 14 months met or exceeded standards for valid test construction in three of four patients. Several observations in this pilot sample demonstrate the prospective utility of this method to confirm surgical target engagement and instruct parameter selection. The topography of an orbital frontal component on the head surface showed specificity for patterns of forceps minor activation, which may provide a means to confirm DBS location with respect to key white matter structures. A divergent cortical response to unilateral stimulation of left (vs. right) hemisphere underscores the need for feedback acuity on the level of a single electrode, despite bilateral presentation of therapeutic stimulation. Results demonstrate viability of this method to explore patient‐specific cortical responsivity to DBS for brain‐circuit pathologies.
Keywords: cortico‐cortical evoked potential, deep brain stimulation, forceps minor, stimulation evoked potential, subcallosal cingulate cortex, treatment resistant depression, white matter tractography
1. INTRODUCTION
Deep brain stimulation (DBS) to the subcallosal cingulate (SCC) has shown promise as an intervention for treatment resistant depression (Holtzheimer et al., 2012; Lozano et al. 2008; Mayberg et al., 2005; Puigdemont et al., 2012). A challenge to effective testing, optimization, and dissemination of this intervention is the absence of objective biomarkers to standardize the treatment protocol (Holtzheimer et al., 2017). This is in contrast with DBS for motor dysfunction, such as tremor, wherein the target symptom itself provides a predictable behavior to guide DBS parameter selection (e.g., optimal stimulation amplitude) at initiation or maintenance phases of treatment. Although transient effects of unilateral SCC DBS on mood have been reported during surgical procedures (Choi, Riva‐Posse, Gross, & Mayberg, 2015), no measure of depression symptomatology has provided instantaneous feedback of adequate consistency and acuity to confirm target engagement or guide DBS parameter adjustment outside the operating room.
Notably, intraoperative behavioral effects of SCC DBS have been linked to stimulation of specific white matter fiber tracts (Choi et al., 2015) and SCC DBS treatment is thought to modulate pathological circuits (Deniau, Degos, Bosch, & Maurice, 2010; McIntyre, Savasta, Kerkerian‐Le Goff, & Vitek, 2004) via specific axonal pathways (Lujan et al., 2012). Riva‐Posse et al. (2014) demonstrated that patients who respond to chronic SCC DBS treatment share a common pattern of white matter connectivity from the site of stimulation in the SCC region. This tractography‐based “connectome blueprint” reliably defines the optimal site for chronic stimulation in individual patients and now guides precision surgical implantation of the electrode to the confluence point of four white matter fiber bundles in each hemisphere: forceps minor, cingulum, frontal‐striatal fibers, and uncinate fasciculus (Riva‐Posse et al., 2017). A crucial next step is to develop a biometric for post‐operative confirmation of white matter target engagement in individuals, as well as a means to optimize (via DBS parameter selection over the course of treatment) electrical signal propagation through these key white matter bundles.
The present research tested the feasibility and reliability of the stimulation evoked potential, which is time‐locked to the stimulation pulse, as a method to observe patient‐specific patterns of signal propagation from SCC‐DBS targets. The read‐out is constructed using a “perturb and measure” method: a target cortical or subcortical location is electrically stimulated at low frequency and the resulting between‐pulse signal propagation is recorded throughout brain (reviewed in Keller et al., 2014). The recorded signal is time‐locked to each perturbation (e.g., pulse of DBS); a trial begins with the stimulation event, which is followed by a cortical response on the order of milliseconds. What remains after averaging across multiple trials is a pattern of signal propagation from a locus of stimulation throughout the brain (e.g., Entz et al., 2014). Although precise activation of long‐range fiber tracts is now central to the model of SCC DBS treatment efficacy (Riva‐Posse et al., 2017), this “perturb and measure” approach has yet to be tested as a candidate brain read‐out to augment the current DBS treatment protocol.
The stimulation evoked potential has received various labels, including a cortico‐cortical evoked potential (CCEP), which is typically associated with invasive stimulation and invasive measurement, or a transcranial magnetic stimulation evoked potential (TEP), which involves noninvasive methods of stimulation and measurement (Baker, Montgomery, Rezai, Burgess, & Lüders, 2002; Ilmoniemi et al., 1997; Matsumoto et al., 2004). Both CCEP and TEP have been explored as a measure of effective connectivity, which refers to the causal influence between cortical regions and the propagation of electrical signal across large‐scale brain networks (Entz et al., 2014; Keller et al., 2011, 2014; Massimini et al., 2005; Matsumoto et al., 2006; Miniussi & Thut, 2010). Importantly, a growing literature also links CCEP and TEP features to white matter integrity and structural connectivity (Conner et al., 2011; Keller et al., 2018; Ookawa et al., 2017; Yamao et al., 2017).
In addition to these theoretical insights, the viability of a noninvasive approach (i.e., electroencephalography [EEG]) to measuring a DBS evoked potential, which we employed, has been demonstrated by Zumsteg et al. (Zumsteg, Lozano, & Wennberg, 2006a; Zumsteg, Lozano, Wieser, & Wennberg, 2006b). Evidence of within‐subject reproducibility has also been observed in CCEP and TEP research (Kerwin, Keller, Wu, Narayan, & Etkin, 2017; Zumsteg, Lozano, Wieser, et al., 2006b). Taken together, this literature suggest a strategy to develop an objective, physiological alternative to patient self‐report for confirmation of optimal target engagement and parameter selection in individual patients over the course of treatment.
Toward the goal of developing an individualized biometric of signal propagation from a novel white matter target in the SCC region, the aims of this feasibility study were to: (1) confirm the presence of a novel evoked potential resulting from tractography guided SCC DBS implants, (2) characterize the signal, as well as the signal detection method, and (3) assess the stability and test–retest reliability of the signal in individual patients over months in the treatment.
2. MATERIAL AND METHODS
2.1. Participants and procedure
From June 1, 2015 to January 1, 2016, four consecutive patients were enrolled at Emory University in a study of the safety and efficacy of SCC DBS for treatment resistant depression (TRD; http://clinicaltrials.gov #NCT01984710). Inclusion and exclusion criteria are described by Riva‐Posse et al. (2017) and provided in Supporting Information Materials. The four participants (two males) ranged in age from 53 to 66. The average HDRS‐17 score over a 4‐week baseline period was 21.44 (SD = 1.8). As the hypothesis under examination was not contingent on clinical features, such as medication status, additional case information is provided in Supporting Information Materials, Table S1. All patients provided written informed consent to participate in the study. The protocol was approved by the Emory University Institutional Review Board and the US Food and Drug Administration under an Investigational Device Exemption (G130107 held by H.S.M.) and is monitored by the Emory University Department of Psychiatry and Behavioral Sciences Data and Safety Monitoring Board.
2.2. Tractography guided DBS implantation and confirmation of contact location
Riva‐Posse et al. (2017) describe the surgical procedures for device implantation, including image acquisition and registration procedures, as well as tractography processing. The present research used an Activa PC+S pulse generator (Medtronic, Minneapolis, MN). Bilateral DBS leads (model 3387), each with 4 contacts (1.5 mm inter‐contact spacing), were implanted in the subcallosal cingulate cortex (SCC) using a prospective connectomic approach and StimVision software (Noecker et al., 2017). This approach uses patient‐specific deterministic tractography and anatomical images to guide ideal placement of the contact used for therapeutic stimulation to a precise location in the SCC of each hemisphere: the confluence point of uncinate fasciculus, forceps minor, cingulum bundle, and fronto‐striatal white matter fibers. Stimulation from this location recapitulates the blueprint of white matter connectivity previously associated with a positive treatment response (Riva‐Posse et al., 2014). Experimental procedures outlined in the present research involved unilateral stimulation from the contact (one per hemisphere) selected for therapeutic stimulation according to these anatomical criteria.
In brief, magnetic resonance imaging (MRI) data, both structural (high‐resolution T1) and diffusion‐weighted (i.e., DTI: 128 noncollinear directions with 11 b0s, b value = 1,000 s/mm2, two opposite phase encoding directions to compensate susceptibility‐induced distortion artifact), were acquired for each patient in a single session on a Siemens 3 T Tim‐Trio scanner (Siemens Medical Solution, Malvern, PA). Post‐surgical, high‐resolution computed tomography (CT) images were acquired on a LightSpeed16 (GE Medical System) to verify that the contacts used for therapeutic stimulation met location criteria with respect to tractography. The CT image was transferred to native T1 space using the AFNI software toolbox (Cox, 1996). Patient‐specific coordinates are provided in Supporting Information Materials, Table S2.
A predictive algorithm (Chaturvedi, Luján, & McIntyre, 2013) was used to estimate the extent of activation of axons in the SCC region (Lujan et al., 2013). The output of this algorithm is generally referred to a volume of tissue activated (VTA). VTAs for the left and right hemisphere contacts were generated using the software tool, StimVision (Noecker et al., 2017). Probabilistic tractography from patient‐specific VTA was performed using the Fdt toolbox (FMRIB, http://www.fmrib.ox.ac.uk/fsl) with the following parameters: 5,000 streamlines were generated per voxel in the seed region, a curvature threshold of 0.2, a step length of 0.5, and a maximum number of steps of 2,000. To compensate for seed size differences, tract maps were generated as the number of streamlines that reached each voxel divided by total number of streamlines sent out.
2.3. EEG recording procedure
Each participant underwent four sessions of EEG recording within the first 14 months of active treatment (Supporting Information Table S2). Of 16 recording sessions (4 per patient) one session was discarded due to excessive nonneural noise contamination (Patient 4; T1). The schedule for repeated data collection to assess retest reliability was not standardized across patients; the inquiry was not designed (or powered) to assess therapeutic effects of chronic stimulation. The goal was to assess the stability of the evoked response following unilateral stimulation from the same contact at multiple time points.
Hardware and software used to acquire and process EEG were products of Electrical Geodesics Inc., (Eugene, OR). Patients were fitted with a 256‐channel Hydrocel Geodesic Sensor Net and seated comfortably in a climate‐controlled environment. An adjustable chin rest was used to reduce movement artifact in the EEG recording. Electrode impedances were maintained below 50 kΩ. Recordings were made at a rate of 1,000 samples per second through a NetAmps 400 amplifier using NetStation software, and the location of EEG channels on the head surface was digitized using a Geodesic Photogrammetry System.
Previous to recording, instructions were read aloud from a script. Instructions included prompts to blink naturally and to relax muscles in shoulders, neck, and face. In two of four sessions, patients were also instructed to monitor their experience and to communicate changes in their subjective mood state with a three‐button response option (better; worse; or different). Buttons were pressed with the right hand. At the end of each recording block, patients were prompted to elaborate on any change in their experience. Responses were video recorded for verification and cataloging as in Choi et al. (2015). The 17‐item Hamilton Depression Rating Scale (HDRS‐17; Hamilton, 1960) was also administered at each of four sessions to provide a control for symptom severity variations within and across subjects over the course of the EEG study.
2.4. Deep brain stimulation parameters
Each EEG recording session involved voltage‐regulated, unilateral DBS to the SCC region at 2 Hz and 6 V. The stimulation pulse width was 90 μs, and current was injected in a monopolar cathodic configuration with the implantable pulse generator (IPG) serving as the anode/return. Unilateral stimulation was administered from the left or right hemisphere lead for 3 min each. The order of conditions was counterbalanced across sessions. Patients were blinded to the nature of each condition. Therapeutic stimulation (130 Hz; 3–5 V; 90 μs) was discontinued prior to the start of each EEG recording session and resumed following experimental procedures.
2.5. Preprocessing and construction of the event‐related potential
EEGs were highpass filtered at 0.1 Hz and re‐referenced to the average reference using a polar average reference effect (PARE) correction to estimate the zero surface potential integral (Junghöfer, Elbert, Tucker, & Braun, 1999). A trained technician identified bad channels (e.g., due to hardware damage) and interpolated data from neighboring channels using spherical splines. On average, less than 3 of 256 channels per session were interpolated following visual inspection (Mean = 2.5, SD = 1.9). All EEG recordings showed a large DBS stimulation artifact, twice per second (2 Hz). Trial time “0 ms” was identified as the first positive peak in the DBS stimulation artifact. Single trials were defined as the 500 ms between pulses of stimulation. Each 3‐min condition yielded up to 360 trials.
To eliminate contamination from eye blinks and eye movements, segmented data were subjected to an artifact detection algorithm using NetStation software (Electrical Geodesics, Eugene, OR). An eye blink was defined as a difference between superior and inferior eye channels greater than 140 μV in a moving average of 80 ms. Similarly, an eye movement was identified in ocular‐adjacent channels as a difference greater than 55 μV in a moving average of 80 ms. Segments containing an eye blink or eye movement were removed. After artifact‐contaminated data was discarded, the mean number of trials in a single subject average was 298 (SD = 66). A paired t‐test was used to assess for statistical differences in data attrition that might confound the condition contrast (i.e., unilateral stimulation to left vs. right hemisphere).
Remaining segments were averaged within conditions and baseline corrected with respect to a pre‐trial period preceding the stimulation artifact (−50 ms to −10 ms). Patient‐level average event‐related potentials (ERPs) were calculated for each condition (i.e., left or right hemisphere stimulation) and session (i.e., T1–T4). Grand average ERPs, containing all subjects and sessions, were also constructed for each condition. The time‐course of voltage at each electrode was plotted on a spherical surface for visual detection of coherent topographical features (see Supporting Information Movie S1).
2.6. Statistical analyses
Given the proposed translational application for the SCC DBS evoked potential as a biomarker of target engagement, we sought to determine adequate reliability of the signal on the level of individual patients. First, we identified stable features of the SCC DBS evoked potential waveform in the distribution of peak maxima and minima and we quantified the extent to which patient‐level measures were predicted by a template waveform (i.e., grand mean evoked potential). To further develop the assessment strategy, we determined the minimum number of trials required to observe a coherent evoked response, and assessed the test–retest reliability of the signal in individuals.
2.6.1. Peak detection
A custom statistical segmentation algorithm was designed in Matlab v2017a (Mathworks, Natick, MA) to identify the latency of ERP component maxima and minima in 15 recording sessions, each with two conditions (unilateral stimulation to left or right hemisphere). The algorithm first applied a cubic smoothing spline to the data to remove minor perturbations in the signal (selected smoothing parameter = 0.001, where 0 = linear fit and 1 = natural interpolant). Component latencies were then defined as time points where a sample is flanked by measures of greater or lesser value, such that maxima/minima were separated by at least 10 ms.
2.6.2. Reliability of peak latency
Reliability of the signal was examined in the distribution of component latencies across 15 sessions, each with two conditions (left or right unilateral stimulation). Detected latencies were also plotted on the waveform for visual inspection. To provide guidelines for future analyses of the evoked potential, we calculated the latency distribution of frequently detected maxima/minima (i.e., mean latency, standard deviation). We also identified single channels with maximal energy (positive and negative amplitude) at each peak and selected these for additional reliability analyses. Channel selection was constrained to the classical 10–10 channel array to facilitate future replications.
2.6.3. Minimum number of trials
For each individual in the study, sub‐sample averages were iteratively constructed using 1:n trials, where n = maximum number of trials in a given session. The distribution of each resulting sub‐sample average was compared with the distribution of the mean of all trials (for a given patient and session) using a Kolmogorov–Smirnov test (K–S test). A K–S test provides a nonparametric assessment of the equality of continuous distributions. The null hypothesis that the sub‐sample average and the single‐subject average were drawn from an equivalent population‐level distribution is typically rejected at a p‐value under a given statistical threshold (e.g., .05). To approximate the minimum number of trials needed to construct a consistent evoked potential for each individual, we plotted the p‐values (y‐axis) and number of trials in the sub‐sample average (n, x‐axis). Type 1 error is mitigated in the iterative nature of the approach, which plots p‐values as a continuous variable as opposed to a cut‐off for statistical significance. A standard statistical threshold was noted for context.
2.6.4. Test–retest reliability
Within‐subject, test–retest reliability of the signal was quantified with an intra‐class coefficient (ICC; Cicchetti, 1994). This approach to reliability assessment is conservative for brain‐based biometrics and comparable to psychometric standards. A perfect correspondence across assessments is indicated when ICC = 1. In the context of psychometric assessment, the following guidelines for interpretation have been suggested: ICC > .75 excellent, ICC > .6 good, ICC > .4 fair, and ICC < .4 poor (Cicchetti, 1994).
A nonparametric correlation analysis (calculation of Spearman's Rho) was used to assess the consistency of the signal; how well one recording predicts another. At the channel identified as maximal (i.e., FP1), time series of individual sessions was first correlated with the grand average. This was an effort to assess the quality of representation provided by the grand average, which was used in subsequent source analyses. The threshold for statistical significance was set at p = .001, using a Bonferroni correction for multiple comparisons.
2.6.5. Means comparison
Given the proposed application of the evoked response as a means to confirm target engagement in each hemisphere, we also contrasted the signal output following unilateral stimulation from right and left SCC. Because SCC DBS target criterion is the same in each hemisphere (i.e., confluence point of white matter fiber bundles), we predicted a mirrored topography and magnitude across these two conditions. Results that contradict this prediction, however, would only underscore the need for a read out that is informative to the location and parameter specifications for one electrode at a time, despite the fact that the treatment is delivered bilaterally.
Statistical difference in the cortical response following unilateral left‐ compared with right‐hemisphere stimulation was assessed at channel Pz at 100 ms, which was identified in the peak detection analysis as maximal across both conditions and thus comparable. Channels FP1 or FP2 were also maximal following left and right stimulation, respectively, but not maximal in both conditions. Average amplitude across sessions was calculated for individual patients. Following a Shapiro–Wilk test, in which rejection of the null hypothesis indicates nonnormality in the data, a paired samples t‐test was used to compare condition means. A confidence interval for the sample average mean difference between conditions was calculated by resampling subject means (1,000 iterations, with replacement) to populate a bootstrapped distribution of the mean difference. To illustrate the effect in figures, we also conducted four within‐subjects, paired‐samples t‐tests, with statistical threshold corrected for multiple comparisons (0.05/4; p = .0125).
2.6.6. Neural source analysis of the evoked potential
A descriptive analysis estimated which cortical regions showed maximal source current density at time points associated with coherent features on the scalp surface: 25, 40, 70, and 100 ms, respectively. Source analysis was performed using GeoSource 2.0 (Electrical Geodesics, Eugene, OR) on the grand average of 15 sessions (n = 4) following unilateral stimulation from the left lead. Detailed methods used by GeoSource software to solve the forward problem are provided in Song et al., (2015) and Li, Papademetris, and Tucker (2016), including: the construction an atlas head model (i.e., Colin27) and volume conductor model, discretization of gray matter volume (2,447 dipoles), registration of standard EEG sensor positions and application of a finite difference method (FDM) to solve Poisson's equation and calculate the lead field (Weinstein, Zhukov, & Johnson, 2000). To solve the inverse problem, we selected a standardized low resolution brain electromagnetic tomography approach, or sLORETA (Pascual‐Marqui, 2002). The (Tikhonov) regularization constant was 1 × 10−3, which represents a conservative (i.e., low‐resolution) approach to source localization. Current density (nA) in a single dipole was represented as the sum root mean square of vectors in the x‐y‐z dimensions. Source maxima were defined as dipoles exceeding 50% of the range in intensity values across all modeled dipoles at a given time point. Maxima were identified visually using a threshold color palette in the GeoSource 2.0 atlas MRI view.
2.6.7. Post‐hoc connectivity analysis with tractography
To aid in interpretation of results (i.e., effects of unilateral stimulation to left vs. right hemisphere), we compared patterns of structural connectivity from left and right stimulating contacts. Patient‐specific structural connectivity maps were combined to create a population map (n = 4) of white connections shared across the sample, as in Noecker et al. (2017), but estimated using the experimental stimulation parameters (i.e., 6 V, pulse width = 90 μs). A detailed description of image acquisition and registration procedures, as well as tractography processing, is provided in Riva‐Posse et al. (2017) and described in Methods, above.
3. RESULTS
3.1. Clinical outcomes
For all four patients, the contact selected from left and right DBS leads remained constant for therapeutic stimulation and repeated EEG assessments (T1–T4). Stimulation voltage used for experimentation was held constant (6 V). Between EEG assessments, however, therapeutic dose remained constant from T1 to T4 for two patients (Patient A: 3.5 V, Patient B: 4.0 V), but was increased for Patient C after T3 (3.5–4.0 V) and Patient D after T2 (3.0–5.0 V). A detailed timeline of therapeutic dose changes is provided in Supporting Information Materials, Table S1, which also provides HDRS‐17 scores at pre‐surgical baseline, consistent with severe depression, as well as HDRS‐17 scores at the time of EEG data collection (4 sessions/patient). Symptom severity scores decreased from T1 to T4 (T = EEG recording session #) and showed three of four patients in remission at T4 (HDRS‐17 ≤ 7). Symptom measures were not utilized as correlates of neural change in the present inquiry, but instead as a means to observe the stability of the signal over repeated measures, despite change in symptomatology.
3.2. Subjective response to unilateral SCC stimulation
No clear pattern emerged relating subjective experience to change in stimulation parameters during the experiment. During recording sessions that involved patient‐reported change in subjective experience (i.e., button press: good, bad, different), Patient 1 and 3 reported no changes. Although Patient 2 and 4 reported some changes, responses were infrequent and response‐type was inconsistent across sessions and conditions (i.e., left hemisphere vs. right hemisphere stimulation). A summary of responses is provided in Supporting Information Materials.
3.3. Cortical evoked response to unilateral SCC stimulation
Across individuals and conditions a sequence of four features with coherent topography was observed within 150 ms following a DBS pulse (Figure 1a; Supporting Information Movie S1). Time “0 ms” was identified as the first positive peak in the stimulation artifact, which was greater than 20 ms wide and removed from figures showing EEG traces. Coming out of the artifact, by 25 ms, a positive focus over anterior cortex was always ipsilateral to the stimulated hemisphere (Figure 2). At 40 ms, a positive‐going wave was observed over orbital frontal cortex, while a broad negativity emerged over posterior cortex. Between 60 and 70 ms, a positive focus formed in dorsal anterior channels and began to migrate along the midline toward posterior channels. By 100 ms, a focal positivity appeared over posterior cortices, coincident with a bilateral negativity in ventral anterior channels.
Figure 1.
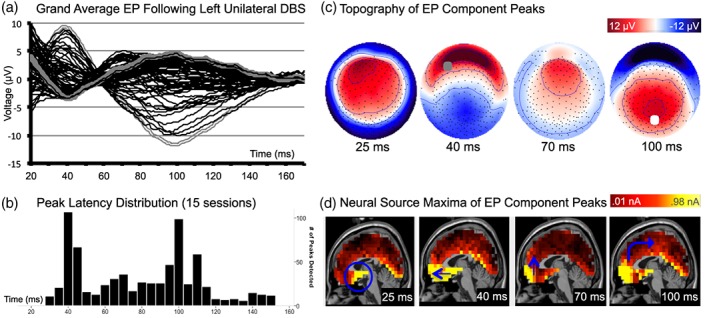
Cortical response to unilateral stimulation of the left subcallosal cingulate. (a) Grand average of recorded voltages in the 10–10 array (n = 4; 15 sessions) following stimulation from left lead. Gray traces show maximum voltage at FP1 (bold) and Pz (hatched). x‐axis = time (ms); y‐axis = amplitude (μV). (b) Distribution of peak latencies (from 15 sessions). (c) Topography of grand average scalp voltages following stimulation from left lead at peak maxima/minima. Topography shown as if looking down on the head surface with nose oriented to the top of the page. Saturated color indicates higher amplitude (red = positive; blue = negative). Gray circle at 40 ms indicates channel FP1; and white circle at 100 ms indicates channel Pz. (d) Neural source model of grand average at peak latencies, including: the locus of stimulation in the SCC (blue circle), mesial temporal lobes and mesial temporal pole (25 ms); anterior ventral medial and orbital frontal dipoles (40 ms); frontal medial BA10 (70 ms); anterior ventral dipoles, including SCC, orbital, and medial frontal pole (100 ms), relative contribution of PCC is maximal at 100 ms (blue arrow)
Figure 2.
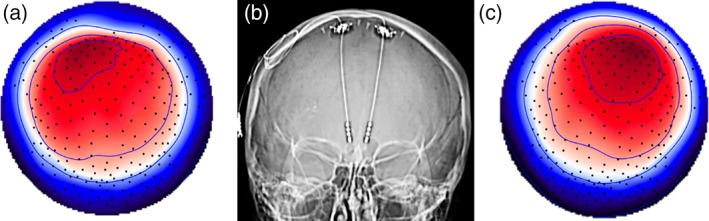
Topography of evoked response at 25 ms reflects the location of unilateral DBS from left or right hemisphere. Topography of grand average scalp potentials (average of 15 sessions, n = 4) 25 ms following stimulation from A. Left hemisphere and C. Right hemisphere. Topography shown as if looking town on the head surface with nose oriented to the top of the page. Saturated color indicates higher amplitude (red = positive; blue = negative). B. CT showing DBS electrodes implanted in bilateral SCC
3.4. Reliability of peak latency
Distribution of peak latency (Figure 1b) showed high reliability of the signal across 15 sessions (4 individuals) with maxima most frequently detected at 40 ms (SD = 4.0), 60–70 ms (SD = 4.6), and 100 ms (SD = 12.7). Topographic patterns across the head surface were visibly consistent across sessions (exemplar provided in Figure 2c) and conditions (stimulation from right vs. left hemisphere). Channels FP1 and Pz showed maximal energy at each maximum following unilateral stimulation to left hemisphere (Figure 1a, gray); channels FP2 and Pz showed maximal energy following stimulation to the right hemisphere. Topography and peak latency distribution of the evoked potential following right hemisphere stimulation is provided in the Supporting Information Materials, Figure S1.
3.5. Test–retest reliability of the EP
Data from channel FP1 following stimulation to left SCC was maximal overall and thus used to quantify the retest reliability of the signal, as well as the quality of representation provided by the grand average (Table 1). Figure 3a shows reproducibility of the signal in patient‐level, repeated measures from channel FP1 over 14 months of treatment. Particularly for three of the four patients, the effect size of correlation between single sessions and the overall grand average was strong (Range: 0.69–0.97), demonstrating that the grand average provides a reliable summary of individual measures for subsequent source analysis, despite the small sample size in this study (Table 1). The relationship was weak to moderate in one of four patients (Range: 0.37–0.49). Using standards applied to the validation of psychometric assessments (Cicchetti, D., 1994), test–retest reliability was excellent in Patient 1 (ICI = 0.93; threshold > 0.75), good in Patient 2 and 3 (ICC = 0.73, .68; threshold > 0.6) and fair in Patient 4 (ICC = 0.58, 0.57; threshold > 0.4). Confidence intervals (95%) for reliability statistics are provided in Table 1. All effects in the correlation and reliability analysis were statistically significant after correcting for multiple comparisons.
Table 1.
Reliability of EP signal across individuals and sessions: Nonparametric correlation (Spearman's rho) and intra‐class coefficient (ICC)
| Grand average | Session 1 | Session 2 | Session 3 | ICC (95% CI) | ||
|---|---|---|---|---|---|---|
| Patient A | Session 1 | 0.938* | ||||
| Session 2 | 0.946* | 0.987* | ||||
| Session 3 | 0.764* | 0.885* | 0.845* | |||
| Session 4 | 0.816* | 0.935* | 0.900* | 0.983* | 0.932 (.911–.948) | |
| Patient B | Session 1 | 0.931* | ||||
| Session 2 | 0.823* | 0.664* | ||||
| Session 3 | 0.968* | 0.911* | 0.795* | |||
| Session 4 | 0.864* | 0.946* | 0.565* | 0.837* | 0.728 (.455–.852) | |
| Patient C | Session 1 | 0.693* | ||||
| Session 2 | 0.973* | 0.741* | ||||
| Session 3 | 0.941* | 0.582* | 0.952* | |||
| Session 4 | 0.921* | 0.901* | 0.930* | 0.816* | 0.680 (.480–.795) | |
| Patient D | Session 1 | 0.422* | ||||
| Session 2 | 0.494* | 0.717* | ||||
| Session 3 | 0.371* | 0.496* | 0.424* | – | 0.584 (.172–.782) | |
p < .0001; Corrected significance threshold, p = .001; ICC, intra‐class coefficient Sessions 1–4 over 15 months.
Figure 3.
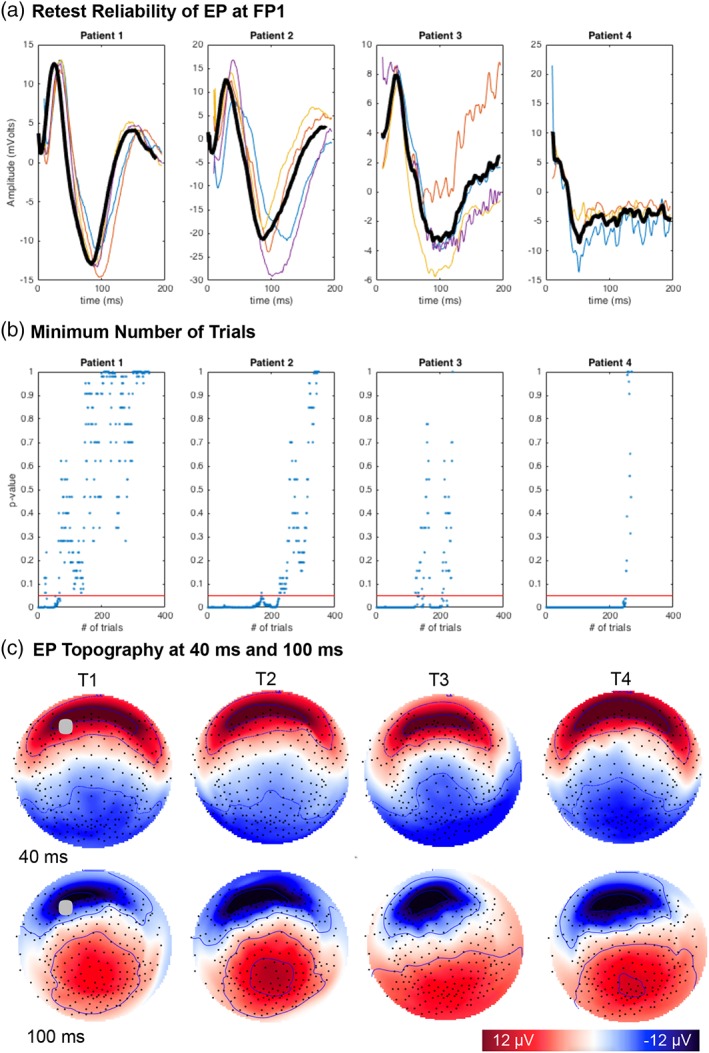
Reliability of the SCC DBS evoked response. A. Repeated measures in four patients shown at channel FP1. Patient‐level average across recording sessions shown in bold. Single session averages: T1 (blue), T2 (red), T3 (orange), T4 (yellow). x‐axis = time (ms), y‐axis = amplitude (μV) B. Minimum number of trials (<250) to derive a consistent signal in individuals. x‐axis = # of trials, y‐axis = K‐S test result, red line indicates p = .05. C. Topography of patient 1 scalp potentials at 40 ms (row 1) and 100 ms (row 2). Results shown for each of four sessions (columns). Topography shown as if looking town on the head surface with nose oriented to the top of the page. Saturated color indicates higher amplitude (red = positive; blue = negative). Gray circle at T1 indicates location of channel FP1
3.6. Minimum number of trials required to generate the evoked potential
Figure 3b provides a patient‐level approximation of the minimum number of single trials required to generate a stable signal: iterative results of a K–S test (p‐value; y‐axis) compared the distribution of a single subject average (~300 trials) with the distribution of an average derived from a subset of trials (1:300; x‐axis). Results across four patients indicate that less than 250 trials are needed to construct a stable signal for an individual. Given that up to 20% of trials were first discarded (using a conservative noise mitigation strategy), these results suggest at a minimum of 315 trials (~ 3 min at 2 Hz) should be recorded to ensure a stable signal in individual patients.
3.7. Hemispheric asymmetry in the cortical response to unilateral stimulation
Average event‐related potentials were constructed for two conditions (following unilateral stimulation from left or right hemisphere) in each session (n = 15). Data attrition due to artifact removal was comparable across conditions, t(14) = 0.247, p = .808. Overall, the average cortical evoked response to unilateral stimulation of left compared with right hemisphere stimulation was consistent in time course and topography (Figure 1; Supporting Information Materials, Figure S1). Both produced a positive focus ipsilateral to the site of stimulation at 25 ms (Figure 2), a frontal positivity at 40 ms, a dorsal medial positivity at 60 ms, and a posterior positivity at 100 ms.
The cortical response was of greater magnitude following unilateral stimulation from left hemisphere, compared with right hemisphere at 100 ms at Pz (Meanleft = 5.56 μV, SD = 3.1; Meanright = 1.29 μV, SD = 0.8). A bootstrapped confidence interval (95% CI: 2.3–6.0) was estimated for the mean difference of 4.2 μV. Results of a Shapiro–Wilk test of normality indicated that data were normally distributed in both conditions: stimulation from left hemisphere (p = .600) and right (p = .937). Paired samples t‐test on subject means indicated that mean amplitude following left hemisphere stimulation was significantly higher, t(3) = 3.5, p = .039. For the purposes of figure construction (Figure 4), a post‐hoc, within‐subjects, means comparison showed the effect to be present in all four patients: Patient 1, t(3) = 17.1, p < .001; Patient 2, t(3) = 5.09, p = .012; Patient 3, t(3) = 10.1, p = .002; Patient 4, t(2) = 6.7, p = .022. Results in three of four patients exceeded a corrected threshold for statistical significance (p = .0125).
Figure 4.
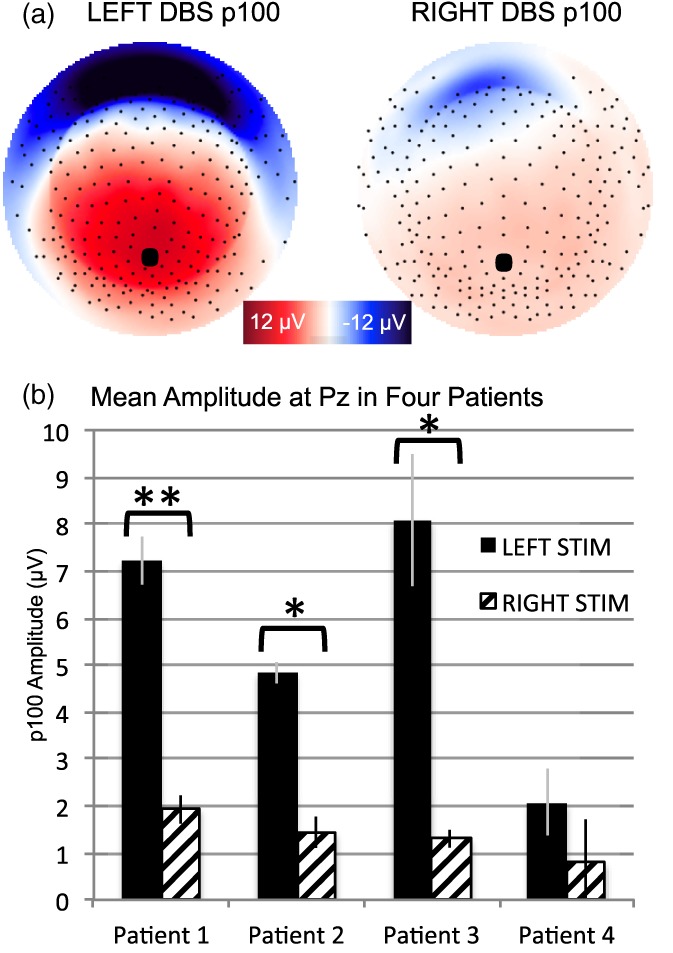
Amplitude of p100 is greater following left versus right hemisphere stimulation. (a) Topography of grand average scalp potentials (average of 15 sessions, n = 4) following stimulation from left hemisphere (left) and right hemisphere (right) at 100 ms. topography shown as if looking town on the head surface with nose oriented to the top of the page. Saturated color indicates higher amplitude (red = positive; blue = negative). (b) Means comparison within subjects. Corrected statistical threshold: *p = .0125, **p < .001
3.7.1. Neural source analysis
Overall, neural source analysis of unilateral DBS implicated source maxima (i.e., exceeding 50% of the range in current source density values) in ventral limbic cortices—specifically, bilateral parahippocampal gyrus/uncus in the mesial temporal lobes and SCC (Figure 1c). A sagittal view (Figure 1d) transected maximal dipoles at each time point of interest. Emerging from the stimulation artifact at 25 ms, source maxima included the locus of stimulation in the SCC region and ventral limbic cortices of the mesial temporal lobe. At 40 ms, the signal maxima also included orbital frontal (medial BA10, BA11) and bilateral insula cortex. Between 60 and 70 ms, as a relatively lower energy feature formed over dorsal anterior cortex and migrated in the posterior direction over the midline, source maxima appeared more exclusively in medial frontal cortex and frontal pole. At 100 ms, source generators were maximal in ventral limbic cortex, including BA25, and a broader swath of orbital, and medial prefrontal cortex.
3.7.2. Post‐hoc tractography analysis
A topographical difference was observed at 40 ms where left hemisphere stimulation (compared with right) generated a more bilateral anterior positivity (Figure 5a). A post‐hoc source analysis suggested an absence of medial frontal maxima following stimulation to right compared with left hemisphere at 40 ms (Figure 5b). Maximal sources identified in the right versus left unilateral stimulation conditions were otherwise comparable (see Supporting Information Figure S1). Post‐hoc tractography analysis showed the following pattern of connectivity in common across the four individuals in the sample (Figure 5c): ipsilateral connectivity to medial frontal cortex from the left and right hemisphere SCC targets but sparse connectivity from the right relative to the left hemisphere SCC target, suggesting relatively less impact of right hemisphere unilateral SCC DBS on ipsilateral medial frontal cortex via forceps minor.
Figure 5.
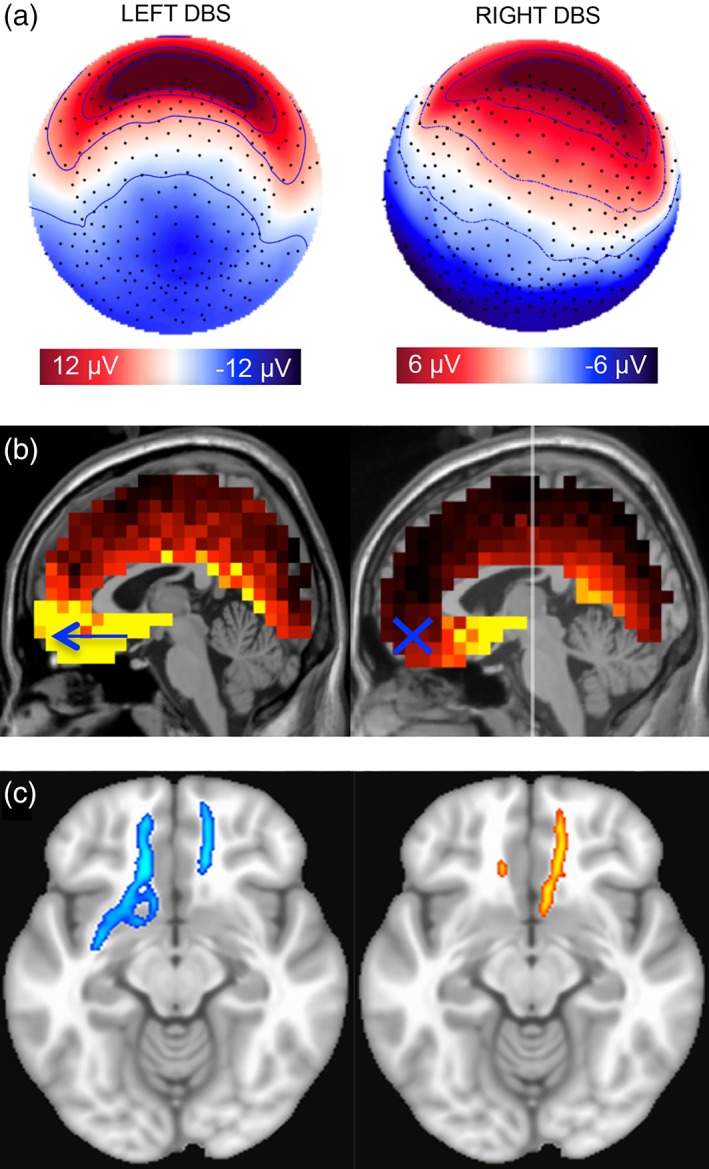
Divergent cortical response to left versus right SCC DBS at 40 ms. (a) Topography of grand average scalp potentials (average of 15 sessions, n = 4) on spherical head surface at 40 ms following stimulation from left hemisphere (left) and right hemisphere (right). Saturated color indicates higher amplitude (red = positive; blue = negative). (b) Differences observed in ventral medial frontal dipoles at p40 maximum: Greater following stimulation from left (left) compared with right (right) hemisphere. Lighter color indicates greater current source density (nA); yellow threshold at 50% of total range. (c) Whole‐brain probabilistic tractography of shared fiber tract maps from left and right unilateral SCC DBS target suggests additional contralateral connectivity from the left SCC target in this sample. Target in left SCC (blue); target in right SCC (orange)
4. DISCUSSION
This study assessed the methodological feasibility and signal reliability of a novel cortical evoked response to deep brain stimulation (DBS) of the subcallosal cingulate (SCC) in patients with treatment resistant depression (TRD). This work represents a first milestone in developing a noninvasive, physiological assay to confirm DBS target engagement and to optimize DBS parameter selection over the course of treatment.
We elicited the SCC DBS evoked potential, recorded noninvasively at four time points over 14 months, by perturbing the brain with unilateral, 2 Hz stimulation to left (or right) SCC white matter. We demonstrated that features of the evoked potential are consistently detected on the level of individual patients (Figure 1). Observing the evoked response as a putative “brain read‐out,” the signal met or exceeded within‐patient, test–retest reliability standards for psychometric assessment (Table 1; Figure 3). These are notably rigorous standards for the engineering of brain‐based biomarkers, which have been greatly anticipated but slow to emerge. We also show that the evoked response can be detected in an individual with passive, noninvasive recording of DBS pulses for less than 3 min (>315 trials at 2 Hz), a feasible time frame for patient assessment.
These efforts position the stimulation evoked potential to provide acute feedback on cortical responsivity, particularly in the absence of overt behavioral change. Consistent with this rationale, self‐reports during the evoked potential experiment revealed no consistent pattern of acute change in subjective mood state. These null findings replicate previous observations that acute changes in subject self‐report may be inadequate for precision tuning of DBS parameters outside the operating room (Choi et al., 2015). More importantly, perhaps, the absence of adverse behavioral effects of supra‐threshold stimulation (i.e., 6 V) demonstrates the safety of this assessment strategy over time.
In addition to characterizing the signal and signal detection method, we provide early evidence to inform DBS programming and target engagement procedures. Of note, the cortical evoked response was greater following left unilateral stimulation, compared with right hemisphere DBS for all four patients in the sample. It is yet unknown whether the asymmetric magnitude in this context reflects an aspect of the brain response at therapeutic frequency (i.e., 130 Hz). We also report preliminary evidence that links the topography of a frontal feature in the evoked potential, maximal at 40 ms, to patterns of white matter tractography (WM) via forceps minor. Our group previously has shown that responders to SCC DBS share a pattern of white matter connectivity from the site of therapeutic stimulation (Riva‐Posse et al., 2014, 2017). This “connectomic blueprint” includes forceps minor, with termini in medial frontal cortices. Thus, setting aside the prospect of a shared mechanism with therapeutic stimulation, the DBS pulse‐locked evoked potential may provide a means to assess relevant white matter engagement, given brain or device changes over the course of treatment. Together, the asymmetric results (i.e., magnitude and topography) underscore a need for feedback acuity on the level of a single electrode to understand the interactive effect of multiple electrodes imposed on a pathological circuit.
4.1. Characterization of SCC DBS evoked potential
Across all four patients, and repeated assessment, we consistently observed four consecutive features within 150 ms following the DBS pulse (Supporting Information Movie S1). Figure 1 shows the following progression: (1) a frontal positivity ipsilateral to stimulation (~25 ms), (2) a broad positivity over orbital frontal cortex (~40 ms; p40), (3) a positive focus over dorsal anterior channels that sweeps down the central midline in the posterior direction (~70 ms), and (4) a broad positivity over posterior cortex with negative inversion in ventral anterior channels (~100 ms; p100).
4.1.1. Orbital frontal positivity (p40)
The first positive peak was detected reliably across all individuals and sessions, with a tight distribution 35–40 ms following the DBS pulse (p40; maximal at FP1, FP2). The scalp topography showed a wide positive focus over orbital frontal cortex (Figures 1 and 5). The latency and shape of this feature appears similar to a negative‐going waveform (i.e., “N1”) observed in cortico‐cortical evoked potentials (CCEP; Matsumoto et al., 2004), which are constructed using an analogous stimulation method, but invasive recording approach (e.g., grids and strips). The opposite polarity recorded on the scalp surface might be explained by the dipolar nature of cortical potentials, which project opposing negative and positive currents to the scalp surface. Conner et al. (2011) report that increases in the CCEP‐N1 amplitude (and latency decreases) predict a greater number of white matter fiber tracts activated by stimulation to thalamic nuclei. Notably, the cortical source model of the p40 peak in the present research was characterized by the onset of maxima in medial frontal pole, which is directly innervated by forceps minor fibers implicated in the profile of treatment response (Riva‐Posse et al., 2014, 2017).
4.1.2. Posterior positivity (p100)
The latest coherent and reliable feature of the evoked response was a broad positivity, maximal at approximately 100 ms (Figure 1c). This p100 was maximal in channels most distal to the site of stimulation, over the posterior cortex (i.e., Pz). The neural source model of the p100, however, emphasized frontal generators closer to a negative‐going peak in anterior channels (Figure 1d). This feature may represent the negative pole of the more diffuse posterior positivity. It closely resembles the “N2” waveform, so identified in CCEP and animal research, which is thought to result from complex cascade of nonlinear events (reviewed in Keller et al., 2014).
4.1.3. Midline sweep (60–70 ms)
A nuance afforded by the whole brain view was observed in a related feature of relatively smaller amplitude between 60 and 70 ms, an apparent transition between the more stable anterior p40 and posterior p100, respectively (Figure 1). This dynamic focus initiated over dorsal frontal cortex and then migrated in the posterior direction along the midline (Supporting Information Movie S1). Notably, the latency of this midline sweep was idiosyncratic across individual patients and sessions, showing a divergent effect of stimulation from left versus right hemisphere. Drawing interpretation from TEP research, it may be that variability in this transitional feature reflects differences in the integrity of network communication following focal stimulation to the SCC region (Borich, Wheaton, Broadie, Lakhani, & Boyd, 2016).
4.2. Divergent response to unilateral stimulation from left versus right hemisphere
Given that SCC DBS target engagement criteria were the same in each hemisphere (i.e., stimulus parameters are symmetrical across hemispheres), we predicted a mirrored topography and magnitude in the evoked response to left versus right unilateral stimulation. We report, however, an overall asymmetry in the magnitude of the evoked response to unilateral stimulation (left greater than right) in all patients (Figure 4). This effect was largest in the posterior component (p100) but was also observed in the magnitude and latency of the midline sweep (60–70 ms), a transition between the anterior p40 and posterior p100 peaks.
Candidate sources of hemispheric differentiation in the present research are varied. In addition to asymmetric behavioral responses in the OR (Choi et al., 2015), we have previously presented evidence of lateralization in the neural response to SCC DBS (Mayberg et al., 2005). The angle of implantation for left and right leads may also diverge, according to surgical constraints (e.g., vasculature) and the anatomical orientation of cortical folds in the SCC region, which will show some asymmetry across hemispheres. Unique to this sample, however, bilateral target engagement criteria are defined (and confirmed post‐operatively) relative to anatomical features within each hemisphere: the therapeutic contact is placed specifically at the confluence point of four white matter bundles (Riva‐Posse et al., 2014, 2017).
It remains unclear whether the notable difference in the magnitude of the cortical response, observed reliably across individuals, reflects a yet unappreciated anatomical asymmetry in the SCC region, observed post‐hoc in tractography models (Figure 5), or a biophysical property of electrocortical signaling. Regardless of mechanism, the observation of divergent cortical responsivity to unilateral stimulation in left (vs. right) hemisphere may be relevant to a protocol that is delivered symmetrically from bilateral electrodes. For example, this opens the possibility that device efficiency (i.e., battery life) could be enhanced with a DBS parameter configuration that is also asymmetric across hemisphere.
4.3. Cortical substrate of SCC DBS evoked potential
A key question in this line of research is how to exploit the signal propagation pattern, observed uniquely in the stimulation evoked response, to enhance the targeting of white matter structures. However, the cellular mechanisms that produce the evoked propagation pattern are not entirely understood. Dominant features are unlikely to reflect passive or monosynaptic propagation, which are expected on the order of 4–8 ms (Keller et al., 2014). Animal research suggests that the evoked features instead capture a more complex response to the disruption of local cellular properties and long‐range axons that traverse the site of stimulation (Ezure and Oshima, 1985; Steriade & Amzica, 1996). Most axonal projections, however, will be orthodromic to the site of stimulation and their excitation contributes to a polysynaptic propagation of signal to distal cortical regions via cortical and cortical–subcortical projections (Deniau et al., 2010). It follows that shorter latency components of the evoked potential result from local excitation and structural features, with possible back‐propagation of signal to local cell somas; while later components reflect more complex and nonlinear mechanisms (Keller et al., 2014; Matsumoto et al., 2006). Importantly, mechanisms of DBS treatment efficacy have also been tied to modulation of local soma and distributed networks via axonal activation (McIntyre et al., 2004).
4.3.1. Topography of p40 may reflect contralateral connectivity via forceps minor
Contrary to our prediction, the cortical response following stimulation to left versus right SCC was not strictly mirrored. In particular, the p40 was symmetric over the frontal pole following stimulation from left hemisphere but observably asymmetric (ipsilateral) following stimulation from right hemisphere (Figure 5a). In both conditions, source analysis of the p40 peak identified key generators in the SCC region, however stimulation to left but not right hemisphere evoked additional source maxima in the medial frontal cortex (medial BA10). A candidate mechanism for this hemispheric difference again implicates forceps minor fibers, which innervate ipsilateral BA10 directly, and contralateral BA10 via the corpus callosum.
To elaborate on this interpretation, we conducted a post‐hoc analysis of white matter connectivity from the volume of tissue activated by unilateral stimulation at 6 V (Figure 5c). The four patients shared a pattern of connectivity from left hemisphere SCC to bilateral medial BA10, consistent with the bilateral p40 topography observed following stimulation from the left lead. In comparison, connectivity was observably less robust from right hemisphere SCC to contralateral BA10, consistent with the asymmetric (i.e., ipsilateral) p40 topography observed following stimulation from the right lead. Though descriptive and not empirical, these results suggest a hypothesis that the p40 feature might provide a surrogate measure forceps minor engagement. This is a notable speculation to pursue because an absence of connectivity to medial cortex via forceps minor previously distinguished nonresponders from responders (Riva‐Posse et al., 2014).
4.4. Limitations and future directions
While it remains unclear to what extent the DBS evoked potential shares biophysical mechanisms with therapeutic stimulation (at 130 Hz), both methods implicate specific patterns of structural connectivity and white matter activation. Future research comparing results across target and nontarget contacts is needed to infer which components of the evoked potential, if any, are therapeutically relevant. In the meantime, our descriptive evidence that the p40 feature may reflect forceps minor activation is consistent with empirical findings that link the stimulation evoked response (e.g., CCEP) to white matter activation (e.g., Conner et al., 2011; Keller et al., 2018; Ookawa et al., 2017, Yamao et al., 2017). Making this leap in the context of SCC‐DBS may require more precise biophysical modeling of neural elements, further exploitation of invasive recording in the OR and utilization of animal models. Should the signal prove adequately specific, however, this approach could provide a much‐needed complement to tractography‐guided white matter targeting, providing verification of target engagement given changes over the course of treatment and in the absence of an acute behavioral response.
The sample average evoked potential (15 sessions) was highly predictive of the signal across individual sessions, particularly for three of four patients (Table 1), suggesting that common phenomenon was observed despite the small sample size and absence of standard replication controls (e.g., split half). This observation is particularly relevant to biomarker research on emerging neuromodulation therapies (i.e., SCC DBS patients), wherein paradoxically, sample sizes will always be small preceding large‐scale trials, the success of which hinge on the viability of an objective treatment protocol. Though our test–retest reliability indicates consistency in the distribution of the signal after 220 trials, it is also possible that more subtle features of the evoked potential will be resolved with longer recordings (>500 trials). Compared with other configurations, monopolar stimulation generates a wide artifact, which may also obscure meaningful features of the propagation profile at short latency. Inspection of the waveform at 25 ms, for example, revealed a downward slope emerging from the stimulation artifact, which likely obscured the peak of this component. Overall, issues related to heterogeneity the in signal will be better overcome with access to a larger sample and increased sampling rate, which might be achieved in the OR.
These limitations acknowledged, our method involved a hybrid of CCEP and TEP methods to maximize benefit from both strategies: paired invasive stimulation for target specificity with noninvasive scalp‐EEG recording. Despite arguably inferior signal acuity, a noninvasive EEG recording strategy meets several key criteria for a viable brain read‐out. Relative to invasive recording strategies (e.g., grids or strips), head surface EEG is amenable to long‐term monitoring (i.e., repeated assessment) over months or years of treatment. Also relative to invasive recording, risk to the patient is reduced and assessment is not limited to a predetermined spatial focus (e.g., prefrontal, motor cortex); the topography of the propagation pattern can be observed across the whole‐head surface.
Given adequate spatial sampling of EEG on the scalp surface, it may be possible to model neural sources of the signal with adequate accuracy. The present approach employed typical model constraints (e.g., sLORETA) for a conservative estimate of neural sources, which was adjunctive and not essential to hypothesis testing. We took care to assess the quality of representation of single subjects by the grand average, which was highly predictive of subject‐level averages in three of four patients (Table 1). Evidence corroborating source model accuracy was observed immediately following the stimulation artifact in the EP recording; maximal source generators of this earliest feature were accurately localized to the SCC region, as well as mesial temporal cortex and temporal pole (Figure 1d). This source activity was coincident with a positive focus over frontal cortex, always ipsilateral to the site of stimulation (Figure 2). However, future research will require additional steps to demonstrate the validity and accuracy of inverse models, including the application of patient‐specific forward models, corroboration with invasive recording strategies and a more comprehensive presentation of results using different modeling parameters.
5. CONCLUSION
As we continue to adapt DBS for motor symptoms to more diffuse psychiatric symptom targets, we will require precision alternatives to clinical phenomenology for immediate feedback guidance. A barrier to effective dissemination and testing of treatment for severe depression with deep brain stimulation to the SCC region is the absence of an objective biomarker to confirm target engagement and guide parameter selection in individuals. It is unknown whether the absence of such objective measures to constrain the SCC DBS treatment protocol explains the discrepant outcomes of a recent multisite trial (Holtzheimer et al., 2017).
Our proposal is to leverage our biophysical models of treatment efficacy (Riva‐Posse et al., 2014) to identify a physiological measure of circuit perturbation. We demonstrate that a noninvasive metric of effective connectivity from a white matter target in the subcallosal cingulate is consistent, reliable, and informative. We provide preliminary evidence that the DBS evoked potential might provide a noninvasive indicator of white matter target engagement. In addition, evidence of divergent responses following stimulation from left versus right leads may challenge assumptions related to symmetry in targeting and dosage. This work represents a necessary first step in developing a patient‐specific measure of acute cortical responsivity to DBS for brain‐circuit pathologies.
DISCLOSURE OF INTERESTS
A.W. None; A.V. None; K.C. None; B.H. None; V.T. None; K.B. None; A.C. None; P.R‐P. None; H.M. has a consulting agreement with St Jude Medical (now Abbott), which has licensed her intellectual property to develop SCC DBS for the treatment of severe depression (US 2005/0033379A1). The terms of this arrangement have been approved by Emory University in accordance with policies to manage conflict of interest.
AUTHOR CONTRIBUTIONS
Experimental design, data analyses: A.W.; Manuscript preparation: A.W., H.M., all authors contributed to and approved the final manuscript; Data collection: A.W., A.V., K.B., A.C., V.T.; Additional analyses: A.V., B.H., K.B., K.C. (DTI tractography); Clinical management: A.C., P.R‐P.
Supporting information
Supporting Information
Supplemental Movie 1 (caption). Signal propagation profile following unilateral stimulation of the left subcallosal cingulate. Topography of grand average scalp voltages (average of 15 sessions, n=4) over 150 ms following stimulation from left lead (time=0). Topography shown as if looking down on the head surface with nose oriented to the top of the page. Saturated color indicates greater amplitude (red=positive; blue=negative, range:‐12 to 12 μV). At 25 ms, a positive focus over anterior cortex is ipsilateral to the locus of stimulation (left SCC). At 40 ms, a positive‐going wave is observed over orbital frontal cortex, while a broad negativity emerges over posterior cortex. Between 60 ‐70 ms, a positive focus forms in dorsal anterior channels. It then migrates along the midline toward posterior channels. By 100 ms, a focal positivity forms over posterior cortices, coincident with a bilateral negativity in ventral anterior channels.
ACKNOWLEDGMENTS
This work was supported by the NIH Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative (UH3NS103550) and the Hope for Depression Research Foundation. The DBS Activa PC+S devices used in this research were donated by Medtronic (Minneapolis, MN). We thank Lydia Dennison, Sinead Quinn and members of the Depression Biometrics Laboratory at Emory University for their effort in support of this work. Thanks also go to Don M. Tucker and Michael Borich for their feedback on study design and results interpretation. Our sincere gratitude goes to the patients who participated in this research.
Waters AC, Veerakumar A, Choi KS, et al. Test–retest reliability of a stimulation‐locked evoked response to deep brain stimulation in subcallosal cingulate for treatment resistant depression. Hum Brain Mapp. 2018;39:4844–4856. 10.1002/hbm.24327
Funding information Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative (UH3NS103550); Hope for Depression Research Foundation
REFERENCES
- Baker, K. B. , Montgomery, E. B. , Rezai, A. R. , Burgess, R. , & Lüders, H. O. (2002). Subthalamic nucleus deep brain stimulus evoked potentials: Physiological and therapeutic implications. Movement Disorders, 17(5), 969–983. [DOI] [PubMed] [Google Scholar]
- Borich, M. R. , Wheaton, L. A. , Brodie, S. M. , Lakhani, B. , & Boyd, L. A. (2016). Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke: A TMS‐EEG investigation. Neuroscience Letters, 618, 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi, A. , Luján, J. L. , & McIntyre, C. C. (2013). Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. Journal of Neural Engineering, 10(5), 056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, K. S. , Riva‐Posse, P. , Gross, R. E. , & Mayberg, H. S. (2015). Mapping the “depression switch” during intraoperative testing of subcallosal cingulate deep brain stimulation. JAMA Neurology, 72(11), 1252–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti, D. V. (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychological Assessment, 6(4), 284–290. [Google Scholar]
- Conner, C. R. , Ellmore, T. M. , DiSano, M. A. , Pieters, T. A. , Potter, A. W. , & Tandon, N. (2011). Anatomic and electro‐physiologic connectivity of the language system: A combined DTI‐CCEP study. Computers in Biology and Medicine, 41(12), 1100–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, R. W. (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Deniau, J. M. , Degos, B. , Bosch, C. , & Maurice, N. (2010). Deep brain stimulation mechanisms: Beyond the concept of local functional inhibition. European Journal of Neuroscience, 32(7), 1080–1091. [DOI] [PubMed] [Google Scholar]
- Entz, L. , Tóth, E. , Keller, C. J. , Bickel, S. , Groppe, D. M. , Fabó, D. , … Mehta, A. D. (2014). Evoked effective connectivity of the human neocortex. Human Brain Mapping, 35(12), 5736–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezure, K. , & Oshima, T. (1985). Lateral spread of neuronal activity within the motor cortex investigated with intracellular responses to distant epicortical stimulation. The Japanese journal of physiology, 35(2), 223–249. [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer, P. E. , Husain, M. M. , Lisanby, S. H. , Taylor, S. F. , Whitworth, L. A. , McClintock, S. , … Rittberg, B. R. (2017). Subcallosal cingulate deep brain stimulation for treatment‐resistant depression: A multisite, randomised, sham‐controlled trial. The Lancet Psychiatry, 4(11), 839–849. [DOI] [PubMed] [Google Scholar]
- Holtzheimer, P. E. , Kelley, M. E. , Gross, R. E. , Filkowski, M. M. , Garlow, S. J. , Barrocas, A. , … Chismar, R. (2012). Subcallosal cingulate deep brain stimulation for treatment‐resistant unipolar and bipolar depression. Archives of General Psychiatry, 69(2), 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi, R. J. , Virtanen, J. , Ruohonen, J. , Karhu, J. , Aronen, H. J. , & Katila, T. (1997). Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport, 8(16), 3537–3540. [DOI] [PubMed] [Google Scholar]
- Junghöfer, M. , Elbert, T. , Tucker, D. M. , & Braun, C. (1999). The polar average reference effect: A bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology, 110(6), 1149–1155. [DOI] [PubMed] [Google Scholar]
- Keller, C. J. , Bickel, S. , Entz, L. , Ulbert, I. , Milham, M. P. , Kelly, C. , & Mehta, A. D. (2011). Intrinsic functional architecture predicts electrically evoked responses in the human brain. Proceedings of the National Academy of Sciences, 108(25), 10308–10313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, C. J. , Honey, C. J. , Mégevand, P. , Entz, L. , Ulbert, I. , & Mehta, A. D. (2014). Mapping human brain networks with cortico‐cortical evoked potentials. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1653), 20130528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, C. J. , Huang, Y. , Herrero, J. L. , Fini, M. , Du, V. , Lado, F. A. , … Mehta, A. D. (2018). Induction and quantification of excitability changes in human cortical networks. Journal of Neuroscience, 38(23), 5384–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin, L. J. , Keller, C. J. , Wu, W. , Narayan, M. , & Etkin, A. (2017). Test‐retest reliability of transcranial magnetic stimulation EEG evoked potentials. Brain Stimulation, 11(3), 536–544. [DOI] [PubMed] [Google Scholar]
- Li, K. , Papademetris, X. , & Tucker, D. M. (2016). BrainK for structural image processing: Creating electrical models of the human head. Computational Intelligence and Neuroscience, 2016, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano, A. M. , Mayberg, H. S. , Giacobbe, P. , Hamani, C. , Craddock, R. C. , & Kennedy, S. H. (2008). Subcallosal cingulate gyrus deep brain stimulation for treatment‐resistant depression. Biological Psychiatry, 64(6), 461–467. [DOI] [PubMed] [Google Scholar]
- Lujan, J. L. , Chaturvedi, A. , Choi, K. S. , Holtzheimer, P. E. , Gross, R. E. , Mayberg, H. S. , & McIntyre, C. C. (2013). Tractography‐activation models applied to subcallosal cingulate deep brain stimulation. Brain Stimulation, 6(5), 737–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan, J. L. , Chaturvedi, A. , Malone, D. A. , Rezai, A. R. , Machado, A. G. , & McIntyre, C. C. (2012). Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Human Brain Mapping, 33(4), 958–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini, M. , Ferrarelli, F. , Huber, R. , Esser, S. K. , Singh, H. , & Tononi, G. (2005). Breakdown of cortical effective connectivity during sleep. Science, 309(5744), 2228–2232. [DOI] [PubMed] [Google Scholar]
- Matsumoto, R. , Nair, D. R. , LaPresto, E. , Bingaman, W. , Shibasaki, H. , & Lüders, H. O. (2006). Functional connectivity in human cortical motor system: A cortico‐cortical evoked potential study. Brain, 130(1), 181–197. [DOI] [PubMed] [Google Scholar]
- Matsumoto, R. , Nair, D. R. , LaPresto, E. , Najm, I. , Bingaman, W. , Shibasaki, H. , & Lüders, H. O. (2004). Functional connectivity in the human language system: A cortico‐cortical evoked potential study. Brain, 127(10), 2316–2330. [DOI] [PubMed] [Google Scholar]
- Mayberg, H. S. , Lozano, A. M. , Voon, V. , McNeely, H. E. , Seminowicz, D. , Hamani, C. , … Kennedy, S. H. (2005). Deep brain stimulation for treatment‐resistant depression. Neuron, 45(5), 651–660. [DOI] [PubMed] [Google Scholar]
- McIntyre, C. C. , Savasta, M. , Kerkerian‐Le Goff, L. , & Vitek, J. L. (2004). Uncovering the mechanism (s) of action of deep brain stimulation: Activation, inhibition, or both. Clinical Neurophysiology, 115(6), 1239–1248. [DOI] [PubMed] [Google Scholar]
- Miniussi, C. , & Thut, G. (2010). Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topography, 22(4), 249–256. [DOI] [PubMed] [Google Scholar]
- Noecker, A. M. , Choi, K. S. , Riva‐Posse, P. , Gross, R. E. , Mayberg, H. S. , & McIntyre, C. C. (2017). StimVision Software: Examples and Applications in Subcallosal Cingulate Deep Brain Stimulation for Depression. Neuromodulation: Technology at the Neural Interface. [DOI] [PMC free article] [PubMed]
- Ookawa, S. , Enatsu, R. , Kanno, A. , Ochi, S. , Akiyama, Y. , Kobayashi, T. , … Kunieda, T. (2017). Frontal fibers connecting the superior frontal gyrus to broca area: A corticocortical evoked potential study. World Neurosurgery, 107, 239–248. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui, R. D. (2002). Standardized low‐resolution brain electromagnetic tomography (sLORETA): Technical details. Methods and Findings in Experimental and Clinical Pharmacology, 24(Suppl D), 5–12. [PubMed] [Google Scholar]
- Puigdemont, D. , Pérez‐Egea, R. , Portella, M. J. , Molet, J. , de Diego‐Adeliño, J. , Gironell, A. , … Serra, M. (2012). Deep brain stimulation of the subcallosal cingulate gyrus: Further evidence in treatment‐resistant major depression. International Journal of Neuropsychopharmacology, 15(1), 121–133. [DOI] [PubMed] [Google Scholar]
- Riva‐Posse, P. , Choi, K. , Holtzheimer, P. E. , Crowell, A. L. , Garlow, S. J. , Rajendra, J. K. , … Mayberg, H. S. (2017). A connectomic approach for subcallosal cingulate deep brain stimulation surgery: Prospective targeting in treatment‐resistant depression. Molecular Psychiatry, 23(4), 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva‐Posse, P. , Choi, K. S. , Holtzheimer, P. E. , McIntyre, C. C. , Gross, R. E. , Chaturvedi, A. , … Mayberg, H. S. (2014). Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment‐resistant depression. Biological Psychiatry, 76, 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz, D. A. , Mayberg, H. S. , McIntosh, A. R. , Goldapple, K. , Kennedy, S. , Segal, Z. , & Rafi‐Tari, S. (2004). Limbic‐frontal circuitry in major depression: A path modeling metanalysis. NeuroImage, 22, 409–418. 10.1016/j.neuroimage.2004.01.015 [DOI] [PubMed] [Google Scholar]
- Song, J. , Davey, C. , Poulsen, C. , Luu, P. , Turovets, S. , Anderson, E. , … Tucker, D. (2015). EEG source localization: Sensor density and head surface coverage. Journal of Neuroscience Methods, 256, 9–21. [DOI] [PubMed] [Google Scholar]
- Steriade, M., & Amzica, F. (1996). Intracortical and corticothalamic coherency of fast spontaneous oscillations. Proceedings of the National Academy of Sciences, 93(6), 2533–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, D. , Zhukov, L. , & Johnson, C. (2000). Lead‐field bases for electroencphalography source imaging. Annals of Biomedical Engineering, 28(9), 1059–1065. [DOI] [PubMed] [Google Scholar]
- Yamao, Y. , Suzuki, K. , Kunieda, T. , Matsumoto, R. , Arakawa, Y. , Nakae, T. , … Shimotake, A. (2017). Clinical impact of intraoperative CCEP monitoring in evaluating the dorsal language white matter pathway. Human Brain Mapping, 38(4), 1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumsteg, D. , Lozano, A. M. , & Wennberg, R. A. (2006a). Rhythmic cortical EEG synchronization with low frequency stimulation of the anterior and medial thalamus for epilepsy. Clinical Neurophysiology, 117(10), 2272–2278. [DOI] [PubMed] [Google Scholar]
- Zumsteg, D. , Lozano, A. M. , Wieser, H. G. , & Wennberg, R. A. (2006b). Cortical activation with deep brain stimulation of the anterior thalamus for epilepsy. Clinical Neurophysiology, 117(1), 192–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supplemental Movie 1 (caption). Signal propagation profile following unilateral stimulation of the left subcallosal cingulate. Topography of grand average scalp voltages (average of 15 sessions, n=4) over 150 ms following stimulation from left lead (time=0). Topography shown as if looking down on the head surface with nose oriented to the top of the page. Saturated color indicates greater amplitude (red=positive; blue=negative, range:‐12 to 12 μV). At 25 ms, a positive focus over anterior cortex is ipsilateral to the locus of stimulation (left SCC). At 40 ms, a positive‐going wave is observed over orbital frontal cortex, while a broad negativity emerges over posterior cortex. Between 60 ‐70 ms, a positive focus forms in dorsal anterior channels. It then migrates along the midline toward posterior channels. By 100 ms, a focal positivity forms over posterior cortices, coincident with a bilateral negativity in ventral anterior channels.


