Abstract
Introduction
Seven low-density lipoprotein (LDL) subclasses are identified, and smaller LDL particles are associated with an increased risk for cardiovascular events. However, there is limited data about the relationship between the acute ischemic stroke (AIS) subtypes and LDL subclasses. The aim of our study is to investigate the relationship between AIS subtypes and LDL subclasses.
Methods
This study consisted of 110 AIS patients and 60 healthy controls. Stroke patients were classified according to the TOAST classification system as cardioembolic infarct (CI), large artery atherosclerosis (LAA), and lacunar infarct (LI). LDL subclasses were distributed as seven bands (LDL-1 and-2 defined as large, and LDL-3 to-7 defined as small-LDL particle), using the LipoPrintª System. Control group and AIS subtypes were compared in terms of LDL subclasses; p<0.05 was considered statistically significant.
Results
AIS patients had higher LDL-2, LDL-3 and LDL-4 subclasses compared to the control groups, while LDL-1 was similar in two groups. In addition, LDL-2 and LDL-3 subclasses were significantly higher in each AIS subtype when compared to the control group. LDL-4 subclasses were significantly higher in LAA and LI subtypes than in the control group, but there was no relationship for CI subtypes. Smaller subclasses LDL-5 to LDL-7 were undetectable in both AIS patients and controls. Using regression analysis; age, LDL-2, LDL-3 and LDL-4 were found to be independent predictors of AIS development.
Conclusion
Our study showed that examination of LDL subclasses may be important in management of AIS patients. LDL-2, LDL-3, and LDL-4 are independent predictors of AIS development. These findings should be supported by further large studies.
Keywords: Cerebrovascular disease, acute ischemic stroke, hyperlipidemia, LDL subclasses
INTRODUCTION
Acute ischemic stroke (AIS) varies among countries in terms of incidence, prevalence, and mortality rates (1, 2). The reason for this variance is that stroke is a multifactorial disease occurring due to various genetic and environmental factors (3–5). One of the important factors responsible for the development of stroke is atherosclerosis.
Atherosclerosis plays a role in the development of both cardiovascular and cerebrovascular diseases. Elevated plasma low-density lipoprotein (LDL) - cholesterol (LDL-C) concentration is a primary risk factor in the development of atherosclerosis (6). In addition to classic atherosclerotic risk factors, lipoprotein subclasses are also considered to be risk factors, and have increasingly drawn attention in recent years. LDL consists of seven subfractions (referred to as LDL-1 to LDL-7) in gradient gel electrophoresis (7). LDL-1 and 2 are defined as large LDL, and LDL-3 to 7 is defined as small dense LDL (sd-LDL). Studies have shown that sd-LDL is associated with an increased risk of coronary artery disease (CAD) and peripheral artery disease (PAD) (8, 9), probably because smaller LDL particles are more easily able to penetrate the endothelium (10). For this reason, high level of sd-LDL has been accepted as a new risk factor for cardiovascular events by the National Cholesterol Education Program Adult Treatment Panel III (NCEP III) (6).
Although the association between LDL subclasses and cardiovascular events are well known, there are limited data regarding the relationship between AIS subtypes and LDL subclasses in the literature (11). The main goal of our study is to investigate the relationship between LDL particle sizes and AIS subtypes.
METHODS
Patients and Control Group Selection
A total of 110 patients diagnosed with the first onset of AIS were included prospectively in our study between February 2013 and November 2013. The diagnosis of ischemic stroke was based on current guidelines and all cranial imaging tests were evaluated by two independent physicians. AIS subgroups were classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system (12). Our study group consisted of 40 patients with cardioembolic infarct (CI), 40 patients with large artery atherosclerosis (LAA) and 30 patients with lacunar infarct (LI). AIS subgroups whose etiology could not be determined according to TOAST classification or was related to other causes were excluded from this study. Informed consent was obtained from participants in the study and/or that the institution’s ethics committee approved the study (approval number: 2013/02/10).
On admission patient characteristics, and risk factors were determined: age, gender, body mass index, smoking, alcohol use, blood pressure and blood glucose. Patients included in this study did not use any lipid-lowering drugs. Co-morbidities: hypertension (HT), according to a previous diagnosis, current anti-hypertensive treatment or measured systolic blood pressure ≥140 mmHg, and/or diastolic blood pressure ≥90 mmHg; presence of cardiovascular disease (previous myocardial infarction, coronary artery disease, valvular heart disease, cardiomyopathy or arrhythmia) and diabetes mellitus (DM), according to a previous diagnosis or current medication for diabetes were also recorded.
The control group consisted of 60 healthy individuals (30 females, 30 males). Individuals with an active infection, previous stroke, brain tumor, systemic malignancies, diabetes mellitus, cardiovascular disease, or who were presently suffering from renal dysfunction or symptomatic peripheral arterial disease were excluded from the control group.
Biochemical Analysis
Blood samples were collected in EDTA tubes and serum sample tubes after 12 hours overnight fasting period. Plasma and serum were separated by immediate centrifugation at 1500 rpm for 10 minutes. Samples were stored at -80ºC until analysis and they were thawed immediately before analyses. Total cholesterol (TC), triglycerides (TG), and high-density lipoprotein (HDL) - cholesterol (HDL-C) were quantified by standard enzymatic-colorimetric methods (13, 14). LDL-cholesterol was calculated using the Friedewald formula (15).
Low-Density Lipoprotein Analysis
The classification and measurement of LDL subgroups were performed with the Lipoprintª System (Quantimetrix Corporation, Redondo Beach, CA, USA) using a polyacrylamide disc gel electrophoresis method (16). The Lipoprintª System is used for clinical and research purposes and is approved by the Food and Drug Administration (FDA). In this system, the electrophoresed gels were scanned to determine the relative area of each lipoprotein subfraction. After electrophoresis, various stained LDL subfractions are identified according to the mobility in the gel (7). Accordingly, LDL subgroups are divided into seven subfractions (LDL-1 to LDL-7). In the analyses of samples included in our study, the first four subfractions of LDL (LDL-1, 2, 3, 4) were detected, but rest of the subfractions (LDL-5, 6, 7) could not be detected.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation and categorical variables were expressed as percentages. Kolmogorov-Smirnov and Shapiro-Wilk tests were used to determine whether the variables were consistent with normal distribution. Since the variables were not found to be normally distributed, nonparametric methods were preferred for analysis. Mann-Whitney U test was used for cases involving two groups and the Kruskal-Wallis test was used for cases involving more than two groups. Mean ranks values were assessed for the reason of differences. Correlation analysis was performed using the Spearman correlation method. Logistic regression analysis was performed to assess independent variables for stroke development. p<0.05 was considered statistically significant.
RESULTS
Clinical and laboratory characteristics of the patient and control groups are shown in Table 1. In the AIS group, mean age (68.5±12.6 vs 61.2±4.2; p<0.001), systolic blood pressure (p<0.001), blood glucose (p<0.001), TG (p<0.001), TC (p<0.001), and LDL-C (p<0.001) were significantly higher compared with the control group, while HDL-C (p<0.001) was significantly lower in AIS patients. There was no significant gender difference between patient and control groups (p=0.137) (Table 1).
Table 1.
Clinical and laboratory characteristics in all subjects
| Patients (n=110) | Controls (n=60) | p | |
|---|---|---|---|
| Age (year) | 68.5±12.6 | 61.2±4.2 | <0.001 |
| Gender M/F | 68/42 | 30/30 | 0.137 |
| BMI (kg/m2) | 27.3±1.5 | 27.0±3.0 | 0.291 |
| Alcohol use (%) | 9 (8.2) | 5 (8.3) | 0.973 |
| Smoking (%) | 35 (31.9) | 30 (50) | 0.020 |
| HT (%) | 78 (70.9) | 33 (55) | 0.037 |
| SBP (mmHg) | 146.5±22.1 | 116.3±10.1 | <0.001 |
| DBP (mmHg) | 85.7±11.3 | 74.3±7.3 | <0.001 |
| Glucose (mg/dL) | 139.3±63.0 | 90.5±15.6 | <0.001 |
| TC (mg/dL) | 190.3±46.2 | 166.5±29.0 | <0.001 |
| TG (mg/dL) | 155.4±106.8 | 100.7±43.5 | <0.001 |
| VLDL-C (mg/dL) | 36.5±16.6 | 26.1±8.7 | <0.001 |
| LDL-C (mg/dL) | 115.4±30.8 | 94.1±20.5 | <0.001 |
| HDL-C (mg/dL) | 38.4±12.0 | 47.4±12.3 | <0.001 |
M, male; F, female; BMI, body mass index; HT, hypertension; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; VLDL-C, very low density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol.
The distribution of LDL subclasses in both patients and controls are presented in Table 2 and Figure 1. As shown in the figure, levels of LDL-2, LDL-3 and LDL-4 subclasses were significantly higher in the AIS group compared to the control group (p<0.001, p<0.001, p=0.011, respectively). But there was no difference between the AIS and control groups in terms of LDL-1 (p=0.602) (Table 2, Figure 1).
Table 2.
The relationship of AIS and control groups with LDL subclasses
| Patients (n=110) | Controls (n=60) | p | AIS subtypes | |||
|---|---|---|---|---|---|---|
| CI (n=40) | LAA (n=40) | LI (n=30) | ||||
| LDL-1 (mg/dL) | 34.6±10.6 | 33.8±8.1 | 0.602 | 32.7±9.8 | 36.2±10.4 | 34.8±12.0 |
| LDL-2 (mg/dL) | 24.9±10.1 | 15.6±9.5 | <0.001 | 24.6±11.1* | 24.4±9.2* | 24.5±13.1* |
| LDL-3 (mg/dL) | 5.5±6.7 | 2.3±3.3 | <0.001 | 5.1±6.2* | 4.8±5.7* | 6.9±8.5* |
| LDL-4 (mg/dL) | 0.9±2.9 | 0.04±0.2 | 0.011 | 0.9±3.3 | 0.9±3.1* | 1.0±2.0* |
LDL, low density lipoprotein; CI, cardioembolic infarct; LAA, large artery atherosclerosis; LI, lacunar infarct.
p <0.05 versus control group
Figure 1.
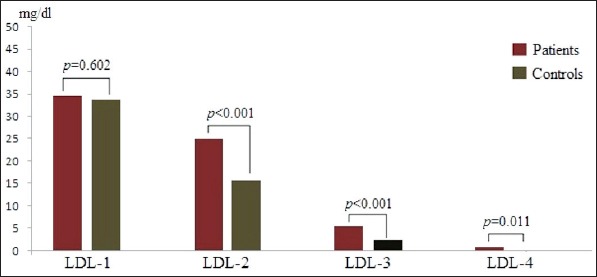
Distributions of LDL subclasses in patients with acute ischemic stroke and control group.
To gain further insight into associations between LDL subclasses and AIS, we evaluated LDL subclasses according to AIS subtypes. In the CI, LAA, and LI subgroups, LDL-2 (p<0.001, p<0.001, p=0.001 respectively) and LDL-3 (p=0.002, p=0.003, p=0.012 respectively) were significantly higher than the control group. In addition, LDL-4 in the LAA and LI groups were significantly higher than in the control group (p=0.006, p=0.006 respectively), but there was no significant difference in the CI subgroup in terms of LDL-4 (p=0.294). There was no relationship between AIS subtypes and the control group in terms of LDL-1 (Figure 2). There was also no significant difference when AIS subtypes were compared with each other in terms of LDL subclasses (Table 3).
Figure 2.
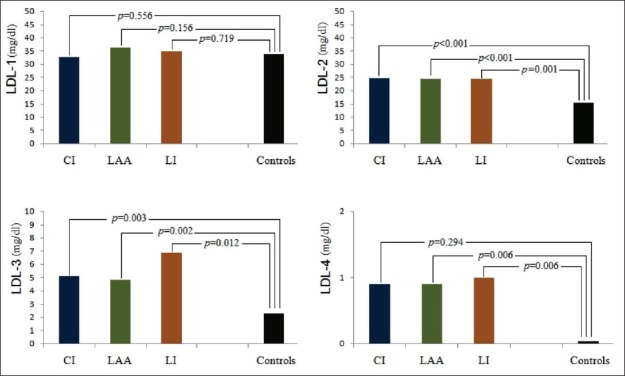
Distributions of LDL subclasses in acute ischemic stroke subtypes and control group.
Table 3.
Comparison of AIS subtypes with each other in terms of LDL subclasses (p values)
| CI-LAA (p value) | CI-LI (p value) | LAA-LI (p value) | |
|---|---|---|---|
| LDL-1 | 0.097 | 0.556 | 0.573 |
| LDL-2 | 0.927 | 0.873 | 0.789 |
| LDL-3 | 0.815 | 0.971 | 0.857 |
| LDL-4 | 0.195 | 0.183 | 0.722 |
LDL, low density lipoprotein; CI, cardioembolic infarct; LAA, large artery atherosclerosis; LI, lacunar infarct
We performed spearman correlation analysis to assess the correlations between LDL and its subclasses. We found positive correlations between LDL-C and LDL-1 (r=0.541, p<0.001) and also between LDL-2 (r=0.682, p<0.001), LDL-3 (r=0.484, p<0.001) and LDL-4 (r=0.347, p<0.001). When we assessed the correlation among LDL subtypes, we found a positive and significant relationship between LDL-2 and LDL-3 (r=0.811, p<0.001) and LDL-4 (r=0.399, p<0.001), and between LDL-3 and LDL-4 (r=0.571, p<0.001). Conversely, there was a significant negative correlation between LDL-1 and LDL-3 (r=_0.186, p=0.015), and between LDL-1 and LDL-4 (r=_0.185, p=0.015) (Table 4).
Table 4.
Correlation analysis of LDL-C and its subclasses
| LDL-1 | LDL-2 | LDL-3 | LDL-4 | |
|---|---|---|---|---|
| LDL-C | r=0.541 p<0.001 |
r=0.682 p<0.001 |
r=0.484 p<0.001 |
r=0.347 p<0.001 |
| LDL-1 | r=0.123 p=0.111 |
r=_0.186 p=0.015 |
r=_0.185 p=0.015 |
|
| LDL-2 | r=0.811 p<0.001 |
r=0.399 p<0.001 |
||
| LDL-3 | r=0.571 p<0.001 |
LDL, low density lipoprotein.
Finally, multivariate logistic regression analysis was performed to assess independent variables for developing AIS, including: LDL-C, LDL-1, LDL-2, LDL-3, LDL-4, VLDL-C, TC, TG, age, BMI, smoking, alcohol use, HT, DM and blood glucose. LDL-2 (OR: 1.2, 95% CI: 1.1–1.3, p=0.001), LDL-3 (OR: 0.7, 95% CI: 0.5–0.9, p=0.009), LDL-4 (OR: 8.8, 95% CI: 1.9–39.8, p=0.005) and age (OR: 1.1, 95% CI: 1.1–1.2, p<0.001) were independent predictors of AIS (Table 5).
Table 5.
Logistic regression analysis to assess possible clinical and laboratory variables independent associated with the presence of AIS
| β | SE | Wald | OR (95% CI) | p | |
|---|---|---|---|---|---|
| LDL-2 | 0.161 | 0.049 | 11.002 | 1.2 (1.1–1.3) | 0.001 |
| LDL-3 | -0.356 | 0.135 | 6.915 | 0.7 (0.5–0.9) | 0.009 |
| LDL-4 | 2.175 | 0.769 | 7.995 | 8.8 (1.9–39.8) | 0.005 |
| Age | 0.119 | 0.030 | 15.463 | 1.1 (1.1–1.2) | <0.001 |
β, β coefficient; SE, standart error; CI, confidence interval; OR, odds ratio; LDL, low density lipoprotein; VLDL-C, very low density lipoprotein cholesterol; DM, diabetes mellitus
Variables: LDL-C, LDL-1, LDL-2, LDL-3, LDL-4, VLDL-C, TC, TG, Age, BMI, Smoking, Alcohol use, HT, DM, Blood glucose.
DISCUSSION
AIS is common all over the world and is one of the leading causes of mortality (17). Various risk factors have been identified for AIS, and one of the most important risk factors is elevated plasma LDL-C. However, findings obtained in studies investigating the relationship between LDL-C and AIS differ from one other. While a positive relationship between LDL-C and AIS risk was detected in the Cardiovascular Health Study (18), in the Atherosclerosis Risk in Communities Study (19) and the Framingham Study (20) a definite relationship was not established. In addition, the relationship between LDL subclasses and AIS subtypes are not yet fully elucidated and data in this respect is limited. In this study, we found a significant relationship between LDL-C and AIS.
The atherogenic effect of LDL-C on major veins, coronary arteries, and peripheral arteries is well known (21). LDL-C is a heterogeneous particle and there are 7 different LDL subfractions detected by means of gel electrophoresis (22, 23). In several studies it has been stated that LDL particles penetrate the endothelium more easily and thus atherogenicity increases as particle size decreases (24, 25). Kwon et al. (8) showed that sd-LDL is independently associated with increased incidence of CAD, and may be a risk factor for CAD and acute coronary syndrome development, while Koba et al. (26) demonstrated that both sd-LDL particles and a high concentration of sd-LDL-C are potent risk factors for CAD. Rizzo et al. (9) also found that patients with PAD had particularly increased levels of sd-LDL particles. Finally, Landray et al. (27) reported that smaller LDL particles are associated with risk for development of carotid atherosclerosis.
Although the relationship of LDL subclasses with CAD and PAD has been well studied, the number of studies examining the relationship between LDL subclasses and AIS subtypes are limited. LDL-2, LDL-3, and LDL-4 were found to be significantly higher in AIS patients than the control group in our study. In addition, LDL-2, LDL-3, and LDL-4 were significantly higher in LAA and LI subgroups compared to the control group. While LDL-2 and LDL-3 were higher in the CI group than the control group, there was no difference in terms of LDL-4 level. Zeljkovic et al. (11) investigated LDL subclasses in AIS and they found that AIS patients had significantly more sd-LDL, but less LDL-1 and 2 particles. The most important difference between this study and ours is that the LDL-2 subclass was also found to be significantly higher in AIS patients. The probable reason may be the exclusion of AIS patients with undetermined etiology or with etiologies from other causes in our study. On the basis of pathogenesis, we included 3 main types of AIS since the relationship between AIS subtypes and LDL subclasses was shown more clearly. To our knowledge, our study is the first prospective study investigating the AIS subtypes and their association with atherosclerosis in terms of LDL subclasses, as well as the relationships between each subclass and the control group.
While some studies in the literature have shown a significant relationship between LDL-C and CI (28, 29), others have not (21, 30), the probable reason of this may be atrial fibrillation (AF) being a major cause of embolism in the CI subgroup (31). In our study, this relationship was examined at the level of LDL subtypes and there was no difference between CI and the control group in terms of LDL-1 and LDL-4, but the LDL-2 and LDL-3 subtypes were significantly higher in the CI group. These contradictory findings may arise since the majority of patients (21 of 40 patients) included in our study had AF or because the overall number of patients in each group was relatively small.
In this study the correlation of LDL subclasses among each other was assessed. While there was a negative correlation between LDL-1 and LDL-3 and between LDL-1 and LDL-4, there was a positive correlation between LDL-2 and LDL-3 and between LDL-2 and LDL-4. These findings suggest the atherogenicity of LDL-2 might be also higher. Indeed, the fact that we found LDL-2 subclasses significantly higher in AIS patients supports that the atherogenicity of LDL-2 might be also higher. When we assessed the effect of lipid parameters in AIS development with logistic regression analysis similar results were obtained. LDL-2 was shown to be a probable independent risk factor in AIS development. Furthermore, LDL-3 and LDL-4 were significant independent predictor of AIS development. Our findings suggest that measuring only plasma LDL level while ignoring subclasses may be insufficient for the comprehensive management of AIS. Although significant results were obtained in our study, further studies with larger sample sizes are needed to elucidate the potential role of LDL subclasses, especially LDL-2, in AIS development.
We tried to ensure patient and control groups had similar patient characteristics. However, the control and patient groups were not similar in terms of age since individuals included in the control group were chosen as healthy controls. Nevertheless, constituting an aged-matched control group could be more appropriate, since incidence of AIS increases by age.
In conclusion, LDL-2, LDL-3 and LDL-4 are independent predictors of AIS development. Therefore, measurement of LDL subclasses should be considered in addition to LDL-C measurement in AIS patients. This may provide additional contribution to the assessment and management of AIS. These findings should be supported by further large studies.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Bakırköy Sadi Konuk Training and Research Hospital (approval number: 2013/02/10).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – YK, VY, MÇ; Design – YK, VY, MÇ, AB, YK, AG; Supervision – YK, VY, MÇ, AB, YK, AG, ZT; Resource – YK, VY, MÇ, AB, YK, AG; Materials –YK, VY, MÇ, AB, YK, AG; Data Collection &/or Processing –YK, MÇ, AB, YK, AG, ZT; Analysis&/or Interpretation – YK, VY, MÇ, AB, YK, AG, ZT; Literature Search – YK, VY, MÇ, AB, YK, AG, ZT; Writing Manuscript– YK, VY, MÇ, AB, YK, AG, ZT; Critical Review – YK, VY, MÇ, AB, YK, AG, ZT.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 2.Aksoy D, İnanır A, Ayan M, Çevik B, Kurt S, Karaer Ünaldı H. Predictors of Mortality and Morbidity in Acute Ischemic Stroke. Arch Neuropsychiatry. 2013;50:40–44. [Google Scholar]
- 3.Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke:importance of population-based studies. Stroke. 2003;34:2050–2059. doi: 10.1161/01.STR.0000079818.08343.8C. [DOI] [PubMed] [Google Scholar]
- 4.Selçuk Ö, Yayla V, Çabalar M, Güzel V, Uysal S, Gedikbaşı A. The Relationship of Serum S100B Levels with Infarction Size and Clinical Outcome in Acute Ischemic Stroke Patients. Arch Neuropsychiatry. 2014;51:395–400. doi: 10.5152/npa.2014.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KılıçÇoban E, Kalyoncu Aslan I, Kırbaş D. Incidence of Metabolic Syndrome in Ischemic Cerebrovascular Disease and the Role of Carotid Intima-Media Thickness. Arch Neuropsychiatry. 2011;48:234–237. [Google Scholar]
- 6.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 7.Kalogirou M, Tsimihodimos V, Gazi I, Filippatos T, Saougos V, Tselepis AD, Mikhailidis DP, Elisaf M. Effect of ezetimibe monotherapy on the concentration of lipoprotein subfractions in patients with primary dyslipidaemia. Curr Med Res Opin. 2007;23:1169–1176. doi: 10.1185/030079907x188062. [DOI] [PubMed] [Google Scholar]
- 8.Kwon SW, Yoon SJ, Kang TS, Kwon HM, Kim JH, Rhee J, Lee SJ, Park JK, Lim JY, Yoon YW, Hong BK. Significance of small dense low-density lipoprotein as a risk factor for coronary artery disease and acute coronary syndrome. Yonsei Med J. 2006;47:405–414. doi: 10.3349/ymj.2006.47.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rizzo M, Pernice V, Frasheri A, Berneis K. Atherogenic lipoprotein phenotype and LDL size and subclasses in patients with peripheral arterial disease. Atherosclerosis. 2008;197:237–241. doi: 10.1016/j.atherosclerosis.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Taskinen MR. LDL-cholesterol, HDL-cholesterol or triglycerides –which is the culprit? Diabetes Res Clin Pract. 2003;61(Suppl 1):S19–S26. doi: 10.1016/s0168-8227(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 11.Zeljkovic A, Vekic J, Spasojevic-Kalimanovska V, Jelic-Ivanovic Z, Bogavac-Stanojevic N, Gulan B, Spasic S. LDL and HDL subclasses in acute ischemic stroke:prediction of risk and short-term mortality. Atherosclerosis. 2010;210:548–554. doi: 10.1016/j.atherosclerosis.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE, 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. [PubMed] [Google Scholar]
- 14.Nägele U, Hägele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, Gruber W. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Hoefner DM, Hodel SD, O'Brien JF, Branum EL, Sun D, Meissner I, McConnell JP. Development of a rapid, quantitative method for LDL subfractionation with use of the Quantimetrix Lipoprint LDL System. Clin Chem. 2001;47:266–274. [PubMed] [Google Scholar]
- 17.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001:systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 18.Psaty BM, Anderson M, Kronmal RA, Tracy RP, Orchard T, Fried LP, Lumley T, Robbins J, Burke G, Newman AB, Furberg CD. The association between lipid levels and the risks of incident myocardial infarction, stroke, and total mortality:The Cardiovascular Health Study. J Am Geriatr Soc. 2004;52:1639–1647. doi: 10.1111/j.1532-5415.2004.52455.x. [DOI] [PubMed] [Google Scholar]
- 19.Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR Atherosclerosis Risk in Communities Study. Plasma lipid profile and incident ischemic stroke:the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2003;34:623–631. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 20.Gordon T, Kannel WB, Castelli WP, Dawber TR. Lipoproteins, cardiovascular disease, and death:the Framingham Study. Arch Intern Med. 1981;141:1128–1131. [PubMed] [Google Scholar]
- 21.Imamura T, Doi Y, Arima H, Yonemoto K, Hata J, Kubo M, Tanizaki Y, Ibayashi S, Iida M, Kiyohara Y. LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population:the Hisayama study. Stroke. 2009;40:382–388. doi: 10.1161/STROKEAHA.108.529537. [DOI] [PubMed] [Google Scholar]
- 22.Packard CJ. Triacylglycerol-rich lipoproteins and the generation of small, dense low-density lipoprotein. Biochem Soc Trans. 2003;31:1066–1069. doi: 10.1042/bst0311066. [DOI] [PubMed] [Google Scholar]
- 23.Superko HR, Nejedly M, Garett B. Small LDL and its clinical importance as a new CAD risk factor:a female case study. Prog Cardiovasc Nurs. 2002;17:167–173. doi: 10.1111/j.0889-7204.2002.01453.x. [DOI] [PubMed] [Google Scholar]
- 24.Berneis KK, Krauss RM. Metabolic origin and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–1379. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 25.Sacks FM, Campos H. Clinical review 163:Cardiovascular endocrinology:Low-density lipoprotein size and cardiovascular disease:a reappraisal. J Clin Endocrinol Metab. 2003;88:4525–4532. doi: 10.1210/jc.2003-030636. [DOI] [PubMed] [Google Scholar]
- 26.Koba S, Hirano T, Ito Y, Tsunoda F, Yokota Y, Ban Y, Iso Y, Suzuki H, Katagiri T. Significance of small dense low-density lipoprotein-cholesterol concentrations in relation to the severity of coronary heart diseases. Atherosclerosis. 2006;189:206–214. doi: 10.1016/j.atherosclerosis.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Landray MJ, Sagar G, Muskin J, Murray S, Holder RL, Lip GY. Association of atherogenic low-density lipoprotein subfractions with carotid atherosclerosis. QJM. 1998;91:345–351. doi: 10.1093/qjmed/91.5.345. [DOI] [PubMed] [Google Scholar]
- 28.Laloux P, Galanti L, Jamart J. Lipids in ischemic stroke subtypes. Acta Neurol Belg. 2004;104:13–19. [PubMed] [Google Scholar]
- 29.Lindgren A, Nilsson-Ehle P, Norrving B, Johansson BB. Plasma lipids and lipoproteins in subtypes of stroke. Acta Neurol Scand. 1992;86:572–578. doi: 10.1111/j.1600-0404.1992.tb05489.x. [DOI] [PubMed] [Google Scholar]
- 30.Shimo-Nakanishi Y, Urabe T, Hattori N, Watanabe Y, Nagao T, Yokochi M, Hamamoto M, Mizuno Y. Polymorphism of the lipoprotein lipase gene and risk of atherothrombotic cerebral infarction in the Japanese. Stroke. 2001;32:1481–1486. doi: 10.1161/01.str.32.7.1481. [DOI] [PubMed] [Google Scholar]
- 31.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]


