The phosphoproteome downstream KOR has been studied in human spermatozoa by applying quantitative MS-based proteomics. This studies have been combined with functional approaches to analyze the role of the receptor in the sperm physiology. Results reported phosphorylation changes in sperm-specific proteins as well as the inhibition of sperm motility and acrosome after the addition of U50488H, the specific ligand.
Keywords: Phosphoproteome, Quantification, Physiology*, Molecular biology*, Cell biology*, GPCR, Human Spermatozoa, KOR, TMT labeling
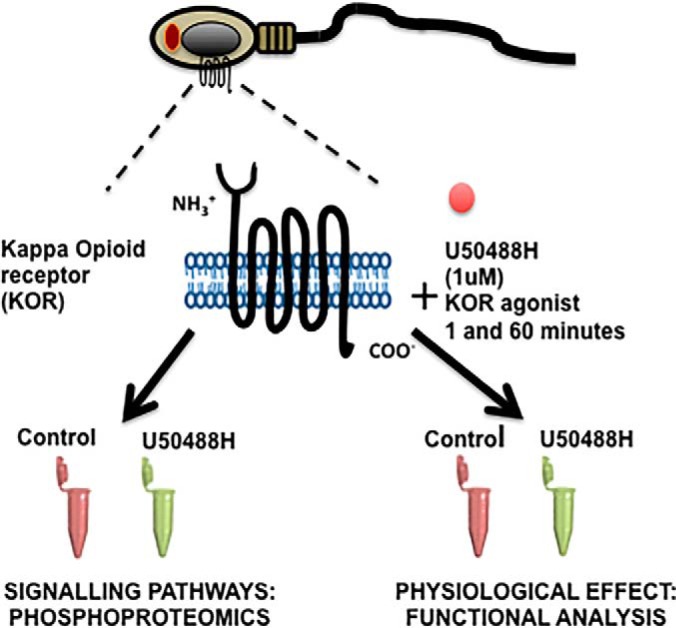
Graphical Abstract
Highlights
Insights into molecular mechanisms underlying GPCR in human spermatozoa.
Quantitative phosphoproteomics downstream Kappa opioid receptor in human spermatozoa.
U50488H agonist induces phosphorylation changes in sperm-specific proteins.
U50488H inhibits human sperm motility and acrosome reaction.
Abstract
G-protein coupled receptors (GPCRs) belong to the seven transmembrane receptor superfamily that transduce signals via G proteins in response to external stimuli to initiate different intracellular signaling pathways which culminate in specific cellular responses. The expression of diverse GPCRs at the plasma membrane of human spermatozoa suggests their involvement in the regulation of sperm fertility. However, the signaling events downstream of many GPCRs in spermatozoa remain uncharacterized. Here, we selected the kappa-opioid receptor (KOR) as a study model and applied phosphoproteomic approach based on TMT labeling and LC-MS/MS analyses. Quantitative coverage of more than 5000 proteins with over 3500 phosphorylation sites revealed changes in the phosphorylation levels of sperm-specific proteins involved in the regulation of the sperm fertility in response to a specific agonist of KOR, U50488H. Further functional studies indicate that KOR could be involved in the regulation of sperm fertile capacity by modulation of calcium channels. Our findings suggest that human spermatozoa possess unique features in the molecular mechanisms downstream of GPCRs which could be key regulators of sperm fertility and improved knowledge of these specific processes may contribute to the development of useful biochemical tools for diagnosis and treatment of male infertility.
Ejaculated mammalian sperm cells are immature and infertile and must undergo many physiological and biochemical modifications to become fertilization competent. These processes as the acquisition of sperm motility, capacitation, hyperactivation and acrosome reaction occur sequentially inside the female reproductive tract and are considered key functions in the control of the reproduction as well as essential for spermatozoa to become fertile (1). It is well documented that ionotropic modulation through rapid responses are the main regulators of sperm physiology (2). However, the presence of a high number of G-protein coupled receptors (GPCRs)1 described over the last decade in human spermatozoa, suggests that metabotropic mechanisms could also be important in the acquisition of the sperm fertilizing capacity (3).
The GPCRs are seven transmembrane receptors that represent approximately the 1% of the human genome and are one of the best therapeutic targets (4). In somatic cells, the canonical (G-protein dependent pathways) and noncanonical (G-protein independent pathways) are the major signaling pathways initiated downstream GPCRs and have an important role in the regulation of basic cellular activities as well as in the coordination of cell actions. Over the last 20 years, several GPCRs have been described in human spermatozoa providing clear evidences of their involvement in the regulation of sperm fertility (5–9). Further, different components from the G-protein dependent transduction pathways such as the cAMP-dependent and the Ca2+/PKC signaling cascades have been described to influence aspects of sperm function such as sperm motility, capacitation and acrosome reaction (10). In addition, in respect to the noncanonical signaling pathway, β-arrestin that promotes desensitization and internalization of the GPCRs via a G-protein independent signaling pathway, has been demonstrated to act as a signal transducer, capable of modulating the sperm motility and acrosome reaction (11). Moreover, because spermatozoa are transcriptionally and translationally silent cells, it has been suggested that they may possess unique, sperm-specific signaling pathways (10, 11). In 2006, although we described for the first time the presence of functional Mu-, delta- and kappa- opioid receptors (MOR, DOR and KOR, respectively) in human spermatozoa (12), the signaling events downstream of these receptors remain uncharacterized.
Taking all this into account, the aim of this study was to elucidate the existence of sperm-specific molecular mechanisms of GPCRs signaling in human spermatozoa. For this purpose we combined for the first time phosphoproteomic approaches together with functional analyses in human spermatozoa, by using the kappa-opioid receptor as a study model.
EXPERIMENTAL PROCEDURES
Spermatozoa Isolation and Treatments
Ethical approval for this study was obtained from the Ethics Committee of the University of the Basque Country (CEISH-UPV/EHU (M10/2016/254)). Freshly ejaculated semen was collected from patients undergoing routine semen analysis at the Cruces University Hospital (Bilbao, Spain). The donors had normal sperm parameters according to World Health Organization standards (13). Semen samples were obtained by masturbation after 3–4 days of sexual abstinence and immediately processed on liquefaction (at 37 °C for 30 min). Spermatozoa were capacitated by the swim-up procedure and resuspended in G-IVF (Vitrolife, Goteborg, Sweden) supplemented with 1% bovine serum albumin for 3 h at 37 °C under 5% CO2.
Isolated spermatozoa were treated at 37 °C and 5% CO2 in G-IVF culture media. The spermatozoa were treated with 1 μm U50488H (the specific agonist of the receptor) (Sigma-Aldrich, Madrid, Spain) for 1 and 60 min independently for proteomic and functional analyses.
For functional analyses the samples were co- incubated with U50488H (Sigma-Aldrich) and the different activators and inhibitors of different signaling pathways. To study the calcium signaling pathway we used U73122 (3 μm) (Sigma-Aldrich), a phospholipase C inhibitor; Mibefradil (30 μm) (Tocris Biosciences, Bristol, UK), a calcium channel activator and NNC55–0395 (10 μm)(Sigma-Aldrich), a CatSper specific calcium channel inhibitor. To study the cAMP/PKA signaling pathway we used the SQ2336 (200 μm) (Sigma-Aldrich), the transmembrane adenylate cyclase (tmAC) inhibitor; Forskolin (50 μm) (Sigma-Aldrich), the tmAC activator and the HCO3− (50 mm)(Sigma-Aldrich), the SACY activator. To study the MAPK signaling pathway we used the BARK1 (126 μm)(Calbiochem, Darmstadt, Germany), the GRK inhibitor and IBMX (0.5 mm)(Sigma-Aldrich), the phosphodiesterases inhibitor.
Experimental Design and Statistical Rationale
Human spermatozoa that had normal parameters were treated with 1 μm U50488H (the specific agonist of the receptor) for 1 and 60 min independently for proteomic and functional analyses. For proteomic analyses, we adopted a Tandem Mass-Tag (TMT) 6-plex isotopic labeling strategy followed by phosphopeptide enrichment by consecutive incubations with titanium dioxide beads (TiO2) and generated samples for LC-MS/MS. The experiment was performed in three biological replicas. Peptide and protein searches were performed using the Andromeda search engine (integrated in MaxQuant, version 1.5.3.30) at a FDR threshold of 1%. Perseus software (v.1.6.0.7) was employed for the calculation of the statistical significance (two-sample student′s t, test) and fold changes between U50488H-treated and Control samples. We considered as U50488H-dependent phosphosites the ones that were consistently regulated presenting a 1.5-fold change (U50488H/Ctr > 1.5 or U50488H/Ctr < 0.67 and p, value < 0.05) in the three replicas for each time point and protein fraction.
Protein Extraction and Digestion
The followed proteomic strategy is summarized in Fig. 1A,. Three biological replicas of each untreated (Ctrl) and treated spermatozoa (U50488H) were used for 1 and 60 min. For soluble and insoluble protein extraction, treated sperm cells were lysed using ice-cold RIPA buffer (50 mm Tris-HCl pH 7.5, 150 mm NaCl, 1% NP-40, 1 mm EDTA, 0.25% sodium deoxycholate, 1 mm sodium pervanadate, 5 mm beta-glycerophosphate, 5 mm NaF, complete protease inhibitor mixture. (Roche Basel, Switzerland). After protein homogenization and sonication (20% amplitude, 10 pulses, three times), proteins were separated in soluble and insoluble fractions after 15 min centrifugation at 13,000 × g,, 4 °C. To solubilize the insoluble fraction, we resuspended proteins into 8 m Guanidinium chloride and sonicated them (20% amplitude, 10 pulses). Both soluble and insoluble protein fractions were reduced and alkylated using 2 mm dithiothreitol and 11 mm chloroacetamide respectively. Then the soluble proteins were precipitated using acetone to further resuspend them in urea buffer (8 m urea, 10 mm TrisHCl pH 7,5) and estimate the protein abundance by bicinchoninic acid (BCA) method (Pierce, Thermo Fisher). After adjusting all the samples to 1:1:1:1:1:1 in each time point and protein fraction (soluble or insoluble), the samples were subjected to in-solution digestion using LysC (4 h) and Trypsin (overnight). Proteolytic digestion products were desalted on a Sep-Pak C18 cartridge (Waters, Milford, MA) for further sample processing.
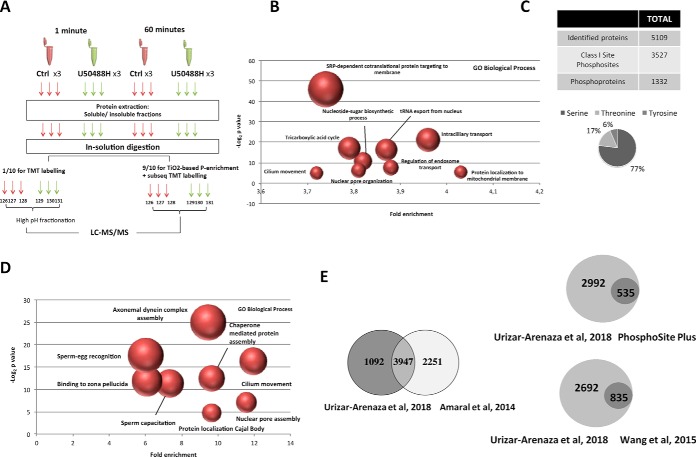
Fig. 1.
Proteomic and phosphoproteomic analysis of human spermatozoa. A,, Schematic representation of the experimental workflow followed in the study. Three biological replicas of each untreated (Ctrl) and treated spermatozoa (U50488H) were used for 1 and 60 min. For both time point, after the protein extraction of each sample, the soluble and insoluble protein fractions were separated and independently subjected to a in-solution digestion. To analyze the whole proteome in human spermatozoa, 1/10 of each sample were used for tandem mass tag (TMT-6plex) labeling and after performing a high pH fractionation, the peptide mixture was analyzed by LC-MS/MS. On the other hand, the other 9/10 of each sample was utilized for TiO2-based phospho-enrichment followed by the subsequent TMT labeling and LC-MS/MS analysis. This procedure was followed independently with both soluble and insoluble protein fractions treated with U50488H for 1 and 60 min. B,, Gene ontology analysis (PANTHER bioinformatic tool) indicating the biological processes of the total proteins identified in the study. The fold enrichment and the statistical significance p, value of the most indicative terms are indicated. The size of the dots correlate with the number of proteins grouped in the same term. C,, Total Class I phosphosite (p-sites) and corresponding proteins quantified in the three biological replicas. Distribution of all quantified phosphorylated serine, threonine and tyrosine residues. D,, Gene ontology analysis (PANTHER bioinformatic tool) indicating the biological processes of the proteins belonging to the Class I site phosphosites. The fold enrichment and the statistical significance p, value of the most indicative terms are indicated. E,, Venn diagrams comparing the number of the identified proteins (left side) and phosphosites (right side) in this study with the published studies.
TMT Labeling
Digested peptides were labeled by Tandem Mass-Tag (TMT) 6-plex isotopic label reagent set (Thermo Scientific, Rockford, IL) to study the total proteome. After the peptide drying, each condition was resuspended in 100 mm Triethylammonium Bicarbonate Buffer (TEAB) (Sigma-Aldrich). Peptides were incubated with each label for 1 h at room temperature (RT) and reactions were quenched using 8 μl of 11% Lysine, following the manufacturer′s recommendations. After further 15 min incubation, the peptide solutions were acidified 1:10 v/v with 10% trifluoroacetic acid (TFA, Sigma-Aldrich). Then we mixed the independent samples in 1:1:1:1:1:1 proportion and fractionated the samples into 17 different fractions following the stepwise high- pH reversed phase fractionation protocol. Each fraction was loaded onto a C18 Stage Tip (made in house using Empore disc -C18 Agilent Life Science, Santa Clara, CA) for further proteomic analyses.
Stepwise High-pH Reversed Phase Fractionation
A part of the digested peptides (1/10) were TMT labeled and then fractionated into 17 different fractions following the Stepwise high-pH reversed phase fractionation protocol. The samples were adjusted to pH 10 with 10 mm ammonium hydroxide and transferred to a home-made tip containing ReproSil-Pur, 1,9 μm, 120A° beads (Dr. Maisch, Ammerbuch-Entringen, Germany). Sequential elutions were made using 1.75, 3.5, 5.25, 7, 8.75, 10.5, 12.5, 14, 15.5, 17.5, 21, 24.5, 28, 31.5, 35, 50, and 70% ACN. Each fraction was loaded onto C18 StageTips. Peptides were eluted with 60% ACN to further continue with mass spectrometry analysis.
Phosphopeptide Enrichment and TMT Labeling
The 9/10s of the peptides were enriched in phosphopeptides by consecutive incubations with titanium dioxide beads (TiO2). After all the samples were brought to 60% acetonitrile (ACN) and 1% TFA, the beads were added and incubated for 15 min rotating. Beads were then transferred to home-made C8 Stage Tips (Empore disc -C8 Agilent Life Science) and washed with 60% ACN/1% TFA. Phosphopeptides were eluted with 5% ammonia and 25% ACN/10% ammonia and loaded onto C18 StageTips. Peptides were eluted with 60% ACN and subjected to TMT labeling as previously described. Finally, the samples were analyzed by mass-spectrometry.
Mass Spectrometry Analysis
Acidified peptide mixtures were separated by online C18- reverse-phase nanoscale liquid chromatography and analyzed by tandem mass spectrometry (LC-MS/MS). MS analysis was performed on an Q-Exactive HF mass spectrometer (Thermo Scientific, Bremen, Germany) connected to an EASY-nanoLC 1000 System (Thermo) using a nanoelectrospray ion source (Proxeon Biosystems, Odense, DK). Survey full-scan MS spectra (m,/z, range, 300–1700; resolution 60,000 at m,/z, 400) were acquired in the Orbitrap followed by the fragmentation of the twelve most intense multiply charged ions. Ions selected for MS/MS were placed on a dynamic exclusion list for 45 s. To improve mass accuracy, internal real time lock mass calibration was enabled. Additional mass spectrometric parameters included a spray voltage of 2.3 kV, no sheath and auxiliary gas flow, and the temperature of the heated capillary was 275 °C. All raw files were searched against combined human database 2015.08 UniProt (with 42,122 sequence entries) and TrEMBL (with 49496 sequence entries) using MaxQuant platform version 1.5.3.30 with an Andromeda search engine.
Precursor and fragment tolerances were 4.5 and 20 ppm, respectively. A peak list was generated using the Quant element of MaxQuant using the following parameters. A maximum of 2 missed cleavages was allowed and enzyme specificity was set to trypsin. In addition, carbamidomethyl (C) was chosen as fixed modification and variable modifications included oxidation (M), deamidation (NQ) and Phospho_STY (STY). The peptide and protein FDR 0.01; site FDR 0.01; max. peptide PEP, 1; min. peptide length 7; min unique peptides and peptides, 1. For protein quantitation, only unmodified peptides and peptides modified by acetyl (protein N terminus), oxidation (Met) and deamidation (NQ) were used. According to the protein group assignment done by MaxQuant, the identified proteins were determined after removing the contaminants, reverses and those proteins only identified by site. Moreover, we considered those proteins with ≥ 2 identified peptides and ≥1 unique peptides. On the other hand, the phosphopeptide data was filtered by FDR < 1% and only the phosphosites displaying a localization probability above 0.75 were considered as confident phosphorylated sites (Class I sites).
Bioinformatic Analysis
The Perseus software (v.1.6.0.7) was employed for the calculation of the statistical significance and Fold changes between U50488H-treated and Control samples. To identify the U50488H-dependent phosphosites only the data of the quantified phosphosites and proteins were considered and used further. First, the data was normalized to correct the labeling variability and then it was normalized depending on phosphosite reporter ion intensities/protein reporter ion intensities. We considered as U50488H-dependent phosphosites the ones that were consistently regulated presenting a 1.5-fold change (U50488H/Ctr > 1.5 or U50488H/Ctr < 0.67 and p, value < 0.05) in the three replicas for each time point and protein fraction.
The PANTHER (v13.1) functional annotation tool (http://geneontology.org/) was used to detect the overrepresented gene ontology (GO) term “biological process” within the total identified proteins and Class I identified phosphoproteins in human spermatozoa. The Homo Sapiens dataset was used as reference. To construct the signaling pathways regulated by the kappa-opioid receptor, GENEMANIA (http://genemania.org/) the interactive functional association network tool was used.
Computer-assisted Motility Analysis
Human spermatozoa were capacitated and adjusted to a concentration of 50 × 106 cells/ml. Motility analysis was conducted by computer-assisted sperm analysis (CASA) (Sperm Class Analyzer, S.C.A., Microptic, Barcelona, Spain) following the WHO guidelines. For that purpose we examined the percent of motile sperm being by the manufacturer as appropriate for human species: progressive motility (rapidly progressive with velocity ≥35 μms/s at 37 °C (grade “a”) + slow sluggish progressive with velocity ≥10 μms/s but <35 μms/s (grade “b”), nonprogressive motility with velocity <10 μms/s and immotile motility. Moreover, the following kinematics parameters were also measured: curvilinear velocity (VCL, μm/s); straight-line velocity (VSL, μm/s), average-path velocity (VAP, μm/s); amplitude of lateral head displacement (ALH, μm); linearity of progression (LIN = VSL/VCL × 100); straightness (STR = VSL/VAP × 100); motility and hyperactivated motility. The percentage of hyperactive cells was defined following parameters above (11): VCL 100 m/s, LIN 60% and ALH 5 μm. To investigate the effects of drugs, sperm samples were divided in several aliquots and treated with a single concentration of U50488H (1 μm) (Sigma). Sperm motility was measured 5 min before U50488H addition (initial value) and after a contact time of 1 and 60 min. Additional experiments were performed in similar conditions to evaluate the effects of Mibefradil (30 μm), U73122 (3 μm) and NNC55–0395 (10 μm) and their co-incubations with the U50488H agonist.
Flow Cytometry
Acrosome reaction was evaluated by using the anti-CD46 antibody by Flow cytometry. The FACScalibur flow cytometer (Becton, Dickinson, San Jose, CA) was used to study the role of the kappa-opioid receptor in human sperm acrosome reaction. Spermatozoa were treated with U50488H for 1 and 60 min, and the different activators and inhibitors of different signaling pathways. The progesterone (10 μm) was used as an internal control for the acrosome reaction. For the staining of the cells the Fluorescein IsoTioCyanate (FITC) antihuman CD46 antibody (BioLegend, CA) (5 μl) was used for 60 min at room temperature as acrosome reaction molecular marker. As indicator of cell viability, we used 0.1 μg/ml Hoechst 33258 for 2 min. Finally, samples were washed twice by centrifugation in PBS at 800 g for 5 min, suspended in PBS and kept in the dark until analysis. Fluorescence data from at least 10.000 alive sperm cells were analyzed and the green fluorescence belonging to spermatozoa was measured. Histograms were analyzed using the Summit v4.3 software (Beckman Coulter, CA).
Western Blotting
For Western blotting analyses, whole cell extracts (500,000 cells) were diluted in 1× Laemmly sample buffer containing Dithiothreitol (DTT) (%10v/v) and boiled for 5 min. Samples were loaded onto 12% resolving gels and separated by one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred to polyvinylidene fluoride membranes (PVDF) (Amersham Biosciences Hybond, Sigma) using the Mini Trans-Blot electrophoretic transfer system (Bio-Rad Laboratories, Hercules, CA). Then, membranes were blocked with Blotto (20 mm Tris-HCl, pH 7.5, 0.15 m NaCl, 1%Triton X-100) containing 5% Bovine Serum Albumin (BSA) for 1 h and then incubated with a 1:1000 dilution of the monoclonal mouse 4G10 anti-phosphotyrosine antibody (05–231, Millipore, Darmstadt, Germany) as a molecular marker of the human sperm capacitation (69), the polyclonal rabbit anti-Kappa opioid receptor (ABN456, Millipore) (1:500), the polyclonal rabbit anti-phosphorylated protein kinase C substrates (#2261, Cell Signaling, Danvers, MA) (1:750), the polyclonal rabbit anti-phosphorylated protein kinase A substrates (#9624,Cell Signaling) (1:750), the monoclonal mouse anti-phosphorylated MAP kinase substrates (#2325, Cell Signaling) (1:750) and the monoclonal mouse anti-alpha tubulin (T5168, Sigma, 1:4000). After washings (3 × 5 min) in Blotto buffer, the membranes were incubated for 1 h at RT with peroxidase-conjugated goat anti-rabbit (Blotto + %5 BSA 1:1000) (Goat anti-rabbit IgG HRP, sc-2004; Santa Cruz Biotechnology) and donkey anti-mouse IgG antibodies (Blotto+ %5 BSA 1:2000) (Donkey anti-mouse IgG HRP, sc-2314; Santa Cruz Biotechnology). After washing (3 × 5 min) blots were revealed for peroxidase activity by enhanced chemoluminiscence (ChemiDoc XRS detector, Bio-Rad). Results were analyzed by semiquantitative Western blots densitometry analysis using Image J (Image Processing and analysis in Java) software.
Indirect Immunofluorescence and Confocal Microscopy
Isolated human sperm cells were fixed in 4% paraformaldehyde for 10 min, permeabilized in 0.5% Triton X-100 for 10 min and blocked for 30 min with 10% (v/v) fetal bovine serum (FBS) in PBS. After, samples were incubated overnight at 4°C with the polyclonal rabbit anti-Kappa opioid receptor (ABN456, Millipore) (1:500). Secondary antibody incubations included Alexa Fluor 488 donkey anti-rabbit IgG (1:200) (Molecular Probes, OR). At the same time, controls for the specificity of the secondary antisera were performed by omitting the primary antiserum before addition of the second antisera. Nuclei were stained with Hoechst 33258 at 10 μg/ml and slides were assembled with Fluoromont G (Molecular Probes). Finally, the samples were examined using confocal microscopy (Zeiss, Apotome 2, Jena, Germany) at the High Resolution Facility (SGIKER UPV/EHU). The image analysis was conducted using the ImageJ software.
Measurements of Sperm Intracellular Ca2+ Concentration
Changes in [Ca2+]i were monitored using the Fura-2 as previously described by Cejudo-Roman et al., (15). Briefly, spermatozoa were adjusted to a concentration of 10 × 106 cell/ml in human serum albumin (HSA) medium. They were then incubated with the acetoxymethyl ester form of Fura-2 (Fura-2/AM 8 × 10−6 m; Molecular Probes) for 60 min at room temperature in the presence of the noncytotoxic detergent pluronic acid (0,1%; Molecular Probes). After loading, the cells were washed and resuspended in G-IVF solution and used within the next 2–7 h. Sperm aliquots (1 ml) were placed in the quartz cuvette of a spectrofluorometer (SLM AMinco-Bowman, Series 2; Microbeam, Barcelona, Spain) and magnetically stirred at 37 °C. The emitted fluorescence was measured at 510 nm. To measure [Ca2+]i, samples were alternatively illuminated with two excitation wavelenghts (340 nm and 380 nm) and the fluorescent light from the two excitation wavelengts was measured by a photomultiplier through a 510 nm filter. After subtracting the autofluorescence signal, obtaining by adding 5 nm MnCl2 at the end of the experiment, the F340/F380 ratio was used an indicator of [Ca2+]i. The effect of U50488H was studied on sperm aliquots incubated with this peptide at different doses (1 μm). Mibefradil (30 μm) was added to the same sperm aliquots to analyze the effect of U50488H of the mibefradil-induced intracellular calcium level. Progesterone (1 μm) was added to the same sperm aliquots as a control. Calibration of [Ca2+]i was achieved adding Triton X-100 (5%) to obtain the maximal response, followed by the addition of ethylene glycol tetra-acetic acid (EGTA) (40 nm) to obtain the minimal response.
Statistical Analysis
Sperm motility data were normalized as [(Treatment -Control)/(Control)] ×100, and acrosome-reacted data were normalized as [(Treatment- Control)/(Progesterone-DMSO)] x100 and evaluated using Students t, test and one-way ANOVA with post-hoc Bonferroni test. These procedures were undertaken using the IBM SPSS Statistics program (version 22). Differences were considered significant at *p, < 0.05 and highly significant at **p, < 0.01. Data are expressed as mean ± S.E. using the GraphPad PRISM (version 6.0) (GraphPad Company, CA) program.
RESULTS
Human Sperm Protein Identification
To gain insights into the human sperm proteome, we followed mass-spectrometry (MS) based proteomic and phosphoproteomic approaches in parallel. Isolated spermatozoa samples, untreated or stimulated with U50488H, were divided into soluble and insoluble protein fractions and subjected to the workflow described in Fig. 1A,. Once we excluded reverse hits and common contaminants and accounting for the reporter ion intensities and number of peptides (≥ 2 identified peptides and ≥ 1 unique peptides), a total of 5109 proteins were identified of which 5070 were confidently quantified (supplemental Table S1).
To obtain an overview of the biological functions of the identified proteins, Gene Ontology (GO) analyses were performed using the Panther classification system (Fig. 1B,). The most enriched functions were related to the protein and tRNA transport, metabolic processes, nuclear organization or processes related to sperm function. Specifically, we found a wide variety of proteins associated to protein folding and targeting to different organelles like the mitochondrion, endoplasmic reticulum or the plasma membrane. Moreover, we identified different enzymes involved in diverse metabolic processes like the glycolysis, the citrate catabolism or nucleobase biosynthetic processes which have an important function in the regulation of sperm fertility. Interestingly, despite mature spermatozoa are unable to translate genes into proteins, we also observed proteins connected to the nuclear envelope disassembly or those related to gene expression and protein synthesis (supplemental Table S1).
Further, we detected key proteins for the flagellar movement and sperm-egg recognition, as well as a broad catalogue of testis- and sperm-proteins involved in different molecular functions that are essential for the accomplishment of cell-specific physiological processes. On the other hand, we also identified proteins belonging to the GPCR signaling pathway. Although we found only few GPCRs in our proteomics dataset, we identified a wide variety of participants of the canonical and noncanonical signaling pathways downstream this family of receptors (supplemental Table S1).
Identification of Novel Phosphosites in Human Spermatozoa
To study the human sperm phosphoproteome, the phosphopeptides belonging to the soluble and insoluble protein fractions were enriched with TiO2 beads followed by TMT labeling (Fig. 1A,) and analyzed by MS, yielding a total of 4367 identified phosphorylation sites. Of those, 3527 had a localization probability above 0.75, considered as confident phosphorylated sites (Class I sites), and mapped to 1332 proteins (Fig. 1C,). The 3527 Class I sites comprised mainly serine phosphorylations (pSer, 77%), followed by phosphorylation on threonine (pThr, 17%) and tyrosine (pTyr, 6%) residues (Fig. 1C,). All the proteins containing Class I sites were categorized according to their “biological process” using the Panther classification system (Fig. 1D,). The most enriched biological processes were related to the cilium or flagellum-dependent cell motility, regulation of protein localization to Cajal Body, axonemal dynein complex assembly, sperm-egg recognition, binding to zona pellucida, sperm capacitation, nuclear organization and chaperone mediated protein assembly.
Although some of the phosphosites we identified have already been described and are reported in the PhosphositePlus database as well as in different studies (32) (supplemental Table S2), we present evidences of many phosphosites (2157) that have not been previously characterized (Fig. 1E,). This information represents a valuable source of novel information to understand the role of phosphorylation events in human spermatozoa.
U50448H-induced Changes in the Phosphoproteome of Human Spermatozoa
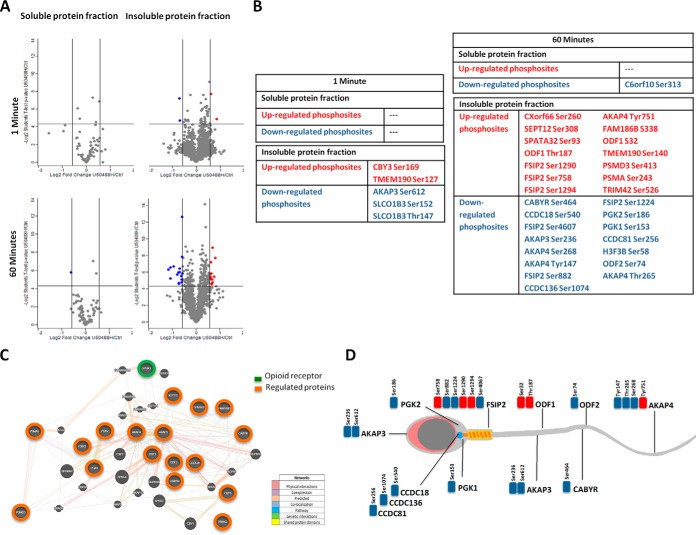
To have a better comprehension of the molecular mechanisms underlying GPCR signaling in human spermatozoa we chose the KOR as a study model (supplemental Fig. S1). After the addition of its specific ligand, U50488H, we followed phosphoproteomic approaches (Fig. 1A,) to decipher the phosphorylation changes downstream KOR. To unravel U50488H-dependent phosphorylation events, phosphosites with at least 1.5-fold increase or 0.67-fold decrease, and with a significant change of p, < 0.05, were considered as regulated. Considering these criteria, in the insoluble protein fraction 1 min U50488H treatment resulted in changes in the phosphorylation levels of five previously undescribed phosphosites belonging to 4 different proteins (Fig. 2A, and 2B,, supplemental Table S3). Treatment with the agonist resulted in decrease in the phosphorylation levels of the threonine 147 and the serine 152 of the solute carrier organic anion transporter family member 13 (Thr147/Ser152 SLCO1B3), and in the serine 612 of the protein kinase A anchoring protein 3 (Ser612 AKAP3), whereas it led to an increase in the phosphorylation on serine 169 of the protein chiby (Ser169 CBY3) and on the serine 127 of the transmembrane 190 protein (Ser127 TMEM190) (Fig. 2A, and 2B,, supplemental Table S3). The phosphorylation levels of the human sperm soluble protein fraction, however, remained unchanged following 1 min treatment with U50488H.
Fig. 2.
Effect of U50488H in the human sperm phosphoproteome. A,, Overall Log2 U50488H/Control TMT fold change as a function of -Log2 statistical significance of U50488H/Control (p, value < 0.05) of the soluble and insoluble protein fractions after 1 and 60 min U50488H treatment. In blue are indicated the down-regulated phosphosites by U50488H. In red are represented the U50488H-up-regulated phosphosites. B,, Tables containing the U50488H-regulated phosphosites following 1 and 60 min treatment in the soluble and insoluble protein fractions. U50488H/Ctrl > 1.5 in red and U50488H/Ctrl < 0.67 in blue. C,, Representation of a working model of the protein interactions belonging to the U50488H-regulated phosphosites. KOR is represented in green color whereas the proteins belonging to the U50488H-regulated phosphosites are colored in orange. The showing model has been constructed based on GENEMANIA (http://genemania.org/) the interactive functional association network tool. D,, Illustration gathering the localization of the proteins belonging to the U50488H-regulated phosphosites within the human spermatozoa. The downregulated phoshosites are represented in blue whereas the up-regulated ones are colorized in red.
At 60 min treatment we detected a single change in the soluble protein fraction, a decreased phosphorylation of the uncharacterized protein C6orf10 (Ser313 C6orf10). In the insoluble fraction, we observed changes in the phosphorylation levels of thirty phosphosites belonging to 21 different proteins. It is worth highlighting that 13 of them were sperm-specific proteins and that 20 phosphosites have not been previously reported in neither in human spermatozoa nor in other cells (Fig. 2A, and 2B,, supplemental Table S3).
Intriguingly, U50488H induced changes in the phosphorylation levels of different sperm-specific proteins mainly involved in the regulation of the sperm fertility. Among other, we saw both increase and decrease in the phosphorylation of different proteins related to sperm motility, capacitation and acrosome reaction like AKAP3 and 4, Outer dense fiber protein 1 and 2 (ODF1 and ODF2), Fibrous sheath interacting protein 2 (FSIP2), the family of the coiled-coil domain containing proteins (CCDC18, CCDC136, and CCDC81) and the Calcium binding tyrosine phosphorylation-regulated protein (CABYR). We also detected changes in different residues of the phosphoglycerate kinase 1 and 2 (PGK1 and PGK2) metabolic enzymes and the 26S proteasome non-ATPase regulatory subunit 3 (PSMD3), involved in protein degradation (supplemental Fig. S2, supplemental Table S3). The biggest changes among the regulated sites were seen for the Ser464 of CABYR, the Ser236 of AKAP3, and the Ser256 of CCDC81 presenting fold changes > 2.
To assess the relationships among the U50488H-regulated phosphoproteins and KOR, we generated a network using the biological network integration platform GeneMANIA. The proposed model predominantly links proteins, which could be regulated by the U50488H with those activated downstream the Kappa-opioid receptor. As seen in Fig. 2C,, KOR, AKAPs, CCDC family, CABYR, ODFs, PGKs, FSIP2, PSMA3, and PSMD3 are connected either directly or indirectly. Based on the data provided by the bioinformatic tool, KOR has been shown to be coexpressed together with AKAP3 and ODF1. At the same time, both proteins tend to coexpress and interact with ODF2, AKAP4 and other cytoskeletal proteins like FSIP2, SEPT12 or the ropporin 1B (ROPNB1). On the other hand, AKAP3 is expressed together with CABYR, which among other, coexpresses with RIIAD1 (Regulatory subunit of type II PKA R-subunit) or the PRKAR1A (protein kinase cAMP-dependent type I regulatory subunit alpha), a direct participant in the kappa-opioid signaling pathway. According to this information, KOR could have the potential to modulate the phosphorylation of sperm-specific proteins that participate in the construction of a functional interaction network based on the information provided by the database. Regarding the localization of the U50488H-regulated phosphoproteins within the spermatozoa, the Fig. 2D, gathers a schematic representation of the distribution of these proteins along the flagella, acrosome, midpiece or axoneme.
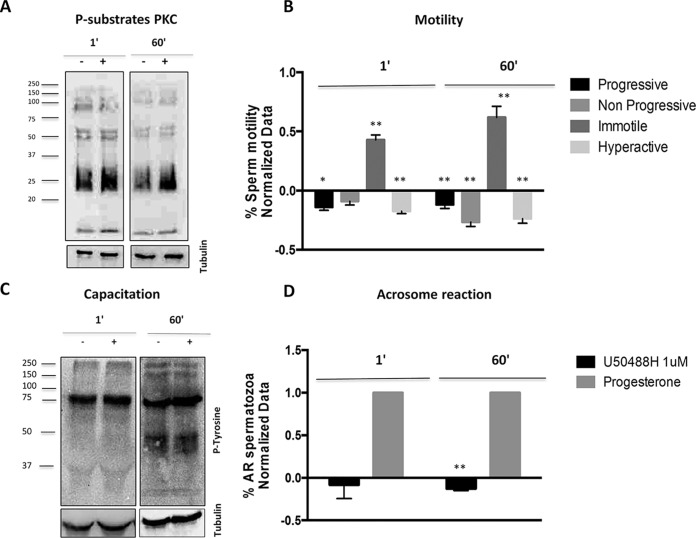
To further investigate the signaling downstream KOR, we studied the phosphorylation of different targets from the canonical and noncanonical signaling pathways by immunoblotting. Considering that the AKAPs act as scaffold proteins forming multi-protein complexes that connect several signaling pathway (16), we analyzed the overall levels of the phosphorylated substrates of protein kinase C (PKC) (Fig. 3A,), protein kinase A (PKA) and MAP kinases (MAPK) (supplemental Fig. S3A, and S3B,) following the ligand addition. In the case of the phosphorylated substrates of PKC, U50488H resulted in an increase after both 1- and 60-min stimulation (p, < 0.01), suggesting that U50488H could participate in the modulation of the Ca2+/PKC signaling pathway. In contrary, we did not observe any substantial change of the overall phosphorylated substrates of PKA and MAPK at either of the time points (supplemental Fig. S3A, and S3B,).
Fig. 3.
Role of kappa-opioid receptor in human sperm physiology. Effect of 1 and 60 min U50488H (1 μm) (Kappa-opioid receptor agonist) treatments in human sperm motility, capacitation and acrosome reaction. A,, Immunoblotting assays showing the expression of the phosphorylated substrates of protein kinase C following 1 and 60 min U50488H treatment. n, = 3. (**p, < 0.01 versus, Control). B,, Motility assays using the CASA (Computer Assisted Sperm Analyzer) system for the study of 1 and 60 minutes U50488H treatment in the progressive, nonprogressive, immotile and hyperactive motilities. X axis shows the motility types measured in the study and the Y axis represents the normalized data of the % of motile spermatozoa. The normalization was performed using the untreated samples (Control). n, = 8. (*p, < 0.05 and **p, < 0.01 versus, Control). C,, Immunoblotting assays for the study of U50488H in human sperm capacitation using the anti-pTyr antibody. D,, Flow citometry experiments for the study of U50488H in the human sperm acrosome reaction. X axis shows the different treatments used in the study and the Y axis represents the normalized data of the % acrosome reacted spermatozoa. The normalization was performed using the untreated samples and the acrosome reacted samples. The progesterone was used as the positive control of the acrosome reaction. n, = 7. (**p, < 0.01 versus, Control) AR: acrosome reaction. P-subtrates PKC: Phosphorylated substrates of PKC.
KOR Regulates Sperm Motility and Acrosome Reaction in Human Spermatozoa
To elucidate the effect of the treatment with the KOR specific ligand on the physiology of human spermatozoa, we analyzed the role of this receptor in sperm motility, capacitation and acrosome reaction. For that purpose, we treated the human spermatozoa with 1 μm of U50488H (KOR agonist) for 1 and 60 min. Cell viability studies using Hoechst 33258 did not show toxicity after U50488H addition of that dose at both time points (results not shown).
To unravel the function of the kappa opioid receptor in human sperm motility, we analyzed the progressive, nonprogressive, immotile and hyperactive motilities following the recommendations by the WHO (13). As it is shown in Fig. 3B,, the percentage of the progressive (p, < 0.05 1 min), nonprogressive (p, < 0.01 60 min) and hyperactive sperm (p, < 0.01) cells decreased at both time points, whereas the percentage of immotile cells was increased (p, < 0.01). In accordance to this result, U50488H inhibited the human sperm motility at 1 and 60 min.
Regarding capacitation, the phosphorylation of tyrosine residues (pTyr) is one of the most important events that occur during the sperm capacitation (17), so to study the role of KOR in this physiological process we analyzed the effect of U50488H on the total pTyr levels. Specifically, the kappa-opioid receptor agonist did not influence the levels of tyrosine phosphorylated proteins at both time points suggesting that U50488H does not induce phosphotyrosine-dependent changes during the process of human sperm capacitation (Fig. 3C,).
To study the role of KOR in the human sperm acrosome reaction, after treating the samples with U50488H we incubated them with the anti-CD46 antibody linked to fluorescein isothiocyanate (FITC). This antibody targets the inner acrosomal membrane for detecting a complete acrosome reaction and allows analyzing the emitted fluorescence by flow cytometry. As it is observed in Fig. 3D,, the agonist showed no significant effect after the U50488H addition for 1 min. However, U50488H inhibited the acrosome reaction following 60 min treatment (p, < 0.01). The progesterone was used as the positive control of the acrosome reaction.
KOR Regulates Hyperactive Sperm Motility and Acrosome Reaction by the Modulation of Calcium Channels in Human Spermatozoa
Once we observed that U50488H provoked an increase in the phosphorylated substrates of PKC and had an inhibitory effect in the hyperactive motility and acrosome reaction, we decided to analyze deeper the calcium signaling pathway as these two physiological processes are mainly regulated by the Ca2+ (18, 19). For that, we co-incubated both U50488H with different activators and inhibitors of diverse key proteins in the calcium cascade, for 1 and 60 min. We used the Mibefradil as a calcium channel activator, NNC55–0395 as the CatSper sperm specific calcium channel selective inhibitor and U73122 as the phospholipase C inhibitor.
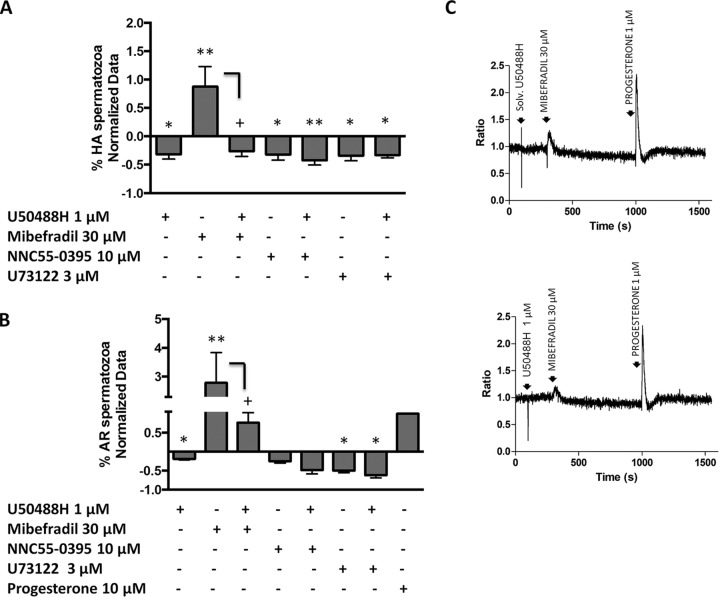
Regarding the motility assays, at 1 min treatment U50488H resulted in inhibition of the hyperactive motility being this effect persistent in all the analyzed samples (n, = 6) (p, < 0.05). As expected, the stimulation with 30 μm of Mibefradil for 1 min, increased the percentage of hyperactive motile sperm cells (p, < 0.01). The co-incubation of Mibefradil with U50488H induced a statistically significant reversion of the effect as the percentage of the hyperactive spermatozoa decreased (p, < 0.05) (Fig. 4A,). Further, 10 μm of NNC55–0395 itself, and together with U50488H had an inhibitory effect in the hyperactive motility after 1 min stimulation (p, < 0.05 and p, < 0.01 respectively). The U73122 treatment and its co-incubation with the KOR agonist, provided similar results to the U50488H, as the percentage of cells with a hyperactive motility was reduced (p, < 0.05) (Fig. 4A,). According to these results, KOR may have the capacity to inhibit the hyperactive motility at 1 min by the modulation of different calcium channels in human spermatozoa. However, at longer times the inhibition of the hyperactive motility would not exclusively go through the same molecular mechanisms (supplemental Fig. S4).
Fig. 4.
Study of the hyperactive motility and acrosome reaction calcium downstream KOR in human spermatozoa. A,, Study of the hyperactive motility by 1 min co-incubation of U50488H (1 μm) and: NNC-55–0395 (10 μm), the Catsper inhibitor; Mibefradil (30 μm), a calcium channel activator and U73122 (3 μm), the PLC inhibitor. X axis shows the different treatments used for this study and the Y axis represents the normalized data of the % of motile spermatozoa. The normalization was performed using the untreated samples. n, = 6. (*p, < 0.05 and **p, < 0.01 versus, Control; +p, < 0.05 versus, Mibefradil). B,, Study of the acrosome reaction by the 60 min co-incubation of U50488H (1 μm) and: NNC-55–0395 (10 μm), Mibefradil (30 μm) and U733122 (3 μm). X axis shows the different treatments used for this study and the Y axis represents the normalized data of the % of acrosome reacted spermatozoa. The normalization was performed using the untreated samples and the acrosome reacted samples. The progesterone was used as the positive control of the acrosome reaction. n, = 7. (*p, < 0.05 and **p, < 0.01 versus, Control; +p, < 0.05 versus, Mibefradil). C,, Intracellular free Ca2+ measurements in human sperm cells loaded with Fura-2 in response to the solvent of U50488H (H2O) (control samples) and U50488H (1 μm). Subsequent addition of 30 μm Mibefradil as a calcium channel activator and 1 μm progesterone to the same sperm aliquots. The progesterone caused a typical biphasic [Ca2+]i progesterone response. The X axis shows time in seconds and the Y axis shows [Ca2+]i expressed by Fura-2 ratio variation. Calibration of [Ca2+]i was achieved adding Triton X-100 (TX), to obtain the maximal response, followed by addition of EGTA to obtain the minimal response. n, = 5. AR = Acrosome reacted. HA = Hyperactive.
Regarding acrosome reaction studies, the stimulation of spermatozoa with Mibefradil, yielded to the activation of the acrosome exocytosis (p, < 0.01). After the co-incubation of U50488H and Mibefradil, the kappa-opioid agonist was able to partially blunt the acrosome reaction induced by the activator (p, < 0.05) (Fig. 4B,). On the other hand, although NNC55–0395 had an inhibitory effect on the acrosome reaction, which was accentuated after the co-incubation with U50488H, these changes were not statistically significant. We observed similar results with the Phospholipase C inhibitor, U73122, as the number of the acrosome reacted spermatozoa were decreased compared with the control samples (p, < 0.05) (Fig. 4B,). This effect was enhanced after the co-incubation of U73122 and U50488H, as we observed a greater decrease in the percentage of the acrosome reacted sperm cells (p, < 0.05). The progesterone was used as the positive control of the acrosome reaction (Fig. 4B,). Considering these results, the U50488H may inhibit the acrosome reaction at 60 min, by blocking calcium channels and PLC and would not involve other canonical and noncanonical mechanisms (supplemental Fig. S3A, and S3B,).
Taking into an account that KOR might regulate the sperm physiology by the blocking of calcium channels, we then analyzed if U50488H provoked changes in the intracellular free calcium concentration. We did not detect any intracellular Ca2+ changes in Fura-2-loaded human sperm cells after the 1 μm U50488H treatment (Fig. 4C,). As we expected, Mibefradil led to an increase in the [Ca2+]i levels in the spermatozoa treated only with the solvent of U50488H (H2O) that was partially reverted in the presence of U50488H (Fig. 4C,). As a positive control, we added 1 μm of progesterone to the same sperm samples that caused the typical biphasic [Ca2+]i response, consisting in a rapid transient peak followed by the decay of the intracellular calcium levels slightly above basal and a lower sustained plateau phase. Our results show that KOR can be involved in the regulation of sperm fertile capacity by the modulation of calcium channels.
DISCUSSION
Identification of Novel Phosphosites in Human Spermatozoa
Proteomic studies based on chemical labeling strategies gave rise to the identification of 5109 sperm proteins of which 5070 were quantified. Although Amaral et al., (20) have presented the most complete catalogue of human sperm proteins with 6198 reported protein IDs, in the present study we have identified over the 68% of the total sperm proteome because the human spermatozoa is estimated to be composed by 7500 different proteins (20). Regarding proteins linked to GPCR, we found proteins associated to different signaling pathways. Concretely, we identified proteins belonging to the canonical and noncanonical signaling pathways as other researchers have previously described.
GPCR activation in somatic cells mainly involves the induction of the Guanine nucleotide-binding proteins for the subsequent activation of the canonical signaling pathways (21). In the present study we identified via proteomics the presence of the Gαs, Gαq, Gα12/13, Gαi, Gαo and Gβ isoforms in human spermatozoa. Although the characterization of Gαs and Gαq was previously reported (22, 23), we have described for the first time the existence of other isoforms in human spermatozoa suggesting that these proteins may also be involved in the regulation of different signaling pathways downstream of GPCRs in spermatozoa.
Regarding the cAMP-dependent signaling pathway, we found the sperm-specific soluble isoform of the adenylate cyclase (SACY or the adenylate cyclase type 10) whereas we did not detect the transmembrane adenylate cyclase (tmAC) in human spermatozoa by our approach. This subject has been a matter of controversy during the last twenty years as although there is one study describing a tmAC in human spermatozoa (23), different researchers have not been able to unambiguously identify it (24–26). This issue reinforces the hypothesis of different authors who suggest the involvement of the soluble form of the protein in the regulation of sperm fertility (27).
In reference to the calcium-signaling pathway, interestingly we identified different isoforms of the phosphoinositide phospholipase C (PLC), such as delta and zeta (PLCD4 and PLCZ1, respectively), and both the calcium-dependent (alpha and delta) and independent (iota and zeta) protein kinase C (PKC), as previously has been described (28–30). According to different reports, PKC iota and zeta could be involved in the regulation of sperm fertility via a specific signaling cascade that does not involve calcium (26–29). At the same time, we also reported by proteomic approaches the presence of different participants of the noncanonical or G-protein independent signaling pathway in human spermatozoa, like the β-arrestin, the GPCR kinases (GRKs) or the ERK1/2 (11, 31–33).
On the other hand, our phosphoproteomic screen allowed us to uncover over 3500 phosphosites coming from 1332 proteins. The identified phosphoproteins in the present work are mainly involved in cilium and flagellum-dependent movement, nuclear pore organization or sperm-egg recognition, among other. According to this, Wang et al., in 2015 (14), previously identified 3303 phosphosites belonging to 965 proteins. Comparing our data with the largest description of the sperm phosphoproteome reported so far (14), we found 2692 novel phosphosites being 835 common in both studies. From these 2692 phosphosites, 535 were previously reported in humans in the PhosphoSitePlus database. Taking all these into account, it is worth highlighting that the present study contains the total of 2157 previously not reported phosphosites in humans and specifically in human spermatozoa.
U50448H-induced Changes in the Phosphoproteome of Human Spermatozoa
To understand the initial steps of the signaling cascades stimulated by KOR, sperm samples were treated with a KOR selective-agonist U50488H (34) for 1 min. Our quantitative phosphoproteomic analyses showed decrease in the phosphorylation levels of four proteins, CBY3, TMEM190, SLCO1B3, and AKAP3, after treatment. Currently, no attributable function has been determined for CBY3, TMEM190, and SLCO3. However, AKAP3 is a scaffold protein, synthesized in round sperm cells and involved in the formation of the basic structure of the fibrous sheath (35). It is localized in the main segments of the sperm flagella and the acrosome region of sperm heads, and different studies have suggested its involvement in the regulation of sperm motility, capacitation and acrosome reaction acting as a scaffold protein that assembles multiprotein signal complexes (35, 36). Although AKAP3 is known to be tyrosine phosphorylated during human sperm capacitation, the agonist showed to decrease the phosphorylation levels of AKAP3 in the Ser612, a phosphosite previously described by Ficarro et al., (37). In 2004, Rieger-Chu et al., (38) reported the co-expression of both KOR and AKAP3 in different cell systems, which suggest that AKAP3 could be involved in different molecular processes induced by KOR in human spermatozoa.
Because spermatozoa are transcriptionally and translationally inactive (20, 39, 40), we also aimed to elucidate the signaling pathways underlying KOR activation at longer time point, 60 min. Interestingly, U50488H induced changes in the phosphorylation levels of 30 phosphosites, belonging to 21 different proteins of which 13 of them are sperm-specific. Among the identified phosphosites we considered 20 as novel, as there are no previous studies describing their presence.
The KOR specific-agonist changed the phosphorylation levels of novel phosphosites belonging to different proteins such as CABYR, AKAP3 and AKAP4 that have different localization within the cell (Fig. 2D,). AKAPs family is one of the main components of sperm fibrous sheath, and it is involved in sperm fertility by sequestering PKA as it has a putative motif for binding cAMP-dependent protein kinase A (RIIa) (16, 36). In fact, this protein family may serve as a platform for the integration of cAMP and other signaling pathways by the binding with other protein kinases, protein phosphatases, ion channels and small GTP binding proteins (41). This is consistent with the fact that AKAP4 is an ERK1/2 substrate and serves as a regulator between the cAMP/PKA and the PKC/ERK1/2 signaling pathways in human spermatozoa to regulate capacitation and acrosome reaction (42). Concretely, these researchers described that ERK2 is the responsible of the in vitro, phosphorylation of the Thr265 of AKAP4, which in our study presents a decrease in the phosphorylation levels after the ligand addition for 60 min. This suggests that AKAP4 could be related to the PKC/ERK1/2 signaling pathway downstream KOR. In fact, we observed that U50488H induced an increase in the phosphorylated substrates of PKC at both time points.
AKAP3 is another component of the AKAPs family, which displayed changes in the phosphorylation level at a specific phosphosite at 60 min U50488H treatment. As already mentioned, there are evidences supporting the coexpression of both KOR and AKAP3 in humans (38), suggesting that in human spermatozoa this GPCR could have a regulatory function in the phosphorylation of the anchoring protein. On the other hand, there are evidences proving the interaction and coexpression of AKAP3 and CABYR in human spermatozoa (43, 44). CABYR is localized throughout the entire length of the midpiece and acts as an important component belonging to the calcium-signaling pathway during the hyperactivation and capacitation. Interestingly, CABYR possesses putative motifs for self-assembly and for binding RIIa and AKAPs (43), and its dephosphorylation abolishes its capacity to bind to calcium (45). As mentioned before, different studies suggest the role of AKAPs serving as a scaffold for integrating cAMP/PKA, Rho and calcium signaling (41) reinforcing that AKAP3 and CABYR could be the linkers between different transduction cascades.
Associated to this, the KOR agonist did not have effect in the phosphorylated substrates of PKA at both time points. In somatic cells, the PKA is activated by the cAMP second messenger (46) which is usually synthetized by the tmAC (47). In human spermatozoa, however, the cAMP is mainly synthetized by SACY activated by HCO3− (48, 49). Functional analyses using tmAC and SACY inhibitors also revealed that the KOR-induced acrosome reaction inhibition is not dependent of both enzymes. This is consistent with the fact that many GPCR ligands fail to increase the cAMP levels suggesting that GPCRs do not stimulate the PKA signaling via the typical transduction pathway in human spermatozoa (50). Moreover, the presence of the tmAC in human spermatozoa is still under doubt because forskolin activation does not involve an increase of cAMP levels (25–27). Although several studies have described the presence of tmAC in mouse spermatozoa (51, 52), we failed in the identification of the tmAC in our proteomic analyses and its presence in human spermatozoa is not reported yet (24, 25, 50).
Regarding the noncanonical signaling pathways, U50488H did not show any effect on the phosphorylated substrates of MAPK at both time points. Although the opioids have been described to promote activation of the MAPK signaling pathway in different cell systems (53, 54) the KOR selective-agonist did not activate this cascade at the selected time of stimulation. It is well studied that the noncanonical signaling pathway involves the GPCR phosphorylation mediated by GRKs to promote the receptor desensitization and internalization with the support of β-arrestin protein family (55, 56). This proteins act as scaffolds activating the MAPKs (55, 56) and recruiting the cAMP phosphodiesterases into a complex with the activated receptor, where they are placed to degrade cAMP (57). Functional studies using U50488H together with GRKs and phosphodiesterases inhibitors fail to induce significant changes in the acrosome reaction suggesting that KOR does not activate the noncanonical transduction-signaling pathway in human spermatozoa.
Although our phosphoproteomics data did not result in a direct signaling pathway downstream KOR, we discovered that most of the proteins having U50488H-regulated phosphosites were related to each other and could be connected indirectly with KOR signaling. ODF1 and 2 are sperm cytoskeletal structures that surround the axoneme in the midpiece and principal piece of sperm tail (58, 59) and their co-expression with KOR has been previously described in cancer (60). The CCDC family (CCDC81, CCDC136 and CCDC18) comprises the main components of the sperm centrosomes (61) and the FSIP2 is a component of the fibrous sheath (35). All these proteins compose the fibrous sheath in human spermatozoa and may be involved in the regulation of the sperm fertility, as the sperm motility.
Finally, the opioid system is known to be involved in regulating carbohydrate metabolism and immune system functions in the periphery (62, 63). This is consistent with the phosphorylation changes we observe in the metabolic enzymes like PGK1 and PGK2, which are implicated in an important step of the glycolysis (64), and hence in sperm fertility (65).
Although there are numerous papers describing the involvement of these proteins in the sperm function, we have not found studies describing the role of the different phosphorylations in none of the proteins. Therefore, further studies are needed to have a better comprehension of the implication of these phosphorylation events in sperm physiology.
KOR Regulates Human Sperm Fertility by Inhibiting the Hyperactive Motility and Acrosome Reaction Through Phosphorylation Changes in Sperm-specific Proteins
Classically, the agonist stimulation of the opioid receptors can inhibit the cyclic adenosine monophosphate (cAMP) production (66), modulate the calcium and potassium channels (66) and recruit alternate signal transduction cascades like the MAPKs (67). Although, opioid receptors are known to be critical in the modulation of pain behavior and antinociception in the central nervous system (63), their presence in human spermatozoa (12) has leaded us to think that may have an important function in the regulation of sperm fertility. In this article we describe for the first time the role of KOR in the hyperactive motility and acrosome reaction in human spermatozoa, via changes in the phosphorylation of sperm-specific proteins.
Considering the phosphoproteomic data and the functional studies, we suggested that at short exposure times the agonist is able to regulate the human sperm hyperactive motility mainly through the calcium ion channel modulation and fast responses. At the same time, the ligand could induce phosphorylation changes in few proteins like AKAP3, which could be involved in the first steps of the inhibition of sperm motility. It is known that, AKAPs are anchor cAMP-dependent protein kinases (PKAs) presented in different subcellular regions where they phosphorylate nearby proteins in response to cAMP signaling and often form complexes with other components of different signaling pathways (16, 41). In human sperm cells AKAPs family may be a link to calcium signaling pathway as they have the capacity to bind to proteins like CABYR and connect to PKC pathway to regulate sperm fertility (42, 45). This is consistent with our results at longer exposures times, in which KOR activation induces changes in the phosphorylation of PKC substrates, in sperm-specific proteins such as AKAP3, AKAP4 and CABYR and other sperm-specific proteins involved in sperm motility and acrosome reaction like ODFs, FSIP2 and CCDCs necessary to provoke the physiological effect by U50488H (Fig. 2D,).
In conclusion, the present data allows us to have a better comprehension of the phosphorylation changes in sperm-specific proteins underlying Kappa-opioid receptor by which the human spermatozoa modulate the hyperactive motility and acrosome reaction. These finding may suggest that human spermatozoa possess unique features in the molecular mechanisms downstream GPCRs which could be key regulators of sperm fertility. Improved knowledge of these specific processes may contribute in the development of useful biochemical tools for the diagnosis and treatment of male infertility. Moreover, it could also represent an innovative opportunity for reproductive management, for either enhancing the probability of fertilization or reducing it through the development of novel target contraceptives.
DATA AVAILABILITY
The mass spectrometry proteomics data have been deposited to ProteomeXchange Cinsortium via the PRIDE partner repository (http://proteomecentral.proteomechange.org) with data identifier PXD011290. Annotated MS/MS spectra can be accessed through MS-Viewer (68) http://msviewer.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msviewer with the following search keys: yfsnn6olid. Project Title: Phosphoproteomic and functional approaches reveal changes in sperm-specific proteins downstream KOR in human spermatozoa. Project ID: PXD011290.
Supplementary Material
Acknowledgments
We thank Dr. S. Sidoli for the help with the analysis of the data. Basque Government and University of the Basque Country (UPV/EHU).
Footnotes
* IU-A is supported by a fellowship from the University of the Basque Country (UPV/EHU). IM-H is supported by a fellowship from the Basque Government. I.K. is supported by a grant from the Danish Medical Research Council.
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- GPCR
- G-protein coupled receptor
- KOR
- Kappa opioid receptor
- SACY
- soluble adenylate cyclase
- tmAC
- transmembrane adenylate cyclase
- cAMP
- cyclic AMP
- PKA
- protein kinase A
- PKC
- protein kinase C
- MAPK
- mitogen activated protein kinase
- TMT
- Tandem mass-tag
- LC-MS/MS
- Liquid chromatography tandem mass spectrometry
- FDR
- False discovery rate.
REFERENCES
- 1. Visconti P. E., Westbrook V. A., Chertihin O., Demarco I., Sleight S., and Diekman A. B. (2002) Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 53, 133–150 [DOI] [PubMed] [Google Scholar]
- 2. Hille B. (1992) Ionic channels of excitable membranes. Sinauer Associates Inc; (Sunderland, MA: ) 2nd edition [Google Scholar]
- 3. Spehr M., Schwane K., Riffell J. A., Zimmer R. K., and Hatt H. (2006) Odorant receptors and olfactory-like signaling mechanisms in mammalian sperm. Mol. Cell. Endocrinol. 250, 128–136 [DOI] [PubMed] [Google Scholar]
- 4. Nambi P., and Aiyar N. (2003) G protein-coupled receptors. Drug Discovery 1, 305–310 [DOI] [PubMed] [Google Scholar]
- 5. Köhn F. M., Dammshäuser I., Neukamm C., Renneberg H., Siems W. E., Schill W. B., and Aumüller G. (1998) Ultrastructural localization of angiotensin-converting enzyme in ejaculated human spermatozoa. Hum. Reprod. 13, 604–610 [DOI] [PubMed] [Google Scholar]
- 6. Schaefer M., Hofmann T., Schultz G., and Gudermann T. (1998) A new prostaglandin E receptor mediates calcium influx and acrosome reaction in human spermatozoa. Proc. Natl. Acad. Sci. U.S.A. 95, 3008–3013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiménez-Trejo F., Tapia-Rodríguez M., Cerbón M., Kuhn D. M., Manjarrez-Gutiérrez G., Mendoza-Rodríguez A., and Picazo O. (2012) Evidence of 5-HT components in human sperm: implications for protein tyrosine phosphorylation and the physiology of motility. Reproduction 144, 677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rossato M., Popa F. I., Ferigo M., Clari G., and Foresta C. (2005) Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J. Clin. Endocrinol. Metab. 90, 984–991 [DOI] [PubMed] [Google Scholar]
- 9. Pinto F. M., Cejudo-Román A., Ravina C. G., Fernández-Sánchez M., Martín-Lozano D., Illanes M., Tena-Sempere M., and Candenas M. L. (2012) Characterization of the kisspeptin system in human spermatozoa. Int. J. Androl. 35, 63–73 [DOI] [PubMed] [Google Scholar]
- 10. Hess K. C., Jones B. H., Marquez B., Chen Y., Ord T. S., Kamenetsky M., Miyamoto C., Zippin J. H., Kopf G. S., Suarez S. S., Levin L. R., Williams C. J., Buck J., and Moss S. B. (2005) The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 9, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Almog T., Lazar S., Reiss N., Etkovitz N., Milch E., Rahamim N., Dobkin-Bekman M., Rotem R., Kalina M., Ramon J., Raziel A., Brietbart H., Seger R., and Naor Z. (2008) Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality. J. Biol. Chem. 283, 14479–14489 [DOI] [PubMed] [Google Scholar]
- 12. Agirregoitia E., Valdivia A., Carracedo A., Casis L., Gil J., Subiran N., Ochoa C., and Irazusta J. (2006) Expression and localization of δ-, κ-, and μ-opioid receptors in human spermatozoa and implications for sperm motility. J. Clin. Endocrinol. Metab. 91, 4969–4975 [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. (2010) WHO laboratory manual for the examination and processing of human semen. Fifth Edition WHO Press, World Health Organization, Geneva, Switzerland [Google Scholar]
- 14. Wang J., Qi L., Huang S., Zhou T., Guo Y., Wang G., Guo X., Zhou Z., and Sha J. (2015) Quantitative phosphoproteomics analysis reveals a key role of insulin growth factor 1 receptor (IGF1R) tyrosine kinase in human sperm capacitation. Mol. Cell. Proteomics 14, 1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cejudo-Roman A., Pinto F. M., Subirán N., Ravina C. G., Fernández-Sánchez M., Pérez-Hernández N., Pérez R., Pacheco A., Irazusta J., and Candenas L. (2013) The voltage-gated sodium channel Nav1.8 is expressed in human sperm. PLoS ONE 8, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michel J. J. C., and Scott J. D. (2002) Akap mediated signal transduction. Annu. Rev. Pharmacol. Toxicol., 42, 235–257 [DOI] [PubMed] [Google Scholar]
- 17. Naz R. K., and Rajesh P. B. (2004) Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod. Biol. Endocrinol. 2, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roldan E. R., and Shi Q. X. (2007) Sperm phospholipases and acrosomal exocytosis. Front. Biosci. 12, 89–104 [DOI] [PubMed] [Google Scholar]
- 19. Yanagimachi R. (1994) Mammalian Fertilization eds Knobil E, Neil J. New York, NY, Raven Press [Google Scholar]
- 20. Amaral A., Castillo Ramalho-Santos J. J., and Oliva R. (2014) The combined human sperm proteome: Cellular pathways and implications for basic and clinical science. Hum Reprod Update. 20, 40–62 [DOI] [PubMed] [Google Scholar]
- 21. Neves S. R., Ram P. T., and Iyengar R. (2002) G protein pathways. Science 296, 1636–1639 [DOI] [PubMed] [Google Scholar]
- 22. Merlet F., Weinstein L. S., Goldsmith P. K., Rarick T., Hall J. L., Bisson J., and Mazancourt P. (1999) Identification and localization of G protein subunits in human spermatozoa. Mol. Hum. Reprod. 5, 38–45 [DOI] [PubMed] [Google Scholar]
- 23. Spehr M., Schwane K., Riffell J. A., Barbour J., Zimmer R. K., Neuhaus E. M., and Hatt H. (2004) Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis. J. Biol. Chem. 279, 40194–40203 [DOI] [PubMed] [Google Scholar]
- 24. Rojas F. J., Bruzzone M. E., and Moretti-rojas I. (1992) Regulation of cyclic adenosine monophosphate synthesis in human ejaculated spermatozoa. II. The role of calcium and bicarbonate ions on the activation of adenylyl cyclase. Hum. Reprod. 7, 1131–1135 [DOI] [PubMed] [Google Scholar]
- 25. Strünker T., Goodwin N., Brenker C., Kashikar N. D., Weyand I., Seifert R., and Kaupp U. B. (2011) The CatSper channel mediates progesterone-induced Ca 2+ influx in human sperm. Nature 471, 382–387 [DOI] [PubMed] [Google Scholar]
- 26. Aitken R. J., Warner P. E., and Reid C. (1984) Factors influencing the success exhibiting of sperm-cervical unexplained mucus infertility interaction in patients infertility technique. J. Androl. 7, 3–10 [DOI] [PubMed] [Google Scholar]
- 27. Jaiswal B. S., and Conti M. (2003) Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl. Acad. Sci. U.S.A. 100, 10676–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rotem R., Paz G., Homonnai Z., Kalina M., Lax J., Breitbart H., and Naor Z. (1992) Ca2+-independent induction protein kinase C in human sperm. Endocrinology 131, 2235–2243 [DOI] [PubMed] [Google Scholar]
- 29. Rotem R., Paz G. F., Homonnai Z. T., Kalina M., and Naor Z. (1990) Protein kinase C is present in human sperm: possible role in flagellar motility. Proc. Natl. Acad. Sci. U.S.A. 87, 7305–7308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon S. Y. Y., Jellerette T., Salicioni A. M., Lee H. C., Yoo M., Coward K., Parrington J., Grow D., Cibelli J. B., Visconti P. E., Mager J., and Fissore R. A. (2008) Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J. Clin. Investig. 118, 3671–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang J., Shi Y., Xiang B., Qu B., Su W., Zhu M., Zhang M., Bao G., Wang F., Zhang X., Yang R., Fan F., Chen X., Pei G., and Ma L. (2005) A nuclear function of β-arrestin1 in GPCR signaling: Regulation of histone acetylation and gene transcription. Cell 123, 833–847 [DOI] [PubMed] [Google Scholar]
- 32. de Lamirande E., and Gagnon C. (2002) The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol. Hum. Reprod. 8, 124–135 [DOI] [PubMed] [Google Scholar]
- 33. Neuhaus E. M. (2006) Novel function of -arrestin2 in the nucleus of mature spermatozoa. J. Cell Sci. 119, 3047–3056 [DOI] [PubMed] [Google Scholar]
- 34. Taub D. D., Eisenstein T. K., Geller E. B., Adler M. W., and Rogers T. J. (1991) Immunomodulatory activity of mu- and kappa-selective opioid agonists. Proc. Natl. Acad. Sci. U.S.A. 88, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brown P. R., Miki K., Harper D. B., and Eddy E. M. (2003) A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol. Reprod. 68, 2241–2248 [DOI] [PubMed] [Google Scholar]
- 36. Vizel R., Hillman P., Ickowicz D., and Breitbart H. (2015) AKAP3 degradation in sperm capacitation is regulated by its tyrosine phosphorylation. Biochim. Biophys. Acta - Gen. Subj. 1850, 1912–1920 [DOI] [PubMed] [Google Scholar]
- 37. Ficarro S., Chertihin O., Westbrook V. A., White F., Jayes F., Kalab P., Marto J. A., Shabanowitz J., Herr J. C., Hunt D. F., and Visconti P. E. (2003) Phosphoproteome analysis of capacitated human sperm: Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J. Biol. Chem. 278, 11579–11589 [DOI] [PubMed] [Google Scholar]
- 38. Rieger K. E., Hong W.-J., Tusher V. G., Tang J., Tibshirani R., and Chu G. (2004) Toxicity from radiation therapy associated with abnormal transcriptional responses to DNA damage. Proc. Natl. Acad. Sci. U.S.A. 101, 6635–6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller C. (1992) Ionic channels of excitable membranes. Second edition. Cell 69, 579 [Google Scholar]
- 40. Castillo J., Amaral A., and Oliva R. (2014) Sperm nuclear proteome and its epigenetic potential. Andrology 2, 326–338 [DOI] [PubMed] [Google Scholar]
- 41. Skroblin P., Grossmann S., Schäfer G., Rosenthal W., and Klussmann E. (2010) Mechanisms of protein kinase A anchoring. Int. Rev. Cell Mol. Biol. 283, 235–330 [DOI] [PubMed] [Google Scholar]
- 42. Ben-Navi L., Almog T., Yao Z., Seger R., and Naor Z. (2016) A-Kinase Anchoring Protein 4 (AKAP4) is an ERK1/2 substrate and a switch molecule between cAMP/PKA and PKC/ERK1/2 in human spermatozoa. Sci. Rep. 6, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li Y. F., He W., Mandal A., Kim Y. H., Digilio L., Klotz K., Flickinger C. J., and Herr J. C. (2011) CABYR binds to AKAP3 and Ropporin in the human sperm fibrous sheath. Asian J. Androl. 13, 266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mallon B. S., Chenoweth J. G., Johnson K. R., Hamilton R. S., Tesar P. J., Yavatkar A. S., Tyson L. J., Park K., Chen K. G., Fann Y. C., and McKay R. D. G. (2013) StemCellDB: The Human Pluripotent Stem Cell Database at the National Institutes of Health. Stem Cell Res. 10, 57–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Naaby-Hansen S., Mandal A., Wolkowicz M. J., Sen B., Westbrook V. A., Shetty J., Coonrod S. A., Klotz K. L., Kim Y. H., Bush L. A., Flickinger C. J., and Herr J. C. (2002) CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev. Biol. 242, 236–254 [DOI] [PubMed] [Google Scholar]
- 46. Tasken K. (2004) Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 84, 137–167 [DOI] [PubMed] [Google Scholar]
- 47. Simonds W. F. (1999) G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 20, 66–73 [DOI] [PubMed] [Google Scholar]
- 48. Wang D., Hu J., Bobulescu I. A., Quill Ta McLeroy P., Moe O. W., and Garbers D. L. (2007) A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc. Natl. Acad. Sci. U.S.A. 104, 9325–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaupp U. B., and Strünker T. (2017) Signaling in sperm: more different than similar. Trends Cell Biol. 27, 101–109 [DOI] [PubMed] [Google Scholar]
- 50. Brenker C., Goodwin N., Weyand I., Kashikar N. D., Naruse M., Krähling M., Müller A., Benjamin Kaupp U., and Strünker T. (2012) The CatSper channel: A polymodal chemosensor in human sperm. EMBO J. 31, 1654–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Livera G., Xie F., Garcia M. A., Jaiswal B., Chen J., Law E., Storm D. R., and Conti M. (2005) Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function. Mol. Endocrinol. 19, 1277–1290 [DOI] [PubMed] [Google Scholar]
- 52. Chien C. L., Wu Y. S., Lai H. L., Chen Y. H., Jiang S. T., Shih C. M., Lin S. S., Chang C., and Chern Y. (2010) Impaired water reabsorption in mice deficient in the type VI adenylyl cyclase (AC6). FEBS Lett. 584, 2883–2890 [DOI] [PubMed] [Google Scholar]
- 53. New D. C., and Wong Y. H. (2002) The ORL1 receptor: Molecular pharmacology and signaling mechanisms. NeuroSignals 11, 197–212 [DOI] [PubMed] [Google Scholar]
- 54. Zhang Z., Xin S. M., Wu G. X., Zhang W. B., Ma L., and Pei G. (1999) Endogenous delta-opioid and ORL1 receptors couple to phosphorylation and activation of p38 MAPK in NG108–15 cells and this is regulated by protein kinase A and protein kinase C. J. Neurochem. 73, 1502–1509 [DOI] [PubMed] [Google Scholar]
- 55. Luttrell L. M., and Lefkowitz R. J. (2002) The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 115, 455–465 [DOI] [PubMed] [Google Scholar]
- 56. Magalhaes A. C., Dunn H., and Ferguson S. S. G. (2012) Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br. J. Pharmacol. 165, 1717–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perry S. J. (2002) Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta -arrestins. Science 298, 834–836 [DOI] [PubMed] [Google Scholar]
- 58. Henkel R., Stalf T., and Miska W. (1992) Isolation and partial characterization of the outer dense fiber proteins from human spermatozoa. Biol. Chem. Hoppe. Seyler. 373, 685–690 [DOI] [PubMed] [Google Scholar]
- 59. Azizi F., and Ghafoun-Fard S. (2017) Outer dense fiber proteins: Bridging between male infertility and cancer. Arch. Iran. Med. 20, 320–325 [PubMed] [Google Scholar]
- 60. Bild A. H., Yao G., Chang J. T., Wang Q., Potti A., Chasse D., Joshi M. B., Harpole D., Lancaster J. M., Berchuck A., Olson J. A., Marks J. R., Dressman H. K., West M., and Nevins J. R. (2006) Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 439, 353–357 [DOI] [PubMed] [Google Scholar]
- 61. Firat-Karalar E. N., Sante J., Elliott S., and Stearns T. (2014) Proteomic analysis of mammalian sperm cells identifies new components of the centrosome. J. Cell Sci. 127, 4128–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eyvazzadeh A. D., Pennington K. P., Pop-Busui R., Sowers M., Zubieta J. K., and Smith Y. R. (2009) The role of the endogenous opioid system in polycystic ovary syndrome. Fertil. Steril. 92, 1–12 [DOI] [PubMed] [Google Scholar]
- 63. Böttcher B., Seeber B., Leyendecker G., and Wildt L. (2017) Impact of the opioid system on the reproductive axis. Fertil. Steril. 108, 207–213 [DOI] [PubMed] [Google Scholar]
- 64. Dolcetta G., Busatta E., Sommacampagna P., Dusi M., Stoppelli I., Tomei F., Schiavon R., Olzer D., and Guidi G. (1986) Adenine nucleotides and some related enzyme activities (adenylate kinase and phosphoglycerate kinase) in normal and abnormal human semen. Andrologia 18, 184–189 [DOI] [PubMed] [Google Scholar]
- 65. Danshina P. V., Geyer C. B., Dai Q., Goulding E. H., Willis W. D., Kitto G. B., McCarrey J. R., Eddy E. M., and O'Brien D. A. (2010) Phosphoglycerate kinase 2 (PGK2) is essential for sperm function and male fertility in mice1. Biol. Reprod. 82, 136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Al-Hasani R., and Bruchas M. R. (2011) Molecular mechanisms of opioid receptor-dependent signalling and behaviour. Anesthesiology 115, 1363–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lefkowitz R. J., and Shenoy S. K. (2005) Transduction of receptor signals by β-arrestins. Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 68. Baker P. R., and Chalkley R. J. (2014) MS-viewer: a web-based spectral viewer for proteomics results. Mol. Cell. Proteomics 13, 1392–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leclerc P., de Lamirande E., and Gagnon C. (1997) Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 22, 643–656 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to ProteomeXchange Cinsortium via the PRIDE partner repository (http://proteomecentral.proteomechange.org) with data identifier PXD011290. Annotated MS/MS spectra can be accessed through MS-Viewer (68) http://msviewer.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msviewer with the following search keys: yfsnn6olid. Project Title: Phosphoproteomic and functional approaches reveal changes in sperm-specific proteins downstream KOR in human spermatozoa. Project ID: PXD011290.