Phosphoproteomic and global proteomic changes that occur during egg activation were quantified for the oocytes and eggs of Drosophila melanogaster, with normal (control) or perturbed (CanB2, knockdown) calcineurin functions. We discovered broad influences of calcineurin on protein phosphorylation states in maturing oocytes and activating eggs. Our results illuminate the molecular mechanisms through which calcineurin regulates meiosis progression, protein translation, and signaling pathways during this critical developmental transition.
Keywords: egg activation, phosphorylation, calcineurin, proteomics, Drosophila melanogaster
Keywords: Phosphoproteins*, Calcium Signaling*, Drosophila melanogaster*, Phosphoproteome, Cell cycle*, calcineurin, Drosophila, egg activation, phosphorylation, proteomics
Graphical Abstract
Highlights
Calcineurin controls proteomic phosphorylation in fly oocytes and activating eggs.
Without CanB2, activated eggs arrest in an M-phase like cell cycle state.
Calcineurin is a key regulator of translation factors during egg activation.
In egg activation, phosphorylation of GSK3β via Akt1 is calcineurin-dependent.
Abstract
In almost all animals studied to date, the crucial process of egg activation, by which an arrested mature oocyte transitions into an actively developing embryo, initiates with an increase in Ca2+ in the oocyte's cytoplasm. This Ca2+ rise sets off a series of downstream events, including the completion of meiosis and the dynamic remodeling of the oocyte transcriptome and proteome, which prepares the oocyte for embryogenesis. Calcineurin is a highly conserved phosphatase that is activated by Ca2+ upon egg activation and that is required for the resumption of meiosis in Xenopus,, ascidians, and Drosophila. The molecular mechanisms by which calcineurin transduces the calcium signal to regulate meiosis and other downstream events are still unclear. In this study, we investigate the regulatory role of calcineurin during egg activation in Drosophila melanogaster,. Using mass spectrometry, we quantify the phosphoproteomic and proteomic changes that occur during egg activation, and we examine how these events are affected when calcineurin function is perturbed in female germ cells. Our results show that calcineurin regulates hundreds of phosphosites and also influences the abundance of numerous proteins during egg activation. We find calcineurin-dependent changes in cell cycle regulators including Fizzy (Fzy), Greatwall (Gwl) and Endosulfine (Endos); in protein translation modulators including PNG, NAT, eIF4G, and eIF4B; and in important components of signaling pathways including GSK3β and Akt1. Our results help elucidate the events that occur during the transition from oocyte to embryo.
Egg activation encompasses a series of major changes through which a resting mature oocyte transitions to an active state that is capable of cell proliferation and embryo development. During this process, the oocyte's vitelline membrane undergoes biochemical modifications, the meiotic cell cycle is released from its species-specific arrest (metaphase II in vertebrates; metaphase I in Drosophila), and the oocyte transcriptome and proteome are remodeled through post-transcriptional and post-translational regulation (1–7). These intricately regulated events transform the oocyte from a highly differentiated gamete into a totipotent zygote.
In vertebrates and many invertebrate species, fertilization triggers egg activation (8–11). But in insects, the trigger is independent of fertilization and is, instead, passage through the female reproductive tract (12–16). Both types of trigger cause a rise in Ca2+ level in the oocyte cytoplasm, which is thought to set off the subsequent events by activating Ca2+/calmodulin-dependent kinase II (CaMKII)1 and the phosphatase calcineurin (CN) (14, 17). These enzymes are presumed to transduce the Ca2+ signal downstream by phosphorylating or dephosphorylating maternally deposited proteins.
Consistent with this scheme, large-scale protein phosphorylation state changes during egg activation have been detected in proteomic studies of vertebrates and invertebrates (18–21). In Drosophila, more than 300 proteins are subject to phosphoregulation during egg activation (20), and orthologs of more than 55% of these proteins are also phosphoregulated during sea urchin egg activation (18). These deeply conserved phosphoproteomic changes suggest their functional importance during this transition, and our genetic data show that these phosphoregulated proteins include numerous factors required for oogenesis and embryogenesis (22). These results support the idea that phosphoregulation is an important mechanism that remodels the oocyte proteome to prepare the cell to undertake development.
Here, we focus on the role of calcineurin in egg activation. This enzyme acts as a heterodimer consisting of a catalytic subunit A and a calcium-sensing regulatory subunit B (23). The Drosophila genome encodes 3 variants of calcineurin subunit A (PP2B-14D, CanA-14F and CanA1) and 2 variants of calcineurin subunit B (CanB and CanB2). Among these variants, only PP2B-14D, CanA-14F, and CanB2 are significantly expressed in the ovary (flyatlas.org). Oocytes depleted of the calcineurin regulatory subunit CanB2 are able to briefly resume meiosis from metaphase I arrest upon egg activation, but eventually they arrest again in anaphase I (24). On the other hand, constitutive activation of the calcineurin catalytic subunit PP2B14D (CnAact) causes a range of meiotic defects in mature oocytes (24). These results indicate that both the function and proper regulation of calcineurin are required for meiosis progression in the activating eggs of Drosophila. Consistent with this model, calcineurin in Xenopus, is transiently activated following the first Ca2+ influx, and it promotes Metaphase II exit by removing inhibitory phosphorylations from the APC/C component Apc3 and its co-activator Fzy (25, 26).
Calcineurin is also involved in other aspects of egg activation in Drosophila. Depletion of CanB2 inhibits the dephosphorylation and activation of the Gnu and YA proteins (27). Gnu is a regulatory subunit of Pan Gu kinase (28, 29), a crucial regulator of maternal mRNA translation and cell cycle progression in the early embryo (30), and YA is a maternal protein essential for the initiation of embryonic mitosis (31–33). In addition, the calcineurin regulator Sarah (Sra; calcipressin) is also required at the transition from oocyte to embryo (24, 34, 35). Depletion of Sra leads to anaphase I arrest in activated eggs and prevents other events including polyadenylation of crucial maternal transcripts and maturation of the male pronucleus (34, 35). These findings together suggest that calcineurin is at the center of the regulatory mechanisms that drive the transition from oocyte to embryo in Drosophila.
Here, we analyze the proteomic and phosphoproteomic changes that take place during egg activation and characterize how these changes are affected by the depletion or constitutive activation of calcineurin in female germ cells. Our data reveal that calcineurin activity is crucial for regulating hundreds of phosphosites during this developmental transition. The proteins whose phosphorylation states are influenced by calcineurin include important regulators of egg activation events, particularly meiotic cell cycle progression and protein translation. Calcineurin-regulated proteins are also enriched for functional groups related to biological processes that are crucial later in development during early embryogenesis. Our results reveal the scale of calcineurin's impact on the remodeling of the oocyte proteome during egg activation.
EXPERIMENTAL PROCEDURES
Fly Stocks
AttP2, (BL36303), UAS-CanB2, shRNA (BL38971) and nos-GAL4, (BL32563) fly stocks were obtained from the Bloomington Stock Center (Bloomington, IN) and maintained on standard yeast-glucose-agar media at 23 ± 2 °C, under a 12-hr light-dark cycle.
To generate flies with germline-specific CanB2, knockdown (kd), 5–10 virgin females from the UAS-CanB2, shRNA stock were mated to 5–10 males from the nos-GAL4, stock. Virgin daughters generated from the cross (referred to as “RNAi females” or “CanB2 kd, females”) were used for egg collection. Virgin daughters generated by AttP2, females mated to nos-GAL4, males (referred to as “control females”) were used as control. RNAi and control females were raised and maintained at 27 °C to increase knockdown efficiency. The efficiency of knockdown was validated with RT-PCR (supplemental Fig. S1A,, S1B,). Embryos from CanB2, RNAi females were stained for DNA and α-tubulin, confirming their arrest in anaphase I (24) using immunofluorescence (supplemental Fig. S1C,). Although more than 90% of 0.5–1.5 h embryos (n, = 38) produced by control females had completed meiosis and initiated the syncytial divisions (based on the presence of the polar body rosette and multiple mitotic spindles), 88% of the 0.5–1.5 h old embryos from the RNAi females were arrested in anaphase of meiosis I (n, = 26). The remaining embryos were unstageable because they lacked a detectable nucleus.
Constitutive activation of calcineurin in female germ cells was achieved using the transgenic fly stock nanos-GAL4, UASp-PP2B-14Dact, (CnAact,) (a kind gift from Dr. Toshiro Aigaki at Tokyo Metropolitan University, Tokyo, Japan) (34).
RT-PCR
Ovaries were dissected from virgin females that had been aged for 3–5 days. RNA extraction and RT-PCR were performed as described in Zhang et al., (22). The CanB2, cDNA was amplified using the primers CanB2_F: 5′-AGCGCGTGATCGACATTT and CanB2_R: 5′-CTTGCCATCCTCATCCTTATCC. As a control, RpL32 cDNA was amplified using the primers RpL32_F: 5′-CACCAGTCGGATCGATATGC and RpL32_R: 5′-CGATCCGTAACCGATGTTG (36).
Immunofluorescence
0.5–1.5 h old fertilized embryos were collected, fixed and stained as described by Zhang et al., (22). Mouse anti- α-tubulin (Sigma, St. Louis, MO, catalogue #T5168) was used at a dilution of 1:400. Alexa Fluor® 488-conjugated anti-mouse (Thermo-Fisher Scientific, Waltham, MA) was used at 1:200. Propidium Iodide was used at 10 μg/ml. Samples were examined and images were generated using a Leica TCS SP2 confocal microscope at the Cornell University Imaging Core.
Sample Preparation for Mass Spectrometry
Mature stage 14 oocytes were dissected from virgin females (aged for 3–5 days) in hypertonic Isolation Buffer (IB) (37). Activated but unfertilized eggs were collected from females (aged for 3–5 days) that had been mated to the spermless “sons of tudor,” males (38). Unfertilized eggs were collected over 30 min intervals using grape juice agar plates.
For each sample, 500–600 oocytes or activated eggs were homogenized in Protease/Phosphatase Inhibition Buffer (PPIHB) as described in Krauchunas et al., (20). The lysates were centrifuged at 20,000 × g, at 4 °C for 30 min, and the supernatants were collected. For each TMT-10plex set, a total of 5 oocyte samples (2 replicates for control and 3 replicates for CanB2 kd, or CnAact,) and 5 activated egg samples (2 replicates for control and 3 replicates for CanB2 kd, or CnAact,) were prepared. 5 pmol of CSN1S1/CSN2 phosphoprotein standard (Sigma Aldrich) was spiked into each activated egg lysates as a positive control for the detection of phosphoproteins (see supplementary Methods).
Protein concentration in each sample was determined using the Bradford assay and further quantified by electrophoresis on a precast NOVEX 10% Bis/Tris mini-gel (Invitrogen, Carlsbad, CA) along with E. coli, lysate in increasing amounts (2.5, 5, 10, 15 μg/lane). The SDS gel was stained with colloidal Coomassie blue (Invitrogen), imaged with a Typhoon 9400 scanner (Thermo-Fisher Scientific) and analyzed with ImageQuant TL 8.1 software (GE Healthcare, Chicago, IL).
Immunoblotting
To confirm preservation of protein phospho-state in our samples, we examined the phosphorylation of the Gnu protein. 15 μl of each of the 10 samples was mixed with 15 μl of SDS loading buffer. Proteins were separated by electrophoresis in 12% polyacrylamide SDS gels. Primary guinea pig anti-Gnu antibody (kindly provided by T. Orr-Weaver, Massachusetts Institute of Technology) was used at 1:10000 (39). Secondary HRP conjugated anti-guinea pig IgG (Jackson Laboratories, Barr Harbor, ME) was used at 1:1000.
Tandem Mass Tag (TMT) Labeling
TMT labeling of proteins was performed according to Thermo-Fisher Scientific's TMT Mass Tagging Kits and Reagents protocol (http://www.piercenet.com/instructions/2162073.pdf) with a slight modification as described in previous publications (40, 41) (see the supplementary Methods for additional details on the proteomics methods). Briefly, 110 μg protein for each sample was alkylated in iodoacetamide in the dark for 1 h, then precipitated in ice-cold acetone overnight at −20°C. Samples were reconstituted in 100 mm triethylammonium bicarbonate buffer (TEAB) and digested with 10 μg trypsin for 18 h at 35°C. The TMT 10-plex labels were added with a 1:2 mass ratio to each of the tryptic digest samples for 1 h at room temperature, and label incorporation was checked using the Orbitrap Fusion (Thermo-Fisher Scientific). Samples were then pooled and cleaned by solid phase extraction. Two aliquots of eluted tryptic peptides (∼550 μg each) were evaporated to dryness, and one aliquot was used for dimensional LC fractionation via a high pH reverse phase (hpRP) method (see below), whereas the other was used for phosphopeptide enrichment with TiO2 beads (see below).
High pH Reverse Phase (hpRP) Fractionation for Global Proteomic Analysis
For global proteomic analysis, hpRP chromatography was carried out on a Dionex UltiMate 3000 HPLC system (Thermo Scientific). The reconstituted TMT 10-plex-tagged tryptic peptides were loaded onto an XTerra MS C18 column (Waters, Milford, MA). Liquid chromatography (LC) was performed using a gradient buffer range of 10–45% in 30 min and a flow rate of 200 μl/min. 48 fractions were collected at 1 min intervals and pooled into 10 fractions. Each of the 10 fractions was dried and reconstituted in 2% acetonitrile (Acn)/0.5% formic acid (FA)) for nanoLC-MS/MS analysis.
Phosphopeptide Enrichment
TiO2 enrichment was conducted using a TiO2 Mag Sepharose kit (GE Healthcare). The reconstituted TMT 10-plex-tagged tryptic peptides aliquot was incubated with the TiO2 slurry for 30 min at 1800 rpm vortex. Beads were then washed with a buffer containing 80% ACN and 1% trifluoroacetic acid (TFA), and phosphopeptides were eluted three times in elution buffer (5% ammonium hydroxide). Eluted fractions were pooled and dried, then reconstituted in 50 μl of 0.5% FA for nanoLC-MS/MS analysis.
Nano-scale Reverse Phase Chromatography and Tandem MS (nanoLC-MS/MS)
The nanoLC-MS/MS analysis was carried out using an Orbitrap Fusion mass spectrometer (Thermo-Fisher Scientific) equipped with a nanospray Flex Ion Source using high energy collision dissociation coupled with the UltiMate3000 RSLCnano (Dionex, Sunnyvale, CA). Each reconstituted fraction (2–3 μl for global proteomics fractions and 10 μl for the enriched phospho-fractions) was injected into a PepMap C-18 RP nano trap column (3 μm, 100 μm × 20 mm, Dionex) at 20 μl/min flow rate for on-line desalting and separated on a PepMap C-18 RP nano column (2 μm, 75 μm × 25 cm). The labeled peptides were eluted in a 120 min gradient of 5% to 35% ACN in 0.1% FA at 300 nL/min, followed by an 8-min ramping to 95% ACN-0.1% FA and a 9 min hold at 95% ACN-0.1% FA. The column was re-equilibrated with 2% ACN-0.1% FA for 25 min prior to the next run. The Orbitrap Fusion was run in positive ion mode with nano spray voltage set at 1.6 kV and source temperature at 275 °C. External calibration for FT, IT and quadrupole mass analyzers were performed.
For global proteomics fractions, the instrument was operated in data-dependent acquisition (DDA) mode using the FT mass analyzer for one survey MS scan to select precursor ions, followed by 3 s “Top Speed” data-dependent HCD-MS/MS scans for precursor peptides with 2–7 charged ions above a threshold ion count of 10,000 with normalized collision energy of 37.5%. MS survey scans at a resolving power of 120,000 (fwhm at m,/z, 200), for the mass range of m/z 400–1600. Dynamic exclusion parameters were set at 1 within 45 s of exclusion duration with ±10 ppm exclusion mass width. All data were acquired under Xcalibur 3.0 operation software (Thermo-Fisher Scientific).
For the phosphoproteomics fractions, the instrument was operated in either the multiplestage activation (MSA)-based Synchronous Precursor Selection MS3 mode or the neutral loss (NL) triggered MS3 mode for phosphopeptides (42). In the MSA SPS MS3 method, the Orbitrap analyzer was used for acquiring CID MS2 spectra to increase confidence of phosphopeptide identifications and site location, whereas the multiple MS2 fragment ions isolated by SPS were subjected to HCD fragmentation at the MS3 level for reporter ion quantitation. In the NL-triggered MS3 method, a regular MS2 spectrum was first generated from the precursor using CID. If the neutral loss peak was detected, then an HCD MS3 scan was acquired on the most abundant ion to obtain both the sequence information and the quantitation.
Data Processing, Protein Identification and Data Analysis
All MS and MS/MS raw spectra from each set of TMT10-plex experiments were processed and searched using Sequest HT software within the Proteome Discoverer 2.2 (PD2.2, Thermo-Fisher). The Drosophila melanogaster, entries (21, 015) in the UniProt database (GCA_000001215.4; www.uniprot.org) were used for database searches. The default search settings for 6-plex TMT quantitative processing and protein identification in PD2.2 were: two mis-cleavage for full trypsin with fixed carbamidomethyl modification of cysteine, fixed 6-plex TMT modifications on lysine and N-terminal amines and variable modifications of methionine oxidation, deamidation on asparagine/glutamine residues, and acetylation of protein N terminus. The peptide mass tolerance and fragment mass tolerance values were 10 ppm and 50 mDa, respectively. Identified peptides were filtered for maximum 1% FDR using the Percolator algorithm in PD 2.2 along with additional peptide confidence set to high. The TMT10-plex quantification method within Proteome Discoverer 2.2 software was used to calculate the reporter ratios with mass tolerance ±10 ppm with applying the isotopic correction factors. Only peptide spectra containing all reporter ions were designated as “quantifiable spectra” and used for peptide/protein quantitation. A precursor co-isolation filter of 50% was applied for minimizing ratio compression caused by co-isolation of precursor ions.
Protein and phosphopeptide abundances were analyzed for differential expression using the LIMMA package in R (v.3.30.13) (43). In the global proteomics analysis, proteins with <2 unique peptides were removed from the analysis. A linear model function was applied to the abundance values, and an empirical Bayes method was used to perform moderated pair-wise t-tests (44). Finally, Gene Ontology enrichment analysis was performed using the DAVID bioinformatics resources (45, 46).
Experimental Design and Statistical Rationale
The experimental design included two sample types (oocyte and activated egg), each with three replicates in the mutant genotypes (CanB2 kd, or CnAact,) and two replicates in the matched control genotype (for CanB2 kd,: female offspring of AttP2, and nos-Gal4,; for CnAact,: non-Sb, female offspring of w1118;;,+/TM3 Sb,). The rationale for the number of replicates was based on the limitation of the 10-plex tagging system, necessitating the choice of 2, rather than 3, replicates for each control sample, although this scheme may reduce statistical power in the control oocyte-to-egg transition compared with the knockdown genotype. To identify biologically meaningful differences between each condition, we calculated a fold-change threshold for each of the protein and phosphopeptide datasets using the abundance ratios between the compared samples (See supplementary Methods). For the global proteomic dataset, we restricted the analysis to proteins that have two identified unique peptides, whereas in the phosphoproteomic analysis we minimized ratio compression by using a 50% precursor co-isolation filter. In addition, phophopeptides that contained shared, excluded modification, or no quantification values were excluded.
Our statistical test employed an empirical Bayes approach, which minimizes a protein's sample variance toward a pooled estimate and is more powerful for detecting significance, even when variability among replicates is high (43, 44). We further performed multiple test corrections on derived p, values to control for false discovery. Finally, comparisons between replicates indicate low inter-replicate variability (all R ≥ 0.92, supplemental Fig. S4A,, S4B,, and S4C,).
RESULTS
TMT-based Quantitative Proteomic Analysis Captures Prevalent Protein Phosphorylation Changes in Egg Activation
We first performed quantitative analysis of phosphoproteomic changes during egg activation in control and CanB2, knockdown (kd) samples. (For simplicity, in this paper, mature stage 14 oocyte samples will be referred to as “oocytes,” and samples from activated unfertilized eggs will be referred to as “eggs”). Control oocyte and egg lysates (2 replicates each) and CanB2 kd, oocyte and egg lysates (3 replicates each) were analyzed together as a TMT-10plex (Fig. 1). To verify that protein phosphorylation states were retained during the sample preparation and analysis procedures, we examined the phosphorylation states of Gnu protein in all 10 samples by Western blotting (supplemental Fig. S2) (27, 39, 47). As expected, Gnu's slower mobility form, corresponding to phosphorylated Gnu, was detected in all the mature oocyte lysates and in the CanB2 kd, activated egg lysates, whereas the faster mobility form of Gnu, corresponding to dephosphorylated Gnu, was detected in the control activated egg lysates but not in CanB2 kd, activated egg lysates (27, 39, 47).
Fig. 1.

Schematic of experimental set up. Please see text for details.
Because the phosphoproteomic analysis is susceptible to ratio compression caused by co-isolation interference, we employed two recently developed acquisition methods, multistage activation (MSA) and neutral loss trigger (NL) (42), in our MS analysis to improve the accuracy of peptide quantitation and the coverage of identified peptides. The MSA method identified a total of 1491 phosphopeptides, whereas the NL method identified 1787 phosphopeptides (Table I, supplemental Table S1 and supplemental Table S2). The two methods together provide information on the abundance changes of 2042 phosphopeptides during egg activation. We observed a range of correspondence between phosphopeptides and their master proteins, as one master protein may have one or several corresponding phosphopeptides quantified (supplemental Fig. S5).
Table I. Summary of phosphopeptides quantifications and abundance changes in TMT 10-plex 1 (CanB2, kd) and TMT 10-plex 2 (CnAact,).
| TMT10plex_1(CanB2) |
TMT10plex_2(CnAact) |
|||||
|---|---|---|---|---|---|---|
| MSA | NL | Combined | MSA | NL | Combined | |
| Total phosphopeptides identified: | 1491 | 1787 | 2042 | 2241 | 2110 | 2672 |
| Upregulated phophopeptides (control) | 114 | 78 | 148 | 154 | 86 | 184 |
| Upregulated phophopeptides (mutant) | 60 | 41 | 75 | 106 | 60 | 130 |
| Downregulated phophopeptides (control) | 175 | 144 | 240 | 161 | 87 | 195 |
| Downregulated phophopeptides (mutant) | 90 | 74 | 128 | 115 | 69 | 147 |
To evaluate the quality of the datasets, we first assessed the variation of peptide abundance among the biological replicates by plotting the abundance values in each sample against those in each of its biological replicates within the 10plex. We found that phosphopeptide/protein abundance was highly consistent among biological replicates (all R2 ≥ 0.92) (supplemental Fig. S4), indicating that our results were highly reproducible.
To identify differences between the phosphoproteomes of mature oocytes and activated eggs, we first calculated the Log2 transformant of egg-to-oocyte peptide abundance ratios for control (4 ratios from 2 replicates) and CanB2 kd, (9 ratios from 3 replicates) sample sets. These ratios were used to calculate the internal errors of the MSA (0.74 fold) and NL (0.99 fold) datasets, which describe 95% of the abundance variation caused by biological and technical differences among replicates in the respective dataset (48). We then identified statistically significant differences in abundance between the conditions using the LIMMA package. Significant peptide abundance changes that are greater than the internal errors likely reflect genuine changes in abundance.
Based on these standards, the MSA and NL methods detected significant abundance changes of 289 and 222 phosphopeptides, corresponding to 200 and 156 proteins, respectively, during egg activation in the control (Fig. 2A, and 2B,, Table I, supplemental Table S1 and supplemental Table S2). Although changes in the abundances of 126 phosphopeptides were detected by both methods, significant changes in 165 and 104 peptides were uniquely detected by MSA and NL, respectively (Fig. 2C,). The substantial overlap between the sets of significantly changed peptides detected by the two methods further verified the reliability of our results.
Fig. 2.
TMT-based quantitative proteomic analysis captures prevalent protein phosphorylation changes in egg activation. A,, Volcano plots of egg/oocyte phosphopeptide abundance foldchange in control and CanB2 kd, using MSA acquisition method. B,, Volcano plots of egg/oocyte phosphopeptide abundance foldchange in control and CanB2 kd, using NL acquisition method. C,, Comparison of significantly changed phosphopeptides (in control) identified by MSA and NL methods. D,, The abundance changes of proteins that contain significantly changed phosphopeptides.
We compared our results with the list of phosphoproteins identified by Krauchunas et al., (20). The phosphoregulated proteins we identified using the two acquisition methods showed limited overlap with that identified by Krauchunas et al.,, likely because of differences in sample processing, enrichment, and acquisition methods (supplemental Fig. S6). The large number of unique phosphoproteins identified by each of these methods suggests that the pool of proteins subject to phosphoregulation upon egg activation may be much larger than previously estimated. Consistent with observations by Krauchunas et al., (20), we detected significantly more peptides whose phosphorylation was downregulated (240 unique phosphopeptides) during egg activation than ones whose phosphorylation was up-regulated (148 unique phosphopeptides; p, value < 0.001, Exact binomial test), suggesting that changes because of protein dephosphorylation are more prevalent than those because of protein phosphorylation during egg activation.
In contrast to what we observed for the control, the number of phosphopeptides with significant abundance changes from mature oocytes to activated eggs was significantly lower when CanB2 is depleted (150 by MSA, p, value < 0.001, Exact binomial test; 115 by NL, p, value < 0.001, Exact binomial test) (Fig. 2A, and 2B,, Table I, supplemental Table S1 and supplemental Table S2). This finding indicates that the loss of calcineurin activity caused aberrant regulation of numerous phosphosites such that many changes that normally occur during egg activation do not take place.
Changes in the abundance of a given phosphopeptide could be caused either by alterations in phosphorylation/dephosphorylation, or alternatively by changed rates of protein synthesis or degradation processes, that are also known to occur during egg activation (30). To distinguish between these possibilities, we performed global proteomic analysis of the sample sets, and quantified the changes in protein levels during egg activation. We identified a total of 4039 proteins with at least 2 unique peptides in our dataset supplemental Table S3). The threshold for the significance of these changes in protein amount was 0.32-fold, determined by internal error. Of these proteins, 249 were significantly up-regulated and 107 were significantly downregulated in control activated eggs compared with mature oocytes (supplemental Fig. S7A,). As described in the Supplementary Methods, because of technical limitations in the analysis of TMT-10plex sets, it is likely that we failed to count some proteins whose levels changed during egg activation, but this undercounting is probably minor because the system is efficient at detecting significant changes of abundance when multiple groups of samples are tested, as we have done. Of the proteins whose levels were significantly up or down regulated during egg activation in the control, the abundance of 108 and 37, respectively, remained constant during this transition in CanB2 kd,.
When comparing the phosphoproteome and the global proteome data, we noted that for phosphopeptides that are significantly up- or down-regulated during egg activation, the corresponding master protein's abundance mostly remained constant (>75% across all categories) during egg activation (Fig. 2D,). This generalization became more apparent when we plotted the phosphopeptide abundance changes against the abundance changes of the protein from which the phosphopeptide was derived (supplemental Fig. S8). This suggests that in most cases, changes in phosphopeptide abundance reflect phosphoregulation, rather than changes in whole protein abundance.
Germline Depletion of CanB2 Leads to Widespread Misregulation of Protein Phosphorylation States During Egg Activation
To visualize the dynamics of phosphoproteomic changes during egg activation in control and CanB2 kd, samples, we plotted the phosphopeptide abundance fold changes in control against those during CanB2 kd, egg activation. In both the MSA and NL datasets, many phosphopeptides appeared to be misregulated in CanB2 kd, samples relative to controls (Fig. 3A,). While the abundance of these phosphopeptides was significantly up (green) or downregulated (orange) during egg activation in the control, these changes were not detected when CanB2 is depleted. This analysis is consistent with our previous observation that fewer phosphopeptides were significantly changed during egg activation in CanB2 kd, samples.
Fig. 3.
Germline depletion of CanB2 causes widespread misregulation of phosphorylation sites during egg activation. A,, Comparison of egg/oocyte phosphopeptide abundance foldchange in control and CanB2 kd, sample sets as quantified by MSA and NL methods. B,, Comparison of phosphopeptides that significantly increased or decreased during egg activation in control and in CanB2 kd, sample sets, as quantified by MSA and NL methods. C,, Enrichment analysis shows the terms that are significantly enriched among proteins whose phosphorylation states are misregulated during egg activation in CanB2 kd, sample sets (MSA and NL combined). n.s. = non-significant.
Importantly, in both the MSA and NL datasets, the phosphopeptides that significantly changed abundance during egg activation in the control are mostly different from those that significantly changed abundance during egg activation in CanB2 kd, (Fig. 3B,). Numbers of phosphopeptides showed significant abundance increases (75 by MSA and 52 by NL) or decreases (120 by MSA and 103 by NL), respectively, during egg activation in the control, but not in the CanB2 kd, samples. It is likely that the phosphorylation state changes of these sites are mediated directly by calcineurin or indirectly by calcineurin's downstream mediators during the egg-to-embryo transition.
Within the class of phosphopeptides that significantly decreased in abundance during egg activation in the control but not in CanB2 kd, are three sites in Gnu (discussed below). This result is consistent with previous observations that Gnu is dephosphorylated during the egg-to-embryo transition in wildtype (39, 47) and that this dephosphorylation is dependent on the activity of calcineurin (27) (and corroborated by our Western blot analysis (supplemental Fig. S2)). Similarly, a phosphopeptide from the nuclear lamina protein YA showed the same pattern of abundance change (supplemental Table S1 and supplemental Table S2). YA is known to be dephosphorylated in wildtype (49) but not in CanB2 kd, during egg activation (27). These findings further validated the ability of our dataset to capture and to reproduce known misregulations of the oocyte phosphoproteome during egg activation caused by CanB2 germline depletion.
Fewer phosphopeptides showed abundance increases [32 (22 by the MSA method and 16 by the NL method)] or decreases [54 (35 by the MSA method and 33 by the NL method)] during egg activation in CanB2 kd, but not in control samples. Clearly, calcineurin normally restrains these phosphosites from significant phosphorylation state changes during egg activation. We would predict that calcineurin does not directly target these classes of phosphosites but instead influences the kinases and phosphatases that act at these locations.
Gene ontology (GO) enrichment analysis of proteins with misregulated phosphopeptide abundance change during egg activation in CanB2 kd, showed enrichment for regulators of biological processes that are relevant to meiosis and early embryogenesis (Fig. 3C,), suggesting that components in these pathways are likely subject to phosphoregulation upon egg activation. A combined list of misregulated peptides from the MSA and NL datasets can be found in supplemental Table S6.
In summary, our data reveal that calcineurin activity is involved in the regulation of hundreds of phosphosites during egg activation, and that many of these sites are likely to be relevant to the egg-to-embryo transition.
Germline Depletion of CanB2 Perturbs the Protein Phosphorylation State of Mature Arrested Oocytes
A tacit assumption in the presentation above was that the abnormal protein phosphorylation state changes observed in CanB2 kd, are the result of aberrant phosphoregulation during egg activation rather than before egg activation (that is, in the mature oocyte). The basis for this assumption was that calcineurin has not previously been reported as essential for oogenesis, and indeed CanB2, knockout oocytes (produced in mutant germline clones) arrest normally at metaphase of meiosis I (24). However, because this has never been explored in detail, we asked whether depletion of CanB2 can cause abnormal phosphorylation in mature oocytes. We identified significant differences in phosphopeptide abundance in CanB2 kd, oocytes relative to control oocytes for the MSA and NL data sets. Surprisingly, we identified 50 phosphopeptides from 42 proteins (MSA and NL datasets combined) with significantly altered abundance in CanB2 kd, oocytes compared with control oocytes (Fig. 4A,, supplemental Table S6), indicating that CanB2 regulates their phosphorylation state in mature oocytes.
Fig. 4.
Perturbation of calcineurin activity in female germline leads to misregulation of protein phosphorylation states in mature oocytes. A,, Volcano plot of CanB2 kd,/control oocyte phosphopeptide abundance ratio as quantified by MSA and NL methods. B,, Terms enriched among proteins whose phosphorylation states are misregulated in CanB2 kd, mature oocytes. C,, Volcano plot of CnAact,/control oocyte phosphopeptide abundance ratio as quantified by MSA and NL methods. Gene Ontology categories: BP = biological process, CC = cellular compartment, MF = molecular function.
We found that the group of misregulated phosphopeptides includes 2 phosphopeptides from the calcineurin catalytic subunit isoforms CanA-14F and Pp2B-14D. One of these peptides encompasses PP2B-14D Ser520, which is conserved in its mammalian orthologue Calcineurin A Alpha, suggesting that CanB2 might influence the phosphorylation state and perhaps even the function of the calcineurin catalytic subunit.
Forty-eight out of the 50 misregulated phosphopeptides were lower in abundance in CanB2 kd, oocytes, indicating that CanB2 is required for maintaining normal levels of phosphorylation at the corresponding phosphosites before egg activation. Because calcineurin is a phosphatase, it is likely that it regulates these sites indirectly, either through one or more kinase effectors, or by antagonizing the activity of another phosphatase in mature oocytes. Our data provide some evidence for the former, because the MSA (but not the NL) data set shows that phosphopeptides from kinase associated protein Akap200 are misregulated in CanB2 kd, oocytes (supplemental Fig. S9). Except for calcineurin, we did not detect abnormal phosphorylation state changes among phosphatases.
The proteins whose phosphorylation states are misregulated in CanB2 kd, are enriched for chromatin regulators and DNA binding proteins (Fig. 4B,), suggesting that calcineurin may be involved in the regulation of chromatin structure before egg activation. On the other hand, the abundances of two phosphopeptides, one from the protein Jabba and one from Trailor hitch (Tral), were lower in CanB2 kd, oocytes compared with those in control oocytes, indicating that the phosphorylations at these sites are likely downregulated by calcineurin directly or indirectly before egg activation.
To further explore the function of calcineurin in mature oocytes, we analyzed the phosphoproteome of mature oocytes produced by CnAact, females, in which a constitutively active form of the calcineurin catalytic subunit PP2B-14D is expressed specifically in the germ cells (34). Fertilized eggs from CnAact, females show severely reduced hatchability and a range of meiotic defects (34). A total of 2672 phosphopeptides (2241 by the MSA method and 2140 by the NL method) were quantified (Table I, supplemental Table S4 and supplemental Table S5). The abundance of 63 phosphopeptides were significantly different between CnAact, oocytes and the control oocytes (Fig. 4C,). Interestingly, 52 of these 63 phosphopeptides showed increased abundance in CnAact, oocytes, again suggesting that calcineurin must regulate the corresponding proteins indirectly through one or more kinases or phosphatases in mature or maturing oocytes.
In addition to phosphoregulation, we also explored the possible role of calcineurin in protein translation in oocyte maturation. We compared the global proteome of control oocytes to that of CanB2 kd, oocytes, and found 37 proteins with abnormal abundance level when CanB2 is depleted (supplemental Fig. S10). Therefore, it is likely that calcineurin does have a role in protein homeostasis during oogenesis. To explore this possibility, we compared our results to an analysis of global proteomic changes that take place during oocyte maturation and egg activation performed by Kronja and colleagues (50). Of the 37 proteins that we observed to have abnormal abundance in CanB2 kd, oocytes, 21 were also captured by Kronja et al., (supplemental Table S7). Among these common proteins, 16 do not show dynamic changes in abundance during oocyte maturation in WT flies, which suggests that calcineurin may affect their production or deposition earlier during oogenesis; 5 are significantly up- or down-regulated during WT oocyte maturation (supplemental Table S7), which suggests that calcineurin may be involved in the regulation of their translation or degradation during the maturation process.
Activated Eggs with CanB2 Knockdown Remain in An M Phase-like Molecular State
Loss of CanB2 in the germline causes the meiotic cell cycle to stall in anaphase of meiosis I (24). To gain further insight into the molecular basis of this unusual meiotic cell cycle arrest in activated eggs deficient in CanB2 activity, we examined the phosphorylation states and protein abundance changes of several cell cycle regulators.
We first searched for regulators of the meiotic cell cycle among the proteins with phosphosites that are misregulated in CanB2 kd,. In metaphase of normal cell cycles, Cyclin-Cdk1 phosphorylates and activates the kinase Greatwall (Gwl) (51, 52), which in turn phosphorylates and activates Endosulfine (Endos), a competitive inhibitor of the Cdk1 antagonist PP2A-B55 (Drosophila Twins, Tws) (53, 54). We found that phosphorylation at the Cdk1 consensus site T606 on Gwl was significantly downregulated in the control but not in CanB2 kd, activated eggs (relative to the mature oocytes in each case), suggesting a high level of Cdk1 activity and low activity of PP2A-B55 in absence of CanB2. Consistent with this interpretation, dephosphorylation of Endos S68, a target of Gwl, was not observed in CanB2 kd, activated eggs (Fig. 5A,). Gnu (47) was also misregulated at several positions, including the predicted Cdk1 consensus sites T16 and S17, in CanB2 kd, activated eggs (Fig. 6A,). These findings provide further evidence that Cdk1 activity in CanB2 kd, activated eggs remains at a high level similar to that of mature oocytes blocked in metaphase of meiosis I.
Fig. 5.

Absence of CanB2 leads to misregulation of phosphorylation, as well as protein level of cell cycle regulators during egg activation. Y-axis is the log2 fold change of abundance in eggs compared with that in oocytes. A,, phosphopeptide abundance change of Endos[S68] and Gwl[T606] during egg activation in control and CanB2 kd,. Error bars reflect standard errors. B,, Mtrm and CycB3 protein level change during egg activation in control and CanB2 kd,. Error bars reflect standard errors. C,, Abundance of various Fzy phosphopeptides and Fzy protein level during egg activation in control and CanB2 kd,. Error bars reflect standard errors.
Fig. 6.

Protein translation is disrupted in activated eggs with CanB2 kd,. A,, Gnu phosphosites that are dephosphorylated upon egg activation in wt but not CanB2 kd, activated eggs. Error bars reflect standard errors. B,, Abundance changes of 3 proteins that are known to be translated upon egg activation under the regulation of PNG. Error bars reflect standard errors. C,, Aberrant phosphoregulation was observed at various phosphosites on translation regulators NAT1, eIF4G and eIF4B in CanB2 kd,. Error bars reflect standard errors.
Because Cdk1 activity requires the binding of cyclins to the kinase subunit, we examined the protein levels of Cyclin B3 (CycB3) and Cyclin B (CycB) in our data set (results for Cyclin A will be presented below). At metaphase exit, activation of the APC/C leads to the destruction of mitotic cyclins (55–58). In controls, the level of CycB3 indeed decreased at the completion of meiosis as expected (Fig. 5B,). Given that at least one aspect of anaphase I (that is, homologous chromosome separation) appears to have been initiated in activated eggs with CanB2 kd,, we also expected to see a decrease in the CycB3 protein level. This decrease was surprisingly not observed in CanB2 kd, eggs after activation (Fig. 5B,). The situation with respect to CycB protein is somewhat more complex. The Cyclin B level did not significantly decrease in the control or in CanB2 kd, activated eggs, and even showed a slight increase in the control (Fig. 6B,). We believe the reason for the result in the control is that most of the control activated eggs had completed meiosis and were arrested in post meiotic interphase. Because the eggs were not fertilized, CycB protein, whose translation is promoted by PNG during egg activation, accumulated in the cytoplasm of the control eggs (59). But most importantly, the data indicate that neither Cyclin B3 nor Cyclin B was destroyed as the result of egg activation in the arrested CanB2 kd, eggs. In the Discussion, we try to reconcile this finding with the fact that meiosis I appears to progress from metaphase to at least the beginning of anaphase in these same eggs.
Because the persistence of the two cyclins may reflect failed APC/C activation, we further queried the activity of APC/C in CanB2 kd, eggs by examining the APC/C target Matrimony (Mtrm), which is a cell cycle regulator that is normally degraded at the onset of anaphase (60). Although only one peptide was captured, our data reflect a strong decrease in Mtrm protein level during control egg activation, consistent with the previous report by Whitfield et al., (60). However, the amount of Mtrm protein remained unchanged in CanB2 kd, activated eggs. This result, coupled with the high level of cyclins that remain in these eggs, together suggest that the APC/C may not be activated in CanB2 kd, eggs.
APC/C activity requires the binding of the coactivator Cdc20. In Drosophila, two Cdc20 family proteins, Cortex (Cort) and Fizzy (Fzy) are involved in meiotic cell cycle regulation (60–62). We could not assess the status of Cort because our phosphoproteomic data did not include any phosphopeptides from this protein. However, we detected five Fzy phosphosites, and the phosphorylation state changes of three of these (T78 T82, and S113) during egg activation are significantly different in control v.s. CanB2 kd, samples (Fig. 5C,). These results suggest the potential involvement of calcineurin in the regulation of Fzy during egg activation, and indeed, calcineurin has been shown to be crucial for the dephosphorylation of Fzy/Cdc20 during egg activation in Xenopus, (25, 26). Inhibition of frog calcineurin prohibits the Fzy dephosphorylation, and causes delayed degradation of CycB. Our data suggest that a similar mechanism occurs in Drosophila through which calcineurin regulates the progression of meiosis by modulating Fzy and potentially Cort.
Finally, we observed strong dephosphorylation of the Cdk1 T14 and Y15 inhibitory phosphosites in control and CanB2 kd, eggs compared with mature oocytes (supplemental Table S1). T14 and Y15 are highly conserved target sites of Wee1, a kinase that is downregulated by Cdks, and of Cdc25, a phosphatase that is up-regulated by Cdks (63–67). Because control activated eggs were expected to be arrested in post-meiotic interphase, it is surprising that Cdk1 T14 and Y15 phosphorylations are downregulated in activated eggs compared with metaphase-arrested mature oocytes. As explained in the Discussion, the dephosphorylation of these sites in controls can perhaps be rationalized by the behavior of the polar bodies, which form a unique structure with arrested cell cycle and condensed chromosomes called a rosette. To explore whether a polar body rosette might explain the Cdk1 phosphorylation changes we detected, we examined control 0.5–1 h activated unfertilized eggs for the presence of polar bodies. A large portion of these eggs (47.6%, n, = 42) indeed contained a polar body rosette with condensed chromosomes (supplemental Fig. S11A, and S11B,). Local activity of regulators associated with the arrested polar body thus may contribute to the observed dephosphorylation of Cdk1 T14 and Y15 in the control activated eggs.
On the other hand, in CanB2 kd, activated eggs, meiosis was not completed. In this case, the dephosphorylation of T14 and Y15 of Cdk1 in activated CanB2, eggs, provides further molecular evidence for the idea that these eggs arrest in a stage of meiosis I in which CdK1 activity is high and in which the APC/C remains unactivated.
CanB2 Knockdown Perturbs the Phosphorylation States of Translation Regulators in Activated Eggs
Another major event of egg activation is the polyadenylation and translation of many maternally deposited mRNAs (30, 68–70). To assess the impact of CanB2, knockdown on protein translation, we examined the global proteomic data, searching for proteins whose abundance levels were misregulated during egg activation in CanB2 kd, compared with controls. Intriguingly, of the 249 proteins whose abundance increases during egg activation in control, 120 did not increase significantly in abundance level during egg activation in CanB2 kd, eggs (supplemental Fig. S7B,, supplemental Table S3), suggesting that calcineurin activity may be required for translation of subsets of proteins during egg activation.
Because Gnu dephosphorylation is inhibited in CanB2 kd, eggs (Fig. 6A,, supplemental Fig. S2), we suspected that PNG, a serine/threonine kinase that regulates the translation of maternal mRNA in early embryos (30, 68, 71–73), was not activated. PNG normally becomes active when it forms a complex with Plutonium (Plu) and Gnu (39). The dephosphorylation of Gnu upon egg activation allows it to bind PNG and Plu, thus activating the complex (39, 47). The obvious hypothesis is that maternal mRNAs whose translation is normally dependent on PNG should not be translated in CanB2 kd, eggs.
We thus examined the abundance changes of several proteins whose translation is known to be up-regulated by PNG upon egg activation. These proteins include Cyclin A (CycA) (74), Smaug (Smg) (68, 75) and CycB (59). As expected, we observed that the levels of all three proteins increased in control activated eggs compared with mature oocytes. However, the abundance of these proteins stayed constant in activated eggs with CanB2 kd, (Fig. 6B,), suggesting that their PNG-mediated translation may be stalled or abolished in absence of calcineurin activity.
We further validated the hypothesis that PNG is not active in eggs knocked down for CanB2 by comparing our list of 120 proteins whose abundance increases were abolished by CanB2 kd, to a published list of 46 proteins that are subject to PNG-mediated translational up-regulation (30). Of this list, we found 7 that were significantly upregulated during egg activation in the control. All 7 of these proteins showed no significant abundance changes in CanB2 kd, eggs, indicating that the PNG-dependent abundance up-regulation of multiple proteins during egg activation is abolished in CanB2 kd, eggs.
In addition to Gnu, multiple phosphosites in several other translation regulators, including NAT1, eIF4G, and eIF4B, were misregulated in CanB2 kd, activated eggs (Fig. 6C,). Intriguingly, all these phosphosites are downregulated during control egg activation. Our combined results suggest widespread calcineurin-mediated dephosphorylation of translation regulators during the egg to embryo transition. Calcineurin may therefore be involved in a variety of mechanisms that mediate the control of protein translation.
Inhibitory Phosphorylation on S9 of GSK3β by Akt1 is Strongly Upregulated in Control but Not in CanB2 kd Eggs
Drosophila Glycogen Synthase Kinase 3β (GSK3β, a.k.a. Sgg) was shown previously to be essential for egg activation (76). Here, we observed that in control egg activation, GSK3β is strongly phosphorylated at N terminus residue S9, an Akt1 kinase-mediated phosphorylation that inhibits GSK3β (77). But we did not observe GSK3β S9 phosphorylation in activated CanB2 kd, eggs (Fig. 7A,), indicating that the phosphorylation of GSK3β by Akt1 is dependent on calcineurin activity.
Fig. 7.

Sgg is strongly phosphorylated at the S9 Akt1 inhibitory phosphorylation site during egg activation in control but not in CanB2 kd,. A,, Y-axis is the log2 fold change of abundance in eggs compared with that in oocytes. Error bars reflect standard errors. B,, Western blotting for Akt1 S505 phosphorylation and Akt1 protein (Akt1-pan) in mature oocytes and activated unfertilized eggs produced by control or CanB2 kd, females.
Akt1 activity is promoted by phosphorylation at S505 by TORC2 (78). We thus assessed the status of Akt1 during egg activation by Western blotting with an S505 phospho-specific antibody. Consistent with a previous study (79), we saw phospho-Akt1 in activated eggs but not in mature arrested oocytes from control females. However, in CanB2 kd,, the phosphorylated form of Akt1 remained absent in activated eggs (Fig. 7B,), indicating that calcineurin activity is important for Akt1 activation during the transition from oocyte to embryo.
DISCUSSION
Phosphoregulation of maternal proteins has been suggested as a major mechanism that drives the transition from oocyte to embryo (18–21). Therefore, the activities and control of protein phosphoregulators are of great importance to understanding egg activation and early embryo development. Calcineurin is a highly conserved phosphoregulator that responds to a Ca2+ rise, the first common step to egg activation pathways in various organisms. Several previous studies showed that calcineurin plays a central role in regulating pathways that mediate egg activation events in Drosophila (2, 24, 34, 35), but the molecular consequences of calcineurin activity at the oocyte-to-egg transition were unknown.
Here, we utilized quantitative proteomic methods to gain a more comprehensive view of calcineurin's influence on global and phospho-proteomes during egg activation. Our data reveal widespread misregulation of protein phosphorylation changes in CanB2 depleted eggs. In particular, the majority of phosphosites whose abundances change during control egg activation are misregulated when CanB2 is depleted, pointing to calcineurin as an upstream master regulator of multiple egg activation events. Calcineurin may dephosphorylate certain of these sites directly, but other cases, particularly those in which the lack of calcineurin is correlated with decreases in the phosphosite's abundance, must be mediated indirectly through other phosphatases and kinases. An additional potential twist is that phosphosites within the calcineurin catalytic subunits PP2B-14D and CanA-14F are misregulated in CanB2 kd, oocytes. One of these sites, found in both proteins, matches the Cdk1 consensus recognition site (80) and is conserved in human calcineurin. Calcineurin autoregulation thus might impart yet another level of regulation to the pathway.
Calcineurin Plays an Unanticipated Role during Oocyte Maturation
Although CanB2-deficient mature oocytes do not show any obvious morphological defects and are arrested in what appears to be a cytologically normal metaphase I state, these oocytes display clear abnormalities in their phosphoproteomes. Almost all of the 50 misregulated phosphopeptides identified are subject to downregulations (in the sense that their abundances were lower in CanB2 kd, than in control oocytes). Conversely, mature oocytes produced by females expressing a constitutively active form of calcineurin (CnAact) also misregulate several phosphopeptides, but here most of these abnormalities are upregulations. These results together imply that calcineurin plays heretofore unexpected active roles in oocyte maturation that must be mediated by other enzymes. Our results that Akap200 is mis-phospho-regulated in CanB2 kd, oocytes suggest Akap200 as a candidate for mediating at least some of calcineurin's regulatory effects in oocytes.
Because the calcineurin's activity usually is dependent on intracellular calcium, it will be important to investigate the mechanism by which calcineurin regulates these phosphorylation sites before the calcium wave initiating egg activation. It will also be of great interest to explore whether and how these misregulated phosphorylations are important for the functions of the target proteins in the context of the developing oocyte.
What Is the Molecular/Cellular State of the Arrested CanB2 kd Eggs?
Our images of the activated eggs produced by CanB2 kd, mothers indicate a consistent arrest in what appears to be anaphase of meiosis I, with homologous chromosomes that have separated from each other supplemental Fig. S1C,) (24). These activated eggs differ from mature oocytes from the CanB2 kd, female, which appear to be in metaphase of meiosis I because all chromosomes are congressed at the metaphase plate (24). Passage through the female's reproductive tract, the trigger for egg activation in insects [12–16, 81], thus causes a change in at least some aspects of the cell cycle status of CanB2 kd, eggs.
Our results indicate however that at the molecular level, activated CanB2 kd, eggs still largely resemble metaphase I (Fig. 8): The phosphoproteome and global proteomes of these calcineurin-deficient eggs are little changed by the activation process. Cdk1 activity remains high; in fact, the Cdk activity may be even higher than prior to activation because the levels of the T14 and Y15 inhibitory phosphorylations on Cdk1 decrease. Activation does not decrease the phosphorylation of Gwl kinase and Endosulfine in the mutant eggs, indicating that the Cdk-countering phosphatase PP2A-B55 remains inactive. In these eggs, the APC/C does not signal the destruction of Matrimony protein as normally occurs during egg activation.
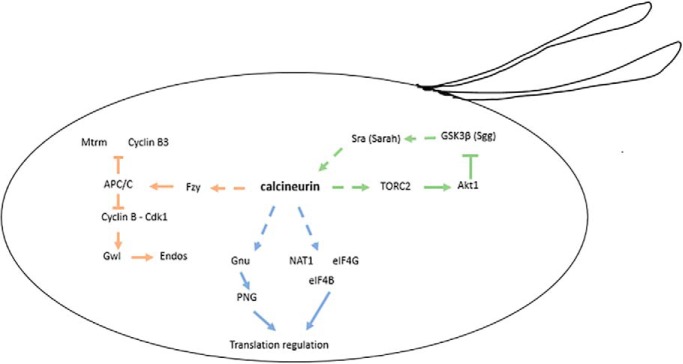
Fig. 8.

Schematic summary of the influences of calcineurin during egg activation on meiotic cell cycle (orange), translation regulation (blue), and GSK3β (green). Dashed lines indicate possible direct or indirect regulatory relationship, solid lines indicate direct regulatory relationship.
These observations explain why activated CanB2 kd, eggs become arrested in a cell cycle state not very far removed from that in mature oocytes. However, our results do not explain the most remarkable feature of this arrest: that homologous chromosomes apparently separate, based on the images of the CanB2 kd, embryos (supplemental Fig. S1C,) (24). The basis for this separation in Drosophila meiosis is somewhat mysterious because the genome has no obvious homologs of the meiotic α-kleisin Rec8. The destruction of Securin and the resultant activation of Separase are still needed in flies to release arm cohesion in anaphase I, even though the target of Separase remains unclear (82). One possibility for how arm cohesion could be partially released independently of calcineurin involves the fact that egg activation may also turn on the calcium-dependent kinase CamKII. This enzyme might potentiate a pathway leading to the destruction of a small population of Securin and thus some degree of homologous chromosome separation. An alternative model involves misregulation of a mechanism mediated by the Wapl protein that can remove cohesin independently of Separase (83) and is known to influence meiotic chromosome segregation in flies (84). This second pathway is normally thought to occur in prophase, but we know of no data that would preclude its function during the metaphase arrest in mature oocytes, particularly in those from CanB2 kd, mothers. Unfortunately, our data do not allow us to discriminate among these or other possible models to explain the separation of homologous chromosomes during the activation of mutant eggs. No known component of cohesin complexes or any other candidate molecules known to be involved in either of these pathways showed significant changes in abundance in control or CanB2 kd, eggs relative to the mature oocytes, so further biochemical studies will be required to solve this puzzle.
Another unexplained observation is the unexpected behavior of two key phosphosites: the T14 and Y15 inhibitory phosphorylations on Cdk1. T14 and Y15 are highly conserved target sites of Wee1, a kinase that is downregulated by Cdks; and of Cdc25, a phosphatase that is up-regulated by Cdks (63–67). One would thus predict that in wildtype, the phospho-occupancy of these sites would be low in mature oocytes (M phase) but high after egg activation (interphase). Yet the phosphorylation of these sites was lower in control (and CanB2 kd,) eggs compared with oocytes (supplemental Table S1). It must be noted that we do not know the absolute occupancy level of these sites, so it is possible that during these developmental stages, the majority of Cdk1 molecules are never phosphorylated at these sites. But whatever the absolute occupancy, T14 and Y15 becomes dephosphorylated in some Cdk1 molecules upon egg activation. At least in wildtype, this behavior can perhaps be rationalized by the status of the polar bodies. In Drosophila, polar bodies generated during meiosis are not expelled from the egg but are instead sequestered at the periphery of the egg and fuse together to form the polar body rosette (85). Because the rosette is maintained in an arrested state with condensed chromosomes in early embryos (86), it is possible that the dephosphorylation of Cdk1 T14 and Y15 in control activated eggs reflects the formation and maintenance of this structure.
Calcineurin Helps Determine the Phosphorylation State of Several Translation Regulators
We have verified the previously reported role of calcineurin on Gnu dephosphorylation, leading to the activation of PNG) (39, 47). Indeed, we found that several proteins whose translation is known to be turned on by PNG were up-regulated during activation of control eggs but not during the activation of eggs deficient in calcineurin. Our results strongly suggest that calcineurin also influences the phosphorylation state of several translation regulators in addition to Gnu (Fig. 6C,) (Fig. 8). These regulatory proteins (as well as Gnu) may be direct substrates of calcineurin because all of these sites become dephosphorylated when Ca2+ levels increase during control egg activation. Further investigation is necessary in order to explore the functional importance of the phosphorylation state of these translation factors.
GSK3β and Calcineurin May Act Within a Regulatory Loop
We were interested to observe that a key inhibitory phosphosite on GSK3β (Ser9) both is up-regulated upon egg activation and is dependent on calcineurin activity. Our data are consistent with, but do not prove, the idea that calcineurin's effect on this phosphosite is indirect and mediated by the kinases Akt1 and possibly TORC2. In turn, GSK3β has been shown to regulate Sra (76), a protein that inhibits calcineurin in mature eggs but that acts as a phosphorylation-dependent calcineurin activator during egg activation. These facts suggest the model that egg activation involves a negative feedback loop that helps create transiency in calcineurin activity during egg activation (Fig. 8). Calcineurin activity initiated by the Ca2+ rise leads indirectly to increased phosphorylation of GSK3β-Ser9, decreased Sra phosphorylation, and thus decreased calcineurin activity. This auto-shutoff model is consistent with a previous report in Xenopus, that calcineurin activity is transient following the calcium wave, and that both the initial calcineurin activation and its transiency are crucial for transition into embryogenesis (26). Future work will be needed to test this negative feedback model for Drosophila and other organisms during the egg activation process.
Data Availability
Mass spectrometry proteomics data have been deposited into the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset accession number PXD010991. The R analysis pipeline is available as a GitHub repository (https://github.com/YazBraimah/Dmel_eggAct_phosphoproteomics).
Supplementary Material
Acknowledgments
We thank Dr. Tim Karr for the opportunity to contribute to this Special Issue, and NIH grant R21-HD088744 to M.F.W. for funding this study. We thank Drs. Terry Orr-Weaver (Massachusetts Institute of Technology) and Toshiro Aigaki for their kind gifts of anti-Gnu primary antibodies and CnAact flies, respectively. We thank Drs. Marcus Smolka (Cornell University), Steve Dorus (Syracuse University), Kathryn Lilley (University of Cambridge), and Sheng Zhang (Cornell BioResource Center) for helpful advice on the relative merits of different quantitative mass spectrometry methods, and Drs. John Schimenti and Ken Kemphues for helpful comments on an early version of this manuscript, and anonymous reviewers for helpful comments on this version. We thank the Proteomic and MS Facility of Cornell University's BioResource Center for running the mass spectrometry on their Orbitrap Fusion mass spectrometer (which had been purchased with NIH grant SIG 1S10 OD017992). We thank the TRiP consortium at Harvard Medical School (funded by NIH grant NIGMS R01-GM084947) and the Bloomington Stock Center for transgenic RNAi fly stocks used in this study.
Footnotes
 This article contains supplemental material.
This article contains supplemental material.
1 The abbreviations used are:
- CamKII
- Ca2+/Calmodulin dependent kinase II
- ACN
- acetonitrile
- APC/C
- anaphase promoting complex/cyclosome
- CanA
- calcineurin A
- CanB2
- calcineurin B2
- CDK
- cyclin-dependent kinase
- CID
- collision induced dissociation
- FA
- formic Acid
- HCD-MS/MS
- higher energy collisional dissociation-MS/MS
- hpRP
- high pH reverse phase
- kd
- knockdown
- MSA
- multistage activation
- NL
- neutral loss trigger
- PP2A-B55
- protein phosphatase 2A at B55
- Pp2B-14D
- protein phosphatase 2B at 14D
- SPS
- synchronous precursor selection
- TMT
- tandem mass tag.
REFERENCES
- 1. Horner V. L., and Wolfner M. F. (2008) Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev. Dyn. 237, 527–544 [DOI] [PubMed] [Google Scholar]
- 2. Krauchunas A. R., and Wolfner M. F. (2013) Molecular changes during egg activation. Curr. Top. Dev. Biol. 102, 267–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Machaty Z., Miller A. R., and Zhang L. (2017) Egg activation at fertilization. Adv. Exp. Med. Biol. 953, 1–47 [DOI] [PubMed] [Google Scholar]
- 4. Marcello M. R., and Singson A. (2010) Fertilization and the oocyte-to-embryo transition in C. elegans,. BMB Rep. 43, 389–399 [DOI] [PubMed] [Google Scholar]
- 5. Laver J. D., Marsolais A. J., Smibert C. A., and Lipshitz H. D. (2015) Regulation and function of maternal gene products during the maternal-to-zygotic transition in Drosophila,. Curr. Top. Dev. Biol. 113, 43–84 [DOI] [PubMed] [Google Scholar]
- 6. Von Stetina J. R., and Orr-Weaver T. L. (2011) Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb. Perspect. Biol. 3, a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aviles-Pagan E. E., and Orr-Weaver T. L. (2018) Activating embryonic development in Drosophila,. Semin. Cell Dev. Biol. 84, 100–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knott J. G., Kurokawa M., Fissore R. A., Schultz R. M., and Williams C. J. (2005) Transgenic RNA interference reveals role for mouse sperm phospholipase C zeta in triggering Ca2+ oscillations during fertilization. Biol. Reprod, 72, 992–996 [DOI] [PubMed] [Google Scholar]
- 9. Saunders C. M., Larman M. G., Parrington J., Cox L. J., Royse J., Blayney L. M., Swann K., and Lai F. A. (2002) PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129, 3533–3544 [DOI] [PubMed] [Google Scholar]
- 10. Steinhardt R., Zucker R., and Schatten G. (1977) Intracellular calcium release at fertilization in the sea urchin egg. Dev. Biol. 58, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yoon S. Y., Eum J. H., Lee J. E., Lee H. C., Kim Y. S., Han J. E., Won H. J., Park S. H., Shim S. H., Lee W. S., Fissore R. A., Lee D. R., and Yoon T. K. (2012) Recombinant human phospholipase C zeta 1 induces intracellular calcium oscillations and oocyte activation in mouse and human oocytes. Hum. Reprod. 27, 1768–1780 [DOI] [PubMed] [Google Scholar]
- 12. Horner V. L., and Wolfner M. F. (2008) Mechanical stimulation by osmotic and hydrostatic pressure activates Drosophila, oocytes in vitro in a calcium-dependent manner. Dev. Biol. 316, 100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaneuchi T., Sartain C. V., Takeo S., Horner V. L., Buehner N. A., Aigaki T., and Wolfner M. F. (2015) Calcium waves occur as Drosophila, oocytes activate. Proc. Natl. Acad. Sci. U.S.A., 112, 791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sartain C. V., and Wolfner M. F. (2013) Calcium and egg activation in Drosophila,. Cell Calcium 53, 10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Went D. F., and Krause G. (1974) Alteration of egg architecture and egg activation in an endoparasitic Hymenopteran as a result of natural or imitated oviposition. Wilhelm Roux Arch. Entwickl Mech. Org. 175, 173–184 [DOI] [PubMed] [Google Scholar]
- 16. York-Andersen A. H., Parton R. M., Bi C. J., Bromley C. L., Davis I., and Weil T. T. (2015) A single and rapid calcium wave at egg activation in Drosophila,. Biol. Open 4, 553–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanders J. R., and Swann K. (2016) Molecular triggers of egg activation at fertilization in mammals. Reproduction 152, R41–R50 [DOI] [PubMed] [Google Scholar]
- 18. Guo H., Garcia-Vedrenne A. E., Isserlin R., Lugowski., A., Morada A., Sun A., Miao Y., Kuzmanov U., Wan C., Ma H., Foltz K., and Emili A. (2015) Phosphoproteomic network analysis in the sea urchin Strongylocentrotus purpuratus, reveals new candidates in egg activation. Proteomics 15, 4080–4095 [DOI] [PubMed] [Google Scholar]
- 19. Roux M. M., Townley I. K., Raisch M., Reade A., Bradham C., Humphreys G., Gunaratne H. J., Killian C. E., Moy G., Su Y. H., Ettensohn C. A., Wilt F., Vacquier V. D., Burke R. D., Wessel G., and Foltz K. R. (2006) A functional genomic and proteomic perspective of sea urchin calcium signaling and egg activation. Dev. Biol., 300, 416–433 [DOI] [PubMed] [Google Scholar]
- 20. Krauchunas A. R., Horner V. L., and Wolfner M. F. (2012) Protein phosphorylation changes reveal new candidates in the regulation of egg activation and early embryogenesis in D. melanogaster,. Dev. Biol., 370, 125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Presler M., Van Itallie E., Klein A. M., Kunz R., Coughlin M. L., Peshkin L., Gygi S. P., Wühr M., and Kirschner M. W. (2017) Proteomics of phosphorylation and protein dynamics during fertilization and meiotic exit in the Xenopus, egg. Proc. Natl. Acad. Sci. U.S.A. 114, E10838–E10847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Z., Krauchunas A. R., Huang S., and Wolfner. M. F. (2018) Maternal proteins that are phosphoregulated upon egg activation include crucial factors for oogenesis, egg activation and embryogenesis in Drosophila, melanogaster. G3 8, 3005–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rusnak F., and Mertz P. (2000) Calcineurin: form and function. Physiol. Rev., 80, 1483–1521 [DOI] [PubMed] [Google Scholar]
- 24. Takeo S., Hawley R. S., and Aigaki T. (2010) Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila,. Dev. Biol., 344, 957–967 [DOI] [PubMed] [Google Scholar]
- 25. Nishiyama T., Yoshizaki N., Kishimoto T., and Ohsumi K. (2007) Transient activation of calcineurin is essential to initiate embryonic development in Xenopus laevis,. Nature 449, 341–345 [DOI] [PubMed] [Google Scholar]
- 26. Mochida S., and Hunt T. (2007) Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature, 449, 336–340 [DOI] [PubMed] [Google Scholar]
- 27. Krauchunas A. R., Sackton K. L., and Wolfner M. F. (2013) Phospho-regulation pathways during egg activation in Drosophila melanogaster,. Genetics, 195, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freeman M., and Glover D. M. (1987) The gnu mutation of Drosophila, causes inappropriate DNA synthesis in unfertilized and fertilized eggs. Genes Dev., 1, 924–930 [DOI] [PubMed] [Google Scholar]
- 29. Renault A. D., Zhang X. H., Alphey L. S., Frenz L. M., Glover D. M., Saunders R. D., and Axton J. M. (2003) Giant nuclei is essential in the cell cycle transition from meiosis to mitosis. Development, 130, 2997–3005 [DOI] [PubMed] [Google Scholar]
- 30. Kronja I., Yuan B, Eichhorn S. W., Dzeyk K., Krijgsveld J., Bartel D. P., and Orr-Weaver T. L. (2014) Widespread changes in the posttranscriptional landscape at the Drosophila, oocyte-to-embryo transition. Cell Rep, 7, 1495–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin H. F., and Wolfner M. F. (1991) The Drosophila, maternal-effect gene fs(1)Ya, encodes a cell cycle-dependent nuclear envelope component required for embryonic mitosis. Cell, 64, 49–62 [DOI] [PubMed] [Google Scholar]
- 32. Liu J., Song K., and Wolfner M. F. (1995) Mutational analyses of fs(1)Ya, an essential, developmentally regulated, nuclear envelope protein in Drosophila,. Genetics, 141, 1473–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lopez J. M., Song K., Hirshfeld A.B., Lin H., and Wolfner M.F. (1994) The Drosophila, fs(1)Ya protein, which is needed for the first mitotic division, is in the nuclear lamina and in the envelopes of cleavage nuclei, pronuclei, and nonmitotic nuclei. Dev. Biol., 163, 202–211 [DOI] [PubMed] [Google Scholar]
- 34. Takeo S., Tsuda M., Akahori S., Matsuo T., and Aigaki T. (2006) The calcineurin regulator sra plays an essential role in female meiosis in Drosophila,. Curr. Biol., 16, 1435–1440 [DOI] [PubMed] [Google Scholar]
- 35. Horner V. L., Czank A, Jang J. K, Singh N, Williams B. C, Puro J, Kubli E, Hanes S. D., McKim K. S., Wolfner M. F., and Goldberg M. L. (2006) The Drosophila, calcipressin sarah is required for several aspects of egg activation. Curr. Biol., 16, 1441–1446 [DOI] [PubMed] [Google Scholar]
- 36. Findlay G. D., Sitnik J. L, Wang W., Aquadro C. F., Clark N. L., and Wolfner M. F. (2014) Evolutionary rate covariation identifies new members of a protein network required for Drosophila melanogaster, female post-mating responses. PLoS Genet. 10, e1004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Page A. W., and Orr-Weaver T. L. (1997) Activation of the meiotic divisions in Drosophila, oocytes. Dev. Biol., 183, 195–207 [DOI] [PubMed] [Google Scholar]
- 38. Boswell R. E., and Mahowald A. P. (1985) tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster,. Cell, 43, 97–104 [DOI] [PubMed] [Google Scholar]
- 39. Lee L. A., Van Hoewyk D., and Orr-Weaver T. L. (2003) The Drosophila cell cycle kinase PAN GU forms an active complex with PLUTONIUM and GNU to regulate embryonic divisions. Genes Dev., 17, 2979–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang Y., Qiang X., Owsiany K., Zhang S., Thannhauser T. W., and Li L. (2011) Evaluation of different multidimensional LC-MS/MS pipelines for isobaric tags for relative and absolute quantitation (iTRAQ)-based proteomic analysis of potato tubers in response to cold storage. J. Proteome Res. 10, 4647–4660 [DOI] [PubMed] [Google Scholar]
- 41. Yang S., Li X., Liu X., Ding X., Xin X., Jin C., Zhang S., Li G., and Guo H. (2018) Parallel comparative proteomics and phosphoproteomics reveal that cattle myostatin regulates phosphorylation of key enzymes in glycogen metabolism and glycolysis pathway. Oncotarget 9, 11352–11370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jiang X., Bomgarden R., Brown J., Drew D. L., Robitaille A. M., Viner R., and Huhmer A. R. (2017) Sensitive and accurate quantitation of phosphopeptides using TMT isobaric labeling technique. J. Proteome Res. 16, 4244–4252 [DOI] [PubMed] [Google Scholar]
- 43. Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., and Smyth G. K. (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kammers K., Cole R. N., Tiengwe C., and Ruczinski (2015) Detecting significant changes in protein abundance. EuPA Open Proteom. 7, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang da W., Sherman B. T., and Lempicki R. A. (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res., 37, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang da W., Sherman B. T., and Lempicki R. A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 [DOI] [PubMed] [Google Scholar]
- 47. Hara M., Petrova B., and Orr-Weaver T. L. (2017) Control of PNG kinase, a key regulator of mRNA translation, is coupled to meiosis completion at egg activation. Elife 2017 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gan C. S., Chong P. K., Pham T. K., and Wright P. C. (2007) Technical, experimental, and biological variations in isobaric tags for relative and absolute quantitation (iTRAQ). J. Proteome Res. 6, 821–827 [DOI] [PubMed] [Google Scholar]
- 49. Yu J., Liu J., Song K., Turner S. G., and Wolfner M. F. (1999) Nuclear entry of the Drosophila melanogaster, nuclear lamina protein YA correlates with developmentally regulated changes in its phosphorylation state. Dev. Biol. 210, 124–134 [DOI] [PubMed] [Google Scholar]
- 50. Kronja I., Whitfield Z.J., Yuan B., Dzeyk K., Kirkpatrick J., Krijgsveld J., and Orr-Weaver T. L. (2014) Quantitative proteomics reveals the dynamics of protein changes during Drosophila, oocyte maturation and the oocyte-to-embryo transition. Proc. Natl. Acad. Sci. U.S.A. 111, 16023–16028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu J., Zhao Y., Li Z., Galas S., and Goldberg M. L. (2006) Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus, egg extracts. Mol. Cell 22, 83–91 [DOI] [PubMed] [Google Scholar]
- 52. Blake-Hodek K. A., Williams B. C., Zhao Y., Castilho P. V., Chen W., Mao Y., Yamamoto T. M., and Goldberg M. L. (2012) Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol. Cell. Biol. 32, 1337–1353, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mochida S., Maslen S. L., Skehel M., and Hunt T. (2010) Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Scienc, 330, 1670–1673 [DOI] [PubMed] [Google Scholar]
- 54. Gharbi-Ayachi A., Labbé J. C., Burgess A., Vigneron S., Strub J. M., Brioudes E., Van-Dorsselaer A., Castro A., and Lorca T. (2010) The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330, 1673–1677 [DOI] [PubMed] [Google Scholar]
- 55. Holloway S. L., Glotzer M., King R. W., and Murray A. W. (1993) Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell 73, 1393–1402 [DOI] [PubMed] [Google Scholar]
- 56. Parry D. H., and O'Farrell P. H. (2001) The schedule of destruction of three mitotic cyclins can dictate the timing of events during exit from mitosis. Curr. Biol. 11, 671–683, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yuan K., and O'Farrell P. H. (2015) Cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition. Curr. Biol. 25, 811–816, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang L., and Barford D. (2014) Insights into the anaphase-promoting complex: a molecular machine that regulates mitosis. Curr. Opin. Struct. Biol. 29, 1–9 [DOI] [PubMed] [Google Scholar]
- 59. Vardy L., and Orr-Weaver T. L. (2007) The Drosophila, PNG kinase complex regulates the translation of cyclin B. Dev. Cell 12, 157–166 [DOI] [PubMed] [Google Scholar]
- 60. Whitfield Z. J., Chisholm J., Hawley R. S., and Orr-Weaver T. L. (2013) A meiosis-specific form of the APC/C promotes the oocyte-to-embryo transition by decreasing levels of the Polo kinase inhibitor matrimony. PLos Biol. 11, e1001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Swan A., and Schupbach T. (2007) The Cdc20 (Fzy)/Cdh1-related protein, Cort, cooperates with Fzy in cyclin destruction and anaphase progression in meiosis I and II in Drosophila,. Development 134, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pesin J. A., and Orr-Weaver T. L. (2007) Developmental role and regulation of cortex, a meiosis-specific anaphase-promoting complex/cyclosome activator. PLoS Genet. 3, e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Den Haese G. J., Walworth N., Carr A. M., and Gould K. L. (1995) The Wee1 protein kinase regulates T14 phosphorylation of fission yeast Cdc2. Mol. Biol. Cell 6, 371–385, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Watanabe N., Broome M., and Hunter T. (1995) Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14, 1878–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Berry L. D., and Gould K. L. (1996) Regulation of Cdc2 activity by phosphorylation at T14/Y15. Prog. Cell Cycle Res. 2, 99–105 [DOI] [PubMed] [Google Scholar]
- 66. Hoffmann I., Clarke P. R., Marcote M. J., Karsenti E., and Draetta G. (1993) Phosphorylation and activation of human cdc25-C by cdc2–cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 12, 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kumagai A., and Dunphy W. G. (1992) Regulation of the cdc25 protein during the cell cycle in Xenopus, extracts. Cell 70, 139–151 [DOI] [PubMed] [Google Scholar]
- 68. Tadros W., Goldman A. L., Babak T., Menzies F., Vardy L., Orr-Weaver T., Hughes T. R., Westwood J. T., Smibert C. A., and Lipshitz H. D. (2007) SMAUG is a major regulator of maternal mRNA destabilization in Drosophila, and its translation is activated by the PAN GU kinase. Dev. Cell 12, 143–155 [DOI] [PubMed] [Google Scholar]
- 69. Cui J., Sackton K. L., Horner V. L., Kumar K. E., and Wolfner M. F. (2008) Wispy, the Drosophila, homolog of GLD-2, is required during oogenesis and egg activation. Genetics 178, 2017–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lim J., Lee M., Son A., Chang H., and Kim V. N. (2016) mTAIL-seq reveals dynamic poly(A) tail regulation in oocyte-to-embryo development. Genes Dev. 30, 1671–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eichhorn S. W., Subtelny A. O., Kronja I., Kwasnieski J. C., Orr-Weaver T. L., and Bartel D. P. (2016) mRNA poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila, oocytes and early embryos. Elife 5, e16955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elfring L. K., Axton J. M., Fenger D. D., Page A. W., Carminati J. L., and Orr-Weaver T. L. (1997) Drosophila PLUTONIUM protein is a specialized cell cycle regulator required at the onset of embryogenesis. Mol. Biol. Cell, 8, 583–593, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shamanski F. L., and Orr-Weaver T. L. (1991) The Drosophila plutonium, and pan gu, genes regulate entry into S phase at fertilization. Cell 66, 1289–1300 [DOI] [PubMed] [Google Scholar]
- 74. Vardy L., Pesin J. A., and Orr-Weaver T. L. (2009) Regulation of Cyclin A protein in meiosis and early embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 106, 1838–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen L., Dumelie J. G., Li X., Cheng M. H., Yang Z., Laver J. D., Siddiqui N. U., Westwood J. T., Morris Q., Lipshitz H. D., and Smibert C. A. (2014) Global regulation of mRNA translation and stability in the early Drosophila, embryo by the Smaug RNA-binding protein. Genome Biol, 15, R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Takeo S., Swanson S. K., Nandanan K., Nakai Y., Aigaki T., Washburn M. P., Florens L., and Hawley R. S. (2012) Shaggy/glycogen synthase kinase 3beta and phosphorylation of Sarah/regulator of calcineurin are essential for completion of Drosophila, female meiosis. Proc. Natl. Acad. Sci. U.S.A., 109, 6382–6389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., and Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 78. Hietakangas V., and Cohen S. M. (2007) Re-evaluating AKT regulation: role of TOR complex 2 in tissue growth. Genes Dev. 21, 632–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sieber M. H., Thomsen M. B., and Spradling A. C. (2016) Electron transport chain remodeling by GSK3 during oogenesis connects nutrient state to reproduction. Cell 164, 420–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Songyang Z., Blechner S., Hoagland N., Hoekstra M. F., Piwnica-Worms H.., and Cantley L. C. (1994) Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 4, 973–982 [DOI] [PubMed] [Google Scholar]
- 81. Heifetz Y., Yu J., and Wolfner M. F. (2001) Ovulation triggers activation of Drosophila, oocytes. Dev. Biol. 234, 416–424 [DOI] [PubMed] [Google Scholar]
- 82. Guo Z., Batiha O., Bourouh M., Fifield E., and Swan A. (2016) Role of Securin, Separase and Cohesins in female meiosis and polar body formation in Drosophila. J. Cell Sci. 129, 531–542 [DOI] [PubMed] [Google Scholar]
- 83. Eichinger C. S., Kurze A., Oliveira R. A., and Nasmyth K. (2013) Disengaging the Smc3/kleisin interface releases cohesin from Drosophila, chromosomes during interphase and mitosis. EMBO J. 32, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Verni F., Gandhi R., Goldberg M. L., and Gatti M. (2000) Genetic and molecular analysis of wings apart-like (wapl), a gene controlling heterochromatin organization in Drosophila melanogaster. Genetics 154, 1693–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mahowald A. P., Goralski T. J., and Caulton J. H. (1983) In vitro activation of Drosophila, eggs. Dev. Biol. 98, 437–445 [DOI] [PubMed] [Google Scholar]
- 86. Su T. T., Sprenger F., DiGregorio P. J., Campbell S. D., and O'Farrell P. H. (1998) Exit from mitosis in Drosophila syncytial embryos requires proteolysis and cyclin degradation, and is associated with localized dephosphorylation. Genes Dev. 12, 1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Mass spectrometry proteomics data have been deposited into the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset accession number PXD010991. The R analysis pipeline is available as a GitHub repository (https://github.com/YazBraimah/Dmel_eggAct_phosphoproteomics).