Abstract
A fast and reliable determination of the ethanol concentration is essential in the analysis of alcoholic beverages. However, different factors like pH value or salt concentration can influence the ethanol measurement. Furthermore, analytical figures of merit for the alcohol sensor, such as limit of detection, sensitivity and measurement uncertainty, are necessary for the application. In this paper, a detailed sensor characterization of a novel sensor based on ethanol-sensitive poly acrylamide hydrogels will be presented. The resulting swelling pressure of the hydrogel was transformed via a piezoresistive pressure sensor into a measurable output voltage. These kinds of sensors can be used over a large measuring range, up to 50 vol% ethanol and more, with a high sensitivity. In the range from pH 7.4 to 4, the pH value had no influence on the sensor signal. Higher salt concentrations can slightly influence the measurement. The detection limit amounts to 0.06–0.65 vol% ethanol. The concentration of a vodka sample was determined with a sufficient measuring uncertainty.
Keywords: stimuli-responsive hydrogel, piezoresistive pressure sensor, ethanol, alcohol, chemical sensor, sensor characterization, ethanol sensitivity, detection limit, cross sensitivity
1. Introduction
The determination of the ethanol concentration is important for numerous industrial and biotechnological processes, such as foodstuffs, cosmetics, or pharmaceuticals [1]. Especially for alcoholic beverages, the accurate ethanol measurement is essential due to the strict regulations of the European Union for the labeling of alcoholic drinks [2]. Methods like hydrometers are often used in brewery industry. However, the alcohol measurement accuracy depends strongly on the expertise of the operator and on the temperature of the sample [3]. Other techniques like high-performance liquid chromatography [4] are often too expensive for small companies. This article focuses on a promising novel sensor approach based on stimuli-responsive hydrogels and piezoresistive pressure sensors for the detection of ethanol. Due to low manufacturing costs, a simple sensor set-up and their in-line process capability, these alcohol sensors have a high potential for industrial applications.
Stimuli-responsive hydrogels are hydrophilic three-dimensional polymer networks that can swell and deswell in dependence on a particular stimulus [5]. Used in combination with a piezoresistive pressure sensor, the resulting swelling pressure of the gel deforms the bending plate of the sensor and changes the resistance of piezoresistors integrated therein [6]. A Wheatstone bridge circuit transforms the resistance change into a measurable output voltage. The resulting change in output voltage depends proportionally on the analyte concentration.
With this concept, different hydrogel-based piezoresistive sensors could be produced by selecting suitable monomers for the preparation of polymerized gels. For example, temperature sensors have already been developed on the basis of the thermosensitive monomer N-isopropylacrylamide [7]. Glucose sensors were demonstrated based on 3-acrylamido phenylboronic acid hydrogels [8]. Furthermore, pH-sensitive hydrogel sensors in a physiological range could be realized by using a copolymer based on 2-(dimethylamino)ethyl methacrylate and hydroxypropyl methacrylate [9].
In [10], we demonstrated the feasibility of an ethanol sensor based on polyacrylamide hydrogels and showed the swelling characteristics in different alcohol-water mixtures. In this article, a detailed characterization of such hydrogel-based ethanol sensors will be presented with respect to sensitivity, detection limit, cross-sensitivities, and measurement uncertainty. In particular, factors that have a decisive role for practical application, such as the measuring range, cross sensitivities to salt concentrations, and pH values are investigated. Furthermore, calibration curves were recorded and used to determine the ethanol concentration in vodka samples.
2. Materials and Methods
2.1. Synthesis of Ethanol-Sensitive Polyacrylamide-Bisacrylamide Hydrogels
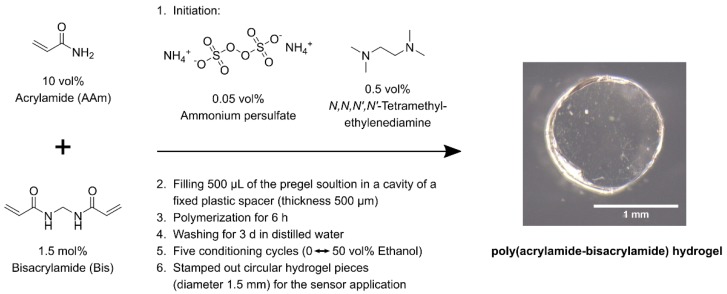
For the polymerization, 10 vol% (0.95 mmol, 67.83 mg) of the monomer acrylamide (Sigma Aldrich, St. Louis, MO, USA) was combined with 1.5 mol% (14.31 μmol, 2.21 mg) of the crosslinker bisacrylamide (Carl Roth, Karlsruhe, Germany) and were dissolved in distilled water. The total volume of each synthesis was 600 µL. After degassing the pregel solution for 5 min with nitrogen, the hydrogels were polymerized by adding the thermal initiator systems ammonium peroxodisulfate (0.05 vol%, 2.60 μmol, 0.59 mg, Sigma Aldrich, St. Louis, MO, USA) and N,N,N′,N′-tetramethylethylenediamine (0.5 vol%, 3.0 µL, Carl Roth, Karlsruhe, Germany). In order to produce sensors with high reproducibility, hydrogels with a defined thickness were synthesized. For this purpose, a plastic spacer with a thickness of 500 µm was fixed between two object slides. The initiated pregel solutions were filled in the cavity of the plastic spacer and were polymerized for 6 h. To remove unreacted monomers, the gels were washed for three days in distilled water. Due to a conditioning process, polymer chains find their optimal arrangement and the measurement accuracy of the sensors increases significantly [11]. For this reason, the gels were conditioned five-fold in an ethanol-water-mixtures (0 vol% ethanol ⇆ 50 vol% ethanol) for a total of five days [10]. The hydrogel synthesis and its preparation for sensor application is shown in Figure 1.
Figure 1.
Synthesis of alcohol-sensitive poly(acrylamide-bisacrylamide) hydrogels and their preparation for the application in a piezoresistive pressure sensor.
2.2. Preparation of Hydrogel-Based Piezoresistive Ethanol Sensors
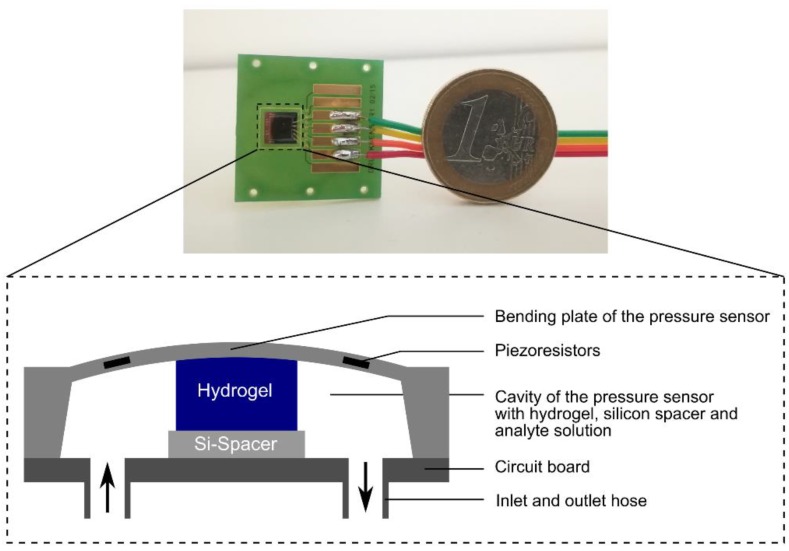
For the preparation of hydrogel sensors, the thin conditioned gel pieces were used to punch out circular hydrogels with a diameter of 1.5 mm. Meanwhile, a silicon spacer with a thickness of approx. 280 μm was fixed with cyanoacrylate in the middle of a circuit board. A circular hydrogel piece was put on the silicon spacer. Afterwards, the pressure sensor chip (TDK Electronics, previously EPCOS, C41-Series, 5.0 × 5.0 × 0.4 mm3, Munich, Germany) was fixed by using cyanoacrylate. This ensured that the hydrogel was located centrally in the cavity of the sensor. After wire bonding providing a signal transmission between sensor and circuit board, the connection cables to the read-out unit (Fluke 45 Multimeter, Glottertal, Germany) were soldered. The inlet and outlet hoses were fixed with cyanoacrylate and two-component epoxy resin on the circuit board. The tubes and a peristaltic pump (Reglo Digital MC-2/6, Ismatec as part of Cole-Parmer, Wertheim, Germany) were used to pump ethanol–water mixtures to the sensor at a constant flow rate of 0.2 mL/min. The supplied voltage of the ethanol sensor was 5 V (DIGI 35, Voltcraft as part of Conrad Electronic, Hirschau, Germany). All sensor measurements were done at room temperature [10]. The resulting hydrogel-based ethanol sensor is shown in Figure 2. For the different measurement tasks, new sensors were always made. The nomenclature of the used hydrogel-based ethanol sensors was summarized in Appendix A.
Figure 2.
Set-up of a hydrogel-based piezoresistive ethanol sensor: The swelling pressure of the gel leads to a deformation of the bending plate and the piezoresistors. The change in resistance due to the piezoresistive effect will be transformed into an output voltage via a Wheatstone bridge circuit. The figure was modified according to [10].
2.3. Determination of the Measuring Range
After the sensor preparation, the hydrogel sensor was conditioned overnight in distilled water. Ethanol–water mixtures between 10 and 50 vol% ethanol with 10 vol%-steps and distilled water were prepared. During the measurements, mixtures were changed to the next higher ethanol concentration every 90 min.
2.4. Cross-Sensitivity to Salt Concentrations
The influence of the salt concentration on the sensitivity of the sensors was exemplarily demonstrated using sodium chloride solutions. Sodium chloride solutions with a concentration of 0.01, 0.05, 0.1, and 0.5 mol/L were prepared using distilled water. First, all sensors were conditioned overnight in distilled water. During the measurement, the solutions were changed every 90 min to the next higher sodium chloride concentration. Finally, a solution with 20 vol% ethanol and 0.5 mol/L sodium chloride was made and measured with the sensors.
2.5. Cross-Sensitivity to pH Value
The cross-sensitivity to the pH value was tested with phosphate-buffered saline (PBS). One PBS tablet (pH 7.4, I = 0.15 mol/L in 200 mL water, Sigma Aldrich, St. Louis, MO, USA) was dissolved in 200 mL distilled water. The pH value of the solutions was adjusted to pH 6.02, 4.97, and 4.01 using a pH meter and 0.1 mol/L hydrochloric acid. After conditioning the sensors overnight in PBS buffer, pH 7.4, the buffer solutions were measured and changed to the next lower pH value every 90 min. At the end, a PBS-buffer solution, pH 4.01, with 20 vol% ethanol was produced and also measured with the sensors.
2.6. Sensor Calibration and Measurements of a Vodka Sample
To determine the alcohol content of vodka (brand: “Wodka Gorbatschow” with 37.5 vol% ethanol, Henkell & Co., Sektkellerei KG, Wiesbaden, Germany), a calibration curve was prepared first. For this purpose, ethanol–water mixtures with 34, 36, 38, and 40 vol% ethanol were made and each sample was measured for 90 min. Subsequently, the vodka sample was analyzed with the help of the derived calibration curves and the alcohol concentration of the vodka could now be determined.
3. Results and Discussion
3.1. Determination of the Measuring Range
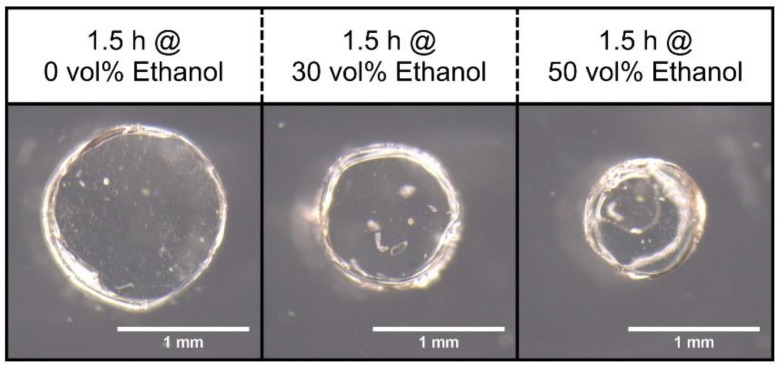
Most alcoholic beverages have an alcohol content of up to 50 vol% ethanol. For this reason, the sensitivity was studied in a range from 0 to 50 vol% ethanol. At first, the hydrogel swelling of a hydrogel dot was tested in different ethanol–water mixtures via microscopy (Figure 3).
Figure 3.
Swelling of a poly(acrylamide-bisacrylamide) hydrogel dot (diameter ca. 1.5 mm) in different ethanol–water mixtures.
Polyacrylamide hydrogels deswell with increasing ethanol concentrations in a wide measuring range. In this context, the solubility of the polymer system was of decisive importance. Polyacrylamide is soluble in water; however, the polymer is insoluble in ethanol. In water, the solvent interacts with the polymer chains and the hydrogel is swollen. In ethanol, the hydrogel chains do not interact with the solvent and, hence, the hydrogel is deswollen [10]. Since the swelling depends on the ethanol concentration, these hydrogel systems are excellently suited to be used in hydrogel-based piezoresistive ethanol sensors (Figure 4).
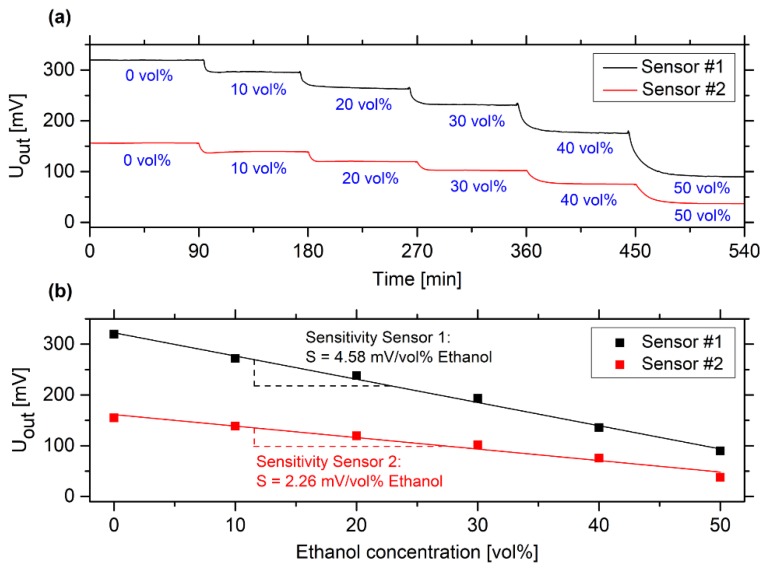
Figure 4.
(a) Time-dependent change of the output voltage for two hydrogel-based ethanol sensors at different ethanol concentrations in a range from 0 to 50 vol% ethanol; and (b) the corresponding calibration curves of these sensors.
In comparison to [10], hydrogels with a higher linker content (1.5 mol% instead of 0.44 mol% bisacrylamide) were used in this application. The composition of the hydrogel influences significantly the sensitivity of the sensor. The higher the concentration of the linker, the more crosslinking points are in the gel. As a result, the hydrogels have a lower swelling degree [10,12,13], which should indicate a lower sensitivity of the gel sensors. However, the swelling pressure of the gel is decisive for the sensitivity of such hydrogel-based pressure sensors. Here, the mechanical stability, in particular the Young’s modulus, is particularly relevant. Due to the higher linker concentration and the resulting enhanced number of crosslinking points, the Young’s modulus will be increased [14]. This leads to a better mechanical stability in the sensor application [15].
All alcohol sensors were highly sensitive to the ethanol concentration over a wide measuring range, from 0 up to 50 vol% ethanol. The sensor characteristic in the investigated measuring range was almost linear. However, both sensors showed different sensitivity values (4.58 and 2.26 mV/vol% ethanol). We assume that this was caused by inhomogeneities during the polymerization of the gel, that lead to areas with higher or lower monomer concentration in the solution and, hence, to a locally varying number of crosslinking points, whereby the elastic properties can vary [14]. The manual assembly of the sensor also influences the sensitivity. If the hydrogel is not placed centrally in the cavity of the pressure sensor or if the gel moves during swelling, force transmission to the bending plate of the pressure sensor can be changed and, thus, influence the sensor signal. In order to minimize this effect, more precise assembly methods should be used for industrial applications. At the moment, each hydrogel-based sensor has to be calibrated individually due to these large deviations in sensitivity.
3.2. Cross-Sensitivity to Salt Concentrations
In alcoholic beverages, there are many different ions due to the used drinking water. In the brewing industry, the addition of carbon dioxide has also an influence on the ionic strength of the sample. However, the ionic concentration in the sensor application could also have a significant influence on the swelling properties depending on the chemical composition of the hydrogels [16,17,18]. This could influence, negatively, the ethanol measurement. For this reason, the influence of the ionic strength was tested with different sodium chloride solutions (Figure 5). With increasing salt concentration, the hydrogel swelled significantly. Sivanantham and Tata investigated the swelling properties and the polymer–solvent interaction parameter of polyacrylamide hydrogels in sodium chloride solutions [19]. The polymer–solvent interaction parameter decreased with increasing the salt concentration. This led to a decrease in polymer–polymer affinity due to electrostatic double-layer formation of the sodium and chloride ions with the polar groups of poly acrylamide (C=O and C–N). Due to the increasing salt concentration and the resulting decrease of polymer–polymer interactions, poly acrylamide swells in sodium chloride solutions [19]. Similar effects can also be expected with other ionic species.
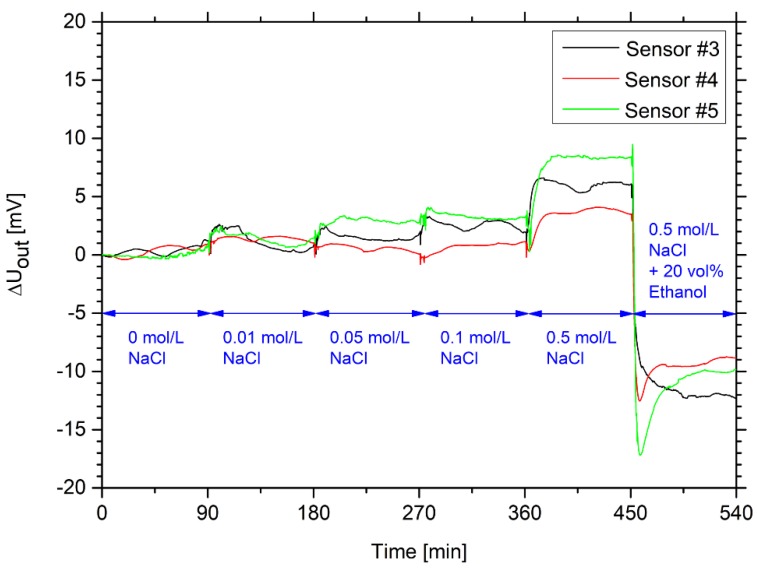
Figure 5.
Time-dependent change of the output voltage for three hydrogel-based sensors in ethanol-free solutions with different values of the ionic strength, in a range from 0.01 to 0.5 mol/L. To underline the ethanol sensitivity of the sensors, a solution with 20 vol% ethanol and 0.5 mol/L sodium chloride was measured at the end of the experiment.
In the sensor measurements, a significant swelling of the hydrogels was detected for salt concentration with equal or more than 50 mmol/L sodium chloride. For these sensors, baseline noise was determined of maximum 1 mV. This uncertainty of measurement is significantly influenced due to the pulsation of the used peristaltic pump to generate a constant flow rate. This uncertainty could be minimized by using a low-pulsation gear pump [20]. Other noise sources, like air bubbles in the sensor system due to dissolved gases in the liquid, can also influence the measuring uncertainty. Other uncertainty factors, such as the background noise of the pressure sensor itself, can be neglected.
In general, drinking water contains many different cations and anions. In practice, for comparing the results of samples with different ion species, the total equivalent concentration is usually used in drinking water analytics. Here, a differentiation was made between the cation- and anion-equivalent concentration, respectively. For example, the cation-equivalent concentration could be calculated as the sum of the product of the absolute value of the charge of a cation species zi,cat and its concentration ci,cat over all cation species Ncat in the sample (Equation (1)). The total anion-equivalent concentration was determined similarly [21,22]. The total anion-equivalent concentration must be equal to the total cation-equivalent concentration due to the electron neutrality in the solution [22,23].
| (1) |
In 2018, drinking water in Dresden (Germany) had total cation- and anion-equivalent concentrations between 2.4 and 6.8 mmol/L [24]. In case of the tested sodium chloride solutions, these values corresponded directly to the concentration of the salt solution. The lowest tested salt concentration (10 mmol/L) contained up to four times higher total ion-equivalent concentration in comparison to drinking water. At this concentration; however, no significant change in output voltage could be observed. Therefore, the cross-sensitivity of the ions in drinking water could be neglected.
3.3. Cross-Sensitivity to pH Value
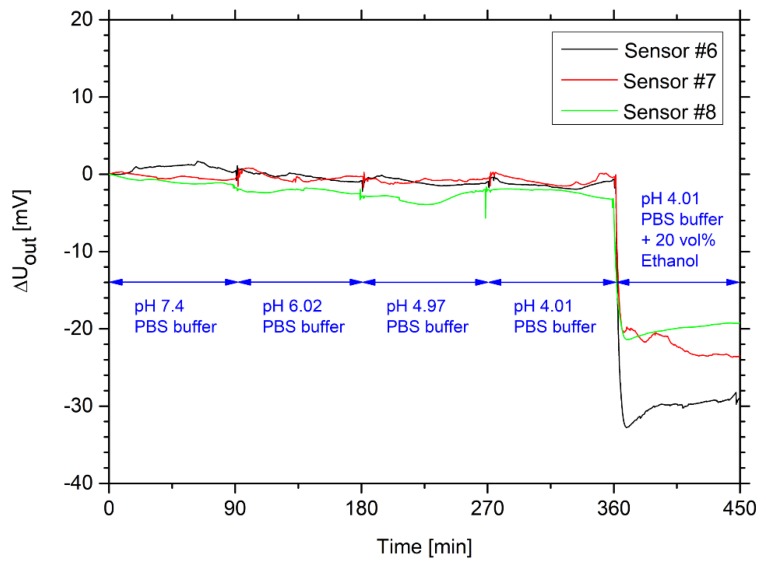
Many different alcoholic beverages have often an acidic pH value. For example, due to the addition of carbonic acid in beer, the pH value of beer lies usually in a range between pH 4 and 4.5 [25,26]. Sweet liquors have a pH value in a range from pH 3.3 to 3.9, and strong alcoholic drinks in a range from pH 6.5 to 6.9 [27]. However, most hydrogel systems show an influence of the pH value on the swelling behavior [28,29,30]. Therefore, the pH sensitivity of hydrogel-based ethanol sensors was tested in an acidic pH range from pH 7.4 to 4.01 (Figure 6).
Figure 6.
Time-dependent change of the output voltage for three hydrogel-based sensors in ethanol-free PBS solution with different pH values in a range from pH 7.4 to 4.01. To underline the ethanol sensitivity of the sensors, a 20 vol% ethanol solution with PBS buffer, pH 4.01, was measured at the end of the experiment.
All sensors showed almost no cross-sensitivity to pH in this range. The baseline noise of less than 1 mV was caused by the same reason as described in Section 3.1. Polyacrylamide is a neutral polymer [31]. In comparison to other pH-sensitive hydrogels, this polymer system has no charged functional groups. This eliminates the repulsive interactions of charged side groups that often explain the pH-dependent swelling of hydrogels [32,33,34]. As a result, neutral polyacrylamide hydrogels do not swell as a function of the pH value. Nesrinne and Djamel have studied the pH-dependent swelling properties of polyacrylamide hydrogels and found no significant influence in the range from pH 2 to 10 [35]. Therefore, the influence of the pH value for the ethanol sensor measurement could also be neglected.
3.4. Calibration Curves and Measurement of a Vodka Sample
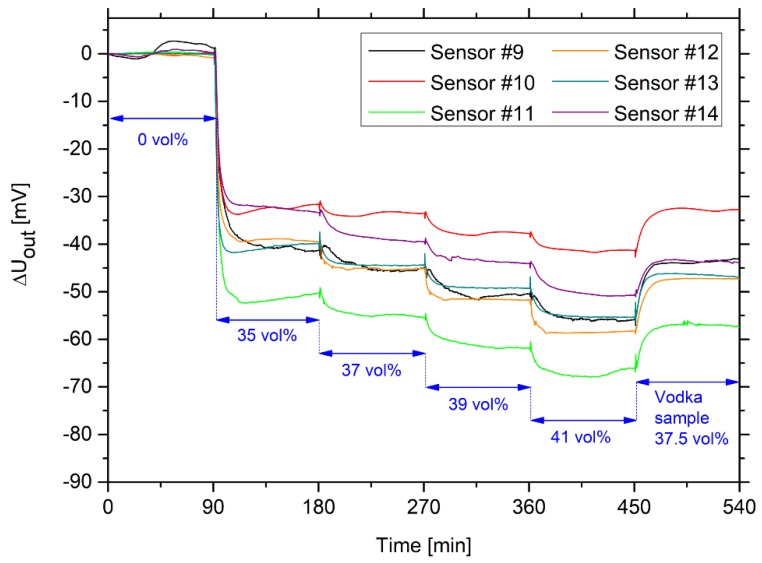
The potential of hydrogel-based ethanol sensors for industrial applications was demonstrated exemplarily by determining the alcohol concentration in a vodka sample (“Wodka Gorbatschow”, Henkell & Co., Sektkellerei KG, Wiesbaden, Germany) with a given value of 37.5 vol% ethanol. For the preparation of calibration curves, ethanol–water mixtures with a known ethanol concentration and, furthermore, the vodka sample were prepared and measured with the sensors (Figure 7).
Figure 7.
Time-dependent change in output voltage for six hydrogel-based sensors in ethanol–water mixtures with defined ethanol concentrations (between 35 and 41 vol% ethanol) for the preparation of calibration curves. At the end of the experiment, a vodka sample (“Wodka Gorbatschow” with 37.5 vol% ethanol) was measured to calculate the ethanol concentration of the vodka using the calibration curves.
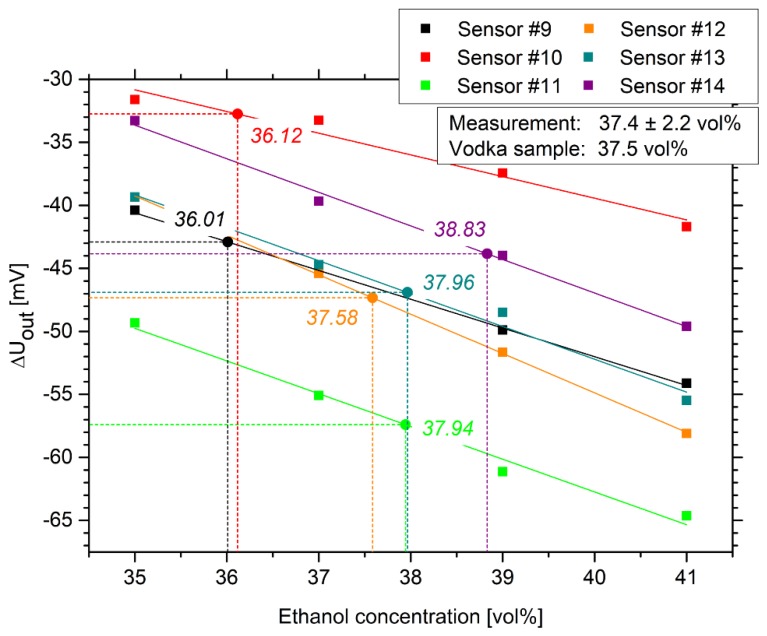
Based on these measurements, an individual calibration curve was prepared for each sensor. Each sample was measured for 90 min until the output voltage was nearly constant. The change in output voltage was plotted as a function of the ethanol concentration. Based on the fitted linear regression curves, the corresponding ethanol concentrations were determined for the measured vodka sample. The measured ethanol concentration is given for each sensor in Figure 8. Furthermore, the uncertainty in measurements was calculated according to the ISO/IEC Guide 98-3: “Guide to the expression of uncertainty in measurement” (GUM) [36]. After the calculation of the mean value and the standard deviation of the determined ethanol concentrations, the standard deviation was then multiplied by the coverage factor k = 2 to ensure a confidence probability of 95%. The results were given as the mean value plus-minus the doubled standard deviation in Figure 8.
Figure 8.
Calibration curves for the hydrogel-based ethanol sensors used for the determination of the ethanol concentration of a vodka sample (“Wodka Gorbatschow” with 37.5 vol% ethanol).
All tested sensors also had a linear calibration curve in the used measuring range, as shown in Figure 4. The sensitivity (see Appendix B) of the ethanol sensors was between 1.72 and 3.13 mV/vol% ethanol. The ethanol concentration of the vodka sample was determined as 37.4 vol% within a standard deviation of 2.2 vol%. According to the manufacturer, the investigated vodka sample has an ethanol concentration of 37.5 vol% ethanol, which is almost identical to the sensor measurements. As shown in Section 3.2 and Section 3.3, the pH value and the salt concentration of the sample did not influence the sensor measurement significantly.
As the measurement results show, hydrogel-based ethanol sensors could be interesting for the usage of ethanol detection in alcoholic beverages. This is particularly due to their advantages, namely the fast sensor response, the low-cost of the sensors, their inline capability, their low cross sensitivities to the pH value or salt concentrations, their low detection limits, and their reliable detection of the ethanol concentration in a vodka sample. Only the measurement uncertainty of the sensors needs to be improved for reliable detection in industrial application. Here, an automated sensor fabrication instead of a manual assembly should lead to a significant improvement of the measurement uncertainties.
3.5. Limit of Detection (LoD) and Limit of Quantification (LoQ)
During the measurements, it was possible to detect concentration changes of down to 2 vol% ethanol for all sensors. This means that the limit of detection (LoD) and the limit of quantification (LoQ) must be below this value. For the determination of the detection and quantification limit in detail, the German standard specification DIN 32654: “Chemical analysis—Decision limit, detection limit and determination limit under repeatability conditions—Terms, methods, evaluation” [37] was used.
The LoD is defined as the lowest analyte concentration that can be detected in the sample. Based on the LoD; however, only a qualitative statement can be made as to whether the solution contains the analyte or not. For a quantitative analysis, the LoQ can be used which is the lowest analyte concentration at which a certain measuring value can be specified. According to DIN 32654, the LoD and the LoQ can be calculated as:
| (2) |
where sL is the standard deviation of the sample without the analyte and b the slope of the calibration curve (sensitivity). The factor f was calculated individually for the LoD (fLoD = 3.30) and LoQ (fLoQ = 5.90), respectively. The detailed calculations for these factors according to DIN 32654 are shown in Appendix B.
The LoD lay, for all sensors, in a range from 0.06 to 0.65 vol% ethanol, whereas the LoQ amounted to 0.10 to 1.17 vol% ethanol. An exception was Sensor #1, which had a significantly higher LoD (2.11 vol% ethanol) and LoQ (3.78 vol% ethanol), respectively. However, as shown in Figure 7, changes in the ethanol concentration of 2 vol% could already be resolved. This means that the LoQ and also the LoD must be lower than 2 vol%. For the determination of the LoD and LoQ, respectively, the signal noise of the sample without ethanol plays a decisive role. Different factors, like air bubbles due to dissolved gases in the sample, can be responsible for this larger deviation of this measurement, which increases the LoD and LoQ according to Equation (2). Like the measurement uncertainty, the LoD and the LoQ could also be decreased by automated sensor production.
4. Summary
In this work, we presented hydrogel-based piezoresistive ethanol sensors and tested their properties for a possible application for the detection of ethanol in alcoholic beverages. The sensor based on the ethanol-sensitive poly acrylamide is highly sensitive to typical ethanol concentrations in alcoholic drinks in the range of up to 50 vol% ethanol. Even small changes in the alcohol concentration can be detected due to the low LoD and LoQ, respectively. Factors like salt concentration or pH value of the sample have no significant influence for the sensor application. Furthermore, the ethanol concentration of a vodka sample (“Wodka Gorbatschow”) with a given value of 37.5 vol% ethanol was determined as 37.4 ± 2.2 vol% ethanol. In comparison to commercially available sensor systems and further sensor concepts in research and development (Table 1), hydrogel-based piezoresistive ethanol sensors could be an alternative for the determination of the ethanol concentration in alcoholic beverages.
Table 1.
Ethanol sensor concepts and their advantages and disadvantages—a comparison.
| Method | Advantages (+) and Disadvantages (−) | |
|---|---|---|
| Chromatographic methods [38,39] | (+) | Most sensitive and accurate method [40,41] |
| (−) | Very high acquisition and operating costs [40,41], especially for smaller companies | |
| (−) | Well-trained operator necessary due to difficult handling of the method [40] | |
| Optical sensors [42,43] | (+) | Wide fields of application due to large measuring ranges (2–50 vol% [42], 5–50 vol% [43]) |
| (−) | High LoD (1.5 vol% [42], 2 vol% [43]) | |
| (−) | Significant cross-sensitivity to pH [42] | |
| (−) | Dye leaching over time possible [41] | |
| Microbial [44] and enzymatic [45] biosensor | (+) | Measuring range: 0.05–5 mmol/L [44], 0.1–5 mmol/L [45], after dilution also usable for alcoholic beverages [44,45] |
| (−) | Microbial and enzymatic activity depends on different factors (e.g., temperature [44,45], pH [45]) | |
| (−) | Poor long-term stability due to loss of microbial and enzymatic activity over time [44,45] | |
| Hydrogel-based sensor (presented in this work) | (+) | Wide measuring range (up to 50 vol%) |
| (+) | Low LoD (0.060–0.56 vol%) | |
| (+) | No relevant salt or pH cross-sensitivity | |
| (+) | Low-cost sensor (~10€/Sensor) | |
| (+) | Small size, even more miniaturizable | |
| (+) | In-line process capability | |
| (−) | Measurement uncertainty must be improved | |
Acknowledgments
The authors would like to thank Ulrike Lehmann for bonding the numerous ethanol sensors and Yuanhe Cui for the sensor measurements.
Appendix A. Nomenclature of the Used Hydrogel-Based Ethanol Sensors
Various hydrogel-based ethanol sensors were used for different research topics. Therefore, all used sensor and their measuring applications are summarized in Table A1.
Table A1.
Overview of all hydrogel-based ethanol sensors including their measurement tasks.
| Sensors | Sensors Were Used for |
|---|---|
| Sensor #1–#2 | Determination of the measuring range (Section 3.1) |
| Sensor #3–#5 | Cross-sensitivity to different salt concentrations (Section 3.2) |
| Sensor #6–#8 | Cross-sensitivity to different pH values (Section 3.3) |
| Sensor #9–#14 | Preparation of calibration curves, measurement of vodka samples (Section 3.4), and calculation of the LoD and LoQ (Section 3.5) |
Appendix B. Determination of the Limit of Detection (LoD) and Limit of Quantification (LoQ) according to DIN 32654
Appendix B.1. Limit of Detection (LoD)
The detection limit is the lowest analyte concentration at which the analyte can be detected in the sample with a certain error probability for the Type I (α) and Type II (β) error. The LoD for a single measurement of the analytical sample can be estimated according to the following equation:
| (A1) |
Here tf;α is the quantile of the one-tailed Student’s t-test, f the degree of freedom (f = n − 1), α the probability for the Type I error, n the number of measurements of the sample without the analyte, sL the standard deviation of the sample without the analyte, and b the slope of the calibration curve (sensitivity).
In all cases, the error probability amounts to 5% (α = β = 0.05). Each analyte solution, including the solutions without ethanol, was measured for 90 min where the measurement signal was recorded every 10 s. However, the last measuring points must be neglected for the determination of the standard deviation sL due to sensor signal fluctuations during solvent changes. For this reason, only the first 500 measuring points (n = 500) were considered for the calculation. Due to the large number of measuring points, the degree of freedom can be simply equated with the number of measuring points (n ≈ f = 500). The quantile of the one-tailed Student’s t-test (α = 0.05, f = 500) is 1.648 [46]. Inserting the known values in Equation (A1) yields:
| (A2) |
The standard deviation sL and the slope of the calibration curve b for each sensor were determined by using the program Excel. Afterwards, the LoD was calculated for each sensor by using Equation (A2). The calculated results for the LoD as well as for the LoQ are presented in Table A2.
Appendix B.2. Limit of Quantification (LoQ)
The limit of quantification is the lowest analyte concentration at which a specific certain value with a certain error probability can be specified. Above the LoD but below the LoQ, the analyte was identified in the sample; however no analyte content may be specified. An indication of the content is only possible above the LoQ value. For a single measurement of the analytical solution, the LoQ can be estimated as:
| (A3) |
where k is the relative result uncertainty for characterization of the determination limit (e.g., 33,3% for k = 3), tf;α/2 the quantile of the two-tailed Student’s t-test, f the degree of freedom (f = n − 1), α the probability for the Type I error, n the number of measurements of the sample without the analyte, sL the standard deviation of the sample without the analyte and b the slope of the calibration curve (sensitivity).
The relative result uncertainty is freely selectable according to DIN 32654, but a value of 33.3% (k = 3) is frequently assumed. The quantile of the two-tailed Student’s t-test (α/2 = 0.05, f = 500) amounts to 1.965 [46]. The other values (α = β = 0.05, n = 500) remain the same compared to the calculation of the detection limit. After insertion in Equation (A3), it yields:
| (A4) |
Knowing the standard deviation sL and the slope of the calibration curve b, which have already been calculated to determine the LoD value, the LoQ can also be calculated by using Equation (A4). The resulting LoD and LoQ for all tested sensors are shown in Table A2.
Table A2.
Determination of the Limit of Detection (LoD) and Limit of Quantification (LoQ) values for all hydrogel-based ethanol sensors in Section 3.4.
| Sensor | sL [mV] | b [mV/vol%] | LoD [vol%] | LoQ [vol%] |
|---|---|---|---|---|
| Sensor #9 | 1.46 | 2.28 | 2.11 | 3.78 |
| Sensor #10 | 0.16 | 1.72 | 0.31 | 0.55 |
| Sensor #11 | 0.13 | 2.60 | 0.17 | 0.31 |
| Sensor #12 | 0.17 | 3.13 | 0.18 | 0.32 |
| Sensor #13 | 0.04 | 2.61 | 0.06 | 0.10 |
| Sensor #14 | 0.53 | 2.66 | 0.65 | 1.17 |
sL: standard deviation of the sample without the analyte, b: slope of the calibration curve (sensitivity).
Author Contributions
Conceptualization, J.E., M.G., and G.G.; investigation, J.E.; writing—original draft preparation, J.E.; writing—review and editing, J.E., M.G., and G.G.; visualization, J.E.; supervision, M.G. and G.G.
Funding
This research was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) within the Research Training Group “Hydrogel-based microsystems” (DFG-GRK 1865).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Wen G., Li Z., Choi M.M.F. Detection of ethanol in food: A new biosensor based on bacteria. J. Food Eng. 2013;118:56–61. doi: 10.1016/j.jfoodeng.2013.01.006. [DOI] [Google Scholar]
- 2.Lachenmeier D.W., Godelmann R., Steiner M., Ansay B., Weigel J., Krieg G. Rapid and mobile determination of alcoholic strength in wine, beer and spirits using a flow-through infrared sensor. Chem. Cent. J. 2010;4:5. doi: 10.1186/1752-153X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osorio D., Ricardo Pérez-Correa J., Agosin E., Cabrera M. Soft-sensor for on-line estimation of ethanol concentrations in wine stills. J. Food Eng. 2008;87:571–577. doi: 10.1016/j.jfoodeng.2008.01.011. [DOI] [Google Scholar]
- 4.Castellari M., Sartini E., Spinabelli U., Riponi C., Galassi S. Determination of Carboxylic Acids, Carbohydrates, Glycerol, Ethanol, and 5-HMF in Beer by High-Performance Liquid Chromatography and UV-Refractive Index Double Detection. J. Chromatogr. Sci. 2001;39:235–238. doi: 10.1093/chromsci/39.6.235. [DOI] [PubMed] [Google Scholar]
- 5.Guenther M., Wallmersperger T., Gerlach G. Piezoresistive Chemical Sensors Based on Functionalized Hydrogels. Chemosensors. 2014;2:145–170. doi: 10.3390/chemosensors2020145. [DOI] [Google Scholar]
- 6.Kumar S.S., Pant B.D. Design principles and considerations for the ‘ideal’ silicon piezoresistive pressure sensor: A focused review. Microsyst. Technol. 2014;20:1213–1247. doi: 10.1007/s00542-014-2215-7. [DOI] [Google Scholar]
- 7.Franke D., Binder S., Gerlach G. Performance of Fast-Responsive, Porous Crosslinked Poly(N-Isopropylacrylamide) in a Piezoresistive Microsensor. IEEE Sens. Lett. 2017;1:1500904. doi: 10.1109/LSENS.2017.2773626. [DOI] [Google Scholar]
- 8.Guenther M., Gerlach G., Wallmersperger T., Avula M.N., Cho S.H., Xie X., Devener B.V., Solzbacher F., Tathireddy P., Magda J.J., et al. Smart Hydrogel-Based Biochemical Microsensor Array for Medical Diagnostics. Adv. Sci. Technol. 2013;85:47–52. doi: 10.4028/www.scientific.net/AST.85.47. [DOI] [Google Scholar]
- 9.Schmidt U., Jorsch C., Guenther M., Gerlach G. Biochemical piezoresistive sensors based on hydrogels for biotechnology and medical applications. J. Sens. Sens. Syst. 2016;5:409–417. doi: 10.5194/jsss-5-409-2016. [DOI] [Google Scholar]
- 10.Erfkamp J., Guenther M., Gerlach G. Hydrogel-based piezoresistive sensor for the detection of ethanol. J. Sens. Sens. Syst. 2018;7:219–226. doi: 10.5194/jsss-7-219-2018. [DOI] [Google Scholar]
- 11.Richter A., Paschew G., Klatt S., Lienig J., Arndt K.-F., Adler H.-J. Review on Hydrogel-based pH Sensors and Microsensors. Sensors. 2008;8:561–581. doi: 10.3390/s8010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Çaykara T., Turan E. Effect of the amount and type of the crosslinker on the swelling behavior of temperature-sensitive poly(N-tert-butylacrylamide-co-acrylamide) hydrogels. Colloid Polym. Sci. 2006;284:1038–1048. doi: 10.1007/s00396-006-1478-7. [DOI] [Google Scholar]
- 13.Caykara T., Kiper S., Demirel G. Thermosensitive poly(N-isopropylacrylamide-co-acrylamide) hydrogels: Synthesis, swelling and interaction with ionic surfactants. Eur. Polym. J. 2006;42:348–355. doi: 10.1016/j.eurpolymj.2005.07.006. [DOI] [Google Scholar]
- 14.Okay O. General Properties of Hydrogels. In: Gerlach G., Arndt K.-F., editors. Hydrogel Sensors and Actuators: Engineering and Technology. Springer; Berlin/Heidelberg, Germany: 2010. pp. 1–14. [Google Scholar]
- 15.Anseth K.S., Bowman C.N., Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- 16.Guenther M., Kuckling D., Corten C., Gerlach G., Sorber J., Suchaneck G., Arndt K.-F. Chemical sensors based on multiresponsive block copolymer hydrogels. Sens. Actuators B Chem. 2007;126:97–106. doi: 10.1016/j.snb.2006.10.054. [DOI] [Google Scholar]
- 17.Guenther M., Gerlach G., Corten C., Kuckling D., Muller M., Shi Z., Sorber J., Arndt K.-F. Application of Polyelectrolytic Temperature-Responsive Hydrogels in Chemical Sensors. Macromol. Symp. 2007;254:314–321. doi: 10.1002/masy.200750846. [DOI] [Google Scholar]
- 18.Guenther M., Gerlach G., Wallmersperger T. Piezoresistive chemical sensors based on hydrogels. SPIE Proc. 7362 Smart Sens. Actuators MEMS IV. 2009:736218. doi: 10.1117/12.821335. [DOI] [Google Scholar]
- 19.Sivanantham M., Tata B.V.R. Swelling/deswelling of polyacrylamide gels in aqueous NaCl solution: Light scattering and macroscopic swelling study. Pramana. 2012;79:457–469. doi: 10.1007/s12043-012-0325-2. [DOI] [Google Scholar]
- 20.Guenther M. Anwendung Polymerer Funktionsschichten in Piezoresistiven Chemischen und Feuchtesensoren. TUDpress, Verl. der Wiss; Dresden, Germany: 2009. Dresdner Beiträge zur Sensorik. [Google Scholar]
- 21.Panteleit B., Hamer K., Kringel R., Kessels W., Schulz H.D. Geochemical processes in the saltwater–freshwater transition zone: Comparing results of a sand tank experiment with field data. Environ. Earth Sci. 2011;62:77–91. doi: 10.1007/s12665-010-0499-1. [DOI] [Google Scholar]
- 22.Tosca N.J., McLennan S.M., Lamb M.P., Grotzinger J.P. Physicochemical properties of concentrated Martian surface waters. J. Geophys. Res. 2011;116 doi: 10.1029/2010JE003700. [DOI] [Google Scholar]
- 23.Lizarralde I., Fernández-Arévalo T., Brouckaert C., Vanrolleghem P., Ikumi D.S., Ekama G.A., Ayesa E., Grau P. A new general methodology for incorporating physico-chemical transformations into multi-phase wastewater treatment process models. Water Res. 2015;74:239–256. doi: 10.1016/j.watres.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Drewag Netz GmbH Medianwerte Reinwasser der Wasserwerke von Januar bis Dezember 2018. [(accessed on 27 February 2019)]; Available online: https://www.drewag.de/wps/wcm/connect/drewag/6056fb2d-fbd0-4e7d-9ea1-54dc3711d285/Durchschnittswerte-Reinwasser-Wasserwerke.pdf?MOD=AJPERES&CVID=m49zF7V&CVID=m49zF7V.
- 25.Vanderhaegen B., Neven H., Verachtert H., Derdelinckx G. The chemistry of beer aging—A critical review. Food Chem. 2006;95:357–381. doi: 10.1016/j.foodchem.2005.01.006. [DOI] [Google Scholar]
- 26.Choi S., Lee J.-K., Shukla S., Kim M. Physiochemical properties and determination of biogenic amines in korean microbrewery beer products. J. Food Biochem. 2012;36:766–773. doi: 10.1111/j.1745-4514.2012.00670.x. [DOI] [Google Scholar]
- 27.Preedy V.R., editor. Comprehensive Handbook of Alcohol Related Pathology. Vol. 1. Elsevier; Amsterdam, The Netherlands: 2005. [Google Scholar]
- 28.Schulz V., Gerlach G., Guenther M., Magda J.J., Solzbacher F. Piezoresistive pH Microsensors Based on Stimuli-Sensitive Polyelectrolyte Hydrogels. Tm-Tech. Mess. Plattf. Für Methoden Syst. Anwend. Messtech. 2010;77:179–186. doi: 10.1524/teme.2010.0045. [DOI] [Google Scholar]
- 29.Trinh Q.T., Gerlach G., Sorber J., Arndt K.-F. Hydrogel-based piezoresistive pH sensors: Design, simulation and output characteristics. Sens. Actuators B Chem. 2006;117:17–26. doi: 10.1016/j.snb.2005.10.041. [DOI] [Google Scholar]
- 30.Sorber J., Steiner G., Schulz V., Guenther M., Gerlach G., Salzer R., Arndt K.-F. Hydrogel-Based Piezoresistive pH Sensors: Investigations Using FT-IR Attenuated Total Reflection Spectroscopic Imaging. Anal. Chem. 2008;80:2957–2962. doi: 10.1021/ac702598n. [DOI] [PubMed] [Google Scholar]
- 31.Neyret S., Candau F., Selb J. Synthesis in microemulsion and characterization of low charge density ampholytic terpolymers. Acta Polym. 1996;47:323–332. doi: 10.1002/actp.1996.010470802. [DOI] [Google Scholar]
- 32.Turan E., Çaykara T. Swelling and network parameters of pH-sensitive poly(acrylamide-co-acrylic acid) hydrogels. J. Appl. Polym. Sci. 2007;106:2000–2007. doi: 10.1002/app.26848. [DOI] [Google Scholar]
- 33.Erfkamp J., Guenther M., Gerlach G. Piezoresistive Hydrogel-Based Sensors for the Detection of Ammonia. Sensors. 2019;19:971. doi: 10.3390/s19040971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin X., Hsieh Y.-L. pH-responsive swelling behavior of poly(vinyl alcohol)/poly(acrylic acid) bi-component fibrous hydrogel membranes. Polymer. 2005;46:5149–5160. doi: 10.1016/j.polymer.2005.04.066. [DOI] [Google Scholar]
- 35.Nesrinne S., Djamel A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017;10:539–547. doi: 10.1016/j.arabjc.2013.11.027. [DOI] [Google Scholar]
- 36.International Organization for Standardization (ISO) International Electrotechnical Commission (IEC) Uncertainty of Measurement—Part 3: Guide to the Expression of Uncertainty in Measurement (GUM:1995) ISO and IEC; Geneva, Switzerland: 2008. ISO-IEC Guide 98-3 2008. [Google Scholar]
- 37.Deutsches Insitiut für Normung e.V. (DIN) Chemische Analytik-Nachweis-, Erfassungs- und Bestimmungsgrenze unter Wiederholbedingungen-Begriffe, Verfahren, Auswertung (Chemical analysis-Decision Limit, Detection Limit and Determination Limit Under Repeatability Conditions-Terms, Methods, Evaluation) DIN; Berlin, Germany: 2008. DIN 32645 2008-11. [Google Scholar]
- 38.Betz J.M., Nikelly J.G. Determination of Ethanol in Alcoholic Beverages by Liquid Chromatography Using the UV Detector. J. Chromatogr. Sci. 1987;25:391–394. doi: 10.1093/chromsci/25.9.391. [DOI] [PubMed] [Google Scholar]
- 39.Buckee G.K., Mundy A.P. Determination of Ethanol in Beer by Gas Chromatography (Direct Injection)—Collaborative Trial. J. Inst. Brew. 1993;99:381–384. doi: 10.1002/j.2050-0416.1993.tb01176.x. [DOI] [Google Scholar]
- 40.Wiśniewska P., Śliwińska M., Dymerski T., Wardencki W., Namieśnik J. Application of Gas Chromatography to Analysis of Spirit-Based Alcoholic Beverages. Crit. Rev. Anal. Chem. 2015;45:201–225. doi: 10.1080/10408347.2014.904732. [DOI] [PubMed] [Google Scholar]
- 41.Petrova S., Kostov Y., Jeffris K., Rao G. Optical Ratiometric Sensor for Alcohol Measurements. Anal. Lett. 2007;40:715–727. doi: 10.1080/00032710601017847. [DOI] [Google Scholar]
- 42.Mohr G.J., Citterio D., Spichiger-Keller U.E. Development of chromogenic reactands for optical sensing of alcohols. Sens. Actuators B Chem. 1998;49:226–234. doi: 10.1016/S0925-4005(98)00132-4. [DOI] [Google Scholar]
- 43.Mohr G.J., Lehmann F., Grummt U.-W., Spichiger-Keller U.E. Fluorescent ligands for optical sensing of alcohols: Synthesis and characterisation of p-N,N-dialkylamino-trifluoroacetylstilbenes. Anal. Chim. Acta. 1997;344:215–225. doi: 10.1016/S0003-2670(97)00113-X. [DOI] [Google Scholar]
- 44.Rotariu L., Bala C., Magearu V. New potentiometric microbial biosensor for ethanol determination in alcoholic beverages. Anal. Chim. Acta. 2004;513:119–123. doi: 10.1016/j.aca.2003.12.048. [DOI] [Google Scholar]
- 45.Kitagawa Y., Kitabatake K., Kubo I., Tamiya E., Karube I. Alcohol sensor based on membrane-bound alcohol dehydrogenase. Anal. Chim. Acta. 1989;218:61–68. doi: 10.1016/S0003-2670(00)80282-2. [DOI] [Google Scholar]
- 46.Schiefer H., Schiefer F. Statistik für Ingenieure: Eine Einführung mit Beispielen aus der Praxis. Springer; Wiesbaden, Germany: 2018. Lehrbuch. [Google Scholar]