Abstract
Background:
Healthy functioning relies on a variety of perceptual, cognitive, emotional, and behavioral abilities that are distributed throughout the normal population. Variation in these traits define the wide range of neurodevelopmental (NDD) and neuropsychiatric (NPD) disorders. Here, we introduce a new measure for assessing these traits in typically developing children and children at risk for NDD and NPD from age 2 to 18 years.
Method:
The Childhood Oxford-Liverpool Inventory of Feelings and Experiences (CO-LIFE) was created as a dimensional, parent- report measure of schizotypal and psychotic traits in the general population. Parents of 2,786 children also self-reported on an adapted version of the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE-US).
Results:
The CO-LIFE resulted in continuous distributions for the total score and for each of three factor analytically-derived subscales. Item response theory (IRT) analyses indicated strong reliability across the score range for the O-LIFE-US and the CO-LIFE. Internal consistency and test-retest reliability were high across all scales. Parent-child intraclass correlations were consistent with high heritability. The scales discriminated participants who reported a lifetime psychiatric diagnosis from those who reported no diagnosis. The O-LIFE-US and CO-LIFE scores correlated positively with the Social Responsiveness Scale 2 (SRS-2) indicating good convergent validity.
Conclusions:
Like the original O-LIFE, the O-LIFE-US and the CO-LIFE are valid and reliable tools that reflect the spectrum of psychiatric and schizotypal traits in the general population. Such scales are necessary for conducting family studies that aim to examine a range of psychological and behavioral traits in both children and adults and are well-suited for the Research Domain Criteria (RDoC) initiative of the NIMH.
Keywords: Dimensional psychiatric traits, schizotypy in children, parent-child psychiatric traits
Introduction
Despite a long history employing a categorical taxonomic system to psychiatric morbidity, scant evidence supports the validity of categorical approach (Insel et al., 2010; Kotov et al., 2017). It has been suggested that the symptoms that define neurodevelopmental (NDD) and neuropsychiatric disorders (NPD) may instead be best conceptualized as variations of quantitative dimensions of sensory, perceptual, cognitive, emotional, social, and behavioral domains that are distributed throughout the general population (Hengartner & Lehmann, 2017; Kotov et al., 2017). The ability to assess variation in such traits along a normal-pathological continuum - and across the life span - is critical for understanding and identifying risk and protective factors associated with NDD/NPD. Here, we introduce new dimensional, quantitative measures for assessing behavioral domains that are implicated in a wide range of NDD/NPD characterized by variations in sensory, perceptual, cognitive, emotional, and social functioning, including, but not limited, to psychotic disorders, mood disorders, and autism spectrum disorders (ASD).
Empirical evidence reveals considerable overlap in the frequency distributions between clinical and nonclinical samples on continuous, quantitative clinical traits once thought to be present only in NDD/NPD (Johns & Van Os, 2001; Kendler & Gardner, 1998). For example, traits characteristic of ASD have been shown to be distributed across the general population (Constantino & Todd, 2003). Even psychotic symptoms such as hallucinations and delusions are relatively common, appearing in some 12%−40% of the general population, and do not necessarily indicate clinical psychiatric morbidity (Johns & Van Os, 2001; Simonoff et al., 2008; Van Os, Linscott, Myin-Germeys, Delespaul, & Krabbendam, 2009; Van’t Wout, Aleman, Kessels, Larøi, & Kahn, 2004). Rather, psychiatric morbidity may be thought of as a ‘shift’ in the continuous distributions of NDD/NPD traits toward greater impairment, while maintaining considerable overlap with the population distribution (Gillberg & Fernell, 2014; Simonoff et al., 2008; Van Os et al., 2009).
Schizotypy refers to the personality continuum of psychotic features that extends into the general population (Van Os et al., 2009; Van’t Wout et al., 2004). A growing body of work has demonstrated that similar genetic and nongenetic factors (Ronald, 2015; Van Os et al., 2009; Zavos et al., 2014) and neural substrates (DeRosse et al., 2015; Ettinger, Meyhofer, Steffens, Wagner, & Koutsouleris, 2014; Evans et al., 2016; Lymer et al., 2006) contribute to schizotypal and psychotic traits across the entire normal-pathological spectrum. For example, structural brain volumes of regions associated with clinically-relevant psychotic experiences (hippocampus, amygdala, caudate, and middle temporal gyrus) are also linked to schizotypal experiences of typically developing children (Evans et al., 2016). In other work, we demonstrated that probands with genomic copy number variations (CNVs) that present risk for ASD and intellectual disability experience significant deficits in social and cognitive functioning relative to noncarrier first-degree relatives - even when diagnostic clinical thresholds are not met (Moreno- De-Luca et al., 2014). A large population-based study in Iceland reports that when control subjects who carry pathogenic CNVs that confer risk for schizophrenia are administered a battery of cognitive tests, their performance is intermediate between patients with schizophrenia and population controls (Stefansson et al., 2014). These studies demonstrate the critical importance of measuring behavioral phenotypes as quantitative dimensions when assessing normal variability in the general population, in terms of both neural correlates and the variable expressivity of genetic syndromes that present risk for NDD/NPD.
Capturing the normal-pathological continuum in the expression of psychotic/schizotypal traits presents significant measurement challenges. Assessment tools need to be sufficiently sensitive to register subtle variation across the whole continuum, in order to avoid floor and ceiling effects. Furthermore, given that schizotypal and psychotic- like experiences may emerge early in typical development, often persisting into adolescence and adulthood, it is necessary to develop measures that are developmentally sensitive across the life span. One of the most widely used measures of the schizotypal and psychosis continuum is the Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE; Mason, Claridge, & Jackson, 1995; Mason & Claridge, 2006), which was normed on typical adults (17–85 years). Validation studies of the O-LIFE have yielded varying results in terms of its structure, including two-factor (negative and positive symptoms), three-factor (negative, positive, and cognitive disorganization), and four-factor solutions (unusual sensory experiences, cognitive disorganization, introvertive anhedonia, and impulsive nonconformity (Mason & Claridge, 2006; Mason, Linney, & Claridge, 2005; see Dembńnska- Krajewska & Rybakowski, 2014; for a review). The empirical consensus favors a three- or four-factor solution over one- or two-factor models (See Fonseca-Pedrero, Ortuno-Sierra, Mason, & Muniz, 2015). The Schizotypy Traits Questionnaire (STA; Cyhlarova & Claridge, 2005) is an adaptation of the O-LIFE that assesses schizotypal traits in early adolescence and reveals that common childhood phenomena such as fantasy proneness are normally distributed and associated with other schizotypal traits (Sánchez-Bernardos & Avia, 2006). Although subtle markers of later-developing schizophrenia can be identified in seemingly healthy children (Isohanni et al., 2000; Jones, Rodgers, Murray, & Marmot, 1994; Niemi, Suvisaari, Haukka, & Lönnqvist, 2005), no quantitative measures have been normed and validated for use with preschool and early school-aged children, despite the importance of early detection of psychiatric conditions, and the ubiquitous presence of fantastical and magical thinking that characterizes early typical childhood.
We present here two, dimensional measures assessing variability in sensory/perceptual, cognitive, social, emotional, and other neurobehavioral traits across the normal-pathological continuum. We adapted the short form of the O-LIFE (Mason & Claridge, 2006; Mason et al., 2005) for use in the United States. We then created a parallel version for assessing schizotypal and psychotic features in children ranging in age from 2 to 18 years. These measures: (a) allow for parallel comparison of children’s and parents’ behaviors that are critical for family pedigree studies, and that will help determine the true impact of genetic variants; (b) identify prodromal symptoms that predict increased risk of later morbidity; (c) enable monitoring of the natural history of schizotypal and other psychotic and psychiatric traits from infancy through adulthood, in a developmentally sensitive manner, and (d) capture the full range of psychotic-like features, from behaviors that represent normal - and even adaptive - aspects of development to the full manifestation of symptoms characteristic of NDDs/ NPDs. Establishing valid and reliable dimensional measures lies at the core of the NIMH Research Domain Criteria (RDoC; Cuthbert & Insel, 2013) initiative, providing an avenue for establishing reliable biomarkers of key dimensional constructs that underlie a wide range of NDD/NPD in children and adults. The measures presented here aim to serve the RDoC and other, similar empirical initiatives.
Materials and methods
Subjects
Participants were recruited through Survey Sampling International (SSI), a scientific survey company that specializes in recruiting representative samples of the US demographic. Participants were 2,786 adults (mean age = 40.4 years, SD = 9.3, range 18–81 years; 1,013 males, 1,773 females) with at least one child ranging in age from 2 years to 17 years 11 months. Offspring of these participants were 1,453 males and 1,299 females (mean age = 10 years 2 months). Demographic data were highly consistent with the general US population for race/ethnicity as well as education and income (Table 1), with a moderate and statistically significant skewing toward greater representation of participants at the lowest income level (<$10,000.00 per annum).
Table 1.
Comparison of socioeconomic demographics between survey participants and National Statistics
| Race/Ethnicity | Survey (%) | 2010 US Censusa (%) |
|---|---|---|
| White | 77.8 | 72.4 |
| African American | 11.0 | 12.6 |
| Hispanic/ Latino | 10.4 | 16.4 |
| Asian | 4.4 | 4.8 |
| Asian-Pacific Islander | 1.1 | 0.2 |
| Native American | 1.8 | 0.9 |
| Other/missing | 0.8 | |
| Total Household Income | Survey (%) | 2014 Congressional Researchb (%) |
| <$10,000 | 22.0 | 7.3 |
| $10,000−$19,999 | 10.4 | 11.5 |
| $20,000−$29,999 | 11.3 | 10.9 |
| $30,000−$39,999 | 12.1 | 10.0 |
| $40,000−$49,999 | 9.7 | 8.9 |
| $50,000−$59,999 | 9.4 | 7.6 |
| $60,000−$69,999 | 5.9 | 6.8 |
| $70,000−$79,999 | 5.0 | 5.9 |
| $80,000−$89,999 | 3.0 | 4.9 |
| $90,000−$99,999 | 3.0 | 4.0 |
| $100,000−$149,999 | 5.5 | 12.4 |
| $150,000 or more | 2.6 | 9.5 |
Measures
The Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE; Mason & Claridge, 2006; Mason et al., 1995, 2005) short form was adapted to include minor conceptual, spelling, and grammatical changes that reflect US English language conventions. We converted the dichotomous response scale to a 5-point Likert scale (Appendix S1). Next, we developed a companion parent-report measure for assessing schizotypal, psychotic, mood, and other neurobehavioral traits in children and adolescents: the Childhood O-LIFE (CO-LIFE; Appendix S2). For each item on the O-LIFE-US, we constructed a comparable CO-LIFE item, cast in a developmental context so as to reflect both normative and potentially symptomatic traits. The items include a range of sensory and perceptual experiences, cognitive disorganization, attention, behavioral control/impulsivity, mood, and social reciprocity.
Both the O-LIFE-US and the CO-LIFE contain 43 items. The measures were formatted for online administration and automated scoring using the Qualtrics Research Suite (Qualtrics, Provo, UT). Raw to t-score conversion tables are available online (Appendix S3). Parents completed a demographics form, and a subset of participants (n = 411) self-reported on the adult Social Responsiveness Scale 2 (SRS 2) and completed the SRS 2 school age or preschool parent-report form for their children (n = 115). We chose the SRS-2 because it is a quantitative measure designed to capture dimensions of symptoms as they manifest in the general population. Recent work with the SRS-2 (Frazier et al., 2014) reports a five-factor solution (Emotion Recognition, Social Avoidance, Interpesonal Relatedness, Insistence on Sameness, and Repetitive Mannerisms) which allows for a comparison of the various scales of the SRS, and those of the O-LIFE-US and the CO-LIFE that resulted from factor analyses reported below.
Procedures
Eligible participants were contacted by SSI and invited to complete the online measures. A primary parent responder self-reported on the O-LIFE-US, then completed the CO-LIFE for their child(ren). A second parent also had the option of self-reporting on the O-LIFE-US. Approximately 3 weeks later, a subset of participants was re-contacted to either complete the O-LIFE-US and CO-LIFE a second time (n = 500) or to complete the SRS-2 adult and child forms (n = 500). Participants were randomly assigned to complete the SRS-2 or O-LIFE-US/CO-LIFE at Time 2 (counterbalanced for order). Participants completed the child forms and adult forms 1 week apart during Time 2 to avoid bias that might result from completing the measures in rapid succession. A maximum of 10% of missing data per participant was allowed.
Results
To evaluate the structure of the O-LIFE-US and COLIFE, principal components analysis (PCA) was conducted using IBM SPSS v24 and the resulting eigenvalues were compared with the upper 95% confidence interval from Horn’s Parallel Analysis (HPA; Glorfeld, 1995; Horn, 1965). After the number of components was selected, exploratory factor analyses were conducted using principal axis factoring and promax (oblique) rotation. Items with loading of ≥.30 were retained.
Initial PCA indicated that for both the O-LIFE-US and the CO-LIFE, six components had eigenvalues over Kaiser’s criterion of 1 and in combination explained 54.05% of the variance for the O-LIFE- US (Factor 1: 32.91%, Factor 2: 7.79%, Factor 3: 4.91%, Factor 4: 3.38%, Factor 5: 2.55%, Factor 6: 2.51%) and 58.77% of the variance for the CO-LIFE (Factor 1: 35.75%, Factor 2: 8.30%, Factor 3: 5.88%, Factor 4: 3.58%, Factor 5: 2.80%, Factor 6: 2.45%).
To provide a fine-grained analysis of reliability as a function of score level (conditional reliability across the latent trait), item response theory (IRT) analyses were conducted using graded response models implemented in Mplus v7.3 (Muthén & Muthén, 2007). IRT analyses indicate the reliability of the instruments for clinical purposes and the ability of scores to differentiate across the various levels of the scale. Separate analyses were conducted for the total scores and for each of the subscales of the O-LIFE- US and the CO-LIFE. Item response theory-derived information values can be converted to reliability coefficients with information values of 3, 5, and 10 reflecting reliability coefficients of .67, .80, and .90, respectively.
Mixture modeling analyses were conducted separately for parent and child ratings using Mplus v7.3. One to eight class solutions were examined with each of the three subscale t-scores as indicators. Optimal class number was determined by examining four fit statistics: Akaike information criterion (Akaike, 1987), Bayesian information criterion (Schwarz, 1978) sample-adjusted Bayesian information criterion (saBIC), and entropy. These statistics are useful for comparing the relative fit of models, with lower values of AIC, BIC, and saBIC indicating better fit and higher values of entropy indicating better fit. Entropy and the base rate of identified classes were given priority in identifying the most parsimonious solution.
These analyses indicated possible three- or four- factor solutions for both the O-LIFE-US and the COLIFE. In both cases, the fourth factor barely exceeded the 95% confidence interval from Horn’s parallel analysis. However, since the loadings for this fourth factor tended to be weak and cross-loaded on other factors, a three-factor solution was considered most robust and parsimonious. The three factors reflect cognitive disorganization/impulsivity (CDI), sensory perception/magical thinking (SPMT), and social anxiety/withdrawal (SAW). Pattern matrix loadings are presented in Appendices S4 and S5. IRT analyses indicated strong reliability across the vast majority of the score range for the full parent and child scales, with only slight trailing off of reliability for very low scores (Figure 1), suggesting excellent sensitivity across the scale range. Parent and child subscales showed good to excellent reliability (>0.80) from low average to very high scores with a slight decrease for low and very low scores. These results suggest that the total scores have excellent reliability for clinical purposes and that the subscales have adequate reliability for distinguishing average from high and very high scores. Reliability of these subscales for use in research is excellent.
Figure 1.
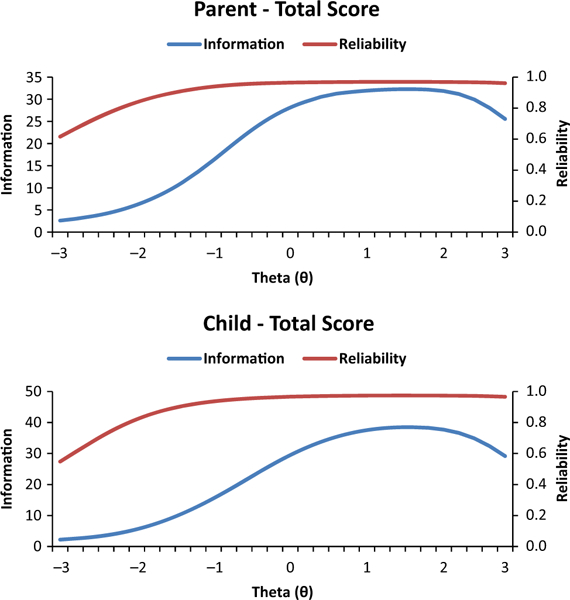
Information and conditional reliability across the full score range for the parent (O-LIFE-US) and child (CO-LIFE) total scores
Fit indices from mixture models continued to decrease through 8-class solutions (1–5 class solutions shown in Appendix S6). However, classification entropy was largest at the 2-class solution for both parent and child ratings. The two-class solutions for both scales included very large classes (class proportions of .90 for parent and .91 for child) with low scores on the CDI and SPMT subscales and small classes (.10 for parent and .09 for child) with high scores on these scales (Figure 1). The difference across classes for SAW subscale was minimal. Higher class solutions produced very small classes (<0.05) and added only groups with more or less extreme CDI or SPMT. Thus, the primary subgroup distinction for parent and child subscales was between elevated and normal range scores.
Reliability: internal consistency, split-half, and test- retest
Analysis of the frequency distributions of the summed scale and subscale scores of the O-LIFE-US revealed mostly normal kurtosis, with normal to positive skew. All but the SPMT subscale showed skewness and kurtosis indices of less than ± 1. The SPMT subscale resulted in the least normal distribution (skewness = 1.62, kurtosis = 2.99), which is not surprising given the nature of many of the items (e.g. hallucinations and delusions). Internal reliabilities (Cronbach’s alpha) for the O-LIFE-US subscales ranged from .74 (SAW) to .93 (CDI) with a total O-LIFE-US reliability of .94. Guttman Split-Half reliability coefficients ranged from 0.71 to 0.91. The test-retest ICCs were as follows: total t-score: ICC(429) = .88; CDI t-score: ICC(429) = .87; SPMT t-score: ICC(429) = .88; SAW t-score: ICC(429) = .79; all p < .0001.
Parallel subscales were created for the CO-LIFE. The SAW and CDI subscales were in the acceptable to excellent range on indices of skewness and kurtosis. The SPMT subscale resulted in the least normal distribution (skewness = 2.21, kurtosis, 5.58). Internal reliabilities (Cronbach’s alpha) ranged from .78 (SAW) to .95 (SPMT); total CO-LIFE scale reliability = 0.95. Guttman Split-Half reliability coefficients ranged from 0.80 (SAW) to 0.93 (CDI). The test-retest ICCs were as follows: total CO-LIFE t-score: ICC (267) = .91; CDI t-score: ICC(267) = .91; SPMT t-score: ICC(267) = .88; SAW t-score: ICC(267) = .88; all p < .0001.
Age effects were explored by comparing CO-LIFE and factor raw scores across the following three age groups (2–5 years, 6–12 years, and 13 years and older) and by child gender. The age group × gender interaction was significant only for the SAW raw score F(2,2746) = 3.43, p = .03. Main effect one-way ANOVAs for age group were as follows: Total CO-LIFE F(2,2749) = 3.07, p = .047, with post hoc tests (Tukey HSD) indicating that 6- to 12-year-old children have higher scores than the 2- to 5-year-old children (p = .04). ANOVA revealed an age effect on the CDI (F(2,2749) = 4.38, p = .013 on CDI, such that the 6- to 12-year-old group had higher scores on the CDI factor than both the younger and older groups. For SPMT, the overall ANOVA was significant, F(2,2749) = 3.66, p = .026, but none of the post hoc tests revealed differences between any two groups, though the 6–12 year olds had the highest means (p = .056 between the middle and oldest group). For SAW, the ANOVA was significant F (2,2749)= 69.54, p < .00001, with significant differences between all three age groups, ranging from the youngest, middle, and oldest age groups, progressively. Males had significantly higher scores than females on all four indices: CO-LIFE F(1,2750) = 39.84, p < .00001; CDI F(1,2750) = 25.28, p < .00001; SPMT F(1,2750) = 9.35, p = .002; SAW F (1,2750) = 104.31, p < .00001. Because of the magnitude of the gender differences, separate raw-to-t- score conversion tables are provided for males and females.
Preliminary tests of validity
Convergent validity.
Because the O-LIFE and CO-LIFE reflect behaviors and traits that are characteristic of ASD and other NDDs, including deficits in social reciprocity, attention, cognitive organization, and emotion regulation, etc., we expected shared variance between these two measures and SRS-2. The O-LIFE-US t-score shared significant variance with the SRS t-score (R2 = .48). Correlations between the O-LIFE-US and each of the five SRS factors ranged from r(411) = .14 (SAW with SRS Repetitive Mannerisms) to r(411) = .63 (CDI with SRS Insistence on Sameness). Mean Pearson correlation among all subscales was .45 or an average of 21% variance shared. For the CO-LIFE, total t-score correlated with total SRS (R2 = .50), with ranges from r(115) = .39 (SAW and SRS Repetitive Mannerisms) to r(115) = .67 (SAW and SRS Social Avoidance).
Discriminant validity.
In total, 35.8% of the adult sample reported that they had received at least one NPD or NDD clinical diagnosis. This is less than the 46.6% reported by the Epidemiological Catchment Area (ECA) survey and the National Comorbidity Survey (NCS; Kessler et al., 2005) and likely results from the fact that our survey included a subset of all the diagnostic categories-only those that were most relevant to the constructs of interest.
Adults who reported at least one NDD/NPD diagnosis (n = 945) differed from those who reported no diagnoses: F(1,2655) = 399.52, p = 6.47 × 10−83, Cohen’s d = .79; CDI t-score: F(1,2655) = 484.41, p = 9.32 × 10−99, Cohen’s d = .87; SPMT t-score: F (1,2655) = 147.35, p = 4.85 × 10−33, Cohen’s d = .47; SAW t-score: F(1,2655) = 59.87, p = 1.43 × 10−14, Cohen’s d = .31. Those indicating a lifetime clinical diagnosis were higher on all scales, with an average shift of +0.75 standard deviations (SDs) between individuals with and without lifetime clinical diagnosis.
Children who were reported to have a neurodevel- opmental or neuropsychiatric diagnosis (n = 679, or 25.7% of the sample) differed from those with no reported diagnoses on all scales: CO-LIFE total t- score: F(1,2637) = 654.28, p = 4.26 × 10−129, Cohen’s d = 1.08; CDI t-score: F(1,2637) = 696.85, p = 1.82 × 10−136, Cohen’s d = 1.11; SPMT t-score: F(1,2637) = 230.54, p = 5.56 × 10−50, Cohen’s d = .61; SAW t-score: F(1,2637) = 158.46, p = 2.49 × 10−35, Cohen’s d = .55. Children with a diagnosis scored 1.03 SDs higher on the total CO-LIFE than those with no diagnosis.
Children at higher risk (parent with a NDD/NPD diagnosis) scored significantly higher on all scales compared with children at lower risk (neither parent with a NDD/NPD diagnosis): CO-LIFE total t-score: F (1,2622) = 162.32, p = 4.05 × 10−36, Cohen’s d = .52; CDI t-score: F(1,2622) = 181.18, p = 5.53 × 10−40, Cohen’s d = .54; SPMT t-score: F(1,2622) = 72.56, p = 2.69 × 10−17, Cohen’s d = .33; SAW t- score: F(1,2622) = 25.91, p = 3.83 × 10−7, Cohen’s d = .21. Children at higher risk scored 0.51 SD higher than those at lower risk (Figure 2).
Figure 2.
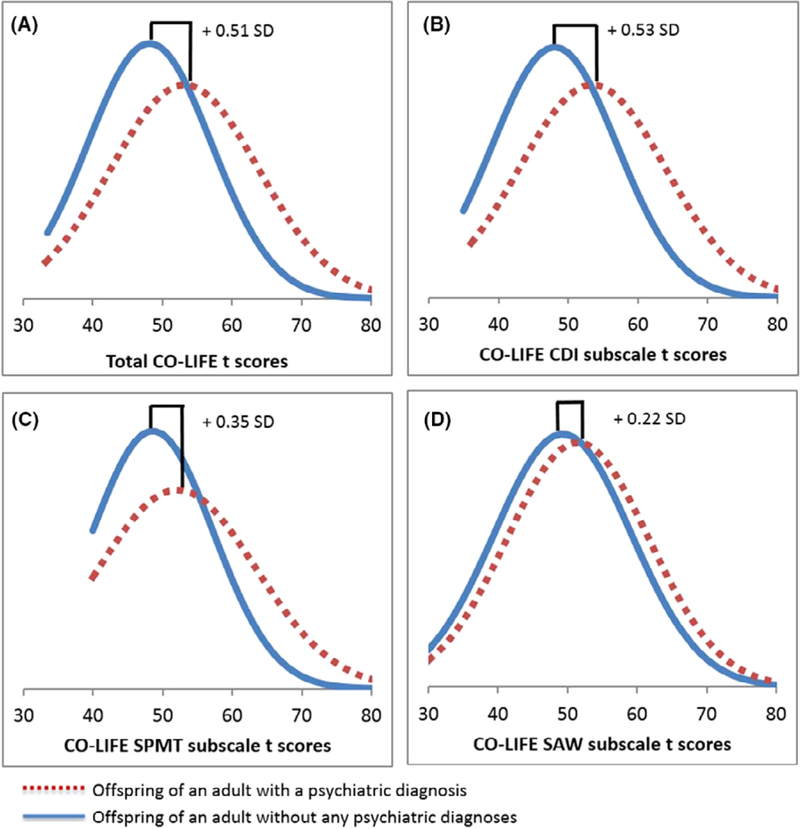
Shift plot comparing CO-LIFE total and subscale t-scores for offspring of adults with a diagnosis (higher risk, represented in dotted line) and offspring of adults without a diagnosis (lower risk, represented in solid line)
Finally, we examined group differences between clinical diagnoses, using clusters of diagnostic categories that share common features and have demonstrated high comorbidity rates. For adults, the clusters were the following: no diagnosis; depression/anxiety; bipolar/schizophrenia; autism/Asper- ger’s/PDD-NOS; ADD/ADHD; obsessive-compulsive disorder/tic disorders. For children, in addition to these clusters, developmental delay/intellectual disability, oppositional defiant/conduct disorders, and speech/language disorders were also present. Adult diagnostic cluster groups differed significantly on the total O-LIFE-US and all three subscales: total t-score: F(5,2652) = 98.29, p = 2.80 × 10−95; CDI t-score: F(5,2652) = 113.55, p = 5.16 × 10−109; SPMT t-score: F(5,2652) = 41.11, p = 7.21 × 10−41; SAW t-score: F(5,2652) = 15.28, p = 7.71 × 10-15. Post hoc tests indicated that the No Diagnosis cluster scored significantly lower than all other groups on the total O-LIFE-US and the CDI subscale, and lower than all other groups except ADD/ADHD on the SPMT and SAW subscale. ASD and bipolar/ schizophrenia clusters had highest scores for total, CDI, and SPMT subscales. For SAW subscale, although bipolar/schizophrenia cluster had the highest score followed by OCD/tic disorder, few of the comparisons were statistically significant. For detailed overview of the post hoc results, see Table 2.
Table 2.
Descriptives and post hoc comparisons for O-LIFE-US
| Total Score Mean (SD) |
CDI Score Mean (SD) |
SPMT Score Mean (SD) |
SAW Score Mean (SD) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Cluster | N | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 |
| No diagnosis | 1,714 | 47.33 | 47.13 | 48.25 | 48.97 | ||||||||||
| (8.66) | (8.6) | (8.91) | (9.85) | ||||||||||||
| ADD/ADHD | 39 | 52.55 | 53.74 | 51.3 | 51.3 | 49.18 | |||||||||
| (9.07) | (8.93) | (9.72) | (9.72) | (9.96) | |||||||||||
| Depression / anxiety | 693 | 53.48 | 53.97 | 51.85 | 51.85 | 51.56 | 51.56 | ||||||||
| (9.47) | (9.42) | (9.91) | (9.91) | (9.33) | (9.33) | ||||||||||
| OCD/tic | 62 | 57.57 | 57.53 | 57.53 | 55.59 | 55.59 | 52.35 | 52.35 | |||||||
| (10.2) | (10.41) | (10.41) | (12.16) | (12.16) | (9.74) | (9.74) | |||||||||
| Bipolar/ schizotypal | 137 | 60.00 | 60.32 | 60.32 | 56.71 | 53.64 | 53.64 | ||||||||
| (10.69) | (10.47) | (10.47) | (11.82) | (10.74) | (10.74) | ||||||||||
| ASD | 14 | 63.63 | 62.2 | 63.85 | 54.65 | ||||||||||
| (10.19) | (10.08) | (14.53) | (11.25) | ||||||||||||
Within each factor (color) means that are in different columns are significantly different from each other.
Significant differences in total CO-LIFE scores and all three subscales were also found between diagnostic clusters in children: total CO-LIFE t-score: F(8,2630) = 92.45, p = 1.20 × 10−135; CDI t-score: F(8,2630) = 95.17, p = 2.77 × 10−139; SPMT t-score: F(8,2630) = 38.48, p = 2.71 × 10−58; SAW t-score: F(8,2630) = 28.26, p = 1.58 × 10-42. The No Diagnosis cluster scored significantly lower than all other clusters on the overall CO-LIFE and the CDI subscale, and lower than all other clusters except ADD/ADHD on the SPMT and SAW subscale. The bipolar/schizophrenia cluster scored significantly higher than all other clusters on the overall CO-LIFE, CDI, and SPMT subscale. There was considerable overlap between most clusters on the SAW subscale, with the ASD/Asperger/PDD-NOS cluster scoring significantly higher than all other clusters. See Table 3 for a summary of these post hoc results.
Table 3.
Descriptives and post hoc comparisons for the CO-LIFE
| Total Score Mean (SD) |
CDI Score Mean (SD) |
SPMT Score Mean (SD) |
SAW Score Mean (SD) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Cluster | N | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 1 | 2 | 3 | 4 |
| No diagnosis | 1,960 | 47.46 | 47.42 | 48.37 | 48.64 | ||||||||||||
| (8.36) | (8.26) | (8.41) | (9.54) | ||||||||||||||
| ADD/ADHD | 160 | 53.95 | 55.21 | 49.98 | 50.76 | 50.76 | |||||||||||
| (8.93) | (10.34) | (9.64) | (9.64) | (9.64) | |||||||||||||
| ID/developmental delay | 59 | 55.3 | 55.3 | 55.63 | 54.28 | 52.3 | 52.3 | 52.3 | |||||||||
| (10.1) | (10.1) | (9.21) | (12.16) | (9.4) | (9.4) | (9.4) | |||||||||||
| Speech disorder | 119 | 57.46 | 57.46 | 57.05 | 57.05 | 54.79 | 52.38 | 52.38 | 52.38 | ||||||||
| (12.04) | (12.04) | (11.64) | (11.64) | (11.97) | (10.37) | (10.37) | (10.37) | ||||||||||
| Depression / anxiety | 122 | 57.93 | 57.93 | 57.41 | 57.41 | 55.43 | 52.97 | 52.97 | 52.97 | ||||||||
| (10.46) | (10.46) | (10.74) | (10.74) | (12.93) | (10.91) | (10.91) | (10.91) | ||||||||||
| OCD/tic | 43 | 59.64 | 59.79 | 55.56 | 54.99 | 54.99 | |||||||||||
| (9.86) | (9.46) | (11.86) | (9.71) | (9.71) | |||||||||||||
| ODD/conduct | 36 | 59.99 | 60.1 | 56.94 | 55.64 | ||||||||||||
| (9.22) | (10.52) | (11.02) | (7.79) | ||||||||||||||
| ASD | 102 | 60.71 | 60.93 | 57.03 | 55.97 | ||||||||||||
| (9.53) | (8.87) | (14.58) | (9.95) | ||||||||||||||
| Bipolar/schizotypal | 38 | 64.17 | 64.8 | 61.21 | 59.51 | ||||||||||||
| (10.48) | (10.96) | (12.15) | (9.3) | ||||||||||||||
Within each factor (different color), means that are in different columns differ significantly from each other. Standard deviations are in parentheses.
Parent-child intraclass correlations
Parent-child intraclass correlations (ICCs) were performed. ICCs are used to compare the strength of association between related pairs, such as sibling- sibling or parent-child dyads, on dimensional traits. ICCs1 were as follows: total t-score: ICC(2749) = .82, p < .0001; CDI t-score: ICC(2749) = .80, p < .0001; SPMT t-score: ICC(2749) = .83, p < .0001; SAW t- score: ICC(2749) = .63, p < .0001. To account for potential response bias resulting from parents completing the self- and child-report measures in rapid succession, subjects who were re-contacted for the test-retest reliability phase were administered the parent and child versions 1 week apart. The ICCs remained high and significant: total t-score: ICC (224) = .71, p < .0001; CDI t-score: ICC(224) = .68, p < .0001; SPMT t-score: ICC(224) = .68, p < .0001; SAW t-score: ICC(224) = .58, p < .0001.
Discussion
We presented data from a nationally representative US cohort on a new measure for assessing psychotic, schizotypal, and other psychiatric traits in children – the CO-LIFE, and an adaptation of an existing measure (the short form of the O-LIFE; Mason et al., 2005). These measures reflect a broad range of traits associated with neurodevelopmental (NDD) and neuropsychiatric (NPD) conditions that extend into the general population, making them excellent candidates for use in cohorts with family history of NDD/NPD, longitudinal research, and for studies aiming to characterize the variable expressivity of genetic variants that confer risk for NDD/NPD.
The O-LIFE-US and CO-LIFE demonstrated excellent range and variability to enable quantitative, dimensional analysis in clinical and nonclinical populations alike. These properties are necessary for studying group and individual differences, when traditional clinical measures would otherwise result in near-floor effects that attenuate relevant gene- brain-behavior links, particularly in studies of subclinical populations. Item response theory analyses support the identification of high levels of schizotypy using the total scores and the CDI and SPMT subscales with differentiation. The results also suggest that elevated SAW is not a core component of schizotypy as identified by the instrument, but rather reflects a distinct construct.
Both the O-LIFE-US and CO-LIFE demonstrated excellent internal consistency, split-half reliability, and test-retest reliability. Total scores for both instruments show very good reliability for clinical purposes and both the O-LIFE-US and CO-LIFE subscales have adequate reliability for distinguishing average from high and very high scores. Correlations between the adapted O-LIFE-US/CO-LIFE and the SRS-2 indicate convergent validity while offering sufficient unique variance to allow substantial discriminant validity.
Adult respondents who reported a lifetime psychiatric diagnosis (35.8%) reported higher scores (greater impairment) across all scales of the O- LIFE-US relative to those with no history of NDD/ NPD. Offspring of parents with a lifetime psychiatric diagnosis (25.7%) were also rated higher across all scales compared with those children whose parents reported no psychiatric history. Children with a reported diagnosis also had higher scores on all scales than children who had not received a diagnosis. The results demonstrate discriminant validity for identifying individuals with psychiatric disorders, as well as offspring who are at risk for psychiatric disorder. As clinical entities, psychotic disorders are generally diagnosed in early adulthood. However, as previously noted, traits such as hallucinations can appear in early childhood (Van Os et al., 2009). Despite that in the majority of cases, these psychotic-like traits in childhood are transitory and do not cause impairment, when exhibited to greater extent they may reflect prodromal psychotic symptoms (Poulton et al., 2000). Therefore, the ability to identify children with elevated schizotypal/psychotic traits is of particular importance. To date, however, few, if any measures, exist that are suitable for capturing the expression of schizotypal/psychotic traits in early childhood in a sensitive and developmentally appropriate manner. Because the norms of the CO-LIFE scales were established for children as young as 2 years, these measures will be useful when examining early/ prodromal psychiatric conditions and at-risk populations.
It is important to consider the work presented here in the context of alternative models in the field of developmental psychopathology, as well as models of schizophrenia and psychotic spectra. One longstanding developmental model is the ‘internalizing- externalizing’ framework of the Child Behavior Checklist (CBCL; Achenbach & Edelbrock, 1978, 1991) and its antecedent, the ‘thought- versus action-orientation’ approach (Zigler & Glick, 1986; Zigler & Phillips, 1961; Werner, 1948). These models characterize symptom expression along a developmental hierarchy, with thought-oriented symptoms (anxiety, depression) reflecting a more sophisticated cognitive-developmental process than action- oriented symptoms (impulsivity, aggression). How-ever, despite its comprehensive and robust evidence base, the CBCL has only few items dedicated to psychotic-like symptoms. We believe that the O- LIFE-US and CO-LIFE provide a fuller picture of the psychosis spectrum, while also capturing symptoms of mood and behavior that comprise the internalizing-externalizing/thought-action continua. More recently, the Hierarchical Taxonomy Of Psychopathology (HiTOP) model (Kotov et al., 2017) has emerged as a result of joint efforts of a number of experts in the field to address limitations of the traditional categorical diagnostic systems. Although it is beyond the scope of this article to provide a detailed overview of the model, it is important to highlight the fact that it combines co-occurring syndromes among six spectra: internalizing/negative affectivity, thought disorder/ psychoticism, disinhibited externalizing, antagonistic externalizing, detachment, and somatoform, each of which are characterized as dimensional. Future research would do well to explore the O-LIFE-US and CO-LIFE in the context of the HiTOP framework.
The three-factor solution we report is consistent with work addressing the broader psychosis spectrum that includes positive symptoms (unusual sensory-perceptual experiences and magical thinking [SMPT factor], negative symptoms (social anxiety/withdrawal [SAW factor] and cognitive disorganization/impulsivity [CDI factor], as well as those reported previously with the O-LIFE (Dem- bińnska-Krajewska & Rybakowski, 2014; Fonseca- Pedrero et al., 2015; Nuechterlein et al., 2004).
When participants were grouped into one of the diagnostic clusters, the O-LIFE-US and CO-LIFE scales differentiated the No Diagnosis group from all other groups, and aligned with our predictions: Symptoms that are most closely associated with psychosis (unusual sensory perceptions/magical thinking) were highest in participants with a reported history of schizophrenia or bipolar disorder in the childhood and adolescent sample. Symptoms most typical of ASD (social anxiety/ withdrawal) were highest in children diagnosed with ASD. Importantly, these findings and IRT analyses revealed that there is a significant range for all symptom types across multiple diagnostic groups. This is consistent with work noting symptom overlap and high comorbidity rates within and between neurodevelopmental and neuropsychiatric disorders, including those representing diverse genotypes (Gillberg, 2010; Moreno-De-Luca et al., 2013; van Os & Reininghaus, 2016).
Heritability estimates of psychotic disorders range from approximately 0.60 to 0.85 (Kendler, Prescott, Myers, & Neale, 2003; Keshavan, Diwadkar, Montrose, Rajarethinam, & Sweeney, 2005; Lichtenstein et al., 2006, 2009; Szatkiewicz et al., 2014) and studies on typically developing monozygotic and dizygotic twins suggest that subclinical psychotic experiences are also genetically influenced (15%- 59%; Zavos et al., 2014). The significant parent- child ICCs on the O-LIFE-US and CO-LIFE are consistent with the heritability of these traits indicating the utility of these scales for family studies. These scales may be particularly useful for examining the variable expressivity of genetic variations that confer risk for a range of psychotic experiences. Comparing probands with noncarrier relatives requires multidimensional, quantitative assessments of proband and family members that are sensitive across the scale range.
As we move away from simplistic dichotomies that obscure the biological realities of comorbidity (Laurens, Hobbs, Sunderland, Green, & Mould, 2012; Owen, 2014) and variable expressivity, quantitative measurement across a range of constructs will be necessary. This approach is reflected in the recent RDoC (Cuthbert & Insel, 2013) initiative, which emphasizes the importance of assessing dimensional, continuous traits in favor of a traditional psychiatric taxonomy. The scales presented here are excellent candidates for such analysis, particularly for the RDoC constructs of Negative Valence Systems, Cognitive Systems, and Social Processes. We acknowledge, however, the inherent limitation of self-reported diagnoses and of subjective reports in general. Thus, it will be important for future research to provide more in-depth comparisons supplemented with well-validated dimensional measures of NDDs/NPDs, such as the CBCL, Strengths and Difficulties Questionnaire, Brief Psychiatric Scale, various diagnostic interviews, and other indices of schizotypy. Further, we acknowledge that dimensionality does not preclude the existence of meaningful diagnostic thresholds. Even when measured as quantitative dimensions, traits such as schizotypy may reach clinical significance when certain thresholds are met (Lenzen- weger, 2015; Van Os et al., 2009). Still, as the fields of psychology, psychiatry, genetics, and neuroscience work to gain a comprehensive, integrated understanding of gene-brain-behavior links, dimensional approaches (such as RDoC) will likely play a prominent role in refining and/or validating long-held traditions in clinical practice and in translational research.
Supplementary Material
The Oxford-Liverpool inventory of feelings and experiences – United States.
The childhood Oxford-Liverpool inventory of feelings and experiences.
Raw to t-score conversion tables for the CO-LIFE and the O-LIFE-US.
Rotated factor matrix for the O-LIFE-US.
Rotated factor matrix for the CO-LIFE.
Fit indices for mixture modeling analyses of parent (O-LIFE-US) and child (CO-LIFE) ratings.
Key Points.
The Childhood Oxford-Liverpool Inventory of Feelings and Experiences (CO-LIFE) - is a new measure for dimensional assessment of schizotypal and psychotic traits in children from 2–18 years of age.
We adapted the Oxford-Liverpool Inventory of Feelings and Experiences as a companion measure to the CO-LIFE enabling parent-child comparisons of the broad psychosis spectrum.
The O-LIFE-US and CO-LIFE demonstrate excellent validity, reliability, clinical sensitivity and specificity.
The O-LIFE-US and CO-LIFE capture the broad spectrum of schizotypal and psychotic-like symptoms as they manifest in the course of typical development, as well as in neurodevelopmental and neuropsychiatric disorders.
The O-LIFE-US and CO-LIFE will be useful for family genetic studies to assess neurodevelopmental and neuropsychiatric risk.
Acknowledgements
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under grant number (RO1MH074090). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared
Note
1. Note that in SPSS, exact p-values are not provided for ICC.
References
- Achenbach TM, & Edelbrock CS (1978). The classification of child psychopathology: A review and analysis of empirical efforts. Psychological Bulletin, 85, 1275–1301. [PubMed] [Google Scholar]
- Achenbach TM, & Edelbrock C (1991). Child behavior checklist. Burlington, VT: University of Vermont. [Google Scholar]
- Akaike H (1987). Factor analysis and AIC. Psychometrika, 52, 317–332. [Google Scholar]
- Constantino JN, & Todd RD (2003). Autistic traits in the general population: A twin study. Archives of General Psychiatry, 60, 524–530. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, & Insel TR (2013). Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine, 11, 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyhlarova E, & Claridge G (2005). Development of a version of the Schizotypy Traits Questionnaire (STA) for screening children. Schizophrenia Research, 80, 253–261. [DOI] [PubMed] [Google Scholar]
- Dembińnska-Krajewska D, & Rybakowski J (2014). The Oxford-Liverpool Inventory of Feelings and Experiences (O- LIFE) schizotypy scale in psychiatry. Archives of Psychiatry and Psychotherapy, 2, 15–22. [Google Scholar]
- DeRosse P., Nitzburg GC., Ikuta T., Peters BD., Malhotra AK., & Szeszko PR. (2015). Evidence from structural and diffusion tensor imaging for frontotemporal deficits in psychometric schizotypy. Schizophrenia Bulletin, 41, 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell CK (2014). The distribution of household income and the middle class (Congressional Research Service; Report, 7–5700). [Google Scholar]
- Ettinger U, Meyhöfer I, Steffens M, Wagner M, & Kout- souleris N (2014). Genetics, cognition, and neurobiology of schizotypal personality: A review of the overlap with schizophrenia. Frontiers in Psychiatry, 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Michael AM, Uljarević M, Lusk LG, Buirkle JM, & Moore GJ (2016). Neural substrates of a schizotypal spectrum in typically-developing children: Further evidence of a normal-pathological continuum. Behavioural Brain Research, 315, 141–146. [DOI] [PubMed] [Google Scholar]
- Fonseca-Pedrero E, Ortuño-Sierra J, Mason O, & Muñiz J (2015). The Oxford-Liverpool Inventory of Feelings and Experiences short version: Further validation. Personality and Individual Differences, 86, 338–343. [Google Scholar]
- Frazier TW, Ratliff KR, Gruber C, Zhang Y, Law PA, & Constantino JN (2014). Confirmatory factor analytic structure and measurement invariance of quantitative autistic traits measured by the Social Responsiveness Scale-2. Autism, 18, 31–44. [DOI] [PubMed] [Google Scholar]
- Gillberg C (2010). The ESSENCE in child psychiatry: Early symptomatic syndromes eliciting neurodevelopmental clinical examinations. Research in Developmental Disabilities, 31, 1543–1551. [DOI] [PubMed] [Google Scholar]
- Gillberg C, & Fernell E (2014). Autism Plus Versus Autism Pure. Journal of Autism and Developmental Disorders, 44, 3274–3276. [DOI] [PubMed] [Google Scholar]
- Glorfeld LW (1995). An improvement on Horn’s parallel analysis methodology for selecting the correct number of factors to retain. Educational and Psychological Measurement, 55, 377–393. [Google Scholar]
- Hengartner MP, & Lehmann SN (2017). Why psychiatric research must abandon traditional diagnostic classification and adopt a fully dimensional scope: Two solutions to a persistent problem. Frontiers in Psychiatry, 8, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn JL (1965). A rationale and test for the number of factors in factor analysis. Psychometrika, 30, 179–185. [DOI] [PubMed] [Google Scholar]
- Humes K, Jones NA, & Ramirez RR (2011). Overview of race and Hispanic origin, 2010. Washington, DC: US Department of Commerce, Economics and Statistics Administration, US Census Bureau, 2011. [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, ... & Wang P. (2010). Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry, 167, 748–751. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Jones P, Kemppainen L, Croudace I, Isohanni I, Veijola S, ... & Rantakallio P. (2000). Childhood and adolescent predictors of schizophrenia in the Northern Finland 1966 birth cohort-a descriptive life-span model. European Archives of Psychiatry and Clinical Neuroscience, 250, 311–319. [DOI] [PubMed] [Google Scholar]
- Johns LC, & Van Os J (2001). The continuity of psychotic experiences in the general population. Clinical Psychology Review, 21, 1125–1141. [DOI] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, & Marmot M (1994). Child development risk factors for adult schizophrenia in the British 1946 cohort. Lancet, 344, 1398–1402. [DOI] [PubMed] [Google Scholar]
- Kendler KS, & Gardner CO, Jr (1998). Boundaries of major depression: An evaluation of DSM-IV criteria. American Journal of Psychiatry, 155, 172–177. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, & Neale MC (2003). The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry, 60, 929–937. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethi- nam R, & Sweeney JA (2005). Premorbid indicators and risk for schizophrenia: A selective review and update. Schizophrenia Research, 79, 45–57. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of- onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby M, ... & Zimmerman M. (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126, 454–477. [DOI] [PubMed] [Google Scholar]
- Laurens KR., Hobbs MJ., Sunderland M., Green MJ., & Mould GL. (2012). Psychotic-like experiences in a community sample of 8000 children aged 9–11 years: An item response theory analysis. Psychological Medicine, 42, 1495–1506. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF (2015). Thinking clearly about schizotypy: Hewing to the schizophrenia liability core, considering interesting tangents, and avoiding conceptual quicksand. Schizophrenia Bulletin, 41 (Suppl. 2), S483–S491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstom E, ... & Pedersen NL. (2006). The Swedish Twin Registry in the third millennium: An update. Twin Research and Human Genetics, 9, 875–882. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, & Hultman CM (2009). Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: A population-based study. The Lancet, 373, 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymer GK, Job DE, William T, Moorhead J, McIntosh AM, Owens DG, ... & Lawrie SM. (2006). Brain- behaviour relationships in people at high genetic risk of schizophrenia. NeuroImage, 33, 275–285. [DOI] [PubMed] [Google Scholar]
- Mason O, & Claridge G (2006). The Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE): Further description and extended norms. Schizophrenia Research, 82, 203–211. [DOI] [PubMed] [Google Scholar]
- Mason O, Claridge G, & Jackson M (1995). New scales for the assessment of schizotypy. Personality and Individual Differences, 18, 7–13. [Google Scholar]
- Mason O, Linney Y, & Claridge G (2005). Short scales for measuring schizotypy. Schizophrenia Research, 78, 293–296. [DOI] [PubMed] [Google Scholar]
- Moreno-De-Luca A, Evans DW, Boomer KB, Hanson E, Bernier R, Goin-Kochel R, ... & Ledbetter D. (2014). The role of parental cognitive, behavioral, and motor profiles in clinical variability in individuals with chromosome 16p11. 2 deletions. JAMA Psychiatry, 72, 199–206. [DOI] [PubMed] [Google Scholar]
- Moreno-De-Luca A., Myers SM., Challman TD., Moreno-De- Luca D., Evans DW., & Ledbetter DH. (2013). Developmental brain dysfunction: Revival and expansion of old concepts based on new genetic evidence. The Lancet Neurology, 12, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen LK, & Muthén BO (2007). Mplus user’s guide. Los Angeles: Author. [Google Scholar]
- Niemi LT, Suvisaari JM, Haukka JK, & Lönnqvist JK (2005). Childhood predictors of future psychiatric morbidity in offspring of mothers with psychotic disorder Results from the Helsinki High-Risk Study. The British Journal ofPsychi- atry, 186, 108–144. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, & Heaton RK (2004). Identification of separable cognitive factors in schizophrenia. Schizophrenia Research, 72, 29–39. [DOI] [PubMed] [Google Scholar]
- Owen MJ (2014). New approaches to psychiatric diagnostic classification. Neuron, 84, 564–571. [DOI] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, & Harrington H (2000). Children’s self-reported psychotic symptoms and adult schizophreniform disorder: A 15-year longitudinal study. Archives of General Psychiatry, 57, 1053–1058. [DOI] [PubMed] [Google Scholar]
- Ronald A (2015). Recent quantitative genetic research on psychotic experiences: New approaches to old questions. Current Opinion in Behavioral Sciences, 2, 81–88. [Google Scholar]
- Sánchez-Bernardos ML, & Avia MD (2006). The relationship between fantasy proneness and schizotypy in adolescents. The Journal of Nervous and Mental Disease, 194, 411–414. [DOI] [PubMed] [Google Scholar]
- Schwarz G (1978). Estimating the dimension of a model. The Annals of Statistics, 6, 461–464. [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry, 47, 921–929. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnus- dottir B, Morgen B, Arnarsdottir S, ... & Stefansson K. (2014). CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature, 316, 361–368. [DOI] [PubMed] [Google Scholar]
- Szatkiewicz JP, O’Dushlaine C, Chen G, Chambert K, Moran JL, Neale BM, ... & Sullivan PF. (2014). Copy number variation in schizophrenia in Sweden. Molecular Psychiatry, 19, 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, & Krabbendam L (2009). A systematic review and metaanalysis of the psychosis continuum: Evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychological Medicine, 39, 179–195. [DOI] [PubMed] [Google Scholar]
- van Os J., & Reininghaus U. (2016). Psychosis as a transdiagnostic and extended phenotype in the general population. World Psychiatry, 15, 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van’t Wout M, Aleman A, Kessels RP, Laroi F, & Kahn RS (2004). Emotional processing in a non-clinical psychosis-prone sample. Schizophrenia Research, 68, 271–281. [DOI] [PubMed] [Google Scholar]
- Werner H. (1948). Comparative psychology of mental development. New York: Follett. [Google Scholar]
- Zavos HM, Freeman D, Haworth CM, McGuire P, Plomin R, Cardano AG, ... & Ronald A. (2014). Consistent etiology of severe, frequent psychotic experiences and milder, less frequent manifestations: A twin study of specific psychotic experiences in adolescence. JAMA Psychiatry, 71, 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler E, & Glick M (1986). A developmental approach to adult psychopathology. New York: Wiley. [Google Scholar]
- Zigler E, & Phillips L (1961). Psychiatric diagnosis: A critique. The Journal of Abnormal and Social Psychology, 63, 607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Oxford-Liverpool inventory of feelings and experiences – United States.
The childhood Oxford-Liverpool inventory of feelings and experiences.
Raw to t-score conversion tables for the CO-LIFE and the O-LIFE-US.
Rotated factor matrix for the O-LIFE-US.
Rotated factor matrix for the CO-LIFE.
Fit indices for mixture modeling analyses of parent (O-LIFE-US) and child (CO-LIFE) ratings.


