Abstract
Background
Bronchopulmonary dysplasia (BPD) is a chronic lung disease common in preterm infants. Montelukast, an effective cysteinyl leukotriene (cysLT) receptor antagonist, has a variety of pharmacological effects and has protective effects against a variety of diseases. Currently, the efficacy and safety of montelukast sodium in treating BPD has been revealed, however, the precise molecular mechanism of the effect of montelukast on BPD development remain largely unclear. Therefore, this study aimed to investigate the effect and mechanism of montelukast on BPD in vivo and in vitro.
Material/Methods
A mouse BPD model and hyperoxia-induced lung cell injury model were established and treated with montelukast. Then mean linear intercept (MLI), radial alveolar count (RAC), lung weight/body weight (LW/BW) ratio, pro-inflammatory factors, and oxidative stress-related factors in lung tissues were determined. Cell viability and apoptosis were detected using MTT assay and flow cytometer respectively.
Results
The results showed that montelukast treatment relieved mouse BPD, evidenced by increased RAC and decreased MLI and LW/BW ratios. We also found that montelukast treatment reduced pro-inflammatory factors (TNF-α, IL-6, and IL-1β) production, enhanced superoxide dismutase (SOD) activity, and reduced malondialdehyde (MDA) content in the lung tissues of BPD mice. Besides, montelukast eliminated the reduced cell viability and enhanced cell apoptosis induced by hyperoxia exposure in vitro. Moreover, the upregulated pro-inflammatory factors production and p-p65 protein level in lung cells caused by hyperoxia were decreased by montelukast treatment.
Conclusions
Montelukast protected against mouse BPD induced by hyperoxia through inhibiting inflammation, oxidative stress, and lung cell apoptosis.
MeSH Keywords: Apoptosis, Bronchopulmonary Dysplasia, Inflammation, Oxidative Stress
Background
Bronchopulmonary dysplasia (BPD) is a chronic lung disease commonly found in premature babies [1–3]. So far, the main treatments for BPD are mechanical ventilation and medication. However, the treatment period is long, and the cure rate is low. There is still no treatment strategy that can effectively treat BPD. In severe cases, many long-term complications, such as pulmonary dysfunction and growth retardation, occur and BPD remains the leading cause of death and disability, causing a heavy burden on families and society [4]. Therefore, exploring and seeking new and effective methods for treating BPD is very urgent and significant.
Histologically, the characteristics of BPD are poor alveolarization, abnormal capillary growth, mesenchymal cell hyperplasia, fibrosis, and abnormal elastin deposition [5]. The pathogenic factors of BPD are complex, and the pathogenesis has not yet been fully elucidated. However, with the deepening of clinical and experimental research on BPD it has been found that oxidative stress and inflammatory response play important roles in the development of BPD [4,6].
Montelukast is a potent cysteinyl leukotriene (cysLT) receptor antagonist with anti-inflammatory effects in bronchial asthma [7,8]. Due to its anti-inflammatory effects, montelukast has also been found to be effective in stroke in vivo [9]. Besides, montelukast was found to have protective effects on other diseases, including rheumatoid arthritis, ischemia-reperfusion (I/R) injury, and Parkinson disease [10–12]. Moreover, studies have reported that montelukast has an activity of inhibiting the growth of tumor cells via inducing cell apoptosis [13,14]. Studies have also revealed the efficacy and safety of montelukast sodium in treating BPD [15,16], however, the precise role and underlying mechanism of montelukast in BPD development remain largely unclear.
Hyperoxic injury impedes key signaling pathways that induce lung development [17]. Exposure to 85% oxygen-induced hyperoxia injury at birth was identified as a well-known neonatal animal BPD model [18]. In the present study, we aimed to investigate the effect of montelukast on 85% oxygen exposure-induced BPD mouse model and lung cell injury model induced by hyperoxia, and further to explore the molecular mechanism.
Material and Methods
Animals and BPD model establishment
A total of 45 neonatal C57BL/6J mice were obtained from Vital River Company (Beijing, China). Mice were housed at 25±5°C, 50% humidity and 12 hours dark/light cycle conditions. All animal experiments were performed as per the Recommended Guideline for the Care and Use of Laboratory Animals issued by Chinese Council on Animal Research. This study was approved by Animal Ethics Committee of the First Hospital Affiliated to Bengbu Medical College.
The mouse model of BPD was conducted according to a previous study [19]. In brief, within 12 hours of birth, the newborn mice were randomly assigned to hyperoxia (85% O2) or room air (21% O2) for 7 consecutive days. Mice were randomly assigned to 3 groups: the control group; the BPD model group; or the BPD+ montelukast group. Mice in the BPD+montelukast group were intraperitoneally injected once every other day with 10 mg/kg montelukast from postnatal day 2 to postnatal day 14. Mice in the Control and BPD model groups were given saline (0.9% NaCl) solution intraperitoneally. At 14 days after treatment, mice were killed by intraperitoneal injection of sodium pentobarbital.
Mean linear intercept (MLI) (representing the average alveolar diameter) and radial alveolar count (RAC) (representing alveolar septation and alveologenesis) were determined as described previously [20]. In addition, the lung weight/body weight (LW/BW) ratio of mice was calculated according to a previous study [20].
Measurement of oxidative stress-related factors in lung tissues
The level of malondialdehyde (MDA) and the activity of superoxide dismutase (SOD) in lung tissues were analyzed by commercially available kits (A003-1, A001-3; JianCheng Bioengineering Institute) following the manufacturer’s instructions of each kit. Experiment was performed in triple.
Cell culture and treatment
A549 cells (human type II alveolar epithelial cells) were obtained from ATCC (CCL-185, USA). A549 cells were grown in RPMI-1640 medium containing 10% FBS and 1% penicillin/streptomycin, and incubated at 37°C with 5% CO2. Then the cells were plated into 6-well plates and incubated for 24 hours. After exposure to RPMI-1640 medium supplemented with 0.1% FBS for 6 hours, the A549 cells were maintained in room air (21% O2) or hyperoxia (85% O2) for 6 hours [21]. Subsequently, the cells were treated with or without montelukast (10 μM) for 24 hours.
ELISA
To determine the levels of TNF-α, IL-6 and IL-1β in cell culture medium, enzyme-linked immunosorbent assay (ELISA) kits (Cell Signaling Technology Inc., Danvers, MA, USA) were used according to the relevant manuals. Experiments were performed in triple.
MTT assay
To measure cell viability, 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay was performed. After certain treatment, the A549 cells (1×104 cells/per well) were plated into a 96-well plate and cultured for 24 hours. Then, 20 μL MTT solution (0.5 mg/mL; Sigma-Aldrich Co., St. Louis, MO, USA) was added to each well, and the cells were further incubated at 37°C for another 4 hours. Cell viability was calculated by detecting the absorbance at 570 nm by using FLUOstar® Omega Microplate Reader (BMG LABTECH, Ortenberg, Germany). Experiments were performed in triplicate.
Apoptosis analysis assay
The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Cat no. 70-AP101-100; MultiSciences, Hangzhou, China) was used to analyze cell apoptosis. After specific treatment, the A549 cells were collected using 0.25% trypsin, washed with phosphate buffered saline (PBS) and then stained with 5 μL Annexin V-FITC and 5 μL PI for 30 minutes at room temperature in the dark. Flow cytometer (FCM, BD Biosciences) was performed to analyze cell apoptosis. Experiments were performed in triple.
Western blot assay
Western blot assay was performed to determine protein expression in our current study. Proteins from lung tissues and the A549 cells were extracted using RIPA buffer (Beyotime Institute of Biotechnology) and quantified using a bicinchoninic acid assay kit (BCA; Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. Equal amounts of protein samples (25 μg/lane) were separated on 12% SDS-PAGE, transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Billerica, MA, USA), blocked in 5% non-fat milk at room temperature for 1 hour, and then incubated with primary antibodies overnight at 4°C. Subsequently, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibody, anti-rabbit IgG, HRP-linked antibody (dilution: 1: 5000; Cell Signaling Technology Inc., Danvers, MA, USA) at room temperature for 2 hours. At last, we performed the enhanced chemiluminescence reagent (Thermo Scientific, Logan, UT, USA) to visualize the immunoreactive bands.
QRT-PCR
Total RNA from cells or tissues was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reversely transcribed into cDNAs by using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) according to the manufacture’s protocol. cDNAs were analyzed using qPCR with the SYBR Premix Ex TaqTM II (TliRNaseH Plus) kit (Takara Bio, Inc., Otsu, Japan). GAPDH was used as internal control. The relative gene expression was calculated using the 2−ΔΔCT method. Experiments were performed in triple.
Statistical analysis
Experimental data were analyzed with SPSS software version 17.0 (IBM Corp., Armonk, NY, USA) and presented as the mean ± standard deviation (SD). Difference between groups were made by one-way analysis of variance with Tukey’s post hoc test or the Student’s t-test. A value of P<0.05 was considered statistically significant.
Results
Effect of montelukast on alveolarization and lung injury
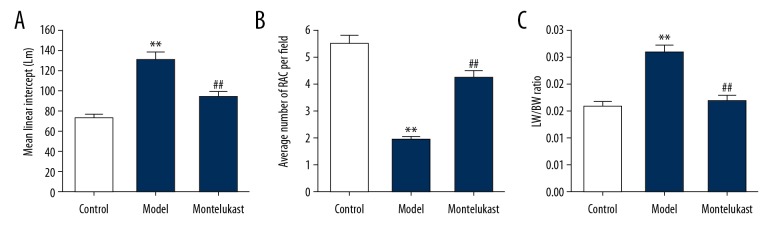
As shown in Figure 1A and 1B, compared with the control group, the lungs obtained from the mice in the BPD model group had an increased MLI and reduced RAC, while montelukast administration decreased MLI and enhanced RAC in the BPD mice. In addition, we further examined the extent of lung injury by measuring the LW/BW ratio, which is an indicator of increased cellular and pulmonary edema. Results showed that LW/BW ratio was significantly increased in the BPD mice compared with the mice in the control group, and this increase was notably eliminated by montelukast treatment (Figure 1C).
Figure 1.
Effect of montelukast on alveolarization and lung injury. The mouse model of bronchopulmonary dysplasia (BPD) was conducted and treated with 10 mg/kg montelukast. Mice in the BPD+montelukast (Montelukast) group were intraperitoneally injected once every other day with 10 mg/kg montelukast from postnatal day 2 to postnatal day 14. Mice in the Control and BPD model (Model) groups were given saline (0.9% NaCl) solution intraperitoneally. 14 days after treatment, the (A) mean linear intercept (MLI), (B) radial alveolar count (RAC), and (C) lung weight/body weight (LW/BW) ratio of mice in different groups were determined. Data were displayed as mean ± standard deviation. ** P<0.01 versus Control; ## P<0.01 versus Model.
Effect of montelukast on inflammatory response in lung tissues of BPD mice
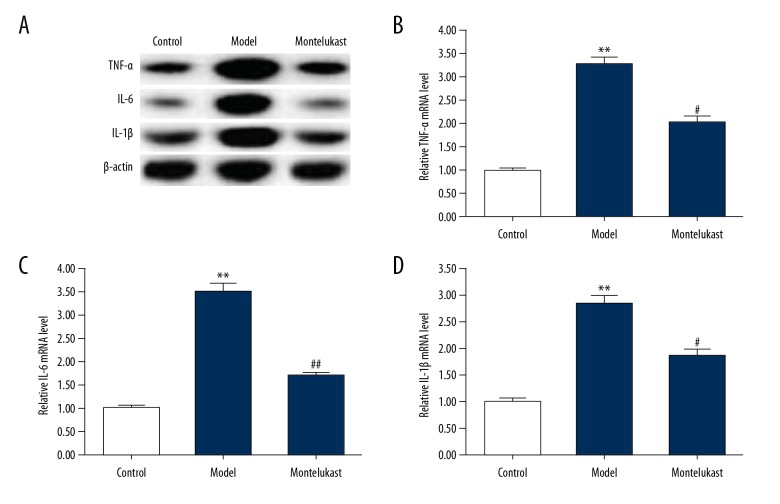
We then determined the effect of montelukast on inflammatory response in the BPD mice, and the protein and mRNA levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β in lung tissues were measured using western blotting and qRT-PCR respectively. As shown in Figure 2, both protein and mRNA levels of TNF-α, IL-6, and IL-1β were significantly enhanced in the lung tissues of the BPD mice compared with the mice in the control group. Montelukast treatment significantly reduced the levels of TNF-α, IL-6, and IL-1β in the lung tissues of the BPD mice.
Figure 2.
Effect of montelukast on inflammatory response in lung tissues of bronchopulmonary dysplasia (BPD) mice. The mouse model of bronchopulmonary dysplasia (BPD) was conducted and treated with 10 mg/kg montelukast. Mice in the BPD+montelukast (Montelukast) group were intraperitoneally injected once every other day with 10 mg/kg montelukast from postnatal day 2 to postnatal day 14. Mice in the Control and BPD model (Model) groups were given saline (0.9% NaCl) solution intraperitoneally. At 14 days after treatment, the protein (A) and mRNA (B–D) levels of TNF-α, IL-6, and IL-1β in lung tissues were measure using western blotting and qRT-PCR respectively. Data were displayed as mean ± standard deviation. ** P<0.01 versus Control; #, ## P<0.05, 0.01 versus Model.
Effect of montelukast on oxidative stress in lung tissues of BPD mice
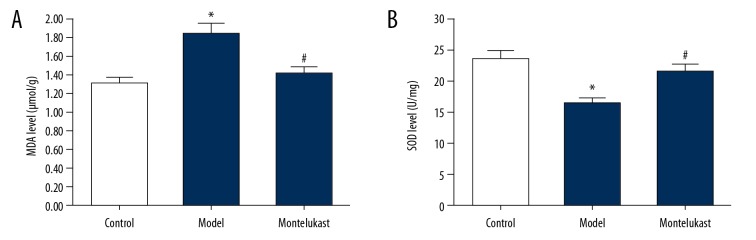
Oxidative stress in lung tissues of mice was also analyzed in the present study. We found that compared with the mice in the control group, the level of MDA significantly increased, and the activity of SOD was notably reduced in the lung tissues in the BPD mice, and montelukast treatment significantly decreased MDA level and enhanced SOD activity in the lung tissues of the BPD mice (Figure 3).
Figure 3.
Effect of montelukast on oxidative stress in lung tissues of bronchopulmonary dysplasia (BPD) mice. The mouse model of bronchopulmonary dysplasia (BPD) was conducted and treated with 10 mg/kg montelukast. Mice in the BPD+montelukast (Montelukast) group were intraperitoneally injected once every other day with 10 mg/kg montelukast from postnatal day 2 to postnatal day 14. Mice in the Control and BPD model (Model) groups were given saline (0.9% NaCl) solution intraperitoneally. At 14 days after treatment, the level of (A) MDA and the activities of (B) SOD in lung tissues of BPD mice were detected. Data were displayed as mean ± standard deviation. * P<0.05 versus Control; # P<0.05 versus Model.
Effect of montelukast on lung cell viability and apoptosis
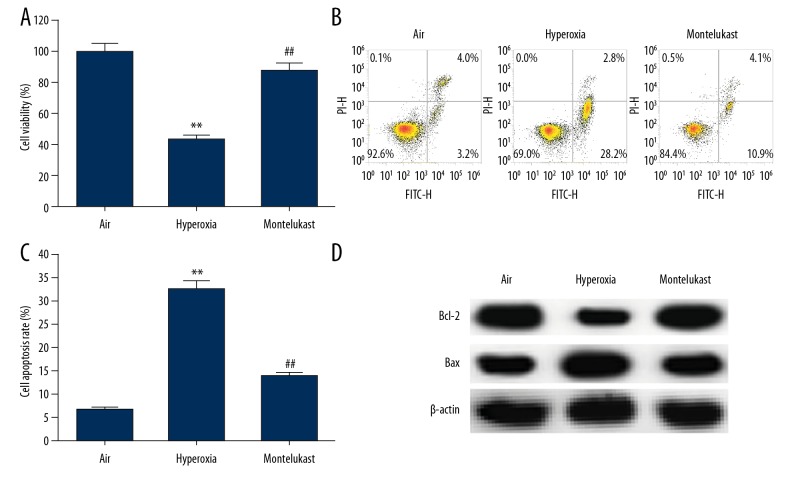
We then investigate the effect of montelukast on hyperoxia-induced lung cell injury in vitro, and human type II alveolar epithelial cells A549 were exposed to room air (21% O2) (air group) or hyperoxia (85% O2) (hyperoxia group) for 6 hours. Our results indicated that compared with the air group, hyperoxia exposure significantly reduced A549 cell viability (Figure 4A) and induced cell apoptosis (Figure 4B, 4C); while the cell viability was notably increased, and cell apoptosis was decreased by 10 μM montelukast administration. Meanwhile, the protein levels of Bcl-2 and Bax were determined, and we found that compared with the air group, Bcl-2 markedly decreased and Bax was enhanced in hyperoxia exposed A549 cells, however, montelukast administration notably increased Bcl-2 and decreased Bax protein level (Figure 4D).
Figure 4.
Effect of montelukast on lung cell viability and apoptosis. A549 cells were maintained in room air (21% O2) or hyperoxia (85% O2) for 6 hours. Subsequently, the cells were treated with or without montelukast (10 μM) for 24 hours. Then, (A) cell viability was detected using MTT assay, (B) cell apoptosis was analyzed by flow cytometer, and (C) the early + late apoptosis was calculated; (D) protein levels of Bcl-2 and Bax were measured by western blotting. Data were displayed as mean ± standard deviation. ** P<0.01 versus Air; ## P<0.01 versus hyperoxia.
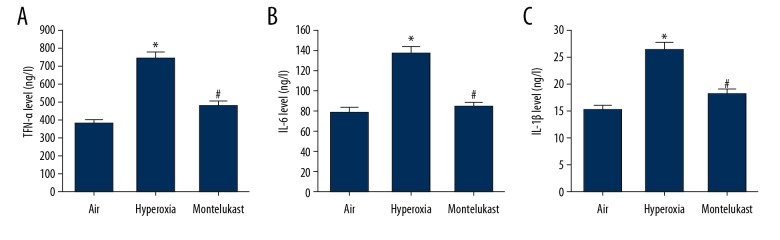
Effect of montelukast on the secretion of pro-inflammatory cytokines in lung cells
A549 cells were maintained in room air (21% O2) or hyperoxia (85% O2) for 6 hours, then the cells were treated with or without montelukast (10 μM) for 24 hours. The cell culture medium was harvested for the levels of TNF-α, IL-6, and IL-1β detection. Findings suggested that compared with the air group, the contents of TNF-α, IL-6, and IL-1β were significantly enhanced by hyperoxia, and these enhancements were reduced by montelukast treatment (Figure 5).
Figure 5.
Effect of montelukast on the secretion of pro-inflammatory cytokines in lung cells. A549 cells were maintained in room air (21% O2) or hyperoxia (85% O2) for 6 hours. Subsequently, the cells were treated with or without montelukast (10 μM) for 24 hours. Then the levels of TNF-α (A), IL-6 (B), and IL-1β (C) in the cell culture medium were detected using ELISA. Data were displayed as mean ± standard deviation. * P<0.05 versus Air; # P<0.05 versus hyperoxia.
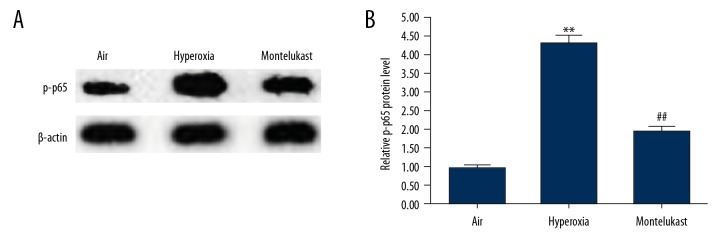
Effect of montelukast on NF-κB pathway in lung cells
Finally, to explore the molecular mechanism of the effect of montelukast on BPD, the NF-κB pathway was analyzed in our study. As shown in Figure 6, the protein level of p-p65 was significantly enhanced in hyperoxia exposed A549 cells in comparison with the air group. As expected, montelukast treatment significantly reduced the protein level of p-p65 in A549 cells.
Figure 6.
Effect of montelukast on NF-κB pathway in lung cells. A549 cells were maintained in room air (21% O2) or hyperoxia (85% O2) for 6 hours. Subsequently, the cells were treated with or without montelukast (10 μM) for 24 hours. Then the protein level of p-p65 was measured by western blotting (A), and the relative protein level of p-p65 was quantified and presented as fold of control (B). Data were displayed as mean ± standard deviation. ** P<0.01 versus Air; ## P<0.01 versus hyperoxia.
Discussion
The current study demonstrated that montelukast relieved mouse BPD induced by hyperoxia through inhibiting inflammation response and oxidative stress. Montelukast also alleviated lung cell viability reduction and apoptosis enhancement caused by hyperoxia treatment. Moreover, we found that montelukast inhibited production of pro-inflammatory factors and NF-κB pathway activation in hyperoxia treated lung cells. Evidence collected in the present study supported that montelukast is a novel therapeutic agent for BPD treatment.
BPD is a lung disease with a high incidence of premature neonates [1–3]. Newborns with BPD usually have respiratory sequelae [22,23] and neurological diseases [24]. Therefore, in-depth study of the pathogenesis of BPD and exploring effective treatment of BPD has become a hot issue in recent years.
Montelukast, an anti-asthmatic drug in a clinical setting, has a powerful anti-inflammatory effect [25]. Studies have revealed the efficacy and safety of montelukast sodium in treating BPD [15,16], but the precise role and underlying mechanism of montelukast in BPD development remain largely unclear. The present study investigated the effect of montelukast on BPD mouse model induced by hyperoxia exposure, and we found that montelukast treatment relieved mouse BPD, evidenced by increased RAC (representing alveolar septation and alveologenesis), and decreased MLI (representing the average alveolar diameter) and LW/BW ratio (an index of lung injury). Alveolar simplification and vascular abnormalities, small and medium blood vessel occlusion, increased inflammation and pulmonary edema are the pathophysiological features of BPD. Inflammatory response plays critical roles in the development of BPD [4,6,26,27]. Oxidative stress leads to alveolar destruction and vascular occlusion of alveolar capillaries, so the imbalance between oxidation and antioxidant systems is considered to be one of the pathogenesis of BPD [4,6,28]. Then we studied the effect of montelukast on inflammatory response and oxidative stress in BPD mice, and we found that montelukast significantly repressed the inflammatory response and oxidative stress.
Previous studies have reported the critical roles of lung cell apoptosis in the occurrence and development of BPD [29,30]. In this study, we also investigated whether montelukast had an effect on lung cell apoptosis in vitro, and human type II alveolar epithelial cell line A549 was used in the present study to establish the lung cell injury model induced by hyperoxia. The findings of this study indicated that montelukast treatment significantly reduced hyperoxia exposure induced A549 cell apoptosis. Contrary to our results, previous studies have reported that montelukast could inhibit the growth of tumor cells via inducing tumor cell apoptosis [13,14]. This may be due to different environmental conditions and different cell types, but further exploration is needed. At the same time, we found that montelukast inhibited pro-inflammatory factors expression in hyperoxia treated A549 cells. In addition, another important finding of our investigation was that montelukast repressed NF-κB pathway activation in hyperoxia treated A549 cells.
In summary, our study showed for the first time that montelukast extenuated hyperoxia induced mouse BPD through inhibiting inflammation response and oxidative stress, and it could also prevent lung cell apoptosis to protect against BPD.
Conclusions
Montelukast presented a protective effect on BPD, thus it may be a promising agent for BPD treatment.
Footnotes
Source of support: The present study was supported by the Funding Project of Bengbu Medical College of Science and Technology Development (No. BYKF1741)
Conflict of interests
None.
References
- 1.Baud O, Maury L, Lebail F, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): A double-blind, placebo-controlled, multicentre, randomised trial. Lancet. 2016;387:1827–36. doi: 10.1016/S0140-6736(16)00202-6. [DOI] [PubMed] [Google Scholar]
- 2.Cui H, He J, Chen H, Chen J, et al. Erythropoietin attenuates hyperoxia-induced lung injury by upregulating epidermal growth factor-like domain in newborn rats. Biomed Rep. 2017;6:32–38. doi: 10.3892/br.2016.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: Chronic lung disease of infancy and long-term pulmonary outcomes. J Clin Med. 2017;6 doi: 10.3390/jcm6010004. pii: E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guaman MC, Gien J, Baker CD, et al. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2015;32:960–67. doi: 10.1055/s-0035-1547326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach KP, Kuschel CA, Hooper SB, et al. High bias gas flows increase lung injury in the ventilated preterm lamb. PLoS One. 2012;7:e47044. doi: 10.1371/journal.pone.0047044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahzad T, Radajewski S, Chao CM, et al. Pathogenesis of bronchopulmonary dysplasia: When inflammation meets organ development. Mol Cell Pediatr. 2016;3:23. doi: 10.1186/s40348-016-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamant Z, van der Molen T. Treating asthma: Is there a place for leukotriene receptor antagonists? Respir Med. 2005;99:655–62. doi: 10.1016/j.rmed.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Riccioni G, Bucciarelli T, Mancini B, et al. Antileukotriene drugs: Clinical application, effectiveness and safety. Curr Med Chem. 2007;14:1966–77. doi: 10.2174/092986707781368522. [DOI] [PubMed] [Google Scholar]
- 9.Saad MA, Abdelsalam RM, Kenawy SA, Attia AS. Montelukast, a cysteinyl leukotriene receptor-1 antagonist protects against hippocampal injury induced by transient global cerebral ischemia and reperfusion in rats. Neurochem Res. 2015;40:139–50. doi: 10.1007/s11064-014-1478-9. [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Liu F, Ma F, et al. Montelukast inhibits inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes. Int Immunopharmacol. 2018;61:215–21. doi: 10.1016/j.intimp.2018.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Bilgiç Mİ, Altun G, Çakıcı H, et al. The protective effect of montelukast against skeletal muscle ischemia reperfusion injury: An experimental rat model. Ulus Travma Acil Cerrahi Derg. 2018;24:185–90. doi: 10.5505/tjtes.2017.22208. [DOI] [PubMed] [Google Scholar]
- 12.Nagarajan VB, Marathe PA. Effect of montelukast in experimental model of Parkinson’s disease. Neurosci Lett. 2018;682:100–5. doi: 10.1016/j.neulet.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 13.Zovko A, Yektaei-Karin E, Salamon D, et al. Montelukast, a cysteinyl leukotriene receptor antagonist, inhibits the growth of chronic myeloid leukemia cells through apoptosis. Oncol Rep. 2018;40:902–8. doi: 10.3892/or.2018.6465. [DOI] [PubMed] [Google Scholar]
- 14.Tsai MJ, Chang WA, Tsai PH, et al. Montelukast induces apoptosis-inducing factor-mediated cell death of lung cancer cells. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18071353. pii: E1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SB, Lee JH, Lee J, et al. The efficacy and safety of montelukast sodium in the prevention of bronchopulmonary dysplasia. Korean J Pediatr. 2015;58:347–53. doi: 10.3345/kjp.2015.58.9.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demir K, Kumral A, Duman N, et al. Clarithromycin, montelukast, and pentoxifylline combination treatment ameliorates experimental neonatal hyperoxic lung injury. J Matern Fetal Neonatal Med. 2008;21:407–13. doi: 10.1080/14767050802045715. [DOI] [PubMed] [Google Scholar]
- 17.Stenmark KR, Abman SH. Lung vascular development: Implications for the pathogenesis of bronchopulmonary dysplasia. Ann Rev Physiol. 2015;67:623–61. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 18.Bhandari V. Hyperoxia-derived lung damage in preterm infants. Semin Fetal Neonatal Med. 2010;15:223–29. doi: 10.1016/j.siny.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park MS, Rieger-Fackeldey E, Schanbacher BL, et al. Altered expressions of fibroblast growth factor receptors and alveolarization in neonatal mice exposed to 85% oxygen. Pediatr Res. 2007;62:652–57. doi: 10.1203/PDR.0b013e318159af61. [DOI] [PubMed] [Google Scholar]
- 20.Maturu P, Wei-Liang Y, Androutsopoulos VP, et al. Quercetin attenuates the hyperoxic lung injury in neonatal mice: Implications for bronchopulmonary dysplasia (BPD) Food Chem Toxicol. 2018;114:23–33. doi: 10.1016/j.fct.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Chen XQ, Wu SH, Luo YY, et al. Lipoxin A4 attenuates bronchopulmonary dysplasia via upregulation of Let-7c and downregulation of TGF-β1 signaling pathway. Inflammation. 2017;40:2094–108. doi: 10.1007/s10753-017-0649-7. [DOI] [PubMed] [Google Scholar]
- 22.Bolton CE, Stocks J, Hennessy E, et al. The EPICure study: Association between hemodynamics and lung function at 11 years after extremely preterm birth. J Pediatr. 2012;161:595–601. doi: 10.1016/j.jpeds.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gough A, Spence D, Linden M, et al. General and respiratory health outcomes in adult survivors of bronchopulmonary dysplasia: A systematic review. Chest. 2012;141:1554–67. doi: 10.1378/chest.11-1306. [DOI] [PubMed] [Google Scholar]
- 24.Short EJ, Kirchner HL, Asaad GR, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: Analysis using a severity-based classification system. Arch Pediatr Adolesc Med. 2007;161:1082–87. doi: 10.1001/archpedi.161.11.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borish L. The role of leukotrienes in upper and lower airway inflammation and the implications for treatment. Ann Allergy Asthma Immunol. 2002;88:16–22. doi: 10.1016/s1081-1206(10)62024-8. [DOI] [PubMed] [Google Scholar]
- 26.Bhandari V. Postnatal inflammation in the pathogenesis of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100:189–201. doi: 10.1002/bdra.23220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsiao CC, Chang JC, Tsao LY, et al. Correlates of elevated interleukin-6 and 8-hydroxy-2′-deoxyguanosine levels in tracheal aspirates from very low birth weight infants who develop bronchopulmonary dysplasia. Pediatr Neonatol. 2017;58:63–66. doi: 10.1016/j.pedneo.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Xu W, Zhao Y, Zhang B, et al. Resveratrol attenuates hyperoxiainduced oxidative stress, inflammation and fibrosis and suppresses Wnt/β-catenin signalling in lungs of neonatal rats. Clin Exp Pharmacol Physiol. 2015;42:1075–83. doi: 10.1111/1440-1681.12459. [DOI] [PubMed] [Google Scholar]
- 29.Das KC, Wasnick JD. Biphasic response of checkpoint control proteins in hyperoxia: Exposure to lower levels of oxygen induces genome maintenance genes in experimental baboon BPD. Mol Cell Biochem. 2014;395:187–98. doi: 10.1007/s11010-014-2124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai C, Qiu J, Qiu G, et al. Long non-coding RNA MALAT1 protects preterm infants with bronchopulmonary dysplasia by inhibiting cell apoptosis. BMC Pulm Med. 2017;17:199. doi: 10.1186/s12890-017-0524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]