Abstract
Background and Purpose
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most-common single gene disorder of cerebral small vessel disease. There is no definite evidence of genotype-phenotype correlation in CADASIL. However, recent studies have shown the unique phenotypic feature of NOTCH3 R544C mutation.
Methods
We investigated the phenotypic spectrum of NOTCH3 R544C mutation in 73 CADASIL patients in Jeju between April 2012 and January 2014.
Results
Of the 73 subjects from 60 unrelated families included in this study, 40 (55%) were men. The mean age of the subjects was 62.2±12.2 (range 34–86 years). Cerebral infarction was the most frequent manifestation (37%), followed by cognitive impairment (32%), headache (17%), psychiatric symptom (16%), intracerebral hemorrhage (12%), transient ischemic attack (7%), and seizure (1%). The mean age of the subjects with ischemic or hemorrhagic episodes was 64.9±10.9 (range 41–86 years). A diagnosis of dementia was made in 12 subjects (16%). The mean age of the subjects with dementia was 75.6±6.5 (range 62–86 years). About 3% of subjects were unable to walk without assistance at assessment. Only one subject had developed chronic headache before the 40s.
Conclusions
Our data support the hypothesis that CADASIL patients with R544C mutation in Jeju have relatively late onset disease.
Keywords: cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy, genotype, phenotype, NOTCH3, R544C mutation
INTRODUCTION
Cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an inherited cerebral small vessel disease caused by mutations in the NOTCH3 gene.1 NOTCH3 gene encodes a cell surface receptor on smooth muscle cell (SMC) and pericyte.1 The mutant Notch3 accumulated in pericyte and SMC causes non-amyloid, non-artherosclerotic angiopathy.2 The main clinical manifestations are recurrent stroke, cognitive decline, chronic headache, mood disturbances, and seizure.3
NOTCH3 R544C mutation accounts for the majority of CADASIL subjects in Jeju and Taiwan.4,5 Of all the reported mutation, most mutations are located in exon 3 and 4.6 However, in subjects of Jeju and Taiwan, the R544C mutation is located in exon 11.4,5 A recent Taiwanese study suggested that R544C mutation has later age at symptom onset and higher frequency of cognitive dysfunction.5 However, genotype-phenotype correlation in CADASIL remains unclear.7 Thus, we sought to elucidate the unique phenotypic feature of NOTCH3 R544C mutation.
METHODS
We studied 86 consecutive subjects with CADASIL between April 2012 and January 2014 at the Department of Neurology, Jeju National University Hospital. Asymptomatic as well as symptomatic subjects were included in the study. Symptomatic subjects had been diagnosed with CADASIL before the study, based on CADASIL symptoms (ischemia or hemorrhagic episode, cognitive impairment, chronic headache, and seizure). Asymptomatic subjects had at least one symptomatic family member. The diagnosis was confirmed by genetic testing or skin biopsy in all subjects. Of 86 CADASIL subjects, 8 subjects were excluded because they had been diagnosed by skin biopsy alone. We further excluded two subjects with an R578C mutation, two subjects with an R75P mutation, and one subject with C452A mutation. After these exclusions, 73 subjects from 60 unrelated families remained. All the subjects underwent detailed clinical evaluation, neuropsychological tests and brain MRI.
The vascular risk factors were recorded, including hypertension, diabetes mellitus, and hypercholesterolemia. Hypertension was defined as blood pressure >140/90 mm Hg on different occasions or use of an antihypertensive agent. Diabetes mellitus was defined as fasting glucose level ≥126 mg/dL or PP2 test level ≥200 mg/dL or use of anti-diabetes medication. Hypercholesterolemia was defined as total serum cholesterol level >240 mg/dL. This study was approved by the Institutional Review Board of Jeju National University Hospital and informed consent was obtained from patients.
We assessed clinical features including transient ischemic attack (TIA), cerebral infarction, intracranial hemorrhage, headache, dementia, seizure, psychiatric disorder and Parkinsonism. Dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorder fourth edition (DSM-IV). The cognitive examination includes the following tests: the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Clinical Assessment Battery,8 Digit Span Forward Test, Digit Span Backward Test and Stroop tests. All scans were acquired on a 3T MRI scanner (Achieva, Philips Healthcare, Best, the Netherlands) by using a 32-channel array head coil (Fig. 1).
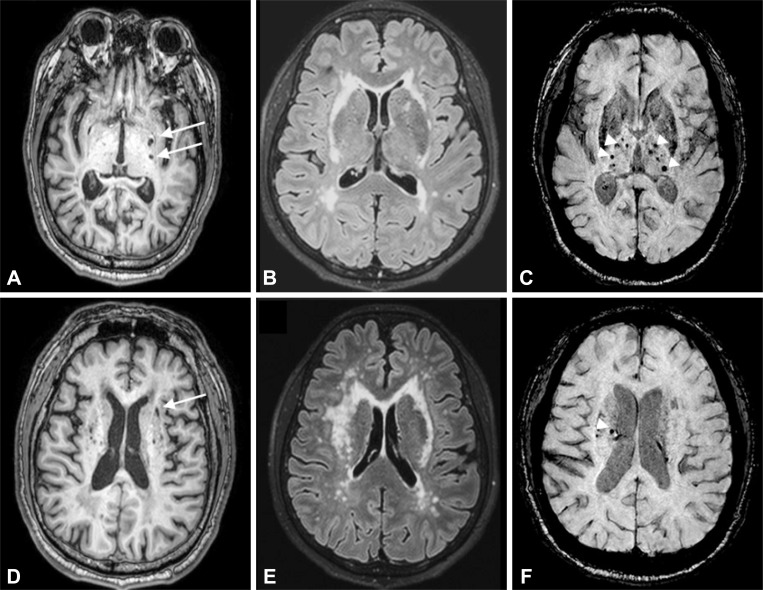
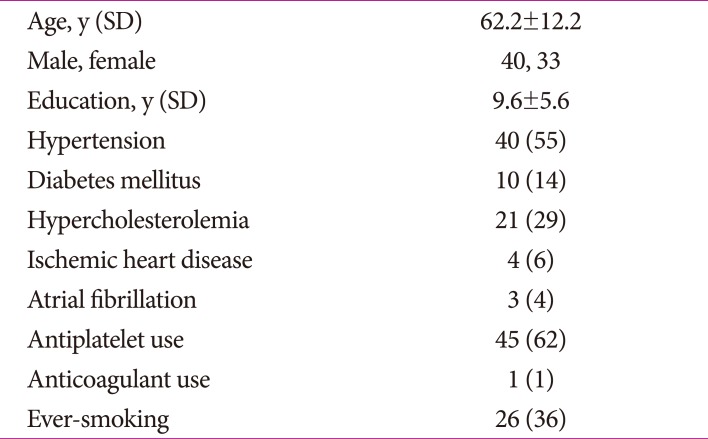
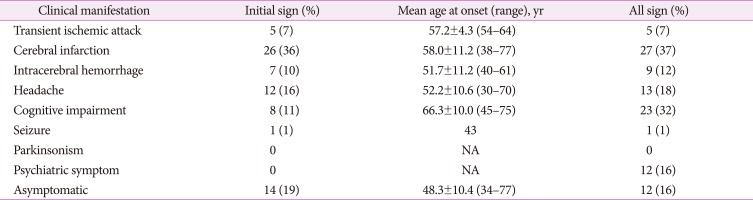
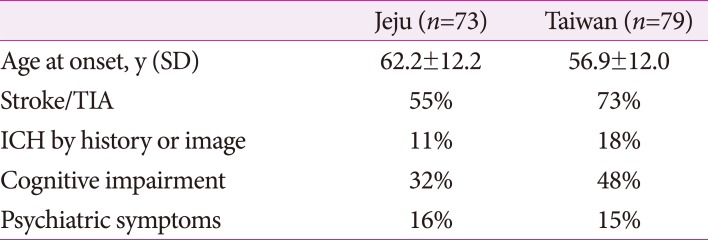
Fig. 1. Axial MR images in the subjects with R544C mutation. A and D: 3D-T1 weighted image showing multiple lacunes in the basal ganglia and corona radiate. B and E: Fluid-attenuated inversion recovery image showing confluent hyperintensity of external capsule. C and F: Susceptibility Weighted Image showing multiple cerebral microbleeds in the thalamus and corona radiate. Arrow indicates lacunes. Arrow-head points to cerebral microbleeds.
RESULTS
Demographic findings of the CADASIL subjects with R544C mutation were presented in Table 1. Of the 73 subjects included in this study, 40 (55%) were men. The mean age of the subjects was 62.2±12.2 (range 34–86 years). Hypertension was present in 40 patients (55%); diabetes mellitus, in 10 patients (14%); hypercholesterolemia, in 21 patients (29%); and ever-smoking, in 26 patients (36%).
Table 1. Demographic data of 73 CADASIL subjects with R544C mutation.
Data are mean±SD or n (%) values.
CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, SD: standard deviation.
Clinical features of the CADASIL subjects with R544C mutation were presented in Table 2. At initial sign, 59 subjects (81%) were symptomatic and 14 subjects (19%) were asymptomatic. Cerebral infarction was the most frequent manifestation (n=26, 36%), followed by headache (n=12, 16%), cognitive impairment (n=8, 11%), intracerebral hemorrhage (n=7, 10%), TIA (n=5, 7%), and seizure (n=1, 1%). Thirty-eight (53%) had experienced ischemic or hemorrhagic episodes (stroke or TIA). The mean age of the subjects at the onset of ischemic or hemorrhagic episodes was 56.7±10.3 (range 38–77 years). The mean age of the subjects at the onset of cognitive impairment was 66.3±10.0 (range 45–75 years). The mean age of the asymptomatic subjects was 48.3±10.4 (range 34–77 years). Two asymptomatic subjects had developed symptoms at the time of investigation. One subject developed lacunar infarction, and the other cognitive impairment during waiting period between the registration and the actual assessment.
Table 2. Clinical features of 73 CADASIL subjects with R544C mutation.
CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.
At the time of investigation, 61 subjects (84%) were symptomatic and 12 subjects (16%) were asymptomatic. Cerebral infarction was the most frequent manifestation (n=27, 37%), followed by cognitive impairment (n=23, 32%), headache (n=13, 18%), psychiatric symptom (n=12, 16%), intracerebral hemorrhage (n=9, 12%), TIA (n=5, 7%), and seizure (n=1, 1%). Forty (55%) had experienced ischemic or hemorrhagic episodes (stroke or TIA). The mean age of the subjects with ischemic or hemorrhagic episodes was 64.9±10.9 (range 41–86 years). Based on DSM-IV criteria, a diagnosis of major depressive disorder was made in 13 subjects, and a diagnosis of dementia in 12 subjects. The mean age of the subjects with dementia was 75.6±6.5 (range 62–86 years). According to their clinical dementia rating (CDR) scores, 12 subjects were classified into mild (CDR1; n=9), moderate (CDR2; n=1) and severe (CDR3; n=2) dementia patients. Two CDR3 patients were unable to walk unassisted.
Comparison of clinical features between the CADASIL patients with R544C mutation in Jeju and those in Taiwan was presented in Table 3.
Table 3. Comparison between CADASIL subjects with R544C mutation in Jeju and those Taiwan.
Data are mean±SD or % values. For comparison purpose using a Taiwanese CADASIL study.5
CADASIL: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, ICH: intracerebral hemorrhage, SD: standard deviation, TIA: transient ischemic attack.
DISCUSSION
This study described the phenotypic characteristics of 73 CADASIL patients with R544C mutation in Jeju Island. Although Caucasian CADASIL studies have shown no relationship between genotype and phenotype, Jejus' CADASIL subjects with R544C mutation showed several features. The clinical features of our cohort were similar to those in Taiwan and our data supported the hypothesis that R544C mutation has relatively late onset disease.
The R544C mutation in exon 11 were initially demonstrated in the Dutch CADASIL series.9 However, R544C mutation has not been reported in Europe since the first Dutch study in 1999. It was not found in the large case series of CADASIL in French and German patients (n=411),10 UK patients (n=200),7 Finnish patients (n=60),11 or Italian patients (n=229).12 Moreover, according to recent reports, R544C mutation is rarely reported in East Asia. Of 52 Chinese mainland subjects with CADASIL, only 2 subjects had R544C mutation.13 It was not found in the Japanese CADASIL series (n=70).14 Therefore, R544C mutation has been mainly reported in Jeju and Taiwan.4,5 These results suggest that founder effect of R544C mutation may exist between the two islands. Unlike many developed countries, Korea does not have a referral center for CADASIL. Thus, it is impossible to differentiate between Korean mainlands' CADASIL patients and Jejus' CADASIL patients.
We found that our subjects had later age at symptom onset. Of 73 CADASIL subjects with R544C mutation of mean age 62.2±12.2, only two subjects (3%) were unable to walk without assistance. However, most Caucasian patients cannot walk at around 60 years, and are bed-ridden at around 65 years.10 In a Taiwan series of 79 CADASIL patients with R544C mutation, their age at symptom onset was later by 9.1 years, as compared to patients with other NOTCH3 mutation.5 In contrast, one Korean mainland study found no differences between 7 CADASIL patients with R544C mutation and 6 CADASIL patients with R75P in clinical and MRI features.15 However, the number of patients in this analysis (n=13) was too small to draw conclusions. The conclusion that 73 Jeju CADASIL subjects with R544C mutation had later age at symptom onset, as compared to Caucasian CADASIL patients remains valid.
We also found that the most common age of onset of chronic headache was in the 50s in our cases. Only one patient, who was classified as migraine without aura, had developed chronic headache before the 40s. In contrast, Caucasian CADASIL studies have shown that migraine with aura is usually the first symptom of CADASIL with a mean age of 26 years in women, and 36 years in men.16,17 20–40% of Caucasian CADASIL subjects are known to have migraine with aura.6 However, our previous study showed that CADASIL subjects with R544C mutation showed no migraine with aura.18
Our data demonstrated that the second most common feature was cognitive impairment. Of 23 subjects with cognitive impairment, 12 subjects (16%) had dementia, and the mean age at assessment was 75.6 years (standard deviation: 6.5). Only 3 out of 12 patients with dementia had moderate to severe dementia. In contrast, Caucasian CADASIL studies showed that dementia starts between 50 and 60 years.3,19
Limitation of this study is that we focus on the clinical presentation of the patients with R544C mutation without imaging data. Moreover, the vascular risk factors may modulate clinical features in CADASIL.20 Further prospective studies with a uniform imaging protocol are needed to elucidate the clinical features of CADASIL patients with R544C mutation.
In conclusion, we reported the phenotypic feature of CADASIL patients with R544C mutation in Jeju to add up the evidence of later symptom onset in the NOTCH3 R544C mutation.
Acknowledgements
This work was supported by the research grant from Jeju National University Hospital.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh M, Balbi M, Hellal F, Dichgans M, Lindauer U, Plesnila N. Pericytes are involved in the pathogenesis of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Ann Neurol. 2015;78:887–900. doi: 10.1002/ana.24512. [DOI] [PubMed] [Google Scholar]
- 3.Chabriat H, Vahedi K, Iba-Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet. 1995;346:934–939. doi: 10.1016/s0140-6736(95)91557-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Kang CH, Park SQ, Choi HA, Sim KB. Clinical significance of cerebral microbleeds locations in CADASIL with R544C NOTCH3 mutation. PLoS One. 2015;10:e0118163. doi: 10.1371/journal.pone.0118163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liao YC, Hsiao CT, Fuh JL, Chern CM, Lee WJ, Guo YC, et al. Characterization of CADASIL among the Han Chinese in Taiwan: distinct genotypic and phenotypic profiles. PLoS One. 2015;10:e0136501. doi: 10.1371/journal.pone.0136501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 7.Adib-Samii P, Brice G, Martin RJ, Markus HS. Clinical spectrum of CADASIL and the effect of cardiovascular risk factors on phenotype: study in 200 consecutively recruited individuals. Stroke. 2010;41:630–634. doi: 10.1161/STROKEAHA.109.568402. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Lee KU, Lee DY, Kim KW, Jhoo JH, Kim JH, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. 2002;57:P47–P53. doi: 10.1093/geronb/57.1.p47. [DOI] [PubMed] [Google Scholar]
- 9.Oberstein SA, Ferrari MD, Bakker E, van Gestel J, Kneppers AL, Frants RR, et al. Diagnostic Notch3 sequence analysis in CADASIL: three new mutations in Dutch patients. Dutch CADASIL Research Group. Neurology. 1999;52:1913–1915. doi: 10.1212/wnl.52.9.1913. [DOI] [PubMed] [Google Scholar]
- 10.Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127(Pt 11):2533–2539. doi: 10.1093/brain/awh282. [DOI] [PubMed] [Google Scholar]
- 11.Mykkänen K, Savontaus ML, Juvonen V, Sistonen P, Tuisku S, Tuominen S, et al. Detection of the founder effect in Finnish CADASIL families. Eur J Hum Genet. 2004;12:813–819. doi: 10.1038/sj.ejhg.5201221. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi S, Zicari E, Carluccio A, Di Donato I, Pescini F, Nannucci S, et al. CADASIL in central Italy: a retrospective clinical and genetic study in 229 patients. J Neurol. 2015;262:134–141. doi: 10.1007/s00415-014-7533-2. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Zuo Y, Sun W, Zhang W, Lv H, Huang Y, et al. The genetic spectrum and the evaluation of CADASIL screening scale in Chinese patients with NOTCH3 mutations. J Neurol Sci. 2015;354:63–69. doi: 10.1016/j.jns.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Ueda A, Ueda M, Nagatoshi A, Hirano T, Ito T, Arai N, et al. Genotypic and phenotypic spectrum of CADASIL in Japan: the experience at a referral center in Kumamoto University from 1997 to 2014. J Neurol. 2015;262:1828–1836. doi: 10.1007/s00415-015-7782-8. [DOI] [PubMed] [Google Scholar]
- 15.Kim YE, Yoon CW, Seo SW, Ki CS, Kim YB, Kim JW, et al. Spectrum of NOTCH3 mutations in Korean patients with clinically suspicious cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging. 2014;35:726.e1–726.e6. doi: 10.1016/j.neurobiolaging.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Vahedi K, Chabriat H, Levy C, Joutel A, Tournier-Lasserve E, Bousser MG. Migraine with aura and brain magnetic resonance imaging abnormalities in patients with CADASIL. Arch Neurol. 2004;61:1237–1240. doi: 10.1001/archneur.61.8.1237. [DOI] [PubMed] [Google Scholar]
- 17.Chabriat H, Tournier-Lasserve E, Vahedi K, Leys D, Joutel A, Nibbio A, et al. Autosomal dominant migraine with MRI white-matter abnormalities mapping to the CADASIL locus. Neurology. 1995;45:1086–1091. doi: 10.1212/wnl.45.6.1086. [DOI] [PubMed] [Google Scholar]
- 18.Choi JC, Song SK, Lee JS, Kang SY, Kang JH. Headache among CADASIL patients with R544C mutation: prevalence, characteristics, and associations. Cephalalgia. 2014;34:22–28. doi: 10.1177/0333102413497598. [DOI] [PubMed] [Google Scholar]
- 19.Dichgans M, Mayer M, Uttner I, Brüning R, Müller-Höcker J, Rungger G, et al. The phenotypic spectrum of CADASIL: clinical findings in 102 cases. Ann Neurol. 1998;44:731–739. doi: 10.1002/ana.410440506. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Chio JC, Kang SY, Na HR, Kang JH. Vascular risk factors in CADASIL patients with Notch R544C mutation. Dement Neurocogn Dis. 2009;8:98–103. [Google Scholar]