Abstract
Background and Purpose
Apathy is one of the most common neuropsychiatric symptoms in patients with Alzheimer's disease (AD). It may have adverse impacts on the progression of AD. However, its neurobiological underpinnings remain unclear. The objective of this study was to investigate differences in regional cerebral blood flow (rCBF) between AD patients with apathy and those without apathy.
Methods
Sixty-six apathetic AD patients and 66 AD patients without apathy completed Neuropsychiatric Inventory (NPI) and underwent technetium-99m hexamethylpropylene amine oxime single-photon emission computed tomography (SPECT) scans. Voxel-wise differences in rCBF between the 2 groups were examined. Association between rCBF and levels of apathy in the apathetic group was also assessed.
Results
AD patients with apathy showed lower rCBF in the bilateral orbitofrontal cortex, left putamen, left nucleus accumbens, left thalamus, and bilateral insula than those without (all p<0.005). Mean perfusion across all significant clusters showed a negative linear correlation with NPI apathy score in AD patients with apathy (β=−0.25; p=0.04).
Conclusions
Hypoperfusion in the prefrontal, striatal, and insular areas may be neural correlates of apathy in AD patients.
Keywords: Alzheimer's Disease; Apathy; Tomography, Emission-Computed, Single-Photon; Regional Blood Flow
INTRODUCTION
Besides cognitive impairment, Alzheimer's disease (AD) is often accompanied by various neuropsychiatric symptoms in both early and late stages.1 For instance, up to 88% of AD patients suffer from one or more neuropsychiatric symptoms over the course of the disease.2 These symptoms can complicate the course of AD and adversely affect quality of life of both patients and caregivers.3
Apathy characterized by reduced motivation, decreased initiative and interest, and blunting of emotions4 is one of the most frequent neuropsychiatric symptoms in AD patients.2 Apathy in AD is related to faster cognitive deterioration, reduced daily functioning, early institutionalization, increased mortality risk, higher caregiver distress, and increased economic burden.5 In addition, apathy is a major barrier to the management of AD patients since they may receive less benefit from non-pharmacological therapies.6
Previous neuroimaging studies have identified neurobiological correlates of apathy in AD during the past decades. Among various imaging modalities, single-photon emission computed tomography (SPECT) has been used extensively to examine brain perfusion. Although hypoperfusion in prefrontal-subcortical regions including the anterior cingulate and orbitofrontal cortices in AD patients with apathy has been suggested,7 this remains controversial. Moreover, most studies were performed with relatively limited number of patients.7
The objective of the present study was to investigate differences in regional cerebral blood flow (rCBF) between AD patients with apathy and those without apathy using perfusion SPECT. Furthermore, correlation between rCBF and levels of apathy in apathetic AD patients was examined.
METHODS
Participants
Patients with AD were recruited from Incheon St. Mary's Hospital (Incheon, South Korea). The diagnosis for probable AD was made based on the Diagnostic and Statistical Manual of Mental Disorders-IV8 and the National Institute of Neurological and Communicative Disorders and Stroke-AD Related Disorders Association criteria.9 AD patients who scored 0 on apathy item of the Neuropsychiatric Inventory (NPI)10 were assigned to “AD without apathy” group while those who scored 1 or above on apathy item were assigned to “AD with apathy” group. Patients with history of head trauma, stroke, epilepsy, mixed or vascular dementia, other neurological or psychiatric disorders were excluded from this study. This study was approved by the Institutional Review Board of Incheon St. Mary's Hospital (OC11OISI0098), and all participants provided written informed consent.
Clinical assessment
Neurologists conducted clinical examinations including medical history and physical assessment. Cognitive performance and neuropsychiatric symptoms were evaluated with Mini-Mental State Examination (MMSE)11 and NPI,10 respectively. Global severity of dementia was assessed with Clinical Dementia Rating (CDR)12 and CDR-Sum of Boxes (CDR-SOB).
SPECT image acquisition
Brain perfusion SPECT images were acquired using a dual-headed rotating gamma camera (Discovery NM630, GE Healthcare, Milwaukee, WI, USA) equipped with a low-energy fan-beam collimator. Patients were administered intravenously with 555 MBq of technetium-99m hexamethylpropylene amine oxime approximately 20 minutes before scanning. Patients were in supine resting state with their eyes open during the scan. Images were taken at a rate of 12 seconds per frame by rotating the camera a total of 720° at 6-degree intervals. Continuous transaxial images were reconstructed using the standard ordered subset expectation maximization (OSEM 6 iterations, 10 subsets) algorithm with a Butterworth filter (cutoff frequency of 0.5 cycles/pixel, power 10) to reduce noise. Matrix size, pixel size, field of view, slice thickness, and energy windows of reconstructed images were 128×128, 1.95 mm×1.95 mm, 250 mm, 2.08 mm, and 140 keV±20%, respectively. Chang's attenuation correction method was used for correction of tissue attenuation.13
SPECT image analysis
Statistical Parametric Mapping 12 (SPM; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) was used for image analysis. All SPECT images were spatially normalized to SPM SPECT template (Montreal Neurological Institute, McGill University, Montreal, Canada) and resliced with a voxel size of 2 mm×2 mm×2 mm. Images were then smoothed with an isotropic Gaussian kernel (12 mm full-width at half-maximum). Voxel intensities were scaled to the mean intensity of the cerebellum using Automated Anatomical Labeling atlas.14,15,16 Global counts were normalized using proportional scaling to a mean value of 50 mL/100 g/min. Group differences in rCBF were assessed using analysis of covariance with age, sex, and MMSE score as covariates. Height threshold was set at p<0.005 while extent threshold was 100 or more contiguous voxels.
Statistical analysis
Independent t-test and χ2 test were used for comparisons of continuous and categorical variables between the 2 groups, respectively. In AD with apathy group, linear regression was performed to examine the association between mean perfusion across all significant clusters and NPI apathy score. All statistical analyses were conducted with Stata version 13.1 (StataCorp., College Station, TX, USA). A 2-tailed p<0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics
A total of 66 apathetic AD patients and 66 AD patients without apathy were included in the analysis. Demographic and clinical characteristics of these patients are summarized in Table 1. Age (t=−0.09; p=0.92), sex (χ2=1.84; p=0.18), or education (t=0.40; p=0.69) did not differ between the 2 groups. There were no significant differences in scores of MMSE (t=0.28; p=0.78), CDR (χ2=0.33; p=0.57), or CDR-SOB (t=0.68; p=0.50) between the 2 groups. NPI scores except apathy score (t=11.28; p<0.001) did not differ between the 2 groups either.
Table 1. Demographic and clinical characteristics of participants.
| Characteristics | AD with apathy (n=66) | AD without apathy (n=66) | Statistics | |
|---|---|---|---|---|
| Age (yr) | 76.1±7.2 | 76.2±9.2 | t=−0.09; p=0.92 | |
| Sex (male/female) | 22/44 | 15/51 | χ2=1.84; p=0.18 | |
| Years of education (yr) | 5.9±4.5 | 5.6±4.3 | t=0.40; p=0.69 | |
| MMSE | 19.0±4.6 | 18.8±4.6 | t=0.28; p=0.78 | |
| CDR | χ2=0.33; p=0.57 | |||
| 0.5 | 48 | 45 | ||
| 1 | 18 | 21 | ||
| CDR-SOB | 3.3±1.9 | 3.1±2.0 | t=0.68; p=0.50 | |
| NPI | ||||
| Delusions | 0.6±1.7 | 0.9±2.3 | t=−0.64; p=0.52 | |
| Hallucinations | 0.4±1.3 | 0.6±2.0 | t=−0.63; p=0.53 | |
| Agitation | 0.9±1.4 | 1.1±2.2 | t=−0.75; p=0.45 | |
| Depression | 1.6±2.4 | 1.1±1.7 | t=1.33; p=0.18 | |
| Anxiety | 1.3±2.1 | 1.0±1.9 | t=0.81; p=0.42 | |
| Elation | 0.2±0.8 | 0.1±0.5 | t=1.05; p=0.30 | |
| Apathy | 4.3±3.1 | 0.0±0.0 | t=11.28; p<0.001 | |
| Disinhibition | 0.6±1.6 | 0.5±1.2 | t=0.12; p=0.90 | |
| Irritability | 1.8±2.4 | 1.4±2.1 | t=1.00; p=0.32 | |
| Aberrant motor behavior | 1.3±2.9 | 1.7±3.6 | t=−0.66; p=0.51 | |
| Sleep disorders | 1.8±3.0 | 1.5±2.9 | t=0.65; p=0.51 | |
| Eating disorders | 2.2±3.0 | 1.4±2.5 | t=1.51; p=0.13 | |
AD: Alzheimer's disease, MMSE: Mini-Mental State Examination, CDR: Clinical Dementia Rating, CDR-SOB: Clinical Dementia Rating-Sum of Boxes, NPI: Neuropsychiatric Inventory.
Differences in brain perfusion
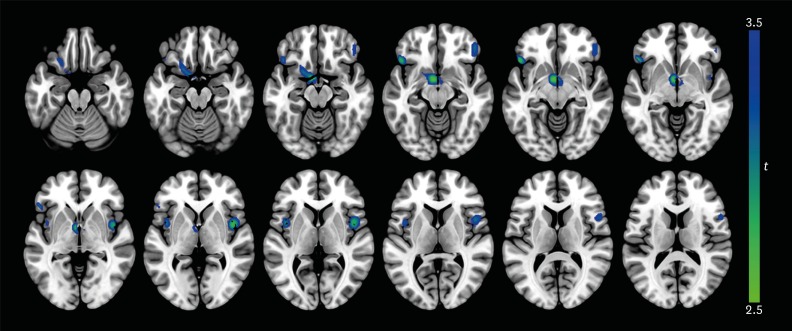
AD patients with apathy showed lower rCBF in the bilateral orbitofrontal cortex, left putamen, left nucleus accumbens, left thalamus, and bilateral insula (all p<0.005) than those without apathy (Table 2 and Fig. 1). However, higher rCBF was not found in AD with apathy group. The mean perfusion across all significant clusters showed a negative correlation with NPI apathy score in AD patients with apathy (β=−0.25; p=0.04).
Table 2. Differences in regional cerebral blood flow between AD patients with apathy and those without apathy.
| Regions | t | p | Coordinates* (x, y, z) | Cluster size (voxels) | |
|---|---|---|---|---|---|
| AD with apathy > AD without apathy | |||||
| None | |||||
| AD with apathy < AD without apathy | |||||
| L orbitofrontal cortex, putamen, nucleus accumbens, thalamus | 3.47 | <0.001 | −6, 0, −10 | 557 | |
| R insula | 3.31 | 0.001 | 44, 4, 2 | 334 | |
| L orbitofrontal cortex | 3.27 | 0.001 | −48, 26, −10 | 179 | |
| L insula | 3.07 | 0.001 | −42, 4, 4 | 102 | |
| R frontal pole, orbitofrontal cortex | 2.92 | 0.002 | 46, 40, −12 | 121 | |
AD: Alzheimer's disease, L: left, R: right.
*The coordinates refer to the Montreal Neurological Institute coordinate system.
Fig. 1. Differences in regional cerebral blood flow between AD patients with apathy and those without apathy. At each voxel, decreases in brain perfusion in AD patients with apathy appear in blue-green. The height threshold is set at p<0.005 and extent threshold is 100 or more contiguous voxels. Images are shown in neurological convention. Color bar represents voxel-level t-values.
AD: Alzheimer's disease.
DISCUSSION
In the present study, we investigated differences in rCBF between AD patients with apathy and those without apathy using brain perfusion SPECT. AD patients with apathy demonstrated lower cerebral perfusion in the orbitofrontal cortex, putamen, nucleus accumbens, thalamus, and insula compared to those without apathy. Moreover, reduced rCBF in those areas was linearly associated with more severe apathy. These results suggest that hypoperfusion in the prefrontal, striatal, and insular regions may be implicated in the pathophysiology of apathy in AD patients.
Previous studies have consistently reported hypoperfusion, hypometabolism, and atrophy of the orbitofrontal cortex in AD patients with apathy.7 The orbitofrontal cortex is a part of the prefrontal-striatal network. It evaluates action and reward by integrating sensory, affective, and motivational information.17 Specifically, this area recognizes salient stimuli and modulates responses to these stimuli based on one's motivation. Dysfunction of the orbitofrontal cortex may lead to impairment of decision-making and response inhibition that are characteristics of apathy.7
In this study, AD patients with apathy were found to have lower rCBF in the putamen, nucleus accumbens, and thalamus than those without apathy, similar to results of previous studies showing hypoperfusion of the basal ganglia18 and hypometabolism of the thalamus19 in apathetic AD patients. Other studies have suggested that gray matter volume losses in the putamen and nucleus accumbens are correlated with apathy in patients with AD20 and Parkinson's disease (PD),21 respectively. The prefrontal-striatal network is organized as feed-forward loop from the prefrontal cortex to the striatum which in turn projects to the thalamus and backs to the prefrontal region. This network is important for goal-directed behavior and executive function. Damage to this circuit may lead to apathy through cognitive, emotional, and motivational impairments.22 For instance, striatal dopamine transporter levels are negatively associated with apathy in patients with AD or dementia with Lewy body.23 The commonest behavioral symptom in patients with basal ganglia lesions is also apathy.24 Furthermore, dysfunction of the prefrontal-striatal network has been implicated in apathy among patients with other neuropsychiatric disorders including frontotemporal dementia, schizophrenia, and mood disorders.25
Associations between the insula and apathy have been reported in previous studies on various neurodegenerative disorders. Apathy is related to atrophy of the insula in AD and progressive supranuclear palsy26 as well as PD.27 Patients with PD also show apathy-related hypometabolism in the insula.28 In healthy volunteers, insula activation during volitional behavior tasks has been observed.29 The insula is closely involved not only in high-level cognitive control, attentional processes, and regulation of emotion and behavior, but also in metacognitive process of self-awareness by integrating external sensory stimuli with internal states.30 Although the exact role of the insula in apathetic patients remains unclear, dysfunction of the insula may lead to impaired insight and self-awareness which in turn may result in apathy.31
Potential limitations of this study are as follows. First, the cross-sectional design of the present study does not permit causal inferences. Further longitudinal studies are warranted to disentangle pre-existing vulnerabilities and acquired deficits. Second, the definition of apathy depends on specific assessment tools used. Thus, generalization across other evaluation methods could be important. Third, psychotropic medication was not considered in this study. It might influence the results.
In conclusion, the results of this study demonstrated that hypoperfusion in the prefrontal, striatal, and insular areas was associated with apathy in AD patients, controlling for demographic characteristics, cognitive performance, and other neuropsychiatric symptoms. The findings of this study suggest that dysfunction in these regions may be underlying neural correlates of apathy in AD patients.
Footnotes
Funding: This research was supported by a grant (NRF-2015M3C7A1064832) of the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, Republic of Korea.
Conflicts of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Jeong H, Chung YA, Song IU.
- Data curation: Jeong H, Im JJ, Park JS, Na SH, Heo Y, Yang Y.
- Formal analysis: Jeong H, Kang I, Im JJ.
- Funding acquisition: Chung YA.
- Writing - original draft: Jeong H, Kang I, Im JJ.
- Writing - review & editing: Jeong H, Kang I, Im JJ, Park JS, Na SH, Heo Y, Yang Y, Chung YA, Song IU.
References
- 1.Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement. 2011;7:532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer's disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 3.Shin IS, Carter M, Masterman D, Fairbanks L, Cummings JL. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:469–474. doi: 10.1176/appi.ajgp.13.6.469. [DOI] [PubMed] [Google Scholar]
- 4.Starkstein SE, Petracca G, Chemerinski E, Kremer J. Syndromic validity of apathy in Alzheimer's disease. Am J Psychiatry. 2001;158:872–877. doi: 10.1176/appi.ajp.158.6.872. [DOI] [PubMed] [Google Scholar]
- 5.Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer's disease. J Am Geriatr Soc. 2001;49:1700–1707. doi: 10.1046/j.1532-5415.2001.49282.x. [DOI] [PubMed] [Google Scholar]
- 6.Brodaty H, Burns K. Nonpharmacological management of apathy in dementia: a systematic review. Am J Geriatr Psychiatry. 2012;20:549–564. doi: 10.1097/JGP.0b013e31822be242. [DOI] [PubMed] [Google Scholar]
- 7.Theleritis C, Politis A, Siarkos K, Lyketsos CG. A review of neuroimaging findings of apathy in Alzheimer's disease. Int Psychogeriatr. 2014;26:195–207. doi: 10.1017/S1041610213001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Forth edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 9.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 10.Choi SH, Na DL, Kwon HM, Yoon SJ, Jeong JH, Ha CK. The Korean version of the neuropsychiatric inventory: a scoring tool for neuropsychiatric disturbance in dementia patients. J Korean Med Sci. 2000;15:609–615. doi: 10.3346/jkms.2000.15.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 12.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 13.Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;25:638–643. [Google Scholar]
- 14.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 15.Pickut BA, Dierckx RA, Dobbeleir A, Audenaert K, Van Laere K, Vervaet A, et al. Validation of the cerebellum as a reference region for SPECT quantification in patients suffering from dementia of the Alzheimer type. Psychiatry Res. 1999;90:103–112. doi: 10.1016/s0925-4927(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 16.Soonawala D, Amin T, Ebmeier KP, Steele JD, Dougall NJ, Best J, et al. Statistical parametric mapping of (99m)Tc-HMPAO-SPECT images for the diagnosis of Alzheimer's disease: normalizing to cerebellar tracer uptake. Neuroimage. 2002;17:1193–1202. doi: 10.1006/nimg.2002.1259. [DOI] [PubMed] [Google Scholar]
- 17.Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 18.Lopez OL, Zivkovic S, Smith G, Becker JT, Meltzer CC, DeKosky ST. Psychiatric symptoms associated with cortical-subcortical dysfunction in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 2001;13:56–60. doi: 10.1176/jnp.13.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64:1015–1020. doi: 10.1001/archneur.64.7.1015. [DOI] [PubMed] [Google Scholar]
- 20.Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer's disease. Brain. 2008;131:2455–2463. doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- 21.Carriere N, Besson P, Dujardin K, Duhamel A, Defebvre L, Delmaire C, et al. Apathy in Parkinson's disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Mov Disord. 2014;29:897–903. doi: 10.1002/mds.25904. [DOI] [PubMed] [Google Scholar]
- 22.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 23.David R, Koulibaly M, Benoit M, Garcia R, Caci H, Darcourt J, et al. Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases A SPECT study with partial volume effect correction. Clin Neurol Neurosurg. 2008;110:19–24. doi: 10.1016/j.clineuro.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. doi: 10.1093/brain/117.4.859. [DOI] [PubMed] [Google Scholar]
- 25.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanton BR, Leigh PN, Howard RJ, Barker GJ, Brown RG. Behavioural and emotional symptoms of apathy are associated with distinct patterns of brain atrophy in neurodegenerative disorders. J Neurol. 2013;260:2481–2490. doi: 10.1007/s00415-013-6989-9. [DOI] [PubMed] [Google Scholar]
- 27.Reijnders JS, Scholtissen B, Weber WE, Aalten P, Verhey FR, Leentjens AF. Neuroanatomical correlates of apathy in Parkinson's disease: a magnetic resonance imaging study using voxel-based morphometry. Mov Disord. 2010;25:2318–2325. doi: 10.1002/mds.23268. [DOI] [PubMed] [Google Scholar]
- 28.Robert G, Le Jeune F, Lozachmeur C, Drapier S, Dondaine T, Péron J, et al. Apathy in patients with Parkinson disease without dementia or depression: a PET study. Neurology. 2012;79:1155–1160. doi: 10.1212/WNL.0b013e3182698c75. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- 30.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aalten P, van Valen E, Clare L, Kenny G, Verhey F. Awareness in dementia: a review of clinical correlates. Aging Ment Health. 2005;9:414–422. doi: 10.1080/13607860500143075. [DOI] [PubMed] [Google Scholar]