Abstract
Background and Purpose
During the Vietnam War, many Korean soldiers were exposed to Agent Orange. Until now, there existed only limited evidence of association between exposure to Agent Orange and Alzheimer's disease (AD). The main pathological feature of AD is brain amyloidosis. To explore the pathophysiological characteristic of AD with Agent Orange exposure, we compared newly developed amyloid beta (Aβ) oligomer levels in plasma between AD with Agent Orange exposure and without exposure.
Methods
We recruited 48 AD patients with Agent Orange exposure and 66 AD patients without Agent Orange. Using the Multimer Detection System technique, which was based on an enzyme-linked immunosorbent assay, we measured Aβ oligomers in the plasma of study subjects.
Results
Compared to normal control patients, plasma Aβ oligomer levels were higher in AD patients regardless of history of Agent Orange exposure. However, AD patients with Agent Orange exposure showed higher plasma Aβ oligomer levels than AD patients without Agent Orange.
Discussion
This study showed higher plasma Aβ oligomer levels in AD patients with Agent Orange exposure compared to AD patients without Agent Orange. This finding suggests the possibility of a different pathophysiology of AD patients with Agent Orange exposure from AD patients without Agent Orange.
Keywords: Agent Orange, Alzheimer's Disease, Plasma Aβ Oligomer
INTRODUCTION
The U.S. Armed Forces sprayed considerable amounts of Agent Orange, a defoliant, from 1962 to 1971, in order to achieve strategic objectives in the Vietnam War. At the time, the Korean army fought in the Vietnam War as an ally of the U.S. Armed Forces and many soldiers and civilians were exposed to Agent Orange. Several mechanical studies and animal experiments revealed that 2,3,7,8-tetrachlorodibenzodioxin (TCDD), one of the main components of Agent Orange, is a very toxic substance that has various effects on the human body. Further TCDD use was then prohibited. However, it was reported that various diseases occurred in people who were exposed to TCDD. Degenerative diseases of the central nervous system (CNS), such as Parkinson's disease and Alzheimer's disease (AD), were frequently observed among those who were exposed to TCDD in the half century after the Vietnam War ended. Whether occurrence of such degenerative diseases of the CNS is related to Agent Orange, or is just happening as a result of aging, is not known. One study reported that a person exposed to Agent Orange had a higher risk of AD than a person not exposed to Agent Orange (odds ratio, 1.64).1 A recent study showed that patients with Parkinson's disease, who were exposed to Agent Orange, were different from patients with idiopathic Parkinson's disease, as identified from a fluorinated-N-3-fluoropropyl-2-b-carboxymethoxy-3-b-(4-iodophenyl) nortropane (FP-CIT) positron emission tomography (PET).2 However, another study reported that whether TCDD has an effect on the CNS is not certain yet.3
It has been reported that the number of patients with AD rose sharply among those who were exposed to Agent Orange. It is, however, not certain whether Agent Orange, which they were exposed to a long time ago, was correlated with AD. It is ideal to check biological markers from those who were exposed to Agent Orange and, using this registry, follow up for various disease occurrences including AD. However, practically, it is not possible in our patient groups. Instead, we compared characteristics of AD patients who were exposed to Agent Orange with AD patients who were not exposed to it and checked the difference, in order to find out the level of Agent Orange involvement in AD development.
Amyloidosis, that occurs in the brain, is a main characteristic of AD.4 Many experts suggest that amyloids are connected with AD and amyloid plaques (Ab; mainly b-1-40 and b-1-42) are closely related to the pathophysiology of AD. According to recent studies, amyloid beta (Aβ) oligomers, soluble small aggregates, rather than amyloid fibril, are more related to the pathophysiology of AD.5,6,7,8,9
Blood bio-marker testing, which has been commonly used, is safe, simple and inexpensive but there is a limit in using blood bio-marker testing because it is not directly related to the pathological mechanism of AD.10,11,12 However, it has become possible to detect and quantify Aβ oligomers, of patients with AD, which have passed the BBB reliably.26 This suggests amyloid beta 1-42 (Aβ42) of CSF, which is used as an amyloid biomarker, or PET images of amyloid, can be used in studying the diagnosis of AD, pathology of AD and drug efficacy, by measuring the levels of it in plasma.
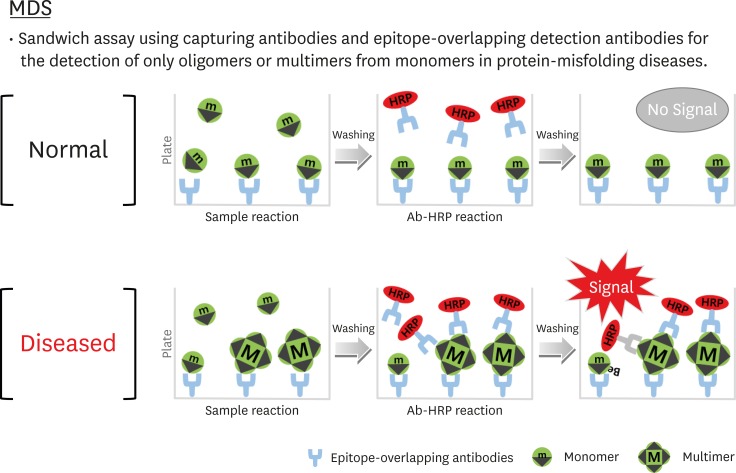
The Multimer Detection System (MDS) is an enzyme-linked immunosorbent assay (ELISA) that is used for measuring Aβ oligomer in plasma. The MDS was originally developed to detect the prion oligomer in blood which was infected with scrapie.13,14 The MDS is similar to the Sandwich ELISA in that it is used to detect polymers instead of detection and capture antibodies (Fig. 1). One epitope exists as a form of a polymer while one exists as a monomer protein. The MDS was developed to detect only the Aβ oligomer by using an epitope which overlaps with N-end Aβ. A recent study reported that the sensitivity and specificity of diagnosis of AD using the method were 79.17% and 82.76%, respectively, which suggests that the MDS is useful.26
Fig. 1. The MDS concept. (A) Monomers form proteins that have a single epitope that is captured by an antibody (capturing antibody: 6E10) attached to the surface of the plate. After adding a detection antibody (FF51-HRP), monomer proteins cannot be detected because the single epitope is already occupied. (B) Multimers with numerous epitopes can be detected by detection antibodies. The capturing and detection antibodies are different, but their epitopes overlap.
MDS: Multimer Detection System.
This study aims to compare Aβ oligomer levels, which is a main pathophysiology of AD, among patients with AD who were exposed to Agent Orange, patients with AD who were not exposed to Agent Orange, and people who do not have AD.
METHODS
Subjects
Forty-eight people with AD who were exposed to Agent Orange and visited VHS Medical Center, 66 people with AD who were not exposed to Agent Orange and visited VHS Medical Center, and 60 people who do not have AD and visited VHS Medical Center, from December 2015 to November 2016, were registered for this study. All participants underwent a clinical assessment, a neuropsychological test, a magnetic resonance imaging (MRI) test and a fluorodeoxyglucose (FDG)-PET computed tomography (CT), in order to accurately diagnose AD.
The AD group met the following inclusion criteria: 1) probable AD by National Institute on Aging-Alzheimer's Association (NIA-AA) criteria; 2) age between 50 and 90 years; 3) ≥6 years of education; 4) Clinical Dementia Rating (CDR) from 0.5 to 2, and CDR - sum of box ≥2.5; 5) modified Hachinski Ischemia Score ≤4; and 6) having a caregiver who knew the patient well.
The inclusion criteria of the normal controls (NC) group were as follows: 1) subjects who are between the ages 50 and 90 years, and who do not have health factors which influence cognitive performance 2) score on the Mini-Mental State Examination (MMSE) above 1.0 standard deviation below the mean for their age and education-matched norm; 3) ≥6 years of education; 4) Short Form Geriatric Depression score ≤7; and 5) not exposed to defoliant.
We excluded subjects if they had: 1) significant or unstable medical problems; 2) psychiatric problems; 3) a cardiac pacemaker; 4) a history of substance abuse or dependence within the past 10 years, and/or 5) an occupational cluster that is likely to be exposed to an herbicide or insecticide containing dioxin.
A perceived exposure index, determined by a veteran's self-report survey and military records, was used to define AD patients that were exposed to Agent Orange.15 Varying degrees of being exposed to Agent Orange were classified as group 1, 2, 3, and 4 and were determined through the use of a self-reporting questionnaire. In order to minimize possibilities in the exposure group, only ‘moderate’ and ‘high’ exposure groups were defined as the “Agent Orange Exposure” group. The group that was not exposed to Agent Orange included a group that was not exposed to Agent Orange and a group that was not exposed to dioxin. This study was approved by the Institutional Review Board (IRB) of the VHS Medical Center. Participants of this study submitted written consent.
Blood sampling
We collected 10 milliliters of venous blood in sodium heparin-containing tubes and centrifuged them at 850×g for 30 minutes. The plasma supernatant was aliquoted and stored in 0.5 mL polypropylene tubes at −80°C until analysis.
MDS
The MDS technique is a sandwich ELISA using epitope-overlapping N-terminal Aβ antibodies for capturing and detecting the antigen. A monoclonal mouse antibody (6E10; BioLegend, San Diego, CA, USA), that was coated on a plate, and an FF51-HRP antibody (PeopleBio, Seoul, Korea) were used for capturing and detecting the antigen, respectively. The 6E10 antibody (3 µg/mL in carbonate-bicarbonate buffer, pH 9.6) was coated overnight at 4°C on a 96-well black plate. The plate was blocked for 2 hours with 100 µL 0.4% Block Ace (AbD Serotec, Raleigh, NC, USA) at room temperature. After washing three times with phosphate-buffered saline (PBS), the plate was stored at 4°C until use. Prior to the assay, each plasma sample was thawed at 37°C for 15 minutes. Then, 10 µL of plasma and 4.04 µL of heterophilic blocking reagent-1 (Scantibodies Laboratory, Santee, CA, USA), PBR-1, and phosphate buffered saline with Tween 20 (PBST) were mixed well. After incubation for 144 hours, plasma samples and control solutions (100 µL) were added to each well. The plate was incubated at room temperature for 1 hour. After washing three times with tris buffered saline with Tween 20 (TBST), the FF51-HRP in TBST containing 0.4% Block Ace was added and the plate was incubated for 1 hour at room temperature. To increase the detection sensitivity, we used 100 µL/well of enhanced chemiluminescent substrate. The developed signal was quantified with a Victor 3 luminometer (PerkinElmer, Waltham, MA, USA). Luminescent reaction data are expressed in relative luminescence units (RLU), which are typically proportional to the amount of analyte present in a sample.
Statistical analyses
Baseline characteristics were compared among groups using a 1-way analysis of variance test and chi-squared tests. For variables with a non-normal distribution (MMSE and MDS RLU), we used the Wilcoxon rank-sum test and a median value was calculated. All statistical analyses were conducted using STATA 14.0 software (StataCorp., College Station, TX, USA).
RESULTS
Characteristics of base value of patients
Table 1 shows the basic demographic characteristics of the AD patient group with Agent Orange exposure (n=48), without Agent Orange (n=66), and the group of people who did not have AD (n=60). There was no significant difference among the three groups in terms of age, gender, and years of education. There was no significant difference between AD patients with Agent Orange exposure and AD patients without Agent Orange in apolipoprotein E ε4 genetic variance (homozygote or heterozygote) and MMSE. There was a significant difference between the group with AD (regardless of Agent Orange exposure) and the NC group (group that did not have AD) in apolipoprotein E ε4 genetic variance and MMSE (Table 1).
Table 1. Baseline characteristics.
| Characteristics | AD with AO (n=48) | AD without AO (n=66) | NC (n=60) | p value |
|---|---|---|---|---|
| Age (yr) | 70.6±7.6 | 69.8±6.4 | 68.1±7.3 | 0.7145 |
| Male (%) | 60 (90.9%) | 47 (97.9%) | 53 (88.3%) | 0.064 |
| Education (yr) | 10.1±3.0 | 9.9±4.8 | 11.1±2.8 | 0.297 |
| MMSE score (median) | 17 | 18 | 29 | <0.0001 |
| ApoE ε4 carrier | 38 (57.5%) | 27 (56.2%) | 8 (13.3%) | 0.011 |
AD: Alzheimer's disease, AO: Agent Orange exposure, NC: normal controls, MMSE: Mini-Mental State Examination.
Difference in relative luminescence units of plasma Aβ oligomer among groups
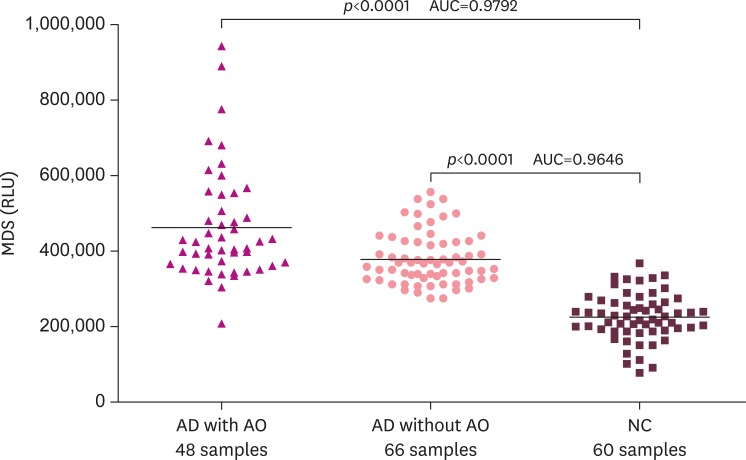
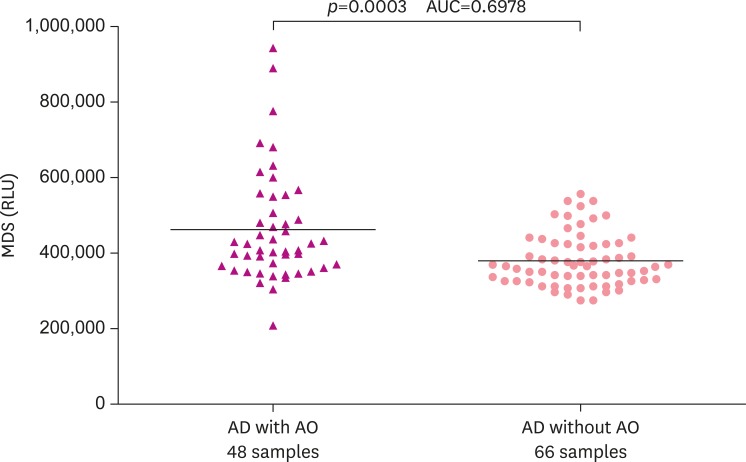
The group with AD was significantly higher than the control group in regards to the relative luminance units of Aβ oligomer in plasma regardless of exposure to Agent Orange (p<0.0001; Fig. 2). Plasma Aβ oligomer relative luminance units of AD patients with Agent Orange exposure were significantly higher than those of AD patients with no Agent Orange exposure (p<0.0003; Fig. 3).
Fig. 2. The distribution pattern of plasma Aβ oligomers determined by the MDS in the AD (AD with or without AO) and NC groups. The MDS RLU were higher in the AD without AO than the NC group (p<0.0001). The MDS RLU were higher in the AD with AO than the NC group (p<0.0001). The horizontal bar is the median MDS RLU. However, there was overlap between the 2 groups, suggesting that further optimization of the MDS is needed.
Aβ: amyloid beta, MDS: Multimer Detection System, AD: Alzheimer's disease, AO: Agent Orange exposure, NC: normal control, RLU: relative luminescence units, AUC: area under the curve.
Fig. 3. The distribution pattern of plasma Aβ oligomers determined by the MDS in AD without AO and AD with AO groups. The MDS RLU were higher in the AD with AO than AD without AO group (p<0.0003). The horizontal bar is the median MDS RLU. However, there was overlap between the 2 groups suggesting that further optimization of the MDS is needed.
Aβ: amyloid beta, MDS: Multimer Detection System, AD: Alzheimer's disease, AO: Agent Orange exposure, RLU: relative luminescence units, AUC: area under the curve.
DISCUSSION
There are few studies which report that Agent Orange or similar chemicals cause AD. Gauthier et al.16 reported that long exposure to herbicides and insecticides did not have a significant correlation with AD. Baldi et al.17 reported that the relationship between exposure to pesticides and AD was significant for males but not females (relative risk, 2.39; 95% confidence interval, 1.02–5.63). 17 A epidemiological study reported that Agent Orange is significantly related to the occurrence of AD (odds ratio, 1.64).1 In Parkinson's disease, which is classified as a neurodegenerative disease like AD, the FP-CIT PET of patients with Parkinson's disease who were exposed to Agent Orange was different from the FP-CIT PET of patients with Parkinson's disease who were not exposed to Agent Orange.2 However, there are few studies that report that Agent Orange is related to degenerative diseases of the CNS.
It has been reported that exposure to TCDD during developmental stages results in damage of the CNS.18 However, there are lots of studies which reported that TCDD had difficulties passing the BBB, although it had small molecular weight and lipophilic character. However, other studies reported that one of main targets of TCDD was the cerebral endothelium. Damage to the endothelium affects the BBB and the CNS may be exposed to this toxic substance.18,19 A study reported that patients who were exposed to TCDD had related neurocognitive dysfunction19,20 and the degree of exposure to this substance was proportional to level of neurocognitive dysfunction.15,21,22
Toxicologically, TCDD, which is the main component of Agent Orange, causes oxidative stress. Increased oxidative stress is related to AD, as well as normal aging. It has been reported that oxidative stress is closely related to the formation of the amyloid seen in AD. Accumulation of amyloid causes oxidative stress which then leads to the formation of more amyloid.23
It has been reported that Aβ oligomer, rather than amyloid, has an important role in the occurrence of AD. There are three types of Aβ oligomers (Aβ dimer, Aβ trimers, and Aβ*56) in brain tissue. When analyzing the correlation between the formation of Aβ oligomer and tau protein, it was found that Aβ*56 was closely related to the pathology of AD.6 It has been reported that Aβ oligomer plays a major role in the occurrence of AD by restricting the long synergy of the hippocampus, relating to the impairment of synapses and changing neuroplasticity.7,8
In this study, Aβ oligomer, which was reported to have a close relation to the pathology of AD, was measured in plasma by using an antibody-mediated detection system.24,25 Some previous studies reported that there was no significant difference between a group of patients with AD and a group of patients without AD, when measuring Aβ40 and Aβ42 in plasma.26 In this study, it was found that the amount of Aβ oligomer in plasma, in the group of patients with AD, was more than that of the control group. Interestingly, the Aβ oligomer plasma of AD patients with Agent Orange exposure was significantly higher than that of AD patients without Agent Orange. Such a tendency was also observed in patients with Parkinson's disease and Agent Orange exposure. A recent FP-CIT PET (biomarker of Parkinson's disease) study, reported that Parkinson's disease with Agent Orange exposure showed more impairment than Parkinson's disease without Agent Orange.2
Generally, acute exposure to a toxic substance has a wide effect on all parts of the nervous system while chronic exposure to a toxic substance may cause lesions on specific parts of the nervous system. Once Agent Orange is absorbed into the body, due to a long half-life (approximately 8 years), it is accumulated widely in fatty tissue. This wide accumulation, slow release, and degradation can cause affects to the CNS and brain-nervous system over long-term that may result in AD or Parkinson's disease, depending on the genetic characteristics or lifestyle of each patient.
The present study had several limitations. First, it is desirable to use reliable biological markers in order to check whether there was exposure to Agent Orange. Due to technical and economic problems, we used an indirect method of a self-report survey and military records to obtain this information. Because of these methods, falsified study inclusion cannot be rule out as a possibility, though the author classified the data carefully. Second, the number of subjects in this study was relatively small. Last, this study is based on only one hospital and therefore, studies that include more institutions are needed.
In conclusion, this study found that Aβ oligomer in plasma of AD patients with Agent Orange exposure was significantly higher than that of the control group, as well as that of AD patients with Agent Orange no-exposure. This suggests that Agent Orange might affect the pathology of AD. To further clarify this finding, it is necessary to conduct further studies on patients with normal cognitive function or mild cognitive impairment, who were exposed to Agent Orange.
Footnotes
Funding: This study was supported by a VHS Medical Center Research Grant, Republic of Korea (grant number: VHSMC 16001).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Yang Y, Kim S, An SS.
- Data curation: Yang Y.
- Formal analysis: Yang Y.
- Funding acquisition: Yang Y.
- Investigation: Yang Y.
- Methodology: Yang Y.
- Project administration: Yang Y.
- Resources: Yang Y.
- Software: Yang Y.
- Supervision: Kim S, An SS.
- Validation: Kim S, An SS.
- Visualization: Kim S, An SS.
- Writing - original draft: Yang Y.
- Writing - review & editing: Yang Y.
References
- 1.Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56–65. doi: 10.1016/j.envres.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Cheon M, Kwak YT. Is Parkinson's disease with history of Agent Orange exposure different from idiopathic Parkinson's Disease? Dement Neurocognitive Disord. 2016;15:75–81. doi: 10.12779/dnd.2016.15.3.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zober A, Ott MG, Messerer P. Morbidity follow up study of BASF employees exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) after a 1953 chemical reactor incident. Occup Environ Med. 1994;51:479–486. doi: 10.1136/oem.51.7.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, et al. Alzheimer's disease-affected brain: presence of oligomeric A β ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesné SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, et al. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain. 2013;136:1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson ME, Lesné SE. Soluble Aβ oligomer production and toxicity. J Neurochem. 2012;120(Suppl 1):125–139. doi: 10.1111/j.1471-4159.2011.07478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Abeta oligomers. FEBS J. 2010;277:1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 9.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 10.Apostolova LG, Hwang KS, Avila D, Elashoff D, Kohannim O, Teng E, et al. Brain amyloidosis ascertainment from cognitive, imaging, and peripheral blood protein measures. Neurology. 2015;84:729–737. doi: 10.1212/WNL.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder HM, Carrillo MC, Grodstein F, Henriksen K, Jeromin A, Lovestone S, et al. Developing novel blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10:109–114. doi: 10.1016/j.jalz.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doecke JD, Laws SM, Faux NG, Wilson W, Burnham SC, Lam CP, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69:1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An SS, Lim KT, Oh HJ, Lee BS, Zukic E, Ju YR, et al. Differentiating blood samples from scrapie infected and non-infected hamsters by detecting disease-associated prion proteins using Multimer Detection System. Biochem Biophys Res Commun. 2010;392:505–509. doi: 10.1016/j.bbrc.2010.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Lim K, Kim SY, Lee B, Segarra C, Kang S, Ju Y, et al. Magnetic microparticle-based multimer detection system for the detection of prion oligomers in sheep. Int J Nanomedicine. 2015;10:241–250. doi: 10.2147/IJN.S88377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decouflé P, Holmgreen P, Boyle CA, Stroup NE. Self-reported health status of Vietnam veterans in relation to perceived exposure to herbicides and combat. Am J Epidemiol. 1992;135:312–323. doi: 10.1093/oxfordjournals.aje.a116285. [DOI] [PubMed] [Google Scholar]
- 16.Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Environmental pesticide exposure as a risk factor for Alzheimer's disease: a case-control study. Environ Res. 2001;86:37–45. doi: 10.1006/enrs.2001.4254. [DOI] [PubMed] [Google Scholar]
- 17.Baldi I, Lebailly P, Mohammed-Brahim B, Letenneur L, Dartigues JF, Brochard P. Neurodegenerative diseases and exposure to pesticides in the elderly. Am J Epidemiol. 2003;157:409–414. doi: 10.1093/aje/kwf216. [DOI] [PubMed] [Google Scholar]
- 18.Konjuh C, García G, López L, de Duffard AM, Brusco A, Duffard R. Neonatal hypomyelination by the herbicide 2,4-dichlorophenoxyacetic acid. Chemical and ultrastructural studies in rats. Toxicol Sci. 2008;104:332–340. doi: 10.1093/toxsci/kfn085. [DOI] [PubMed] [Google Scholar]
- 19.Urban P, Pelclová D, Lukás E, Kupka K, Preiss J, Fenclová Z, et al. Neurological and neurophysiological examinations on workers with chronic poisoning by 2,3,7,8-TCDD: follow-up 35 years after exposure. Eur J Neurol. 2007;14:213–218. doi: 10.1111/j.1468-1331.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 20.Peper M, Klett M, Frentzel-Beyme R, Heller WD. Neuropsychological effects of chronic exposure to environmental dioxins and furans. Environ Res. 1993;60:124–135. doi: 10.1006/enrs.1993.1021. [DOI] [PubMed] [Google Scholar]
- 21.Byers JP, Masters K, Sarver JG, Hassoun EA. Association between the levels of biogenic amines and superoxide anion production in brain regions of rats after subchronic exposure to TCDD. Toxicology. 2006;228:291–298. doi: 10.1016/j.tox.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan C, Liu J, Nie X, Zhao J, Zhou S, Duan Z, et al. 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS One. 2014;9:e89811. doi: 10.1371/journal.pone.0089811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veloso AJ, Chow AM, Ganesh HV, Li N, Dhar D, Wu DC, et al. Electrochemical immunosensors for effective evaluation of amyloid-beta modulators on oligomeric and fibrillar aggregation processes. Anal Chem. 2014;86:4901–4909. doi: 10.1021/ac500424t. [DOI] [PubMed] [Google Scholar]
- 24.Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, et al. Monoclonal antibodies that target pathological assemblies of Abeta. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- 25.Shanthi KB, Krishnan S, Rani P. A systematic review and meta-analysis of plasma amyloid 1-42 and tau as biomarkers for Alzheimer's disease. SAGE Open Med. 2015;3:2050312115598250. doi: 10.1177/2050312115598250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SY, An SS, Youn YC, Kang SM, Lim KT, Yang YS, et al. Measurement of Aβ oligomer in plasma and a significant correspondence to diagnosis of CSF biomarkers and PIB-PET; Poster presented at the Alzheimer's Association International Conference; 2015 July 18??3; Washington, D.C.. Cambridge, MA: Alzforum; 2015. [Google Scholar]