Abstract
Background and Purpose
Neuropsychiatric symptoms (NPS) such as anxiety, depression, and delusions affect up to 90% of all patients with Alzheimer's disease (AD). NPS is associated with significant caregiver burden and patient distress. Given the severe burden of NPS in AD, it is critical to know potential modifiable risk factors of NPS in AD. This study explores the association between hypertension and NPS in patients with drug-naïve AD.
Methods
We reviewed medical records of 149 patients with AD with (n=80) and without (n=69) hypertension. NPS were assessed using the Korean version of Neuropsychiatric Inventory (K-NPI). Affective, psychotic, and behavior symptom clusters were assessed separately.
Results
The total score of K-NPI was not significantly different between patients with AD with and without hypertension. Among K-NPI domains, scores of depression/dysphoria (p=0.045), anxiety (p=0.022), and apathy/indifference (p=0.037) were significantly higher in patients with AD with hypertension. Systolic blood pressure (BP) was associated with higher total K-NPI and affective symptom cluster scores. Diastolic BP was associated with affective symptom cluster scores.
Conclusions
Results suggest that hypertension increases risk of specific NPS in patients with AD. Among NPS, hypertension was associated with affective symptom cluster.
Keywords: hypertension, Alzheimer's disease, neuropsychiatric symptoms
INTRODUCTION
Hypertension and dementia are common diseases in the elderly population. Approximately 8–10% of people age 65 or older suffer from dementia and 65% suffer from hypertension.1,2 In recent epidemiological studies, vascular risk factors have been associated with Alzheimer's disease (AD) as well as vascular dementia. In particular, hypertension is a major vascular risk factor, and studies on the relationship between hypertension, cognitive dysfunction, and onset of dementia are actively underway. Some cross-sectional observational studies have reported cognitive decline occurs frequently when blood pressure (BP) is high, especially when uncontrolled.3 Other studies have reported hypertension and cognitive function are correlated as U-curves, and that both very high and very low BP increase cardiovascular disease and consequently cognitive decline.4,5 In most follow-up studies, middle-aged hypertension was an independent risk factor for senile cognitive impairment and dementia.6,7,8,9
However, little is known about the relationship between modifiable risk factors and neuropsychiatric symptoms (NPS) in AD. Vascular risk factors, such as hypertension, hyperlipidemia, and stroke, are of interest because they are risk factors for NPS among individuals without AD.10 We investigated prevalence of NPS and the degree of Korean version of Neuropsychiatric Inventory (K-NPI) domain according to presence or absence of hypertension in patients with AD.
METHODS
Participant screening
This study was designed to retrospectively review medical records to evaluate prevalence of dementia at the Veterans Health Service Medical Center in Korea August 2011–August 2014, for a maximum of 3 years. Data were reviewed from the time of the first diagnosis of dementia. Patients with AD were selected according to criteria for ‘probable AD’ of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association.11 Patients with a history of head injury, mental illness, alcoholism, substance abuse, or other neurological or medical disorders that may impair cognitive function were excluded from the study. Patients were divided into two groups according to presence or absence of hypertension, and sociodemographic differences between patients and caregivers, memory, depression, and behavioral psychological symptoms were compared in each group. Presence of hypertension was documented by medical history. Participants with systolic BP more than 140 mm Hg and/or a diastolic BP more than 90 mm Hg were classified as hypertensive. A total of 149 subjects were enrolled in this study, 80 patients with AD with hypertension and 69 patients with AD without hypertension.
Clinical assessments
Patients were assessed by the Korean version of the Mini Mental State Examination (K-MMSE) to determine overall cognitive function.12,13 Overall disease progression was assessed using the Clinical Dementia Rating Scale Sum of Boxes (CDR-SOB) score.14 For evaluation of NPS, 12 domains were analyzed using the K-NPI.15 The 12 domains were delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behavior, night-time behavior disturbances, and appetite/eating abnormalities. We examined the total score of K-NPI, the score of each domain of K-NPI, and prevalence. For primary endpoints, we assessed prevalence of NPS in patients with AD with or without hypertension. For secondary endpoints, we examined the extent to which domains of K-NPI correlated with hypertension in patients with AD. This study was approved by the Institutional Review Board of Veteran Health Service Medical Center and met standards established by the Declaration of Helsinki (IRB No. 2015-04-001).
Data analysis
Collected data were analyzed using Statistical Package for the Social Sciences (SPSS) version 21.0 (IBM Corp., Armonk, NY, USA). Two-group homogeneity tests were analyzed by χ2-test, Fisher's exact test, and Paired t-test, and differences in NPS between the two groups were analyzed by Paired t-test. Principal component factor analysis was used to test validity of K-NPI. The relationship between NPS, domains of K-NPI, and hypertension was analyzed by Pearson's correlation. The p values of less than 0.05 were considered to indicate statistically significant differences.
RESULTS
Homogeneity test of general characteristics and dependent variables between two groups
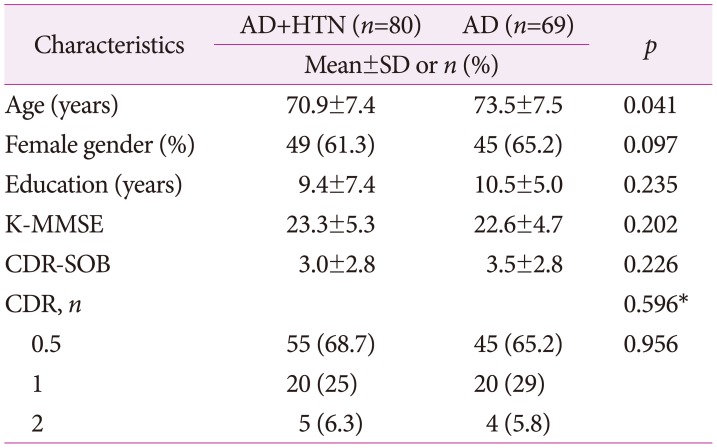
A total of 149 subjects were enrolled in this study, 80 of whom were patients with AD with hypertension and 69 patients with AD without hypertension. Table 1 compares clinical characteristics of patients with AD with and without hypertension. There was no statistically significant difference in sex ratio, duration of education, progression of dementia, cognitive function test between two groups. Both groups revealed homogeneity in general characteristics except for age.
Table 1. Homogeneity test of general characteristics and dependent variables between two groups (n=149).
*Fisher's exact test.
AD: Alzheimer's disease, CDR: Clinical Dementia Rating Scale, CDR-SOB: Clinical Dementia Rating Scale Sum of Boxes, HTN: hypertension, K-MMSE: Korean version of the Mini-Mental State Examination, SD: standard deviation.
Prevalence of domain scores of K-NPI in patients within two groups
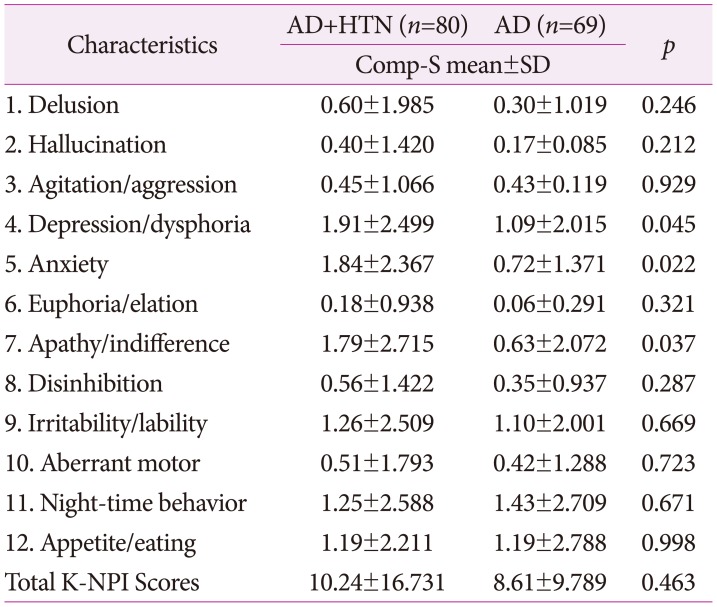
Mean total score of K-NPI was higher in patients with AD with hypertension than without hypertension, but was statistically insignificant. In all domains except of night-time behavior disturbances, mean score was higher in patients with AD with hypertension than that in patients with AD without hypertension. In particular, there was a statistically significant difference in the domains of depression/dysphoria (p=0.045), anxiety (p=0.022), and apathy/indifference (p=0.037) (Table 2).
Table 2. Prevalence of domain scores of K-NPI in patients with two groups (n=149).
AD: Alzheimer's disease, Comp-S: composite score (frequency × severity), HTN: hypertension, K-NPI: Korean version of Neuropsychiatric Inventory, SD: standard deviation.
Exploratory factor analysis with an extraction of three factors
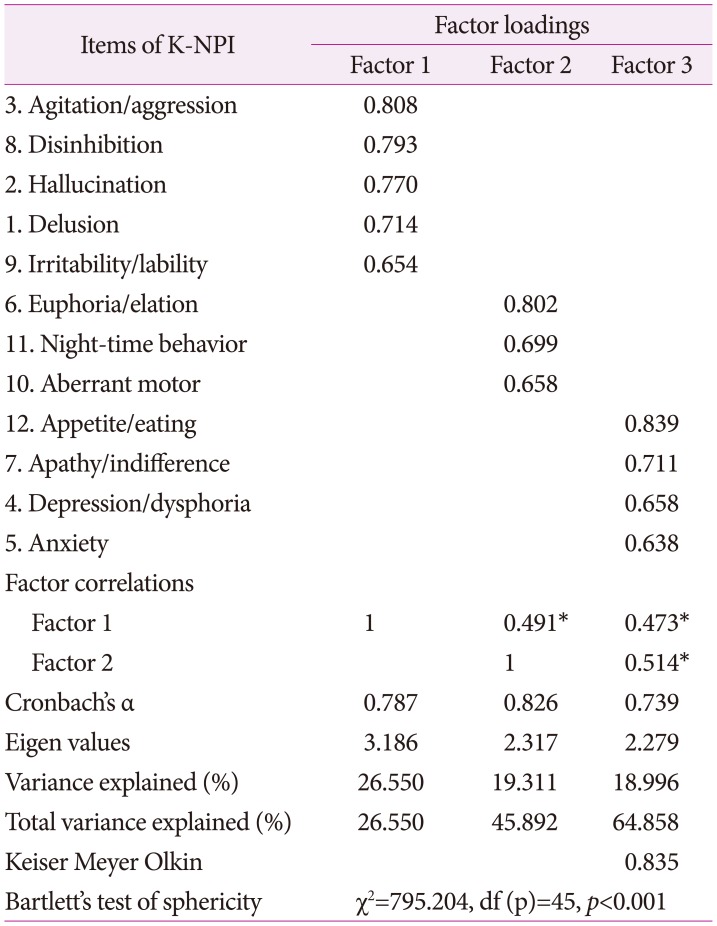
To verify construct validity and classify a number of variables by homogeneous factor, an exploratory factor analysis was conducted. As the goodness-of-fit of the sample (Kaiser-Meyer-Olkin, KMO) was 0.818 and Bartlett sphericity test was statistically significant, factor analysis was conducted. Active ingredient factor analysis was used, and because correlation between factors was not assumed, orthogonal rotation and Varimax method were used. Since commonness by question was not less than 0.4, no deleted question was found. As these criteria were applied, factors of which characteristic value determining number of factors not less than 1 was identified, and the final 3 factors were extracted. Factor 1 described 26.5% of distribution percentage with 5 questions, and the level of confidence was α=0.787, named psychotic symptom cluster. Factor 2 described 19.3% of distribution percentage with 3 questions, and level of confidence was α=0.826, named behavior symptom cluster. Factor 3 describes 18.9% of distribution percentage with 4 questions, level of confidence is α=0.739, named affective symptom cluster. Each factor was named K-NPI factor. Total accumulated distribution percentage was 64.858 (Table 3).
Table 3. Exploratory factor analysis with an extraction of three factors (n=149).
*p<0.01. K-NPI: Korean version of Neuropsychiatric Inventory.
Correlation between K-NPI/K-NPI factor and variables
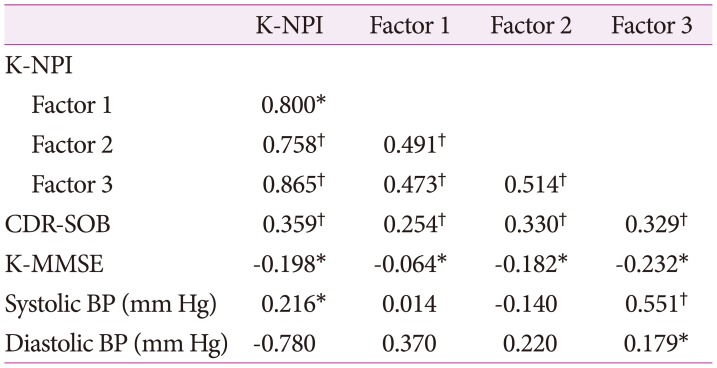
There was a strong correlation between K-NPI and K-NPI factors. There was correlation between K-NPI and CDR-SOB, and between K-NPI and K-MMSE. Systolic hypertension was correlated with K-NPI, and in particular, to the affective symptom cluster of the three factors. Diastolic hypertension was also correlated with the affective symptom cluster. Therefore, hypertension was related to NPS in AD patients and was associated with affective symptom cluster of the NPS (Table 4).
Table 4. Correlation between K-NPI/K-NPI factors and variables.
*p<0.05, †p<0.01.
BP: blood pressure, CDR-SOB: Clinical Dementia Rating Scale Sum of Boxes, K-NPI: Korean version of Neuropsychiatric Inventory, K-MMSE: Korean version of the Mini-Mental State Examination.
DISCUSSION
In this study, total score of K-NPI was not significantly different between patients with AD with and without hypertension. However, hypertension was associated with specific domains of K-NPI, depression/dysphoria (p=0.045), anxiety (p=0.022), and apathy/indifference (p=0.037). It was also associated with the affective symptom cluster but not with psychotic or behavior symptom clusters. A previous study in a crosssectional sample of 254 participants with AD followed in the Cache County Study on Memory in Aging, hypertension was associated with 2–3 times increased risk for delusions, anxiety, and agitation/aggression.10 A recent study has found that hypertensive patients with AD had increased NPS burden compared with normotensive patients with AD.16 Another recent study in a large cohort of 457 patients with AD revealed a history of hypertension was associated with worse NPS as measured by the Neuropsychiatric Inventory Questionnaire at the time of AD diagnosis.17 However, in one longitudinal study in a community-based AD cohort, no clear relationship was found between individual vascular risk factor and NPS in AD, but use of antihypertensive medication more than four times per week was associated with higher total neuropsychiatric inventory and affective cluster scores in AD.18 Different results between these studies are probably due to differences in study methods, including study subjects (normotensive vs. hypertensive subjects), BP measurement (single outpatient monitoring vs. mobile BP monitoring), antihypertensive use, presence of other vascular risk factors, and differences in cognitive function and dementia selection criteria.
Pathophysiology of how hypertension relates to NPS in AD is unclear. A possible explanation for association between hypertension and NPS is that cerebral blood flow may be decreased in prefrontal and temporal cortices.19 Another study suggests that hypertension can increase risk of cerebrovascular disease, that may increase incidence of depression in dementia.20 Hypertension is related to cerebrovascular disease and vascular dementia based on vascular remodeling.21 However, much is unknown about mechanisms of linking hypertension to AD. Although exact mechanisms are not fully understood, increasing evidence suggests vascular risk factors including hypertension may be associated with AD.22,23,24 Pathophysiological processes between hypertension and AD may involve inflammatory processes, blood-brain barrier dysfunction, and hypoperfusion.25,26,27 Evidence suggests that as a result of hypertension, chronic oligemia may downregulate synthesis of proteins necessary for synaptic plasticity and memory formation, and promote neuronal tau phosphorylation, β-amyloid oligomerization, and upregulation of amyloidogenic amyloid precursor protein.28,29 Each of these neurophysiological changes likely contributes to development of AD.
Results of this study suggest hypertension increases risk of specific NPS in patients with incident of AD. Among NPS, hypertension was associated with affective symptom cluster. Therefore, if an affective symptom is observed in patients diagnosed with AD, it may be necessary to investigate whether the patient has vascular risk factor such as hypertension. Effective management of hypertension can potentially play a therapeutic role in mitigation of NPS in AD. Given the severe burden of NPS in AD, further study on effects of modifiable risk factors and antihypertensive medications in AD are of interest. In addition, identifying potential modifiable risk factors for AD is crucial for primary prevention and may reduce incidence of AD.
Several limitations of this study exist. First, this was a limited study using single centre dementia registry. However, composition and ratio of NPS was consistent with many other studies. Second, there was insufficient control of antihypertensive therapy and vascular comorbidities. Several patients in our sample were on antihypertensive medication that may underestimate negative impact of hypertension on NPS. Other vascular risk factors such as diabetes or obesity may independently influence AD pathology. Finally, this was a retrospective, cross-sectional, and observational study. Further longitudinal studies whether prevention of hypertension could attenuate NPS are needed.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Fratiglioni L, De Ronchi D, Agüero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 3.Seux ML, Thijs L, Forette F, Staessen JA, Birkenhäger WH, Bulpitt CJ, et al. Correlates of cognitive status of old patients with isolated systolic hypertension: the Syst-Eur Vascular Dementia Project. J Hypertens. 1998;16:963–969. doi: 10.1097/00004872-199816070-00009. [DOI] [PubMed] [Google Scholar]
- 4.Morris MC, Scherr PA, Hebert LE, Bennett DA, Wilson RS, Glynn RJ, et al. Association between blood pressure and cognitive function in a biracial community population of older persons. Neuroepidemiology. 2002;21:123–130. doi: 10.1159/000054809. [DOI] [PubMed] [Google Scholar]
- 5.Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- 6.Elias MF, Wolf PA, D'Agostino RB, Cobb J, White LR. Untreated blood pressure level is inversely related to cognitive functioning: the Framingham Study. Am J Epidemiol. 1993;138:353–364. doi: 10.1093/oxfordjournals.aje.a116868. [DOI] [PubMed] [Google Scholar]
- 7.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- 8.Tzourio C, Dufouil C, Ducimetière P, Alpérovitch A EVA Study Group. Epidemiology of Vascular Aging. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. Neurology. 1999;53:1948–1952. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- 9.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 10.Treiber KA, Lyketsos CG, Corcoran C, Steinberg M, Norton M, Green RC, et al. Vascular factors and risk for neuropsychiatric symptoms in Alzheimer's disease: the Cache County Study. Int Psychogeriatr. 2008;20:538–553. doi: 10.1017/S1041610208006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Kang Y, Na DL, Hahn SH. A validity study on the Korean minimental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 14.Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, et al. Estimating the validity of the Korean version of expanded clinical dementia rating (CDR) scale. J Korean Neurol Assoc. 2001;19:585–591. [Google Scholar]
- 15.Choi SH, Na DL, Kwon HM, Yoon SJ, Jeong JH, Ha CK. The Korean version of the neuropsychiatric inventory: a scoring tool for neuropsychiatric disturbance in dementia patients. J Korean Med Sci. 2000;15:609–615. doi: 10.3346/jkms.2000.15.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jutkowitz E, MacLehose RF, Gaugler JE, Dowd B, Kuntz KM, Kane RL. Risk factors associated with cognitive, functional, and behavioral trajectories of newly diagnosed dementia patients. J Gerontol A Biol Sci Med Sci. 2017;72:251–258. doi: 10.1093/gerona/glw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg M, Hess K, Corcoran C, Mielke MM, Norton M, Breitner J, et al. Vascular risk factors and neuropsychiatric symptoms in Alzheimer's disease: the Cache County Study. Int J Geriatr Psychiatry. 2014;29:153–159. doi: 10.1002/gps.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez OL, Smith G, Becker JT, Meltzer CC, DeKosky ST. The psychotic phenomenon in probable Alzheimer's disease: a positron emission tomography study. J Neuropsychiatry Clin Neurosci. 2001;13:50–55. doi: 10.1176/jnp.13.1.50. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien J, Perry R, Barber R, Gholkar A, Thomas A. The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Ann N Y Acad Sci. 2000;903:482–489. doi: 10.1111/j.1749-6632.2000.tb06403.x. [DOI] [PubMed] [Google Scholar]
- 21.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luchsinger JA, Gustafson DR. Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis. 2009;16:693–704. doi: 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 25.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu L, Ng G, Tan EK, Liao P, Kandiah N, Zeng L. Chronic cerebral hypoperfusion enhances Tau hyperphosphorylation and reduces autophagy in Alzheimer's disease mice. Sci Rep. 2016;6:23964. doi: 10.1038/srep23964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett SA, Pappas BA, Stevens WD, Davidson CM, Fortin T, Chen J. Cleavage of amyloid precursor protein elicited by chronic cerebral hypoperfusion. Neurobiol Aging. 2000;21:207–214. doi: 10.1016/s0197-4580(00)00131-7. [DOI] [PubMed] [Google Scholar]
- 28.Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-β. Am J Pathol. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Xing A, Xu C, Cai Q, Liu H, Li L. Cerebrovascular hypoperfusion induces spatial memory impairment, synaptic changes, and amyloid-β oligomerization in rats. J Alzheimers Dis. 2010;21:813–822. doi: 10.3233/JAD-2010-100216. [DOI] [PubMed] [Google Scholar]