Abstract
Background and Purpose
Although acetyl-L-carnitine (ALC) treatment may have beneficial effects on Alzheimer's disease (AD), its underlying neural correlates remain unclear. The purpose of this study was to investigate cerebral perfusion changes after ALC treatment in AD patients using technetium-99m hexamethylpropylene amine oxime single photon emission computed tomography (SPECT).
Methods
A total of 18 patients with early AD were prospectively recruited and treated with ALC at 1.5 g/day for 1.4±0.3 years. At baseline and follow-up, brain SPECT, Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), Global Deterioration Scale (GDS), and Neuropsychiatric Inventory (NPI) were used to assess participants. After ALC administration, changes in brain perfusion, severity of dementia, cognitive performance, and neuropsychiatric disturbances were examined.
Results
After ALC administration, changes in scores of MMSE, CDR, GDS, and NPI were not statistically significant (p>0.05). Voxel-wise whole-brain image analysis revealed that perfusion was significantly (p<0.001) increased in the right precuneus whereas perfusion was reduced in the left inferior temporal gyrus (p<0.001), the right middle frontal gyrus (p<0.001), and the right insular cortex (p=0.001) at follow-up.
Conclusions
Although previous studies have suggested that AD patients generally demonstrate progressive deterioration in brain perfusion and clinical symptoms, this study reveals that the perfusion of the precuneus is increased in AD patients after ALC administration and their cognitive and neuropsychiatric symptoms are not aggravated. Further studies are warranted to determine the potential association between perfusion increase in the precuneus and clinical symptoms after ALC treatment in AD patients.
Keywords: Alzheimer's disease, acetyl-L-carnitine, single photon emission computed tomography, brain perfusion, cognitive function
INTRODUCTION
Alzheimer's disease (AD), the most prevalent type of dementia, is characterized by chronic and progressive memory loss. It can advance to impairments of higher cognitive functions across multiple domains.1
Acetyl-L-carnitine (ALC) is an acetylated form of L-carnitine. It can be actively transported across the blood-brain barrier.2 Carnitine plays an important role in β-oxidation of fatty acids while acetyl moiety maintains acetyl-CoA levels.3 ALC is involved in the modulation of brain energy production and phospholipid metabolism, cellular macromolecules such as neurotrophic factors and neurohormones, and synaptic morphology and transmission.3 A previous meta-analysis of mild cognitive impairment (MCI) and mild AD has suggested that ALC is beneficial to these patients in accordance with both clinical and psychometric tests.4 For instance, in a double-blind, placebo-controlled, parallel-group, and randomized clinical trial among mild or moderate AD patients, one year of ALC administration has resulted in slower rate of deterioration in various domains of cognitive function with good tolerability.5 However, brain responses to ALC remains unknown. Although an early 31P magnetic resonance spectroscopy study in AD has reported that decreased levels of phosphomonoester and high-energy phosphate at baseline were normalized after ALC treatment, these changes in chemical levels have only been examined in the dorsal prefrontal area.6
The objective of the current study was to investigate changes in brain perfusion and clinical symptoms including dementia severity, cognitive performance, and neuropsychiatric disturbances after ALC administration in AD patients using single photon emission computed tomography (SPECT). Regional cerebral blood flow (rCBF) is considered as a valid biomarker for neuronal activity since it is closely coupled to neuronal metabolism.7
With regard to possible modes of action of ALC in AD, restoring cell membranes and synaptic function, enhancing cholinergic activity and brain energy metabolism, protecting against toxins, and exerting neurotrophic effects have been previously suggested.3 Previous in vivo imaging studies in mild AD patients have revealed that cholinergic activities in typical AD-affected brain areas including precuneus/posterior cingulate cortex are decreased.8,9 It has been reported that metabolic reduction is the most prominent in precuneus/posterior cingulate cortex region in very early AD.10 Based on these findings, we hypothesized that ALC might be able to improve rCBF primarily in the medial parietal area.
METHODS
Participants
Patients who met the Diagnostic and Statistical Manual of Mental Disorders-IV criteria for AD11 and the National Institute of Neurologic and Communicative Disorders and Stroke-AD and Related Disorders Association criteria for probable AD12 were prospectively recruited at Incheon St. Mary's Hospital (Incheon, South Korea). Patients did not use acetylcholinesterase inhibitor or other cognitive-enhancers due to side effects or patient refusal. Exclusion criteria were: history of head trauma, epilepsy, stroke, mixed or vascular dementia, radiological findings on magnetic resonance imaging (MRI), and other neurological or psychiatric disorders.
All patients were treated with ALC at 1.5 g/day for 1.4±0.3 years. Safety assessments included adverse events, physical examinations, vital signs, electrocardiography, and laboratory tests. Written informed consent was obtained from all participants. The study protocol was approved by the Research Ethics Committee of Incheon St. Mary's Hospital.
Clinical assessment
Evaluation of medical history and physical examinations were conducted by board-certified neurologists. Clinical Dementia Rating (CDR),13 CDR-Sum of Boxes (CDR-SB), and Global Deterioration Scale (GDS)14 were used to examine the overall severity of dementia and functional status. Global cognitive performance and neuropsychiatric symptoms were evaluated with Mini-Mental State Examination (MMSE)15 and Neuropsychiatric Inventory (NPI),16 respectively.
Image acquisition and analysis
Brain perfusion SPECT scans were conducted with a dual-head gamma camera (Discovery NM640; GE Healthcare, Milwaukee, WI, USA) at baseline and follow-up. An intravenous administration of 1110 MBq of technetium-99m hexamethylpropylene amine oxime was performed for each participant at 40 minutes before the scan in a dark and quiet room. All images were attenuation corrected and reconstructed in 128×128 matrices with a voxel size of 3.9×3.9×3.9 mm3 using filtered back projection.
Statistical Parametric Mapping 12 (SPM; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) was used for image analysis and statistical modelling. For each patient, baseline and follow-up images were realigned to create a single mean image. Realigned images were spatially normalized to SPM SPECT template (Montreal Neurological Institute, McGill University, Montreal, Canada) using transformation parameters estimated from the mean image. All images were then resliced with a voxel size of 2×2×2 mm3 and smoothed with a 12 mm full-width half-maximum isotropic Gaussian kernel. A voxel-wise paired t-test was conducted to examine changes in rCBF between baseline and follow-up. Perfusion values were normalized to those of the cerebellum using automated anatomical labeling atlas.17,18,19 A voxel-level threshold of p<0.005 with a cluster size threshold of 150 contiguous voxels was applied.
Statistical analysis
Shapiro-Wilk's tests were used to test the normality of variables. Changes in continuous variables between baseline and follow-up were assessed with paired t-tests or Wilcoxon signed rank tests as appropriate. A two-tailed p value of less than 0.05 was considered statistically significant. All analyses were conducted with Stata version 13.1 (StataCorp., College Station, TX, USA).
RESULTS
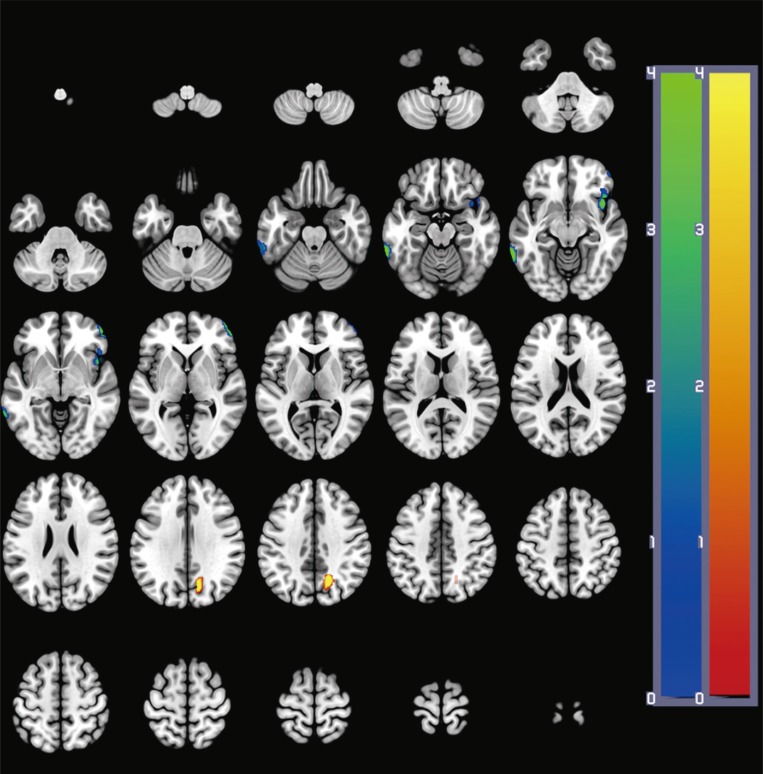
A total of 18 patients with early AD were recruited, including 2 males and 16 females. Demographic and clinical characteristics of these participants are summarized in Table 1. Their mean age at baseline was 77.9±5.3 years (67–86 years). At follow-up, changes in MMSE (t=0.69, p=0.50), CDR-SB (z=0.65, p=0.52), GDS (z=1.00, p=0.32), and NPI (z=0.54, p=0.59) were not significant. In addition, CDR rating at follow-up was not changed compared to that at baseline.
Table 1. Demographic and clinical characteristics of participants.
*Paired t-test, †Wilcoxon signed rank test.
CDR: Clinical Dementia Rating, CDR-SB: Clinical Dementia Rating-Sum of Boxes, GDS: Global Deterioration Scale, MMSE: Mini-Mental State Examination, NPI: Neuropsychiatric Inventory.
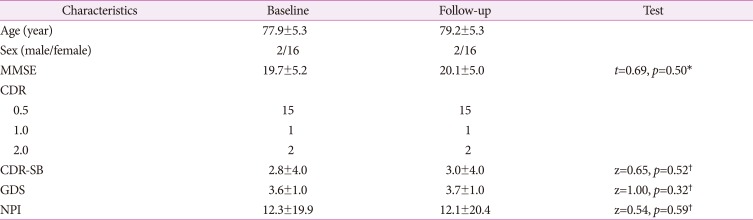
Results from image analysis (Table 2 and Fig. 1) revealed that perfusion in the right precuneus was increased (t=4.09, p<0.001, cluster size= 152 voxels) at follow-up. On the other hand, rCBF was decreased in the left inferior temporal gyrus (t=4.76, p<0.001, cluster size=232 voxels), the right middle frontal gyrus (t= 4.44, p<0.001, cluster size=197 voxels), and the right insular cortex (t=3.65, p=0.001, cluster size=164 voxels).
Table 2. Changes in regional cerebral blood flow.
*Coordinates refer to the Montreal Neurological Institute coordinate system.
Fig. 1. Changes in regional cerebral blood flow at follow-up. At each voxel, increase or decrease in brain perfusion is indicated by red-yellow or blue-green color, respectively. Images are shown in neurological convention. Color bars represent voxel-level t-values.
DISCUSSION
This perfusion SPECT study investigated changes of rCBF in early AD patients after administration of ALC for approximately 1.4 years. Changes in rCBF at follow-up compared to that at baseline were analyzed. Changes in cognitive function, dementia severity, and neuropsychiatric symptoms were also examined.
At follow-up, changes in clinical characteristics for all outcome measures were not significant. Previous longitudinal studies in AD patients have reported that deterioration in cognitive performance20 and neuropsychiatric symptoms21 over one year is highly significant. Our results suggest that ALC at least can delay or prevent the progression of AD, indirectly supporting results of a previous meta-analysis indicated that ALC has beneficial effects on AD.4
As disease progressed, patients demonstrated perfusion decreases in inferior temporal, middle frontal, and insular areas. These findings are in agreement with previous studies on AD.22,23,24,25,26,27 The inferior temporal gyrus is closely involved in verbal fluency that is affected in the early course of AD.27 Moreover, postmortem evidence in AD patients has indicated that synaptic loss in this region is significantly associated with impairment in verbal fluency.27 Other postmortem and in vivo neuroimaging studies in AD have found that neuronal counts and perfusion in the middle frontal gyrus are reduced compared to those of healthy controls.24,25 In the pathophysiology of AD, the insular cortex contributes to neuropsychiatric symptoms, changes in cardiovascular and autonomic regulation, and mortality.26 Our findings are consistent with results of previous structural MRI studies demonstrating gray matter loss in the insular region of AD patients.22,23
On the other hand, rCBF was increased in the precuneus after the administration of ALC in this study. The precuneus exhibits widespread connectivities with both cortical and subcortical structures. It plays a pivotal role in a variety of highly integrated functions, including visuo-spatial imagery, retrieval of episodic memory, self-processing, and consciousness.28 Furthermore, the precuneus is considered a central hub in default mode network (DMN).29 It is specifically vulnerable to amyloid pathology.30 Previous neuroimaging studies have reported hypoperfusion,31,32 hypometabolism,33 gray matter loss,34 and increased amyloid burden35 of the precuneus in AD patients and suggested that progressive structural or functional deficits in this region are implicated in the worsening of early AD. In a longitudinal functional MRI study, DMN connectivity has successfully predicted progression from MCI to AD.36 Further studies are needed to investigate the potential association between perfusion increase in the precuneus and clinical symptoms after ALC treatment in AD patients. Interestingly, treatment with transcranial direct current stimulation has improved both glucose metabolism in the precuneus and subjective memory function in MCI patients.37
Potential weaknesses of the current study include its relatively small sample size and the lack of comparison subjects. The high ratio of female to male patients was another limitation. In addition, comprehensive assessments of cognitive abilities using detailed neuropsychological batteries were not conducted to detect subtle cognitive decline during the follow-up period. Finally, correlations between perfusion increase in the precuneus and clinical symptoms were not demonstrated.
In spite of these limitations, this is the first SPECT study that examines cerebral perfusion changes associated with ALC administration in early AD patients. After ALC administration, cognitive performances, severity of dementia, and levels of neuropsychiatric symptoms were not significantly changed, whereas rCBF in the precuneus was significantly increased. Further studies with larger samples and longer follow-up periods are warranted to investigate whether a perfusion increase in the precuneus after ALC administration has beneficial effects on clinical symptoms of AD.
Acknowledgements
This study was supported by a grant (NRF-2015M3C7A1064832) of the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning, Republic of Korea.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Helmes E, Østbye T. Beyond memory impairment: cognitive changes in Alzheimer's disease. Arch Clin Neuropsychol. 2002;17:179–193. doi: 10.1016/s0887-6177(00)00109-8. [DOI] [PubMed] [Google Scholar]
- 2.Burlina AP, Sershen H, Debler EA, Lajtha A. Uptake of acetyl-L-carnitine in the brain. Neurochem Res. 1989;14:489–493. doi: 10.1007/BF00964865. [DOI] [PubMed] [Google Scholar]
- 3.Pettegrew JW, Levine J, McClure RJ. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: relevance for its mode of action in Alzheimer's disease and geriatric depression. Mol Psychiatry. 2000;5:616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery SA, Thal LJ, Amrein R. Meta-analysis of double blind randomized controlled clinical trials of acetyl-L-carnitine versus placebo in the treatment of mild cognitive impairment and mild Alzheimer's disease. Int Clin Psychopharmacol. 2003;18:61–71. doi: 10.1097/00004850-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Spagnoli A, Lucca U, Menasce G, Bandera L, Cizza G, Forloni G, et al. Long-term acetyl-L-carnitine treatment in Alzheimer's disease. Neurology. 1991;41:1726–1732. doi: 10.1212/wnl.41.11.1726. [DOI] [PubMed] [Google Scholar]
- 6.Pettegrew JW, Klunk WE, Panchalingam K, Kanfer JN, McClure RJ. Clinical and neurochemical effects of acetyl-L-carnitine in Alzheimer's disease. Neurobiol Aging. 1995;16:1–4. doi: 10.1016/0197-4580(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 7.Henderson TA. The diagnosis and evaluation of dementia and mild cognitive impairment with emphasis on SPECT perfusion neuroimaging. CNS Spectr. 2012;17:176–206. doi: 10.1017/S1092852912000636. [DOI] [PubMed] [Google Scholar]
- 8.Mazère J, Prunier C, Barret O, Guyot M, Hommet C, Guilloteau D, et al. In vivo SPECT imaging of vesicular acetylcholine transporter using [123 I]-IBVM in early Alzheimer's disease. Neuroimage. 2008;40:280–288. doi: 10.1016/j.neuroimage.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Kendziorra K, Wolf H, Meyer PM, Barthel H, Hesse S, Becker GA, et al. Decreased cerebral α4β2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer's disease assessed with positron emission tomography. Eur J Nucl Med Mol Imaging. 2011;38:515–525. doi: 10.1007/s00259-010-1644-5. [DOI] [PubMed] [Google Scholar]
- 10.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to Establish a Registry for Alzheimer's disease (CERAD Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Jeong Y, et al. The validity of the Korean version of Global Deterioration Scale. J Korean Neurol Assoc. 2002;20:612–617. [Google Scholar]
- 15.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 16.Choi SH, Na DL, Kwon HM, Yoon SJ, Jeong JH, Ha CK. The Korean version of the neuropsychiatric inventory: a scoring tool for neuropsychiatric disturbance in dementia patients. J Korean Med Sci. 2000;15:609–615. doi: 10.3346/jkms.2000.15.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 18.Pickut BA, Dierckx RA, Dobbeleir A, Audenaert K, Van Laere K, Vervaet A, et al. Validation of the cerebellum as a reference region for SPECT quantification in patients suffering from dementia of the Alzheimer type. Psychiatry Res. 1999;90:103–112. doi: 10.1016/s0925-4927(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 19.Soonawala D, Amin T, Ebmeier KP, Steele JD, Dougall NJ, Best J, et al. Statistical parametric mapping of (99m)Tc-HMPAO-SPECT images for the diagnosis of Alzheimer's disease: normalizing to cerebellar tracer uptake. Neuroimage. 2002;17:1193–1202. doi: 10.1006/nimg.2002.1259. [DOI] [PubMed] [Google Scholar]
- 20.Burns A, Jacoby R, Levy R. Progression of cognitive impairment in Alzheimer's disease. J Am Geriatr Soc. 1991;39:39–45. doi: 10.1111/j.1532-5415.1991.tb05904.x. [DOI] [PubMed] [Google Scholar]
- 21.Benoit M, Robert PH, Staccini P, Brocker P, Guerin O, Lechowski L, et al. One-year longitudinal evaluation of neuropsychiatric symptoms in Alzheimer's disease. The REAL.FR study. J Nutr Health Aging. 2005;9:95–99. [PubMed] [Google Scholar]
- 22.Baron JC, Chételat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 23.Frisoni GB, Testa C, Zorzan A, Sabattoli F, Beltramello A, Soininen H, et al. Detection of grey matter loss in mild Alzheimer's disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73:657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, et al. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851–859. doi: 10.1148/radiol.2343040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mountjoy CQ, Roth M, Evans NJ, Evans HM. Cortical neuronal counts in normal elderly controls and demented patients. Neurobiol Aging. 1983;4:1–11. doi: 10.1016/0197-4580(83)90048-9. [DOI] [PubMed] [Google Scholar]
- 26.Royall DR. Insular Alzheimer disease pathology and the psychometric correlates of mortality. Cleve Clin J Med. 2008;75(Suppl 2):S97–S99. doi: 10.3949/ccjm.75.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 27.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2011;24:547–557. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 29.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 30.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26:231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley KM, O'Sullivan VT, Soper ND, Nagy Z, King EM, Smith AD, et al. Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer's disease. Brain. 2002;125(Pt 8):1772–1781. doi: 10.1093/brain/awf185. [DOI] [PubMed] [Google Scholar]
- 32.Sakamoto S, Matsuda H, Asada T, Ohnishi T, Nakano S, Kanetaka H, et al. Apolipoprotein E genotype and early Alzheimer's disease: a longitudinal SPECT study. J Neuroimaging. 2003;13:113–123. [PubMed] [Google Scholar]
- 33.Salmon E, Collette F, Degueldre C, Lemaire C, Franck G. Voxel-based analysis of confounding effects of age and dementia severity on cerebral metabolism in Alzheimer's disease. Hum Brain Mapp. 2000;10:39–48. doi: 10.1002/(SICI)1097-0193(200005)10:1<39::AID-HBM50>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryu SY, Kwon MJ, Lee SB, Yang DW, Kim TW, Song IU, et al. Measurement of precuneal and hippocampal volumes using magnetic resonance volumetry in Alzheimer's disease. J Clin Neurol. 2010;6:196–203. doi: 10.3988/jcn.2010.6.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology. 2011;76:511–517. doi: 10.1212/WNL.0b013e31820af94e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yun K, Song IU, Chung YA. Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimers Res Ther. 2016;8:49. doi: 10.1186/s13195-016-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]