Abstract
Background and Purpose
Evaluating instrumental activities of daily living (IADL) is an important part of procedure to diagnose dementia. The Korean-Instrumental Activities of Daily Living (K-IADL) has been used extensively in Korea. However, its cut-off score has not been reformulated since 2002. The purpose of this study was to yield a new optimal cut-off score for the K-IADL and confirm the validity of this new cut-off score with various dementia groups.
Methods
We retrospectively collected a total of 2,347 patients' K-IADL data from 6 general hospitals in Korea. These patients had mild cognitive impairment (MCI) or dementia with various etiologies for cognitive impairment. We also recruited a normal control group (n=254) from the community. Korean-Mini Mental State Examination, Short version of the Geriatric Depression Scale, Clinical Dementia Rating, and Global Deterioration Scale were administered to all participants. Caregivers completed K-IADL and Barthel Index.
Results
K-IADL scores were significantly different among dementia subgroups, but not significantly different among MCI subgroups. Based on internal consistency, correlations with other scales, and factor analysis, K-IADL showed excellent reliability and validity. The new optimal cut-off score to diagnose dementia was 0.40, which gave a sensitivity of 0.901 and a specificity of 0.916. Positive predictive value for dementia using the new cut-off score was 94.2% for Alzheimer's disease, 100% for vascular dementia, and 84% for Parkinson's disease.
Conclusions
Our results illustrate that the new K-IADL cut-off score of 0.40 is reliable and valid for screening impairments of daily functioning resulting from various etiologies.
Keywords: Activities of Daily Living, Dementia, Mild Cognitive Impairment
INTRODUCTION
Evaluation of instrumental activities of daily living (IADL) is an important part of dementia diagnostic procedure.1,2 Various activities — including using telephone, shopping, preparing food, housekeeping, using transportation, taking medicine, conducting finance, enjoying hobbies, doing laundry, and watching television — are included in IADL.3,4 That is, complex activities representing instrumental self-maintenance, effectance, and social behavior are included in IADL scales.
IADL is important in the care of dementia patients. Evaluation of IADL is essential to the diagnosis of dementia.1,2 Although new diagnostic research criteria including pathological markers for dementia due to Alzheimer's disease (AD) have been established,5 impairment of daily living activities as well as cognition is practically necessary to diagnose dementia in clinical setting. If a patient shows cognitive decline without having significant disturbance in activities of daily living, the patient is not diagnosed with dementia.1,2 For this case, normal cognitive aging or mild cognitive impairment (MCI), not dementia, might be a possible reason for cognitive decline.
To evaluate activity of daily living (ADL) objectively, formal ADL scales should be used to quantify and exteriorize the level of functioning in IADL and physical activities of daily living (PADL).6 A few IADL scales have been standardized in Korea, including the Korean-Instrumental Activities of Daily Living (K-IADL),7 the Korean version of Bayer ADL,8 and the Korean version of Disability Assessment of Dementia Scale.9 In addition, Seoul-Instrumental Activities of Daily Living10 and K-IADL11 were newly developed in Korea.
Among them, K-IADL7 has been most widely used in Korea for over 16 years. It was developed through reviewing various IADL instruments. It was composed of suitable items in consideration of Korean culture.7 Reliability and validity of the K-IADL were determined in 2002. Its optimal cut-off was 0.43, with sensitivity and specificity of 83% and 82%, respectively.7 Although K-IADL was developed 16 years ago, its cut-off score of 0.43 has been used in clinical setting without any revision. Considering dramatic changes in lifestyle and enhanced education level of the geriatric population over the past 16 years, its cut-off score needs to be revised to improve diagnostic accuracy. Furthermore, it would be important to examine differences in IADL characteristics with various dementia groups.
Therefore, the purpose of this study was to develop a new optimal cut-off score of K-IADL for diagnosing dementia and investigate variations in cut-off scores of various dementia groups, including AD, vascular dementia (VaD), and Parkinson's disease dementia (PDD) not conducted in the previous study. We also performed a cross-validation study by examining the positive predictive value of the newly developed cut-off score with various dementia groups.
METHODS
Procedure of data collection
We retrospectively collected patients' clinical data from the Departments of Neurology of 6 general hospitals located in Seoul and Gyeonggi-do of South Korea. We reviewed medical records and database of the Seoul Neuropsychological Screening Battery, second edition (SNSB-II)12 including K-IADL. Institutional Review Boards of the 6 hospitals approved this study respectively.
Participants
All participants aged 45–90 years old. The normal control group consisted of 254 elderly people who lived in the local community and met the criteria for normal cognition.13 The patient group had 2,347 people with K-IADL that was administered to their caregivers in the Departments of Neurology from January 2013 to December 2015. Among these 2,347 patients, we randomly selected data of 1,877 patients (80%) and used these data to conduct a receiver operating characteristic (ROC) curve analysis to obtain new optimal score, sensitivity, and specificity of the K-IADL. We used data from the remaining 470 patients (20%) for a cross-validation study to identify the positive predictive value of the new cut-off score.
We confirmed the cognitive dysfunction of patients through comprehensive neuropsychological assessment and verified the impairment of ADL based on medical records that contained reports of patients and their caregivers. The final diagnosis was based on the diagnostic decision of neurologists. A total of 759 patients with AD were diagnosed as probable AD based on the criteria of the National Institute on Aging-Alzheimer's Association (NIA-AA) workgroups.5 There were 841 patients with amnestic MCI (aMCI) whose diagnoses were based on Peterson's criteria.14 To recruit the prodromal stage of dementia with AD as much as possible, we only selected aMCI patients. There were 164 patients with VaD. They were diagnosed using the diagnostic criteria for probable VaD of the American Heart Association-American Stroke Association.15 A total of 108 patients with vascular MCI (VaMCI) were diagnosed based on criteria for probable VaMCI of the American Heart Association-American Stroke Association.15 A total 475 patients with Parkinson's disease (PD) were diagnosed using UK Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria.16 Among these patients with PD, 128 patients were diagnosed as PDD17 while the remaining 278 patients with PD-MCI were confirmed to have cognitive deficit without ADL impairment.
Measures
The Korean-Mini Mental State Examination (K-MMSE),18 Clinical Dementia Raging (CDR),19 and Global Deterioration Scale (GDS)20 were administered to all participants. Short version of the Geriatric Depression Scale (S-GDS)21 was administered to 1,956 patients in 5 out of 6 hospitals.
Caregivers completed K-IADL and Barthel Index (BI).22 K-IADL was developed for evaluating impairment of complex ADL, including shopping, using transportation, conducting financial affairs, housekeeping, preparing food, using the telephone, taking medicine, remembering recent event, enjoying hobbies, watching TV, and conducting home repair. K-IADL used a 4-point scale: “independently performed/normal” = 0, “need some help/mild impairment” = 1, “need a lot of help/moderate impairment” = 2, and “impossible” = 3. Activities that were not performed before the onset of dementia were rated as “not applicable”. They were not included in the scoring. To obtain the total score, sum of scores was divided by the number of rated items except for “not applicable” items.7
Statistical analysis
To confirm whether there were significant differences among patient groups, we compared scores of various measures including K-IADL in 1,877 patients. First, we examined whether there were significant differences in demographics. Then we performed an analysis of covariance (ANCOVA) to compare scores of K-MMSE, CDR, GDS, S-GDS, K-IADL, and BI after controlling for demographic variables that showed significant differences.
To confirm the reliability of K-IADL, we assessed the internal consistency with Cronbach's α and conducted correlation analysis between item scores and total score. To confirm concurrent validity of K-IADL, we performed correlation analysis between K-IADL and other related measures. Construct validity was examined using exploratory factor analysis (EFA) on K-IADL items, in which extraction was done on the correlation matrix using principle axis factoring analysis. Factors were rotated using direct oblimin method. We also performed confirmatory factor analysis (CFA) to test whether our data fit the measurement model of K-IADL that was hypothesized based on result of EFA. CFA was conducted with data of 470 patients who were randomly selected for the cross-validation study. We investigated whether there were significant differences in K-IADL scores according to levels of CDR and GDS.
Additionally, we conducted ROC curve analyses to identify optimal cut-off score of K-IADL to differentiate between dementia and non-dementia patients as well as between MCI patients and normal control group. Furthermore, we performed ROC curve analyses for various dementia groups such as AD, VaD, and PDD to yield new optimal cut-off scores. We determined the optimal cut-off score to maximize both sensitivity and specificity. With 470 patients, we performed a cross-validation study to identify the positive predictive value of the new cut-off score for dementia differentiation in various dementia groups.
RESULTS
Demographic data and means and standard deviations of measures
There were significant differences in sex, age, and education level among normal control, MCI, and dementia groups. Overall, the percentage of women was higher in aMCI and AD groups. Patient groups were older with lower education level than the normal control group. For MCI groups, patients with PD-MCI were younger than those in VaMCI and aMCI groups while patients with aMCI had significantly higher levels of education than those in the VaMCI group. In the dementia group, AD patients were older than PDD patients. There was no significant difference in education level among patient groups.
Among all patients, there were significant differences in K-MMSE, CDR, GDS, S-GDS, BI, and K-IADL scores after controlling for demographics. For BI, there was no significant difference between VaMCI and PD-MCI groups. However, aMCI group showed higher score than VaMCI and PD-MCI groups. There was no significant difference in BI between VaD and PDD. However, AD had a higher score. For K-IADL score, there was no significant difference among MCI groups However, PDD patients had lower scores the AD and VaD patients. These results are presented in Table 1.
Table 1. Demographic data and means and standard deviations of K-MMSE, CDR, GDS, S-GDS, K-IADL, and BI.
| Variables | Normal (n=254) | MCI | Dementia | F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCI (n=673) | VaMCI (n=88) | PD-MCI (n=278) | F | Post hoc | AD (n=604) | VaD (n=131) | PDD (n=103) | F | Post hoc | |||
| Sex§ (male/female) | 105/149 | 259/414 | 40/48 | 132/146 | 7.19* | 185/419 | 60/71 | 49/54 | 18.92‡ | 34.14‡ | ||
| Age | 64.66±9.25 | 73.11±8.02 | 73.74±8.04 | 70.31±8.20 | 13.19‡ | aMCI=VaMCI>PD-MCI | 76.70±8.19 | 74.82±8.36 | 74.46±7.95 | 5.29† | AD>VaD=PDD | 69.96‡ |
| Education (yr) | 9.88±4.02 | 9.51±5.29 | 7.61±5.19 | 9.03±5.49 | 5.19† | aMCI>VaMCI | 8.54±5.29 | 7.69±5.81 | 7.67±5.24 | 2.20 | 6.58‡ | |
| PD-MCI=aMCI, VaMCI | ||||||||||||
| K-MMSE | 27.99±1.89 | 24.72±3.55 | 25.05±3.65 | 25.29±3.48 | 2.31 | 18.80±4.58 | 19.02±4.34 | 20.17±4.47 | 2.27 | 246.50‡ | ||
| CDR | - | 0.51±0.13 | 0.49±0.12 | 0.44±0.21 | 19.63‡ | aMCI=VaMCI>PD-MCI | 1.07±0.52 | 1.13±0.51 | 0.94±0.47 | 3.79* | AD=VaD>PDD | 217.21‡ |
| GDS | - | 3.09±0.35 | 2.91±0.45 | 2.87±0.48 | 27.07‡ | aMCI>VaMCI=PD-MCI | 4.45±0.75 | 4.35±0.73 | 4.12±0.77 | 8.36‡ | AD=VaD>PDD | 482.36‡ |
| S-GDS | 2.31±2.85 | 4.81±4.06 | 5.65±4.58 | 5.93±4.01 | 2.04 | 5.65±4.64 | 7.84±4.64 | 8.38±3.90 | 15.94‡ | AD<VaD=PDD | 24.53‡ | |
| K-IADL | 0.05±0.12 | 0.20±0.21 | 0.20±0.22 | 0.20±0.26 | 0.28 | 1.23±0.75 | 1.29±0.66 | 1.03±0.66 | 3.78* | AD=VaD>PDD | 344.24‡ | |
| BI | - | 19.79±1.19 | 19.38±2.65 | 19.33±2.26 | 10.60‡ | aMCI>VaMCI=PD-MCI | 18.72±2.65 | 17.26±3.90 | 17.21±3.84 | 22.58‡ | AD>VaD=PDD | 39.79‡ |
K-MMSE: Korean-Mini Mental State Examination, CDR: Clinical Dementia Raging, GDS: Global Deterioration Scale, S-GDS: short version of the Geriatric Depression Scale, K-IADL: Korean-Instrumental Activities of Daily Living, BI: Barthel Index, MCI: mild cognitive impairment, VaMCI: vascular mild cognitive impairment, PD-MCI: Parkinson's disease mild cognitive impairment, AD: Alzheimer's disease, VaD: vascular dementia, PDD: Parkinson's disease dementia, aMCI: amnestic mild cognitive impairment.
*p<0.05, †p<0.01, ‡p<0.001. §χ2 tests were used for sex ratio comparisons.
Reliability
The internal consistency of K-IADL measured by Cronbach's α was 0.968, indicating high reliability. Item-total correlation coefficients ranged from 0.781 to 0.884, with an average coefficient of 0.844.
Validity
Results of EFA revealed one factor retrieved from 11 items. It accounted for 76.61% of total variance of K-IADL. All factor loadings of 11 items were greater than 0.790 (see Table 2).
Table 2. Factor loadings of K-IADL.
| Items | Factor loading |
|---|---|
| 1. Shopping | 0.898 |
| 2. Using transportation | 0.895 |
| 3. Conducting financial affairs | 0.896 |
| 4. Housekeeping (use of electronic devices) | 0.880 |
| 5. Preparing food | 0.884 |
| 6. Using the telephone | 0.854 |
| 7. Taking medicine | 0.835 |
| 8. Remembering recent event | 0.790 |
| 9. Enjoying hobbies | 0.877 |
| 10. Watching TV | 0.828 |
| 11. Conducting home repair | 0.840 |
| Variance explained (%) | 76.61 |
K-IADL: Korean-Instrumental Activities of Daily Living.
Correlations between K-IADL and scales representing dementia severity such as CDR (r=0.788, p<0.001), GDS (r=0.783, p<0.001), and K-MMSE (r=−0.646, p<0.001) were significant. The correlation between K-IADL and the BI measuring basic PADL was also significant (r=−0.495, p<0.001). The correlation coefficient between K-IADL and S-GDS was statistically significant, but not strongly (r=0.193, p<0.001). Results of these correlations are presented in Table 3.
Table 3. Correlations between K-IADL and other measures.
| Variables | K-IADL | BI | K-MMSE | CDR | GDS | S-GDS |
|---|---|---|---|---|---|---|
| K-IADL | - | |||||
| BI | −0.495* | - | ||||
| K-MMSE | −0.646* | 0.296* | - | |||
| CDR | 0.788* | −0.426* | −0.601* | - | ||
| GDS | 0.783* | −0.347* | −0.656* | 0.765* | - | |
| S-GDS | 0.193* | −0.207* | −0.272* | 0.112* | 0.104* | - |
K-IADL: Korean-Instrumental Activities of Daily Living, BI: Barthel Index, K-MMSE: Korean-Mini Mental State Examination, CDR: Clinical Dementia Rating, GDS: Global Deterioration Scale, S-GDS: short version of the Geriatric Depression Scale.
*p<0.001.
Furthermore, we assessed whether there were significant differences in K-IADL according to CDR as well as GDS levels in all patients (see Table 4). Total K-IADL score was significantly different according to CDR. The total score of K-IADL was also significantly different according to GDS except that between GDS 1 and GDS 2.
Table 4. K-IADL total scores (mean±SD) according to CDR and GDS level.
| Variables | No. | K-IADL | F | |
|---|---|---|---|---|
| CDR level | 899.06* | |||
| CDR 0 | 51 | 0.09±0.22 | ||
| CDR 0.5 | 1,176 | 0.28±0.30 | ||
| CDR 1 | 496 | 1.14±0.59 | ||
| CDR 2 | 144 | 2.12±0.56 | ||
| CDR 3 | 9 | 2.50±0.92 | ||
| GDS level | 674.26* | |||
| GDS 1 | 7 | 0.05±0.09 | ||
| GDS 2 | 59 | 0.12±0.20 | ||
| GDS 3 | 966 | 0.23±0.25 | ||
| GDS 4 | 492 | 0.81±0.53 | ||
| GDS 5 | 296 | 1.61±0.64 | ||
| GDS 6 | 57 | 2.25±0.63 | ||
K-IADL: Korean-Instrumental Activities of Daily Living, SD: standard deviation, CDR: clinical dementia rating, GDS: global deterioration scale.
*p<0.05.
Sensitivity and specificity
Total participants
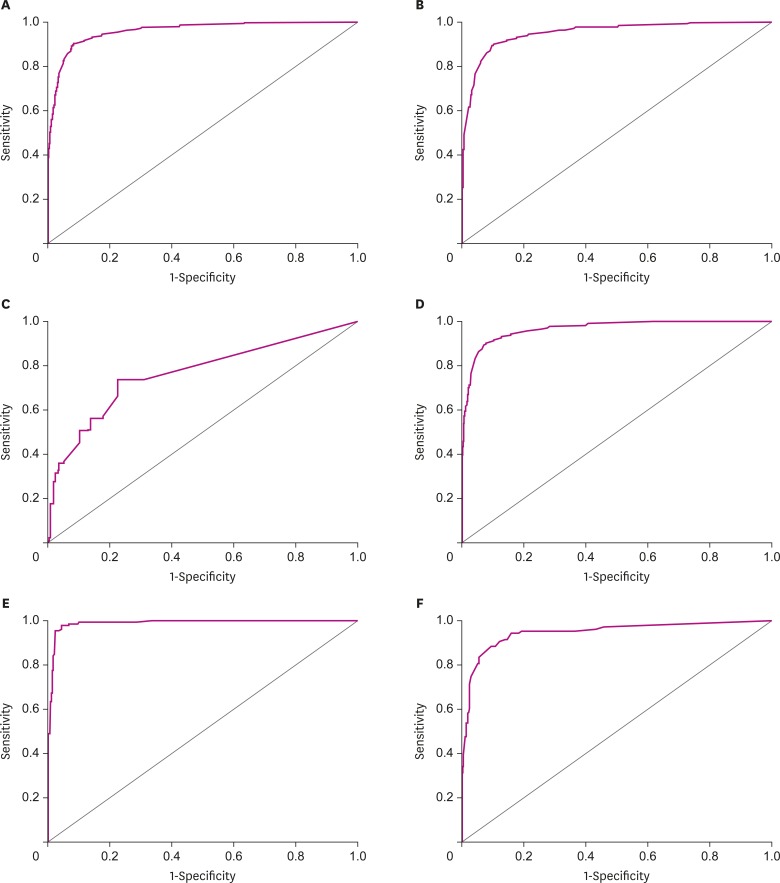
We conducted an ROC curve analysis and calculated the area under the curve (AUC) to confirm the power of discrimination of K-IADL as a diagnostic tool for dementia (see Fig. 1A). When we discriminated dementia patients from non-dementia patients in all participants, K-IADL had a sensitivity of 0.901 and a specificity of 0.916 with a cut-off score of 0.40. The AUC of K-IADL was 0.959, indicating that the power of dementia discrimination using K-IADL was excellent. For discriminating dementia from MCI, the AUC of K-IADL was 0.952. K-IADL had a sensitivity of 0.901 and a specificity of 0.898 when we used the same cut-off score of 0.40 (see Fig. 1B). For discriminating MCI from the normal control group, the AUC of K-IADL was 0.765, with a sensitivity of 0.736 and a specificity of 0.776 when a cut-off score of 0.09 was used (see Fig. 1C).
Fig. 1.
ROC curve for K-IADL to diagnose dementia or MCI. (A) ROC curve for K-IADL for diagnosing dementia in all participants (AUC = 0.959; p<0.001). (B) ROC curve for K-IADL to differentiate dementia from MCI in all participants (AUC = 0.952; p<0.001). (C) ROC curve for K-IADL to differentiate MCI from normal control in all participants (AUC = 0.765; p<0.001). (D) ROC curve for K-IADL for diagnosing dementia in patients with AD, MCI, and normal control (AUC = 0.964; p<0.001). (E) ROC curve for K-IADL for diagnosing dementia in the patients with VCI and normal control (AUC = 0.989; p<0.001). (F) ROC curve for K-IADL for diagnosing dementia in patients with PD and normal control (AUC = 0.944; p<0.001).
ROC: receiver operating characteristic, K-IADL: Korean-Instrumental Activities of Daily Living, MCI: mild cognitive impairment, AUC: area under the curve, AD: Alzheimer's disease, VCI: vascular cognitive impairment, PD: Parkinson's disease.
Patients with AD or aMCI
To discriminate dementia from non-dementia in patients with AD, aMCI, and the normal control group, the AUC of K-IADL was 0.964. K-IADL had a sensitivity of 0.901 and a specificity of 0.921 with an optimal cut-off score of 0.40 which was the same as the global cut-off score derived from all participants (see Fig. 1D).
Patients with vascular cognitive impairment (VCI)
In patients with VaD or VaMCI and normal control group, we used ROC curve analysis and calculated AUC. The AUC of K-IADL was 0.989 and the optimal cut-off score was 0.37 for distinguishing dementia from non-dementia in patients, with a sensitivity of 0.962 and a specificity of 0.956 (see Fig. 1E). When the global cut-off score of 0.40 was applied to discriminate dementia from non-dementia in the group of VCI patients, the sensitivity was slightly lower (0.962 to 0.954) while the specificity was slightly higher (0.956 to 0.977) than those when an optimal cut-off score of 0.37 was applied.
PD
To discriminate dementia from non-dementia in patients with PDD or PD-MCI and the normal control group, the AUC of K-IADL was 0.944, with a sensitivity of 0.883 and a specificity of 0.891 when an optimal cut-off score of 0.35 was used (see Fig. 1F). When the global cut-off score of 0.40 was applied to discriminate dementia from non-dementia in the group with PD, the sensitivity was slightly lower (0.883 to 0.835) while the specificity was slightly higher (0.891 to 0.942) than those when an optimal cut-off score of 0.35 was applied.
Cross validation
A total of 470 patients (20%) were randomly selected out of 2,347 patients for the cross-validation study. They did not show significant differences in sex, age, education, or scores of K-MMSE, CDR, GDS, S-GDS, BI, or K-IADL compared to the other 1,877 (80%) patients.
When we diagnosed patients with a K-IADL total score above the new cut-off score of 0.40 to determine whether they had dementia, the positive predictive value was 93.9% for the 470 patients. On the other hand, when we used the previous cut-off score of 0.43 for dementia diagnosis, the positive predictive value was 92.5%. For various dementia groups, we also found that positive predictive values were slightly higher when we used the new cut-off score of 0.40 than the previous one of 0.43. These results are summarized in Table 5.
Table 5. Optimal cut-off scores of K-IADL to diagnosis dementia and MCI, AUC, sensitivity, and specificity.
| Variables | Optimal cut-off score | AUC | p-value | Sensitivity | Specificity | Positive predict value for dementia with 0.40 | Positive predict value for dementia with 0.43* | |
|---|---|---|---|---|---|---|---|---|
| In all participants | 93.9% | 92.5% | ||||||
| Dementia vs. non-dementia | 0.40 | 0.959 | <0.001 | 0.901 | 0.916 | |||
| Dementia vs. MCI | 0.40 | 0.952 | <0.001 | 0.901 | 0.898 | |||
| MCI vs. normal | 0.09 | 0.765 | <0.001 | 0.736 | 0.776 | |||
| In the patients with AD and MCI | 94.2% | 93.5% | ||||||
| AD vs. MCI | 0.40 | 0.964 | <0.001 | 0.901 | 0.921 | |||
| In the patients with VCI | 100% | 97% | ||||||
| VaD vs. VaMCI | 0.37 | 0.989 | <0.001 | 0.962 | 0.956 | |||
| In the patients with PD | 84% | 80% | ||||||
| PDD vs. PD-MCI | 0.35 | 0.944 | <0.001 | 0.883 | 0.891 | |||
K-IADL: Korean-Instrumental Activities of Daily Living, MCI: mild cognitive impairment, AUC: area under the curve, AD: Alzheimer's disease, VCI: vascular cognitive impairment, VaD: vascular dementia, VaMCI: vascular MCI, PD: Parkinson's disease, PDD: Parkinson's disease dementia.
*The cut-off score in the previous study.7
We performed CFA with these 470 patients to test data fit 1 factor model of K-IADL. Goodness-of-fit index of 1 factor model results were: Tucker-Lewis Index (TLI)=0.945, Comparative Fit Index (CFI)=0.963, and Root Mean Square Error of Approximation (RMSEA)=0.082. These values indicated a good fit between the measurement model of K-IADL and data of these 470 patients (χ2=184.04, p<0.001).
DISCUSSION
Evaluating IADL is an important procedure for dementia diagnosis. It is critical for differentiating dementia and MCI in clinical setting. Furthermore, evaluation of IADL can be useful for early detection of dementia. In patients with neurodegenerative disease such as AD, PADL can be maintained until the end stage of the disease. However, IADL can decline in the early stage of dementia. In some cases, caregivers can detect an abnormality very early in patients when they notice problems in IADL before patients' cognitive dysfunction is evaluated through neuropsychological tests in the clinic.
K-IADL was developed in 2002. Its reliability and validity study was performed with 114 dementia patients (64 AD, 46 VaD, and 4 mixed dementia) and 106 normal controls.7 In that study, the authors reported good test-retest reliability, internal consistency, and construct and concurrent validities. Additionally, they conducted ROC curve analyses to identify the optimal cut-off score for K-IADL to discriminate dementia from non-dementia patients. In the previous study, the optimal cut-off was 0.43, with sensitivity and specificity of 83% and 82%, respectively.7
Our results showed that the new optimal cut-off score of K-IADL was 0.40, which was slightly lower than the original cut-off score of 0.43. The difference between the new cut-off score and the original one was small. However, when we discriminated dementia patients from non-dementia patients, both sensitivity and specificity were improved with the new cut-off score of 0.40 compared to the original cut-off score of 0.43. Our results suggest that the new cut-off score of 0.40 is more appropriate in a clinical setting.
To confirm the validity of K-IADL, we also assessed whether there were significant differences in K-IADL according to the CDR and GDS levels in all patients. Mean scores of K-IADL were significantly different according to CDR levels. Mean scores of K-IADL were also significantly different according to GDS level except for scores between GDS 1 and GDS 2. In the previous study, the cut-off score of 0.43 was found between mean scores of K-IADL in CDR 0 (0.11±0.15) and CDR 0.5 (0.52±0.42), and between mean scores of K-IADL in GDS 2 (0.16±0.17) and GDS 3 (0.52±0.42).7 However, in our results, the new cut-off score of 0.40 occurred between mean scores of K-IADL in CDR 0.5 (0.28±0.30) and CDR 1 (1.14±0.59), and between mean scores of K-IADL in GDS 3 (0.23±0.25) and GDS 4 (0.81±0.53). These results indicated that the new cut-off score might be more optimal for differentiating dementia (usually CDR 1 or above and GDS 4 or above) from MCI (usually CDR 0.5 or less and GDS 3 or less). In addition, the higher positive value with cut-off score of 0.40 suggests that the new cut-off score is appropriate, although the difference between the 2 cut-off scores was small.
The reason for the lower cut-off score for dementia in this study compared to the original one7 seemed to be due to difference in characteristics of control groups. The control group in this study was composed of normal elderly in the community and MCI patients. However, the control group in the previous study consisted of caregivers and patients who did not have disease of the central nervous system or complaint of cognitive impairment. We could expect that IADL of normal controls from the community would be better than IADL of controls recruited in hospital. As a result, the mean K-IADL score of the control group (normal elderly and MCI patients) in this study was 0.17±0.21, which was lower than that in the previous study (0.21±0.28). Thus, the cut-off score of this study was lower than the previous study due to the lower mean score of the control group in this study.
When we yielded cut-off scores in various dementia subgroups, the cut-off score for dementia due to AD was the same for discriminating dementia in all participants (cut-off score in AD: 0.40). However, the cut-off scores for discriminating dementia in patients with VCI or PD were slightly lower than cut-off scores in patients with AD (cut-off score in VCI: 0.37; in PD: 0.35), although K-MMSE total scores were not significant different among the three dementia groups. When we used the same cut-off score of 0.40 for K-IADL to discriminate dementia in patients with VCI or PD, the sensitivity was slightly lower (VCI: 0.962 to 0.954; PD: 0.883 to 0.835) while the specificity was slightly higher (VCI: 0.956 to 0.977; PD: 0.891 to 0.942). Based on these results, it is possible that some mild VaD and PDD patients could be missed from diagnosis if global cut-off score of 0.40 is applied.
One possible reason for a lower cut-off score in VCI and PD might be that VaD or PDD patients were marked as “not applicable” for many items because of their physical or movement problems. In fact, further analysis of this study revealed that the number of items marked as “not applicable” were significantly higher in patients with VaD and PDD than AD (AD: 1.30±1.32; VaD: 2.12±2.01; PDD: 2.19±1.94). As the number of items marked “not applicable” would increase, the total score of the K-IADL would decrease. Based on these results, we can conclude that a new IADL instrument should be developed for correct diagnosis of dementia in patients with physical limitations such as VaD and PDD. Cheon et al.23 have also argued that the impairment of IADL in PDD should be assessed with items specifically impaired due to cognitive disabilities in t patients with PD (e.g., keeping appointments, talking about recent events, managing money, and using a telephone). Therefore, further research is needed to find specifically tailored IADL items for target groups to attenuate the effect of physical or movement problems.
Recent studies have reported that intact ADL could not be used as a criterion to diagnose MCI because impairment of ADL already appears in MCI state.24-26 We also conducted ROC curve analysis to differentiate MCI from the normal control group using K-IADL. However, the AUC of K-IADL was 0.765, which was lower than that when differentiating dementia patients from non-dementia ones. Its sensitivity of 0.736 and specificity of 0.776 were also lower than those when differentiating dementia. These results could be attributed to contents of K-IADL for discriminating dementia patients from non-dementia patients instead of discriminating MCI patients from normal controls. If an IADL inventory was developed to diagnose patients with MCI, items with more complex activities should be included. Additionally, changes in elderly lifestyle such as development of information technology and rapid increase in the use of smartphones and the internet should be considered when developing a new IADL inventory for MCI patients. For instance, the Alzheimer's Disease Cooperative Study Scale for ADL (ADCS-ADL) was newly developed for MCI diagnosis with a 24-item scale, in which six MCI-specific items, such as driving a car and organizing medication, were added to the original ADCS-ADL scale.27 On the other hand, Jekel et al.28 have suggested that an ADL scale for MCI diagnosis should include activities that require higher cognitive processes. The use of performance-based measures and technology-related items seems to be promising.
In summary, this study was a large-scale collaborative research in which six major general hospitals participated and more than 2,300 patient data with 254 normal elderlies were collected. Our results supported that the newly developed cut-off score of 0. 40 for K-IADL was valid. This new cut-off score showed higher positive predict value than the previous one. We believe that this new cut-off score is clinically useful by improving diagnostic accuracy with high sensitivity and specificity while reducing false negative in dementia diagnosis.
Footnotes
Funding: This study was supported by the Korean Dementia Association Grant for Research Group (2016).
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Chin J, Kang Y.
- Data curation: Chin J, Park J, Yang S, Yeom J, Ahn Y, Baek MJ, Ryu HJ, Lee BH, Han NE, Ryu KH, Kang Y.
- Formal analysis: Chin J, Park J, Kang Y.
- Funding acquisition: Kang Y.
- Methodology: Chin J, Park J, Yang S, Yeom J, Ahn Y, Baek MJ, Ryu HJ, Lee BH, Han NE, Ryu KH, Kang Y.
- Project administration: Chin J.
- Writing - original draft: Chin J.
- Writing - review & editing: Chin J, Kang Y.
References
- 1.Cumming JL, Benson DF. Dementia: a Clinical Approach. 2nd ed. Boston, MA: Butterworth-Heinemann; 1992. [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Hindmarch I, Lehfeld H, de Jongh P, Erzigkeit H. The Bayer activities of daily living scale (B-ADL) Dement Geriatr Cogn Disord. 1998;9(Suppl 2):20–26. doi: 10.1159/000051195. [DOI] [PubMed] [Google Scholar]
- 4.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 5.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HW, Kang SJ, Choi SH. Korean Dementia Association, editors. Dementia: Clinical Approach. 2nd ed. Anyang: Academia; 2011. Assessment of activities of daily living and abnormal behavior; pp. 125–143. [Google Scholar]
- 7.Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH, et al. The reliability and validity of the Korean Instrumental Activities of Daily Living (K-IADL) J Korean Neurol Assoc. 2002;20:8–14. [Google Scholar]
- 8.Choi SH, Na DL, Lee BH, Kang SJ, Ha CK, Han SH, et al. Validation of the Korean version of the Bayer activities of daily living scale. Hum Psychopharmacol. 2003;18:469–475. doi: 10.1002/hup.505. [DOI] [PubMed] [Google Scholar]
- 9.Suh GH. Development of the Korean version of Disability Assessment for Dementia Scale (DAD-K) to assess function in dementia. J Korean Geriatr Soc. 2003;7:278–287. [Google Scholar]
- 10.Ku HM, Kim JH, Kwon EJ, Kim SH, Lee HS, Ko HJ, et al. A study on the reliability and validity of Seoul-Instrumental Activities of Daily Living (S-IADL) J Korean Neuropsychiatr Assoc. 2004;43:189–199. [Google Scholar]
- 11.Won CW, Rho YG. SunWoo D, Lee YS. The validity and reliability of Korean Instrumental Activities of Daily Living (K-IADL) Scale. J Korean Geriatr Soc. 2002;6:273–280. [Google Scholar]
- 12.Kang Y, Jahng S, Na DL. Seoul Neuropsychological Screening Battery II. Seoul: Human Brain Research & Consulting Co.; 2012. [Google Scholar]
- 13.Christensen KJ, Multhaup KS, Nordstrom S, Voss K. A cognitive battery for dementia: development and measurement characteristics. Psychol Assess. 1991;3:168–174. [Google Scholar]
- 14.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 15.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22:1689–1707. doi: 10.1002/mds.21507. [DOI] [PubMed] [Google Scholar]
- 18.Kang Y. A normative study of the Korean-Mini Mental State Examination (K-MMSE) in the elderly. Korean J Psychol Gen. 2006;25:1–12. [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 21.Cho MJ, Bae JN, Suh GH, Hahm BJ, Kim JK, Lee DW, et al. Validation of Geriatric Depression Scale, Korean version (GDS) in the assessment of DSM-III-R major depression. J Korean Neuropsychiatr Assoc. 1999;38:48–63. [Google Scholar]
- 22.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 23.Cheon SM, Park KW, Kim JW. Identification of daily activity impairments in the diagnosis of Parkinson disease dementia. Cogn Behav Neurol. 2015;28:220–228. doi: 10.1097/WNN.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 24.Aretouli E, Brandt J. Everyday functioning in mild cognitive impairment and its relationship with executive cognition. Int J Geriatr Psychiatry. 2010;25:224–233. doi: 10.1002/gps.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. J Clin Exp Neuropsychol. 2012;34:11–34. doi: 10.1080/13803395.2011.614598. [DOI] [PubMed] [Google Scholar]
- 26.Perneczky R, Pohl C, Sorg C, Hartmann J, Tosic N, Grimmer T, et al. Impairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndrome. Int J Geriatr Psychiatry. 2006;21:158–162. doi: 10.1002/gps.1444. [DOI] [PubMed] [Google Scholar]
- 27.Pedrosa H, De Sa A, Guerreiro M, Maroco J, Simoes MR, Galasko D, et al. Functional evaluation distinguishes MCI patients from healthy elderly people--the ADCS/MCI/ADL scale. J Nutr Health Aging. 2010;14:703–709. doi: 10.1007/s12603-010-0102-1. [DOI] [PubMed] [Google Scholar]
- 28.Jekel K, Damian M, Wattmo C, Hausner L, Bullock R, Connelly PJ, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimers Res Ther. 2015;7:17. doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]