Abstract
Cerebral microbleeds (CMBs) are increasingly recognized neuroimaging findings, occurring with cerebrovascular disease, dementia, and aging. CMBs are associated with subsequent hemorrhagic and ischemic stroke, and also with an increased risk of cognitive deterioration and dementia. They occur in the setting of impaired small vessel integrity due to hypertension or cerebral amyloid angiopathy. This review summarizes the concepts, cause or risk factors, histopathological mechanisms, and clinical consequences of CMBs.
Keywords: Cerebral Microbleeds, Dementia, Stroke
INTRODUCTION
Cerebral microbleeds (CMBs) are a chronic accumulation of small blood products in the brain tissue.1 When brain magnetic resonance imaging (MRI) began to apply a high magnetic susceptibility technique in the mid-1990s, CMBs became well known.2 With further development of MRI techniques, the detection rate of CMBs increased significantly, and it has become of great interest and importance to certain conditions, such as dementia and stroke, as well as normal aging.
The prevalence of CMB varies according to the subject and disease being investigated. In an extensive study of community-dwelling middle-aged or older adults,3,4 the prevalence of CMB was 11.1%–15.3%. The incidence of CMB was 6% in the late 40s, and 38% after age 80.5,6 The CMB prevalence has been reported to be 18%–32% in Alzheimer's disease, 20%–43% in mild cognitive impairment, and 68%–85% in subcortical vascular dementia.7,8,9 And, it occurred in 20%–70% of patients with cerebral hemorrhage, and 30%–40% of patients with cerebral infarction.10
CMBs have been considered to be asymptomatic lesions that may be found incidentally on the brain MRI.11,12 Recently, CMBs were associated with an increased the risk of cognitive decline, dementia, intracerebral hemorrhage (ICH), cerebral infarction, or recurrence of transient ischemic attack,7,13 and mortality rate.14 And it has been shown to play the role of a biomarker in small vessel disease (SVD), such as hypertension, cerebral amyloid angiopathy (CAA), and chronic kidney disease.5,15
Despite the growing interest and investigations in CMB, many issues remain to be clarified, such as the precise developmental mechanism, clinical significance, prognosis, and treatment of CMBs. In this paper, we review clinical research on CMBs and discuss the future directions for study.
DEFINITION OF CMB
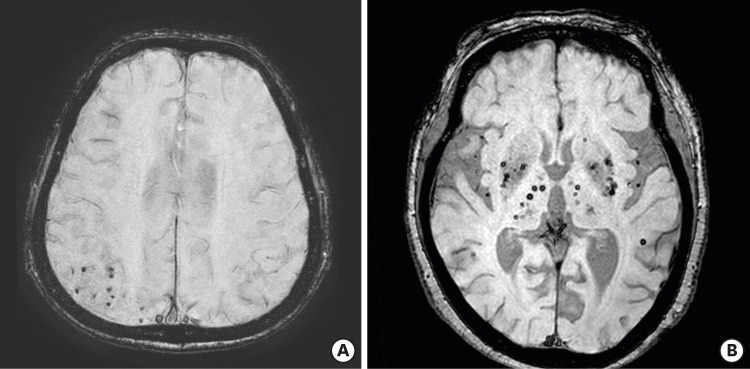
CMB is a small circular or elliptical lesions with a size of 2 to 10 mm in a gradient-recalled echo (GRE) image or susceptibility-weighted image (SWI) (Fig. 1).2,16 Histopathologically, CMB shows hemosiderin accumulation in the surrounding blood vessels, in the same region that appears as a hypointense lesion on brain MRI.17 Greenberg suggested criteria (Table 1) to differentiate among diseases that are easily confused with the neuroimaging features of CMBs.2 In addition, Wardlaw et al.16 proposed a standardization neuroimaging definition of CMBs, to be used for SVD studies.
Fig. 1. CMBs on gradient-recalled echo imaging in brain magnetic resonance imaging. (A) Multiple CMBs in the parietal lobe (B) multiple deep CMBs in bilateral basal ganglia and thalamus.
CMB: cerebral microbleed.
Table 1. Recommended criteria for cerebral microbleed identification.
| Greenberg's criteria1 | |
| - Black on GRE T2*-weighted MRI | |
| - Round or ovoid (rather than linear) | |
| - Blooming on T2*-weighted MRI | |
| - Devoid of signal hyperintensity on T1- or T2-weighted sequences | |
| - At least half surrounded by brain parenchyma | |
| - Distinct from other potential mimics such as iron/calcium deposits, bone, or vessel flow voids | |
| - Clinical history, excluding traumatic diffuse axonal injury | |
| Wardlaw's definition16 | |
| Small (generally 2–5 mm in diameter, but sometimes up to 10 mm) areas of the signal void with associated blooming seen on T2*-weighted MRI or other sequences that are sensitive to susceptibility effects. | |
GRE: gradient-recalled echo, MRI: magnetic resonance image.
CMB requires differentiation from other hypointense lesions in GRE and SWI. For instance, calcium or iron depositions appear as hypointensities in the bilateral basal ganglia, choroidal plexus, and pineal gland. It can be distinguished from CMB by the location and very high-signal intensity on computed tomography. The blood flow of the pial vessels also is seen with the same signal intensity of CMB, with round shape on the T2-weighted image and GRE. It is possible to discriminate the 2 by continuity with other slices.2 Artifacts in the frontal or temporal lobes, due to the orbital or mastoid bone7 and cavernous malformations, occur in the curved space in which stagnant blood and multiple stages of blood products produce various signals.18 Melanin can be confused with CMB because of its hemorrhagic tendency, but melanin can be distinguished by high signal intensity on T1-weighted images and edema around the lesion.19
CMB DETECTION USING BRAIN MRI
Findings of CMBs are affected by echo time, spatial resolution, and intensity of the magnetic field.20,21 As the MRI magnetic field strength increases and the cross section becomes thinner, the blooming effect, which produces an image larger than the actual amount of accumulated blood iron, becomes so large that the CMB can be confirmed with greater sensitivity.20,22 GRE and SWI are commonly used in clinical practice to detect microbleeds in the brain. SWI is recommended for quantifying numbers of CMBs because it showed more inter-rater reliability and higher sensitivity for detection CMB, than GRE in previous studies.13,22,23
For evaluating CMB, visual scoring systems and an automated or a semi-automated brain imaging analysis method are used.24,25 Visual scoring systems include the Microbleed Anatomical Rating Scale (MARS)26 and the Brain Observer MicroBleed Scale (BOMBS).27 In both methods, CMB is scored by location, such as right or left infra-tentorial, deep, and cerebral hemisphere; and by certain or ambiguous. The MARS scale is different in that the cerebral regions are divided into each lobe, whereas the BOMBS is divided into the cortex and subcortical regions (Fig. 2). Reliability of the assessed number and location of CMBs is low without the use of an evaluation tool, and these visual scoring systems do not show any significant difference in results, even when applied to other MRIs with different echo times.
Fig. 2. Visual rating of cerebral microbleeds. (A) The Microbleed Anatomical Rating Scale,26 (B) The Brain Observer MicroBleed Scale.27.
B: brain stem, Bg: basal ganglia, C: cerebellum, Cc:corpus callosum, DPWM: deep periventricular white matter, Ec: external capsule, F: frontal lobe, I: insula, Ic: internal capsule, O: occipital lobe, P: parietal lobe, T: temporal lobe, Th: thalamus.
Therefore, it is helpful to identify the presence, number and location of CMB systematically to provide more clinical information on microbleeds.
RISK FACTORS OF CMB
Age is an important risk factor for the development of microbleeds in the brain, and an increase in CMBs is associated with advancing age, white matter hyperintensities, and lacunar infarction. A recent cohort study of more than 2,500 community-dwelling people, done over a 5-year period, showed a 2.6-fold increase in the risk of lobar microbleeds in participants with brain atrophy.28
Hypertension is the most reliable risk factor for CMB. It causes increasing vascular endothelial cell damage, and inflammation due to tumor necrosis factor.29,30 It is also associated with lacunar infarction, white matter hyperintensities and left ventricular hypertrophy. When two or more retinal microvascular signs, such as retinal arteriovenous stenosis due to hypertension are observed, the risk of microbleeds in the brain is increased 3-fold.28 Therefore, it can be assumed that vascular injury due to hypertension causes CMBs.2,22,31
Cholesterol, diabetes mellitus, and smoking have also been reported as risk factors for microbleeds, but the results differ according to the study. Although some studies have shown hypercholesterolemia lowered the risk of microbleeds and statin therapy increased the risk of cerebral hemorrhage,32,33 meta-analysis results showed the opposite.34 Cystatin C, a new renal function indicator, has been associated with deep and infratentorial CMB, as well as chronic kidney disease, implicating it as a risk factor for CMB.35
The incidence of microbleeds is less than 1 per year, but it is high in cases of: 1) more than 5 CMBs in the early stage of disease, 2) severe SVD pathology, or 3) CAA with apolipoprotein ε2 or ε4 genotype.32,36,37 Of the apolipoprotein E genotypes, ε2 is associated with fibrinoid necrosis, and ε4 is characterized by accumulations of β-amyloid, absence of smooth muscle fiber, and vessel wall thickening, resulting in microbleeds.6,38 In a pathology study, more than 50 counts of CMBs in a patient were associated with thicker amyloid-deposited vessels than with less than 3 number of CMBs.39 This indicates that the number of CMBs reflects the underlying vascular disease state and is a biomarker of future microbleed occurrence.
PATHOPHYSIOLOGY OF CMB
The pathophysiology of CMB has not yet been fully elucidated. However, hypertensive microangiopathy and CAA could damage the blood-brain barrier and neurovascular unit, to cause CMBs with blood leakage and hemosiderin deposits in the brain parenchyma. Hypertension causes lipohyalinosis, and fibrohyalinosis which affect the deep penetrating arterioles and accumulation of Aβ in the cortical and leptomeningeal arteries, resulting in inflammation, oxidative stress, and apoptosis. Also, cerebral amyloid disease, chronic kidney disease, and cardiac disease, commonly damage arteriolar smooth muscle cells and capillaries, which comprise the blood-brain barrier and neurovascular unit.40
Recently, a relationship between CMB and enlargement of the perivascular space in the centrum semiovale seen by MRI, was proposed. It was speculated that amyloid accumulation in CAA may cause enlargement of vascular space and microbleeds.41,42,43 In Alzheimer's disease, CMB occurs mainly in the cortical-subcortical boundary and is caused by CAA associated with Alzheimer's disease. It is accompanied by blood-brain barrier dysfunction, as well as Aβ deposition in blood vessels. More than 87% of CMBs located strictly in the lobar regions and predominantly in the posterior cortex of the brain and not in the deep gray matter or brainstem, are likely caused by CAA.44 On the other hand, hypertensive microbleeds predominantly occur in deep gray matter or the brainstem.
DISEASES ASSOCIATED WITH CMBs
Dementia and cognitive decline
CMB has been shown to increase the risk of dementia and cognitive decline in patients with cognitive impairment, as well as in the normal community-dwelling population.6,19,42 Although the number of CMB needed to affect cognitive function has not yet been determined, some studies have examined the relationship between number of CMB and cognitive function. In the Rotterdam study,6,43 the number of CMBs was associated with a greater cognitive decline, with more pronounced cognitive dysfunction at 4 or more CMBs, and decline of all cognitive functions, except memory at 5 or more CMBs.
CMB could damage function at the bleed location or cause various non-specific cognitive declines. Lobar microbleeds have been related to the Mini-Mental Status Examination score (general cognitive function), executive function, process speed, and memory impairment. Deep and infratentorial microbleeds have been associated with motor or psychomotor speed, and attention deficits.7 CMBs in the temporal lobe have been associated with memory and attention deficits, and frontal CMBs with dysfunction of memory, mental speed, set shifting, and attention.36
In general, CMBs are associated with a higher risk of dementia or cognitive decline in patients with cognitively normal, mild cognitive impairment, Alzheimer's disease or vascular dementia. Although there have not been many longitudinal studies analyzing the effect of CMBs on cognitive function over time, microbleeds in patients with ICH have been associated with a higher likelihood of cognitive decline.
CMB distribution is different among dementia types. CMBs in CAA, and in AD patients showed lobar distribution predominantly. CMBs in patients with Lewy-body dementia or fronto-temporal lobar degeneration, also occurred at lobar locations predominantly. However, CMBs in vascular dementia patients were distributed equally in the lobar, deep and infratentorial regions.45
Stroke
Microbleeds accompany strokes with lacunar infarction more often than cerebral infarction by atherothrombosis or cardioembolism. This suggests that microbleeds are associated with small vessel lipohyalinosis.46 CMB is used as a predictor of future stroke. In patients with lacunar infarction or transient ischemic attack, CMB is more likely to cause cerebral hemorrhage, as well as ischemic stroke.12,22,47,48 In particular, if there are more than 10 CMBs or multiple CMBs in the lobes, the risk of bleeding is higher.31,49 Stroke mortality is significantly higher in patients with CMBs, than with other associated vascular risk factors.36,50,51
Cerebral hemorrhage including CMB is classified as lobar hemorrhage. It is can be located in the cortex due to damage of cortical/leptomeningeal vessels by CAA; seen as deep hemorrhage caused by hypertension with damaging deep perforating artery; or mixed hemorrhage, a combination of lobar and deep hemorrhage.41 As lobar hemorrhage shows more severe white matter change in the occipital area, and has a higher frequency of ε4 than deep hemorrhage, this type of bleed is more likely to occur due to CAA.52 Deep or infratentorial microbleeds occur when there is a high systolic blood pressure, a high blood pressure fluctuation, a lacunar infarction, or severe white matter hyperintensities.3,53 Mixed hemorrhage has been associated with more lacunar infarction and lower risk of recurrence of hemorrhage, compared to only lobar hemorrhage or deep hemorrhage. Mixed hemorrhage was also associated more with older age, number of CMBs, and higher recur risk than deep hemorrhage, suggesting that location, mechanism, and prognosis are different between deep and mixed hemorrhage types.41
Genetic diseases
CMBs can appear in various diseases, such as cerebellar autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), autoimmune encephalitis, traumatic brain injury, cerebral degeneration after radiation therapy, and Moyamoya disease.21,22,23 The incidence of CMB in CADASIL ranges from 30% to 70%, and increases with age, mainly in the thalamus.21 COL4A1 mutation carriers have a variety of brain lesions, such as white matter hyperintensities, perivascular enlargement, and microbleeds. More than half of the patients have CMBs that develop mainly in the deep white matter, gray matter, brainstem, and cerebellum.24 The sortilin-related receptor gene was reported to correlate with microbleeds in populations with hypertension, and involved the amyloid cascade process.54
MANAGEMENT AND PROGNOSIS
Although there is no proven therapy for CMB, management of hypertension may be helpful in microbleeds because blood pressure control can reduce not only hypertensive deep hemorrhage, but also CAA-induced lobar hemorrhage.10 Although different results have been seen, depending on study design and medications, the use of antiplatelet, antithrombotic, and thrombolytic agents for prolonged periods may cause CMBs.44,46
Risk of CMB may influence the treatment of accompanying disease. There is insufficient evidence to limit the use of anticoagulants unconditionally for the treatment of atrial fibrillation in patients with CMBs. However, use of anticoagulants is not recommended in patients over 60 years of age with atrial fibrillation who require anticoagulant therapy, but may have: 1) a history of cerebral hemorrhage and superficial siderosis, 2) an intracranial hemorrhage in mid-life, 3) a family history of early-onset dementia and MRI findings suggest CAA, or 4) a multiple CMBs after taking 2 antiplatelet agents for acute cardiovascular disease.55 Caution should be exercised when selecting intravenous thrombolytic agents for management of acute cerebral infarction accompanied by CMBs. Studies have shown that the risk of cerebral hemorrhage is relatively greater after thrombolysis in the presence of CMB, and more symptomatic ICH occurs with higher numbers of microbleeds. However, there is not enough evidence yet to determine the appropriate use of thrombolytic agents in the cases of patients with CMBs. In relation to the treatment of Alzheimer's disease, some researchers have recommended that patients with more than 4 CMBs be excluded from treatment with Aβ immunotherapy.9 This is because an inflammatory reaction could be triggered by the immunotherapy agent used to target amyloid in the tissue and vessels.56
CONCLUSIONS
CMB increases the risk of cognitive decline or dementia, as well as increases the risk of stroke and stroke mortality. In addition, it plays a role as a biomarker of SVDs, such as arteriosclerosis and CAA, and the extent of CMBs can be used as an index to estimate the status of underlying disease. Therefore, systemic and quantitative evaluation should be used to assess prognosis. Further studies are needed on treatment guidelines that consider the risk of cerebral hemorrhage when there is CMB in stroke patients, and appropriate blood pressure therapy to prevent CMB occurrence.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Lee J, Sohn EH, Oh E, Lee AY.
- Investigation: Oh E.
- Resources: Sohn EH.
- Supervision: Sohn EH, Oh E.
- Validation: Sohn EH, Oh E.
- Writing - original draft: Lee J, Lee AY.
- Writing - review & editing: Lee J, Lee AY.
References
- 1.Roob G, Schmidt R, Kapeller P, Lechner A, Hartung HP, Fazekas F. MRI evidence of past cerebral microbleeds in a healthy elderly population. Neurology. 1999;52:991–994. doi: 10.1212/wnl.52.5.991. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poels MM, Ikram MA, van der Lugt A, Hofman A, Krestin GP, Breteler MM, et al. Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study. Stroke. 2011;42:656–661. doi: 10.1161/STROKEAHA.110.607184. [DOI] [PubMed] [Google Scholar]
- 4.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 6.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–S106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 7.Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–943. doi: 10.1001/jamaneurol.2016.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo SW, Lee BH, Kim EJ, Chin J, Cho YS, Yoon U, et al. Clinical significance of microbleeds in subcortical vascular dementia. Stroke. 2007;38:1949–1951. doi: 10.1161/STROKEAHA.106.477315. [DOI] [PubMed] [Google Scholar]
- 9.Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. 2011;134:335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- 10.Koennecke HC. Cerebral microbleeds on MRI: prevalence, associations, and potential clinical implications. Neurology. 2006;66:165–171. doi: 10.1212/01.wnl.0000194266.55694.1e. [DOI] [PubMed] [Google Scholar]
- 11.Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CL, et al. Edinburgh Stroke Study Group. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41:1222–1228. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 12.Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, et al. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: a meta-analysis. Neurology. 2016;87:1501–1510. doi: 10.1212/WNL.0000000000003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park MY, Park HJ, Shin DS. Distribution analysis of cerebral microbleeds in Alzheimer's disease and cerebral infarction with susceptibility weighted MR imaging. J Korean Neurol Assoc. 2017;35:72–79. [Google Scholar]
- 14.Akoudad S, Ikram MA, Koudstaal PJ, Hofman A, van der Lugt A, Vernooij MW. Cerebral microbleeds and the risk of mortality in the general population. Eur J Epidemiol. 2013;28:815–821. doi: 10.1007/s10654-013-9854-3. [DOI] [PubMed] [Google Scholar]
- 15.Peng Q, Sun W, Liu W, Liu R, Huang Y CASISP Study Group. Longitudinal relationship between chronic kidney disease and distribution of cerebral microbleeds in patients with ischemic stroke. J Neurol Sci. 2016;362:1–6. doi: 10.1016/j.jns.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis. 2011;32:528–534. doi: 10.1159/000331466. [DOI] [PubMed] [Google Scholar]
- 18.Al-Shahi Salman R, Berg MJ, Morrison L, Awad IA Angioma Alliance Scientific Advisory Board. Hemorrhage from cavernous malformations of the brain: definition and reporting standards. Stroke. 2008;39:3222–3230. doi: 10.1161/STROKEAHA.108.515544. [DOI] [PubMed] [Google Scholar]
- 19.Gaviani P, Mullins ME, Braga TA, Hedley-Whyte ET, Halpern EF, Schaefer PS, et al. Improved detection of metastatic melanoma by T2*-weighted imaging. AJNR Am J Neuroradiol. 2006;27:605–608. [PMC free article] [PubMed] [Google Scholar]
- 20.Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haller S, Vernooij MW, Kuijer JP, Larsson EM, Jäger HR, Barkhof F. Cerebral microbleeds: imaging and clinical significance. Radiology. 2018;287:11–28. doi: 10.1148/radiol.2018170803. [DOI] [PubMed] [Google Scholar]
- 22.Haacke EM, DelProposto ZS, Chaturvedi S, Sehgal V, Tenzer M, Neelavalli J, et al. Imaging cerebral amyloid angiopathy with susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2007;28:316–317. [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, et al. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke. 2013;44:2782–2786. doi: 10.1161/STROKEAHA.113.002267. [DOI] [PubMed] [Google Scholar]
- 24.Seghier ML, Kolanko MA, Leff AP, Jäger HR, Gregoire SM, Werring DJ. Microbleed detection using automated segmentation (MIDAS): a new method applicable to standard clinical MR images. PLoS One. 2011;6:e17547. doi: 10.1371/journal.pone.0017547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuijf HJ, Brundel M, de Bresser J, van Veluw SJ, Heringa SM, Viergever MA, et al. Semi-automated detection of cerebral microbleeds on 3.0 T MR images. PLoS One. 2013;8:e66610. doi: 10.1371/journal.pone.0066610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 27.Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, et al. improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS) Stroke. 2009;40:94–99. doi: 10.1161/STROKEAHA.108.526996. [DOI] [PubMed] [Google Scholar]
- 28.Qiu C, Ding J, Sigurdsson S, Fisher DE, Zhang Q, Eiriksdottir G, et al. Differential associations between retinal signs and CMBs by location: the AGES-Reykjavik Study. Neurology. 2018;90:e142–e148. doi: 10.1212/WNL.0000000000004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H923–H924. doi: 10.1152/ajpheart.01278.2005. [DOI] [PubMed] [Google Scholar]
- 30.Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, et al. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. doi: 10.1212/WNL.0000000000001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher M. Cerebral microbleeds and thrombolysis: clinical consequences and mechanistic implications. JAMA Neurol. 2016;73:632–635. doi: 10.1001/jamaneurol.2016.0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeon SB, Kang DW, Cho AH, Lee EM, Choi CG, Kwon SU, et al. Initial microbleeds at MR imaging can predict recurrent intracerebral hemorrhage. J Neurol. 2007;254:508–512. doi: 10.1007/s00415-006-0406-6. [DOI] [PubMed] [Google Scholar]
- 33.Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Statin treatment and the occurrence of hemorrhagic stroke in patients with a history of cerebrovascular disease. Stroke. 2008;39:497–502. doi: 10.1161/STROKEAHA.107.488791. [DOI] [PubMed] [Google Scholar]
- 34.Hackam DG, Woodward M, Newby LK, Bhatt DL, Shao M, Smith EE, et al. Statins and intracerebral hemorrhage: collaborative systematic review and meta-analysis. Circulation. 2011;124:2233–2242. doi: 10.1161/CIRCULATIONAHA.111.055269. [DOI] [PubMed] [Google Scholar]
- 35.Oh MY, Lee H, Kim JS, Ryu WS, Lee SH, Ko SB, et al. Cystatin C, a novel indicator of renal function, reflects severity of cerebral microbleeds. BMC Neurol. 2014;14:127. doi: 10.1186/1471-2377-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol. 2014;4:205. doi: 10.3389/fneur.2013.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee SH, Lee ST, Kim BJ, Park HK, Kim CK, Jung KH, et al. Dynamic temporal change of cerebral microbleeds: long-term follow-up MRI study. PLoS One. 2011;6:e25930. doi: 10.1371/journal.pone.0025930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarron MO, Nicoll JA, Stewart J, Ironside JW, Mann DM, Love S, et al. The apolipoprotein E ε2 allele and the pathological features in cerebral amyloid angiopathy-related hemorrhage. J Neuropathol Exp Neurol. 1999;58:711–718. doi: 10.1097/00005072-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg SM, Nandigam RN, Delgado P, Betensky RA, Rosand J, Viswanathan A, et al. Microbleeds versus macrobleeds: evidence for distinct entities. Stroke. 2009;40:2382–2386. doi: 10.1161/STROKEAHA.109.548974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Z, Soo YO, Mok VC. Cerebral microbleeds: is antithrombotic therapy safe to administer? Stroke. 2014;45:2811–2817. doi: 10.1161/STROKEAHA.114.004286. [DOI] [PubMed] [Google Scholar]
- 41.Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology. 2018;90:e119–e126. doi: 10.1212/WNL.0000000000004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charidimou A, Boulouis G, Pasi M, Auriel E, van Etten ES, Haley K, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88:1157–1164. doi: 10.1212/WNL.0000000000003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez-Ramirez S, Pontes-Neto OM, Dumas AP, Auriel E, Halpin A, Quimby M, et al. Topography of dilated perivascular spaces in subjects from a memory clinic cohort. Neurology. 2013;80:1551–1556. doi: 10.1212/WNL.0b013e31828f1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renard D. Cerebral microbleeds: a magnetic resonance imaging review of common and less common causes. Eur J Neurol. 2018;25:441–450. doi: 10.1111/ene.13544. [DOI] [PubMed] [Google Scholar]
- 45.Unetani H, Hirai T, Hashimoto M, Ikeda M, Kitajima M, Sakamoto F, et al. Prevalence and topography of small hypointense foci suggesting microbleeds on 3T susceptibility-weighted imaging in various types of dementia. AJNR Am J Neuroradiol. 2013;34:984–989. doi: 10.3174/ajnr.A3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato H, Izumiyama M, Izumiyama K, Takahashi A, Itoyama Y. Silent cerebral microbleeds on T2*-weighted MRI: correlation with stroke subtype, stroke recurrence, and leukoaraiosis. Stroke. 2002;33:1536–1540. doi: 10.1161/01.str.0000018012.65108.86. [DOI] [PubMed] [Google Scholar]
- 47.Imaizumi T, Horita Y, Hashimoto Y, Niwa J. Dotlike hemosiderin spots on T2*-weighted magnetic resonance imaging as a predictor of stroke recurrence: a prospective study. J Neurosurg. 2004;101:915–920. doi: 10.3171/jns.2004.101.6.0915. [DOI] [PubMed] [Google Scholar]
- 48.Bokura H, Saika R, Yamaguchi T, Nagai A, Oguro H, Kobayashi S, et al. Microbleeds are associated with subsequent hemorrhagic and ischemic stroke in healthy elderly individuals. Stroke. 2011;42:1867–1871. doi: 10.1161/STROKEAHA.110.601922. [DOI] [PubMed] [Google Scholar]
- 49.van Etten ES, Auriel E, Haley KE, Ayres AM, Vashkevich A, Schwab KM, et al. Incidence of symptomatic hemorrhage in patients with lobar microbleeds. Stroke. 2014;45:2280–2285. doi: 10.1161/STROKEAHA.114.005151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soo YO, Yang SR, Lam WW, Wong A, Fan YH, Leung HH, et al. Risk vs benefit of anti-thrombotic therapy in ischaemic stroke patients with cerebral microbleeds. J Neurol. 2008;255:1679–1686. doi: 10.1007/s00415-008-0967-7. [DOI] [PubMed] [Google Scholar]
- 51.Altmann-Schneider I, Trompet S, de Craen AJ, van Es AC, Jukema JW, Stott DJ, et al. Cerebral microbleeds are predictive of mortality in the elderly. Stroke. 2011;42:638–644. doi: 10.1161/STROKEAHA.110.595611. [DOI] [PubMed] [Google Scholar]
- 52.Zhu YC, Chabriat H, Godin O, Dufouil C, Rosand J, Greenberg SM, et al. Distribution of white matter hyperintensity in cerebral hemorrhage and healthy aging. J Neurol. 2012;259:530–536. doi: 10.1007/s00415-011-6218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, et al. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916–2922. doi: 10.1161/STROKEAHA.112.658369. [DOI] [PubMed] [Google Scholar]
- 54.Schuur M, van Swieten JC, Schol-Gelok S, Ikram MA, Vernooij MW, Liu F, et al. Genetic risk factors for cerebral small-vessel disease in hypertensive patients from a genetically isolated population. J Neurol Neurosurg Psychiatry. 2011;82:41–44. doi: 10.1136/jnnp.2009.176362. [DOI] [PubMed] [Google Scholar]
- 55.Selim M, Diener HC. Atrial fibrillation and microbleeds. Stroke. 2017;48:2660–2664. doi: 10.1161/STROKEAHA.117.017085. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg SM, Bacskai BJ, Hyman BT. Alzheimer disease's double-edged vaccine. Nat Med. 2003;9:389–390. doi: 10.1038/nm847. [DOI] [PubMed] [Google Scholar]