Abstract
Background and Purpose
Although the clock drawing test (CDT) is a widely used cognitive screening instrument, there have been inconsistent findings regarding its utility with various scoring systems in patients with mild cognitive impairment (MCI) or dementia. The present study aimed to identify whether patients with MCI or dementia exhibited impairment on the CDT using three different scoring systems, and to determine which scoring system is more useful for detecting MCI and mild dementia.
Methods
Patients with amnestic mild cognitive impairment (aMCI), vascular mild cognitive impairment (VaMCI), mild Alzheimer's disease (AD), mild vascular dementia (VaD), and cognitively normal older adults (CN) were included. All participants were administered the CDT, the Korean-Mini Mental State Examination (K-MMSE), and the Clinical Dementia Rating scale. The CDT was scored using the 3-, 5-, and 15-point scoring systems.
Results
On all three scoring systems, all patient groups demonstrated significantly lower scores than the CN. However, while there were no significant differences among patients with aMCI, VaMCI, and AD, those with VaD exhibited the lowest scores. Area under the Receiver Operating Characteristic curves revealed that the three CDT scoring systems were comparable with the K-MMSE in differentiating aMCI, VaMCI, and VaD from CN. In differentiating AD from CN, however, the CDT using the 15-point scoring system demonstrated the most comparable discriminability with K-MMSE.
Conclusions
The results demonstrated that the CDT is a useful cognitive screening tool that is comparable with the Mini-Mental State Examination, and that simple CDT scoring systems are sufficient for differentiating patients with MCI and mild dementia from CN.
Keywords: Clock Drawing Test, Mini Mental State Examination, Mild Cognitive Impairment, Alzheimer's Disease, Vascular Dementia
INTRODUCTION
Cognitive screening tests have long been used as an initial step in the assessment of dementia. Ideally, a cognitive screening test should be brief, acceptable to patients, easy to score, independent of educational/cultural/language confounders, psychometrically robust, and broad in its coverage of cognitive domains.1,2 In this regard, the value of the clock drawing test (CDT) as a screening instrument for global cognitive deficits has been recognized by many studies due to its ease of use and brief administration time for patients with mild cognitive impairment (MCI) and/or various types of dementia.3,4 Proficiency in the CDT requires a wide range of cognitive domains, such as auditory comprehension, sustained attention, visuospatial ability, memory, abstract thinking, planning, motor execution, and executive function.1,2,3,4
Researchers have used slightly different instructions and methodologies to administer the CDT to cognitively impaired patients. These include using a pre-drawn circle, additional copying or time-reading commands, as well as free-drawing, which is the most commonly used method.4,5,6,7,8 In addition, several different scoring systems have been used, such as the two-point system in the Saint Louis University Mental Status9; the 3-point system in the Montreal Cognitive Assessment (MoCA)10; the five-point system in the Alzheimer's Disease Neuroimaging Initiative (ADNI)'s cognitive assessment; the 15-point system in the Behavioural Neurological Assessment (BNA)11; and the 20-point system in the Mendez's scoring system.12 Although there is no consensus on which CDT scoring system is the most effective, recent reviews suggest that simpler scoring systems are better because of their ease of use and their strong correlations with more complex systems.2,3
The utility of the CDT for screening patients with dementia as compared to normal participants has been widely accepted, and many studies have found that the positive and negative predictive values of the CDT are good.13,14,15 Additionally, the value of the CDT for differentiating patients with MCI from those with dementia has been recognized in many studies.16,17 However, there have been inconsistent findings regarding the utility of the CDT in discriminating between MCI and healthy normal controls. Some researchers have reported that the CDT is not useful for differentiating MCI from normal participants,18,19,20 while others have found that the CDT is a valuable screening tool for MCI patients.16,17
Previous studies examining whether the CDT could differentiate types of dementia have yielded conflicting results.21,22,23,24 For example, one study found that patients with Alzheimer's disease (AD) demonstrated poorer performance on the CDT than those with vascular dementia (VaD),22 whereas other studies reported that VaD patients scored lower than AD patients.23,24 Considering the subtypes of MCI, no difference was found in the performances on the CDT between amnestic mild cognitive impairment (aMCI) and vascular mild cognitive impairment (VaMCI) patients.25,26 However, to date, no studies have examined the validity of the CDT in differentiating these two MCI groups.
Collectively, we assumed that these inconsistent findings regarding the utility of the CDT may be attributed to differences in the scoring systems used, the severity of cognitive impairment, and the subtypes of MCI and dementia among study participants. Therefore, the present investigation aimed to identify whether an impairment in the CDT exists for MCI as well as dementia using three different scoring systems. In addition, we examined which scoring system is most useful for detection in patients with MCI (aMCI and VaMCI) and mild dementia (AD and VaD).
METHODS
Participants
The subjects were selected from a group of patients who were diagnosed by neurologists in the Department of Neurology at Hallym University Sacred Heart Hospital (Anyang, Korea). There were 42 patients with aMCI who were diagnosed based on Petersen's criteria.27 A total of 40 patients with mild AD were diagnosed based on the criteria described by the National Institute on Aging-Alzheimer's Association (NIA-AA) workgroups.28 Forty-one patients with VaMCI were diagnosed with criteria for probable VaMCI from the American Heart Association-American Stroke Association (AHA-ASA).29 Forty patients with VaD were diagnosed based on criteria from the AHA-ASA.29 All patients underwent a comprehensive neuropsychological assessment and brain magnetic resonance imaging (MRI). Forty-five community-dwelling older adults were recruited through community outreach. They were screened based on Christensen's health screening criteria30 and on having a total score higher than the 16th percentile on the Korean-Mini Mental State Examination (K-MMSE)31 (cognitively normal older adults; CN). Clinical psychology graduate students who had been trained by the author (YK) visited welfare centers for older adults in the community, then administered the CDT to older adults. The present study is a retrospective study using the data already collected for clinical use and other purposes. Thus, informed consents from the participants could not be obtained.
Administration of the CDT
The CDT was conducted using the free-drawn command. The instruction was as follows: “Draw a face of the clock. Put in all the numbers and set the time to 10 past 11.” Participants were not permitted to look at a clock or wristwatch when drawing this clock. The instructions were repeated for individuals who could not understand them, and there was no time limitation.
Three CDT scoring systems
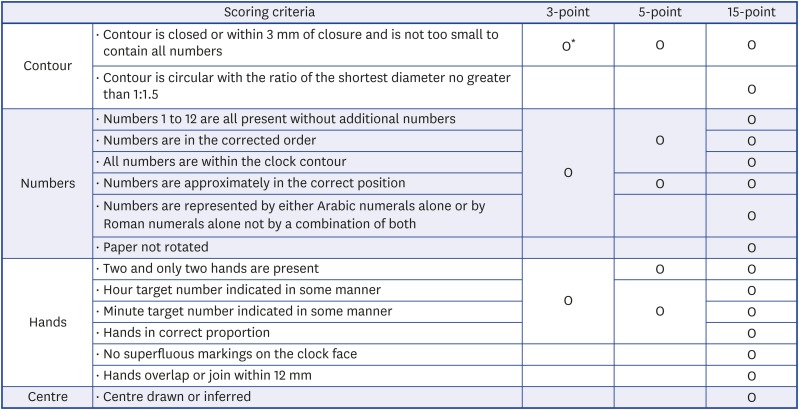
The three most popular scoring systems used in the clinical field were selected. The 3-point scoring system adopted for the MoCA10 assigns one point each for drawing a closed circle, placing all expected numbers in their correct positions, and correctly placing the clock hands to reflect the requested time. The 5-point scoring system was adopted from the ADNI's cognitive assessments, and was developed and modified by Goodglass and Kaplan.32 One point each is assigned for drawing an approximately circular face, the symmetry of number placement, the correctness of the numbers, the presence of two hands, and the hands showing correct the lengths and time. The 15-point scoring system, developed by Freedman et al.,33 was used in the BNA.11 Two points are given for contour, 6 points for numbers, 6 points for clock hands, and 1 point for the center. These three scoring systems are summarized in Fig. 1. The scoring for the CDT was performed by a clinical neuropsychologist (SK).
Fig. 1. Scoring systems of the clock drawing test.
*“O” all of the scoring criteria in the corresponding column must be satisfied in order to assign points in each scoring system.
Other measures
Several studies have reported that the Mini-Mental State Examination (MMSE) is insufficiently sensitive to detecting MCI, and is particularly insensitive to deficits in executive functioning and visual constructive ability34,35; nevertheless, the MMSE remains the most widely used cognitive screening instrument and is considered to be the “gold standard” for general cognitive function.1 Therefore, the K-MMSE31 was administered in comparing the utility of the CDT as a cognitive screening tool. The Clinical Dementia Rating (CDR)36 scale was also rated in all patients to obtain information regarding the severity of dementia.
Statistical analysis
After examining whether there were significant differences in demographic characteristics, multivariate analysis of covariance was performed to compare group differences in total K-MMSE scores and the three CDT scores after controlling for demographic variables. Receiver operating characteristic (ROC) curve analyses were performed in order to examine the ability of the three CDT scoring systems to differentiate aMCI, VaMCI, AD, and VaD from CN, while demographic variables were controlled as covariates. Areas under the receiver operating characteristic curves (AUCs) for the three CDT scoring systems were compared with those for the K-MMSE to ensure that they were comparable with the K-MMSE as a cognitive screening instrument. Additionally, multiple comparison tests of the AUCs were performed to identify which scoring system was the most useful for each patient group.
RESULTS
Characteristics of demographic variables, K-MMSE, and CDR
Demographic variables as well as K-MMSE and CDR scores for CN, aMCI, VaMCI, AD, and VaD are presented in Table 1. There were no significant differences among the groups in levels of education (F[4, 203]=1.26, ns). However, there were significant group differences in age, with the VaMCI group being the youngest (F[4, 203]=3.55, p<0.05). There were also significant group differences in sex (X2[4, 203]=10.64, p<0.05). The average scores of the K-MMSE in AD and VaD groups were significantly lower than those in the other three groups, while the CN group exhibited higher scores than the aMCI and VaMCI groups (F[4, 203]=34.30, p<0.001). There were significant group differences in the CDR-Global Score (CDR-GS) and CDR-Sum of Boxes (CDR-SB), with the AD and VaD groups exhibiting higher scores than the aMCI and VaMCI groups (CDR-GS: F[3, 160]=212.93, p<0.001; CDR-SB: F[3, 160]=115.92, p<0.001).
Table 1. Demographic characteristics of the CN, mild cognitive impairment, and mild dementia patient groups.
| Variable | CN (n=45) | aMCI (n=42) | VaMCI (n=41) | AD (n=40) | VaD (n=40) | F or Χ2 | Post-hoc* (Tukey) |
|---|---|---|---|---|---|---|---|
| Age (yr) | 70.67 (8.39) | 71.19 (8.60) | 67.20 (10.46) | 73.85 (8.96) | 73.07 (9.20) | 3.55† | a=b=d=e, a=b=c, c<d=e |
| Sex (M/F) | 24/21 | 24/18 | 29/12 | 14/26 | 21/19 | Χ2=10.6† | - |
| Education (yr) | 8.09 (3.56) | 8.88 (4.82) | 8.07 (4.46) | 9.69 (4.31) | 9.43 (4.17) | 1.26 | ns |
| K-MMSE | 28.40 (1.40) | 25.19 (3.03) | 25.63 (3.14) | 21.50 (3.84) | 22.10 (3.79) | 34.30‡ | a>b=c>d=e |
| CDR-GS | - | 0.50 (0.00) | 0.46 (0.13) | 0.95 (0.15) | 0.96 (0.13) | 212.93‡ | b=c<d=e |
| CDR-SB | - | 1.92 (0.88) | 1.43 (1.10) | 5.29 (1.46) | 5.26 (1.41) | 115.92‡ | b=c<d=e |
CN: cognitively normal older adults, aMCI: amnestic mild cognitive impairment, VaMCI: vascular mild cognitive impairment, AD: Alzheimer's disease, VaD: vascular dementia, K-MMSE: Korean Mini-Mental State Examination, CDR-GS: clinical dementia rating-global score, CDR-SB: clinical dementia rating-sum of boxes.
*a: CN, b: aMCI, c: VaMCI, d: AD, e: VaD; †p<0.05; ‡p<0.001.
CDT results scored using three CDT scoring systems
The scoring results for the CDT based on the 3-, 5-, and 15-point scoring systems are summarized in Table 2. The patients in the aMCI, VaMCI, AD, and VaD groups exhibited significantly lower scores than the CN group in all of the CDT scores recorded using the 3 scoring systems (F[4, 203]=9.53, p<0.001; F[4, 203]=17.45, p<0.001; F[4, 203]=11.60, p<0.001, respectively). Post-hoc analyses for the three-point scoring system revealed that the VaMCI, AD, and VaD groups performed worse than the CN group, although there was no significant difference between the aMCI and CN groups. There were no significant differences between patients with VaMCI and those with mild dementia. With the 5-point scoring system, the aMCI, VaMCI, AD, and VaD groups each exhibited poorer performance than the CN group. The VaD group had the lowest score, while there were no significant differences among the aMCI, VaMCI, and AD groups. Using the 15-point scoring system, the aMCI, VaMCI, AD, and VaD groups demonstrated significantly worse scores than the CN group. There were no significant differences between the aMCI and VaMCI, or the AD and VaD groups. The aMCI group exhibited significantly higher scores than the VaD, although the VaMCI did not demonstrate any differences with the AD or VaD groups.
Table 2. Clock drawing test performances of the CN, mild cognitive impairment, and mild dementia patient groups.
| Variable | CN (n=45) | aMCI (n=42) | VaMCI (n=41) | AD (n=40) | VaD (n=40) | F | Post-hoc* (Bonferroni) |
|---|---|---|---|---|---|---|---|
| 3-point | 2.87 (0.40) | 2.55 (0.67) | 2.38 (0.71) | 2.15 (0.83) | 1.93 (0.83) | 9.53† | a=b>e, a>c=d=e, b=c=d |
| 5-point | 4.89 (0.32) | 3.76 (0.98) | 4.27 (0.95) | 3.70 (1.07) | 3.20 (1.29) | 17.45† | a>c=b=d, a>c=d>e, e=b |
| 15-point | 14.87 (0.34) | 13.40 (2.21) | 13.68 (2.07) | 12.40 (2.53) | 11.93 (2.81) | 11.60† | a>c=d=e, a>b>e, b=c=d |
CN: cognitively normal older adults, aMCI: amnestic mild cognitive impairment, VaMCI: vascular mild cognitive impairment, AD: Alzheimer's disease, VaD: vascular dementia.
*a: CN, b: aMCI, c: VaMCI, d: AD, e: VaD; †p<0.001.
Comparisons of AUCs
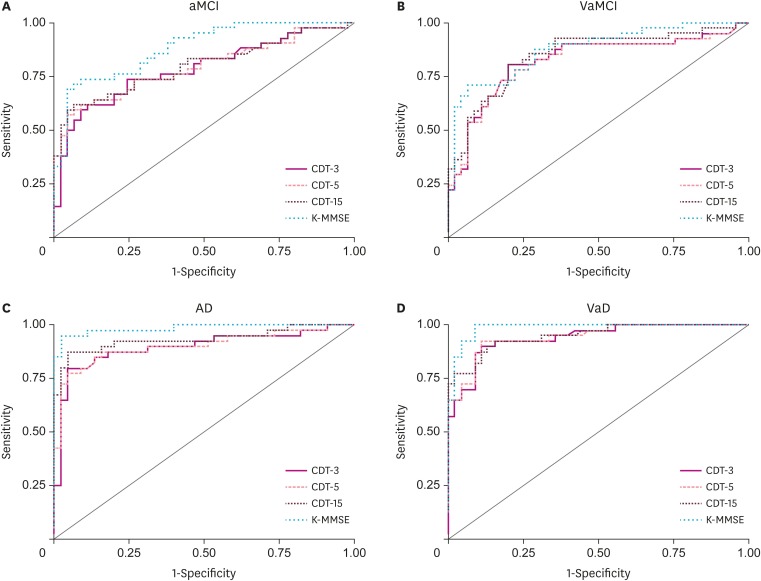
The AUCs for the three scoring systems and the K-MMSE total score are shown in Fig. 2 and Supplementary Table 1. ROC curve analysis revealed that all three CDT scores derived from the three scoring systems were comparable with the K-MMSE in differentiating patients with aMCI, VaMCI, and VaD from those were CN. Multiple comparison tests revealed that the AUCs of the 3 CDT scores in each comparison (aMCI vs. CN, VaMCI vs. CN, and VaD vs. CN) did not significantly differ from one another. However, it was found that the CDT scored using the 15-point scoring system differentiated AD from CN significantly better than that scored using the three-point scoring system (AUC difference, 0.037; 95% confidence interval [CI], 0.002–0.071; p<0.05). Moreover, the K-MMSE discriminated AD from CN significantly better than the CDT scored using the three-point scoring system (AUC difference, 0.088; 95% CI, 0.016–0.159; p<0.05) and the five-point scoring system (AUC difference, 0.083; 95% CI, 0.015–0.151; p<0.05), whereas there was no significant difference between the CDT scored using the 15-point scoring system and the K-MMSE.
Fig. 2. Receiver operating characteristic curves of CDT and K-MMSE for mild cognitive impairment and dementia patient groups.
CDT: clock drawing test, K-MMSE: Korean Mini-Mental State Examination, aMCI: amnestic mild cognitive impairment, VaMCI: vascular mild cognitive impairment, AD: Alzheimer's disease, VaD: vascular dementia.
DISCUSSION
The present study aimed to examine whether an impairment in the CDT exists for MCI and/or dementia with the three different scoring systems commonly used for the CDT. Our results demonstrated that CDT scores in the aMCI and VaMCI groups were significantly lower than those of the CN group when the CDT was scored using the 5- or 15-point scoring systems. However, when using the three-point scoring system, no significant differences were found between the aMCI and CN groups. In addition, there were no significant differences in CDT scores between the aMCI and VaMCI groups between all of the three scoring systems. Some recent studies have reported that there is no significant difference in CDT performance between MCI and CN subjects,37,38 while others have found that MCI patients demonstrate worse scores than cognitively unimpaired (i.e., CN) participants.39 The results of the present study suggest that deficits do exist in the performance of the CDT, even for aMCI and VaMCI patients, although a simple scoring system, such as the three-point system, was not able to detect subtle deficits in patients with aMCI.
The results also demonstrated that the CDT scored using any of the three scoring systems could not provide any information regarding the severity of dementia (i.e., MCI vs. dementia). There were no significant differences among the aMCI, VaMCI, and AD groups in CDT scores as scored using all three scoring systems. However, the VaD group always demonstrated the lowest CDT scores, and a significantly poorer performance than AD was found for the VaD group using the 5-point scoring system. The CDT has been known to assess various cognitive functions with more focus on executive control and visuospatial functions.40 Although deficits in executive functions and episodic memory occur in the very early stages of AD,41 many studies have demonstrated that VaD exhibits more impaired executive function and visuoconstructional skills.42 A recent study performed a qualitative analysis of error types in CDT and found that, compared to AD patients, VaD patients exhibited more stimulus-bound responses, difficulty in planning, and perseveration errors that reflected frontal lobe dysfunction.43 In this regard, our results demonstrating that the VaD group exhibited the lowest CDT scores in all three scoring systems re-confirms that the CDT is a good measure of frontal/executive functions.
Our results also demonstrated that all CDTs scored using the three different scoring systems yielded satisfactory AUCs comparable with the K-MMSE in the MCI and VaD groups, although we did not observe significant differences between the aMCI and CN groups in the total mean scores of the CDT using the 3-point scoring system. This result indicates that the CDT is a useful cognitive screening instrument for aMCI, VaMCI, and VaD, regardless of the scoring system used. Recently, review studies have suggested that elaborating the detail and complexity of CDT scoring systems does little to improve the test's ability to identify significant cognitive impairment.2,3 Our results were also consistent with these findings. Thus, we conclude that simple scoring systems (i.e., 3- and 5-point) for the CDT can be as good as complex scoring systems (i.e., 15-point) for differentiating MCIs and VaD from CN.
However, the 15-point scoring system was found to have significantly higher discriminatory power than the 3-point scoring system for differentiating AD from CN, although the 3- and 5-point scoring systems also had significant discriminability. The 15-point scoring system includes more items relating to visuospatial skills, such as “contour is circular with the ratio of the shortest diameter no greater than 1:1.5,” “paper not rotated,” “hands overlap or join within 12 mm,” and “center drawn or inferred”, which are not scored in the 3- or 5-point systems. Visuospatial function relies on the parietal lobe, which is affected in the early stages of AD.44,45,46 Therefore, it appears that the better discriminability of the 15-point scoring system for AD was due to the fact that it evaluated these items relating to visuospatial functions, while the other systems did not. Moreover, only the CDT scored using the 15-point scoring system demonstrated comparable discriminability with the K-MMSE for differentiating AD from CN. Therefore, these results support the use of more complex scoring systems, including more items relating to visuospatial functions, for differentiating AD from CN. However, in the case that there is some reason that a simple scoring system must be used, we recommend using a combination of the CDT and MMSE, similar to what some previous studies have suggested.47,48
The present study had several limitations. First, it was a retrospective investigation with a relatively small sample size. Second, we could not control the effects of medication on CDT performance in the patient groups. Third, we did not distinguish aMCI into two groups, such as single-domain or multiple-domain; therefore, further research classifying the different subtypes of aMCI is necessary. Fourth, we only applied the “free-drawing to verbal command” method and quantitative scoring, as they are the ones most commonly used in clinical settings. Thus, further research using other conditions, such as copying and qualitative analysis (e.g., error type analysis), is needed in order to further discern the utility of the CDT.
In summary, our results demonstrated that deficits in CDT performance are present even in patients with aMCI and VaMCI as well as individuals with mild dementia, indicating that the CDT is a useful cognitive screening tool with results comparable to those of the MMSE. The clinical implications of the results suggest that simple scoring systems for the CDT, such as a three-point system, are sufficient for differentiating VaMCI and VaD from CN, although more detailed scoring systems, such as a 15-point system, are better for aMCI and AD.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Kim S, Kang Y.
- Data curation: Kim S, Kang Y.
- Formal analysis: Kim S, Kang Y.
- Methodology: Kim S, Kang Y, Jahng S.
- Project administration: Kim S, Kang Y.
- Writing - original draft: Kim S, Kang Y.
- Writing - review & editing: Jahng S, Yu KH, Lee BC.
SUPPLEMENTARY MATERIAL
AUCs of the CDT and K-MMSE
References
- 1.Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry. 2010;25:111–120. doi: 10.1002/gps.2306. [DOI] [PubMed] [Google Scholar]
- 2.Shulman KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000;15:548–561. doi: 10.1002/1099-1166(200006)15:6<548::aid-gps242>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Mainland BJ, Amodeo S, Shulman KI. Multiple clock drawing scoring systems: simpler is better. Int J Geriatr Psychiatry. 2014;29:127–136. doi: 10.1002/gps.3992. [DOI] [PubMed] [Google Scholar]
- 4.Pinto E, Peters R. Literature review of the clock drawing test as a tool for cognitive screening. Dement Geriatr Cogn Disord. 2009;27:201–213. doi: 10.1159/000203344. [DOI] [PubMed] [Google Scholar]
- 5.Tuokko H, Hadjistavropoulos T, Rae S, O'Rourke N. A comparison of alternative approaches to the scoring of clock drawing. Arch Clin Neuropsychol. 2000;15:137–148. [PubMed] [Google Scholar]
- 6.Manos PJ, Wu R. The ten point clock test: a quick screen and grading method for cognitive impairment in medical and surgical patients. Int J Psychiatry Med. 1994;24:229–244. doi: 10.2190/5A0F-936P-VG8N-0F5R. [DOI] [PubMed] [Google Scholar]
- 7.Lam LC, Chiu HF, Ng KO, Chan C, Chan WF, Li SW, et al. Clock-face drawing, reading and setting tests in the screening of dementia in Chinese elderly adults. J Gerontol B Psychol Sci Soc Sci. 1998;53:353–357. doi: 10.1093/geronb/53b.6.p353. [DOI] [PubMed] [Google Scholar]
- 8.Cacho J, García-García R, Arcaya J, Vicente JL, Lantada N. A proposal for application and scoring of the clock drawing test in Alzheimer's disease. Rev Neurol. 1999;28:648–655. [PubMed] [Google Scholar]
- 9.Tariq SH, Tumosa N, Chibnall JT, Perry MH, 3rd, Morley JE. Comparison of the Saint Louis University mental status examination and the mini-mental state examination for detecting dementia and mild neurocognitive disorder--a pilot study. Am J Geriatr Psychiatry. 2006;14:900–910. doi: 10.1097/01.JGP.0000221510.33817.86. [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 11.Darvesh S, Leach L, Black SE, Kaplan E, Freedman M. The behavioural neurology assessment. Can J Neurol Sci. 2005;32:167–177. doi: 10.1017/s0317167100003930. [DOI] [PubMed] [Google Scholar]
- 12.Mendez MF, Ala T, Underwood KL. Development of scoring criteria for the clock drawing task in Alzheimer's disease. J Am Geriatr Soc. 1992;40:1095–1099. doi: 10.1111/j.1532-5415.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 13.Esteban-Santillan C, Praditsuwan R, Ueda H, Geldmacher DS. Clock drawing test in very mild Alzheimer's disease. J Am Geriatr Soc. 1998;46:1266–1269. doi: 10.1111/j.1532-5415.1998.tb04543.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Swanwick GR, Coen RF, Lawlor BA. Use of the clock drawing task in the diagnosis of mild and very mild Alzheimer's disease. Int Psychogeriatr. 1996;8:469–476. doi: 10.1017/s1041610296002827. [DOI] [PubMed] [Google Scholar]
- 15.Seigerschmidt E, Mösch E, Siemen M, Förstl H, Bickel H. The clock drawing test and questionable dementia: reliability and validity. Int J Geriatr Psychiatry. 2002;17:1048–1054. doi: 10.1002/gps.747. [DOI] [PubMed] [Google Scholar]
- 16.Ricci M, Pigliautile M, D'Ambrosio V, Ercolani S, Bianchini C, Ruggiero C, et al. The clock drawing test as a screening tool in mild cognitive impairment and very mild dementia: a new brief method of scoring and normative data in the elderly. Neurol Sci. 2016;37:867–873. doi: 10.1007/s10072-016-2480-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Xu S, Jin X, Lu X, Liu L, Lou Y, et al. A comparison of six clock-drawing test scoring methods in a nursing home. Aging Clin Exp Res. 2018;30:775–781. doi: 10.1007/s40520-017-0843-3. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS, Kim EA, Hong CH, Lee DW, Oh BH, Cheong HK. Clock drawing test in mild cognitive impairment: quantitative analysis of four scoring methods and qualitative analysis. Dement Geriatr Cogn Disord. 2008;26:483–489. doi: 10.1159/000167879. [DOI] [PubMed] [Google Scholar]
- 19.Ehreke L, Luppa M, König HH, Riedel-Heller SG. Is the clock drawing test a screening tool for the diagnosis of mild cognitive impairment? A systematic review. Int Psychogeriatr. 2010;22:56–63. doi: 10.1017/S1041610209990676. [DOI] [PubMed] [Google Scholar]
- 20.Ehreke L, Luck T, Luppa M, König HH, Villringer A, Riedel-Heller SG. Clock drawing test - screening utility for mild cognitive impairment according to different scoring systems: results of the Leipzig Longitudinal Study of the Aged (LEILA 75+) Int Psychogeriatr. 2011;23:1592–1601. doi: 10.1017/S104161021100144X. [DOI] [PubMed] [Google Scholar]
- 21.Tan LP, Herrmann N, Mainland BJ, Shulman K. Can clock drawing differentiate Alzheimer's disease from other dementias? Int Psychogeriatr. 2015;27:1649–1660. doi: 10.1017/S1041610215000939. [DOI] [PubMed] [Google Scholar]
- 22.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Ten-point clock test: a correlation analysis with other neuropsychological tests in dementia. Int J Geriatr Psychiatry. 2002;17:347–353. doi: 10.1002/gps.600. [DOI] [PubMed] [Google Scholar]
- 23.Heinik J, Solomesh I, Raikher B, Lin R. Can clock drawing test help to differentiate between dementia of the Alzheimer's type and vascular dementia? A preliminary study. Int J Geriatr Psychiatry. 2002;17:699–703. doi: 10.1002/gps.678. [DOI] [PubMed] [Google Scholar]
- 24.Sallam K, Amr M. The use of the mini-mental state examination and the clock-drawing test for dementia in a tertiary hospital. J Clin Diagn Res. 2013;7:484–488. doi: 10.7860/JCDR/2013/4203.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jager CA. Changes over time in memory, processing speed and clock drawing tests help to discriminate between vascular cognitive impairment, mild cognitive impairment and Alzheimer's disease. Neurol Res. 2004;26:481–487. doi: 10.1179/016164104225016209. [DOI] [PubMed] [Google Scholar]
- 26.Allone C, Lo Buono V, Corallo F, Bonanno L, Palmeri R, Di Lorenzo G, et al. Cognitive impairment in Parkinson's disease, Alzheimer's dementia, and vascular dementia: the role of the clock-drawing test. Psychogeriatrics. 2018;18:123–131. doi: 10.1111/psyg.12294. [DOI] [PubMed] [Google Scholar]
- 27.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christensen KJ, Multhaup KS, Nordstrom S, Voss K. A cognitive battery for dementia: Development and measurement characteristics. J Consult Clin Psychol. 2004;19:759–767. [Google Scholar]
- 31.Kang Y. A normative study of the Korean-Mini Mental State Examination (K-MMSE) in the elderly. Korean J Psychol. 2006;25:1–12. [Google Scholar]
- 32.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 33.Freedman M, Leach L, Kaplan E, Winocur G, Shulman KI, Delis D. Clock Drawing: a Neuropsychological Analysis. Oxford: Oxford University Press, Inc.; 1994. [Google Scholar]
- 34.Blake H, McKinney M, Treece K, Lee E, Lincoln NB. An evaluation of screening measures for cognitive impairment after stroke. Age Ageing. 2002;31:451–456. doi: 10.1093/ageing/31.6.451. [DOI] [PubMed] [Google Scholar]
- 35.Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, Kappelle LJ, de Haan EH. Restrictions of the Mini-Mental State Examination in acute stroke. Arch Clin Neuropsychol. 2005;20:623–629. doi: 10.1016/j.acn.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 37.Beinhoff U, Hilbert V, Bittner D, Gron G, Riepe MW. Screening for cognitive impairment: a triage for outpatient care. Dement Geriatr Cogn Disord. 2005;20:278–285. doi: 10.1159/000088249. [DOI] [PubMed] [Google Scholar]
- 38.Sager MA, Hermann BP, La Rue A, Woodard JL. Screening for dementia in community-based memory clinics. WMJ. 2006;105:25–29. [PubMed] [Google Scholar]
- 39.Ehreke L, Luppa M, Luck T, Wiese B, Weyerer S, Eifflaender-Gorfer S, et al. Is the clock drawing test appropriate for screening for mild cognitive impairment?--Results of the German study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe) Dement Geriatr Cogn Disord. 2009;28:365–372. doi: 10.1159/000253484. [DOI] [PubMed] [Google Scholar]
- 40.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L. Executive function deficits in early Alzheimer's disease and their relations with episodic memory. Arch Clin Neuropsychol. 2006;21:15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. J Neurol Neurosurg Psychiatry. 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- 43.Lee AY, Kim JS, Choi BH, Sohn EH. Characteristics of clock drawing test (CDT) errors by the dementia type: quantitative and qualitative analysis. Arch Gerontol Geriatr. 2009;48:58–60. doi: 10.1016/j.archger.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Salimi S, Irish M, Foxe D, Hodges JR, Piguet O, Burrell JR. Can visuospatial measures improve the diagnosis of Alzheimer's disease? Alzheimers Dement (Amst) 2017;10:66–74. doi: 10.1016/j.dadm.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iachini I, Iavarone A, Senese VP, Ruotolo F, Ruggiero G. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci. 2009;2:43–59. doi: 10.2174/1874609810902010043. [DOI] [PubMed] [Google Scholar]
- 46.Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain. 2006;129:729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- 47.Kato Y, Narumoto J, Matsuoka T, Okamura A, Koumi H, Kishikawa Y, et al. Diagnostic performance of a combination of Mini-Mental State Examination and Clock Drawing Test in detecting Alzheimer's disease. Neuropsychiatr Dis Treat. 2013;9:581–586. doi: 10.2147/NDT.S42209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cacho J, Benito-León J, García-García R, Fernández-Calvo B, Vicente-Villardón JL, Mitchell AJ. Does the combination of the MMSE and clock drawing test (mini-clock) improve the detection of mild Alzheimer's disease and mild cognitive impairment? J Alzheimers Dis. 2010;22:889–896. doi: 10.3233/JAD-2010-101182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AUCs of the CDT and K-MMSE