Abstract
Primary progressive aphasia (PPA) is a clinical syndrome diagnosed when three core criteria are met. First, there should be a language impairment (i.e., aphasia) that interferes with the usage or comprehension of words. Second, the neurological work-up should determine that the disease is neurodegenerative, and therefore progressive. Third, the aphasia should arise in relative isolation, without equivalent deficits of comportment or episodic memory. The language impairment can be fluent or non-fluent and may or may not interfere with word comprehension. Memory for recent events is preserved although memory scores obtained in verbally mediated tests may be abnormal. This distinctive clinical pattern is most conspicuous in the initial stages of the disease, and reflects a relatively selective atrophy of the language network, usually located in the left hemisphere. There are different clinical variants of PPA, each with a characteristic pattern of atrophy. Clinicoanatomical correlations in patient with these variants have led to new insights on the organization of the large-scale language network in the human brain. For example, the left anterior temporal lobe, which was not part of the classic language network, has been shown to play a critical role in word comprehension and object naming. Furthermore, patients with PPA have shown that fluency can be dissociated from grammaticality. The underlying neuropathological diseases are heterogeneous and can include Alzheimer's disease as well as frontotemporal lobar degeneration. The clinician's task is to recognize PPA and differentiate it from other neurodegenerative phenotypes, use biomarkers to surmise the nature of the underlying neuropathology, and institute the most fitting multimodal interventions.
Keywords: language, network, dementia, Alzheimer, frontotemporal
INTRODUCTION
Primary progressive aphasia (PPA) is a neurodegenerative syndrome that is diagnosed in patients who present with an initially isolated and progressive impairment of language. The term was introduced in 19871 and led to a classification into 3 variants in 2011.2 First considered rare, PPA is now being recognized with increasing frequency as a major form of dementia. In the 5-year interval from 2001 to 2005, 180 scientific papers were published with “PPA” in the title or abstract. This number has increased to 900 during the subsequent 5 years from 2011 to 2015. Despite this increasing awareness, however, patients with PPA remain underserved when compared to those with typical amnestic dementias and typical stroke-induced aphasias. There is skepticism of the value of providing speech and language therapy services to individuals with progressive disease;3 and although reports of language treatment in PPA are being published with increasing frequency, the accounts are mostly based on single cases or small groups so that there is a scarcity of data on generalization and maintenance effects.4,5 There are equally complex challenges at the medical level, where clinicians and families may be unduly preoccupied with the question of whether this is ‘PPA or Alzheimer's disease (AD),’ not realizing that it can frequently be both. In fact, an atypical form of AD accounts for approximately 40% of the cases.6 Even specialists tend to overlook this fact so that PPA patients become excluded from clinical trials designed to treat AD.7
Within the language-dominant hemisphere (usually left), a distributed network of interconnected regions subserves language-related functions. Each network component displays a relative specialization for individual aspects of language. Depending on the anatomical location of peak neuronal loss, PPA can lead to impairments of naming (anomia), word finding (logopenia), grammar (agrammatism), repetition (impairment of phonological loop), spelling (dysgraphia), reading (dyslexia), and comprehension (impaired verbal semantics). Variations in the site of peak atrophy and corresponding variations in the cluster of aphasic features provide the basis for the subtyping of PPA patients.
CRITERIA FOR THE DIAGNOSIS OF PPA
Age of symptom onset in PPA is most frequently in the 50s and early 60s, with an even representation of males and females. The diagnosis is made when the following three core criteria are met.8
1) Insidious onset and gradual progression of language impairment (i.e., aphasia). Word finding pauses during speech, uninformative output dominated by fillers, grammatically abnormal or disordered sentences, inability to name parts of objects, failure to understand the meaning of words, and spelling errors are common manifestations of PPA. An impairment of speech (dysarthric, apraxic, aphemic) alone is not sufficient for the diagnosis.
2) A neurodegenerative, and therefore progressive, process as the only underlying cause. This criterion is established through standard neurodiagnostic procedures that rule out cerebrovascular, space occupying, post-traumatic, or other potential causes of a language disorder.
3) Prominence of the aphasia, which arises as the most consequential (i.e., primary) impairment and progresses to become the principle cause of disrupted daily living activities.
Patients with typical amnestic forms of AD [dementia of the Alzheimer-type (DAT)] are brought to medical attention because of forgetfulness, patients with posterior cortical atrophy (PCA) because of visuospatial disorientation, and patients with frontal-type dementias (FTD) because of aberrant behaviors or apathy. In contrast, patients with PPA come with a history of word finding impairments, difficulty remembering names of persons and objects, inability to communicate in complete sentences, and errors in interpreting the meaning of words.
Before the PPA diagnosis can be finalized, it is necessary to establish that episodic memory, visuospatial skills, executive functions and behavior were relatively preserved during an initial period of at least 1–2 years. This can be done in part through history from a reliable informant and in part through cognitive testing. As scores on verbally mediated tasks are difficult to interpret in an aphasic patient, non-verbal standardized tests should be used whenever possible. These include the Visual Verbal Test for executive functions, the 3 Words-3 Shapes Test for memory, and the Judgment of Line Orientation Test for visuospatial orientation.9,10,11
The evaluation of patients with motor speech impairment is particularly challenging. Dysarthria, aphemia and apraxia of speech (AOS) need to be distinguished from paraphasias, word retrieval impairments and agrammatism. Asking the patient to write can help to make the distinction. If the patient cannot write accurately and fluently, the verbal output impairment cannot be attributed to speech abnormalities alone. When a patient has a prominent AOS and the aphasic component is of lesser magnitude, a diagnosis of progressive AOS rather than PPA should be made.12
Progressive aphasic impairments can arise in patients with DAT, PCA, FTD and even the corticobasal syndrome (CBS). None of these patients, however, would qualify for a diagnosis of PPA because their language impairments constitute secondary features overshadowed by the memory loss (as in DAT), visuospatial disorientation (as in PCA), behavioral abnormalities (as in FTD) and movement abnormalities (as in CBS). Such patients can be said to have a progressive aphasia, but not a PPA. The PPA diagnosis is justified only when the aphasia, having emerged in relative isolation, remains salient during most of the course of the disease.13 It is also important to avoid excessive reliance on test results. If a patient with a prominent progressive aphasia, whose only complaint is an inability to find words, also happens to have subnormal scores in some frontal lobe test battery but without corresponding impairments of daily activities (e.g., disinhibition, perseveration, apathy), a PPA diagnosis is justified.
The diagnosis of PPA is based on the initial features of a neurodegenerative syndrome. As will be described below, all neurodegenerative diseases progress and eventually encompass other networks. When a patient with a history and/or medical record consistent with an initial PPA diagnosis seeks consultation at a stage when the aphasia is no longer the only prominent impairment, the diagnosis of PPA+ (PPA plus) can be made. The underlying assumption in this diagnosis is that the disease had the characteristic of PPA at onset. The spread of neurodegeneration beyond the language network happens in almost all PPA patients but the rate of progression varies greatly, with a range of 2–10 years before the aphasia stops becoming the most salient and consequential feature.
ANATOMY OF ATROPHY (Fig. 1)
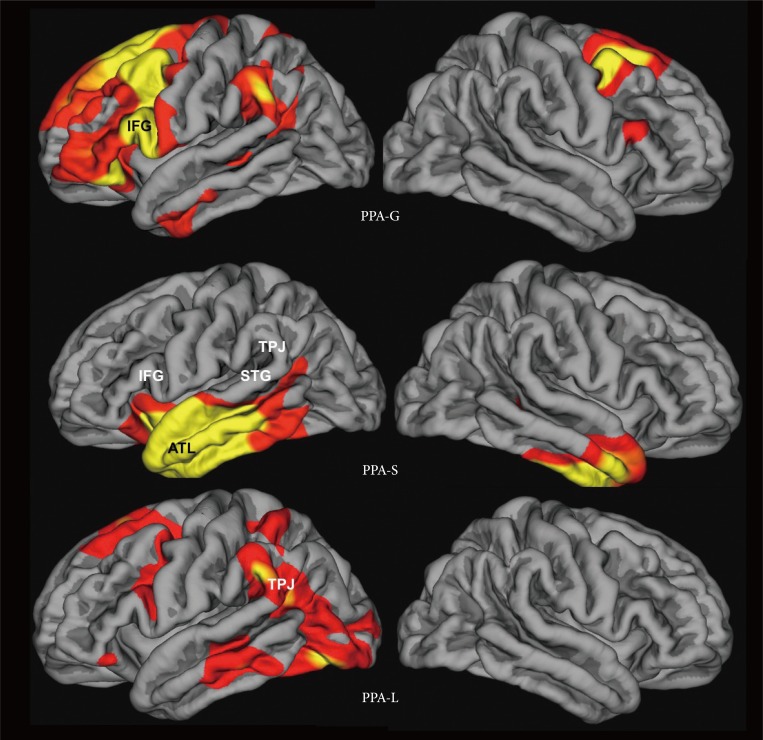
Fig. 1. Atrophy maps in PPA subtypes from Mesulam et al.28 PPA-G, PPA-L, PPA-S- agrammatic, logopenic, and semantic variants of primary progressive aphasia. ATL: anterior temporal lobe, IFG: inferior frontal gyrus, STG: superior temporal gyrus, TPJ: temporoparietal junction. Adapted from Mesulam et al. Arch Neurol 2009;66:1545-1551.28.
The defining feature of PPA is the asymmetrical neurodegeneration of the left hemisphere. The asymmetry can be quite pronounced and the contralateral hemisphere may appear intact during the initial years. Within the language network, neurodegeneration can encompass ‘Broca's area’ in the inferior frontal gyrus (IFG) and ‘Wernicke's area’ in the superior temporal gyrus (STG) and the TPJ. The anterior temporal lobe (ATL) can also be involved, all the way into the polar region. The distribution of neurodegeneration, usually identified in the form of atrophy sites by structural imaging modalities, determines the nature of the resultant aphasia. In patients whose structural scans are normal at the time of symptom onset, SPECT or FDG PET scans may show decreased blood flow and metabolism in language-related areas of the dominant hemisphere. However, MRI, CT and even PET scans at such early stages may be completely normal and may lead the clinician to attribute the symptoms to anxiety, laryngeal dysfunction or other non-neurological factors.14 In approximately 40% of left-handers, language dominance is located in the right hemisphere and may lead to PPA caused by asymmetrical atrophy of the right hemisphere.15 Rarely, right hemisphere dysfunction can cause ‘crossed’ PPA in right-handers.16 Cortical components of the language network are interconnected through the arcuate fasciculus, the uncinate fasciculus, the inferior longitudinal fasciculus, the aslant tract and the inferior frontooccipital fasciculus. These axonal pathways can also undergo degenerative changes in PPA.17,18,19
THE ASSESSMENT OF LANGUAGE
A practiced clinician can diagnose aphasia at the bedside. However, the further subtyping of PPA and the formulation of clinicopathologic correlations related to the language network require the use of specialized testing instruments. In our laboratory, word comprehension is tested with a subset of 36 moderately difficult items (157–192) of the Peabody Picture Vocabulary test, PPVT-IV.20 Each item requires the patient to match an auditory word representing an object, action or attribute to one of 4 picture choices. Although the PPVT-IV is a word-picture matching task, less than half of the items represent concrete objects. The majority of the remaining words (e.g., salutation, perplexed, culinary) require extensive associative interpretation (i.e., comprehension) of the words so that they can be matched to pictorial representations of the corresponding concept. Performance in the PPVT-IV correlates with word-word association tasks but not with tests of fluency.21 The Boston Naming Test is used to assess the naming of objects.22 It is a 60-item standardized test in which items are administered in order of decreasing frequency of occurrence in the language. Non-verbal object knowledge is assessed with the three picture version of the Pyramids and Palm Trees test23 where the patient is asked to decide which of two pictures is conceptually more closely associated with a target object. The ability to understand sentences is assessed with the most difficult non-canonical items of the Sentence Comprehension Test of the Northwestern Assessment of Verbs and Sentences (NAVS).24 In this test, the subject is shown a pair of reversible action pictures and asked to point to the one that matches a grammatically complex (i.e., non-canonical) sentence. The verbs and nouns used in the test of sentence comprehension (boy, girl, dog, cat, kiss, chase) are of high enough frequency in American English to be understood by all subjects so that poor performance indicates a specific impairment of sentence comprehension. Sentence production scores on the Northwestern Anagram Test (NAT)25,26 and the Sentence Production Priming Test of the NAVS24 were averaged to derive a composite score of grammaticality of sentence production.14 The NAT does not require verbal responses and was specifically designed to dissociate agrammatism from lack of fluency. Repetition of phrases and sentences is tested with the 6 most difficult items of the WAB Repetition subtest.14 To control for differently scaled variables, quantitative performance scores are transformed into a percentage of the total possible score. Control subjects of similar ages and education levels perform all of these tests with accuracy levels of 98% or higher.14 Analogous tests can be used for languages other than English. The greatest challenge lies in finding comparable tests of grammar since different languages differ to a much greater extent in sentence structure than in other aspects of language function.
THE MAJOR CLINICAL SUBTYPES
Syndromes of classic aphasiology were based almost entirely on patients with cerebrovascular accidents where onset is abrupt and where gray and deep white matter may both be damaged with equal severity. In contrast, many neurodegenerative diseases selectively destroy the cerebral cortex and also display a progressive course that encompasses degenerative as well as compensatory phenomena. Furthermore, PPA may arise through the selective neurodegeneration of areas that are relatively immune to cerebrovascular accidents. These are some of the reasons why the characterization of language disturbances in PPA necessitates a new classification system and a slightly revised view of the language network.
According to current practice, the language impairment in PPA is classified into one of three principal patterns- agrammatic, logopenic and semantic.2,14 The core feature of the agrammatic subtype (PPA-G) is a distortion of word and sentence construction as manifested by abnormal word order (syntax), distorted use of word endings, misuse of pronouns, and the omission of small grammatical words such as articles and prepositions. In mild cases, these abnormalities may only emerge in writing. Most agrammatic patients will also have low speech fluency, as if every word required extra effort to be retrieved and produced, even in the absence of dysarthria. Word comprehension is spared but grammatically complex sentences may fail to be deciphered. The most distinctive anatomical feature of PPA-G is the presence of peak atrophy sites within the language-dominant IFG, where Broca's area is located (Fig. 1). Additional peak atrophy sites may be found in more dorsal and medial parts of posterior frontal cortex, in the TPJ.
The core features of the semantic subtype (PPA-S) include profound impairments of object naming and word comprehension on a background of preserved fluency, repetition and grammar. Initially, the relatively preserved comprehension of casual conversation (in part due to the patient's ability to catch contextual cues) contrasts sharply with severe deficits in understanding nouns that denote objects, especially animals, fruits and vegetables. Initial comprehension and naming impairments display the phenomenon of taxonomic interference whereby words are understood at a generic but not specific level of meaning.27 An analogous impairment at the stage of translating percepts and thoughts into words leads to the semantic paraphasias and vagueness of speech content. As the disease progresses, the comprehension impairment extends to all word classes and sentences. Surface dyslexia and dysgraphia (inability to read or spell words that have irregular phonology) are commonly seen. The distinctive peak atrophy sites in PPA-S are concentrated within the ATL of the left hemisphere (Fig. 1).
The logopenic subtype (PPA-L) is characterized by variable interruptions of fluency on a background of intact grammar and comprehension. The patient may appear fluent if allowed to engage in small talk and generalities but starts to display frequent word-finding hesitations when access to specific terms and infrequently used words becomes necessary. Many patients will circumvent these retrieval blocks through circumlocutions but a careful listener will detect a simplification of output and vagueness of meaning. Object naming impairments (anomia), based on word retrieval failures, are usually present and may elicit phonological paraphasias. Abnormal repetition has been included as a necessary criterion for the research-based diagnosis of this subtype.2 However, this feature can be so mild that its inclusion as a core criterion may need to be reconsidered.14,28 The distinctive anatomical pattern in PPA-L is one where the atrophy is much more pronounced in the posterior than anterior parts of the language network (Fig. 1). Peak atrophy sites encompass posterior temporal cortex, including the STG and the TPJ where the inferior parietal lobule joins the posterior parts of the superior and middle temporal gyri.
In a few PPA patients, combined impairments of grammar and comprehension arise early in the course of the disease. These patients constitute a fourth group of ‘mixed’ PPA (PPAM).14,28 Peak atrophy sites in these patients include the IFG as well as the ATL of the language-dominant hemisphere.
CLASSIFICATION SYSTEMS
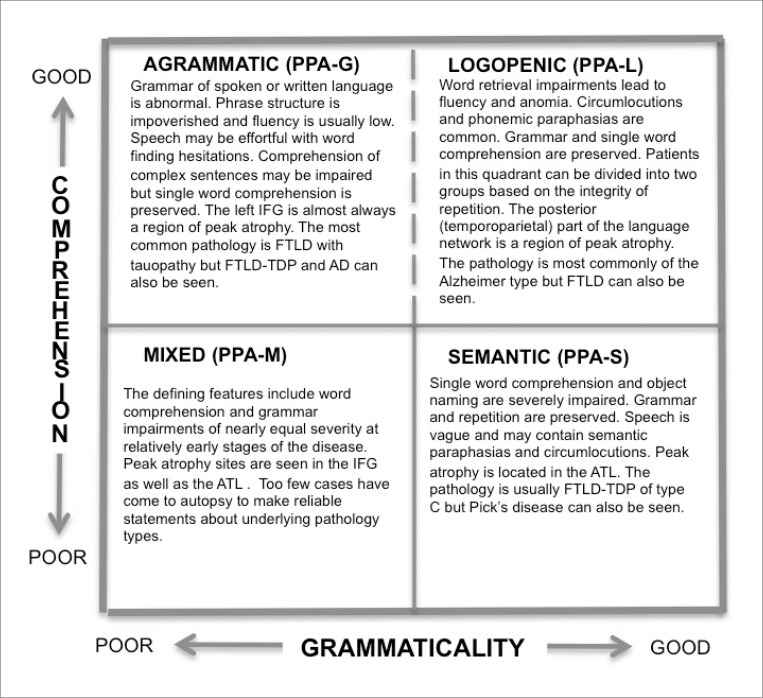
Consensus guidelines for subtyping PPA have been compiled.2 These guidelines have greatly improved the status of research on PPA but they also have some shortcomings. In their present version, the guidelines do not account for all language impairment patterns of PPA so that approximately 30% of patients may remain unclassified. Furthermore, some patients may fulfill criteria for more than one subtype. Minor revisions to correct these problems are being considered.29 The consensus guidelines also require the assessment of 10 different domains of language function. Their routine implementation even in research settings would be burdensome. A simpler, albeit less rigorous, alternative is to use the 2-dimensional template based on word-comprehension and grammar function as shown in Fig. 2.
Fig. 2. A 2-dimensional template for the rapid clinical classification of PPA patients. PPA-G, PPA-L, PPA-M, PPA-S- agrammatic, logopenic, mixed and semantic variants of primary progressive aphasia.
The upper left and lower right quadrants of this template closely correspond to the PPA-G and PPA-S subtypes delineated by the 2011 consensus guidelines. The lower left quadrant incorporates the PPA-M patients, a subtype that is not part of the 2011 guidelines. The upper right quadrant is the most heterogeneous. It contains not only the PPA-L subtype as defined by the 2011 guidelines (i.e., combination of word retrieval and repetition impairments), but also patients who are descriptively logopenic (i.e., have word retrieval impairments) but without abnormalities of repetition. The template approach is most meaningful if it is used within 1–3 years after symptom onset. If it is used too early in the disease, the upper right quadrant will contain patients in the prodromal stages of PPA-G and PPA-S; if it is used at advanced stages, many patients will have developed language production as well as comprehension impairments and will gravitate toward the lower left quadrant.
NEW INSIGHTS INTO THE LANGUAGE NETWORK
The classic language network revolves around two epicenters known as Broca's area (located within parts of the IFG) and Wernicke's area (involving subsectors of the STG, TPJ and adjacent areas), interconnected through the arcuate fasciculus.30 The anterior cortical parts of this network were traditionally associated with fluency and grammar, the posterior parts with language comprehension, and the arcuate fasciculus with language repetition. As noted above, this model and the associated aphasia syndromes were based on case studies of patients with cerebrovascular accidents. In contrast to cerebrovascular accidents, neurodegenerative diseases that cause PPA selectively target specific layers and regions of cortex. Deep white matter is usually spared. Even within peak cortical atrophy sites, neuronal destruction is never complete, and remaining neurons can still participate in language function, albeit with distorted patterns of network connectivity.31,32,33 The partial and gradual neuronal loss in PPA sets the stage for extensive reorganization of cerebral circuitry, at least some of which may be compensatory.34 These partial and progressive perturbations of the underlying network induce dissociations of language function that can differ from those that arise in patients with cerebrovascular disease. Additionally, selective neurodegeneration may target areas such as the ATL that are rarely destroyed by focal cerebrovascular accidents. These are some of the reasons why the language disturbances in PPA have generated new insights into the anatomy of language.
Investigations of PPA-S, for example, showed that the classic model is incomplete and that the left ATL, including the temporal pole, should be included within the language network as a third hub that plays a critical role in single word comprehension and object naming.35,36,37,38 The right ATL displays a different pattern of specialization related to the non-verbal recognition of objects and faces. Neurodegeneration of the right ATL can therefore lead to progressive associative agnosias.39 In cases of bilateral ATL atrophy, word, object and face recognition and jointly impaired, giving rise to the syndrome of semantic dementia (SD).40 PPA-S and SD are therefore distinct syndromes with different distributions of neuronal dysfunction.
Another major deviation from the classic account comes from Investigations on PPA-L, where cortical atrophy encompasses the TPJ and posterior STG, a territory that falls within the traditional boundaries of Wernicke's area. These studies have shown that the region designated “Wernicke's area” by classic aphasiology is important for language repetition (i.e., phonological loop function) and sentence comprehension but not single word comprehension.35,41 The classic language model had considered Wernicke's area to be critical for all kinds of language comprehension, word as well as sentence, probably because the cases that were used to generate the model were based on cerebrovascular accidents that destroyed not only the cortex of ‘Wernicke's area’ but also the deep white matter that linked otherwise spared temporoparietal areas to the ATL.35
Classic aphasiology considered grammar and fluency to have a common substrate so that the terms such as ‘agrammatic aphasia’ and ‘nonfluent aphasia’ tended to be used interchangeably. Work on PPA showed this not to be the case as grammaticality and fluency can be dissociated from one another, clinically and anatomically.42,43 One relevant finding comes from clinicoanatomical correlations based on diffusion tensor imaging which showed that grammaticality is associated with the integrity of the arcuate fasciculus whereas fluency is associated with the integrity of the aslant tract.18
The traditional account of the language network tends to advocate an anterior-posterior axis where grammar/fluency is mapped anteriorly and sentence/word comprehension posteriorly. Work on PPA is helping to transform this view into a dorsal-ventral axis where grammar/fluency/repetition/sentence comprehension is mapped dorsally while object naming/word comprehension is mapped ventrally. These new perspectives introduced by PPA do not negate what has been learned from the classic account. They simply show that the methodology of observation (in this case cerebrovascular versus degenerative lesions) influences the inferences that are drawn. Realistic accounts will require the integration of results obtained using multiple lesion types and multiple testing procedures.
NEUROPATHOLOGY AND DIFFERENTIAL DIAGNOSIS
The PPA syndrome can be caused by Alzheimer pathology or frontotemporal lobar degeneration (FTLD). The two major classes of FTLD most relevant to PPA are designated FTLD-tau and FTLD-TDP.44 In FTLD-tau the abnormal protein is a hyperphosphorylated and misfolded form of tau with morphologic and molecular features that are different from the neurofibrillary tauopathy of AD. In FTLD-TDP the pathology revolves around abnormal precipitates of the 43 kd transactive response DNA-binding protein TDP-43. FTLD-TDP comes in 4 types (A–D), each characterized by a distinctive intracortical location and morphology. Major FTLD-tau subtypes include Pick's disease (PiD), tauopathy of the corticobasal degeneration (CBD) type, and tauopathy of the progressive supranuclear palsy (PSP) type, each identified according to the molecular forms or morphology of the hyperphosphorylated tau precipitates.
According to multiple autopsy series, approximately 30% of PPA patients are found to have the neuropathology of FTLD-tau while an additional 30% have FTLD-TDP. The remaining 40% of patients display Alzheimer pathology but with neurofibrillary degeneration that can be more intense in the language-dominant hemisphere.6,45,46,47,48 Rarely, PPA can be caused by diffuse Lewy body disease.6 Although Jacob-Creutzfeldt disease can also lead to a relatively isolated aphasia, the course is usually much faster and inconsistent with the temporal course of a neurodegenerative process.49 This heterogeneity shows that the clinical specificity of the PPA syndrome is not determined by the histopathology of the disease but, rather, by its anatomical predilection for the language network of the brain.
Accurate clinical classification of PPA increases the precision with which the clinician can predict the nature of the underlying pathology. In PPA-S, 75–80% of patients will be found at autopsy to have FTLD-TDP pathology of type C, with most of the remaining having PiD.50,51 Approximately 60–70% of PPA-G patients have FTLD-tau with the remaining having Alzheimer pathology or FTLD-TDP of type A. The pattern is different in PPA-L where approximately 75% of patients have Alzheimer pathology and the remainder FTLD-T or FTLD-TDP of type A.6
Within the PPA-G and PPA-L subtypes patients with and without Alzheimer pathology are nearly indistinguishable-they both have asymmetric atrophy, progressive aphasia and relative preservation of memory for recent events. The question of differential diagnosis in these patients can be addressed with the help of biomarkers such as amyloid imaging and determinations of tau and beta amyloid in the CSF. A positive amyloid PET scan or high phosphotau with low beta amyloid in the CSF signals a high likelihood of Alzheimer pathology while a negative amyloid scan with normal CSF beta amyloid and phosphotau excludes Alzheimer pathology. In the future, tau imaging with PET will help to differentiate FTLD-tau from FTLD-TDP. Patients will frequently ask whether the diagnosis is ‘PPA or AD’. The clinician needs to state that it could be both, and then explain that in this context the term ‘PPA’ refers to the clinical features experienced by the patient while the term ‘Alzheimer’ refers to the nature of the microscopic changes that damage the language-related parts of the brain.
The Alzheimer pathology associated with the logopenic and agrammatic subtypes of PPA has prominent features that set it apart from the typical amnestic form of this disease. 1) Onset is most commonly before the age of 65, explaining why the female predominance of typical AD is not present. 2) Peak atrophy shows an asymmetric predilection for the language-dominant left hemisphere and display only partial overlap with the atrophy signature of typical AD.52 3) The ApoE ε4 allele is not a risk factor.6 4) Learning disabilities, including dyslexia, are risk factors.53,54 5) Neurofibrillary tangles can display atypical asymmetric distributions that violate the Braak and Braak pattern by favoring language cortex over mediotemporal limbic areas.45 6) Neuritic amyloid plaques may be asymmetrically distributed, favoring the left hemisphere.55 7) TDP-43 abnormalities, seen in at least 30% of typical AD cases, are less frequently encountered in the AD pathology associated with PPA.56,57 These features indicate that the AD associated with PPA has temporal, anatomical, neuropathologic and genetic factors that diverge from those of the far more common late-onset and amnestic forms of AD.
Patients with atypical non-amnestic dementias tend to be excluded from AD clinical trials, where outcome measures tend to emphasize memory function. The advent of biomarkers now makes it possible to identify the contingent of PPA patients with underlying AD pathology. Their inclusion in clinical trials will offer these patients equal access to novel agents but will require the introduction of new outcome measures designed to assess the relevant non-amnestic domain of primary cognitive impairment.
TRAJECTORY OF PROGRESSION
Gradual intensification of impairment is the hallmark of PPA.11,58,59 Each clinical subtype displays a somewhat different progression trajectory. In PPA-S, the extension of ATL atrophy into the anterior insula, posterior orbitofrontal cortex and contralateral ATL may lead to the emergence of behavioral abnormalities characteristic of FTD syndromes or associative agnosias characteristic of the SD syndrome. In PPA-G, the spread of atrophy to motor areas and basal ganglia may lead to the emergence of movement disorders characteristic of the CBD or PSP syndromes. The trajectory of PPA-L is the most variable. In some patients, additional IFG atrophy leads to the emergence of features characteristic of PPA-G. In others, the spread of atrophy into additional parts of the temporal lobe leads to word comprehension and memory impairments. Some cases of PPA-L can show an unusually indolent pace of progression for up to a decade or more during which daily living activities that do not depend on language are preserved.
PATIENT CARE
Patient care in PPA can be divided into symptomatic and etiological components. The symptomatic approach starts with an evaluation by a knowledgeable speech therapist who can work with the patient and family to maximize communicative effectiveness. The etiological component of patient care revolves around the judicious use of clinical subtyping and biomarker information to surmise the nature of the underlying disease process. Based on this information, the clinician can decide whether to use Alzheimer medications or to channel the patient into clinical trials relevant to AD or FTLD.
For several years following symptom onset, the non-dominant hemisphere may show no significant atrophy or loss of metabolism.60 Within the affected dominant hemisphere atrophic components of the language network may continue to participate in language tasks.32 The question has therefore been raised whether activation of either hemisphere with transcranial magnetic stimulation might prove beneficial. Anecdotal reports have described positive results.61
CONCLUSIONS
Although it is being diagnosed with increasing frequency, PPA is still a rare syndrome so that patients, families and clinicians usually find it difficult to access appropriate resources. A dedicated website, the international PPA connection (ppaconnection.org), has been established to serve as an international registry for patients and resources. Its goal is to link patients and clinicians to relevant local resources around the world and, in the future, help launch collaborative clinical trials when promising therapeutic interventions become available. The site also contains demonstrations of key diagnostic features and a section on frequently asked questions.
The field of PPA is enjoying considerable progress in diagnostic accuracy, clinical characterization, differential diagnosis, and clinicopathologic correlation. Much additional work, however, remains to be done to identify more creative interventions for addressing clinical symptoms as well as more effective therapeutic agents aimed at the underlying disease process. These are challenges that PPA shares with nearly all other neurodegenerative syndromes. While these challenges are being addressed, PPA will continue to offer unique opportunities for exploring the neurobiology of language and the molecular bases of selective vulnerability.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Mesulam M. Primary progressive aphasia–differentiation from Alzheimer’s disease. Annals of neurology. 1987;22:533–534. doi: 10.1002/ana.410220414. [DOI] [PubMed] [Google Scholar]
- 2.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray LL, Paek EJ. Behavioral/nonpharmacological approaches to addressing cognitive-linguistic symptoms in individuals with dementia. Perspect ASHA Spec Interest Groups. 2016;1:12–25. [Google Scholar]
- 4.Croot K, Nickels L, Laurence F, Manning M. Impairment–and activity/participation-directed interventions in progressive language impairment: clinical and theoretical issues. Aphasiology. 2009;23:125–160. [Google Scholar]
- 5.Farrajota L, Maruta C, Maroco J, Martins IP, Guerreiro M, de Mendonça A. Speech therapy in primary progressive aphasia: a pilot study. Dement Geriatr Cogn Dis Extra. 2012;2:321–331. doi: 10.1159/000341602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137(Pt 4):1176–1192. doi: 10.1093/brain/awu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogalski E, Sridhar J, Rader B, Martersteck A, Chen K, Cobia D, et al. Aphasic variant of Alzheimer disease: clinical, anatomic, and genetic features. Neurology. 2016;87:1337–1343. doi: 10.1212/WNL.0000000000003165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mesulam M. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 9.Wicklund AH, Johnson N, Weintraub S. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer’s disease and the behavioral presentation of frontotemporal dementia. J Clin Exp Neuropsychol. 2004;26:347–355. doi: 10.1080/13803390490510077. [DOI] [PubMed] [Google Scholar]
- 10.Weintraub S, Rogalski E, Shaw E, Sawlani S, Rademaker A, Wieneke C, et al. Verbal and nonverbal memory in primary progressive aphasia: the Three Words-Three Shapes Test. Behav Neurol. 2013;26:67–76. doi: 10.3233/BEN-2012-110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- 12.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135(Pt 5):1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesulam MM, Weintraub S. Primary progressive aphasia and kindred disorders. In: Duyckaerts C, Litvan I, editors. Handbook of Clinical Neurology. New York: Elsevier; 2008. pp. 573–587. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135(Pt 5):1537–1553. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mesulam M, Weintraub S, Parrish T, Gitelman D. Primary progressive aphasia: reversed asymmetry of atrophy and right hemisphere language dominance. Neurology. 2005;64:556–557. doi: 10.1212/01.WNL.0000150545.46351.DE. [DOI] [PubMed] [Google Scholar]
- 16.Demirtas-Tatlidede A, Gurvit H, Oktem-Tanor O, Emre M. Crossed aphasia in a dextral patient with logopenic/phonological variant of primary progressive aphasia. Alzheimer Dis Assoc Disord. 2012;26:282–284. doi: 10.1097/WAD.0b013e31823346c6. [DOI] [PubMed] [Google Scholar]
- 17.Catani M, Piccirilli M, Cherubini A, Tarducci R, Sciarma T, Gobbi G, et al. Axonal injury within language network in primary progressive aphasia. Ann Neurol. 2003;53:242–247. doi: 10.1002/ana.10445. [DOI] [PubMed] [Google Scholar]
- 18.Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, et al. A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain. 2013;136(Pt 8):2619–2628. doi: 10.1093/brain/awt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D'Anna L, Mesulam MM, Thiebaut de Schotten M, Dell'Acqua F, Murphy D, Wieneke C, et al. Frontotemporal networks and behavioral symptoms in primary progressive aphasia. Neurology. 2016;86:1393–1399. doi: 10.1212/WNL.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn LA, Dunn LM. Peabody Picture Vocabulary Test-4. 4th ed. Minneapolis: Pearson; 2006. [Google Scholar]
- 21.Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132(Pt 9):2553–2565. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan E, Goodglass H, Weintraub S. Boston naming test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 23.Howard D, Patterson K. Pyramids and palm trees: a test of semantic access from pictures and words. Bury St. Edmunds, Suffolk: Thames Valley Test Company; 1992. [Google Scholar]
- 24.Thompson CK. Northwestern Assessment of Verbs and Sentences (NAVS) [cited 2016 May 3]. Available from: http://flintbox.com/public/project/9299.
- 25.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24:408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson CK, Weintraub S, Mesulam MM The Northwestern Anagram Test (NAT) [cited 2016 May 3]. Available from: http://flintbox.com/public/project/19927.
- 27.Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136(Pt 2):601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesulam MM, Weintraub S. Is it time to revisit the classification guidelines for primary progressive aphasia? Neurology. 2014;82:1108–1109. doi: 10.1212/WNL.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 30.Geschwind N. Language and the brain. Sci Am. 1972;226:76–83. doi: 10.1038/scientificamerican0472-76. [DOI] [PubMed] [Google Scholar]
- 31.Sonty SP, Mesulam MM, Thompson CK, Johnson NA, Weintraub S, Parrish TB, et al. Primary progressive aphasia: PPA and the language network. Ann Neurol. 2003;53:35–49. doi: 10.1002/ana.10390. [DOI] [PubMed] [Google Scholar]
- 32.Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. J Neurosci. 2007;27:1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesulam MM, Rogalski EJ, Wieneke C, Hurley RS, Geula C, Bigio EH, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10:554–569. doi: 10.1038/nrneurol.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenbulcke M, Peeters R, Van Hecke P, Vandenberghe R. Anterior temporal laterality in primary progressive aphasia shifts to the right. Ann Neurol. 2005;58:362–370. doi: 10.1002/ana.20588. [DOI] [PubMed] [Google Scholar]
- 35.Mesulam MM, Thompson CK, Weintraub S, Rogalski EJ. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;138(Pt 8):2423–2437. doi: 10.1093/brain/awv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seckin M, Mesulam MM, Voss JL, Huang W, Rogalski EJ, Hurley RS. Am I looking at a cat or a dog? Gaze in the semantic variant of primary progressive aphasia is subject to excessive taxonomic capture. J Neurolinguistics. 2016;37:68–81. doi: 10.1016/j.jneuroling.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurley RS, Paller KA, Rogalski EJ, Mesulam MM. Neural mechanisms of object naming and word comprehension in primary progressive aphasia. J Neurosci. 2012;32:4848–4855. doi: 10.1523/JNEUROSCI.5984-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurley RS, Wang X, Mesulam MM. Asymmetric physiology of the perisylvian language network: evidence from resting state fMRI. J Cogn Neurosci. 2013;2013:194. [Google Scholar]
- 39.Tyrrell PJ, Warrington EK, Frackowiak RS, Rossor MN. Progressive degeneration of the right temporal lobe studied with positron emission tomography. J Neurol Neurosurg Psychiatry. 1990;53:1046–1050. doi: 10.1136/jnnp.53.12.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 41.Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson CK, Cho S, Hsu CJ, Wieneke C, Rademaker A, Weitner BB, et al. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012;26:20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135(Pt 5):1554–1565. doi: 10.1093/brain/aws076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris JM, Gall C, Thompson JC, Richardson AM, Neary D, du Plessis D, et al. Classification and pathology of primary progressive aphasia. Neurology. 2013;81:1832–1839. doi: 10.1212/01.wnl.0000436070.28137.7b. [DOI] [PubMed] [Google Scholar]
- 47.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 48.Mesulam M, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mandell AM, Alexander MP, Carpenter S. Creutzfeldt-Jakob disease presenting as isolated aphasia. Neurology. 1989;39:55–58. doi: 10.1212/wnl.39.1.55. [DOI] [PubMed] [Google Scholar]
- 50.Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75:2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol. 2008;65:244–248. doi: 10.1001/archneurol.2007.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogalski EJ, Rademaker A, Wieneke C, Bigio EH, Weintraub S, Mesulam MM. Association between the prevalence of learning disabilities and primary progressive aphasia. JAMA Neurol. 2014;71:1576–1577. doi: 10.1001/jamaneurol.2014.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martersteck A, Murphy C, Rademaker A, Wieneke C, Weintraub S, Chen K, et al. Is in vivo amyloid distribution asymmetric in primary progressive aphasia? Ann Neurol. 2016;79:496–501. doi: 10.1002/ana.24566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bigio EH, Mishra M, Hatanpaa KJ, White CL, 3rd, Johnson N, Rademaker A, et al. TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol. 2010;120:43–54. doi: 10.1007/s00401-010-0681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, et al. TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol. 2015;78:697–709. doi: 10.1002/ana.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickerson BC. Quantitating severity and progression in primary progressive aphasia. J Mol Neurosci. 2011;45:618–628. doi: 10.1007/s12031-011-9534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chawluk JB, Mesulam MM, Hurtig H, Kushner M, Weintraub S, Saykin A, et al. Slowly progressive aphasia without generalized dementia: studies with positron emission tomography. Ann Neurol. 1986;19:68–74. doi: 10.1002/ana.410190112. [DOI] [PubMed] [Google Scholar]
- 61.Trebbastoni A, Raccah R, de Lena C, Zangen A, Inghilleri M. Repetitive deep transcranial magnetic stimulation improves verbal fluency and written language in a patient with primary progressive aphasia-logopenic variant (LPPA) Brain Stimul. 2013;6:545–553. doi: 10.1016/j.brs.2012.09.014. [DOI] [PubMed] [Google Scholar]