Abstract
Understanding the intracellular distribution and trafficking of nanoparticle drug carriers is necessary to elucidate their mechanisms of drug delivery and is helpful in the rational design of novel nanoparticle drug delivery systems. The traditional immunofluorescence method to study intracellular distribution of nanoparticles using organelle-specific antibodies is laborious and subject to artifacts. As an alternative, we developed a new method that exploits ratiometric fluorescence imaging of a pH-sensitive Lysosensor dye to visualize and quantify the spatial distribution of nanoparticles in the endosomes and lysosomes of live cells. Using this method, we compared the endolysosomal distribution of cell-penetrating peptide (CPP)-functionalized micelles to unfunctionalized micelles and found that CPP-functionalized micelles exhibited faster endosome-to-lysosome trafficking than unfunctionalized micelles. Ratiometric fluorescence imaging of pH-sensitive Lysosensor dye allows rapid quantitative mapping of nanoparticle distribution in endolysosomes in live cells while minimizing artifacts caused by extensive sample manipulation typical of alternative approaches. This new method can thus serve as an alternative to traditional immunofluorescence approaches to study the intracellular distribution and trafficking of nanoparticles within endosomes and lysosomes.
Keywords: Fluorescence imaging, lysosome, nanoparticle, cell-penetrating peptide
Grphical Abstract:

Nanoparticles have been widely used in anticancer drug delivery to increase drug accumulation in tumors and enhance therapeutic efficiency of their drug cargo.1 Understanding the intracellular distribution and trafficking of nanoparticle drug carriers is necessary to elucidate their drug delivery mechanisms and is important for the rational design of the next-generation of nanoparticle drug delivery systems.2,3
The primary cellular internalization pathway of nanoparticles is endocytosis with subsequent distribution to endosomes and then lysosomes.4 The traditional method to study this intracellular distribution of nanoparticles is immunofluorescence (IF) using organelle-specific antibodies, such as early endosome-specific anti-EEA1 antibody and lysosome-specific anti-LAMP1 antibody.5 The IF method includes the following sample preparation steps (Figure S1): fixing cells with paraformaldehyde or methanol, permeabilizing cells with Triton X-100, blocking cells with bovine serum albumin (BSA) or milk, incubating cells with primary antibodies, and subsequently incubating cells with fluorescent secondary antibodies prior to imaging. This method is prone to artifacts due to its need for rigorous sample processing, whereby fixation, permeabilization, and copious cell washing can cause artificial redistribution of internalized material within the cell that can complicate the interpretation of imaging results.
To overcome drawbacks of the IF method, we developed a new approach to spatially map the intracellular distribution of nanoparticles as a function of their local pH. This method distinguishes the location of nanoparticles in endosomes and lysosomes according to their local pH, where we define endosome pH in the 5–6 range and lysosome pH below 5. Local pH in the endolysosomal compartments was monitored with Lysosensor yellow/blue DND-160, a fluorescent small molecule pH indicator that partitions into acidic intracellular compartments.6 Lysosensor exhibits pH-dependent dualemission spectral peaks, where the ratio of its blue and green fluorescence (IBlue/IGreen; I, fluorescence intensity) exhibits a linear relationship with pH in the endolysosomal compartments of live cells (Figure 1A).
Figure 1.
Method of studying spatial distribution of fluorescent nanoparticles as a function of local pH using ratiometric fluorescence imaging of Lysosensor and pixel-by-pixel analysis. (A) Fluorescence emission spectra of Lysosensor at pH 4 and pH 7 excited at 405 nm and the fluorescence emission spectrum of Alexa Fluor 488 (AF488) excited at 488 nm. Lysosensor exhibits two pH-dependent emission peaks at 440 and 530 nm measured using a blue filter (447 ± 30 nm) and a green filter (525 ± 15 nm), respectively, while the fluorescence of AF488 is measured using a green filter (525 ± 15 nm). The blue and green bars indicate the emission filters used for fluorescence emission measurements. (B) Spinning disk confocal fluorescence images of Lysosensor-Blue, Lysosensor-Green, and AF488 peaks captured in live cells. The white scale bar indicates 2 μm. (C) All fluorescence images are analyzed as 512 pixels × 512 pixels grids where the origin (0, 0) is set at the bottom-left and every pixel is assigned a coordinate (x, y). Fluorescent endolysosomal areas (shown as gray pixels) are identified and distinguished from the largely nonfluorescent cytosol using ImageJ software. (D) The ratio of Lysosensor’s two emission peaks (IBlue/IGreen) in endolysosomal compartments shows a linear relationship with pH in live cells equilibrated with a series of calibration buffers ranging from pH 4 to pH 7.5. Using this IBlue/IGreen ratio versus pH plot, the IBlue/ IGreen ratio of each pixel can be converted to a pH value. (E) Using this method to map endolysosomal pH, normal cells have an endolysosomal pH ranging from pH 4 to pH 7 while chloroquine-treated cells exhibit increased endolysosomal pH. The black scale bar indicates 2 μm and the color bar indicates pH value.
As an improvement over the IF method, our new approach involves only a single incubation and wash step and requires less than an hour to collect and analyze the imaging results (Figure S1). To quantitatively map the location of nanoparticles in endolysosomes, live cells that have been preincubated with fluorescent nanoparticles are incubated with 2 μM Lysosensor yellow/blue DND-160 for 30 min at 37 °C. Cells are washed and then immediately imaged on a spinning disk confocal microscope equipped with an electron multiplier charged-coupled device (EMCCD) camera that provides quantitative images of both Lysosensor and the fluorescently labeled nanoparticles (Figure 1A,B).
The fluorescence images are captured and analyzed at a resolution of 512 pixels ×512 pixels, where every pixel in the two-dimensional grid is defined by a coordinate (x, y) that is accordingly assigned fluorescence intensity values (Figure 1C). The IBlue/IGreen fluorescence ratio of Lysosensor at each pixel in a cell is then assigned a pH value based on the calibration of IBlue/IGreen against pH in live cells (Figure 1D). These pixels are then categorized into distinct compartments based on their pH, where pixels with a pH of 5–6 are defined as endosomes and those pixels with a pH below 5 are defined as lysosomes. The distribution of nanoparticles in the endolysosomal compartments can then be quantified by correlating the pH value with the fluorescence intensity of the nanoparticles at each pixel. The detailed image analysis process is shown as a flowchart in Figure S2. Measurement of endolysosome pH was confirmed by imaging cells treated with chloroquine, which is known to prevent endosomal acidification and hence raises the lysosomal pH.7 Consistent with the effect of chloroquine, the pH in the endolysosomal compartments approached neutral, as compared with untreated cells (Figure 1E).
Using this method, we studied the effect of cell-penetrating peptides (CPPs) on the intracellular distribution of nanoparticles. We chose CPP-functionalized nanoparticles as a system of interest because CPPs are known to greatly enhance the cellular uptake of nanoparticles and are consequently of considerable interest for the intracellular delivery of drug cargo.8 Cationic CPPs, such as arginine-rich peptides, dramatically increase the cellular uptake of conjugated molecules through efficient binding to surface proteoglycans.9 The impact of CPP functionalization on intracellular trafficking, however, is far less understood. The specific nanoparticle system we analyzed was CPP-functionalized elastin-like polypeptide diblock copolymer (ELPBC) micelles. ELPs are a class of recombinant peptide polymers composed of a XGVPG pentapeptide repeat derived from human tropoelastin, where the guest residue (X) can be any amino acid except proline.10 ELPs exhibit lower critical solution temperature (LCST) phase transition behavior such that they are soluble at temperatures below their inverse transition temperature (Tt) and coacervate into insoluble aggregates above their Tt.
The ELPBC we studied consists of two ELP segments that are genetically fused in a linear arrangement. Each block when synthesized as a separate polymer has a distinctly different Tt, which is controlled by the guest residue composition and polymer chain length.11 When the two ELP segments are genetically fused, the ELPBCs can self-assemble into micelles above a critical micellization temperature (CMT) that is a consequence of the selective desolvation of the more hydrophobic block as the solution temperature is increased. ELPBCs can be functionalized at the genetic level with peptides that are presented at the hydrophilic terminus of the ELPBC such that the peptides are displayed on the corona of the self-assembled micelle.12–15 This is a simple and versatile approach to present targeting ligands on the surface of the micelle.
Previously, we reported a CPP-ELPBC where the ELPBC consisted of 40 repeats of hydrophobic VGVPG and 30 repeats of hydrophilic AGVPGGGVPG functionalized with arginine (Arg)-rich CPPs including Arg5, Arg8, and TAT (transactivator of transcription from human immunodeficiency virus). This construct acted as a thermally triggered amplifier of cellular uptake by modulating the density of the CPP on the nanoparticle surface. This CPP-ELPBC self-assembled into micelles above 39 °C and showed low cellular uptake of the unimer (low CPP density) at 37 °C but greatly enhanced cellular uptake of the micelle (high CPP density) at 42 °C.14,15
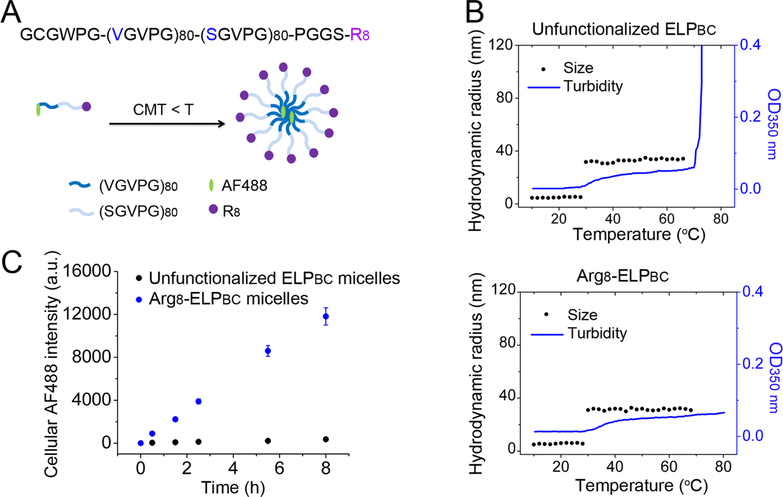
In this study, we used an ELPBC that consists of 80 repeats of hydrophobic VGVPG and 80 repeats of hydrophilic SGVPG. These ELPBCs are functionalized with eight arginine (Arg8) residues at the hydrophilic end of the ELPBC with an intervening PGGS peptide linker and are labeled with Alexa Fluor 488 (AF488) by covalent conjugation to a cysteine residue at the hydrophobic end of the ELPBC (Figure 2A). We chose this ELPBC sequence because both unfunctionalized ELPBC and Arg8-ELPBC self-assemble into ~30 nm hydrodynamic radius (Rh) spherical micelles that are stable over a wide temperature range in aqueous solution, as characterized by temperature-programmed dynamic light scattering (DLS) and turbidity measurements (Figure 2B). Because these micelles have a CMT of 30 °C and are stable up to 70 °C (unfunctionalized ELPBC) or 80 °C (Arg8-ELPBC), they are compatible with cell culture experiments that are performed at 37 °C. Flow cytometry demonstrated that Arg8-ELPBC micelles had dramatically greater uptake than unfunctionalized ELPBC micelles in FaDu squamous cell carcinoma cells at 37 °C (Figure 2C), which agrees with our previous reports.14,15 The raw data of flow cytometry analysis of cellular uptake is shown in Figure S3.
Figure 2.
Physical characterization and cellular internalization of ELPBC micelles. (A) Scheme of AF488-labeled Arg8-ELPBC self-assembling into micelles triggered by an increase in solution temperature above the CMT. (B) Temperature-programed DLS and turbidimetry of unfunctionalized ELPBC and Arg8-ELPBC. Both self-assemble into ~30 nm hydrodynamic radius micelles in aqueous solution between 30 and 70 °C (unfunctionalized ELPBC) or between 30 and 80 °C (Arg8-ELPBC) with an OD350 nm value (optical density at 350 nm) close to 0.06. When the temperature is above 70 °C, the unfunctionalized ELPBC aggregates and its OD350 nm value dramatically increases. (C) AF488-labeled micelles were incubated with FaDu cells at 37 °C and cellular AF488 fluorescence intensity was quantified by flow cytometry over time. Cellular uptake was much greater for Arg8-ELPBC micelles as a function of time compared with unfunctionalized ELPBC micelles.
Next, we evaluated the intracellular distribution of Arg8ELPBC micelles and unfunctionalized ELPBC micelles by IF and ratiometric fluorescence imaging of Lysosensor dye. For IF, FaDu cells were first incubated with 20 μM AF488-labeled ELPBC micelles for 6 h. After fixation, permeabilization, and blocking, cells were incubated with anti-EEA1 primary antibody or anti-LAMP1 primary antibody and then incubated with Alexa Fluor 555 (AF555)-conjugated secondary antibody. Stained cells were then imaged with a SP5 inverted confocal fluorescence microscope. Figure 3A,B shows the distinct distribution of unfunctionalized ELPBC micelles and Arg8ELPBC micelles in early endosomes and lysosomes after 6 h. A large fraction of Arg8-ELPBC micelles (green fluorescence) colocalized with lysosome-specific anti-LAMP1 antibody (red fluorescence), as seen by the overlapping yellow fluorescence. In contrast, unfunctionalized ELPBC partly overlapped with early endosome-specific anti-EEA1 antibody and lysosome-specific anti-LAMP1 antibody at 6 h. These results are consistent with those obtained with ratiometric fluorescence imaging of Lysosensor dye. As shown in Figure 3C,D, after incubation with 20 μM AF488-labeled micelles for 3 or 6 h, Arg8-ELPBC micelles showed preferential accumulation in lower pH lysosomes, while unfunctionalized ELPBC micelles showed an invariant spatial distribution from pH 4 to pH 7, reflecting equal distribution across endosomes and lysosomes.
Figure 3.
Intracellular distribution of AF488-labeled ELPBC micelles. (A) Location of unfunctionalized ELPBC micelles and (B) Arg8-ELPBC micelles in endosomes and lysosomes determined by IF. The white scale bar indicates 2 μm. DIC: differential interference contrast. (C) Distribution of unfunctionalized ELPBC micelles and (D) Arg8-ELPBC micelles in endolysosomes as a function of pH, determined by ratiometric fluorescence imaging of Lysosensor dye.
When FaDu cells were washed with micelle-free cell culture media to stop further cellular uptake of the ELPBC micelles and then imaged after an additional 18 h, the accumulation of unfunctionalized ELPBC in the endolysosomes also adopts an asymmetric distribution with increased accumulation in lower pH lysosomes as compared with endosomes (Figure 4A). IF also shows a large fraction of the unfunctionalized ELPBC overlapping with the lysosome-specific antibody (Figure 4B), corroborating the above results. Taken together, we conclude that the ultimate trafficking of unfunctionalized ELPBC micelles from higher pH endosomes to lower pH lysosomes is similar to Arg8-ELPBCs but occurs with much slower kinetics than for Arg8-ELPBC micelles.
Figure 4.
Prolonged incubation induced endosome-to-lysosome trafficking of unfunctionalized ELPBC micelles and macropinocytosis inhibitors blocked the endosome-to-lysosome trafficking of Arg8-ELPBC micelles. (A,B) When FaDu cells were incubated with AF488-labeled unfunctionalized ELPBC micelles for 6 h, washed, and imaged 18 h later, the unfunctionalized ELPBC was preferentially distributed in lower pH lysosomes, as quantified by fluorescence ratiometric imaging (A) and confirmed by IF (B). The while scale bar indicates 2 μm. (C) When macropinocytosis was inhibited in FaDu cells by heparinase III inhibitor or by PAK1 siRNA, the accumulation of Arg8-ELPBC micelles in endolysosomes decreased and their distribution became invariant across pH.
Previously, we have shown that ELPBC micelles functionalized with arginine-rich CPPs use macropinocytosis as the predominant mechanism of cellular uptake.14 Macropinocytosis is a complex process that includes binding to the cell membrane, signal activation, membrane protrusion, macropinosome closure, and trafficking.16,17 In the macropinocytosis of CPPs, the membrane-associated heparin sulfate proteoglycan (HSPG) has been identified as a receptor for arginine-rich CPPs.9 Additionally, p21-activated kinase 1 (PAK1) has been identified as an important factor in macropinosome closure and formation by regulating cytoskeleton dynamics and mobility.18 We thus blocked macropinocytosis by treating FaDu cells with heparinase III to deplete HSPG. In a separate experiment, we also knocked down PAK1 expression by siRNA treatment to inhibit macropinocytosis and the decreased PAK1 protein expression after siRNA treatment is shown in Figure S4. The cellular uptake of Arg8-ELPBC micelles decreased greatly in response to both treatments, and their distribution in endolysosomes changed from preferential accumulation in lower pH lysosomes in untreated cells to invariant accumulation with respect to pH in treated cells (Figure 4C). These results strongly suggest that macropinocytosis mediates the fast endosome-to-lysosome trafficking of Arg8-ELPBC micelles.
It is not clear why CPP-functionalized nanoparticles that extensively exploit macropinocytosis to enter cells traffic to lower pH lysosomes faster than unfunctionalized nanoparticles. One possible reason is that macropinosomes have a faster acidification rate than other kinds of internalizing vesicles. In macropinocytosis, macropinosomes undergo fast acidification and finally fuse with lysosomes, reflected by the gain and loss of endosome-specific or lysosome-specific markers.17 It has been reported that greater than 60% of newly formed macropinosomes stain positively with a lysosome-specific antibody within 2–4 min after internalization and almost all of them stain positively with the lysosome-specific antibody in 9–12 min, suggesting their rapid acidification.19
We speculate that unfunctionalized ELPBC micelles, in contrast to Arg8-ELPBC micelles, are probably taken up randomly by a range of endocytosis pathways, which encompass a set of internalization processes including macropinocytosis as well as clathrin-dependent endocytosis and caveolae-dependent endocytosis.20 Compared with macropinocytosis, alternative internalization mechanisms tend to involve intracellular vesicles that progress toward acidification at variable rates. In clathrin-mediated endocytosis, a large fraction of clathrin-coated vesicles are recycled back to the plasma membrane, thereby avoiding significant acidification, while the other fraction undergoes slow acidification and finally fuses with lysosomes.21–23 Furthermore, unlike macropinosomes and clathrin-coated vesicles, caveolae do not undergo acidification, whereby internalized vesicles by caveolin-dependent endocytosis travel to pH-neutral caveosomes and avoid normal lysosomal degradation.24 Thus, mechanisms of uptake other than macropinocytosis may result in initial accumulation within compartments of diverse pH that then undergo acidification at a rate that is on average slower than that in macropinosomes alone. If unfunctionalized ELPBC micelles are taken up randomly by a variety of endocytosis mechanisms, this may explain their slower trafficking to lower pH lysosomes, which is in contrast to Arg8-ELPBC micelles.
In summary, we have developed a new method to spatially map the intracellular distribution of fluorescent nanoparticles in endolysosomes according to their local pH in live cells using ratiometric fluorescence imaging of a pH-sensitive Lysosensor dye. Using this method we observed faster endosome-to-lysosome trafficking of Arg8-functionalized micelles than unfunctionalized micelles, which suggests that CPP-functionalization of nanoparticles can efficiently deliver drug cargo to lysosomes. In contrast to the traditional IF method to study the intracellular distribution of nanoparticles in endolysosomes, our approach offers the advantages of simplified sample preparation and live cell imaging, which decrease the possibility of artifacts caused by redistribution of internalized nanoparticles during sample preparation. As a fast and simple approach, this new method should have broad use in studying the endolysosomal trafficking of fluorescent nanoparticles in live cells, which can help to better understand the intracellular distribution of nanoparticle drug carriers and their trafficking within cells with implications for the rational design of nanoparticles for drug delivery.
Materials.
Lysosensor yellow/blue DND-160, Alexa Fluor 488 C5 maleimide, and Lipofectamine 2000 were purchased from Life Technology. Anti-EEA1 rabbit antibody, anti-LAMP1 rabbit antibody, and Alexa Fluor 555-conjugated anti-rabbit IgG were from Cell Signaling Technology. Anti-PAK1 rabbit antibody was from Kerafast. PAK1 siRNA was ordered from GenScript. Chloroquine, heparinase III, and all other reagents were from Sigma.
Cell Culture.
Human pharynx squamous cell carcinoma FaDu cells were grown in Eagle’ s Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/ml penicillin, and 100 μg/mL streptomycin. Cells were maintained at 37 °C and 5% CO2.
Ratiometric Fluorescence Imaging and Analysis.
An Andor XD revolution spinning disk confocal microscope equipped with a high-sensitivity electron multiplier charge-coupled device (EMCCD) camera was used to quantitatively measure the fluorescence of Lysosensor and AF488-labeled ELPBC micelles in live cells. An excitation wavelength of 405 nm with a blue emission filter (447 ± 30 nm) and a green emission filter (525 ± 15 nm) was used to measure Lysosensor’s two fluorescence emission peaks at 440 and 530 nm, respectively. An excitation wavelength of 488 nm with a green emission filter (525 ± 15 nm) was used to measure the fluorescence of AF488.
To study the relationship between Lysosensor’s IBlue/IGreen intensity ratio (I: fluorescence intensity) and pH in live cells, 105 FaDu cells which had been seeded onto 35 mm glass bottom dishes (MatTek) were incubated with 2 μM Lysosensor at 37 °C for 30 min and then washed twice with ice-cold 2-(Nmorpholino)ethanesulfonic acid (MES) calibration buffer at different pH (pH 4 to pH 7.5 at intervals of 0.5 pH units) and equilibrated for 2 min. The MES buffers contained 5 mM NaCl, 115 mM KCl, 1.2 mM MgSO4, 25 mM MES, 10 μM monensin, and 10 μM nigericin.25 The blue and green fluorescence of Lysosensor was then immediately measured in live cells by a spinning disk confocal microscope. After image acquisition, the fluorescent pixels that represent endolysosomal compartments were distinguished from the largely nonfluorescent cytosol by setting a fluorescence intensity threshold in ImageJ software. A calibration curve was then created by plotting Lysosensor’s IBlue/IGreen ratio against buffer pH. According to this calibration curve, Lysosensor’s IBlue/IGreen ratio at each pixel throughout endolysosomes in live cells was converted to a pH value and then the spatial map of endolysosomal pH in live cells was plotted by Matlab software (Mathworks).
In the study of endolysosomal distribution of fluorescent nanoparticles associated with pH, 105 FaDu cells were seeded onto 35 mm glass bottom dishes. Live cells were incubated with 20 μM AF488-labeled ELPBC micelles for the desired time and then incubated with 2 μM Lysosensor at 37 °C for 30 min. Cells were rinsed with phosphate buffered saline (PBS) three times and observed immediately by a spinning disk confocal microscope. The sample preparation process is shown as a flowchart in Figure S1. The blue and green fluorescence images of Lysosensor and the green fluorescence image of AF488 were acquired at a resolution of 512 pixels × 512 pixels. For each sample, at least 10 images were captured and every image included 10 to 20 cells. These images were analyzed as a 512 pixels × 512 pixels grid where the origin (0, 0) was set at the bottom-left and every pixel was assigned a coordinate (x, y).
Image analysis then proceeded in the following five steps: (1) The spatial overlap of Lysosensor with fluorescent nanoparticles was evaluated to confirm the endocytosis of nanoparticles and their accumulation in endolysosomes. (2) Fluorescent pixels in endolysosomal compartments were identified and distinguished from the largely nonfluorescent cytosol by setting an intensity threshold in ImageJ software. All fluorescent pixels identified as belonging to endolysosomal compartments were subject to further analysis. (3) Lysosensor’s IBlue/IGreen ratio at each pixel throughout the endolysosomal compartments was obtained using image calculator in ImageJ software. (4) Lysosensor’s IBlue/IGreen ratio at each pixel was converted to a pH value according to the calibrated linear relationship between IBlue/IGreen ratio and pH obtained through live cell experiments explained above. (5) Pixels with the same pH value throughout the endolysosomal compartments were binned, and the corresponding AF488 fluorescence intensity of the nanoparticles at these pixels was averaged. This average AF488 intensity was plotted as a function of pH by Matlab software. The Matlab code is shown in the Supporting Code file. The detailed image analysis process is shown as a flowchart in Figure S2.
Immunofluorescence.
A Leica SP5 inverted confocal fluorescence microscope was used to visualize the colocalization of AF488-labeled ELPBC micelles with AF555-labeled early endosome-specific anti-EEA1 antibody and AF555-labeled lysosome-specific anti-LAMP1 antibody. An excitation wavelength of 488 nm with a green emission filter (515 ± 15 nm) and an excitation wavelength of 561 nm with a red emission filter (585 ± 15 nm) were used to measure the fluorescence of AF488 and AF555, respectively.
105 FaDu cells were seeded onto 35 mm glass bottom dishes and were incubated with 20 μM AF488-labeled ELPBC micelles at 37 °C for 6 h. Cells were then washed with PBS and fixed with 4% paraformaldehyde at room temperature for 15 min to be stained with anti-EEA1 antibody or fixed with ice-cold 100% methanol at −20 °C for 10 min to be stained with anti-LAMP1 antibody. Fixed cells were washed with PBS and then incubated with 0.3% Triton X-100 at room temperature for 5 min. Fixed cells were blocked by incubating with 5% goat serum for 1 h at room temperature. After incubation with anti-EEA1 or antiLAMP1 rabbit antibody (dilution 1:100) at 4 °C overnight, cells were rinsed with PBS three times and then incubated with AF555-conjugated anti-rabbit IgG (dilution 1:500) at room temperature for 1 h. Fixed and stained cells were then rinsed with PBS again and imaged by an inverted SP5 confocal fluorescence microscope. The detailed sample preparation process is shown as a flowchart in Figure S1.
Pharmacological Inhibitor and siRNA Experiment.
Measurement of the pH in endolysosomes was confirmed by imaging cells treated with chloroquine, which is known to disrupt endocytosis by inhibiting endolysosomal acidification and thereby raising the pH in endosomes and lysosomes.7 To observe the effects of chloroquine on endolysosomal pH, FaDu cells were preincubated with 50 μM chloroquine at 37 °C for 30 min, rinsed, and then stained with Lysosensor and observed with a spinning disk confocal microscope.
To investigate the involvement of macropinocytosis in the asymmetric distribution of Arg8-ELPBC micelles in endolysosomes, macropinocytosis was inhibited in FaDu cells by depleting HSPG with heparinase III or by knocking down PAK1 gene expression by siRNA. The sequence of PAK1 siRNA was 5′-AUAACGGCCUAGACAUUCAdTdT-3′.18 To deplete HSPG, FaDu cells were preincubated with 1.5 U/ml heparinase III for 30 min, washed with PBS, and then incubated with 20 μM AF488-labeled Arg8-ELPBC micelles at 37 °C for 6 h. To knock down PAK1 gene expression, FaDu cells were seeded in a 24-well plate at 60% confluence. A premixed complex of 30 pmol siRNA and 1.5 μL Lipofectamine 2000 was added to FaDu cells in Opti-MEM reduced serum medium and incubated at 37 °C for 6 h. The medium was then replaced with complete cell culture medium and the incubation was continued at 37 °C for 48 h. Decreased PAK1 protein expression after siRNA treatment was confirmed by Western blotting (Figure S4).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH through Grants R01 EB-00188 and R01 EB-007205 to A.C. We appreciate the assistance of Dr. Yasheng Gao and Dr. Sam Johnson in the Duke Light Microscopy Core Facility.
ABBREVIATIONS
- CPP
cell-penetrating peptide
- ELPBC
elastin-like polypeptide diblock copolymer
- LCST
lower critical solution temperature
- CMT
critical micellization temperature
- Arg
arginine
- AF488
Alexa Fluor 488
- EMCCD
electron multiplier charged-coupled device
- DLS
dynamic light scattering
- OD
optical density
- DIC
differential interference contrast
- HSPG
heparin sulfate proteoglycan
- PAK1
p21-activated kinase 1
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.nanolett.6b05041.
(Figure S1) Flowchart of sample preparation process for ratiometric fluorescence imaging and for immunofluorescence (IF) imaging; (Figure S2) Flowchart of image analysis process for ratiometric fluorescence imaging; (Figure S3) Raw data of flow cytometry analysis of FaDu cancer cells following incubation with AF488-labeled unfunctionalized ELPBC micelles (A) or Arg8-ELPBC micelles (B) at 37 °C; (Figure S4) Western blotting confirmed the decreased PAK1 protein expression in FaDu cells after knocking down PAK1 by siRNA (PDF) The Matlab code to group pixels with the same pH value and to plot average AF488 fluorescence intensity against pH (TXT)
REFERENCES
- (1).Peer D; Karp JM; Hong S; Farokhzad OC; Margalit R; Langer R Nat. Nanotechnol 2007, 2, 751–760. [DOI] [PubMed] [Google Scholar]
- (2).Watson P; Jones AT; Stephens DJ Adv. Drug Delivery Rev 2005, 57, 43–61. [DOI] [PubMed] [Google Scholar]
- (3).Panariti A; Miserocchi G; Rivolta I Nanotechnol., Sci. Appl 2012, 5, 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hillaireau H; Couvreur P Cell. Mol. Life Sci 2009, 66, 2873–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Georgieva JV; Kalicharan D; Couraud P-O; Romero IA; Weksler B; Hoekstra D; Zuhorn IS Mol. Ther 2011, 19, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Diwu Z; Chen C-S; Zhang C; Klaubert DH; Haugland RP Chem. Biol 1999, 6, 411–418. [DOI] [PubMed] [Google Scholar]
- (7).Misinzo G; Delputte PL; Nauwynck HJ J. Virol 2008, 82, 1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Melikov K; Chernomordik L Cell. Mol. Life Sci 2005, 62, 2739–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nakase I; Tadokoro A; Kawabata N; Takeuchi T; Katoh H; Hiramoto K; Negishi M; Nomizu M; Sugiura Y; Futaki S Biochemistry 2007, 46, 492–501. [DOI] [PubMed] [Google Scholar]
- (10).Meyer DE; Chilkoti A Nat. Biotechnol 1999, 17, 1112–1115. [DOI] [PubMed] [Google Scholar]
- (11).Dreher MR; Simnick AJ; Fischer K; Smith RJ; Patel A; Schmidt M; Chilkoti AJ Am. Chem. Soc 2008, 130, 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Simnick AJ; Valencia CA; Liu R; Chilkoti A ACS Nano 2010, 4, 2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Hassouneh W; Fischer K; MacEwan SR; Branscheid R; Fu CL; Liu R; Schmidt M; Chilkoti A Biomacromolecules 2012, 13, 1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).MacEwan SR; Chilkoti A Nano Lett. 2012, 12, 3322–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).MacEwan SR; Chilkoti A Nano Lett. 2014, 14, 2058–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Mercer J; Helenius A Curr. Opin. Microbiol 2012, 15, 490–499. [DOI] [PubMed] [Google Scholar]
- (17).Jones AT J. Cell. Mol. Med 2007, 11, 670–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Al Soraj M; He L; Peynshaert K; Cousaert J; Vercauteren D; Braeckmans K; De Smedt S; Jones AT J. Controlled Release 2012, 161, 132–141. [DOI] [PubMed] [Google Scholar]
- (19).Racoosin EL; Swanson JA J. Cell Biol 1993, 121, 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Conner SD; Schmid SL Nature 2003, 422, 37–44. [DOI] [PubMed] [Google Scholar]
- (21).Huotari J; Helenius A EMBO J. 2011, 30, 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lakadamyali M; Rust MJ; Zhuang X Cell 2006, 124, 997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sieczkarski SB; Whittaker GR Traffic 2003, 4, 333–343. [DOI] [PubMed] [Google Scholar]
- (24).Parton RG; Simons K Nat. Rev. Mol. Cell Biol 2007, 8, 185–194. [DOI] [PubMed] [Google Scholar]
- (25).Wang Z; Zhang J; Wang Y; Xing R; Yi C; Zhu H; Chen X; Guo J; Guo W; Li W; Wu L; Lu Y; Liu S Carcinogenesis 2013, 34, 128–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.