Abstract
Background:
Cardiovascular disease (CVD) is the leading cause of mortality in type 1 diabetes (T1D) and relates strongly to insulin resistance (IR). Lean and obese T1D adolescents have marked IR. Metformin improves surrogate markers of IR in T1D, but its effect on directly-measured IR and vascular health in T1D youth is unclear. We hypothesized that 1) T1D adolescents have impaired vascular function, and 2) metformin improves this IR and vascular dysfunction.
Methods:
T1D adolescents and controls underwent MRI of the ascending (AA) and descending aorta (DA) to assess pulse wave velocity (PWV), relative area change (RAC), maximal (WSSMAX) and time-averaged wall shear stress (WSSTA). T1D participants also underwent assessment of carotid intima-media thickness (cIMT) by ultrasound, brachial distensibility (BrachD) by DynaPulse, fat and lean mass by DXA, fasting labs following overnight glycemic control, and insulin sensitivity by hyperinsulinemic-euglycemic clamp (glucose infusion rate/insulin, [M/I]). T1D adolescents were randomized 1:1 to 3 months of 2000 mg metformin or placebo daily, after which baseline measures were repeated.
Results:
Forty-eight T1D adolescents ages 12–21 years (40% BMI ≥ 90th%ile; 56% female) and twenty-four nondiabetic controls of similar age, BMI and sex distribution were enrolled. T1D adolescents demonstrated impaired aortic health vs. controls, including elevated AA and DA PWV, reduced AA and DA RAC and elevated AA and DA WSSMAX and WSSTA. T1D adolescents in the metformin vs. placebo group had improved M/I (12.2±3.2 vs. −2.4±3.6 [mg/kg/min]/uIU/uL, p=0.005; 18.6±4.8 vs. −3.4±5.6 [mg/lean kg/min]/uIU/uL, p=0.005) and reduced weight (−0.5±0.5 vs. 1.6±0.5 kg, p=0.004), BMI (−0.2±0.15 vs. 0.4±0.15 kg/m2, p=0.005) and fat mass (−0.7±0.3 vs. 0.6±0.4 kg, p=0.01). M/I also improved in normal-weight participants (11.8±4.4 vs. −4.5±4.4 [mg/kg/min]/uIU/uL, p=0.02, 17.6±6.7 vs. −7.0±6.7 [mg/lean kg/min]/uIU/uL, p=0.02). The metformin group had reduced AA WSSMAX (−0.3±0.4 vs. 1.5±0.5 dyne/cm2, p=0.03), AA PWV, (−1.1±1.20 vs. 4.1±1.6 m/s, p=0.04) and far-wall diastolic cIMT (−0.04±0.01 vs. −0.00±0.01 mm, p=0.049) vs. placebo.
Conclusions:
T1D adolescents demonstrate IR and impaired vascular health vs. controls. Metformin improves IR, regardless of baseline BMI, and BMI, weight, fat mass, insulin dose, aortic and carotid health in T1D adolescents. Metformin may hold promise as a cardioprotective intervention in T1D.
Keywords: type 1 diabetes, cardiac MRI, insulin sensitivity, metformin, diabetic vasculopathy
Introduction
The incidence of youth-onset type 1 diabetes (T1D) is increasing worldwide 1, translating to a lifetime of exposure and increased risk for early death from cardiovascular disease (CVD). Vascular dysfunction starts early after the onset of T1D and relates to metabolic risk factors, including glycemic control, blood pressure (BP) and dyslipidemia 2. While control of hyperglycemia, hypertension and hyperlipidemia are beneficial, normalization of these risk factors only partially protects against CVD 2. In fact, clinical trials focused on mitigating these traditional risk factors have yielded disappointing results. The recently published Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT) trial demonstrated that use of an angiotensin converting enzyme inhibitor (ACEi) and a statin over 2–4 years each failed to change carotid intima–media thickness (cIMT) and other CVD risk markers in youth with T1D 3. Thus, evaluating alternative therapeutic targets to impede the development of CVD remains a public health priority.
Using gold-standard techniques, we and others have demonstrated that insulin resistance (IR) is a prominent feature of T1D in adolescents 4, 5 and adults 6, even of normal weight, and that evidence of cardiac and vascular dysfunction is already present during adolescence in T1D 4, 7, 8. Moreover, IR has been increasingly recognized to confer higher risk for a more atherogenic lipoprotein profile 9, 10 and microvascular and macrovascular complications in T1D youth 11 and adults 12, 13. Further, the Diabetes Control and Complications Trial (DCCT) emphasized that intensive glycemic control reduces, but does not eliminate T1D complications, and was associated with weight gain in a subset of participants, along with worsening lipid profiles, BP, central adiposity and inflammation, all of which may negate positive effects of improved HbA1c 14. Central aortic stiffness is an early predictor of overt CVD in adults with T1D and type 2 diabetes (T2D) 15, 16 and is reported in children and young adults with T1D 17–19. However, most previous studies applied tonometric-based methods, which can underestimate central aortic stiffness by disregarding aortic wave reflections and making assumptions regarding intravascular length and homogeneous aortic wall morphology 20. Phase-contrast MRI-derived PWV from the flow-area method offers more specific comparison of aortic extrinsic properties 21. Phase contrast MRI is also a technique that can non-invasively and simultaneously provide multiple direct measures of aortic health and may be more sensitive to early cardiovascular changes in T1D than traditional vascular measures. Phase-contrast MRI has not yet been comprehensively investigated in this population.
Metformin has been shown to reduce CVD risk and improve body composition in adults with T2D 22. Similarly, we previously demonstrated that metformin lowered the daily insulin dose in overweight/obese T1D adolescents and in T1D adolescents with high HbA1c, implying improved insulin sensitivity 23, 24. However, these studies did not evaluate whether metformin directly improves insulin sensitivity or CVD in T1D, nor whether its effects are different in normal-weight versus obese patients. Normal-weight patients are important to consider, as both normal-weight and obese T1D adolescents are significantly insulin resistant when compared to BMI-matched normoglycemic peers, yet have a unique phenotype that differs from youth with T2D 4.
Accordingly, we had the following objectives 1) assess MRI-based aortic health in adolescents with T1D vs. non-diabetic controls of similar age, sex and BMI distribution and 2) perform a 3-month, placebo-controlled, double-blinded, randomized trial to determine the effect of the addition of metformin to basal-bolus insulin treatment on the primary outcome of whole-body insulin sensitivity, measured by gold-standard hyperinsulinemic euglycemic clamp, as well as comprehensive central and peripheral measures of vascular health.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure and is available by contacting this article’s corresponding author. This study was approved by the Colorado Multiple Institutional Review Board (COMIRB) and all participants and guardians provided written informed assent and consent as appropriate for age. An IND exemption was obtained from the U.S. FDA for the use of metformin. In addition to the study investigators and COMIRB, an independent Safety Officer also monitored the study.
Participants and Study Design
As part of the EMERALD study (Effects of MEtformin on CardiovasculaR function in AdoLescents with Type 1 diabetes) (ClinicalTrials.gov Identifier: NCT01808690), 52 adolescents with T1D were recruited from the Barbara Davis Center for Childhood Diabetes and from private endocrinology practices between 2013–2017. Inclusion criteria included age 12–21 years, Tanner Stage >1, diabetes duration of ≥ 1 year and T1D defined as: 1) diabetes by American Diabetes Association (ADA) criteria and 2) ≥ 1 diabetes-associated autoantibody and 3) insulin requirement since diagnosis. Exclusions included resting blood pressure (BP) > 140/90 mmHg, hemoglobin <9 g/L, serum creatinine >106 μmol/L, HbA1c > 108 mmol/mol, smoking, medications affecting insulin sensitivity (oral steroids, immunosuppressants, non-insulin antidiabetic agents, atypical antipsychotics), anti-hypertensive medications, pregnancy, breastfeeding, implanted metal, weight > 136 kg, BMI < 5th%ile, severe illness or DKA within 60 days, inability to tolerate ≥500 mg of metformin twice per day and plans to alter amount of exercise, mode of insulin delivery mode or diet during the study. Contraceptive medications were not excluded. Urine pregnancy tests were performed in female participants prior to randomization and hyperinsulinemic euglycemic clamp visits.
After an initial screening visit, 48 eligible T1D adolescents underwent a baseline comprehensive evaluation of insulin sensitivity, body composition and vascular health (see methods below) at Children’s Hospital Colorado (CHCO) and were then randomized to 3 months of metformin or an identical-appearing placebo. All study measures were then repeated in the T1D adolescents after completing the 3-month intervention. Twenty-four nondiabetic controls of similar age, BMI and sex distribution also underwent an identical comprehensive MRI of the ascending (AA) and descending aorta (DA) for comparison.
In the T1D group, the cardiovascular assessment and DXA and MRI examinations were performed on one day, followed by an admission that evening with an overnight insulin drip to control glycemia. If the participant was on insulin injections, the last long-acting insulin dose was given 24 hours prior to starting the overnight insulin drip. The following morning, fasting labs were drawn, followed by a hyperinsulinemic euglycemic clamp to measure insulin sensitivity. All assessments were performed in the morning fasting for at least 10 hours, in the early follicular phase for menstruating females where possible and preceded by 3 days of no caffeine or strenuous physical activity. Visits were rescheduled if severe hypoglycemia occurred in the prior 24 hours or if hyperglycemia with large ketones or signs of acute metabolic decompensation were present. Participants gave a correction bolus of insulin at home if their waking AM blood sugar was above their target range to minimize hyperglycemia on arrival. Blood glucose was checked again on arrival, treated if low, and documented to have resolved before assessments were initiated.
Anthropometrics
BMI z-score was calculated from BMI, sex and age by the LMS method using 2000 Center for Disease Control and Prevention growth charts. Body composition by DXA (Hologic) was performed to determine fat mass and lean mass 4.
Cardiovascular Assessments
BP was measured with a manual cuff immediately prior to MRI evaluation using the pressure oscillometer technique (Invivo, Model 1400 MRI). BP and heart rate were taken seated after at least 5 minutes of rest. cIMT was assessed with ultrasound, as previously described 25. Briefly, far-wall cIMT was measured from a longitudinal two-dimensional (2D) B-mode image obtained with a Vivid 7 (General Electric, Waukesha, WI, USA) ultrasound system and analyzed using Vascular Analysis Tools software version 5.0 (MIA, Coralville, IA, USA). cIMT was defined as the distance from the leading edge of the media–adventitia interface of the near-wall to the media–adventitia interface of the far-wall. Lumen diameter was measured as the distance between the vessel far-wall boundary (corresponding to the interface between the lumen and intima) and a near-wall boundary (corresponding to the interface of the adventitia and media). The average of five separate lumen and IMT measurements was utilized for analysis. All images were coded by number, blinded to group assignment, and analyzed by a single cardiologist with extensive experience in arterial imaging (U.T.). The Dynapulse 5200A Pathway system (PulseMetric, Inc., San Diego, CA), a non-invasive portable system, was used to measure brachial artery distensibility (BrachD), utilizing pulse waveform analysis of arterial pressure signals obtained from a sphygmomanometer 26.
Cardiovascular MRI
All subjects underwent MRI evaluation with a 3T magnet system (Skyra, Siemens, Erlangen, Germany) using a 32-channel coil. A free breathing phase contrast sequence was applied with Cartesian encoding and retrospective sorting (TR: 14–28 ms, 30 to 50 phases, TE: 2.2–3.5 ms, matrix: 160 × 256, flip angle: 25°) with 100% k-space sampling and no further temporal interpolation. Depending on participant size and field of view (128–225 × 210–360 mm), the cross-sectional pixel resolution was 0.82 × 0.82–1.56 ×1.56 mm2 with a slice thickness of 5 mm. Phase contrast acquisition time for each plane varied per heart rate, between 2–3 min. Velocity-encoding values were adjusted according to the maximum velocities encountered during scout sequences to avoid aliasing artifact (typically 100–250 cm/s).
This phase-contrast sequence was applied orthogonally to the AA at approximately one centimeter above the sino-tubular junction; a plane which also corresponded to an orthogonal plane across the DA, approximately 3–5 cm below the origin of the left subclavian artery. Both magnitude (tissue intensity) and phase-velocity maps were obtained, and raw data was transferred to an off-line processing system, MatLab (Mathworks, Inc., Natick, MA), to determine the pulse wave velocity (PWV), relative area change (RAC) and wall shear stress (WSS). Using flow-area (dQ/dA) method, flow and area waveforms were generated from time-frame-segmented respective phase and magnitude images 20, 27. Flow-area diagrams assessed regional PWV by computing dQ/dA slope from early systolic data points. Central aortic distensibility was measured using the RAC ([maximum area-minimum area]/maximum area) × 100%). WSS was calculated from segmented phase and magnitude images as described previously using an 8-point model to calculate through-plane WSS from the shear curve, yielding maximum systolic WSS (WSSMAX) and time-averaged WSS (WSSTA).
Insulin Sensitivity
T1D participants were admitted to the inpatient Clinical and Translational Research Center (CTRC) for 12 hours of overnight monitored fasting and bedrest, following a fixed macronutrient (55% carbohydrate, 30% fat, 15% protein, no caffeine, no regular soda), weight-maintenance meal provided and labeled for carbohydrate counting with short-acting insulin by the CTRC metabolic kitchen. Subcutaneous insulin was then replaced with a variable-rate overnight intravenous insulin infusion to gradually normalize glucose as previously described 4, 28. The following morning, a multi-phase hyperinsulinemic euglycemic clamp (insulin doses of 10 mU/m2/min for 60 min, 16 mU/m2/min for 90 min, 80mU/m2/min insulin for 120 min, paired with a variable rate of 20% dextrose) was performed as previously described to quantify insulin sensitivity 4, 29. One IV was used for infusion of dextrose and insulin, and the contralateral, heated, arterialized IV was used for blood sampling and bedside monitoring of blood glucose (BG) every 5 minutes with a steady-state goal BG of 5.3 mmol/L. Glucose infusion rate [GIR or M] in mg·kg·min and mg·lean kg ·min was calculated during steady-state of the final stage 29. Insulin sensitivity was calculated as M/I, where I is the insulin concentration at steady state.
Laboratory measures
Fasting serum labs were drawn in the AM prior to the hyperinsulinemic clamp during normoglycemia, including: HbA1c, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lipid panel, glucose, insulin and creatinine. Total cholesterol (total-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides were assessed via enzymatic assays (Beckman Coulter); low-density lipoprotein cholesterol (LDL-C) levels were calculated with the Friedewald equation. HbA1c was measured by DCCT-calibrated ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Hercules, CA). Other laboratory tests were performed by standard methods in the CTRC laboratory.
Intervention
After completion of baseline assessments, T1D participants were randomized 1:1 by the CHCO research pharmacist in a double-blinded fashion using the Microsoft Excel randomization function to 3 months of active metformin or identical-appearing placebo (compounded by Belmar Compounding Pharmacy, Lakewood, CO). The research pharmacist maintained the blinding code and assigned participants to interventions. Blinded investigators enrolled and followed participants, who were also blinded. Randomization was stratified by: 1) HbA1c above or below 69 mmol/mol and 2) BMI above or below the 85th percentile. Study medication was titrated to a final dose of 1000 mg twice daily, over a period of 4 weeks (minimum dose required was 1000 mg per day). Following the 12-week treatment phase, all T1D participants underwent repeat outcome assessments identical to the baseline assessments.
Throughout the study, participants were asked to check their home BG ≥ 4 times per day, urine ketones if BG > 16.7 mmol/L on ≥ 3 consecutive readings, and to contact the study staff for assistance for: large urine ketones, ≥2 BG < 3.6 mmol/L in any week, hypoglycemia requiring assistance or other concerns. Participants adjusted insulin doses as routinely recommended by their diabetes provider. An interim phone call occurred weekly during metformin/placebo titration, at 10 weeks and as needed; in-person visits occurred at 6 and 12 weeks to assess BG, insulin dose, pill count, side effects, hemoglobin, ALT, AST, and serum creatinine. Adverse events were queried at all scheduled and unscheduled phone calls and visits. The primary pre-defined severe adverse events included lactic acidosis, anemia, severe hypoglycemia, and diabetic ketoacidosis. Gastrointestinal symptoms were also evaluated, as well as changes in serum creatinine, AST, ALT and unanticipated adverse events.
Power calculations
Power calculations were based on data from a small study in youth with poorly controlled T1D 30, which reported a significant increase in M/I in the 11 participants treated with metformin. Based on the effect size reported in that study, a sample size of 25 per group and an alpha of 0.05 provided us with 93% power to detect a difference of 1 standard deviation in the primary outcome of M/I.
Statistical analyses
Analyses were performed in SAS (version 9.4 or higher; SAS Institute, Cary, NC). Variables were checked for the distributional assumption of normality using normal plots, in addition to Kolmogorov-Smirnov and Shapiro Wilks tests. Variables that were skewed were natural log transformed, and skewed variables which included negative values were log-modulus transformed for the analyses. Demographic and clinical characteristics in adolescents with and without T1D and in the metformin and placebo groups were compared using t-tests for normally distributed continuous variables and χ2 for categorical variables. General linear regression models were used to determine the effect of metformin vs. placebo therapy on the primary outcome of M/I per kg and M/I per lean kg, secondary outcomes of near and far-wall cIMT, BrachD and MRI-derived aortic PWV and WSS, and tertiary measures of body composition, HbA1c, LDL-C and BP. The change in the outcomes from baseline to 3 months were the dependent variables in the models, and all models were adjusted for the baseline values of the dependent variables. We also fit multivariable models, which included sex, pubertal status and change in BMI for the primary outcome (M/I), and change in SBP, change in BMI, and change in M/I for the secondary outcomes (vascular markers). We also performed sub-group analysis for M/I stratified by BMI category (lean and obese). Significance was based on an α-level of 0.05.
Results
Aortic Stiffness and Wall Shear Stress in Youth with and without T1D
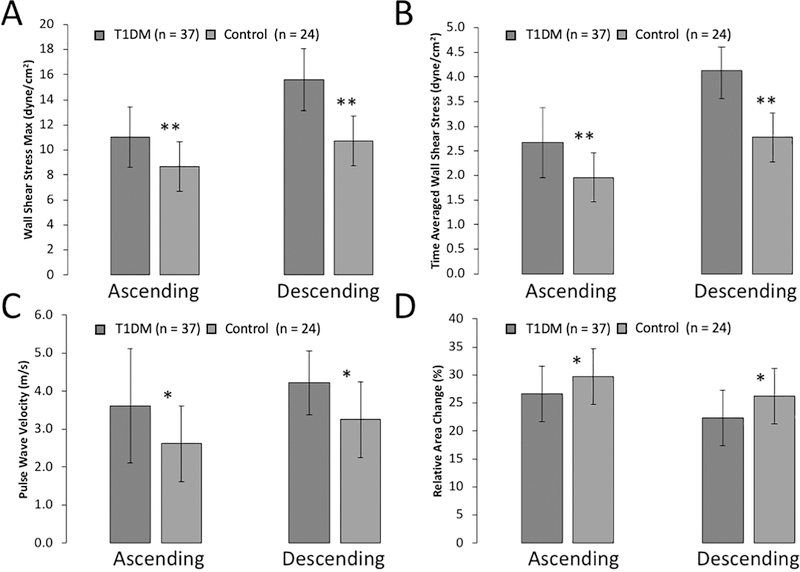
T1D and nondiabetic control participant’s clinical and MRI parameters are summarized in Table 1. The groups were similar in age (p=0.10) and sex distribution (p=0.53). Although the BMI z-score was not different between groups, the BMI percentile was slightly higher in the T1D group, and therefore comparisons of the baseline MRI measures were controlled for BMI percentile as well as for the pre-determined variables of age and sex. Participants with diabetes had significantly elevated WSSMAX (Figure 1A) and WSSTA (Figure 1B) in both the AA and DA. Youth with T1D also demonstrated greater PWV in both aortic segments, indicating increased vascular stiffness (Figure 1C). Similarly, RAC was significantly lower in the AA and DA in T1D youth (Figure 1D), indicating decreased central aortic distensibility.
Table 1:
Baseline Characteristics of Adolescents with and without T1D with Baseline Aortic MRI Data
| Variables | T1D (n = 37) | Controls (n = 24) | p-value |
|---|---|---|---|
| Age (years) | 16.8 ± 2.6 | 15.6 ± 2.6 | 0.10 |
| Sex (% female) | 46% | 54% | 0.53 |
| BMI Z-score | 0.95 ± 1.01 | 0.34 ± 0.74 | 0.01 |
| Diabetes duration (years) | 7.8 ± 4.2 | -- | -- |
| HbA1c (mmol/mol) | 72 ± 9.4 | -- | -- |
| SBP (mmHg) | 122 ± 9 | 114 ± 9 | 0.002 |
| DPB (mmHg) | 72 ± 8 | 67 ± 7 | 0.02 |
| SBP percentile | 72 ± 25 | 64 ± 22 | 0.20 |
| DBP percentile | 67 ± 21 | 56 ± 18 | 0.17 |
| Heart rate (BPM) | 71 ± 10 | 71 ± 15 | 0.97 |
| MRI Measures | |||
| AA WSSMAX (dyne/cm2)* | 10.9 ± 0.4 | 8.7 ± 0.5 | 0.002 |
| AAWSSTA (dyne/cm2)* | 2.7 ± 0.1 | 1.9 ± 0.1 | < 0.001 |
| AA PWV (m/s)* | 3.7 ± 0.3 | 2.5 ± 0.4 | 0.045 |
| AA RAC (%) | 27 ± 5 | 30 ± 5 | 0.002 |
| DA WSSMAX (dyne/cm2)* | 15.5 ± 0.6 | 10.7 ± 0.7 | < 0.001 |
| DA WSSTA (dyne/cm2)* | 4.1 ± 0.1 | 2.7 ± 0.2 | < 0.001 |
| DA PWV (m/s)* | 4.2 ± 0.2 | 3.1 ± 0.3 | 0.02 |
| DA RAC (%) | 22 ± 4 | 26 ± 6 | 0.01 |
Limited to participants who had phase-contrast MRI data at baseline. Data are n (%), means ± SD unless otherwise noted.
Sex, age and BMI percentile adjusted means presented as least square means ± SEM
BMI = body mass index, SBP =systolic blood pressure, DBP =diastolic blood pressure, BPM=beats per minute, WSSMAX = maximal wall shear-stress, WSSTA = time-averaged wall shear-stress, PWV =pulse wave velocity, RAC = relative area change
Figure 1A-D: Phase-Contrast MRI Reveals Abnormal Aortic Function in T1D Youth vs. Controls.
Figure 1. Limited to participants who had phase-contrast MRI data at baseline. Phase-contrast derived MRI measures of the ascending and descending aorta are shown, including: (A) Maximal systolic wall shear stress; (B) Time-averaged wall shear stress; (C) Pulse wave velocity; (D) Relative area change (aortic distensibility). All data sets were adjusted for age, sex, and BMI percentile. *p < 0.05; †p < 0.001
Baseline Characteristics of T1D Youth Randomized into the Clinical Trial
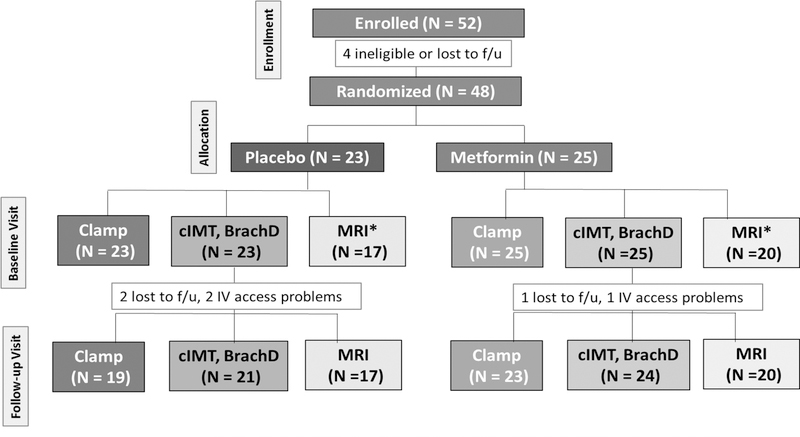
A Consort Diagram depicting the randomization and follow-up visit sample sizes is shown in Figure 2. The baseline characteristics of the youth with T1D randomized into the clinical trial, stratified by treatment group (placebo vs. metformin) is shown in Table 2. Participants assigned to the placebo vs. metformin group were similar in age, T1D duration, sex distribution, pubertal status and ethnicity. HbA1c, systolic and diastolic BP, total-C, HDL-C, LDL-C, triglycerides, total daily insulin dose (TDI), M/I per kg and M/I per lean kg were also similar across the two treatment groups at baseline.
Figure 2. Consort Diagram of Clinical Trial in participants with T1D.
*MRI repairs led to smaller sample size for MRI than for other outcomes
Table 2.
Baseline Characteristics of T1D Participants by Treatment Group
| Variables | Placebo | Metformin |
|---|---|---|
| Age (years) | 16.0±2.7 | 17.3±2.3 |
| Sex (% female) | 46% | 56% |
| Ethnicity (% NHW) | 83% | 88% |
| Tanner Stage* | 5 (4–5) | 5 (5–5) |
| Weight (kg) | 69.9±14.6 | 72.3±13.7 |
| Fat mass (kg) | 22.4±9.3 | 22.9±9.3 |
| BMI (kg/m2) | 25.3±4.9 | 25.4±4.4 |
| BMI percentile | 76.8±23.5 | 74.6±26.1 |
| Waist Circumference (cm) | 80±14 | 79±12 |
| Glycemia and IR | ||
| HbA1c (mmol/mol) | 69±8.3 | 72±10.5 |
| Diabetes Duration (years) | 7.8±4.4 | 8.0±3.7 |
| TDI (units/day) | 65.2±25.4 | 60.0±22.4 |
| TDI/kg (units/kg/day) | 0.93±0.27 | 0.83±0.25 |
| GIR (mg/kg/min) | 7.5±3.7 | 7.7±3.7 |
| GIR (mg/lean kg/min) | 11.2±4.9 | 11.8±4.8 |
| Insulin (uIU/uL) | 171.3±62.9 | 192.9±93.6 |
| M/I* (mg/kg/min)/uIU/uL | 3.9 (2.1–8.5) | 3.5 (2.4–6.3) |
| M/I Lean (mg/lean kg/min)/uIU/uL * | 6.2 (3.6–10.9) | 6.0 (3.8–8.7) |
| Lipids | ||
| Total-C (mmol/L) | 3.81±0.88 | 3.73±0.75 |
| LDL-C (mmol/L) | 2.23±0.49 | 2.12±0.52 |
| HDL-C (mmol/L) | 1.17±0.26 | 1.19±0.26 |
| Triglycerides (mmol/L) | 0.92±0.35 | 0.89±0.45 |
| Peripheral Vascular Measures | ||
| SBP (mm Hg) | 122±10 | 119±8 |
| DBP (mm Hg) | 71±8 | 71±7 |
| Average far-wall cIMT (diastole) (mm) | 0.46±0.06 | 0.47±0.06 |
| Average far-wall cIMT (systole) (mm) | 0.44±0.06 | 0.44±0.06 |
| Average carotid diameter (diastole) (mm) | 6.12±0.45 | 6.28±0.46 |
| Average carotid diameter (systole) (mm) | 6.95±0.46 | 7.06±0.55 |
| Average carotid distension (mm) | 0.14±0.03 | 0.12±0.03 |
| Brachial Distensibility (%/mmHg) | 5.8±0.9 | 6.1±1.2 |
| Aortic MRI Measures | ||
| AA WSSMAX (dyne/cm2) | 11.0±2.3 | 11.0±2.6 |
| AA WSSTA (dyne/cm2) | 2.6±0.7 | 2.7±0.8 |
| DA WSSMAX (dyne/cm2) | 14.5±4 | 16.6±4.3 |
| DA WSSTA (dyne/cm2) | 4.2±1.2 | 4.1±1.2 |
| AA PWV (m/s) | 3.0±1.3 | 4.0±2.2 |
| DA PWV (m/s) | 4.1±1.6 | 4.3±1.4 |
| AA RAC (%) | 26.5±3.0 | 26.8±6.2 |
| DA RAC (%) | 22.8±4.4 | 21.9±4.6 |
Data are presented as means ± SD unless otherwise noted.
Median, p25–p75
TDI = Total Daily Insulin Dose, GIR = glucose infusion rate, M = steady-state glucose infusion rate, I = steady-state insulin, Total-C = total cholesterol, LDL-C = low-density lipoprotein cholesterol, HDL-C = high-density lipoprotein cholesterol, SBP = systolic blood pressure, DBP = diastolic blood pressure, cIMT = carotid intima media thickness, AA = ascending aorta, DA = descending aorta, WSSMAX = maximal wall shear-stress, WSSTA = time-averaged wall shear-stress, PWV = pulse wave velocity, RAC = relative area change
Primary Outcome (Figure 3 and Table 3)
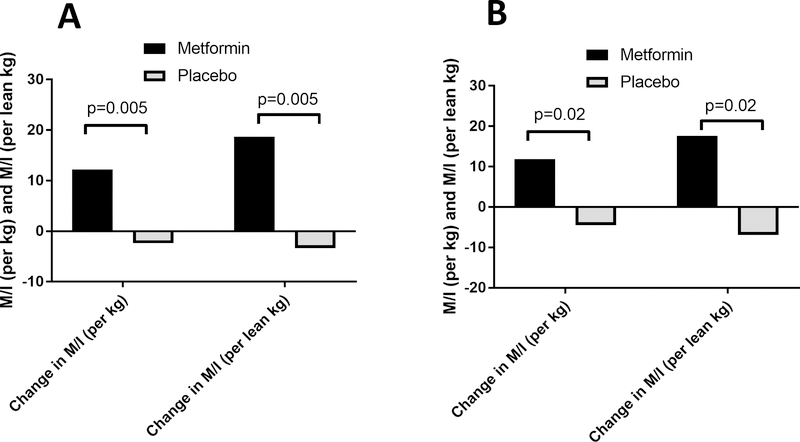
Figure 3A and 3B: Metformin Improves Insulin Sensitivity in Adolescents with Type 1 Diabetes.
Figure 3A. Change in M/I (per kg) in response to metformin vs. placebo, in all T1D participants, adjusted for baseline M/I (per kg) and change in M/I (per lean kg) in response to metformin vs. placebo in all T1D participants, adjusted for baseline M/I (per lean kg)
Figure 3B. Change in M/I (per kg) and M/I (per lean kg) in response to metformin vs. placebo in n=28 T1D youth with BMI <90th %ile, adjusted for baseline M/I (per kg) or baseline M/I (per lean kg)
Table 3.
The Effect of Metformin on Vascular Health Markers
| Variables | Placebo | Metformin | Metformin vs. Placebo P-value | Placebo | Metformin | Metformin vs. Placebo P-value |
|---|---|---|---|---|---|---|
| LSM±SE | LSM±SE | LSM±SE | LSM±SE | |||
| Crude* | Adjusted† | |||||
| Carotid Ultrasound | ||||||
| Δ Average far-wall cIMT (diastole) (mm) | −0.00±0.01 | −0.04±0.01 | 0.06 | −0.00±0.02 | −0.03±0.01 | 0.30 |
| Δ Average far-wall cIMT (systole) (mm) | 0.00±0.01 | −0.02±0.01 | 0.16 | −0.01±0.02 | −0.01±0.01 | 0.83 |
| Δ Average diameter (diastole) (mm) | 0.00±0.06 | −0.01±0.05 | 0.91 | −0.04±0.07 | −0.02±0.05 | 0.88 |
| Δ Average diameter (systole) (mm) | −0.03±0.06 | 0.01±0.05 | 0.66 | −0.13±0.8 | 0.03±0.05 | 0.12 |
| Δ Average distension (mm) | −0.00±0.01 | 0.00±0.01 | 0.77 | −0.01±0.01 | 0.01±0.01 | 0.34 |
| Aortic MRI | ||||||
| Δ AA WSSMAX (dyne/cm2) | 0.83±0.35 | 0.00±0.32 | 0.10 | 1.51±0.52 | −0.34±0.40 | 0.03 |
| Δ AA WSSTA (dyne/cm2) | 0.10±0.09 | −0.04±0.08 | 0.30 | 0.14±0.15 | −0.09±0.11 | 0.29 |
| Δ DA WSSMAX (dyne/cm2) | 0.26±0.72 | −0.77±0.63 | 0.30 | 0.94±1.09 | −1.59±0.78 | 0.13 |
| Δ DA WSSTA (dyne/cm2) | 0.10±0.24 | −0.22±0.21 | 0.32 | 0.32±0.35 | −0.35±0.26 | 0.19 |
| Δ AA PWV (m/s) | 1.80±1.32 | 0.15±1.08 | 0.35 | 4.07±1.60 | −1.14±1.20 | 0.04 |
| Δ DA PWV (m/s) | −0.32±0.21 | −0.39±0.17 | 0.80 | −0.11±0.30 | −0.56±0.22 | 0.30 |
| Δ AA RAC (%) | 0.35±1.46 | −1.79±1.34 | 0.29 | −0.99±2.25 | −0.89±1.75 | 0.97 |
| Δ DA RAC (%) | 4.75±1.97 | 3.73±1.80 | 0.71 | 1.85±2.44 | −6.04±1.84 | 0.24 |
| Dynapulse | ||||||
|
ΔBrachial Distensibility (%/mmHg) |
0.16±0.20 | 0.23±0.19 | 0.80 | 0.27±0.27 | 0.13±0.23 | 0.71 |
Delta (Δ) value = 3-month value – baseline value.
Adjusted for baseline values
Adjusted for baseline values, change in BMI, change in M/I (lean mass) and change in SBP
cIMT = carotid intimal medial thickness, AA = ascending aorta, DA = descending aorta, WSSMAX = maximal wall shear-stress, WSSTA = time-averaged wall shear-stress, PWV = pulse wave velocity, RAC = relative area change
Data are presented as least square means (LSM) and standard errors (SE) from generalized linear regression models
M/I per kg improved over the 3-month intervention in the metformin group compared with placebo (12.2±3.2 vs. −2.4±3.6 [mg/kg/min]/uIU/uL, p=0.005) (Figure 3A and Supplementary Table 1). The difference remained significant after adjusting for sex, pubertal status and change in BMI (11.5±3.4 vs. −1.3±3.91 [mg/kg/min]/uIU/uL, p=0.03). M/I per lean kg also improved in the metformin group compared with placebo (18.6±4.8 vs. −3.4±5.6 [mg/lean kg/min]/uIU/uL, p=0.005) (Figure 3A), and remained significant after adjustment for sex, pubertal status and change in BMI (7.4±9.6 vs. −14.3±9.8 [mg/lean kg/min]/uIU/uL, p=0.01). When limiting analyses to the 28 participants with baseline BMI <90th percentile, significant improvements in M/I per kg (11.8±4.4 vs. −4.5±4.4 [mg/kg/min]/uIU/uL, p=0.02) and M/I per lean kg (17.6±6.7 vs. −7.0±6.7 [mg/lean kg/min]/uIU/uL, p=0.02) were still observed in the metformin group compared with placebo (Figure 3B) and both remained significant after adjustment for sex, pubertal status and change in BMI (p=0.03 for both M/I per kg and M/I per lean kg).
Secondary Outcomes
i. Markers of body composition and total daily insulin doses:
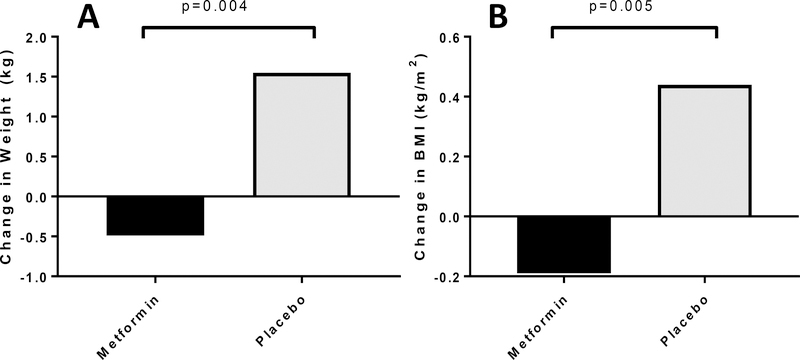
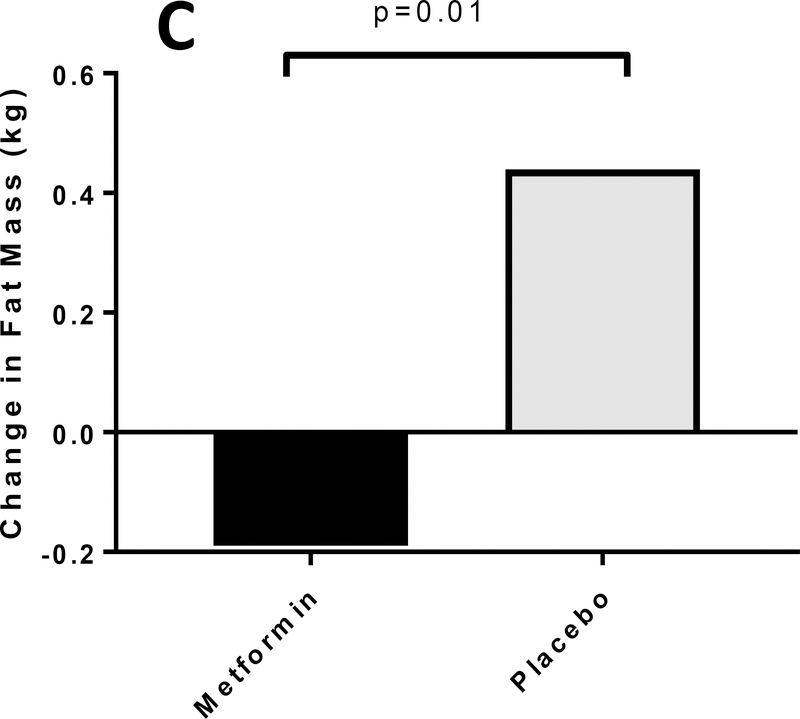
Body weight (−0.5±0.5 vs. 1.6±0.5 kg, p=0.004) (Figure 4A), BMI (−0.2±0.15 vs. 0.4±0.15 kg/m2, p=0.005) (Figure 4B) and fat mass (−0.7±0.3 vs. 0.6±0.4 kg, p=0.01) (Figure 4C) all improved in the metformin group compared with placebo. These improvements remained significant after adjusting for age and pubertal status. Greater reductions in TDI (−6.4±2.5 vs. −0.06±2.6 units/day, p=0.09) and TDI per kg (−0.07±0.04 vs. −0.02±0.04 units/kg/day, p=0.31) were observed in metformin group compared with placebo but did not reach statistical significance.
Figure 4A, 4B and 4C: Metformin Improves Weight and Body Composition in T1D Adolescents.
Figure 4A Change in weight (kg) in response to metformin vs. placebo, adjusted for baseline weight (kg)
Figure 4B Change in BMI (kg/m2) in response to metformin vs. placebo, adjusted for baseline BMI (kg/m2)
Figure 4C Change in fat mass (kg) in response to metformin vs. placebo, adjusted for baseline fat mass (kg)
ii. Traditional CVD risk factors:
There were no significant changes in HbA1c, systolic BP, diastolic BP, total-C, LDL-C, HDL-C or triglycerides in the metformin vs. placebo group with and without adjustment for sex, Tanner stage and change in BMI (Supplementary Table 2).
iii. Markers of vascular health:
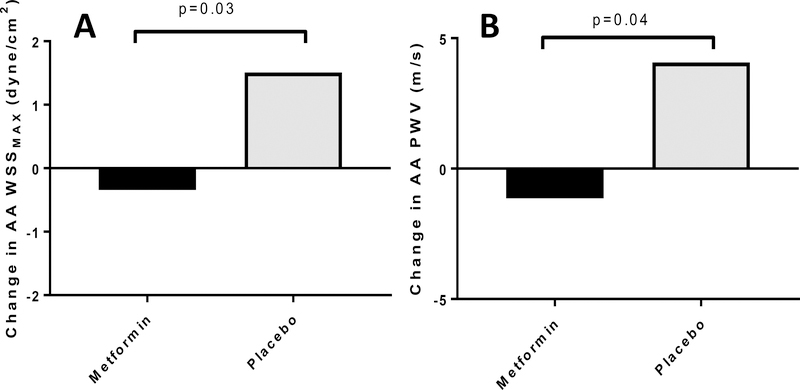
(Table 3) Phase contrast MRI-derived maximal aortic wall shear stress was decreased in the AA in the metformin vs. placebo group (change in AA WSSMAX: −0.3±0.4 vs. 1.5±0.5 dyne/cm2, p=0.03) (Figure 5A) as did aortic stiffness (change in AA PWV: −1.1±1.20 vs. 4.1±1.6 m/s, p=0.04) (Figure 5B), in models adjusting for change in BMI, change in M/I per lean kg and change in SBP. No significant improvements were observed for AA WSSTA, DA WSSMAX, DA WSSTA or DA PWV. Mean diastolic far wall cIMT was significantly improved in the metformin group after adjusting for change in BMI (−0.04±0.01 vs. −0.00±0.10 mm, p=0.04) and change in BMI and change in SBP (−0.04±0.01 vs. −0.00±0.01 mm, p=0.049), but lost significance after further adjustment for change in M/I per lean kg) (p=0.30). No significant improvements were observed for BrachD.
Figure 5A and 5B: Metformin Improves Vascular Health in Adolescents with Type 1 Diabetes.
Figure 5A Change in AA WSSMAX in response to metformin vs. placebo, adjusted for baseline AA WSSMAX, change in BMI, change in M/I (per lean kg) and change in SBP
Figure 5B Change in AA PWV in response to metformin vs. placebo, adjusted for baseline AA PWV, change in BMI, change in M/I (per lean kg) and change in SBP
Safety data
Of the 48 randomized participants, only 3 participants (6%) did not finish the study protocol. Medication adherence was 93% in the metformin group vs. 93% in the placebo group at 6 weeks (p=ns) and 97% in metformin group vs. 91% in placebo group at 12 weeks (p=ns). There were no severe adverse events. Adverse events included only minor gastrointestinal side effects (nausea, diarrhea, reduced appetite, stomach pain) which were reported in 10 participants in the metformin group (40%) compared to two participants in the placebo group (8%) (p<0.01). De-escalation in metformin dose was applied in three participants (12%), but all participants tolerated at least 1000 mg of metformin per day. There were no significant changes between groups in hemoglobin, ALT, AST or serum creatinine (data not shown).
Discussion
Our T1D cohort demonstrated vascular dysfunction compared to controls of similar age, BMI and sex distribution, and as in our previous studies, adolescents with T1D had lower insulin sensitivity measured by hyperinsulinemic euglycemic clamp than our previous cohorts of nondiabetic controls 4, 5, 9. The primary finding of our current randomized, double-blinded, controlled trial is that metformin improves directly-measured insulin sensitivity. Additionally, we demonstrated that metformin reduces weight, BMI and fat mass and improves measures of aortic and carotid vascular health over 3 months. The improvement in insulin sensitivity was not limited to obese participants and was significant both when assessing per kg of body weight or per kg of fat-free mass. This is the first report demonstrating improvement in insulin sensitivity by the gold standard hyperinsulinemic euglycemic clamp technique with metformin versus placebo in lean and obese youth with T1D. In addition, to our knowledge, there are no other pediatric or adult studies investigating the impact of metformin therapy on aortic wall-shear stress or aortic stiffness.
T1D increases CVD risk 4–8-fold 31, yet there has been a lack of progress in the management of macrovascular complications. Moreover, obesity is now increasing in youth with T1D 32, further augmenting CVD risk. While overt CVD is rare until adulthood, precursors of CVD are increasingly documented in youth with T1D. For example, youth with T1D have evidence of a more atherogenic LDL-C and HDL-C distribution 9, peripheral arterial stiffness 17, 33, elevated BP 2, increased aortic 34 and carotid 35 IMT, endothelial dysfunction 4, 7, diastolic dysfunction 36, left ventricular hypertrophy 4, reduced cardiac strain 8 and lower VO2peak 4. Accordingly, to reduce CVD risk, it may be beneficial to begin intervention in childhood. While glycemic control is important to CVD risk, less than 21% of adolescents currently are able to meet glycemic control guidelines 37. Further, the recent randomized, placebo-controlled AdDIT study examined the impact of the traditional adjunctive therapies, ACE-inhibitors and statins, on CVD risk over 2–4 years in 443 T1D adolescents. Statins lowered LDL-C and triglycerides and also lowered HDL-C; however neither ACE-inhibitors nor statins improved urinary albumin excretion, cIMT, high-sensitivity C-reactive protein, asymmetric dimethylarginine, glomerular filtration rate or retinopathy 3. Therefore, novel approaches to CVD risk-reduction in T1D are needed, especially in youth. Here, we demonstrate improvement in central aortic stiffness and wall shear stress as well as carotid intimal medial thickness following short term metformin therapy.
Insulin resistance is an increasingly recognized component of T1D 4, 6, 38, and relates to CVD development 13. Insulin resistance in T1D is not due to obesity alone, as normal-weight youth and adults with T1D are both significantly insulin resistant compared to well-matched non-diabetic controls 4, 6, 11, 38. Insulin resistance in youth with T1D relates to markers of increased CVD risk, including reduced VO2peak 4 forearm blood flow 4, blood pressure, total-C, high sensitivity C-reactive protein 11 and a more atherogenic lipoprotein profile9. Moreover, in adults with T1D, insulin sensitivity, not HbA1c, relates to markers of CVD risk including a more atherogenic lipoprotein profile10, presence, extent6 and progression of coronary artery calcification and albuminuria 12 and predicts definitive coronary artery disease (CAD) end points (angiographic stenosis, revascularization, Q waves, nonfatal myocardial infarction and CAD death) 13. In earlier studies, cohorts showed a relationship between insulin sensitivity and HbA1c 38, 39 whereas newer data in youth and adults using modern insulin analogs and stricter glycemic control show no relationship between insulin sensitivity and either CGM or HbA1c-assessed hyperglycemia 4, 6, 28. Thus, treatments that target IR in T1D hold promise as a novel method of preventing CVD.
We assessed aortic PWV and RAC by phase-contrast-MRI and found significant arterial stiffness and reduced distensibility in both the AA and DA in adolescents with T1D compared to normal controls. Further, we observed significantly elevated shear forces in the same aortic segments, which are known to be responsible for triggering endothelial signaling cascades and remodeling of the systemic vasculature 40, suggesting that flow hemodynamics may contribute to accelerated vasculopathy in T1D. Endothelial cells in the AA have different responsiveness to shear abnormalities than those at the level of the DA or thoraco-abdominal aorta 41, demonstrating the importance of assessing segment-specific aortic health as we did in the present study. Our findings are supported by Samyn et al. who described reduced distensibility and elevated WSS derived by computational fluid dynamic simulation in T1D youth 42. However, our phase-contrast MRI approach has advantages over computational fluid dynamics including the well-accepted spatial-temporal resolution associated with phase-contrast MRI imaging and is a directly-measured approach that avoids the anatomical and flow hemodynamic assumptions inherent in computational modeling techniques. Further, we found improvements in the AA PWV and WSS as well as cIMT with metformin, showing both the potential beneficial impact of metformin on CVD in T1D and supporting the link between CVD and IR in T1D. Metformin is the only oral medication approved for pediatric use in T2D and remains the most widely-prescribed insulin-sensitizing agent. Metformin’s effects in T2D are incompletely understood but are likely mediated by mechanisms including decreasing hepatic glucose output and increasing glucose disposal in myocytes 43, its more recently recognized gastrointestinal effects 44, 45, as well as direct impacts on the heart, endothelium and vasculature 4, 46. The UK Prospective Diabetes Study (UKPDS) trial demonstrated cardiovascular benefits with metformin in adults with T2D 22. However, a recent meta-analysis of randomized controlled trials in T2D adults demonstrated that while all CVD outcomes, with the exception of stroke, favored metformin, none achieved statistical significance due to the limited number of metformin studies with CVD outcomes 47. Since the phenotype of IR in T1D differs considerably from the metabolic syndrome phenotype of T2D 4, it is not clear whether metformin would have similar effects in T1D as in T2D, hence the need for the current study.
The data on the effects of metformin in T1D vary somewhat, but the majority of studies, including our two larger studies of the impact of metformin on glycemic control in T1D youth 23, 24 and a Cochrane Systematic Review 48 demonstrate that metformin lowers BMI and insulin dose in T1D youth, implying improved insulin sensitivity, but no improvement in HbA1c. The current study confirms and takes these findings a step further by demonstrating that IR measured by gold-standard hyperinsulinemic euglycemic clamp improves in T1D youth with metformin vs. placebo, as does body composition by DXA. A smaller study in Swedish adolescents in the 1990’s did assess insulin sensitivity with metformin use in T1D but was not powered to compare effects between the metformin and placebo group 30. Moreover, the Swedish cohort’s inclusion criteria required both a Hb1c>64 mmol/mol and high insulin requirements, limiting generalizability, and the insulin sensitivity measurement was non-standard, with an inadequate clamp insulin dose to suppress hepatic glucose output in adolescents and an inadequate clamp insulin infusion time to achieve steady-state.
Existing T1D studies of metformin with CVD outcomes are sparse, with inconsistent results. Recently published data from the REducing with MetfOrmin Vascular Adverse Lesions in T1D (REMOVAL) study of adults with T1D did not show reduction in the primary outcome of progression of mean cIMT, but did show significant improvement in maximal cIMT, LDL-C, weight and estimated glomerular filtration rate 49. In contrast, a randomized controlled trial by Anderson et al. found no significant effect of metformin on mean cIMT in youth (8–18 years), but observed improvement in glyceryl trinitrate mediated-dilatation, indicating improved vascular smooth muscle function 50. A potential explanation for the differences in cIMT findings in this latter study is the inclusion of 8–11 year-olds, as younger and prepubertal youth are less likely to have measurable baseline abnormalities in cIMT.
There are important strengths and limitations of our study. First, this carefully designed clinical trial included both normal-weight and overweight/obese participants with T1D, allowing sub-analysis in the normal-weight group. Second, we controlled for the acute impact of diet and physical activity and performed an overnight admission to control acute glycemia, to limit the impact of these variables on insulin sensitivity and vascular measures. Third, we used the gold-standard hyperinsulinemic euglycemic technique to directly measure whole-body insulin sensitivity, DXA to measure body composition and aortic phase contrast MRI to assess central aortic health, in addition to more traditional measures of vascular health including brachial distensibility and cIMT. Moreover, our participant adherence with metformin and visit attendance was excellent. Limitations of this study include the fact that our results may not be applicable to pre-pubertal youth, and that treatment duration was limited to 3 months. In addition, our institution obtained a new MRI scanner during the study, and during the replacement process, MRI’s were unavailable. Thus, our MRI sample size was smaller than our other outcomes, a potential explanation for the lack of significant response to metformin in the descending aortic segments. In addition, because our control group was a convenience sample of youth undergoing the identical MRI protocol for another study, we lack the other study outcomes in these participants. Hypoglycemia and marked hyperglycemia were avoided throughout all assessments, but we lacked a measure such as continuous glucose monitoring to repeatedly monitor blood sugars during vascular assessments. Finally, not all girls were studied in the traditionally-defined follicular phase because adolescents often have anovulatory, irregular periods in the first 2 years following menarche. However, because such girls sit in a pseudofollicular phase, most of our female participants were in a follicular or pseudofollicular phase.
In conclusion, youth with T1D have reduced insulin sensitivity, aortic stiffness, reduced central aortic distensibility, elevated wall shear-stress, increased cIMT and compared to similarly-age normoglycemic peers. Metformin therapy for 3 months improved each of these abnormalities, in addition to improving BMI and fat mass. Further, the impact of metformin on insulin sensitivity was not limited to overweight youth with T1D. Insulin resistance is an important contributor to CVD disease in T1D, and metformin may hold promise in reducing CVD risk. Future directions include evaluating the impact of metformin on renal, cardiac, mitochondrial and exercise function as additional markers of CVD, as well as longer-term trials assessing CVD outcomes.
Supplementary Material
Clinical Perspective.
1). What is new?
We detected early signs of cardiovascular disease with MRI in adolescents with type 1 diabetes (T1D) compared to controls.
Insulin resistance is increasingly being recognized as a contributor to the excess risk of cardiovascular disease (CVD) in T1D.
We found that 3 months of metformin therapy improved insulin sensitivity, assessed by a gold-standard hyperinsulinemic euglycemic clamp, in both normal-weight and obese adolescents with T1D.
Moreover, metformin improved carotid intima medial thickness and aortic wall shear stress and stiffness.
2). What are the clinical implications?
Glycemic control is most difficult during adolescence.
Adolescents with type 1 diabetes (T1D) are also more insulin resistant than their peers, increasing their risk for cardiovascular disease (CVD).
Insulin resistance is further worsened by the rising obesity prevalence in T1D, creating a need for adjunctive therapies in T1D to lower CVD risk.
We demonstrated that metformin, a safe oral medication, can improve insulin resistance, BMI and fat mass in T1D adolescents.
We were also able to detect improvements in vascular health after 3 months of metformin.
These effects of metformin suggest potential CVD risk protection, arguing for future, longer-term trials.
Acknowledgements
We would like to thank the participants and their families, CTRC nurses, CTRC pharmacy, Belmar Pharmaceuticals for compounding the metformin and identical placebo and Nova Biomedical for supplying Stat Strip glucometer strips.
Funding
We would like to thank the following funding sources: NIH/National Institute of Diabetes and Digestive and Kidney Diseases T32 DK063687, NIH/National Institute of Diabetes and Digestive and Kidney Diseases K23 DK116720; NIH Building Interdisciplinary Research Careers in Women’s Health K12 5K12HD057022; American Diabetes Association 7-11-CD-08; Juvenile Diabetes Research Foundation Award #11-2010-343; NIH/National Institute of Diabetes and Digestive and Kidney Diseases K23 DK107871; NIH/National Center for Advancing Translational Sciences, Colorado Clinical and Translational Sciences Institute UL1 TR001082.
Footnotes
Disclosures
None
Clinical Trial Registration
References:
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, Imperatore G, Linder B, Marcovina S, Pettitt DJ, Pihoker C, Saydah S, Wagenknecht L. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, Kelly AS, Nadeau KJ, Martyn-Nemeth P, Osganian SK, Quinn L, Shah AS, Urbina E. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130:1532–1558. [DOI] [PubMed] [Google Scholar]
- 3.Marcovecchio ML, Chiesa ST, Bond S, Daneman D, Dawson S, Donaghue KC, Jones TW, Mahmud FH, Marshall SM, Neil HAW, Dalton RN, Deanfield J, Dunger DB. ACE Inhibitors and Statins in Adolescents with Type 1 Diabetes. N Engl J Med. 2017;377:1733–1745. [DOI] [PubMed] [Google Scholar]
- 4.Nadeau KJ, Regensteiner JG, Bauer TA, Brown MS, Dorosz JL, Hull A, Zeitler P, Draznin B, Reusch JE. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cree-Green M, Newcomer BR, Brown MS, Baumgartner AD, Bergman B, Drew B, Regensteiner JG, Pyle L, Reusch JE, Nadeau KJ. Delayed skeletal muscle mitochondrial ADP recovery in youth with type 1 diabetes relates to muscle insulin resistance. Diabetes. 2015;64:383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schauer IE, Snell-Bergeon JK, Bergman BC, Maahs DM, Kretowski A, Eckel RH, Rewers M. Insulin resistance, defective insulin-mediated fatty acid suppression, and coronary artery calcification in subjects with and without type 1 diabetes: The CACTI study. Diabetes. 2011;60:306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimaki T, Ronnemaa T, Viikari J, Raitakari OT. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–1755. [DOI] [PubMed] [Google Scholar]
- 8.Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Regensteiner JG, Reusch JE, Nadeau KJ. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: A RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. J Diabetes Complications. 2016;30:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cree-Green M, Maahs DM, Ferland A, Hokanson JE, Wang H, Pyle L, Kinney GL, King M, Eckel RH, Nadeau KJ. Lipoprotein subfraction cholesterol distribution is more atherogenic in insulin resistant adolescents with type 1 diabetes. Pediatr Diabetes. 2016;17:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maahs DM, Hokanson JE, Wang H, Kinney GL, Snell-Bergeon JK, East A, Bergman BC, Schauer IE, Rewers M, Eckel RH. Lipoprotein subfraction cholesterol distribution is proatherogenic in women with type 1 diabetes and insulin resistance. Diabetes. 2010;59:1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Specht BJ, Wadwa RP, Snell-Bergeon JK, Nadeau KJ, Bishop FK, Maahs DM. Estimated insulin sensitivity and cardiovascular disease risk factors in adolescents with and without type 1 diabetes. J Pediatr. 2013;162:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornstad P, Maahs DM, Duca LM, Pyle L, Rewers M, Johnson RJ, Snell-Bergeon JK. Estimated insulin sensitivity predicts incident micro- and macrovascular complications in adults with type 1 diabetes over 6 years: the coronary artery calcification in type 1 diabetes study. J Diabetes Complications. 2016;30:586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–1379. [DOI] [PubMed] [Google Scholar]
- 14.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280:140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511–1522. [DOI] [PubMed] [Google Scholar]
- 16.Townsend RR. Arterial Stiffness: Recommendations and Standardization. Pulse (Basel). 2017;4:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C, Schwartz RF, Atkinson M, Shuster JJ, Pierce GL, Silverstein JH. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004;27:2911–2917. [DOI] [PubMed] [Google Scholar]
- 18.Llaurado G, Ceperuelo-Mallafre V, Vilardell C, Simo R, Freixenet N, Vendrell J, Gonzalez-Clemente JM. Arterial stiffness is increased in patients with type 1 diabetes without cardiovascular disease: a potential role of low-grade inflammation. Diabetes Care. 2012;35:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romney JS, Lewanczuk RZ. Vascular compliance is reduced in the early stages of type 1 diabetes. Diabetes Care. 2001;24:2102–2106. [DOI] [PubMed] [Google Scholar]
- 20.Quail MA, Short R, Pandya B, Steeden JA, Khushnood A, Taylor AM, Segers P, Muthurangu V. Abnormal Wave Reflections and Left Ventricular Hypertrophy Late After Coarctation of the Aorta Repair. Hypertension. 2017;69:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wentland AL, Grist TM, Wieben O. Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc Diagn Ther. 2014;4:193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 23.Libman IM, Miller KM, DiMeglio LA, Bethin KE, Katz ML, Shah A, Simmons JH, Haller MJ, Raman S, Tamborlane WV, Coffey JK, Saenz AM, Beck RW, Nadeau KJ. Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/Obese Adolescents With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2015;314:2241–2250. [DOI] [PubMed] [Google Scholar]
- 24.Nadeau KJ, Chow K, Alam S, Lindquist K, Campbell S, McFann K, Klingensmith G, Walravens P. Effects of low dose metformin in adolescents with type I diabetes mellitus: a randomized, double-blinded placebo-controlled study. Pediatr Diabetes. 2015;16:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel SS, Truong U, King M, Ferland A, Moreau KL, Dorosz J, Hokanson JE, Wang H, Kinney GL, Maahs DM, Eckel RH, Nadeau KJ, Cree-Green M. Obese adolescents with polycystic ovarian syndrome have elevated cardiovascular disease risk markers. Vasc Med. 2017;22:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urbina EM, Wadwa RP, Davis C, Snively BM, Dolan LM, Daniels SR, Hamman RF, Dabelea D. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156:731–737. [DOI] [PubMed] [Google Scholar]
- 27.Schafer M, Ivy DD, Abman SH, Barker AJ, Browne LP, Fonseca B, Kheyfets V, Hunter KS, Truong U. Apparent Aortic Stiffness in Children With Pulmonary Arterial Hypertension: Existence of Vascular Interdependency? Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan CL, Pyle L, Morehead R, Baumgartner A, Cree-Green M, Nadeau KJ. The role of glycemia in insulin resistance in youth with type 1 and type 2 diabetes. Pediatr Diabetes. 2017;18:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadeau KJ, Zeitler PS, Bauer TA, Brown MS, Dorosz JL, Draznin B, Reusch JEB, Regensteiner JG. Insulin Resistance in Adolescents with Type 2 Diabetes Is Associated with Impaired Exercise Capacity. J Clin Endocrinol Metab. 2009;94:3687–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarnblad S, Kroon M, Aman J. Metformin as additional therapy in adolescents with poorly controlled type 1 diabetes: randomised placebo-controlled trial with aspects on insulin sensitivity. Eur J Endocrinol. 2003;149:323–329. [DOI] [PubMed] [Google Scholar]
- 31.Libby P, Nathan DM, Abraham K, Brunzell JD, Fradkin JE, Haffner SM, Hsueh W, Rewers M, Roberts BT, Savage PJ, Skarlatos S, Wassef M, Rabadan-Diehl C. Report of the National Heart, Lung, and Blood Institute-National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation. 2005;111:3489–3493. [DOI] [PubMed] [Google Scholar]
- 32.Minges KE, Whittemore R, Weinzimer SA, Irwin ML, Redeker NS, Grey M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: The T1D Exchange Clinic Registry. Diabetes Res Clin Pract. 2017;126:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah AS, Wadwa RP, Dabelea D, Hamman RF, D’Agostino R Jr., Marcovina S, Daniels SR, Dolan LM, Fino NF, Urbina EM. Arterial stiffness in adolescents and young adults with and without type 1 diabetes: the SEARCH CVD study. Pediatr Diabetes. 2015;16:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington J, Pena AS, Gent R, Hirte C, Couper J. Aortic intima media thickness is an early marker of atherosclerosis in children with type 1 diabetes mellitus. J Pediatr. 2010;156:237–241. [DOI] [PubMed] [Google Scholar]
- 35.Urbina EM, Dabelea D, D’Agostino RB Jr., Shah AS, Dolan LM, Hamman RF, Daniels SR, Marcovina S, Wadwa RP. Effect of type 1 diabetes on carotid structure and function in adolescents and young adults: the SEARCH CVD study. Diabetes Care. 2013;36:2597–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunvand L, Fugelseth D, Stensaeth KH, Dahl-Jorgensen K, Margeirsdottir HD. Early reduced myocardial diastolic function in children and adolescents with type 1 diabetes mellitus a population-based study. BMC Cardiovasc Disord. 2016;16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, Quinn M, Tamborlane WV, Woerner SE. Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36:2035–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arslanian S, Nixon PA, Becker D, Drash AL. Impact of physical fitness and glycemic control on in vivo insulin action in adolescents with IDDM. Diabetes Care. 1990;13:9–15. [DOI] [PubMed] [Google Scholar]
- 39.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49:626–632. [DOI] [PubMed] [Google Scholar]
- 40.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samyn MM, Dholakia R, Wang H, Co-Vu J, Yan K, Widlansky ME, LaDisa JF, Simpson P, Alemzadeh R. Cardiovascular magnetic resonance imaging-based computational fluid dynamics/fluid-structure interaction pilot study to detect early vascular changes in pediatric patients with type 1 diabetes. Pediatr Cardiol. 2015;36:851–861. [DOI] [PubMed] [Google Scholar]
- 43.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karhus ML, Bronden A, Sonne DP, Vilsboll T, Knop FK. Evidence connecting old, new and neglected glucose-lowering drugs to bile acid-induced GLP-1 secretion: A review. Diabetes Obes Metab. 2017;19:1214–1222. [DOI] [PubMed] [Google Scholar]
- 45.Bauer PV, Duca FA. Targeting the gastrointestinal tract to treat type 2 diabetes. J Endocrinol. 2016;230:R95–R113. [DOI] [PubMed] [Google Scholar]
- 46.Nesti L, Natali A. Metformin effects on the heart and the cardiovascular system: A review of experimental and clinical data. Nutr Metab Cardiovasc Dis. 2017;27:657–669. [DOI] [PubMed] [Google Scholar]
- 47.Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60:1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al Khalifah RA, Alnhdi A, Alghar H, Alanazi M, Florez ID. The effect of adding metformin to insulin therapy for type 1 diabetes mellitus children: A systematic review and meta-analysis. Pediatr Diabetes. 2017;18:664–673. [DOI] [PubMed] [Google Scholar]
- 49.Petrie JR, Chaturvedi N, Ford I, Brouwers M, Greenlaw N, Tillin T, Hramiak I, Hughes AD, Jenkins AJ, Klein BEK, Klein R, Ooi TC, Rossing P, Stehouwer CDA, Sattar N, Colhoun HM. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson JJA, Couper JJ, Giles LC, Leggett CE, Gent R, Coppin B, Pena AS. Effect of Metformin on Vascular Function in Children With Type 1 Diabetes: A 12-Month Randomized Controlled Trial. J Clin Endocrinol Metab. 2017;102:4448–4456. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.