Abstract
Background
Desmoplastic small round cell tumor (DSRCT) is a rare, aggressive sarcoma. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) may improve survival.
Methods
A retrospective review of the anesthetic management and postoperative pain control strategies after CRS/HIPEC for DSRCT from 2013 to 2017 was performed.
Results
Ten CRS/HIPEC procedures were performed on 9 patients with DSRCT with a median age 19 years (10–24), 6 Caucasian, 7 male. Median operative and anesthesia duration were 551 minutes (510–725) and 621 minutes (480–820), respectively. Postoperative mechanical ventilation was necessary in 5 patients for a median duration of 1 days (0–2). Median intraoperative intravenous fluid administration was 13 ml/kg/hr (6.3–24.4) and colloid administration was 12 ml/kg (0.0–53.0). Median blood loss was 15 ml/kg (6.3–77.2). Nine patients received intraoperative transfusion with a median red blood cell transfusion volume of 14 ml/kg (10.1–58.5). Median intraoperative urine output was 2.0 ml/kg/hr (0.09–8.40) and half of the patients received intraoperative diuretics. Cisplatin was utilized during HIPEC for 8 surgeries. Acute kidney injury was observed in 2 patients; 1 required short-term dialysis. Epidural infusions were utilized in 8 cases for a median of 4 days (3–5). Postoperative intravenous opioid use (morphine equivalent) was 0.67 mg/kg/day (0.1–9.2) and administered for a median of 11 days (2–35).
Conclusion
Cytoreduction and HIPEC for DSRCT are associated with significant perioperative fluid requirements and potentially challenging pain management. Renal protective strategies should be considered for reduction of cisplatin-associated nephrotoxicity. Further investigation for a more effective, less systemically toxic HIPEC agent is warranted.
Keywords: Cytoreduction, HIPEC, Desmoplastic Small Round Cell Tumor, Anesthesia, Pain Management
BACKGROUND
Desmoplastic small round cell tumor (DSRCT) is a mesenchymal soft tissue sarcoma that spreads on peritoneal surfaces, affecting primarily children, adolescents, and young adults. The most common primary tumor sites include the right diaphragm, omentum and pelvis, with the liver, lungs, mediastinum, and pleura being the most common sites of metastasis.[1] First characterized in 1989 by Gerald and Rosai, DSRCT is a rare and highly aggressive sarcoma, and 60–70% of patients survive less than 3 years despite multi-modality treatment protocols.[2, 3]
Current treatment of DSRCT consists of multi-agent systemic chemotherapy, including cyclophosphamide, doxorubicin, and vincristine, followed by alternating ifosfamide and etoposide, with subsequent debulking surgery followed by adjuvant chemotherapy and whole abdomen radiation therapy.[4] The prognosis remains poor, however, despite such an aggressive, complex multimodality treatment approach. Recurrence after resection and disease progression contribute to early treatment failure and the overall survival rate remains 15–30% at 5 years.[5]
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have emerged as treatment options for the management of DSRCT.[6] CRS removes all visible tumor within the abdominal cavity and HIPEC is administered to eradicate any residual microscopic disease. Heated chemotherapy is administered as a peritoneal lavage which allows for increased regional delivery of chemotherapy with minimal systemic absorption or toxicity.[7] Hyperthermia denatures proteins, inhibits DNA repair processes, and activates heat shock proteins which produces a cytotoxic environment and elicits an immune response against tumor cells.[8]
The use of HIPEC in children has been proven to be safe and for DSRCT is associated with a disease free survival benefit.[6] CRS/HIPEC has also been investigated for other malignancies including rhabdomyosarcoma, Sertoli-Leydig tumor, Wilms tumor, colon adenocarcinoma, liposarcoma, and mesothelioma.[9] Despite this growing experience, the perioperative management of adolescents and young adults who undergo CRS/HIPEC has not been extensively described. Therefore, the aims of this study were to retrospectively review the anesthetic, fluid and pain management characteristics in children and young adult patients who undergo CRS/HIPEC for DSRCT.
METHODS
Patients
A retrospective review of patients who underwent CRS/HIPEC for DSRCT from 2013 to 2017 was performed. Patients received neoadjuvant and adjuvant therapy at St. Jude Children’s Research Hospital (SJCRH) and underwent CRS/HIPEC at Methodist University Hospital (N=5) or SJCRH (N=5). Institutional review board approval was obtained from each institution.
Description of CRS/HIPEC Technique
After induction of anesthesia, patients were placed in lithotomy position and urinary catheters and esophageal temperature probes were placed. A midline laparotomy was performed and the abdomen was inspected to determine the extent and distribution of disease and to calculate the peritoneal cancer index (PCI) score.[10] The operation consisted of attempted resection of all visible disease with a goal of performing complete cytoreduction (CCR 0/1).[11]
Upon completion of CRS, in-flow and out-flow catheters were placed within the pelvis and upper abdomen, respectively, and were connected to a Belmont Perfusion Pump (Billerica, MA). The abdomen was filled with saline and was temporarily closed with a running nylon suture. The saline solution was circulated and heated to a target temperature of 42°C. Once target temperature was achieved, the chemotherapeutic agent was added to the perfusate and circulated at a flow rate of one liter per minute. The abdomen was gently agitated during HIPEC to improve the distribution of chemotherapy throughout the abdominal cavity. Systemic hyperthermia was prevented with cold packs, cooling blankets, and forced cool ambient air, as needed. At the completion of HIPEC, the catheters were removed, gastrointestinal continuity was restored and drains were placed. All patients were admitted to the intensive care unit (ICU) for initial care after CRS/HIPEC.
Anesthesia and Pain Management Details
General anesthesia was induced with propofol, opioid and a non-depolarizing muscle relaxant and maintained with an inhalational agent and intermittent opioid doses. Standard monitoring included pulse oximetry, electrocardiogram, arterial blood pressure, temperature and urine output. Intravenous fluids were administered using crystalloids (ringers lactate and normal saline) and colloid (5% albumin). Blood transfusion and postoperative extubation were performed at the discretion of the anesthesiologist.
Anesthesia-related data collection included the duration of anesthesia, fluid management, blood loss, urine output, diuretic usage, and administration of blood products. Intraoperative hemodynamics and arterial blood gas data were collected at 4 time points: T1-beginning of cytoreduction, T2-end of cytoreduction, T3-start of HIPEC, T4-end of CRS/HIPEC. Fluids administered are described in total volume (milliliters-ml), volume per kilogram (ml/kg) and volume per kilogram per hour (ml/kg/hr).
Epidural analgesia was performed at the discretion of the anesthesiologist and consisted of either bupivacaine 0.1–0.2% or ropivacaine 0.125–0.2% titrated at 5–12 ml/hr. A patient-controlled analgesia (PCA) pump was utilized after epidural catheter removal for additional pain control, as needed. Intravenous opioid administration was analyzed and a conversion factor of 5:1 (5 mg morphine equals 1 mg of hydromorphone) was used to calculate the morphine equivalent dose (MED) when hydromorphone was used for pain relief. For consistency in opioid administration reporting, intravenous opioid dosages are reported as morphine equivalent doses (mg/kg/day).
Morbidity and Statistical Analysis
Complications were graded according to the Clavien-Dindo classification schema.[12] Minor complications included Clavien-Dindo 1–2 morbidity while major complications included Clavien-Dindo 3–5 morbidity. Renal toxicity was defined per standard criteria as acute kidney injury or increase in creatinine above baseline or when hemodialysis was indicated and by the KDIGO criteria.[13, 14] KDIGO criteria for acute kidney injury in the pediatric population are described as Stage I-increase in serum creatinine 0.3 mg/dL in 48 hours or increased150–200% over 7 days, Stage II-increase ≥ 200–300%, Stage III-increase of serum creatinine ≥ 4 mg/dL, increase ≥ 300% or needing dialysis. Categorical variables were summarized using percentages while continuous variables were summarized using the median with range. All statistical analysis was performed using SPSS software, version 24 (IBM Corporation, Armonk, NY).
RESULTS
Patient Characteristics
Nine patients underwent 10 CRS/HIPEC procedures between 1/2013 and 12/2017, including seven males and two females. All patients had an ASA physical status score of 3 and the median body mass index was 21 (range, 16.7–29.2). At the time of initial diagnosis and CRS/HIPEC, 2 patients were under the age of 12 years and 7 patients were 13–24 years.
Operative Characteristics
The length of anesthesia was 621 minutes (range, 480–820) and the operative length was 551 minutes (range 410–725) (Table 1). The median PCI score was 16 (range, 5–20) and complete cytoreduction was performed in 9 CRS/HIPEC procedures. Multivisceral resection of 4 or more organs was performed in 6 patients with the omentum, rectum, pelvic peritoneum and right diaphragm peritoneum being the most frequently resected organs. HIPEC was performed using cisplatin (100 mg/m2 for 60 minutes, N=8), mitomcyin C (40 mg for 90 minutes, N=1) and melphalan (50 mg/m2 for 90 minutes, N=1).
Table 1.
Operative and Perioperative Characteristics
| Variable | All |
|---|---|
| N=10 | |
| Anesthesia Length (minutes) | 621 (480–820) |
| Operative Length (minutes) | 551 (410–725) |
| Intraoperative Fluid Management | |
| Intravenous Fluid Administered (ml) | 6450 (4000–16500) |
| Intravenous Fluid Administered (ml/kg) | 122 (53.69–288.06) |
| Intravenous Fluid Administered (ml/kg/hr) | 13 (6.3–24.2) |
| Colloid Administered (g) | 50 (25.0–150.0) |
| Colloid Administered (ml) | 1000 (500–3000) |
| Colloid Administered (ml/kg) | 12 (0.0–53.0) |
| Urine Output (ml) | 1200 (360–4000) |
| Urine Output (ml/kg) | 3 (0.84–73.53) |
| Urine Output (ml/kg/hr) | 2 (0.09–8.40) |
| Red Blood Cell Transfusion (ml) | 800 (580–3200) |
| Red Blood Cell Transfusion (ml/kg) | 14 (10.13–58.52) |
| Fresh Frozen Plasma Transfusion (ml) | 570 (500–630) |
| Fresh Frozen Plasma Transfusion (ml/kg) | 10 (9.19–11.33) |
| Blood Loss (ml) | 900 (300–4300) |
| Blood Loss (ml/kg) | 15 (6.33–77.2) |
| Postoperative Fluid Management | |
| Median (ml/kg/day) | 33 (6.62–384.60) |
| Duration of Fluid Management (days) | 8 (3–35) |
| Baseline Weight (kg) | 58.4 (40.0–89.8) |
| Day 1 (kg) | 65.5 (45.4–89.8) |
| Final (kg) | 57.8 (38.3–84.0) |
| Difference (kg) | −2.0 (−7.0-+5.0) |
| Postoperative Mechanical Ventilation | 5 (50) |
| Day of Extubation | 0.5 (0–2) |
| Re-intubation | 1 (10) |
| Day of Nasogastric Tube Removal | 3.0 (1–6) |
| Nasogastric Tube Re-Insertion | 3 (30) |
| Day of Ambulation | 2 (1–3) |
| Day of Foley Catheter Removal | 5 (3–8) |
Results presented as frequency (percent) or median (range).
Hemodynamically, heart rate increased over each measured time point from the start of cytoreduction (T1) to the start of HIPEC (T3) while systolic blood pressure steadily decreased from T1-T3 (Figure 1A-B). Temperature started out low and reached the highest values during the HIPEC portion (T3) of the procedure (Figure 1C). Blood gas measurements were characterized by a steady decrease in the pH (Figure 1D) and a worsening of the base deficit (Figure 1E) with an increase in the serum lactate (Figure 1F) over each measured time point. A detailed description of hemodynamic and blood gas measurements is presented in Supplemental Table 1. Five patients required postoperative mechanical ventilation but all were extubated the following day. The median ICU length of stay was 3 days (range, 1–25) and hospital length of stay was 9 days (range, 8–38). Details of the hemodynamic characteristics are presented in Table 2.
Figure 1A-F:
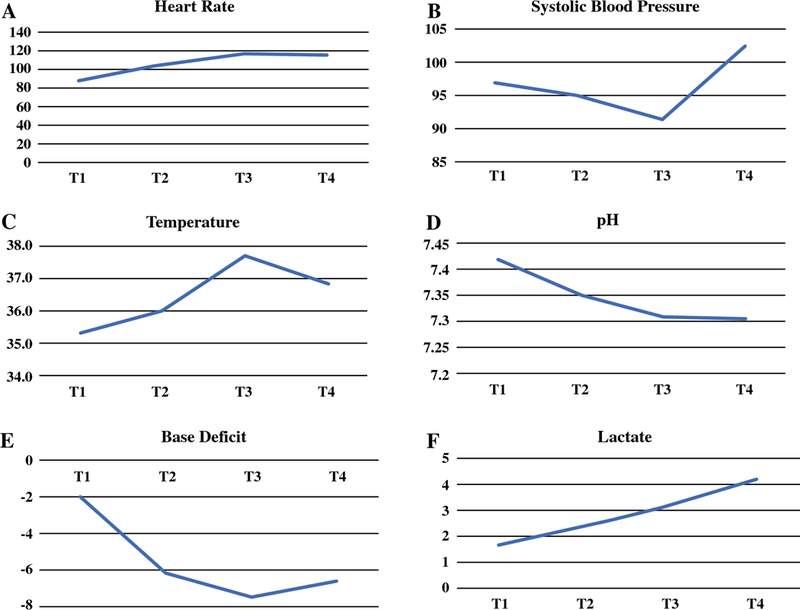
Hemodynamic and blood gas measurements during the beginning of cytoreduction (T1), end of cytoreduction (T2), start of HIPEC (T3), and the end of cytoreduction/HIPEC (T4). Results reported as median value for each time point.
Table 2.
Intraoperative Fluid Management
| Patient | Wt | Anes | Op | IVF | COL | RBC | UOP | EBL |
|---|---|---|---|---|---|---|---|---|
| (kg) | (min) | (min) | (mL/kg/hr) | (mL/kg) | (mL/kg) | (mL/kg/hr) | (mL/kg) | |
| 1 | 54.4 | 551 | 525 | 14.9 | 27.6 | 58.8 | 8.40 | 27.6 |
| 2 | 89.8 | 665 | 608 | 13.2 | 11.1 | 17.8 | 1.31 | 10.0 |
| 3 | 56.5 | 680 | 582 | 7.7 | 53.1 | 14.2 | 6.11 | 9.7 |
| 4α | 74.5 | 575 | 513 | 6.3 | 20.1 | 10.7 | 1.50 | 13.4 |
| 5 | 79.0 | 494 | 420 | 10.9 | 19.0 | 10.1 | - | 6.3 |
| 6β | 44.5 | 480 | 410 | 13.2 | 11.2 | 13.8 | 0.34 | 6.7 |
| 7 | 55.7 | 820 | 707 | 24.4 | 17.9 | 45.5 | 0.22 | 77.2 |
| 8 | 40.0 | 639 | 543 | 14.9 | 20.0 | 14.5 | 0.09 | 18.8 |
| 9* | 76.0 | 697 | 559 | 12.6 | 13.2 | 11.2 | 0.16 | 11.8 |
| 10 | 60.3 | 603 | 536 | 15.1 | 12.4 | 10.25 | 0.19 | 16.6 |
-Melphalan administered during HIPEC,
-Mitomycin C administered during HIPEC
-Repeat HIPEC
Abbreviations: Wt-Weight (kg); Anes-Anesthesia Length (minutes); Op-Operative Length (minutes); IVF-Intravenous Fluid Infusion Rate (mL/kg/hr); COL-Colloid Infusion Rate (mL/kg); RBC-Red Blood Cell Infusion Rate (mL/kg); UOP-Urine Output (mL/kg/hr); EBL- Estimated Blood Loss (mL/kg)
Intraoperative and Perioperative Fluid Management
Details of the intraoperative fluid management are presented in Table 2. The amount of intraoperative IV fluids administered was 122 ml/kg (range, 54.7–288.1) which equated to 13 ml/kg/hr (range, 6.3–24.4), intraoperative colloid was 12 ml/kg (range, 0–53.0) intraoperative red blood cells transfused was 14 ml/kg (range, 10.1–58.5) and intraoperative fresh frozen plasma transfused was 10 ml/kg (range, 9.2–11.3). The median intraoperative urine output (UOP) was 2 ml/kg/hr (range, 0.09–8.40) and blood loss was 15 ml/kg (range, 6.3–77.2).
Postoperative IV fluids were administered for a median of 8 days (range, 3–35) with a median of 33 ml/kg/day administered (range 23.4–65.6). Two patients received fluid therapy prior to CRS/HIPEC and 4 patients received a diuretic during the intraoperative or postoperative time periods.
Morbidity
Minor postoperative morbidities occurred in 6 patients, the most frequent being abdominal wound infection (N=3). Major morbidities occurred in 4 patients: one patient developed a pneumothorax requiring thoracostomy tube placement, two developed intraabdominal abscesses requiring percutaneous drainage and two developed acute kidney injury (AKI) from cisplatin-nephrotoxicity, one of whom required re-intubation and short-term hemodialysis. There were no operative or 30-day mortalities.
Alterations in renal function, as reflected by an increase in the creatinine level at >50% of preoperative baseline, were noted in 5 of 10 cases. All but one patient experienced a transient rise in serum creatinine. The impact on postoperative serum creatinine levels (mg/dL) was most pronounced in those who received cisplatin (N=8) as opposed to those who received melphalan (N=1) or mitomycin C (N=1) (Table 3).
Table 3.
Perioperative Fluid Management
| Patient | Diuretics | Postoperative IVF (ml/kg/day) | Serum Creatinine (mg/dL) | KDI GO |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lasix | Mannitol | Preoperative Hydration |
Min | Max | Median | Days | Pre- op |
Post- op Max |
% Increase |
||
| 1 | Y | Y | - | 6.62 | 44.98 | 33.75 | 9 | 0.70 | 0.80 | 14.3 | - |
| 2 | - | - | - | 19.49 | 42.71 | 27.77 | 6 | 0.70 | 1.30 | 42 | I |
| 3 | - | - | - | 9.79 | 50.29 | 30.70 | 6 | 0.40 | 1.10 | 175 | I |
| 4α | - | - | - | 13.13 | 47.76 | 32.79 | 5 | 0.40 | 0.70 | 75 | I |
| 5 | - | - | - | 12.97 | 33.59 | 23.41 | 3 | 1.10 | 1.10 | - | - |
| 6β | - | - | - | 17.96 | 157.53 | 28.61 | 8 | 0.33 | 0.38 | 15.2 | - |
| 7 | - | - | - | 34.78 | 384.6 | 65.64 | 14 | 0.54 | 1.02 | 88.9 | I |
| 8 | Y | - | - | 58.53 | 218.80 | 59.71 | 35 | 0.27 | 5.76 | 2033.3 | III |
| 9* | Y | Y | 1.5 x MIVF | 32.91 | 166.79 | 51.07 | 20 | 1.61 | 1.92 | 19.3 | I |
| 10 | Y | Y | 2 x MIVF | 34.48 | 197.73 | 43.55 | 30 | 0.51 | 5.05 | 890.2 | III |
-Melphalan administered during HIPEC,
-Mitomycin C administered during HIPEC,
-Repeat HIPEC
Abbreviations: IVF-intravenous fluid; Min-minimum; Max-maximum; Pre-op-preoperative serum creatinine; Post-op Max-postoperative maximum serum creatinine; MIVF-maintenance intravenous fluid, KDIGO-acute kidney injury criteria in pediatric population
Postoperative Pain Management
Postoperative pain management consisted of epidural analgesia, intravenous opioid agonists and non-opioid analgesics. Epidural analgesia was utilized in 8 surgeries for a median of 4 days (range, 3–5). No epidural analgesia related complications were observed. Intravenous opioids were administered to all patients for a median of 11 days (range, 2–35) with a median morphine equivalent dose of 0.67 mg/kg/day (range, 0.06–9.2). A detailed description of the postoperative pain management parameters are presented in Table 4.
Table 4.
Postoperative Pain Management
| Patient | Postoperative Epidural | Postoperative Opioids (MED; mg/kg/day) | ||||||
|---|---|---|---|---|---|---|---|---|
| Agent | Fentanyl | Rate | Days | Min | Max | Median | Days | |
| 1 | Ropivicaine 0.2% | 5 | 7 | 3 | 0.27 | 0.32 | 0.30 | 2 |
| 2 | Ropivicaine 0.2% | 5 | 5 | 3 | 0.01 | 0.75 | 0.30 | 6 |
| 3 | - | - | - | - | 0.04 | 1.03 | 0.13 | 12 |
| 4 | - | - | - | - | 0.07 | 1.40 | 0.73 | 9 |
| 5 | Bupivicaine 0.2% | - | 6 | 4 | 0.0 | 0.35 | 0.06 | 7 |
| 6 | Ropivicaine 0.125% | 2 | 8 | 4 | 0.45 | 1.18 | 0.73 | 8 |
| 7 | Ropivicaine 0.125% | 2 | 10 | 4 | 0.09 | 1.38 | 0.61 | 14 |
| 8 | Ropivicaine 0.125% | 2 | 8 | 3 | 0.56 | 6.38 | 2.46 | 35 |
| 9* | Bupivicaine 0.1% | - | 10 | 5 | 1.39 | 19.67 | 9.64 | 20 |
| 10 | Ropivicaine 0.125% | 2 | 12 | 4 | 0.04 | 6.22 | 1.47 | 33 |
-Repeat HIPEC
Abbreviations: Fentanyl-mcg/hr; Postoperative Epidural Rate-mL/hr; MED-morphine equivalent dose (7 mg morphine equivalent to 1 mg hydromorphone); Min-minimum; Max-maximum
DISCUSSION
The perioperative management of patients undergoing CRS/HIPEC remains a challenge for the anesthesiologists, surgeons and intensivists alike. While a significant body of literature exist for adults, only one study regarding the anesthetic management of pediatric patients undergoing CRS/HIPEC has been reported.[15] Owusu-Agyemang et al reported on 10 patients who underwent HIPEC in a phase 1 trial of escalating doses of cisplatin for sarcomatosis. The anesthetic management, intraoperative fluid and blood requirements and postoperative renal function were described. Aggressive intraoperative fluid administration was required to maintain satisfactory urine output and all experienced a transient rise in postoperative serum creatinine. In the current study, despite significant intraoperative and perioperative fluid administration, transient rise in creatinine occurred in all but one with significant acute kidney injury occurring in two patients. Moreover, despite epidural analgesia, a high consumption of opioid therapy was necessary for a prolonged period to maintain adequate pain control.
Perioperative fluid management and volume therapy are important aspects of anesthetic management in maintaining hemodynamic stability during CRS/HIPEC.[16] A restrictive fluid approach is detrimental to outcome and is associated with increased risk of complications, while liberal fluid administration risks fluid overload, tissue edema and pulmonary complications.[17– 19] In adults, fluid administration at an average rate of 9–12 ml/kg/h is recommended to maintain satisfactory urine output.[15, 16] In children and young adults, however, the most appropriate rate of fluid administration remains undetermined. Patients received approximately 133 ml/kg of crystalloids intraoperatively in this series, which equated to 13 ml/kg/hr. Blood loss was significant (15 ml/kg), however, and intraoperative urine output was adequate, approximately 2 ml/kg/hr. Despite being administered what appears to be adequate intraoperative volume replacement in adults, in a younger aged population, however, it is possible that more vigorous fluid resuscitation may be necessary to avoid oliguria and potentiate cisplatin-related nephrotoxicity. Owusu-Agyemang et al observed that intraoperative fluid administration at a rate of 6–15 ml/kg/hr was necessary to maintain urine output at 3 ml/kg/h.[15]
The incidence of grade 3–4 nephrotoxicity after CRS/HIPEC in adults is 6% and as high as 25% in pediatric and young adults.[20, 21] In a report of 50 patients who underwent CRS/HIPEC, Hayes-Jordan et al reported two patients experienced grade III AKI while three patients developed grade IV acute renal failure. [9] Thong et al, in an adult study of 111 patients undergoing 113 CRS/HIPEC procedures, noted that 6 patients experienced renal impairment with one experiencing AKI and 2 patients requiring hemodialysis.[22] Postoperative serum creatinine rose above baseline in all but one patient in the current series with two patients developing severe AKI. The two patients who developed severe AKI (patient 8 & 10) also were significantly under-resuscitated based on intraoperative urine output (Table 2). One required short-term hemodialysis and both eventually recovered renal function. While cisplatin is known to be nephrotoxic, inadequate fluid resuscitation with oliguria may potentiate the renal injury observed after HIPEC.
Cisplatin causes acute tubular necrosis within the proximal collecting system and a reduced glomerular filtration rate.[23] The nephrotoxic effects of cisplatin can be mitigated with preoperative hydration, osmotic diuresis and administration of renal protective agents such as theophylline.[24, 25] In a study of 54 patients who underwent 58 HIPEC procedures with cisplatin, Green et al. noted a reduction in the incidence of grade III/IV nephrotoxicity from 25% to 0% after implementation of a renal protective protocol.[21] In that protocol, patients received preoperative hydration at 150% of the standard maintenance fluid rate with simultaneous administration of sodium thiosulfate during HIPEC. Sodium thiosulfate was administered as a continuous infusion for 24 hours after HIPEC and continued for 48 hours after HIPEC. In the current series, adoption of a formalized renal protective strategy may have minimized the degree of nephrotoxicity observed after HIPEC. While these efforts are necessary and helpful to minimize cisplatin-associated nephrotoxicity, development of a more effective, less toxic HIPEC agent for DSRCT warrants further investigation.
Postoperative pain management in children and young adults who undergo CRS/HIPEC has not been previously described. Eight patients received epidural analgesia in this study and there were no epidural related complications necessitating early removal. Thoracic epidural analgesia is an excellent option for postoperative pain management and is commonly utilized in many HIPEC centers.[26, 27] Epidural analgesia use is associated with reduced opioid requirements, decreased need for postoperative mechanical ventilation, reduced ICU length of stay and can be safely performed with minimal morbidity.[28–31] However, despite epidural analgesia use in this series, intravenous opioid use after CRS/HIPEC was higher and for longer duration than anticipated. The median duration of intravenous opioid use was 11 days with a median morphine equivalent dose of 0.67 mg/kg/day. This opioid dose is greater than previously reported after pediatric abdominal surgery and contrasts with the data reported from adult studies in which epidural analgesia is associated with opioid use for a shorter duration.[27]
There are several limitations to this study. The results reflect a single institution experience for a rare pathology with a limited number of patients. Although the surgeons were the same, patients were treated at two locations by two separate anesthesia provider practice groups. Inconsistent pre-hydration, intermittent diuretic use and lack of a formalized renal protective protocol may have impacted cisplatin-associated nephrotoxicity. Lastly, lack of a standard perioperative pain management regimen may have contributed to significant opioid use.
CONCLUSION
CRS/HIPEC for DSRCT is associated with significant intravenous fluid requirements along with increased and prolonged opioid use. Adoption of a renal protective strategy may minimize cisplatin-associated nephrotoxicity. Further investigation for a more effective, less toxic HIPEC agent is warranted.
Supplementary Material
SYNOPSIS.
Cytoreduction and hyperthermic intraperitoneal chemotherapy for desmoplastic small round cell tumor are associated with significant perioperative fluid requirements and potentially challenging pain management. Renal protective strategies should be considered for reduction of cisplatin- associated nephrotoxicity.
ACKNOWLEDGEMENTS
The authors wish to thank Lynn Wynn, Amy Kimble and Dina Darby for care of the patients with DSRCT.
FUNDING SOURCES: This work was supported in part by the R25CA23944 grant from the National Cancer Institute.
This work was presented in part at the 13th Annual Regional Therapies Meeting, February 17–19, 2018, Jacksonville, FL.
Footnotes
DISCLOSURES: All authors declare no conflicts of interest.
REFERENCES
- 1.Hayes-Jordan A Cytoreductive Surgery Followed by Hyperthermic Intraperitoneal Chemotherapy in DSRCT: Progress and Pitfalls. Curr Oncol Rep 2015; 17: 38. [DOI] [PubMed] [Google Scholar]
- 2.Hayes-Jordan A, Green HL, Lin H et al. Complete cytoreduction and HIPEC improves survival in desmoplastic small round cell tumor. Ann Surg Oncol 2014; 21: 220–224. [DOI] [PubMed] [Google Scholar]
- 3.Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol 1989; 9: 177–183. [DOI] [PubMed] [Google Scholar]
- 4.Kushner BH, LaQuaglia MP, Wollner N et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 1996; 14: 1526–1531. [DOI] [PubMed] [Google Scholar]
- 5.Hayes-Jordan A, LaQuaglia MP, Modak S. Management of desmoplastic small round cell tumor. Semin Pediatr Surg 2016; 25: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayes-Jordan A, Green H, Fitzgerald N et al. Novel treatment for desmoplastic small round cell tumor: hyperthermic intraperitoneal perfusion. J Pediatr Surg 2010; 45: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 7.Jacquet P, Vidal-Jove J, Zhu B, Sugarbaker P. Peritoneal carcinomatosis from gastrointestinal malignancy: natural history and new prospects for management. Acta Chir Belg 1994; 94: 191–197. [PubMed] [Google Scholar]
- 8.Dedrick RL. Theoretical and experimental bases of intraperitoneal chemotherapy. Semin Oncol 1985; 12: 1–6. [PubMed] [Google Scholar]
- 9.Hayes-Jordan A, Green H, Lin H et al. Cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for children, adolescents, and young adults: the first 50 cases. Ann Surg Oncol 2015; 22: 1726–1732. [DOI] [PubMed] [Google Scholar]
- 10.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996; 82: 359–374. [DOI] [PubMed] [Google Scholar]
- 11.Glehen O, Kwiatkowski F, Sugarbaker PH et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004; 22: 3284–3292. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCI. Common Terminology Criteria for Adverse Events (CTCAE). 20009; 4. [Google Scholar]
- 14.Selewski DT, Cornell TT, Heung M et al. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med 2014; 40: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 15.Owusu-Agyemang P, Arunkumar R, Green H et al. Anesthetic management and renal function in pediatric patients undergoing cytoreductive surgery with continuous hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin. Ann Surg Oncol 2012; 19: 2652–2656. [DOI] [PubMed] [Google Scholar]
- 16.Raspe C, Flother L, Schneider R et al. Best practice for perioperative management of patients with cytoreductive surgery and HIPEC. Eur J Surg Oncol 2017; 43: 1013–1027. [DOI] [PubMed] [Google Scholar]
- 17.Colantonio L, Claroni C, Fabrizi L et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg 2015; 19: 722–729. [DOI] [PubMed] [Google Scholar]
- 18.Varadhan KK, Lobo DN. A meta-analysis of randomised controlled trials of intravenous fluid therapy in major elective open abdominal surgery: getting the balance right. Proc Nutr Soc 2010; 69: 488–498. [DOI] [PubMed] [Google Scholar]
- 19.Abraham-Nordling M, Hjern F, Pollack J et al. Randomized clinical trial of fluid restriction in colorectal surgery. Br J Surg 2012; 99: 186–191. [DOI] [PubMed] [Google Scholar]
- 20.Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FA. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol 2004; 85: 61–67. [DOI] [PubMed] [Google Scholar]
- 21.Green H, Lin H, Owusu-Agyemang P et al. Perioperative renal protective treatment avoids renal toxicity in pediatric and adult patients undergoig HIPEC with cisplatin. Journal of Pediatric Oncology 2014; 2: 10–16. [Google Scholar]
- 22.Thong SY, Chia CS, Ng O et al. A review of 111 anaesthetic patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Singapore Med J 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol 2003; 23: 460–464. [DOI] [PubMed] [Google Scholar]
- 24.Santoso JT, Lucci JA 3rd, Coleman RL et al. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: a randomized trial. Cancer Chemother Pharmacol 2003; 52: 13–18. [DOI] [PubMed] [Google Scholar]
- 25.Benoehr P, Krueth P, Bokemeyer C et al. Nephroprotection by theophylline in patients with cisplatin chemotherapy: a randomized, single-blinded, placebo-controlled trial. J Am Soc Nephrol 2005; 16: 452–458. [DOI] [PubMed] [Google Scholar]
- 26.Maciver AH, Al-Sukhni E, Esquivel J et al. Current Delivery of Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery (CS/HIPEC) and Perioperative Practices: An International Survey of High-Volume Surgeons. Ann Surg Oncol 2017; 24: 923–930. [DOI] [PubMed] [Google Scholar]
- 27.Osseis M, Weyrech J, Gayat E et al. Epidural analgesia combined with a comprehensive physiotherapy program after Cytoreductive Surgery and HIPEC is associated with enhanced post-operative recovery and reduces intensive care unit stay: A retrospective study of 124 patients. Eur J Surg Oncol 2016; 42: 1938–1943. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt C, Creutzenberg M, Piso P et al. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia 2008; 63: 389–395. [DOI] [PubMed] [Google Scholar]
- 29.Bell JC, Rylah BG, Chambers RW et al. Perioperative management of patients undergoing cytoreductive surgery combined with heated intraperitoneal chemotherapy for peritoneal surface malignancy: a multi-institutional experience. Ann Surg Oncol 2012; 19: 4244–4251. [DOI] [PubMed] [Google Scholar]
- 30.Owusu-Agyemang P, Soliz J, Hayes-Jordan A et al. Safety of epidural analgesia in the perioperative care of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2014; 21: 1487–1493. [DOI] [PubMed] [Google Scholar]
- 31.Piccioni F, Casiraghi C, Fumagalli L et al. Epidural analgesia for cytoreductive surgery with peritonectomy and heated intraperitoneal chemotherapy. Int J Surg 2015; 16: 99–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


