Summary
Gene regulation requires selective targeting of DNA regulatory enhancers over megabase (Mb) distances. Here, we show that Evf2, a cloud-forming Dlx5/6 ultraconserved enhancer (UCE) lncRNA simultaneously localizes to activated (Umad1, 1.6Mb distant) and repressed (Akr1b8, 27Mb distant) chr6 target genes, precisely regulating UCE-gene distances and cohesin binding in mouse embryonic forebrain GABAergic interneurons (INs). Transgene expression of Evf2 activates Lsm8 (12Mb distant), but fails to repress Akr1b8, supporting trans-activation and long-range cis repression. Through both short-range (Dlx6 anti-sense) and long-range (Akr1b8) repression, the Evf2–5’UCE links homeodomain and mevalonate pathway regulated enhancers to interneuron diversity. The Evf2–3’ end is required for long-range activation, but dispensable for RNA cloud localization, functionally dividing the RNA into 3’-activator and 5’UCE-repressor/targeting regions. Together, these results support that Evf2 selectively regulates UCE interactions with multi-megabase distant genes through complex effects on chromosome topology, linking lncRNA-dependent topological and transcriptional control with interneuron diversity and seizure susceptibility.
Introduction
Enhancers (Enhs) are defined as DNA sequences capable of regulating genes at a distance, independent of orientation. Early studies showed regulatory interactions between the sonic hedgehog (Shh) limb Enh (ZRS) and the Shh gene, despite 1Mb distance (Lettice et al., 2003). Technological advances in understanding chromosome topology (referred to as topology) (Dekker, 2016) reveal that the majority of promoter interactions (~93%) are distal, rather than proximal, many exhibiting asymmetry in the number of interactions (Sanyal et al., 2012). While validated Enh regulatory landscapes in vertebrates span ~1Mb and facilitate tissue-specific and/or developmentally programmed gene expression, selective regulation of genes over long distances remains a fundamental question in biology.
Our early work on Evf2, a spliced and polyadenylated UCE-lncRNA (Feng et al., 2006) showed that Evf2 is transcribed from the Dlx5/6UCE (referred to hereafter as UCE, (Zerucha et al., 2000)), and regulates UCE activity in trans (Feng et al., 2006). UCE regions are defined as highly conserved >200 base pairs segments in the genome (Bejerano et al., 2004; Sandelin et al., 2004). Evf2 is expressed at sites of sonic hedgehog-activated interneuron (IN) birth in mouse embryonic forebrain (E13.5 medial and caudal ganglionic eminences [MGE, CGE] (Anderson et al., 1997a; Kohtz et al., 1998; Nery et al., 2002) recruits transcription factors (TFs) to the UCE (Bond et al., 2009), forms a large DLX1 homeodomain containing ribonucleoprotein complex (Evf2-RNP), and directly inhibits BRG1(SMARCA4) ATPase and chromatin remodeling activities (Cajigas et al., 2015). In adult mice, Evf2 loss causes hippocampal GABAergic circuitry defects, supporting UCE-lncRNA biological significance (Bond et al., 2009).
Evf2 forms one-two RNA clouds per nucleus in developing INs (Feng et al., 2006), similar to clouds described for imprinting and dosage compensation lncRNAs (Brockdorff, 2011; Redrup et al., 2009). While the dosage compensation lncRNA Xist controls topology across the entire inactivated X-chromosome (Giorgetti et al., 2016; Nora et al., 2012), the Airn lncRNA regulates imprinting of genes in the Igf2r cluster through distinct mechanisms including long-range cis silencing via recruitment of the histone methyltransferase G9a and short-range transcriptional interference (Nagano et al., 2008; Pauler et al., 2012). Although a recent report indicates that Airn regulated imprinting extends to Arid1b (7.7Mb distant), (Andergassen et al., 2017), the mechanistic basis for Airn imprinting at such a distance is unknown. Long-range actions of lncRNAs in dosage compensation and imprinting raise questions about whether cloud-forming autosomal lncRNAs regulate multi-megabase distant genes in other biological contexts. In this report, we show that Evf2 targets UCE interactions (UCEins) across chr6 (0–40Mb), affecting gene expression and chromosome topology across a ~27Mb region, thereby extending beyond the limitation of ~1Mb for the majority of Enh regulatory landscapes. Thus, UCE-lncRNA-dependent topological and transcriptional control occurs across surprisingly long (multi-megabase) distances during development in events critical for IN diversity.
Results
The Evf2–5’ UCE-containing region regulates interneuron subtype genes
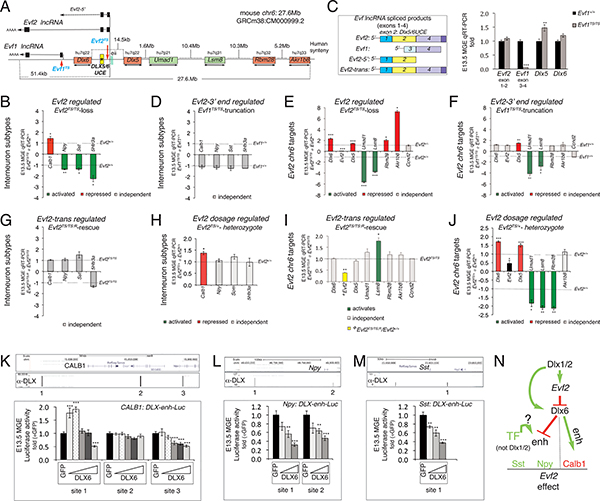
INs in the adult brain are the most diverse of any cell type, partly due to expression of IN subtype specific genes (IN-SGs) (DeFelipe et al., 2013). In mice, Dlx homeodomain TFs, originally identified by homology to fly dll, play critical roles throughout IN development, beginning during their birth in the GEs (Anderson et al., 1997a; Price et al., 1991). In mouse GEs, Shh induces IN specification, activating Dlxs, Evf1 (an alternatively spliced form of Evf2) and Evf2 (Feng et al., 2006; Kohtz et al., 1998). While embryonic Shh and Dlx genes contribute to IN diversity (Cobos et al., 2005; Long et al., 2007; Xu et al., 2010), the role of Evf2 is unknown. Using mice lacking Evf2 (Evf2TS/TS (Bond et al., 2009), transcription stop insertion (TS) in Evf exon 1, Fig 1A), we determined the effects of Evf2 loss on IN-SG expression in MGE. Evf2 activates and represses IN-SGs in MGE, with greater than two-fold changes in serotonin receptor 3a (5Htr3a), and subtle changes in calbindin 1 (Calb1), neuropeptide Y (Npy), and somatostatin (Sst, Som) (Fig 1B). While Sst and 5Htr3a constitute two of the three major IN subclasses (Rudy et al., 2011), additional subtype genes (parvalbumin, vasoactive intestinal peptide (VIP), calretinin) are not expressed this early in development, not shown). The Evf gene contains 4 exons, generating lncRNAs by alternative transcriptional initiation, splicing and polyadenylation (Evf1, lacking the UCE sequence, (Kohtz and Fishell, 2004), and Evf2, containing the UCE sequence at 5’ end, Fig 1A). In order to distinguish between Evf2–5’ (UCE-containing) and Evf2–3’ (UCE-lacking) regions, Evf1TS/TS mice were generated (inserting TS into Evf exon 3, Evf1TS, Fig 1A). Evf1TS insertion truncates Evf2, generating an Enh-containing form (Evf2–5’) that lacks the Evf2–3’ end, and prevents transcription of Evf1 (Fig 1C). Evf1 and Evf2 are co-expressed with Dlx5/6 genes in E13.5 GE subventricular zone, and nuclear RNA cloud forming. In Evf1TS/TS mice, the truncated Evf2–5’ transcript retains transcription across UCE and Dlx6 anti-sense transcription, the latter consistent with the finding that Dlx6 expression does not change in Evf1TS/TS (Fig 1C). However, similar to Evf2TS/TS, Dlx5 is subtly increased in Evf1TS/TS (Fig 1C). Therefore, Evf2–5’ is necessary and sufficient for Dlx6 repression, while Evf2–3’ is required for Dlx5 repression. In Evf1TS/TS, IN-SG expression is not affected (Fig 1D). Given our previous evidence that Evf1 continues to be expressed in Evf2TS/TS, Evf1 is not sufficient to regulate IN-SGs in Evf2TS/TS MGE. Therefore, Evf2 truncation, rather than Evf1 loss is responsible for IN-SG regulatory differences between Evf1TS/TS and Evf2TS/TS (Fig S1A, pink star). The combined genetic data indicates that the Evf2–5’ UCE region is both necessary and sufficient for regulating Dlx6 and IN-SGs.
Figure 1. Evf2 UCE-lncRNA regulation of genes across 27Mb and Dlx6 dosage regulated interneuron subtype enhancers.
A. Schematic of the 27Mb region of mouse chr6 encompassing Evf2 and Evf1 lncRNAs (transcribed over ~51kb), target genes, Dlx5/6 intergenic region and Enhs (UCE*, yellow box, eii), (Zerucha et al., 2000)). Red arrows; sites of triple polyA transcription stop insertions, preventing Evf2 (Evf2TS) and Evf1 (Evf1TS) transcription in mice. Evf2-chr6 target-human chr7 synteny is labeled. B-H. E13.5 MGE qRT-PCR gene expression analysis from mice lacking Evf2 (Evf2TS/TS, Evf2-regulated), lacking Evf1 and expressing truncated Evf2–5’ (Evf1TS/TS, Evf2–3’-regulated), expressing an Evf2 transgene (Evf2TS/TS;R trans-regulated), expressing Evf2 from one allele (Evf2TS/+, Evf2 dosage-regulated), or wild-type littermates (Evf1+/+, Evf2+/+). B. Calb1, Npy, Sst, 5Htr3a in Evf2TS/TS normalized to Evf2+/+. C. Left: schematic of differentially spliced Evf transcripts (exons1–4), and Evf2 generated by transgenic rescue (Evf2-trans). Right: E13.5 MGE qRT-PCR gene expression analysis showing relative expression of Evf2–5’ (exon1–2), Evf1 (exon3–4), Dlx5, and Dlx6, in Evf1TS/TS (grey bars), normalized to Evf1+/+ (black bars). D. Calb1, Npy, Sst, 5Htr3a in Evf1TS/TS normalized to Evf1+/+, E. chr6 targets: Evf2TS/TS normalized to Evf2+/+, F. chr6 targets: Evf1TS/TS normalized to Evf1+/+. G. Calb1, Npy, Sst, 5Htr3a in Evf2TS/TS;R normalized to Evf2TS/TS. H. Calb1, Npy, Sst, 5Htr3a in Evf2TS/+ normalized to Evf2+/+. I. chr6 targets: Evf2TS/TS;R normalized to Evf2TS/TS (except for yellow bar, normalized to Evf2+/+). J. chr6 targets: in Evf2TS/+ normalized to Evf2+/+. B-J: n=4–7 of each genotype, values normalized to Evf2+/+, Evf1+/+, or Evf2TS/TS (dotted lines), red (repressed genes), green (activated genes), grey (Evf2 independent gene). K-M. DLX BSs identified by ChIPseq in E13.5GE chromatin. Dlx6 dosage-dependent regulation of DLX BS is tested in luciferase reporter assays, using primary E13.5 MGE cells. Triangles represent increasing concentrations of Dlx6 plasmid; results are normalized to plasmid expressing GFP. K. Calb1 gene, Dlx6-regulated 2/3 DLX BSs, L. Npy gene, Dlx6-regulated 2/2 DLX BSs. M. Sst (Som) gene, Dlx6-regulated 1/1 DLX BS. K-M, n=12/condition, values from two experiments, Student’s t-test, *p<0.05, **p<0.01, ***p<0.001, error bars (S.E.M). N. Model summarizing the Dlx-Evf2 regulatory pathway leading to IN-SG activation and repression in E13.5MGE. Only effects that are consistent with both genetic and Enh assays are included.
Evf2 activation and repression, in trans and cis
In order to identify genes involved in Evf2-IN-SGs regulation, we compared gene expression between Evf2+/+ and Evf2TS/TS MGE using microarray analysis (Table S1, Table S2). The majority of validated targets are located on mouse chr6 (Evf2-chr6 targets) (Table S1). With the exception of overlapping Dlx6 (anti-sense), Evf2-chr6 target genes are organized asymmetrically, 5’ of the Evf2 transcription start site across ~27Mb (Fig 1A). Evolutionarily conserved organization of 5/6 of the Evf2-chr6 target genes on human chr7 supports a potentially significant biological role (Fig 1A), leading to further focus on the significance and mechanism of Evf2-chr6 target gene regulation.
Evf2 represses short-range targets Dlx5/6, long-range targets Rbm28, Akr1b8, and activates long-range targets Umad1, Lsm8 (Fig 1A, 1E, Table S1). Comparisons of Evf2-chr6 targets in Evf2TS/TS and Evf1TS/TS MGE show that Evf2–5’ is both necessary and sufficient for Rbm28 and Akr1b8 repression, while Evf2–3’ is required for Umad1 and Lsm8 activation (Fig 1E, F). Therefore, Evf2 repression of Dlx6 and long-range targets requires the Evf2–5’UCE region, while activation requires the Evf2–3’ region. In order to distinguish between Evf2 trans and cis regulation, we next compared gene expression in Evf2 rescue (Evf2TS/TS;R) and Evf2 heterozygotes (Evf2TS/+), models that express similar levels of Evf2 (Evf2TS/TS;R ~38% wildtype levels (Berghoff et al., 2013), and Evf2TS/+ at ~48% wildtype levels), but differ by source (Evf2TS/TS;R:Evf2 expressed from a transgene versus Evf2TS/+:Evf2 expressed from a single endogenous locus). In Evf2TS/TS;R MGE, Evf2 is unable to rescue IN-SGs when compared to expression levels in Evf2TS/TS (Fig 1G), but activates Lsm8 (Fig 1I). In Evf2TS/+, Evf2 is haplosufficient for Npy, Sst, 5Htr3a, and Akr1b8 and haploinsufficient for Calb1, Dlx5/6, Umad1, Lsm8, and Rbm28 (Fig 1H, J). While transgenic rescue supports that Evf2 activates Lsm8 in trans, Evf2 haplosufficiency for genes that are not rescued, supports Evf2 cis-regulation. However, given Evf2 haploinsufficiency for genes that are not rescued, we cannot distinguish between a failure to rescue due to dosage effects (Evf2 transgene rescue expression at ~38% wildtype) or cis-regulation. Together, these data support that Evf2 regulates Lsm8 in trans, and Akr1b8, Npy, Sst, and 5Htr3a, in cis.
The Evf2-antisense target Dlx6, regulates multiple interneuron subtype genes
Gene expression analysis correlates Evf2–5’ repression of Evf2-chr6 targets (Dlx6, Rbm28, Akr1b8) with regulation of IN-SG expression (Fig 1, compare B, E, and compare D, F); contributions of Evf2–5’ UCE region, −3’ end, and trans effects are summarized in Fig S1A, with pink star highlighting repression). Using a genetic approach, we next analyzed gene expression from 6 mouse mutants with different combinations of Evf2TS, Evf1TS and Dlx5/6KO (Merlo et al., 2002) alleles (Fig S1B). Dlx5/6KO removes the entire Dlx5 and Dlx6 region, deleting UCE, eii, and Evf2–5’ exons. In Dlx5/6KO; Evf2TS mice, rescue of Dlx6 and Dlx5, also rescues IN-SG effects (Fig S1C). Furthermore, 4/35 possible dose-dependent relationships between five IN subtype and seven Evf2-chr6 target genes are detected at an r2>0.8, including Dlx6 and Calb1 (r2=0.81) (Table S3). In order to test whether Dlx6 dosage directly regulates Calb1 expression, we used anti-DLX ChIPseq to identify potential Enhs in E13.5GE, and found three DLX binding sites (BSs) within ~50kb of the Calb1 gene (Fig 1K). Transfection into primary E13.5 MGE cultures shows that Dlx6 dosage regulates Calb1-Enhs (sites 1 and 3) in luciferase reporter assays (Fig 1K). Evf2-dependent changes in H3K4me3 are observed in the promoter regions of Calb1, Npy, and Sst (Fig S1D). Anti-DLX ChIPseq also identifies DLX BSs near Npy and Sst genes, repressed by Dlx6 in a dose-dependent manner (Fig 1L, M). Reporter assays together with genetic data support that Evf2 repression of Calb1 and activation of Npy and Sst occurs through antisense regulation of Dlx6, in a dose-dependent manner (Fig 1N). Furthermore, in Dlx1/2−/− E13.5 MGE, Dlx6 is significantly reduced (Berghoff et al., 2013) and Npy increases (Fig S1E), supporting that Dlx6 repression of Npy is independent of Dlx1/2. Therefore, while differential Dlx6 activator and repressor activities may involve antagonism of a transcriptional activator, the precise mechanism remains to be determined.
Regional control of the Evf2-Akr1b8-5Htr3a axis involves the mevalonate pathway and Akr1b8 regulated enhancers
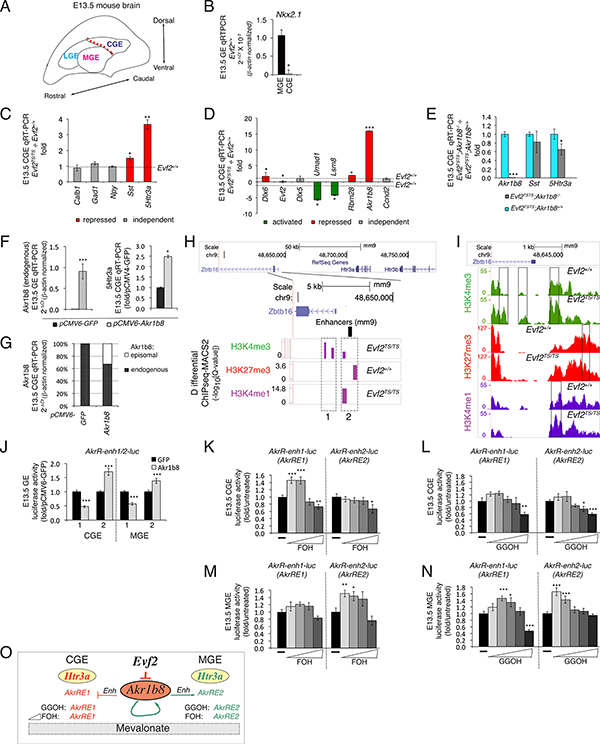
Morphologically and molecularly distinct lateral, medial and caudal ganglionic eminences (LGE, MGE and CGE, Fig 2A) are sites of IN birth in the embryonic brain, and differentially contribute to IN subtypes in the adult brain (Gelman and Marin, 2010; Nery et al., 2002; Wichterle et al., 2001). In addition to differences in Nkx2.1 expression (MGE-Nkx2.1+, CGE-Nkx2.1−, confirmed in Fig 2B), Npy+ and Sst+ INs derive from MGE, while 5Htr3a+ INs derive from CGE (Rudy et al., 2011). Based on the identification of CGE as the primary source of 5Htr3a+IN and Evf2 regulation of 5Htr3a in E13.5 MGE (Fig 1B), we next determined whether Evf2 IN-SG regulation differs between CGE and MGE. In Evf2TS/TS CGE, Sst and 5Htr3a increase, with no changes in Calb or Npy levels (Fig 2C). However, Evf2-chr6 targets show a similar profile of activation and repression compared to MGE (compare Fig 1E and Fig 2D), except that subtle Dlx5 repression is not observed in CGE and Akr1b8 repression is greater in CGE compared to MGE. Therefore, although Evf2 regulation of Evf2-chr6 targets is similar in MGE and CGE, IN-SG expression differs for all four IN-SGs as follows: (1) Evf2 represses Calb in MGE, but not CGE, (2) Evf2 activates Npy in MGE, but not CGE, (3) Evf2 activates Sst and 5Htr3a in MGE, but represses Sst and 5Htr3a in CGE (compare Fig 1B and 2C). Together, these data show that Evf2 control of IN-SG expression in embryonic brain is regionally determined, depending on MGE or CGE origin.
Figure 2. The Evf2-Akr1b8–5Htr3a axis: Akr1b8 and mevalonate pathway-regulated enhancers in the Zbtb16–5Htr3a region.
A. Schematic of E13.5GE mouse embryonic brain showing subdivisions of embryonic ganglionic eminences (LGE, MGE, CGE). Red dotted line: region dissected to separate MGE from CGE. B. qRT-PCR analysis of Nkx2.1 confirms the accuracy of dissections between E13.5MGE (Nkx2.1+), and CGE (Nkx2.1-), n=2 pools each region, *p=0.02, Evf2+/+ tissues. C-E. CGE qRT-PCR, Evf2TS/TS normalized to Evf2+/+, red (repressed genes), green (activated genes) and grey (independent genes). C. Calb1, Npy, Sst, 5Htr3a and Gad1. D. Evf2-chr6 targets. E. qRT-PCR analysis of CGE Evf2TS/TS; Akr1b8−/− normalized to Evf2TS/TS; Akr1b8+/+. CGE (Akr1b8, Sst, 5Htr3a), n=6–15 of each genotype. F. qRT-PCR of endogenous Akr1b8 and 5Htr3a expression in primary CGE transfected with Akr1b8 (pCMV6-Akr1b8, grey bars), normalized to GFP transfected (pCMV-GFP, black bars), n=3–6. G. Relative contributions of endogenous Akr1b8 and episomal Akr1b8 in CGE transfections. H. Zbtb16-Htr3a/b (5Htr3a/b) region, differential H3Kme (ChIPseq-MACS2, purple bars) in the promoter region of Zbtb16 of Evf2+/+ vs Evf2TS/TS GE, identifies potential Akr1b8-regulated Enhs (AkrRE1/2). I. Traces from H3Kme ChIPseq visualize peak differences between Evf2+/+ and Evf2TS/TS. H3K4me3 (green), H3K27me3 (red), H3K4me1 (purple). J. Regulation of AkrRE1/2 in luciferase reporter assays, using primary CGE and MGE cells. K-N. Dosage effects of MV pathway metabolites FOH and GGOH (grey bars) on AkrRE1/2 luciferase reporters, normalized to buffer alone (−, black bars). Triangles indicate increasing concentrations (FOH: 0.1, 1, 10, or 100 μM, GGOH: 0.01, 0.1, 1, 10, or 100 μM). J-N. CGE (n=10) and MGE (n=12)/condition, averaged from two experiments, Student’s t-test, *p≤0.05, **p≤0.01, ***p≤0.001, error bars (S.E.M). O. Model describing Evf2-Akr1b8 tissue-specific and metabolite-specific regulation of AkrRE1 and AkrRE2 activities. Only the effects on AkrRE1 repression in CGE and AkrRE2 activation in MGE are included, as these are consistent with both genetic outcomes (1B, 2C) and gain-of-function results in (J).
We next investigated the basis for correlations between Akr1b8 and 5Htr3a, the most highly regulated Evf2-chr6 target and IN-SG, respectively. Although loss of Akr1b8 (Akr1b8−/−) does not affect IN-SG expression in CGE (Fig S2A), loss of Akr1b8 from Evf2TS/TS partially rescues 5Htr3a levels in Evf2TS/TS;Akr1b8−/− double homozygote CGE (Fig 2E), but not MGE (Fig S2B). Thus, Evf2 represses 5Htr3a, in part, through Akr1b8 repression in CGE. Furthermore, Akr1b8 transfection into CGE primary cultures increases endogenous Akr1b8 and 5Htr3a levels (Fig 2F, G). Differential analysis of ChIPseq peaks identifies Evf2-dependent changes in H3K4me3 (active promoters) (sites 1/2), H3K27me3 (silent chromatin) (site 2), and H3K4me1 (Enhs) (site 2) located at the Zbtb16 5’ end (~63kb from 5Htr3a gene, Fig 2H, I, Fig S2C). Co-transfection of Akr1b8 with site 1 or site 2 luciferase reporters into primary CGE and MGE shows that Akr1b8 represses Akr1b8-regulated Enh1 (AkrRE1) and activates AkrRE2 (Fig 2J). As part of the mevalonate (MV) pathway, Akr1b8 controls farnesol (FOH) and geranylgeraniol (GGOH) levels, affecting protein prenylation (Endo et al., 2011), but also converts all-trans-retinaldehyde to retinol (Gallego et al., 2007). However, given the lack of evidence to support the involvement of retinoids in Evf2 regulation, (Table S1, S2) confirmed in Fig S2D, we tested the involvement of MV pathway metabolites in AkrRE1/2 regulation. In CGE and MGE, FOH and GGOH display dose-dependent, Enh- and region-specific effects (Fig 2K-N, schematic in 2O). Thus, the Evf2-Akr1b8–5Htr3a axis reveals a mechanism involving MV pathway regulation of Enhs that distinguish E13.5 MGE from CGE.
Evf2 regulates chromosome topology in interneuron subpopulations
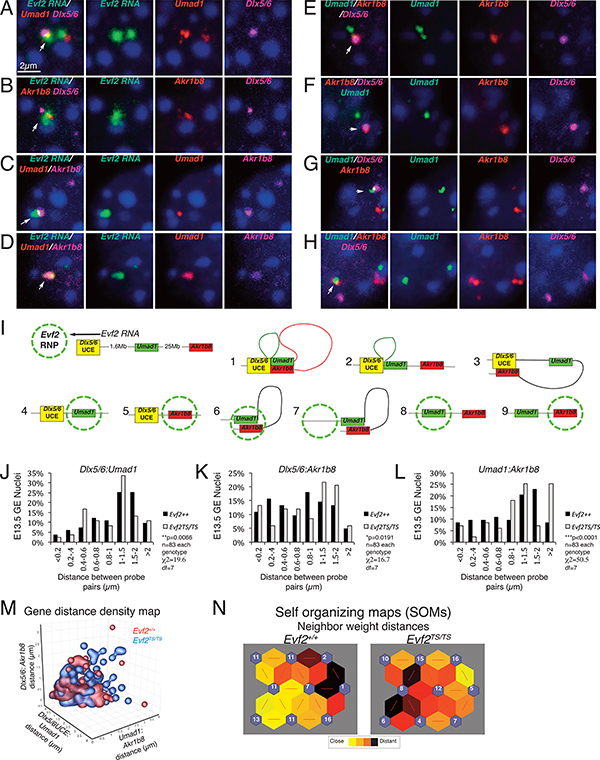
We next used Evf2 RNA/DNA fluorescence in situ hybridization (FISH) in E13.5 GE nuclei to investigate the relationship between the Evf2 RNA cloud, UCE, and long distance repressed (Akr1b8) and activated (Umad1) target genes (Fig 3A-I). Evf2 RNA clouds co-localize with Akr1b8 or Umad1 near UCE (Fig 3A, B), and with Akr1b8/Umad1 associated loci (Fig 3C, D). DNA FISH analysis of Umad1-Akr1b8-UCE detects all possible relationships (Fig 3E-H), suggesting that, although UCE associates with both repressed and activated target genes, Evf2 RNA clouds can also localize with targets when UCE is not associated (Fig 3I, [4,5] schematic). Canonical FISH analysis methods compare distances between Dlx5/6:Umad1 (X), Dlx5/6:Akr1b8 (Z), and Umad1:Akr1b8 (Y) in 83 nuclei from Evf2+/+ and Evf2TS/TS, and by binning gene distances into 8 groups (<2μm−>0.2μm, Fig 3J-L), determines that Evf2 regulates distance profiles in all pairs (Fig 3J-L). A density map visually represents the same data by superimposing Evf2+/+ and Evf2TS/TS values in each nucleus, revealing differential clustering of Evf2+/+ and Evf2TS/TS nuclei (Fig 3M). Furthermore, an optimized clustering analysis program (self-organizing map, SOM, see methods section and Fig S3A-D), reveals that 42/83 Evf2+/+ nuclei have close neighbor weight distances compared to 21/83 Evf2TS/TS (Fig 3N) and provides an unbiased multivariable analysis tool that considers all three distances (or more), in contexts where cellular heterogeneity masks subpopulation differences. Thus, Evf2 directly associates with gene targets, and regulates topology of the 27Mb region by altering Dlx5/6:Umad1:Akr1b8 gene-distance relationships in a heterogeneous manner, reflecting differences among IN subpopulations.
Figure 3. Evf2 RNA cloud associates with Umad1 and Akr1b8, and regulates UCE-Umad1-Akr1b8 distances in interneuron subpopulations.
A-D. Fluorescent in situ hybridization (FISH) of GE nuclei showing Evf2 RNA (green), Umad1, Akr1b8 (red or magenta) and DAPI (blue). White arrows indicate co-localization. Scale bar =2μm, dark blue spots indicate heterochromatin. E-H. DNA FISH of GE nuclei showing examples of UCE-gene interactions. I. Schematic showing: UCE (yellow), Umad1 (green, activated target gene), Akr1b8 (red, repressed target gene), direction of Evf2 lncRNA transcription, and formation of the Evf2 RNA cloud (green dashed circle); schematics (1–9) summarizing Evf2 RNA cloud localization and UCE-Umad1-Akr1b8 interactions. J-L: Comparison of distances between UCE-Umad1-Akr1b8 in Evf2+/+ and Evf2TS/TS GE nuclei (n=83, each genotype). Gene distances from single nuclei binned in 8 groups (<0.2μm−>2μm), and percentages of nuclei in each bin plotted. Chi-square (χ2), (*p<0.05), degrees of freedom (df=7), Evf2+/+ (black bars), Evf2TS/TS (grey bars), M. 3-D density plot of gene distances visualizes the relationship between values from Evf2TS/TS and Evf2+/+ nuclei, showing greater density of values from Evf2TS/TS nuclei (blue) outside of main cluster Evf2+/+ nuclei (red). N. Self-organizing maps (SOMs) in the Matlab neural network toolbox (NNT) and three training iterations optimally cluster gene-distance data and visualization. SOMs allow visualization of complex numerical relationships, in this case 3 values from 83 nuclei in two genotypes can be compared. Neighbor weight distance SOMs show that ~2-fold more Evf2+/+ nuclei clusters are connected by closer distances (yellow hexagons) compared to Evf2TS/TS (darker hexagons).
Evf2-dependent UCEins, cohesin binding and histone methylation at activated target genes: Umad1 and Lsm8
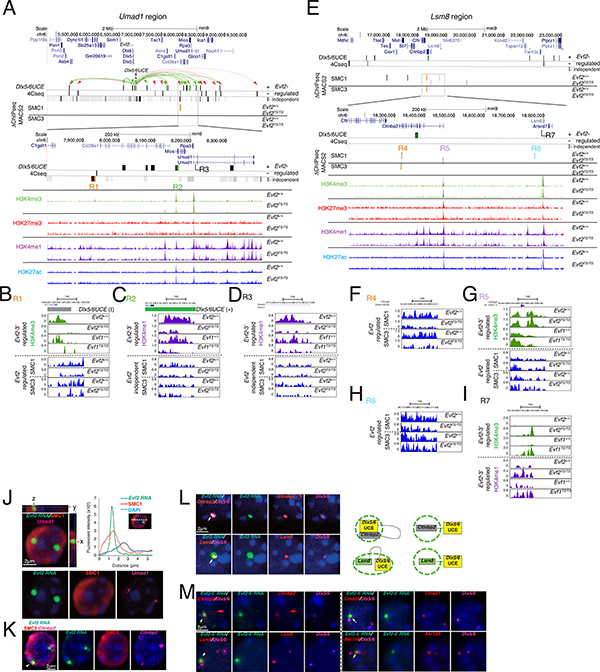
Given that FISH localization does not allow precise determination of UCEins, we next used chromosome conformation capture sequencing (4Cseq) (van de Werken et al., 2012) with UCE as the bait sequence to compare UCEins from Evf2+/+ and Evf2TS/TS E13.5 GE. 4Cseqpipe domainograms visualize enrichment of UCEins and support Evf2 positive regulation of UCEins across a ~500kb region surrounding the activated target gene Umad1 (Fig S3E). The intersection of FourCseq (Klein et al., 2015) and DESeq computational methods (ICM) increases the confidence of computational predictions, and identifies UCEins across chr6 (Fig S4A). Across chr6 0–40Mb region, UCEins are categorized into Evf2 (+) regulated (UCE+=36 sites), (−) regulated (UCE−=42 sites), and Evf2-independent (UCE-I=162), (Fig S4A). Evf2 regulated UCEins in 4Mb regions surrounding activated target genes Umad1 and Lsm8 reveal potentially important relationships with Evf2-regulated cohesin binding (SMC1 and SMC3) and HMs (summarized in Table S4). The Umad1 4Mb region includes Evf2 and UCE (bait region), and multiple UCE+/− (green and red arrows, respectively, Fig 4A). Differential ChIPseq-MACS2 analysis (ΔChIPseq) of cohesin binding in Evf2+/+ vs. Evf2TS/TS E13.5 GE identifies one Evf2 regulated SMC1 site across 4Mb (R1, orange bar, Fig 4B) that overlaps with UCE-I, and near UCE-. Upstream of Umad1, traces from R1 indicate that Evf2 regulates both SMC1 and SMC3 binding 3’ of Col28a (Fig 4B), while R2 contains a UCE+ in Mios (green bar, Fig 4C). ΔChIPseq of histone modifications (HMs: H3K4me3, H3K4me1, H3K27me3) in Evf2+/+, Evf2TS/TS, Evf1+/+, and Evf1TS/TS E13.5 GE identifies three histone methylation sites regulated by both Evf2 and Evf2–3’ (R1, R2, and R3, Fig 4B-D, Table S4). Given that Evf2–3’ is required for Umad1 and Lsm8 activation (Fig 1F), overlap of Evf2–3’ regulated histone methylation sites with SMC1 and UCE-regulated interactions support involvement of these sites in transcriptional regulation.
Figure 4. Analysis of Evf2-regulated UCEins, histone lysine methylation, and SMC1/3 binding at activated targets Umad1 and Lsm8.
4Mb regions surrounding activated target genes Umad1 and Lsm8 are shown. 4Cseq using UCE as the bait sequence in Evf2+/+ and Evf2TS/TS E13.5GE, with 4Cseq-ICM sites shown. Grey/black bars indicate genomic locations of UCEins (+/−/I). ΔChIPseq of HMs (H3K4me3, H3K4me1, H3K27me3) in Evf2+/+, Evf2TS/TS, Evf1+/+, and Evf1TS/TS E13.5 GE identifies potentially significant Evf2- and Evf2–3’ regulated HMs. ΔChIPseq of cohesin (SMC1 and SMC3) in Evf2+/+, Evf2TS/TS E13.5 GE identifies Evf2 regulated SMC1 BSs. Umad1 (A-G) A. Green arrows indicate UCE+. Red arrows indicate UCE−. Orange bar: Evf2 regulated SMC1 BS that overlaps with UCE-I. Green bar: UCE+ overlap with Evf2 and Evf2–3’-regulated H3K4me1. B. R1 (3’ of Col28a): Evf2-regulated SMC1/3, Evf2- and Evf2–3’ regulated H3K4me3, and UCE-I site. C. R2 (Mios -intron1), Evf2- and Evf2–3’ regulated H3K4me1 and Evf2 regulated UCE+. D. R3 (Umad1- intron 1), Evf2- and Evf2–3’ regulated H3K4me1, adjacent to UCE-I site. Lsm8 (E-I) E. A 4Mb region includes Lsm8 and adjacent genes, and indicates UCEins (I, +, −). F-I. ΔChIPseq of SMC1 and SMC3 in Evf2+/+, Evf2TS/TS E13.5GE identifies 3 Evf2 regulated SMC1 sites and one Evf2-regulated SMC3 site (colored bars in the boxed region), and one UCE interacting site (+) located in the middle of Cttnbp2. R4 (orange), R5 (lavender), and R6 (blue) are regions where Evf2-regulates cohesin binding. In R7, located upstream of Lsm8, both Evf2 and Evf2–3’ regulate histone methylation (H3K4me3/me1), supporting potential significance. F. R4 (Cttnbp2 intron 22/22), Evf2 positively regulates SMC1 and SMC3 binding. G. R5 (Cttnbp2 intron 1/22), Evf2 negatively regulates SMC1 binding, and both Evf2 and Evf2–3’ regulate H3K4me3. H. R6: Evf2 positively regulates SMC1 binding. I. R7: Evf2 and Evf2–3’ negatively regulate H3K4me3 and H3K4me1. J. FISH of Evf2+/+ GE nuclei probed with anti-sense Evf2 RNA (green), anti-SMC1 antibody (red), and Umad1 DNA (magenta). Graph of fluorescent intensity map of x-axis is shown on the right, indicating co-localization of Evf2 RNA cloud peak and SMC1 peak. K. FISH of Evf2+/+ GE nuclei probed with anti-sense Evf2 RNA (green), anti-SMC3 antibody (red), and Cttnbp2 DNA (magenta) showing co-localization, white arrow. L. FISH of Evf2+/+ GE nuclei probed with anti-sense Evf2 RNA (green), and DNA probes (Cttnbp2 or Lsm8, red), (Dlx5/6, magenta). White arrows indicate co-localization. Right: schematic showing Evf2 RNA cloud/UCE/gene relationships. M. FISH of Evf1TS/TS E13.5GE nuclei probed with anti-sense Evf2 RNA (green), and DNA probes. White arrows indicate co-localization.
In the 4Mb region surrounding Lsm8, the closest UCE interaction site is located in the intron of the adjacent Cttnbp2 gene (Fig 4E). Four additional sites may contribute to transcriptional or topological control: Evf2 regulation of SMC1 and/or SMC3 binding at sites R4, R5, R6, and Evf2–3’ regulation of histone methylation at sites R5 and R7. Similar to Umad1, Evf2 positively regulates UCEins, histone methylation and SMC1/3 binding in the Lsm8 region. However, in the Lsm8 region, Evf2 regulates the position of SMC1 binding, shifting from 5’ (R5) to 3’ (R4) of Cttnbp2. SMC1 is localized near the nuclear periphery, with the highest intensity peak of SMC1 co-localizing with Evf2 RNA (Fig 4J). Consistent with 4Cseq-ICM predictions that identify Cttnbp2-UCE+, Evf2 and UCE co-localize with Cttnbp2 (Fig 4E, L). Although Evf2 co-localizes with Lsm8 and UCE, Lsm8 and UCE are adjacent rather than overlapping (Fig 4L). Thus, topological, SMC1 and HM regulation support the potential significance of UCE/Cttnbp2 interactions in Evf2-dependent transcriptional activation of Lsm8.
We next determined how Evf2 truncation affects RNA cloud formation and co-localization with UCE and Evf2-chr6 target genes. Analysis of Evf1TS/TS E13.5 GE nuclei, shows that the Evf2–5’ is sufficient to form RNA clouds, and retains the ability to co-localize with UCE and Evf2-chr6 target genes (Fig 4M). Thus, despite the ability of Evf2–5’ to localize at activated target genes Umad1 and Lsm8, Evf2–3’ is necessary for gene activation, distinguishing between RNA localization and transcriptional activation mechanisms. Together, these data suggest that Evf2 regulates UCEins, cohesin binding and HMs at activated, long-range target genes, identifying potential regulatory sites.
Cohesin distinguishes between Evf2 (+) and (−) regulated UCEins across chr6:0–40Mb
In order to determine the relationship between Evf2 -dependent topological and epigenetic effects, we aligned 4Cseq-ICM and histone methylation ChIPseq data using Circos plots across chr6 (0–40Mb) (Fig 5A-C) and across chr6: 40–150Mb, (Fig S4B-D) (UCE+, orange, UCE−, blue, UCE-I, grey). Whereas Evf2 positively regulates UCEins near Lsm8 and Akr1b8, Evf2 shifts the position of UCEins at Umad1 and Rbm28 (Fig 5B, C, Table S4). In addition, the majority of UCE-I are near Evf2, while Evf2-regulated UCE(+/−) are distributed across chr6 (0–40Mb), supporting a role for Evf2 in multi-megabase interactions. Relative counts of UCE(+/−) at repressed (Rbm28, 6651) and activated (Lsm8, 4094) target genes, and at Umad1 (+, 8826, vs. −, 6835) are in a similar range, and therefore appear unbiased with respect to positive or negative transcriptional effects (Fig 5D, E).
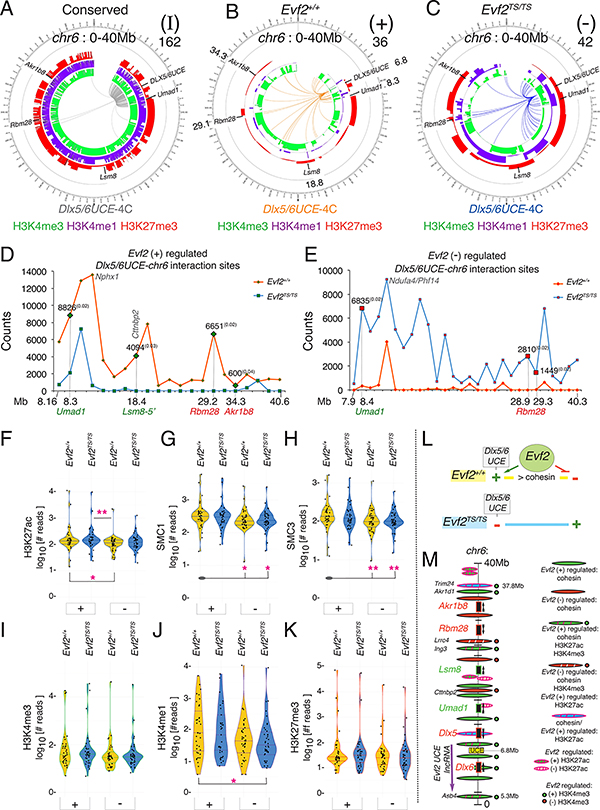
Figure 5. Evf2-regulated UCEins, histone modifications and cohesin (SMC1/SMC3) binding across chr6 (0–40Mb).
A-C. Integrated Circos plots indicating UCEins across a 40Mb region of chr6 (inner panels showing interactions identified by 4Cseq-ICM, and corresponding H3Kme profiles (identified by native ChIPseq of GE, H3K4me3 [green], H3K4me1 [purple], H3K27me3 [red] peaks). chr6 0–40Mb includes the 27Mb region containing the UCE bait and transcriptional regulated target genes: Umad1, Lsm8, Rbm28, Akr1b8. A. UCEins detected in both Evf2+/+ and Evf2TS/TS (I, Evf2-independent), B. UCEins enriched in Evf2+/+. (+, Evf2 positively regulated), C. UCEins enriched in Evf2TS/TS (−, Evf2 negatively regulated), D, E. Scaled counts of 4Cseq-ICM regions are plotted (y-axis), representing overlapping positions of FourCSeq significant sites (Q values <0.05, midpoint +/−5Kb) and DESeq2 identified significant regions at restriction enzyme site resolution. The 4Cseq coverage of the Evf2+/+ sample is scaled to Evf2TS/TS coverage to normalize for sequencing depth differences. F-K. Violin plots representing ChIPseq results from E13.5GE, where the y-axis indicates normalized read counts of H3K4me3, H3K4me1, H3K27me3, and H3K27ac, or SMC1/3 at ±2kb distance from UCEins across chr6 (0–40Mb). Different genotypes are colored (Evf2+/+ in yellow, and Evf2TS/TS in blue). Evf2 regulated UCE+/−: (+) and (−). Mann Whitney U test (*p<0.05, **p<0.01) on pairs of samples are analyzed, where pink stars indicate significant differences (p<0.05) between (+/−) UCEins. Grey ovals represent multiple pair-wise comparisons to one sample for simplified visualization. L. Model representing the relationship between Evf2 and cohesin in UCE site selection across chr6:0–40Mb. M. Schematic of Evf2-regulated cohesin and H3K27ac sites in a 31Mb region of chr6: the Evf2 transcription start (start of purple arrow) to Evf2 regulated cohesin and H3K27ac sites downstream of long-range target gene Akr1b8. Of 10 Evf2 regulated H3K27ac sites (ovals w/pink stripes), 5/10 sites overlap with cohesin (blue oval w/ pink stripes); 4/5 sites are near the edges of the 31Mb domain (Evf2–5’, Trim24–5’), in Dlx5 intron, or ~300kb from Rbm28. Green/red circles label cohesin bound sites where Evf2 regulates H3K27ac and H3K4me3, common to both domain edge sites.
4Cseq experiments show that transcriptional effects are not detected at genes near the majority of UCE+/− sites, including those associated with the highest counts. Together with cohesin localization near (18kb from Col28a intron) or overlapping (Nphx1, Ndufa4/Phx14) UCE+/− sites, these data raise the possibility that Evf2 plays a topological role that is distinct from transcription. Violin plot analysis of cohesin (SMC1/3) and histone modification counts at ±2kb distances from all UCE+/− sites across chr6:0–40Mb shows enrichment of cohesin at UCE+ sites compared to UCE− (Fig 5 F-K, chr6:40–150Mb, Fig S4E-J). Thus, in Evf2+/+, Evf2 directs UCE+ sites, and prevents UCE− sites, coincident with greater cohesin enrichment at UCE+ compared to UCE− sites (Fig 5L). However, in Evf2TS/TS, Evf2 is absent, resulting in UCE interacting with (−) sites instead of (+) sites, equalizing cohesin binding between (+/−) sites.
Unlike overall cohesin differences linking Evf2 UCE site selection and cohesin enrichment, analysis of HMs (H3K27ac, H3K4me3, H3K27me3, H3K4me1) indicates that only the range of H3K27ac counts at UCE+ sites increases compared to UCE− sites (Fig 5F). Analysis of specific Evf2 regulated H3K27ac sites across chr6:0–40Mb identifies only 13 regulated sites, 10/13 located 5’ of Evf2 across 31Mb (Fig 5M). Notably, 5/10 Evf2 regulated H3K27ac sites overlap with cohesin; 4/5 are located at potentially significant sites (Fig S5). In addition, Evf2 regulates H3K4me3 at 4/4 of these sites (green/red circles, Fig 5M), with correlations between (+) regulated cohesin and (+) regulated H3K4me3, and (−) regulated cohesin and (−) regulated H3K4me3. Thus, Evf2 regulated H3K27ac and H3K4me3 is site-specific, occurs at key cohesin BSs, and defines the edges of the Evf2-chr6 target gene domain as large as ~31Mb (Fig 5M).
Evf2 decreases seizure susceptibility in adult mice
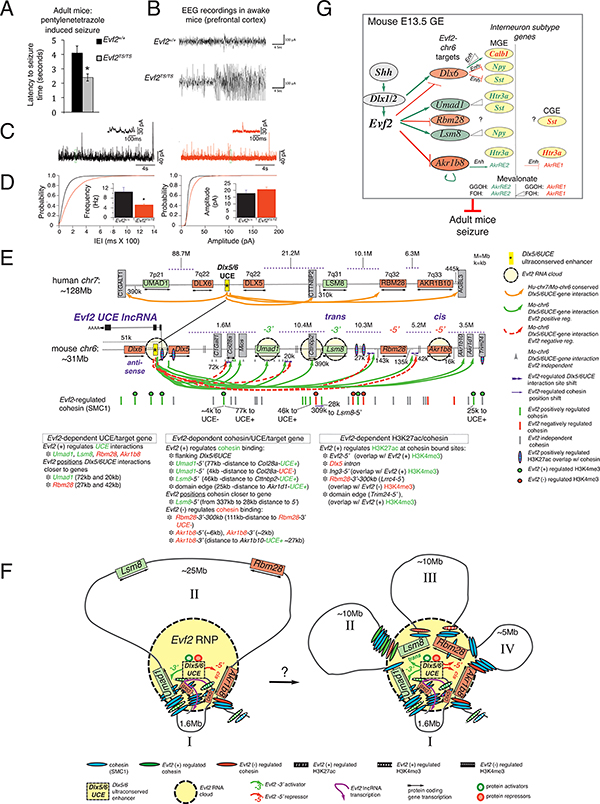
Evf2-dependent IN-SG regulation may contribute to control of GABAergic synaptic transmission in adult brain hippocampus, as previously reported (Bond et al., 2009). However, given the subtlety of IN-SG expression effects in the embryo, it is not known whether circuitry defects in live mice are detectable. Here, we show that mice lacking Evf2 exhibit increased seizure susceptibility after administration of a GABA antagonist pentylenetetrazole (PTZ, reduced latency times, Fig 6A), as well as increased seizure severity (electroencephalogram [EEG] recordings from the medial prefrontal cortex [mPFC], Fig 6B). Patch-clamp recordings from deep layers (layer V) of Evf2TS/TS cingulate cortex (CG1) indicate that seizures are likely to be caused by decreased frequency but not amplitude of GABAergic synaptic transmissions (spontaneous inhibitory postsynaptic currents [Fig 6C, D], miniature inhibitory postsynaptic currents [Fig S6A-C]), similar to previously reported defects in Evf2TS/TS hippocampal pyramidal neurons (Bond et al., 2009). However, Evf2 loss affects GABAergic synaptic transmissions in CG1 deep layer (V), but not superficial layers (II/III), (Table S4). Thus, Evf2 regulatory events are critical for GABAergic synaptic transmission in two different adult brain regions (hippocampus and mPFC), controlling seizure susceptibility in adult mice.
Figure 6. Significance and mechanisms involved in Evf2 UCE-lncRNA transcriptional regulation of interneuron diversity.
A. Latency to seizures in Evf2+/+ (black bars) and Evf2TS/TS mice (grey bars). Times of seizure onset indicate latency to seizure (in seconds), measured after injection with PTZ (25mg/kg), n=4 pairs of male littermates for each genotype, error bars (S.E.M.). B. Representative trace EEG recordings in freely moving awake mice were obtained from electrodes placed in the prefrontal cortex (BREGMA 2.0mm) of Evf2+/+ and Evf2TS/TS (adult male littermates) after injection. Traces from one of 3 littermate male pairs are shown. Recordings capture epileptiform discharges, measuring both frequency (x-axis, seconds) and amplitude (y-axis, μA). C, D. Whole-cell, voltage-clamp recordings from pyramidal cells in deep layer V of acute cortical slices compare Evf2+/+ (black) and Evf2TS/TS (red) mice. C. Recordings were obtained holding the cells at 0 mV, in the presence of bath-applied DNQX (20 μM) and AP5 (5 μM). The inset traces show the trace segments included in the green boxes on expanded time and current scales. D. Cumulative distributions of the inter-event intervals (IEI) of spontaneous IPSCs. The inset bar graph shows the mean IPSC frequency, 10.6±1.8 Hz (Evf2+/+) and 5.2±0.4 Hz (Evf2TS/TS)(7 and 8 cells, respectively; *p<0.05. Cumulative distributions of the IPSC amplitudes. The inset bar graph shows the mean event amplitudes (17.8±2.4 pA vs. 20.8±1.6 pA, Evf2+/+ n= 7 and Evf2TS/TS n=8 cells); Mann Whitney test, frequency: p=0.03, amplitude: p=0.13. error bars (S.E.M.) E. Across Dlx6-Akr1b8 region of mouse chr6: Evf2-chr6 target gene organization is conserved with human chr7, except UMAD1 is located 88Mb 3’ of UCE (7p21). UCE-Umad1, -Lsm8, -Rbm28, -Akr1b8/10 UCEins conserved in mouse E13.5GE and developing human brain (orange arrows). UCE+/− (solid green and red dashed arrows), distance of Evf2-dependent UCE position shifts (near red dashed lines), UCE-I (grey triangles), Evf2-regulated cohesin (SMC1) binding (red/green lines), Evf2-independent cohesin binding (grey lines), cohesin bound-Evf2 regulated H3K27ac sites (blue ovals with pink stripes), Evf2 RNA cloud localization (yellow circles, as verified by RNA/DNA FISH), lncRNA mechanisms involved at specific target genes: Evf2–3’ activator (−3’), Evf2–5’ repressor (−5’), trans target Lsm8, and cis target Akr1b8. UCE interactions and cohesin binding between double lines are omitted for clarity. F. Possible spatial relationships between Evf2 UCE-lncRNA cloud-UCE and target genes-aligned with Evf2 regulated cohesin, and cohesin sites that overlap with Evf2 (+) regulated H3K27ac (white lines), Evf2 (+) regulated H3K4me3 (large white dots), and Evf2 (−) regulated H3K4me3 (small white dots). Left: Model showing activated target Umad1 and repressed target Akr1b8 associated with UCE and Evf2 RNA cloud. Right: Model showing all four target genes associated with UCE and Evf2 RNA cloud. Loops I-IV (representing intergenic distances) may form sequentially, in a combinatorial manner, or at the same time. G. Schematic showing the relationship between Evf2 chr6 targets and IN-SG regulation, including the involvement of specific enhancers (Enh), dosage dependence (triangles), and MV metabolites GGOH and FOH. Evf2 represses Dlx6, Rbm28 and Akr1b8 (red ovals) through Evf2–5’ UCE-containing region. Dlx6 dosage regulates Enhs in IN-SGs (Calb1, Npy, Sst), contributing to IN diversity. Akr1b8, an aldoketoreductase and MV pathway metabolites (FOH and GGOH) regulate Enhs at the promoter of Zbtb16, downstream of the IN-SG (5Htr3a). Evf2 activates Umad1 and Lsm8 (green ovals), activating Lsm8 through trans-mechanisms. Umad1 and Lsm8 dosage are genetically linked to IN-SG dosage (Umad1:5Htr3a, Umad1:Sst, Lsm8:Npy), through unknown mechanisms. Evf2 regulation of IN-SGs depends on embryonic brain region (MGE vs. CGE). Evf2-dependent events in the developing brain control seizure susceptibility in adult mice.
Discussion
In this work, we show that a cloud-forming UCE-lncRNA selects UCE DNA interaction sites to multi-megabase distant genes through complex effects on topology. These studies link lncRNA-dependent topological and transcriptional control with cohesin binding, HMs, and the regulation of embryonic IN diversity, supporting that synergistic interactions between specific RNA, DNA and protein components organize and regulate gene expression. We propose the following roles for the Evf2 UCE-lncRNA: (1) Precise organization of the UCE and cohesin relative to target genes, enabling gene selectivity spanning a multi-megabase domain. (2) Precise organization of an RNA cloud subnuclear domain, providing a source for diverse regulatory factors including chromatin structural proteins (cohesin), key TFs (Dlx1), remodeling proteins (Smarca4/Brg1), and additional Evf2-RNPs, as previously reported (Cajigas et al., 2015), contributing to both gene selectivity and enhancer activity. (3) Determination of UCE repressor and activator activities through cis and trans-acting mechanisms and differential utilization of the lncRNA −5’ (UCE) and −3’ ends. (4) Topological and transcriptional control of a gene network that regulates embryonic interneuron diversity and adult seizure susceptibility.
Mechanisms distinguishing UCE RNA cloud gene targeting, cis-repression, and trans-activation
The Evf2 RNA cloud localizes to long-range activated and repressed target genes, supporting a direct role of the UCE-lncRNA in regulating UCE activity (yellow circles, Fig 6E). The Evf2–5’ UCE-containing region (Evf2–5’) is sufficient to regulate IN-SGs through antisense repression of Dlx6 and long-range repression of Akr1b8, demonstrating a functional role for the UCE region, in vivo. Consistent with Evf2–5’ repression of Akr1b8, Evf2–5’ forms RNA clouds that localize to Akr1b8. However, despite Evf2–5’ RNA cloud co-localization at long-range activated target genes (Umad1, Lsm8), Evf2–5’ is not sufficient for transcriptional activation, indicating that the Evf2–3’ end is required for activation. Differential contributions of Evf2–5’- and −3’ to long-range repression and activation, respectively, support lncRNA functional constraints outside of the Evf2 ultraconserved sequence. Given that Evf2 recruits both transcriptional activators and repressors to the UCE (Bond et al., 2009; Cajigas et al., 2015), and that the Evf2-RNP contains both activators and repressors, it is possible that distribution of activity-specific factors within the cloud depends on the Evf2–5’ vs. −3’ ends. Possible configurations of simultaneous RNA cloud/UCE association with repressed and activated target genes support a model in which the RNA cloud functions as an organizer of UCE DNA, UCE-lncRNA and proteins for target gene selection and UCE activity (Fig 6F).
Evf2–5’ truncation studies show that RNA cloud localization at target genes is not sufficient for regulating UCE activation. The rules governing UCE-lncRNA/UCE activity relationships are further complicated by transgenic rescue experiments indicating that Evf2 activates Lsm8 in trans, unexpected because Lsm8 is located on the same chromosome. In addition, failure of the transgene to rescue Akr1b8 supports Evf2 repression of Akr1b8 through cis-mechanisms, also unexpected because Akr1b8 is further from Evf2 than Lsm8 (by ~15Mb). It is possible that specific 3-dimensional configurations place Akr1b8 closer to Evf2–5’ and UCE, while Lsm8 is further requiring Evf2–3’ trans effects, as shown in Fig 6F. However, several key questions still remain regarding Evf2-trans regulation. Importantly, while the rescue model in this work could not determine whether trans or cis mechanisms regulate Umad1 and Rbm28, differential regulation of Lsm8 and Akr1b8 supports functional separation of the UCE-lncRNA into Evf2–5’UCE (cis-repression and RNA cloud gene targeting) and Evf2–3’ (trans-activation) regions. Precise definition of the regions of Evf2–5’ and 3’ that contribute to cis-repression vs. trans-activation, and RNA cloud gene targeting will be critical to further understanding UCE-lncRNA-dependent regulation of UCE activity.
Selective regulation of multi-megabase distant genes through complex effects on chromosome topology
How does Evf2 select genes over multi-megabase distances? 3D-chromosomal analysis shows that Evf2 positively regulates UCEins near all chr6 target genes (green arrows, Fig 6E), supporting a link between topological and transcriptional control. Evf2 shifts UCEins towards both activated and repressed target genes, with shifted distances ranging from 20–72kb. However, given that multiple UCEins are also Evf2-independent at Umad1 (grey triangles, Fig 6E), the significance of precisely regulated Evf2-dependent positioning of UCE in relation to transcriptional regulation remains to be determined.
The Evf2 RNA cloud co-localizes with SMC3 and regulates SMC1/3 binding at Cttnbp2, supporting the potential significance of the Cttnbp2-UCE+ to Lsm8 activation. In addition, Evf2 shifts cohesin binding ~141kb within Cttnbp2, and positively regulates cohesin binding ~25kb 5’ of Lsm8. Importantly, Evf2 regulation of cohesin binding correlates with UCE activity, raising the possibility that Evf2 regulated cohesin binding may distinguish between activation and repression mechanisms. In addition, Evf2 regulated cohesin positioning (near Lsm8, a trans-activated target) is unique to Lsm8, and differs from positioning of UCEins at activated target gene Umad1.
Together, these data support the idea that Evf2 regulates individual long-range targets through shared and distinct mechanisms that may involve differentially regulated UCE and cohesin positioning and/or binding. Cohesin-dependent chromosome looping (Rao et al., 2017; Schwarzer et al., 2017) and ThymoD lncRNA regulation of cohesin and Enh interactions (Isoda et al., 2017), combined with our data on Evf2 support the idea that lncRNA regulated cohesin contributes to both topological (UCE selectivity) and transcriptional (UCE activity) control.
An important observation from our studies is that the majority of cohesin bound sites and UCE interactions are Evf2-independent. Thus, consistent with gene expression effects on a small number of genes, it is likely that Evf2 precisely organizes UCE/target genes/proteins, directing relationships within a pre-existing topology. However, a major question arising from our work is how the boundaries of such a large (~27Mb) domain are established. Analysis of cohesin overlap with Evf2 regulated H3K27ac and H3K4me3 defines the edges of the 31Mb domain raising the possibility that boundaries are established at Evf2-dependent and independent cohesin BSs through a unique combination of mechanisms involving both histone acetylation and methylation. Given that SMC1 is a component of the Evf2-RNP (Cajigas et al., 2015), that contain chromatin remodelers known to bind RNA and acetylated histones (SMARCA4, BRG1), future experiments involving the contribution of individual Evf2-RNP proteins in topological and transcriptional control will be important.
Although the majority of Evf2-regulated UCEins do not cause detectable changes in gene expression (permissive sites), it is possible that cellular heterogeneity masks transcriptional changes. Combined RNA/DNA and DNA FISH support heterogeneity in topology among IN progenitors. Given reports of highly heterogeneous chromosome 3D structures in single cells (Nagano et al., 2013; Nagano et al., 2017; Stevens et al., 2017), it will be important to determine whether heterogeneity results from transient, unsynchronized interactions that occur in the majority of cells, or interactions that are limited to specific GE subpopulations. While the ability of a single Enh to contact and regulate multiple genes simultaneously has been shown (Fukaya et al., 2016), simultaneous association of UCE with a repressed and activated target gene supports the possibility that differential regulation may also occur at a single site. As discussed above, repression but not activation requires the Evf2-3’ end, supporting that distribution of activity-regulating factors to specific genes occurs in an RNA-dependent manner. The distribution of regulatory factors from a centrally localized RNA cloud and the presence of both transcriptional activators and repressors in the Evf2-RNP are consistent with a recently proposed phase-transition model for organizing Enh-RNA-protein complexes (Hnisz et al., 2017). While it remains to be determined whether the Evf2 RNA cloud or Evf2-RNPs mediate phase-transitions, our data support the idea that the Evf2 RNA cloud is an Enh organizer, acting through both chromosome looping and protein recruitment. Unlike UCE+/−, the majority of UCE-I’s cluster within chr6:0–9Mb, (ending at the edge of a cluster of genes containing Umad1), supporting a role for Evf2 in super-looping events (distances greater than >2Mb from the Enh). Future experiments designed to answer whether RNA clouds are mobile, DNA is looped through stationary clouds, or RNA clouds undergo cycles of assembly/disassembly will be important for understanding the mechanistic basis for RNA-dependent selective looping/gene regulation over multi-megabase domains.
Another possible explanation for permissive UCEins is that topological changes change during developmental and or cell cycle transitions, as previously reported (Hug et al., 2017; Nagano et al., 2017; Noordermeer et al., 2011; Phillips-Cremins et al., 2013). Therefore, Evf2 may establish a topology required for future regulatory events, similar to that proposed for ZRS-Shh interactions in the developing limb (Williamson et al., 2016). Future experiments to determine the roles of heterogeneity, transient vs. stable associations, and developmental timing in linking transcriptional effects and Evf2-regulated UCEins will be important for distinguishing between RNA-dependent instructive and permissive topological control.
Identification of regulatory pathways contributing to interneuron diversity and function
Evf2 target gene-UCEins are conserved in mouse E13.5 GE (Fig 6E) and human developing brain (Won et al., 2016), and along with Evf2 ~6000-fold enrichment in human embryonic brain INs compared to other lncRNAs (Liu et al., 2016), support potential significance to human brain development. Analysis of multiple mouse genetic models identifies in vivo dosage relationships between Evf2-chr6 target genes and IN-SGs, linking five of six Evf2-chr6 target genes to IN-SG regulation, supporting the idea that Evf2 is a master regulator of IN diversity. While involvement of Evf2-Dlx6 in regulating IN-SGs is not surprising, identification of Dlx6-regulated Enhs at Calb1, Npy, and Sst, as well as repressive activities unexpectedly support a major role for Dlx6 dosage. In addition, the Evf2-Akr1b8–5Htr3a axis links the MV pathway to Akr1b8 regionally controlled Enh activity, identifying a role for lncRNA regulation of lipid metabolism in IN diversity. This is important, as the embryonic 5Htr3a+ IN population gives rise to a major subclass of adult INs (VIP+) involved in disinhibition, and control of adult brain circuitry and behavior (Lee et al., 2013; Letzkus et al., 2011; Pi et al., 2013). In addition, 5Htr3a itself controls the migration of IN progenitors from CGE to adult destinations, modulating growth cone dynamics in CGE-derived INs, but not MGE-derived INs (Murthy et al., 2014). Our data sheds light on gene regulatory differences between CGE and MGE, revealing regional control of the Evf2-Akr1b8–5Htr3a axis and identifying CGE- and MGE-specific effects of MV pathway-regulated Enhs. While Akr1b8 (AKR1B10 in human) belongs to a large family of aldo-keto reductases with links to diabetes and cancer (Penning, 2015), to our knowledge, roles in neuronal development or brain circuitry have not been reported. These studies are significant as they reveal a pathway for modulating 5Htr3a in developing neurons, demonstrating the potential for identifying signaling pathways through studies of UCE-lncRNA gene regulation in vivo.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to Jhumku D. Kohtz (j-kohtz@northwestern.edu)
Experimental Model and Subject Details
Generation of Evf1 TS/TS Mice
The Evf1 targeting construct was generated using lambda phage based recombineering in E.coli as described (Liu et al., 2003). The retrieval vector was constructed as follows. Using high fidelity Taq (Roche), homology arms of approximately 500 bp were PCR amplified (with restriction sites added) from BAC DNA. Using a three-fragment ligation, homology arms were cloned into ClaI and NheI sites of PL253, with a HindIII site engineered between them. A 19.4 kb region (corresponding to position 6,809,651–6,825,742 on mouse chromosome 6, NCBI assembly) was retrieved from pBAC e3.6 M8 (M. Ekker, U. Ottawa) into the retrieval plasmid using recombination-induced EL250 cells (Liu et al., 2003). Further targeting was performed on the retrieved plasmid. The polyadenylation targeting vector was constructed in PL452, a floxed-Neo containing plasmid. The triple polyadenylation signal (Soriano, 1999) was cloned into EcoRI and SalI sites of PL452. Approximately 500bp of targeting homology arms were cloned sequentially on either side of the polyA-floxed-Neo insert. Briefly, fragments were PCR amplified as above and cloned into either ClaI and KpnI sites or NotI and SacII sites. This triple polyA-floxed-Neo cassette was targeted into the retrieved 19.4kb region using recombination-induced EL250 cells. Successful targeting was confirmed by Southern blot analysis of the completed construct using internal probes (NEBlot kit, NEB).
Mouse ES cells were targeted by homologous recombination using standard procedures. Successful targeting in ES cells was confirmed by Southern blot, verifying proper recombination at both the 5’ and 3 ends. Probes were generated outside the 19.4kb homologous region. EL250 cells and recombineering plasmids PL253 and PL452 were provided by Dr. Neal Copeland.
Evf1TS (floxed neo)/+ heterozygotes were verified by Southern, crossed to EIIAcre (Jackson Labs) for two generations, and crossed to the Evf2TS/TS background. Neo removal was verified by PCR (not shown). Mice are maintained on the same mixed background as Evf2TS/TS strain; all mice are housed according to IACUC guidelines.
Additional mouse strains
Evf2TS/TS (Bond et al., 2009) were crossed to C57/Bl6 for one generation, and maintained on a mixed background (C57/Bl6, 129Sv, FVB), source: Kohtz lab
Evf2TS/TS;R (Berghoff et al., 2013), maintained on the same background as Evf2TS/TS, source: Kohtz lab
Akr1b8+/− (Akr1b8tm1.1(KOMP)Vlcg), source: Jackson (strain 024334, https://www.jax.org/strain/024334)
Akr1b8−/−; Evf2TS/TS: crossed to Evf2TS/TS for three generations, and maintained on the Evf2TS/TS mixed background, source: Kohtz lab
Dlx5/6KO/TS: Dlx5/6KO/+ mice (Merlo et al., 2002) were maintained on Evf2TS/+ background, and crossed to Evf2TS/TS mixed background, source of Dlx5/6KO/+ (A. Bendall)
Dlx1/2+/−: mice were obtained from K. Campbell (Cincinnati Children’s), as reported in (Anderson et al., 1997b).
Method Details
Microarray data and validation
E13.5 medial and caudal ganglionic eminences were isolated from embryos using fine microdissection scissors (Lumsden bioscissors), in L15 medium. In Fig 2A, a schematic of E13.5 mouse brain shows ganglionic eminences (LGE, MGE, CGE, based on schematic (Gelman and Marin, 2010), and dorsal/ventral and rostral/caudal axes. Dotted red line shows the boundary between MGE/LGE and CGE where tissues are dissected. At E13.5, the sulcus between MGE and LGE is well defined, allowing precise definition of LGE/MGE/CGE regions under a dissecting microscope. RNA isolation, cDNA production, qPCR were performed, as previously described (Berghoff et al., 2013). For microarray analysis, 5 pools of E13.5 MGEs from two brains per genotype from males (5) and females (5) were hybridized to 10 Affymetrix. GeneChIP.Mouse430_2 arrays, and the results analyzed using GeneSpring software. Genes showing a minimum of 2-fold differences, and p-values of ≤0.05 were validated further by TaqMan qRT-PCR.
Luciferase Reporter and Expression Vectors
For all luciferase experiments, enhancers were cloned into the pGL3 promoter vector (Promega) using the KpnI and NheI restriction sites. The Calb1 enhancers (site 1, site 2, and site 3), AkrR enhancers (site 1 and site 2), NPY enhancers (site 1 and site 2), and SST enhancer (site 1) were identified by MACS2 peak analysis of ChIPseq (DNA sequences obtained from the UCSC genome browser). Enhancer sequences from C57BL/6J mouse genomic DNA were PCR amplified for subcloning into expression plasmids.
Expression plasmids for pCMV6-Akr1b8, pcDNA3-EGFP, or pENTR223.1-Dlx6, were purchased from Origene, Addgene, or DNASU, respectively. Dlx6 was amplified by PCR and cloned into the BamHI and EcoRI restriction sites on the pcDNA3 backbone. To generate the pCMV6-EGFP control plasmid, EGFP was PCR amplified and cloned into the pCMV6 empty using the restriction sites AscI and NotI.
Primary embryonic brain GE transfections
MGE and CGE tissues were dissected from E13.5 Swiss Webster embryos, dissociated in L15 media by pipetting several times, and spun through a cell strainer for single cell preparations. Briefly, cells were seeded at a density of 2.5 × 105 cells per cm2 (Flandin et al., 2011). One day prior to seeding cells, 24-well plates were coated with poly-L-lysine (30 μg/mL; Sigma) and laminin (5 μg/mL; Sigma), while 96-well plates were coated with poly-L-lysine (3 μg/mL; Sigma) and laminin (5 μg/mL; Sigma). Initially, cells were seeded in neurobasal medium (DMEM/F-12 supplemented with L-glutamate, B-27 (Gibco), N2 supplement (Gibco), bovine pituitary extract (35 μg/mL; Life Technologies), mito+ serum extender (BD Biosciences), penicillin (100 U/mL; Gibco), streptomycin (100 μg/mL; Gibco), and glutamax (0.8 mM; Gibco)).
Specifically, for the Akr1b8 gene expression study, cells were seeded at 470,000 cells per well in a 24-well plate. 24 hours after culturing cells, the medium was changed to neuralbasal media without antibiotics and 1.4 μg of expression vector (pCMV6-AKR1b8) or control vector (pCMV6-EGFP) was transfected using Fugene 6 (Promega), as recommended in the user manual. Cells were harvested 48 hours after transfection for RNA isolation (PicoPure RNA isolation kit; Applied Biosystems). Akr1b8 and 5Htr3a levels were quantified by RT-PCR (Assay ID: Mm00484314, and Assay ID: Mm00442874, respectively) and normalized to β-actin. To determine the contribution of endogenous and episomal expression to the total Akr1b8 levels, we used RT-PCR quantification with primers specific for endogenous Akr1b8 (Akr1b8-F: 5′-CCTGCCTGACATCCTGCTAT-3’, Akr1b8-R: 5′-GGAGATGTCCGTTCGCTTCT-3’). These primers amplify a region of Akr1b8 exon 10 that is not present in the pCMV6-AKR1b8 plasmid sequence. The Akr1b8 Taqman Gene Expression Assay (ID Mm00484314) amplifies a region of Akr1b8 (exon 9–10) that is present in both endogenous and episomal Akr1b8 cDNA.
For all luciferase experiments, cells were cultured at a density of 78,300 cells per well in a 96-well microplate treated for tissue culture. Cells were allowed to attach for 24 hours before changing the medium to neuralbasal media without antibiotics. Transfections using Fugene 6 (Promega) were performed as recommended. Cells were harvested 48 hours after transfection with 1X passive lysis buffer (Promega) supplemented with 0.1% Digitonin (Sigma) for cell lysis. To ensure thorough cell lysis, lysates were subjected to two freeze-thaw cycles prior to performing Dual Luciferase Reporter assays (Promega). All transfections were normalized to the internal control expressing Renilla luciferase, performed at least in triplicate and a minimum of two times.
For Calb1 enhancer transfections, we used five concentrations ranging from 20 ng to 240 ng of pcDNA3-Dlx6, where the total amount of expression plasmids was maintained at 240 ng using pcDNA3 EGFP as the control; 50 ng of pGL3 luciferase reporter containing Calb1 site 1, site 2, or site 3; and 5 ng of pRL null. For NPY and SST transfections, three concentrations ranging from 40 ng to 160 ng of pcDNA3-Dlx6 were tested, where the total amount of expression plasmids was maintained at 280 ng using pcDNA3 EGFP as the control, along with 50 ng of pGL3 luciferase reporter containing NPY site 1, NPY site 2, or SST site 4, and 5 ng of pRL null. For AkrR-enhancer transfections, optimal effects were obtained with 160 ng of pCMV6-Akr1b8 for CGE AkrRE1/2 and MGE AkrRE1, and 80 ng of pCMV6-Akr1b8 for MGE AkrRE2. The total amount of expressed plasmid DNA was maintained at 240 ng using pCMV6 EGFP as the control. For reporters, we used 50 ng of pGL3 luciferase reporter containing AkrRE1/2 and 5 ng of pRL null.
For Farnesol (FOH; Sigma) and Geranylgeraniol (GGOH; Sigma) treated cells, 50 ng of each AkrR enhancer reporter plasmid and 5 ng of pRL null were used. FOH and GGOH were freshly prepared in DMSO (Sigma) at varying concentrations using serial dilutions. Neurobasal media without antibiotics was supplemented with a final concentration of 0.01, 0.1, 1, 10, or 100 μM for GGOH and 0.1, 1, 10, or 100 μM for FOH. Prior to adding transfection reagent/DNA mixture, the media was changed to that containing the respective concentration of metabolite.
Chromatin immunoprecipitation (ChIP)
For ChIP experiments whole ganglionic eminences were dissected from 10 Evf2+/+ and 10 Evf2TS/TS E13.5 embryos. Tissues were pooled for each genotype, triturated by pipetting, and filtered through a cell-strainer capped 5 ml polystyrene round- bottom tube (BD Falcon) to make single-cell suspensions. Duplicate ChIP experiments were performed to determine reproducibility, generating libraries as described below.
Native ChIP
Native ChIP protocol has been described in detail previously (Brind’Amour et al., 2015), and detailed for E13.5 GE cells as follows. Cells from the single cell suspension described above were split into 1 × 106 cell aliquots, and pelleted through centrifugation at 1000 × g for 10 min. Cell pellets were flash frozen in liquid nitrogen, and stored at −80°C. Nuclei were isolated using EZ Nuclei Isolation Lysis Buffer (N3408, SIGMA). Chromatin was digested in 2U/μl Micrococcal nuclease (M0247S, NEB) at 37°C for 7 min. The reaction was quenched with EDTA (10 mM final concentration). Triton X-100 and Sodium Deoxycholate were added (0.1% final concentration). Samples were incubated on ice for >15 minutes. Immunoprecipitation buffer (20 mM Tris-HCl pH 8.0, 2 mM EDTA, 150 mM NaCl, 0.1% Triton X-100, 1x Protease inhibitor cocktail, 1 mM PMSF) was added to a final volume of 200 μl and the samples were rotated at 4°C for 1 hour. The chromatin was pre-cleared by rotating at 4°C with 15 μl of Protein G–Agarose beads for 1 hour. After centrifugation to pellet the beads, the supernatant was further pre-cleared by rotating at 4°C with 15 μl rabbit IgG conjugated Protein G–Agarose beads for 1 hour. The pre-cleared chromatin was incubated with rabbit IgG (1 μg), or antibodies targeting histone modifications (1 μg) at 4°C for 1–2 hours with rotation. 15 μl of Protein G-Agarose beads blocked with 1% BSA in 1X PBS were added to each sample and incubated at 4°C overnight with rotation. The beads were pelleted by centrifugation and washed twice with 200 μl Low Salt Wash buffer (20 mM Tris-HCl pH 8.0, 2 mM EDTA, 150 mM NaCl, 1% Triton X-100, 0.1% SDS) and twice with 200 μl High Salt Wash buffer (20 mM Tris-HCl pH 8.0, 2 mM EDTA, 500 mM NaCl, 1% Triton X-100, 0.1% SDS). Immunoprecipitated DNA was eluted in 100 μl of ChIP elution buffer (100 mM NaHCO3, 1% SDS) at 65°C for 1–1.5 hour. The DNA was purified using phenol chloroform extraction and ethanol precipitated. The pellet was resuspended in 10 mM Tris-HCl pH 8.5. The DNA was incubated with 20 mg of RNAse A at 55°C for 1 hour. 40 mg Proteinase K were added and incubated at 55°C for 1 hour. The immunoprecipitated DNA was purified using the Qiaquick PCR Purification Kit.
Antibodies: ChIP antibodies targeting histone modifications are Encode verified: H3K4me3 (Abcam ab8580), H3K4me1 (Abcam ab8895), H3K27me3 (Active Motif 39155).
Cross-linked ChIP
For anti-DLX and anti-H3K27ac ChIP cells were fixed in 1% paraformaldehyde for 10 min. For anti-SMC1 and anti-SMC3 ChIP cells were fixed in 1% paraformaldehyde for 90 min. Cells were lysed in SDS lysis buffer (1% SDS, 50 mM Tris-HCl pH 8, 10 mM EDTA) with protease inhibitors (11836153001, Roche). The lysates were sonicated with a Bioruptor Pico (Diagenode) for 10 cycles (30 sec On, 30 sec Off). The lysates were then centrifuged to pellet cellular debris and the supernatant collected for ChIP. 25 μg of chromatin were diluted 1:10 in RIPA Buffer (10mM Tris pH 7.6, 1mM EDTA, 0.1% SDS, 0.1% Sodium Deoxycholate, 1% Triton X-100) with protease inhibitors (B14002, Biotool). The chromatin was pre-cleared by rotating at 4°C with 50 μl of Protein G–Agarose beads (11719416001, Roche) for 1 hour. After centrifugation to pellet the beads, the supernatant was further pre-cleared by rotating at 4°C with 50 μl rabbit IgG conjugated Protein G–Agarose beads for 1 hour. The precleared chromatin was incubated with rabbit IgG (2.5 μg), previously validated anti-pan-DLX (2.5 μg, (Bond et al., 2009; Cajigas et al., 2015; Feng et al., 2006)), anti-H3K27ac (1 μg, Abcam Ab4729), anti-SMC1 (1 μg, Bethyl A300–055A) or anti-SMC3 (1 μg, Abcam ab9263) at 4°C for 4 hours with rotation. 50 μl of Protein G-Agarose beads blocked with 1% BSA in 1X PBS were added to each sample and incubated at 4°C overnight with rotation. Beads were pelleted by centrifugation and washed twice with Low Salt Buffer (20 mM Tris-HCl pH 8.1, 2 mM EDTA, 150 mM NaCl, 0.1% SDS, 1% Triton X-100), three times with High Salt Buffer (20 mM Tris-HCl pH 8.1, 2 mM EDTA, 500 mM NaCl, 0.1% SDS, 1% Triton X-100), four times with LiCl buffer (0.25M LiCl, 10 mM Tris-HCl pH 8.1, 1 mM EDTA, 1% sodium deoxycholate and 1% NP-40), twice with 0.1% Tween-20 in 1X PBS, and once with TE buffer (10 mM Tris-HCl pH 8.1 and 1 mM EDTA). Immunoprecipitated DNA was eluted from the beads by incubation with 200 μl of elution buffer (50 mM Tris-HCl pH 8, 10 mM EDTA, 1% SDS) at 65°C for 1 hour. The beads were removed by centrifugation and DNA crosslinking was reversed at 65°C for 4 hours. The DNA was incubated with 20 mg of RNAse A at 55°C for 1 hour. 40 mg Proteinase K (3115879001, Roche) were added and incubated at 55°C for 1 hour. The Immunoprecipitated DNA was purified using the Qiaquick PCR Purification Kit (Qiagen).
ChIPseq library preparation and sequencing
Quantity of ChIP’d DNA was determined using Picogreen Reagent (Quant-iT™ PicoGreen dsDNA Assay Kit, Thermo Fisher P11496) and a fluorometer instrument. 150ng to 1ug of DNA was prepared into Illumina libraries, according to manufacturer’s instructions, using the TruSeq Nano DNA Library Prep Kit (Illumina, FC-121–4003). Resulting libraries were deep sequenced, using the Illumina HiSeq2500 system in Rapid Run mode, obtaining between 10M and 15M of 100-bp length, single-end reads per library.
ChIPseq read alignment
Raw sequencing reads for all the individual ChIPseq datasets were aligned using bwa (Li and Durbin, 2009) (version 0.7.12) mapper with the following settings ‘aln −t 8 samse’. We allowed two mismatches relative to the reference and only retained the unique alignments with Phred quality score greater than 30 as done in the previous study (Marinov et al., 2014). The datasets were mapped against mm9 version of the mouse genome.
ChIPseq data analysis
Quality assessment
ChIPseq quality assessment was carried out using the strategy described by ENCODE ChIPseq data analysis guidelines (Landt et al., 2012). Cross-correlation analysis was performed using SPP package (Kharchenko et al., 2008) using the parameter ‘−s=−100:5:600’. The analysis is essential to assess the NSC (Normalized Strand Correlation) and RSC (Relative Strand Correlation) values as recommended by ENCODE (Landt et al., 2012). As per the guideline, all of our selected ChIPseq datasets are above NSC value (> 1.05) and RSC value (> 0.8) threshold, and subsequent QC scores equal to or above 1 (Landt et al., 2012; Marinov et al., 2014).
Peak calling and differential ChIPseq analysis
After quality assessment, we used “irreproducible discovery rate” (IDR) framework to call the peaks against their respective input ChIP libraries using MACS2 program (Feng et al., 2011) as described in the ENCODE guidelines (Landt et al., 2012). MACS2 peak calling was performed using the following settings ‘-p 1e-3 --tolarge --nomodel --shiftsize’ while the other parameters were set to their default mode. The final conservative set of peaks for all the samples were called across technical replicates with an IDR threshold of 0.01.
Differential ChIPseq analysis
Differential ChIPseq analysis between two conditions was performed using MACS2 program (Feng et al., 2011) by treating one of the samples as the control for the other. The peak identification by MACS2 was carried out using the same parameter settings as previously described in the ChIPseq data analysis section. The cross-correlation analysis step (Kharchenko et al., 2008) was also performed on the respective datasets to determine the ‘--shiftsize’ parameter essential for peak identification by MACS2.
Chromosome conformation capture (4Cseq)
Whole ganglionic eminences (GEs) were dissected from 10 Evf2+/+ and 10 Evf2TS/TS E13.5 embryos (schematic in Fig 2A, GE=LGE+MGE+CGE). Tissues were pooled for each genotype, triturated by pipetting, and filtered through a cell-strainer capped 5 ml polystyrene round- bottom tube (BD Falcon) to make single-cell suspensions. Cells were fixed in 2% paraformaldehyde/10% Fetal Bovine Serum (FBS) at room temperature for 10 min with rotation. 125 mM glycine was used to quench the formaldehyde. The 4C method used has been described in detail (van de Werken et al., 2012). EcoRI was used for the primary restriction digestion and DpnII was used for the secondary restriction digestion.
The following steps were performed to generate the 4C library for sequencing. First, overhangs were added to the 4C template using PCR amplification with primers containing the bait sequence. Primers:
Dlx5/6UCE Forward:
5’TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGATGCCAAACCACTGTGAGTGTA3’
Dlx5/6UCE Reverse:
5’GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGTCCCCAATGTCTGCTTCAA 3’.
PCR reaction: 200 ng 4C template, 0.2 mM dNTPs, 35 pmol Primer Dlx5/6UCE-Fwd, 35 pmol Primer Dlx5/6UCE-Rev, 1.75 U Expand Long Template Enzyme Mix (Roche), 1X Buffer I. PCR cycles: 94°C - 2 min, 94°C – 10 sec, 55°C - 1min, 68°C - 3 min, 29 cycles, 68°C - 5min. The PCR product was purified using the High Pure PCR Product Purification Kit (Roche). Then, the 4C DNA containing the overhangs was used as template for a second PCR that adds index sequences and Illumina sequencing adapters to generate the 4C library for sequencing. PCR reaction (50 μl): 225 ng DNA template, 0.5 mM dNTPs, 5μl Nextera XT Index1 primer (N7XX, Illumina), 5 μl Nextera Index 2 primer (S5XX, Illumina), 3.5 U Expand Long Template Enzyme Mix (Roche), 1X Buffer I. PCR cycles: 94°C - 5 min, 94°C – 10 sec, 55°C – 30 sec, 68°C - 1 min, 8 cycles, 68°C - 7min.The PCR product was purified using the High Pure PCR Product Purification Kit (Roche).
4Cseq reads mapping
4C sequencing reads for all the samples were aligned on a reduced mm9 version of mouse genome using bowtie2 alignment program (Langmead and Salzberg, 2012). The reduced genome consists of only EcoRI (+/− 50 base-pair) cut-sites. These EcoRI sites were selected based on the presence of a second restriction enzyme cut-site i.e. DpnII, within +/− 500 base-pairs. We trimmed the 5’ end of the raw reads to remove the bait sequence before mapping on to the reduced genome. We allowed two mismatches outside the EcoRI sequence in the reduced genome during mapping and only retained chromosome 6 specific unique alignments with Phred quality score greater than 30.
4Cseq differential data analysis
4C reads mapped at the EcoRI restriction site resolution on chromosome 6, were further filtered based on their reproducibility in each pair of replicates. An EcoRI cut-site was deemed reproducible if the two replicates in a given condition (Evf2+/+ and Evf2TS/TS) have either both non-zero counts or both zero counts. We identified 1108 and 1266 non-zero count EcoRI restriction cut-sites that are reproducible in both replicates of Evf2+/+ and Evf2TS/TS, respectively. Across the two conditions (Evf2+/+ and Evf2TS/TS), we retained a total of 997 reproducible 4C sites that either have reproducible interactions in the two replicates of either case or in both conditions. We then performed a DESeq2 (Love et al., 2014) based differential contact count analysis on these sites to identify Evf2-regulated sites (p-adjusted value ≤ 0.05 and a log2 fold change ≥ 2 for positively regulated (+) or ≤ −2 for negatively regulated (−)) and Evf2-independent (I) (p-adjusted value > 0.05 and an absolute log2 fold change < 2) 4C interaction sites.
We also performed the 4Cseq analysis using FourCSeq program (Klein et al., 2015). FourCSeq program models the overall decreasing interaction frequency with genomic distance by fitting a smooth monotonically decreasing function to suitably transformed count data. With this transformed and normalized count data, FourCSeq performs differential analysis between conditions to get significant differential interactions. We applied FourCSeq on our Evf2+/+ and Evf2TS/TS 4Cseq samples and retrieved (Dlx5/6UCE+) and (Dlx5/6UCE−) Evf2 regulated 4C interactions (p-adjusted value ≤ 0.05 and a log2 fold change ≥ 2) and Dlx5/6UCE-I, Evf2-independent interactions (p-adjusted value > 0.05 and an absolute log2 fold change < 2).
In order to avoid method-specific biases, interaction sites that were assigned the same label (+, −, I) by the two different approaches (DESeq2 and FourCSeq) were called 4Cseq-intersectional computational method sites (4Cseq-ICMs).
Histone lysine methylation determination at Dlx5/6UCE interaction sites
To interrogate the interplay between changes in chromatin contacts and changes in local chromatin landscape (e.g., histone modifications), we computed normalized ChIPseq signal of four different histone marks (H3K4me3, H3K4me1, H3K27me3, H3K27Ac) and SMC1/3 near each reproducible EcoRI cut-site. E13.5GE chromatin from different Evf genotypes was generated and, “bedtools intersect” (Quinlan and Hall, 2010) was used to average ChIPseq signals for each mark based on surrounding EcoRI cut-sites +/− 2Kb of (Dlx5/6UCE+, −, and I). Violin plots compare average ChIPseq signals between Evf2+/+ vs. Evf2TS/TS genotypes, with statistically significant differences determined by Mann Whitney U test.
Human/Mouse specific Dlx5/6UCE - gene interaction and conservation analysis
We used the preprocessed and hiclib (*https://bitbucket.org/mirnylab/hiclib) normalized human cortex Hi-C data (Won et al., 2016) (GSE77565; ftp://ftp.ncbi.nlm.nih.gov/geo/series/GSE77nnn/GSE77565/suppl/GSE77565_FBD_IC-heatmap-chr-10k.hdf5.gz) at 10kb resolution to first extract all the Evf2 (Dlx5/6UCE-containing) (chr7:96,594,838–96,643,377 in hg19) interacting genic regions (gene +/− 500Kb) on human chromosome 7. At 10kb resolution, the human Evf2/Dlx5/6UCE region is distributed within five Hi-C bins (9660 to 9664). Evf2/Dlx5/6UCE bins with non-zero normalized interaction counts with a genic region were considered for further processing in the downstream analysis. Evf2+/+ and Evf2TS/TS 4C interacting genic regions were extracted from mouse chromosome 6 in a similar manner. Next, the “liftOver” (Kent et al., 2002) tool was used to obtain the list of conserved Evf2/Dlx5/6UCE interacting genic regions among human cortex (Hi-C), mouse- Evf2+/+ (4C) and mouse-Evf2TS/TS (4C) conditions. We then applied FitHiC program (Ay et al., 2014) on the whole chromosome 7 normalized contact count map to assign statistical significance to each pair-wise interaction. FitHiC assigns statistical confidence estimates to intra-chromosomal contacts from Hi-C data by jointly modeling the random polymer effect and assay-specific technical biases. The FitHiC p-values were corrected for multiple testing for the subset of interactions with human-Evf2/Dlx5/6UCE on one side and retrieved significantly interacting 10kb bins with human Evf2/Dlx5/6UCE region at an FDR of 5%. In order to go from 10kb bins to gene coordinates, +/−50Kb was added to each significant bin and checked the overlap of 529 such extended unique bins with coordinates of annotated genes on hg19 human genome to determine the Evf2/Dlx5/6UCE interacting genes from human Hi-C data.
Circular visualization, density, histone peak plots and gene ontology analysis
Circular visualization of integrated 4C and histone mark data were generated using circos software package (Krzywinski et al., 2009). Wash U Epigenome Browser (Zhou et al., 2011) was used to plot the histone peaks and their signal intensities.
Self-organizing maps
SOMs were generated in the Matlab neural network toolbox (NNT) using three training iterations to optimally cluster gene-distance data and visualization: https://www.mathworks.com/help/nnet/gs/cluster-data-with-a-self-organizing-map.html). The NNT provides algorithms and applications to create and visualize neural networks, including methods for clustering data https://www.mathworks.com/help/nnet/index.html.
A self-organizing algorithm is an unsupervised neural network that, in a generic sense, finds patterns in data. The result is visualized through a self-organizing map (SOM). The very first self-organization formation was created in 1982 by T. Kohonen, with a basis stemming from Schelling’s 1963 segregation model. Schelling’s study concludes that entities with similar attributes tend to relocate and form homogenous groups. Essentially, self-organization is very similar to clustering. This principle has been utilized in diverse fields, including but not limited to biology, geology, business, and psychology. Most self-organization algorithms are utilized to analyze complex systems; where traditional statistics and math methods are not conclusive, or impossible to execute. Unlike traditional mapping, SOM can plot data points that have multiple dimensions. In this dataset, there are 3 dimensions represented by 3 distances, with “nodes” based on a 3 by 3 grid, or 9 clusters. The grid is plotted on the same map as the data points, initially at a random position. At a randomly selected data point, the closest node is determined, moved closer towards that data point, while the rest of the nodes also move slightly closer to that data point. This step is repeated for the total number of data points, in this case 83, then the whole process is iterated a total of 200 times. The result is the SOM. The main advantage with this method is that the computer bins the data optimally based on the three distances.
DNA Fluorescent in Situ Hybridization (FISH)
The DNA FISH method was adapted from a detailed lab protocol provided by Dr. Jerold Chun (Scripps, LaJolla, CA) (Westra et al., 2008). Single cell suspensions from whole GEs were made as described above. Cell pellets were gently resuspended in 500 μl Nuclear Extraction Buffer (0.32 M sucrose, 5 mM CaCl2, 3 mM Mg(Ac)2, 0.1 mM EDTA, 20 mM Tris-HCl pH 8.0, 0.1 % TritonX-100) and incubated on ice for 10 min. Cells were centrifuged at 100×g for 2.5 min at 4°C and the supernatant was removed. Cells were washed gently with ice-cold 1X PBS with 2 mM EGTA. Cells were centrifuged at 100×g for 2.5 min at 4°C. The supernatant was removed and cells were gently resuspended in 500 μl of ice-cold fixative (3 Methanol: 1 Glacial Acetic Acid). The cells were fixed for 10 min on ice. 5 μl of cells in fixative were transferred to Superfrost Plus microscope slides (Fisher Scientific) and allowed to air dry. The slides were transferred to a slide holder, vacuum-sealed and stored at −80°C.
Slides were incubated with 100 μg/mL RNase at 37°C for 30 min. Cells were washed twice with 2X SSC (0.30 M NaCl buffer, 0.030 M trisodium citrate) for 2 min, treated with 50 μg/mL pepsin in 0.01 M HCl at 37°C for 7 min, and washed twice with 2X SSC for 2 min. Cells were fixed in 1% paraformaldehyde for 10 min at room temperature and washed 3 times with 2X SSC for 5 min. The slides were dehydrated by incubation for 2 min in 70%, 80% and 100% ethanol. 200 μl denaturation solution (70% formamide in 2XSSC) was added and the slides were incubated at 85C°C for 10 min. Slides were dehydrated in ice-cold 70%, 80% and 100% ethanol for 2 min and allowed to air dry. 150 μl pre-hybridization buffer (50% formamide, 0.1% SDS, 300 ng/ml Salmon Sperm DNA, 2x SSC) were added and the slides were incubated overnight at 37°C.
DNA FISH probes were generated by nick translation using the FISH Tag DNA Kit (Thermo Fisher Scientific) following manufacturers instructions. The templates for the nick translation reactions were obtained from the BACPAC Resources Center (Children’s Hospital Oakland Research Institute): Dlx5/6 region: WI1–1693G2, Umad1 region: WI1–1946E1, Akr1b8 region: RP23–120B14, Lsm8 region: RP23–223J9, Cttnbp2 region: RP23–383O13. DNA probes in hybridization buffer (50% formamide, 10% dextran sulfate, 0.1% SDS, 300 ng/ml Salmon Sperm DNA, 2× SSC) were denatured in the presence of 2μg Mouse Hybloc DNA (Applied Genetics Laboratories) at 80°C for 7 min and re-annealed at 37°C for 1 hour. Slides were incubated for 5 min in 2X SSC with 50% formamide, 2 min in 4X SSC with 0.1% Tween-20 and 2 min in 2X SSC at 45°C. The slides were dehydrated in ethanol and denatured as described above. 10μl of FISH probe solution were added, the coverslips were sealed with rubber cement and the slides were incubated overnight at 37°C. Slides were incubated in 2X SSC with 50% formamide for 10 min (3 times), in 2X SSC for 10 min and in 2X SSC with 0.1% NP40 for 5 min at 45°C. The slides were rinsed with 1X PBS, incubated with 5 mg/ml DAPI for 5 min, rinsed again and mounted using SlowFade Gold antifade reagent (Thermo Fisher Scientific).
Combined RNA and DNA FISH
Slides containing cell nuclei were prepared as described above. Slides were incubated with 50 μg/mL pepsin in 0.01 M HCl at 37°C for 7 min, and washed twice with 2X SSC. Cells were fixed in 4% paraformaldehyde for 5 min at room temperature and washed 3 times with 2X SSC for 5 min. The slides were incubated in 1X PBS with 1% hydrogen peroxide for 30 min at room temperature and rinsed twice with 2X SSC. The slides were dehydrated by incubation for 2 min in 70%, 80% and 100% ethanol. 200 μl denaturation solution (70% formamide in 2X SSC) were added and the slides were incubated at 85°C for 10 min. Slides were dehydrated in ice-cold 70%, 80% and 100% ethanol for 2 min and allowed to air dry. 150 μl pre-hybridization buffer (50% formamide, 0.1% SDS, 300 ng/ml Salmon Sperm DNA, 2× SSC) were added and the slides were incubated overnight at 37°C.
DNA FISH probes were generated as described above. The digoxigenin labeled RNA probe was generated as described previously (Feng et al., 2006). DNA probes and RNA probe in hybridization buffer (50% formamide, 10% dextran sulfate, 0.1% SDS, 300 ng/ml Salmon Sperm DNA, 2× SSC) were denatured in the presence of 2 μg Mouse Hybloc DNA (Applied Genetics Laboratories) at 80°C for 7 min and reannealed at 37°C for 1 hour. Slides were incubated for 5 min in 2X SSC with 50% formamide, 2 min in 4X SSC with 0.1% Tween-20 and 2 min in 2X SSC at 45°C. The slides were dehydrated in ethanol and denatured as described above. 10μl of FISH probe solution was added, and coverslips were sealed with rubber cement and the slides were incubated overnight at 37°C.
Slides were incubated in 2X SSC with 50% formamide for 10 min (3 times), in 2X SSC for 10 min and in 2X SSC with 0.1% NP40 for 5 min at 45°C. The slides were rinsed with 1X PBS and incubated in 1% blocking solution (Tyramide Signal Amplification Kit, Thermo Fisher Scientific) for 1 hour. Mouse monoclonal anti-Digoxigenin (DIG, Roche) was diluted 1:500 in blocking reagent, added to the slides and incubated at 4°C overnight. For SMC1 and SMC3 immunofluorescence, anti-SMC1 and anti-SMC3 antibodies (1:500 dilution) were mixed with anti-DIG, added to the slides and incubated at 4°C overnight. Slides were washed 3 times in 1X PBS for 3 min at room temperature, incubated with 1:100 HRP-goat anti-mouse IgG in blocking solution for 1 hour at room temperature and tyramide labeled according to manufacturer’s instructions (TSA Kit, Thermo Fisher Scientific). A second tyramide labeling step was performed for SMC1 and SMC3 immunostaining. Slides were washed 3 times in 1X PBS for 3 min at room temperature after the first round of labeling. Then the slides were incubated with 1:100 HRP-goat anti-rabbit IgG for 1 hour at room temperature and tyramide labeled. The slides were washed 3 times with 1X PBS for 3 min and incubated with 5 mg/ml DAPI for 5 min, rinsed with 1X PBS and mounted using SlowFade Gold antifade reagent (Thermo Fisher Scientific).
Confocal Microscopy
Cells were visualized using a Zeiss Laser Scanning Microscope 880 and a 100X immersion oil objective. Z-stacks of 0.3 μm intervals were taken using the Zen 2.1 software. To measure interprobe distances, a line was traced from the center of one probe to the center of the adjacent probe. Distances were measured only between probes on the same z-slice.
Patch-Clamp Recordings in Acute Brain Slices of Mouse Cingulate Cortex
Adult mice (males and females; 3–7 months-old) were deeply anesthetized with isoflurane and decapitated. The brains were removed from the skull in ice-cold sucrose-ACSF containing (in mM): 87 NaCl, 3 KCl, 1.25 NaH2PO4, 75 sucrose, 16 glucose, NaHCO3, 7 MgCl2, 0.5 CaCl2. 300 μm thick coronal mPFC slices were cut using a vibroslicer (Leica), stored for approximately 20 minutes at 35°C and allowed to recover at room temperature (22–24°C) for at least 30 minutes in ACSF (saturated with 95% O2 and 5% CO2). For recordings, slices were transferred to a recording chamber and continuously superfused with ACSF containing (in mM): 125 NaCl, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 25 glucose, 1.5 CaCl2, 1 MgCl2, saturated with 95% O2 and 5% CO2. Bath temperature was kept between 30 and 32°C using a TC-324B control unit (Warner Instruments). Pyramidal neurons were visualized using an upright microscope (Olympus) with oblique illumination and video microscopy using a CCD camera. Cells were selected based on location, size and shape and filled with biocytin for subsequent histologically processing. Recordings were performed using an Axopatch 200B amplifier. Pipettes were pulled from WPI glass (PG10–165) using a horizontal puller (P97; Sutter Instruments) and were filled with an internal solution consisting of 130 mM Cs-MeSO3, 5 mM CsCl, 4mM MgCl2, 10Na-phosphocreatine, 2 mM Na2ATP, 0.2 EGTA 0.3 mM NaGTP, 10 HEPES, 10 mM QX-314, pH 7.35 (with Cs-OH). Pipette resistances in the working solutions ranged from 3 to 6 MΩ, yielding series resistances of 10–20 MΩ that were compensated 70%. The recording solution contained DNQX (20μM) and AP5 (5μM) to block ionotropic glutamatergic currents. Miniature events were isolated by bath application of TTX (500nM). The GABAergic nature of the recorded currents was verified by blocking them with 0.1 mM picrotoxin.
Recordings were filtered at 2 KHz using the built in 4-pole Bessel filter of the amplifier and digitized at 5 KHz using a Digidata AD/DA converter connected to a PC. Current recordings were analyzed using PClamp 9 software. Synaptic currents were identified using the template search method. Secondary analysis was performed using Igor (Wavemetrics) and SigmaPlot. Summary cumulative histograms shown in the figures were constructed by normalizing the histograms obtained from each individual recording and then averaging them. Statistical analysis to compare between groups was performed using the Mann-Whitney test. Data are presented as mean ±SEM. Error bars in the figures also represent SEM.
Drug stock solutions. DNQX (20 mM) and APV (50 mM) were dissolved in water and stored at −30°C. Picrotoxin (50 mM) was dissolved in dimethylsulfoxide (DMSO) and stored at 4°C. Fresh working solutions were prepared on the day of the experiment. DNQX, APV and picrotoxin were from Abcam.
Seizure susceptibility in mice
Evf2+/+ mice are maintained on a mixed background of 129/BL6/FVBn. In order to determine seizure susceptibility, Evf2+/+ and Evf2TS/TS were first scored for spontaneous or handling induced seizures using the following criteria: (1) no seizure, (2) jerking (muscle spasms), short lasting 1–2 seconds, (3) myoclonic jerking of neck and forelimb, (4) whole body clonus involving all four limbs, (5) generalized clonic seizure episode, tail arching over the body (Straub tail), and vigorous clonic movements. No indication of seizure behavior was observed upon handling or in home-cage. A chemically-induced seizure assay was adopted using pentelenetetrazole (PTZ), as described (Loscher et al., 1991). A suprathreshold dose of PTZ (25 mg/kg) for seizure induction (score 3–5) was determined based on injections into Evf2+/+ male mice using 10, 20, 25, 30, and 40mg/kg pentelenetetrazole (PTZ). No seizures are detected after injection of 10mg/kg PTZ, scores of 2 at 20mg/kg, and scores 3–5 are observed at 25 mg PTZ/kg. Therefore, we used 25 mg/kg PTZ to determine the latency time to seizures, comparing the time of seizure onset between Evf2+/+ and Evf2TS/TS. Trace EEG recordings were obtained from electrodes placed in the prefrontal cortex of Evf2+/+ and Evf2TS/TS (4 male littermate pairs) immediately after 25 mg PTZ injection, as previously reported for 10–12 day old rats (Radzicki et al., 2013). Latency times were based on EEG recordings by H.L., who was blind regarding the genotype.
Quantification and Statistical Analysis
Multiple biological replicates were used for experiments in Figures 1 and 2. For qRT-PCR, n=3–15 of each genotype. For luciferase reporter assays, n=10–12 per condition. p-values were determined using Student’s t-test, *p<0.05, **p<0.01, ***p<0.001, error bars (S.E.M). For gene distances measurements shown in Figure 3J-L, n=83, Chi-square (χ2), (*p<0.05), degrees of freedom (df=7). In Figure 5F and K, Evf2 regulated Dlx5/6UCE-chr6 interacting sites were analyzed using Mann Whitney U test (*p<0.05, **p<0.01) on pairs of samples. For seizure susceptibility experiments shown in Figure 6, n=4 pairs of male littermates for each genotype. For patch clamp experiments shown in Figure 6D, n=7–8 cells, Mann Whitney test, frequency: p=0.03, amplitude: p=0.13.
Data Availability
Imaging data from Figs 3 and 4 is available at Mendeley: http://dx.doi.org/10.17632/hjn9w7bm64.1
The microarray data reported in this paper is GEO:GSE102089. The ChIPseq and 4Cseq data reported in this paper is GEO:GSE117184.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-H3K4me3 | Abcam | Cat#ab8580 |
| Rabbit polyclonal anti-H3K4me1 | Abcam | Cat#ab8895 |
| Rabbit polyclonal anti-K3K27me3 | Active Motif | Cat#39155 |
| Rabbit polyclonal anti-H3K27ac | Abcam | Cat#ab4729 |
| anti-DLX | (Feng et al. 2006; Bond et al. 2009; Cajigas et al. 2015) | N/A |
| Rabbit polyclonal anti-SMC1 | Bethyl | Cat#A300–055A |
| Rabbit polyclonal anti-SMC3 | Abcam | Cat#ab9263 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Farnesol | Sigma | Cat#277541 |
| Geranylgeraniol | Sigma | Cat#G3278 |
| Pentelenetetrazole (PTZ) | Sigma | Cat#P6500 |
| DNQX | Abcam | Cat#ab120169 |
| APV | Abcam | Cat#ab120003 |
| Picrotoxin | Abcam | Cat#ab120315 |
| Critical Commercial Assays | ||
| Fugene 6 | Promega | Cat#E2691 |
| PicoPure RNA isolation kit | Applied Biosystems | Cat#KIT0204 |
| Dual-Luciferase Repporter Assay System | Promega | Cat#E1910 |
| EZ Nuclei Isolation Lysis Buffer | Sigma | Cat#N3408 |
| FISH Tag DNA Kit | Molecular probes | Cat#F32951 |
| Deposited Data | ||
| Microarray Results (GEO) | This study | GSE102089 |
| Imaging data from Figs 3 and 4 (Mendeley) | This study | http://dx.doi.org/10.17632/hjn9w7bm64.1 |
| ChIPseq/4Ceq (GEO) | This study | GSE117184 |
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Evf2TS/TS | Bond et al. 2009 | N/A |
| Evf1 TS/TS | This study | N/A |
| Evf2TS/TS;R | Berghoff et al. 2013 | N/A |
| Akr1b8+/− (Akr1b8tm1.1(KOMP)Vlcg) | Jackson Laboratories | Strain 024334 |
| Akr1b8−/−; Evf2TS/TS | This study | N/A |
| Dlx5/6KO/+ | (Merlo et al. 2002) | N/A |
| Dlx5/6KO/TS | This study | N/A |
| Dlx1/2+/− | (Anderson et al. 1997) | N/A |
| Oligonucleotides (Table S6) | ||
| Recombinant DNA | ||
| pGL3 promoter vector | Promega | Cat# E1761 |
| pCMV6-Akr1b8 | Origene | Cat#MC200763 |
| pcDNA3-EGFP | Addgene | Cat#13031 |
| pENTR223.1-Dlx6 | DNASU | Cat#MmCD00297421 |
| pcDNA-Dlx6 | This study | Addgene Cat#99480 |
| pCMV6-EGFP | This study | N/A |
| Software and Algorithms | ||
| Matlab neural network toolbox (NNT) | MathWorks | https://www.mathworks.com/products/neural-network.html |
| MACS2 | (Feng et al. 2011) | http://liulab.dfci.harvard.edu/MACS/index.html |
| bowtie2 | (Langmead and Salzberg 2012) | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| DESeq2 | (Love et al. 2014) | http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html |
| FourCSeq | (Klein et al. 2015) | http://www.bioconductor.org/packages/release/bioc/html/FourCSeq.html |
| “bedtools intersect” | (Quinlan and Hall 2010) | https://github.com/arq5x/bedtools2/releases/tag/v2.27.1 |
| FitHiC program | (Ay et al. 2014) | http://www.bioconductor.org/packages/release/bioc/html/FitHiC.html |
| bwa mapper (version 0.7.12) | (Li and Durbin 2009) | http://bio-bwa.sourceforge.net/bwa.shtml |
| GeneSpring | Agilent | https://www.agilent.com/en/products/software-informatics/life-sciences-informatics/genespring-gx |
Acknowledgments
We thank: previous lab members for generating mutant tissue essential for these studies, A. Bendall (U. of Guelph, Dlx5/6KO mice with permission from G. Levi), K. Campbell (Dlx1/2−/+ mice, Cincinnati Children’s), A. Kohtz (training in live animal brain stereotactic surgery and EEG implantation surgery, Rutgers Univ.). This work is funded by NIMH R01MH094653 and R01MH111267 to J.D.K.
Footnotes
Declaration of interests
Invention Report Number 1525701-16-0001 (JDK) was filed to iEdison.
References
- Andergassen D, Dotter CP, Wenzel D, Sigl V, Bammer PC, Muckenhuber M, Mayer D, Kulinski TM, Theussl HC, Penninger JM, et al. (2017). Mapping the mouse Allelome reveals tissue-specific regulation of allelic expression. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, and Rubenstein JL (1997a). Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278, 474–476. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, and Rubenstein JL (1997b). Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron 19, 27–37. [DOI] [PubMed] [Google Scholar]
- Ay F, Bailey TL, and Noble WS (2014). Statistical confidence estimation for Hi-C data reveals regulatory chromatin contacts. Genome Res 24, 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, Mattick JS, and Haussler D (2004). Ultraconserved elements in the human genome. Science 304, 1321–1325. [DOI] [PubMed] [Google Scholar]
- Berghoff EG, Clark MF, Chen S, Cajigas I, Leib DE, and Kohtz JD (2013). Evf2 (Dlx6as) lncRNA regulates ultraconserved enhancer methylation and the differential transcriptional control of adjacent genes. Development 140, 4407–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, Vangompel MJ, Sametsky EA, Clark MF, Savage JC, Disterhoft JF, and Kohtz JD (2009). Balanced gene regulation by an embryonic brain ncRNA is critical for adult hippocampal GABA circuitry. Nat Neurosci 12, 1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brind’Amour J, Liu S, Hudson M, Chen C, Karimi MM, and Lorincz MC (2015). An ultra-low-input native ChIPseq protocol for genome-wide profiling of rare cell populations. Nature communications 6, 6033. [DOI] [PubMed] [Google Scholar]
- Brockdorff N (2011). Chromosome silencing mechanisms in X-chromosome inactivation: unknown unknowns. Development 138, 5057–5065. [DOI] [PubMed] [Google Scholar]
- Cajigas I, Leib DE, Cochrane J, Luo H, Swyter KR, Chen S, Clark BS, Thompson J, Yates JR, Kingston RE, et al. (2015). Evf2 lncRNA/BRG1/DLX1 interactions reveal RNA-dependent inhibition of chromatin remodeling. Development 142, 2641–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, and Rubenstein JL (2005). Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nature neuroscience 8, 1059–1068. [DOI] [PubMed] [Google Scholar]
- De Marco Garcia NV, Karayannis T, and Fishell G (2011). Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature 472, 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, et al. (2013). New insights into the classification and nomenclature of cortical GABAergic interneurons. Nature reviews Neuroscience 14, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J (2016). Mapping the 3D genome: Aiming for consilience. Nat Rev Mol Cell Biol 17, 741–742. [DOI] [PubMed] [Google Scholar]
- Endo S, Matsunaga T, Ohta C, Soda M, Kanamori A, Kitade Y, Ohno S, Tajima K, El-Kabbani O, and Hara A (2011). Roles of rat and human aldo-keto reductases in metabolism of farnesol and geranylgeraniol. Chem Biol Interact 191, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bi C, Clark BS, Mady R, Shah P, and Kohtz JD (2006). The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev 20, 1470–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Liu T, and Zhang Y (2011). Using MACS to identify peaks from ChIPseq data. Current protocols in bioinformatics / editoral board, Baxevanis Andreas D [et al. ] Chapter 2, Unit 2 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, Westphal H, and Rubenstein JL (2011). Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron 70, 939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya T, Lim B, and Levine M (2016). Enhancer Control of Transcriptional Bursting. Cell 166, 358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego O, Ruiz FX, Ardevol A, Dominguez M, Alvarez R, de Lera AR, Rovira C, Farres J, Fita I, and Pares X (2007). Structural basis for the high all-trans-retinaldehyde reductase activity of the tumor marker AKR1B10. Proceedings of the National Academy of Sciences of the United States of America 104, 20764–20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, and Marin O (2010). Generation of interneuron diversity in the mouse cerebral cortex. The European journal of neuroscience 31, 2136–2141. [DOI] [PubMed] [Google Scholar]
- Giorgetti L, Lajoie BR, Carter AC, Attia M, Zhan Y, Xu J, Chen CJ, Kaplan N, Chang HY, Heard E, et al. (2016). Structural organization of the inactive X chromosome in the mouse. Nature 535, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnisz D, Shrinivas K, Young RA, Chakraborty AK, and Sharp PA (2017). A Phase Separation Model for Transcriptional Control. Cell 169, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug CB, Grimaldi AG, Kruse K, and Vaquerizas JM (2017). Chromatin Architecture Emerges during Zygotic Genome Activation Independent of Transcription. Cell 169, 216–228 e219. [DOI] [PubMed] [Google Scholar]
- Isoda T, Moore AJ, He Z, Chandra V, Aida M, Denholtz M, Piet van Hamburg J, Fisch KM, Chang AN, Fahl SP, et al. (2017). Non-coding Transcription Instructs Chromatin Folding and Compartmentalization to Dictate Enhancer-Promoter Communication and T Cell Fate. Cell 171, 103–119 e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, and Haussler D (2002). The human genome browser at UCSC. Genome Res 12, 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharchenko PV, Tolstorukov MY, and Park PJ (2008). Design and analysis of ChIPseq experiments for DNA-binding proteins. Nature biotechnology 26, 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein FA, Pakozdi T, Anders S, Ghavi-Helm Y, Furlong EE, and Huber W (2015). FourCSeq: analysis of 4C sequencing data. Bioinformatics 31, 3085–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz JD, Baker DP, Corte G, and Fishell G (1998). Regionalization within the mammalian telencephalon is mediated by changes in responsiveness to Sonic Hedgehog. Development 125, 5079–5089. [DOI] [PubMed] [Google Scholar]
- Kohtz JD, and Fishell G (2004). Developmental regulation of EVF-1, a novel noncoding RNA transcribed upstream of the mouse Dlx6 gene. Gene Expr Patterns 4, 407–412. [DOI] [PubMed] [Google Scholar]
- Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, and Marra MA (2009). Circos: an information aesthetic for comparative genomics. Genome Res 19, 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, Batzoglou S, Bernstein BE, Bickel P, Brown JB, Cayting P, et al. (2012). ChIPseq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res 22, 1813–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G, and Rudy B (2013). A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nature neuroscience 16, 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, and de Graaff E (2003). A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet 12, 1725–1735. [DOI] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C, and Luthi A (2011). A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature 480, 331–335. [DOI] [PubMed] [Google Scholar]
- Li H, and Durbin R (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, and Copeland NG (2003). A highly efficient recombineering-based method for generating conditional knockout mutations. Genome research 13, 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Nowakowski TJ, Pollen AA, Lui JH, Horlbeck MA, Attenello FJ, He D, Weissman JS, Kriegstein AR, Diaz AA, et al. (2016). Single-cell analysis of long non-coding RNAs in the developing human neocortex. Genome biology 17, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, Alvarez-Buylla A, and Rubenstein JL (2007). Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience 27, 3230–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Honack D, Fassbender CP, and Nolting B (1991). The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. III. Pentylenetetrazole seizure models. Epilepsy research 8, 171–189. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinov GK, Kundaje A, Park PJ, and Wold BJ (2014). Large-scale quality analysis of published ChIPseq data. G3 4, 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo GR, Paleari L, Mantero S, Genova F, Beverdam A, Palmisano GL, Barbieri O, and Levi G (2002). Mouse model of split hand/foot malformation type I. Genesis 33, 97–101. [DOI] [PubMed] [Google Scholar]
- Nagano T, Lubling Y, Stevens TJ, Schoenfelder S, Yaffe E, Dean W, Laue ED, Tanay A, and Fraser P (2013). Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature 502, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Lubling Y, Varnai C, Dudley C, Leung W, Baran Y, Mendelson Cohen N, Wingett S, Fraser P, and Tanay A (2017). Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, and Fraser P (2008). The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322, 1717–1720. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, and Corbin JG (2002). The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci 5, 1279–1287. [DOI] [PubMed] [Google Scholar]
- Noordermeer D, Leleu M, Splinter E, Rougemont J, De Laat W, and Duboule D (2011). The dynamic architecture of Hox gene clusters. Science 334, 222–225. [DOI] [PubMed] [Google Scholar]
- Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, et al. (2012). Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauler FM, Barlow DP, and Hudson QJ (2012). Mechanisms of long range silencing by imprinted macro non-coding RNAs. Curr Opin Genet Dev 22, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM (2015). The aldo-keto reductases (AKRs): Overview. Chemico-biological interactions 234, 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Cremins JE, Sauria ME, Sanyal A, Gerasimova TI, Lajoie BR, Bell JS, Ong CT, Hookway TA, Guo C, Sun Y, et al. (2013). Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell 153, 1281–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, and Kepecs A (2013). Cortical interneurons that specialize in disinhibitory control. Nature 503, 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M, Lemaistre M, Pischetola M, Di Lauro R, and Duboule D (1991). A mouse gene related to Distal-less shows a restricted expression in the developing forebrain. Nature 351, 748–751. [DOI] [PubMed] [Google Scholar]
- Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzicki D, Yau HJ, Pollema-Mays SL, Mlsna L, Cho K, Koh S, and Martina M (2013). Temperature-sensitive Cav1.2 calcium channels support intrinsic firing of pyramidal neurons and provide a target for the treatment of febrile seizures. J Neurosci 33, 9920–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SSP, Huang SC, Glenn St Hilaire B, Engreitz JM, Perez EM, Kieffer-Kwon KR, Sanborn AL, Johnstone SE, Bascom GD, Bochkov ID, et al. (2017). Cohesin Loss Eliminates All Loop Domains. Cell 171, 305–320 e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrup L, Branco MR, Perdeaux ER, Krueger C, Lewis A, Santos F, Nagano T, Cobb BS, Fraser P, and Reik W (2009). The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development 136, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, and Hjerling-Leffler J (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Developmental neurobiology 71, 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A, Bailey P, Bruce S, Engstrom PG, Klos JM, Wasserman WW, Ericson J, and Lenhard B (2004). Arrays of ultraconserved non-coding regions span the loci of key developmental genes in vertebrate genomes. BMC Genomics 5, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Lajoie BR, Jain G, and Dekker J (2012). The long-range interaction landscape of gene promoters. Nature 489, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer W, Abdennur N, Goloborodko A, Pekowska A, Fudenberg G, Loe-Mie Y, Fonseca NA, Huber W, C HH, Mirny L, et al. (2017). Two independent modes of chromatin organization revealed by cohesin removal. Nature 551, 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics 21, 70–71. [DOI] [PubMed] [Google Scholar]
- Stevens TJ, Lando D, Basu S, Atkinson LP, Cao Y, Lee SF, Leeb M, Wohlfahrt KJ, Boucher W, O’Shaughnessy-Kirwan A, et al. (2017). 3D structures of individual mammalian genomes studied by single-cell Hi-C. Nature 544, 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Werken HJ, de Vree PJ, Splinter E, Holwerda SJ, Klous P, de Wit E, and de Laat W (2012). 4C technology: protocols and data analysis. Methods Enzymol 513, 89–112. [DOI] [PubMed] [Google Scholar]
- Westra JW, Peterson SE, Yung YC, Mutoh T, Barral S, and Chun J (2008). Aneuploid mosaicism in the developing and adult cerebellar cortex. J Comp Neurol 507, 1944–1951. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, and Alvarez-Buylla A (2001). In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development 128, 3759–3771. [DOI] [PubMed] [Google Scholar]
- Williamson I, Lettice LA, Hill RE, and Bickmore WA (2016). Shh and ZRS enhancer colocalisation is specific to the zone of polarising activity. Development 143, 2994–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won H, de la Torre-Ubieta L, Stein JL, Parikshak NN, Huang J, Opland CK, Gandal MJ, Sutton GJ, Hormozdiari F, Lu D, et al. (2016). Chromosome conformation elucidates regulatory relationships in developing human brain. Nature 538, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, and Anderson SA (2010). Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron 65, 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerucha T, Stuhmer T, Hatch G, Park BK, Long Q, Yu G, Gambarotta A, Schultz JR, Rubenstein JL, and Ekker M (2000). A highly conserved enhancer in the Dlx5/Dlx6 intergenic region is the site of cross-regulatory interactions between Dlx genes in the embryonic forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience 20, 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Maricque B, Xie M, Li D, Sundaram V, Martin EA, Koebbe BC, Nielsen C, Hirst M, Farnham P, et al. (2011). The Human Epigenome Browser at Washington University. Nat Methods 8, 989–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Imaging data from Figs 3 and 4 is available at Mendeley: http://dx.doi.org/10.17632/hjn9w7bm64.1
The microarray data reported in this paper is GEO:GSE102089. The ChIPseq and 4Cseq data reported in this paper is GEO:GSE117184.