Abstract
Prostate cancer stem cells (CSCs) are implicated in tumor initiation, cancer progression, metastasis, and the development of therapeutic-resistant disease. It is well known that the bulk of prostate cancer (PCa) cells express androgen receptor (AR) and that androgens are required for PCa growth, progression and emergence of castration-resistant disease. In contrast, the small subpopulation of self-renewing CSCs exhibit an AR-negative (−) signature. The mechanisms underlying the absence of AR are unknown. Using CSC-like cell models isolated from clinical biopsy tissues, we identify the E3 ligase MDM2 as a key regulator of prostate CSC integrity. First, unlike what has been reported for the bulk of AR(+) tumor cells where MDM2 regulates the temporal expression of AR during transcriptional activity, MDM2 in CSCs promoted the constant ubiquitination and degradation of AR, resulting in sustained loss of total AR protein. Second, MDM2 promoted CSC self-renewal, the expression of stem cell factors, and CSC proliferation. Loss of MDM2 reversed these processes and induced expression of full-length AR (and not AR variants), terminal differentiation into luminal cells, and cell death. Selectively blocking MDM2-mediated activity in combination with androgen/AR-targeted therapy may offer a novel strategy for eliminating AR(−) CSCs in addition to the bulk of AR(+) PCa cells, decreasing metastatic tumor burden and inhibiting the emergence of therapeutic resistance.
Keywords: Cancer stem cells, prostate, androgen receptor, MDM2, ubiquitination
INTRODUCTION
While the androgen receptor (AR) is a master regulator of prostate development and disease, it is commonly believed that normal prostate stem cells and prostate cancer stem cells (CSCs) express no/low AR and that their growth is androgen-independent (1). Our previous study demonstrated that AR(−) pluripotent CSCs isolated from patient biopsies differentiated into prostatic glandular structures containing all three epithelial cell types including AR(+) luminal secretory cells as well as AR(−) basal and neuroendocrine cells when engrafted with embryonic mesenchyme under the renal capsule (2). Similarly, other studies report that AR(−) normal prostate stem-like cells differentiate into three prostatic epithelial cell lineages (3). This pattern of AR(−) expression mimics that observed in human and rodent prostate development where AR(−) epithelial anlagen grow into the urogenital mesenchyme until AR protein is induced by, as yet unknown mechanisms, to initiate glandular lumen formation, epithelial cell specification, and androgen-mediated secretory activity (4,5). Qin and colleagues determined that Prostate Specific Antigen (PSA)−/lo cells, a subpopulation isolated from LNCaP and LAPC9 prostate cancer (PCa) cell lines, were AR−/lo and exhibited stem-like properties including self-renewal and the ability to regenerate PSA+ cells (6). PSA−/lo LAPC9 cells developed into therapeutic-resistant tumors (6).
Together, these observations imply that an AR(−) phenotype is essential for maintaining CSC and normal prostate stem cell homeostasis and for promoting castration resistant prostate cancer (CRPC). The mechanisms underlying this AR(−) phenotype are unknown. Our previous study showed that biopsy-derived PCa CSCs, referred to as HPET (human prostate epithelial cells expressing hTERT), expressed AR mRNA but not AR protein, suggesting that expression was regulated at the posttranscriptional level (2). The Qin study reported that in PSA−/lo cells, both AR mRNA and protein were down-regulated, implying that AR expression was regulated at the transcriptional level (6). Whether inhibition of AR protein expression occurs at the transcriptional and/or posttranscriptional level remains to be established.
Evidence from non-CSC, AR(+) PCa cell lines suggests that the ubiquitin-proteasome system (UPS) modulates the steady state of AR expression. For example in AR(+) LNCaP, CWR-R1, and CWR22Rv1 cells, the E3 ligase MDM2 (mouse double minute 2 homolog) transiently modulates AR stability during transcriptional activity (7). Other E3 ligases, including NEDD4 (neural precursor cell expressed developmentally down-regulated protein 4) (8), CHIP (C-terminus of Hsp70-interacting protein) (9), and SKP2 (S-Phase Kinase-Associated Protein 2) (10) also regulate AR protein degradation in LNCaP, C4–2B and CWR22Rv1 cells. Whether AR(−) CSCs use these same ligases to block total AR expression remains to be explored.
The recent discovery of AR splice variants (AR-Vs) provides insight into the mechanisms promoting emergence of CRPC. These naturally occurring AR-Vs are identified in clinical PCa biopsy specimens and non-CSC PCa cell lines [reviewed in (11,12)]. AR-Vs contain the N-terminal and DNA binding domains; however, they lack a ligand binding domain, resulting in constitutive activation. Several reports demonstrate that AR-Vs are highly expressed in CRPC, metastasis, and PCa cell lines not requiring androgens for cell growth. The most commonly expressed variant AR-V7 (a.k.a. AR3) is associated with development of CRPC and drug resistance. Furthermore, AR-V7 promotes epithelial mesenchymal transition (EMT) and induces expression of signature stem cell genes, including Nanog in LNCaP cells and Lin28B in DU-145 cells (13). Interestingly, MDM2 induces AR-V7 ubiquitination and protein degradation (14). Whether CSCs express AR-Vs remains to be investigated.
Prostate cells with CSC-like properties have typically been isolated as side populations (~0.1–0.3% of PCa cells) from established cell lines, e.g., PC-3 (15), DU-145 (16), and LNCaP (17) cells, and from biopsy tissues (18). Here we use two CSC-like cell models to investigate the AR(−) signature of prostate CSCs. Both HPET (2) and HuSLC (human stem like cells; previously termed HPE for human prostate epithelial) (19) cell lines were isolated from high grade Gleason 9 biopsy tissues from two unrelated individuals. Of note is that the HuSLC line arose spontaneously. Both cell lines express AR mRNA but not AR protein, and exhibit stem-like properties including pluripotency in vivo, differentiating into the three prostatic epithelial cell lineages, i.e., luminal secretory AR(+) cells, basal cells and neuroendocrine cells (2,19). Using these CSC-like cell models, we report that MDM2 is critical for conserving an AR(−) signature and promoting stemness, while loss of MDM2 induces full-length AR expression, terminal CSC differentiation into AR(+) luminal epithelial cells, and cell death.
MATERIALS AND METHODS
Materials
Prostate cancer cells LNCaP, PC3, 22RV1 and VCaP were purchased from American Type Culture Collection (ATCC). Proteasome inhibitors MG132 (cat.#: 3175-v), MG115 (cat.#: 3170-V) and Epoxomicin (cat#: 4381-v) were purchased from the Peptide institute, Osaka, Japan. Matrigel (BD Biosciences, cat.#:10828028) was batch-tested by the Pluripotent Stem Cell Facility at Cincinnati Children’s Hospital Medical Center. The human full-length wild type (wt) AR expression vector pSVARo was a gift from Dr. S. Liao, Chicago. Additional materials and reagents are indicated below.
Cell Culture
The parental HPET cell line was established by transducing primary human prostate epithelial cells (passage 2) cultured from de-identified human prostate cancer surgical waste material (Gleason 9, undifferentiated PCa,) using pLenti-particles expressing the hTERT-EGFP gene (2). The HPET cell lines and their prostate epithelial and stem cell characteristics were authenticated both in vitro and in vivo as described in detail in Gu et al. (2). Vials containing HPET passage 73 were thawed and passaged approximately 5 times to complete this current study. A second cell line, termed HuSLC (Human Stem Cell-Like Cell) line arose spontaneously from human prostate epithelial (HPE) cells cultured from de-identified biopsy tissue (Gleason 9, undifferentiated PCa) from an unrelated male donor. Their HPE-like and PCa-generating characteristics were authenticated both in vitro and in vivo in detail in Williams et al. [Figure 6 (19)]. They were initially named “HPE”, but subsequently renamed “HuSLC” because of their stem-like characteristics. Vials containing HuSLC passage 32 and 34 were thawed and passaged approximately 5 to 6 times to complete this study. Cell lines were recently tested (June, 2018) and found to be negative for mycoplasma (Lonza MycoAlert™ Mycoplasma Detection Kit, cat.#: LT07–218; Lonza Mycoalert™ Mycoplasma Assay Control Set, cat.#: LT07–518).
Both the HPET and HuSLCs cell lines were cultured in under embryonic stem (ES) cell conditions using defined ESC medium, DMEM-F12 (ThermoFisher Scientific, cat.#: 11320033) supplemented with KnockOut™ Serum Replacement (ThermoFisher Scientific, cat.#: 10828028) and 4ng/ml recombinant bFGF (ProSpec, cat.#: CYT-218) on Matrigel coated plates. Prostate cancer cells LNCaP, PC3, 22RV1 and VCaP were purchased from American Type Culture Collection (ATCC) and cultured as recommended by ATCC. Cells from the company were expanded through approximately 4 to 5 passages and frozen down as stock vials. Stock vials were thawed and cells were passaged approximately 2 to 4 times to complete this study.
Sphere formation assay
HPET or HuSLC cells were trypsinized using Trypsin-EDTA Solution (ThermoFisher Scientific, cat.#: 25200056), centrifuged for 5 minutes at 300 × g and the cell pellet resuspended in defined ESC medium (described above). Cells were seeded at 2,500 cells/ml in 6-well ultra-low attachment plates (FisherScientific, Corning™ Costar™ 3471, cat.#: 07–200-601) and cultured for 10 days. Wells were photographed using phase contrast and total number of spheres/well were counted using NIH ImageJ software.
Proliferation assay
Cells were plated at 5×103 cells/well using 24 well plates and either transfected with control plasmid or pSVARo to induce exogenous AR protein expression or treated with MG132 to induce endogenous AR, and treated with/without 10−8 M DHT with/without 10−5 M OHF or vehicle control (95% ethanol) as indicated in each assay. Cell numbers were determined using the Trypan Blue Viability assay (ThermoScientific, HyClone™ Trypan Blue Solution, 0.4%, cat.# SV3008401).
Western Blot Analysis
Cells were harvested using RIPA buffer (Invitrogen Inc., cat.#: R0278) with 1% protease inhibitor cocktail (FisherScientific, cOmplete™ Protease Inhibitor Cocktail tablets/Roche, cat.# NC0939492) and phosphatase inhibitor cocktail (FisherScientific, EMD Millipore™ Calbiochem™ Phosphatase Inhibitor Cocktail Set I, cat.# 53–913-110VL). Lysates were centrifuged at 4°C for 10 min at 14,000 × g; 50 μg protein from each supernatant was subjected to 10% SDS-PAGE, transferred to PVDF membrane and blocked and probed with primary antibody overnight at 4°C. Peroxidase-conjugated secondary antibody was added at a 1:4500 dilution and blots were developed using the Enhanced Chemiluminescence (ECL) kit (Pierce™/ThermoFisher Scientific™, cat.#: 32132). Antibodies used in this study are listed in Supplementary Table S1.
Immunoprecipitation
Cells were lysed in 300 μL of cold lysis buffer [1% Triton X-100, 150 mmol/L NaCl, 10 mmol/L NaHPO4 (pH 7.2), 5 mmol/L NaF, 2 mmol/L EDTA, 1x HALT™ protease inhibitor cocktail (ThermoFisher Scientific, cat.#: 78440)] at 4°C in a cold room. Lysates were cleared by centrifugation and immunoprecipitation was performed by incubating 500 μg total protein with 10 μg rabbit anti-AR antibody or 5 μg mouse anti-hemagglutinin (HA) antibody (Santa Cruz Biotechnology, cat # sc-816 and cat.# sc-805 respectively) overnight at 4°C. ImmunoPure Immobilized Protein G beads (Pierce Biotechnology, cat.#: 44667) were used to pull down protein complexes. The immunoprecipitates were washed in 1x phosphate buffed saline (PBS), resuspended in 1x Laemmli buffer and subjected to Western blot analysis.
Luciferase assay
Cells were transfected with the ARR2PB-luc reporter (20) and Renilla luciferase vectors (Promega, cat.#: E2231) and either treated with increasing concentrations of MG132 (to induce endogenous AR) or co-transfected with increasing concentrations of pSVARo (to induce exogenous AR). Twenty-four hours post AR induction, cells were treated with vehicle control (ethanol) or DHT (10−8 M) with/without OHF (10−5 M) for 24 h, as described previously (21). Cells were lysed and luciferase activity was determined using the Promega Dual-Luciferase® Reporter Assay System kit and protocol (Promega, cat.#: E1910) according to the manufacturer’s protocol. Cell lysate protein concentrations were determined using the Protein BCA Assay kit (Pierce™/ThermoFisher Scientific™, cat.#: 23225).
Total RNA Extraction, Purification, and cDNA Synthesis
Total RNA was extracted from HPET and HuSLCs using TRIzol reagent (Invitrogen™/ThermoFisher Scientific™, cat.#: 15596018) following the manufacturer’s protocol. Total RNA concentrations (260/280 nm) were determined using the NanoDrop system (NanoDrop Technologies Inc., Wilmington, DE). RNAs were treated with DNase I (Invitrogen Inc., cat.#: AM2222) to remove any traces of DNA contamination and cDNAs were synthesized from 1 μg of RNA per sample using the Fermentas Revertaid kit (Fermentas™/ThermoFisher Scientific™, cat.#: K1621), according to the manufacturers’ protocols.
Quantitative Polymerase Chain Reaction and Data Analysis
Primers used in this study are listed in Supplementary Tables S2 and S3. One (1) μg of synthesized cDNA was added to 1μM random-specific primers (synthesized by IDT Inc.), and 12.5 μl of 2x Power SYBR® Green PCR Master Mix (Applied Biosystems™/ThermoFisher Scientific™, cat.#: 4309155) to a final volume of 25 μl. PCR amplification was performed using an Applied Biosystems 7300 Real-Time PCR System [one cycle at 50°C for 2 min, one cycle of 95°C for 10 min, followed by 40 cycles of 15 sec at 95°C and 1 min at 60°C]. The dissociation curve was completed with one cycle of 1 min at 95°C, 30 sec of 55°C, and 30 sec of 95°C. Non-reverse transcription control and no template control were included in the PCR program for quality control. RNA expression for the genes of interest were normalized to expression of the GAPDH gene and analyzed using the ΔΔCt method (22).
QUANTIFICATION AND STATISTICAL ANALYSIS
GraphPad Prism v4.0 was used for all statistical analyses. Statistical parameters, including the types of tests, number of samples (n), descriptive statistics and p values are reported in the figure legends.
RESULTS
Inhibition of the proteasome induces AR expression in CSC-like PCa cells
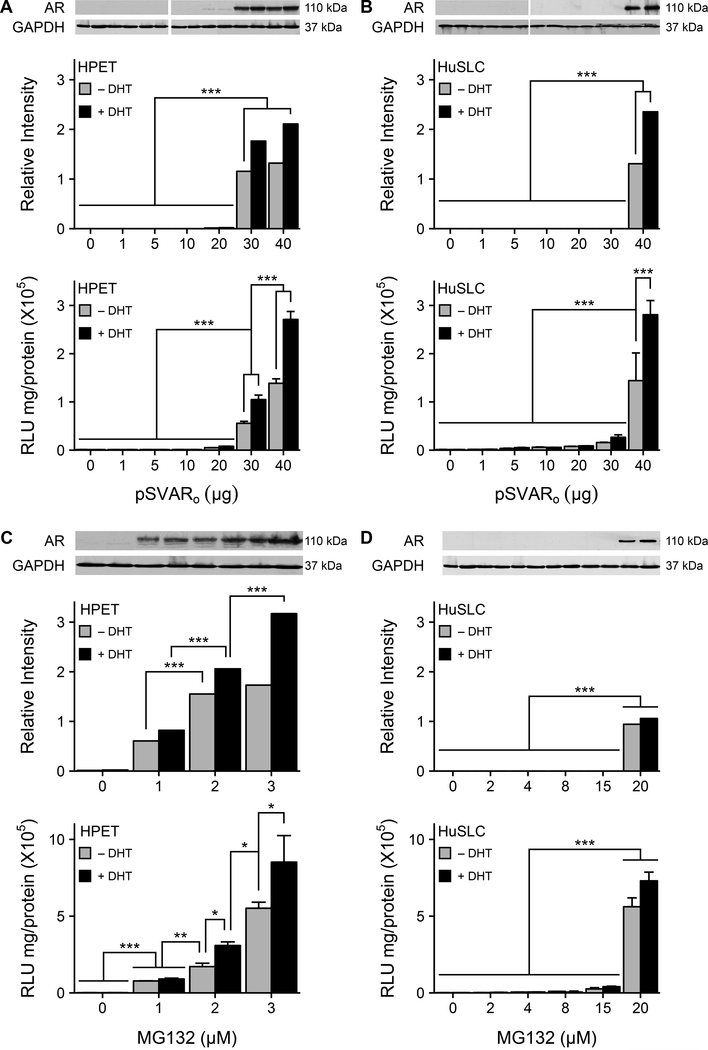
Previously, we reported that HPET cells recapitulated the AR(−) phenotype reported for CSCs (2) (Figure 1A) and similarly, the HuSLC line also expressed AR mRNA but not AR protein (Figure 1B). To determine whether AR protein was constitutively being degraded, HPET and HuSLCs were transfected with increasing concentrations of pSVARo, which expresses human full-length/wt AR at low concentrations (≤ 4 μg) (23). Unexpectedly, 30 μg pSVARo were required for detectable AR protein expression in HPETs (Figure 1A; p<0.0001). Moreover, addition of dihydrotestosterone (DHT, 10−8 M) appeared to stabilize AR protein levels. Similarly, 40 μg pSVARo were required to induce detectable AR protein in HuSLCs (Figure 1B; p<0.0001), suggesting that at lower pSVARo concentrations, AR protein was actively being degraded.
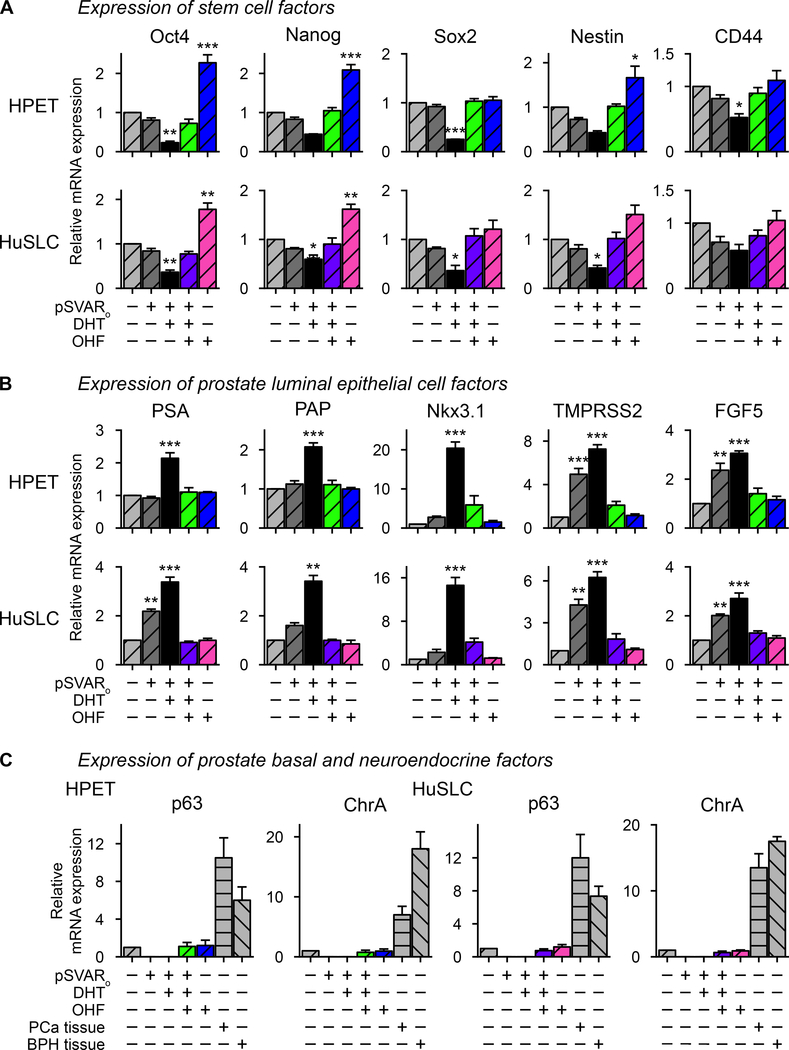
Figure 1. AR expression in CSC-like PCa cells is modulated by the proteasome.
(A, B) AR expression and transcriptional activity are only induced following transfection with high concentrations of pSVARo plasmid. HPET cells (A) and HuSLCs (B) were transfected with increasing concentrations of pSVARo plasmid (expressing full-length, wt human AR). AR protein levels were analyzed by Western blot and semi-quantified by Densitometry. Cells were transfected with ARR2PB-luc (24,49) and Renilla luciferase reporter genes. Luciferase activity was determined using the Promega Dual-Luciferase® Reporter Assay System kit and protocol (Promega, cat.#: E1910).
(C, D) Proteasomal inhibition initiates AR expression and transcriptional activity. HPET cells (C) and HuSLCs (D) were treated with increasing concentrations of MG132 with/without addition of 10−8 M DHT and dual luciferase activity was determined as above. Abbreviations: wt, wild type; DHT, dihydrotestosterone. Data are expressed as mean ± SEM; n = 4. *, p<0.01; **, p<0.001; ***, p<0.0001.
To determine whether AR protein levels were down-regulated by proteasomal degradation, HPET cells (Figure 1C) and HuSLCs (Figure 1D) were treated with increasing concentrations of the proteasome inhibitor MG132. In HPET cells, endogenous full-length AR protein was already detected at the lowest concentration of MG132 (1 μM) tested. Similarly, AR protein was induced in HuSLCs following MG132 treatment (20 μM). Furthermore, treatment with other proteasome inhibitors, MG115 and Epoxomicine, confirmed these observations (Supplementary Figure S1). In transfection assays using the androgen-regulated probasin promoter linked to the luciferase reporter gene (ARR2PB-luc) (24), AR-mediated transcription was only induced when exogenous (Figure 1A, 1B) or endogenous (Figure 1C, 1D) AR protein was expressed.
The E3 ligase MDM2 is reported to regulate AR levels in PCa cells (7). Therefore, MDM2 expression was analyzed in HuSLC and HPET cells and compared to two standard PCa cell lines, LNCaP (where MDM2 modulates AR levels in a temporal manner to regulate AR-mediated transcription) and DU-145 (which do not express AR). Both HuSLC and HPET cells expressed higher levels of MDM2 (2.16- and 1.74-fold respectively) as compared to LNCaP and DU-145 cells (Supplementary Figure S2). Furthermore, MDM2 levels were highest in HuSLCs, implying that AR degradation in HuSLCs was greater than in HPET cells. This is supported by the findings that more AR plasmid and higher amounts of proteasome inhibitor were required to induce HuSLC AR expression. Taken together, these observations support an active role for the proteasome in conserving the AR(−) CSC signature.
HPET and HuSLCs express full length AR but not AR-Vs
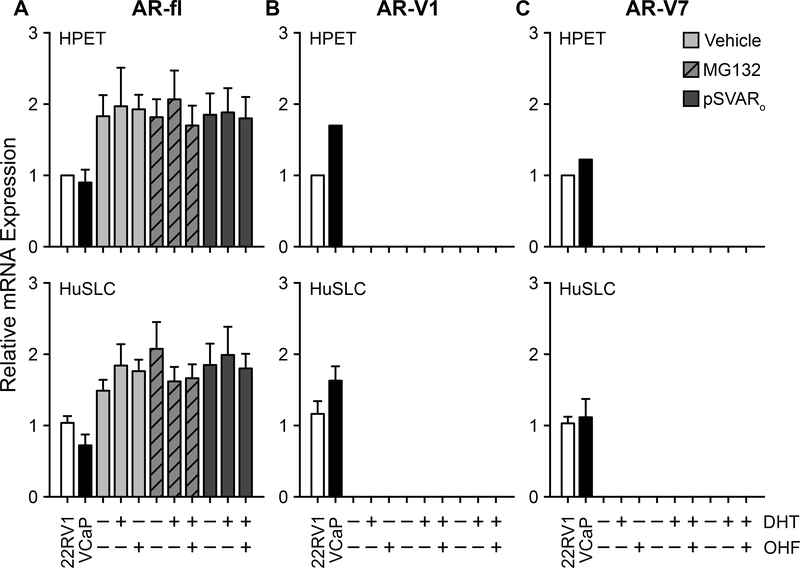
Published primer sets spanning the genomic region from which AR-Vs are transcribed were used to determine whether HPET cells and HuSLCs expressed AR-V transcripts (25,26). A universal forward primer, P1/P2/P3 (F), located in AR exon 2 was paired with one of three reverse primers (P1R, P2R, and P3R) located within in each variant exon (as outlined in Supplementary Table S2). This approach provided coverage for the known AR variants. 22Rv1 and VCaP cell lines served as positive controls.
Both HPET cells and HuSLCs expressed full-length AR transcript (AR-fl) (Figure 2A); however, they did not express any of the other recognized AR-V transcripts (Figure 2, Supplementary Figure S3). We then determined whether androgen-mediated activity was required for induction of AR-V expression. AR-V1 and AR-V7 transcripts were not detected under any conditions tested (Figure 2B and 2C). In contrast, full-length AR mRNA was expressed at similar levels regardless of treatment with vehicle or dihydrotestosterone (DHT) with/without hydroxyflutamide (OHF) (Figure 2A). Western blot analysis using an AR antibody towards the N-terminal (which recognizes AR-fl and AR-Vs) determined that HPET cells and HuSLCs expressed full-length 110 kDa endogenous AR following MG132 treatment, however an 80 kDa band corresponding to AR-Vs (27) was not detected in either cell line under any condition tested (Supplementary Figure S4). Several bands at lower molecular weights (<68 kDa) were observed in HPET cells, however they did not correspond to any known AR-Vs. Since these bands were already present prior to induction of AR expression, they may represent degraded/non-functional AR or non-specific antibody interactions. In summary, CSC-like HPET cells and HuSLCs did not express AR-Vs, but instead, conserved expression of full length AR.
Figure 2. CSC-like HPET cells and HuSLCs express full-length AR, but not AR-Vs.
(A) RT-qPCR analysis of endogenous HPET and HuSLC AR-fl following treatment with DHT with/without OHF. HPET cells (upper panel) were treated with 2 μM MG132 to induce endogenous AR, or 30 μg pSVARo to express exogenous AR-fl, and treated with 10−8 M DHT with/without 10−5 M OHF. Vehicle control, 95% ethanol. HuSLCs (lower panel) were treated with 20 μM MG132 to induce endogenous AR, or 40 μg pSVARo to express exogenous AR-fl, and treated with 10−8 M DHT with/without 10−5 M OHF. Vehicle control, 95% ethanol. VCaP and 22RV1 cells lines served as control PCa cell lines for AR-fl, AR-V1 and AR-V7 expression. Primer sets used to characterize AR-V expression are listed in Supplementary Table S2. Only data for AR-fl, AR-V1 and AR-V7 are shown since HPET and HuSLCs did not express any of the other AR-Vs tested (Supplementary Figure S3).
(B-C) RT-qPCR analysis of endogenous AR-V1 and AR-V7 following treatment with DHT with/without OHF. HPET cells (upper panel) and HuSLCs (lower panel) were treated as in (A). In both HPET and HuSLCs, AR-V1 and AR-V7 expression was not observed under any conditions tested. Abbreviations: fl, full-length; V, Variant; DHT, dihydrotestosterone; OHF, hydroxyflutamide. Data are expressed as mean ± SEM; n = 4.
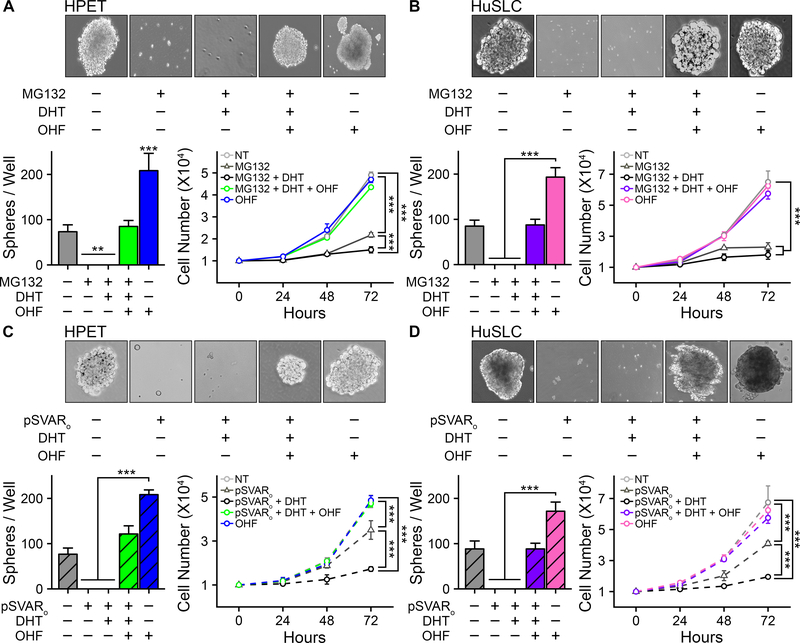
An AR(−) phenotype is essential for prostate CSC self-renewal and proliferation
A standard sphere formation assay was performed to determine whether AR signaling inhibited CSC stemness. HPET cells (Figure 3A) and HuSLCs (Figure 3B) were treated with MG132 with/without DHT to induce AR protein expression and activate AR-mediated signaling. Sphere formation was absent upon AR expression, and remained unaltered by DHT treatment. In contrast, sphere formation was rescued by addition of OHF. Similarly, exogenous expression of pSVARo inhibited sphere formation; and this could be rescued by OHF treatment (Figure 3C, 3D). Furthermore, induction of AR dramatically decreased cell proliferation within 48 h, and addition of DHT decreased cell proliferation even further down to baseline levels. In contrast, OHF-mediated inhibition of AR activity restored cell proliferation (Figure 3C, 3D).
Figure 3. Induction of AR protein down-regulates sphere formation and cell proliferation.
(A) Top panel, sphere formation assay (2). HPET cells were treated with 2 μM MG132 to induce endogenous AR and 10−8 M DHT with/without 10−5 M OHF to increase or inhibit AR activity respectively. Vehicle control, 95% ethanol. Phase contrast images, 20x. Bottom left panel, quantification of the number of spheres/well. Bottom right panel, proliferation assay (19). HPET cells were treated as described in the top panel and total cell numbers/well were determined using the Trypan Blue Viability assay. AR expression inhibited sphere formation and cell proliferation which could be rescued by treatment with OHF.
(B). Assays in HuSLCs were performed as described in (A) with HuSLCs being treated with 20 μM MG132 to induce endogenous AR.
(C) Assays in HPET cells were performed as described in (A) with the modification that AR was exogenously expressed following transfection with 30 μg pSVARo plasmid.
(D) Assay in HuSLCs cells were performed as described in (A) following transfection with 40 μg pSVARo plasmid. Data are expressed as mean ± SEM; n = 4. **, p<0.001; ***, p<0.0001.
Collectively, these observations infer that an AR(−) phenotype is essential for prostate CSC self-renewal and proliferation. Moreover, treatment with OHF alone is sufficient to significantly promote sphere formation. Therefore, absence of AR protein and the potential of antiandrogens to exert as yet undiscovered AR-independent effects on stimulating prostate CSC self-renewal and proliferation could facilitate the emergence of therapeutic resistance.
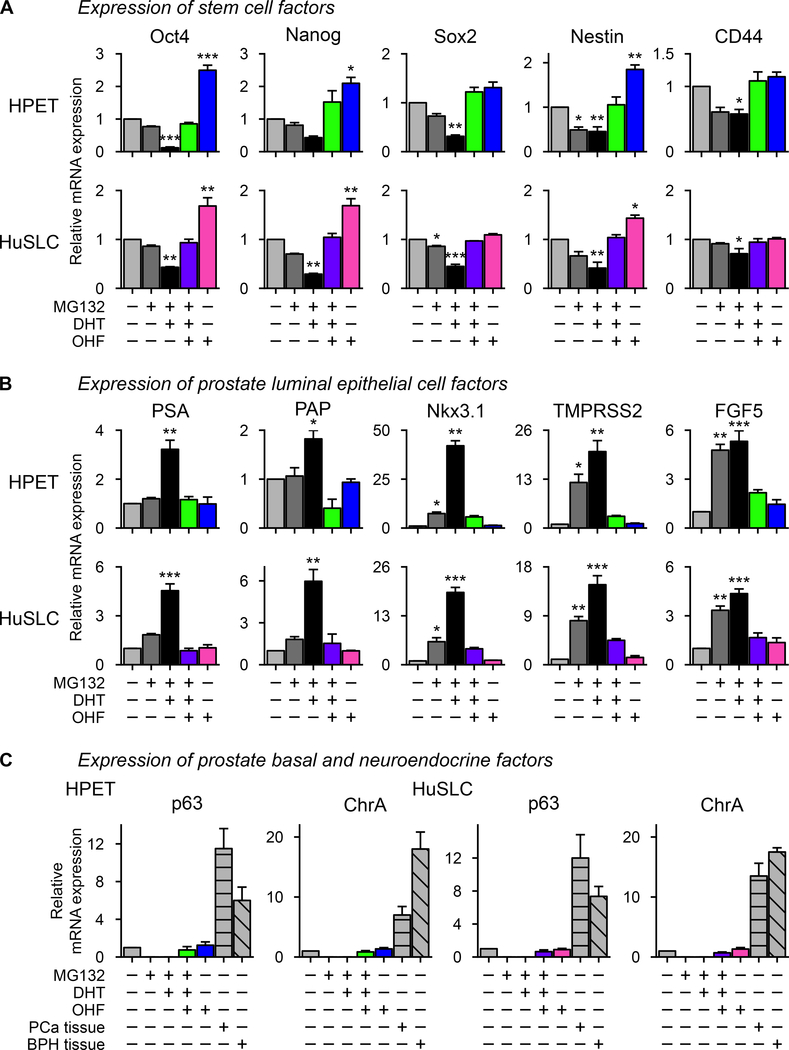
Induction of AR down-regulates stem/progenitor characteristics and promotes luminal epithelial cell fate
HPET and HuSLCs express numerous stem/progenitor cell markers, including the transcription factors Oct-4, Nanog, and Sox2 which regulate pluripotency and self-renewal in human and mouse embryonic stem cells (2). They also express the progenitor cell markers Nestin and CD44 (2). To determine whether AR restricted their expression, cells were treated with MG132. As seen in Figure 4A, induction of endogenous AR decreased Oct-4, Nanog, Sox2, Nestin, and CD44 expression following DHT treatment (p<0.05). In contrast, inhibition of AR signaling by OHF restored their expression. Transfection of HPET and HuSLCs with pSVARo confirmed these observations (Figure 5A). Moreover, in both MG132-treated and pSVARo-transfected cells, OHF alone could up-regulate these factors with Oct4, Nanog, and Nestin increasing >2-fold (p<0.05) as compared to the vehicle control group, suggesting that antiandrogens might exert, as yet, unknown AR-independent actives that support CSC expansion.
Figure 4. MG132 treatment down-regulates expression of stem cell-associated markers and promotes luminal epithelial cell fate.
(A) HPET cells and HuSLCs were treated with 2 μM and 20 μM MG132 respectively and expression of the stem cell factors Oct4, Nanog, Sox2, Nestin and CD44 were determined by RT-qPCR.
(B) Cells were treated with 2 μM and 20 μM MG132 respectively and expression of prostate luminal epithelial cells factors PSA, PAP, Nkx3.1, TMPRSS2 and FGF5 were determined by RT-qPCR.
(C) Cells were treated with 2 μM and 20 μM MG132 respectively and expression of p63 and ChrA were determined by RT-qPCR. Primer sets to characterize expression of all of these factors are listed in Supplementary Table S3. Data are expressed as mean ± SEM; n = 4. *, p<0.01; **, p<0.001; ***, p<0.0001.
Figure 5. Induction of exogenous AR down-regulates expression of stem cell-associated markers and promotes luminal epithelial cell fate.
(A) HPET cells and HuSLCs were transfected with 30 μg and 40 μg of pSVARo respectively and treated with/without DHT with/without OHF as indicated. Expression of the stem cell factors Oct4, Nanog, Sox2, Nestin and CD44 were determined by RT-qPCR.
(B) Cells were transfected and treated as in (A) and expression of prostate luminal epithelial cells factors PSA, PAP, Nkx3.1, TMPRSS2 and FGF5 were determined by RT-qPCR.
(C) Cells were transfected and treated as in (A) and expression of p63 and ChrA were determined by RT-qPCR. Primer sets to characterize expression of all of these factors are listed in Supplementary Table S3. Data are expressed as mean ± SEM; n=4. *, p<0.01; **, p<0.001; ***, p<0.0001.
These observations provide evidence that CSC-like cells lose stemness characteristics upon expression of AR. Since prostatic glandular epithelium consists of luminal secretory, basal, and neuroendocrine epithelial cells (2), we questioned whether loss of these factors would initiate epithelial cell specification. Indeed, androgen-regulated genes associated with luminal epithelial cell fate, including PSA and PAP (p<0.001) as well as Nkx3.1, TMPRSS2, and FGF5 (p<0.05) were induced upon activation of endogenous AR (Figure 4B) or pSVARo (Figure 5B) following treatment with DHT. Furthermore, expression of these luminal cell markers was inhibited by treatment with OHF. Similarly, the AR target genes HES1 and HEY1 were also induced following induction of AR expression (Supplementary Figure S5). In contrast, neither endogenous AR (Figure 4C) nor pSVARo (Figure 5C) induced expression of p63, a common basal epithelial cell marker, or Chromogranin A, typically expressed by prostate neuroendocrine cells, under any condition tested. Thus, AR appears to selectively promote luminal secretory cell fate.
Poly-ubiquitination regulates the dynamic turnover of AR protein
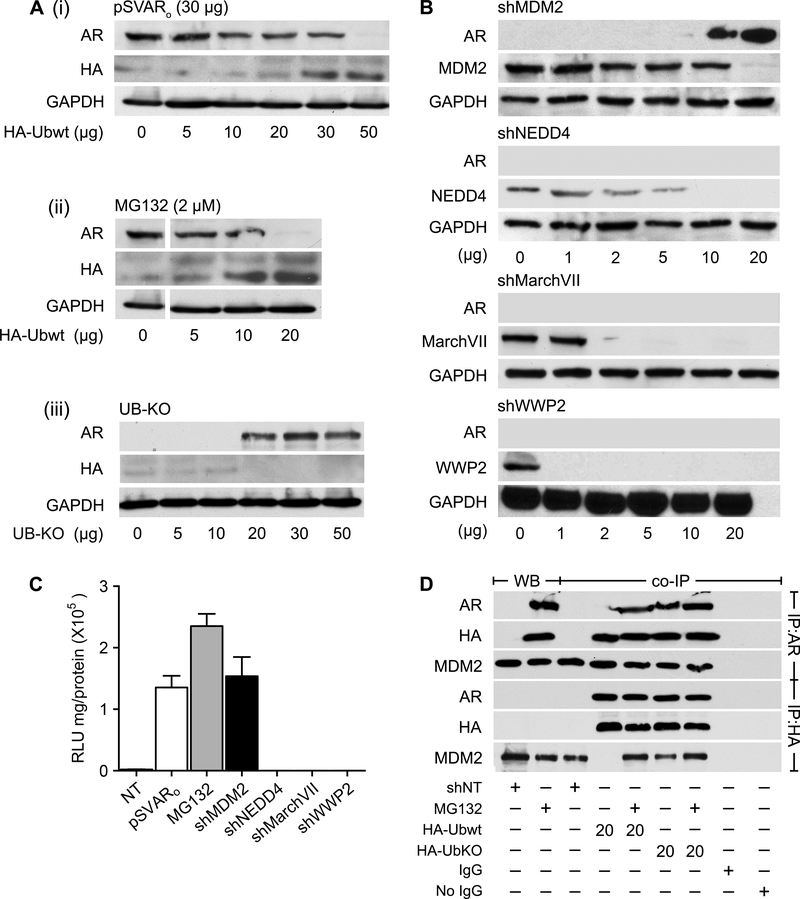
Several studies report that the UPS modulates transcription factor levels to regulate stem cell and CSC maintenance and differentiation (28–30). To determine whether poly-ubiquitination regulates AR protein levels, HPET cells were transfected with 30 μg pSVARo and increasing concentrations of the wild-type ubiquitin expression vector pRK5-HA-Ubiquitin-WT (HA-UbWt; Figure 6Ai). Exogenous AR was degraded in a dose-dependent manner with complete degradation occurring at 50 μg HA-UbWt. In a similar manner, 20 μg HA-UbWt was capable of degrading endogenous AR protein induced with MG132 treatment (Figure 6Aii). Cells were then transfected with a mutant ubiquitin plasmid pRK5-HA-Ubiquitin-KO (HA-UbKO) that is incapable of adding ubiquitin molecules onto its target protein to determine if inhibition of AR poly-ubiquitination prevented AR degradation. Endogenous AR protein was strongly expressed following transfection with HA-UbKO (Figure 6Aiii). Collectively, these observations imply that the dynamic turnover of AR protein in prostate CSC-like cells is regulated by poly-ubiquitination.
Figure 6. The E3 ligase MDM2 selectively degrades AR in prostate CSCs.
(A) HPET cells were transfected with either 30 μg pSVARo (i) or treated with 2 μM MG132 (ii) and transfected with increasing concentrations of the wild-type ubiquitin expression vector pRK5-HA-Ubiquitin-WT (HA-UbWt) to determine whether poly-ubiquitination regulated AR protein levels. In addition, HPET cells were transfected with increasing concentrations of a mutant ubiquitin plasmid pRK5-HA-Ubiquitin-KO (HA-UbKO, iii) which is incapable of adding ubiquitin molecules onto its target protein, to demonstrate that inhibiting AR poly-ubiquitination would prevent AR degradation.
(B) HPET cells were transfected with increasing concentrations of shMDM2, shNEDD4, shMarchVII or shWWP2 plasmid and protein expression was analyzed by Western blot analysis.
(C) Cells were co-transfected with 20 μg of shMDM2, 10 μg of shNEDD4, 2 μg of shMarchVII or 1ug of shWWP2 plasmid and the ARR2PB-luc and Renilla luciferase reporter gene constructs. Luciferase activity was determined. Positive controls, cells transfected with 30 μg pSVARo or treated with 2 μM MG132. NT, non-targeting shRNA control.
(D) IP analysis was performed to determine whether MDM2 directly binds to AR. Cells were transfected with 20 μg non-targeting shNT control plasmid, 20 μg HA-UbWt, or 20 μg HA-UbKO and treated with 2 μM MG132 as indicated. Antibodies for Western blot analysis are listed in Supplementary Table S1. Data are expressed as mean ± SEM; n=4.
MDM2 E3 ligase selectively degrades AR in prostate CSCs
The final step in the ubiquitination cascade is carried out by E3 ligases (31). Several E3 ligases, including MDM2 and NEDD4, are reported to regulate AR and/or AR-V protein levels in non-CSC, AR(+) PCa cells which comprise the bulk of prostate tumor cells and in AR(+) PCa cell lines derived from metastatic lesions. Whether MDM2 and/or NEDD4 degrade AR in prostate CSCs is unknown. Therefore, HPET cells were transfected with pSVARo and increasing concentrations of shMDM2 plasmid to determine the level of MDM2 knock-down and whether AR protein would be induced. As shown in Figure 6B, MDM2 levels decreased in parallel with increasing concentrations of shMDM2; and AR expression was greatest when MDM2 protein was barely detectable. AR-mediated transcription was confirmed using the ARR2PB-luc assay (Figure 6C). In contrast, NEDD4 knockdown to undetectable levels did not induce AR protein, implying that it did not play a role in modulating AR levels in CSC-like cells.
Other E3 ligases not currently known to regulate AR protein include MarchVII, associated with adult stem cells (32), and WWP2, reported to regulate embryonic stem cell factors, e.g., Oct4 (33) and Sox2 (34). Transfecting HPET cells with shMarchVII and shWWP2 in a dose-dependent manner determined that loss of MarchVII and WWP2 expression did not induce AR protein expression under any concentrations of shRNA tested (Figure 6B). Thus, MDM2 appears to selectively degrade AR in CSCs.
Immunoprecipitation (IP) analysis was performed to determine whether MDM2 directly binds AR (Figure 6D). When AR(−) HPET cells were transfected with HA-UbWt alone (lane 4), AR was not observed in the IP:AR fraction and MDM2 was absent in the IP:HA fraction, suggesting that AR expression was required for the formation of an AR/HA-UbWt/MDM2 binding complex. Once endogenous AR was expressed following MG132 treatment, both AR and MDM2 were observed following IP (lane 5), indicating that AR was necessary for AR/HA-UbWt/MDM2 complex formation. Similarly, an AR/HA-UbWt/MDM2 complex was observed in cells where expression of mutant HA-UbKO inhibited the degradation of endogenous AR (lane 6), confirming that AR could form a complex with ubiquitin and MDM2.
MDM2 knockdown inhibits CSC self-renewal and cell proliferation and promotes luminal epithelial cell differentiation
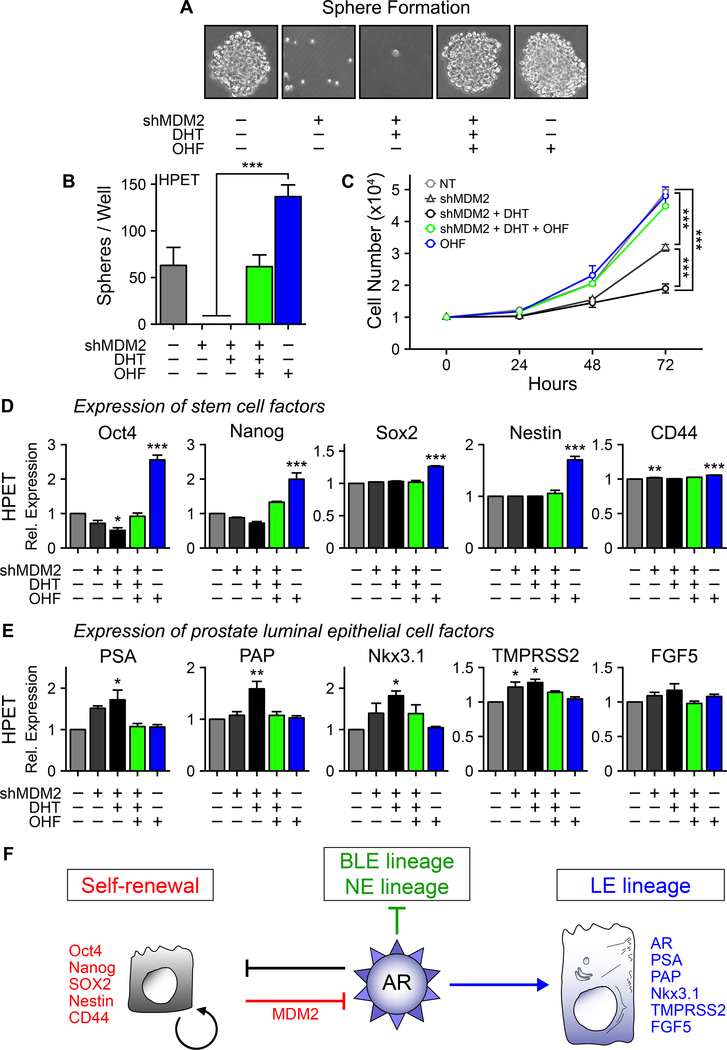
To determine the effects of MDM2 knockdown on CSC stemness, HPET cells were transfected with shMDM2 to prevent AR degradation. Knockdown of MDM2 alone was sufficient to abolish sphere formation and treatment with DHT did not alter these effects (Figure 7A, 7B). Again, sphere formation was rescued by addition of OHF. In addition, MDM2 knockdown alone decreased cell proliferation (p<0.05), addition of DHT inhibited cell proliferation even further, and proliferation was rescued with OHF treatment (Figure 7C). Furthermore, MDM2 knockdown decreased Oct-4 and potentially Nanog expression, and treatment with OHF alone increased their expression significantly (Figure 7D). In parallel, expression of luminal epithelial cell-specific genes, PSA, PAP, Nkx3.1, and TMPRSS2, increased with DHT treatment and decreased to basal levels with addition of OHF, while FGF5 expression was regulated in a similar manner, it did not reach statistical significance (Figure 7E).
Figure 7. MDM2 maintains stemness in human prostate CSC-like cells.
(A) Sphere formation assay. HPET cells were transfected with 20 μg of shMDM2 plasmid and treated with 10−8 M DHT with/without 10−5 M OHF to increase or inhibit AR activity respectively. Control group, transfected with 20 μg of non-targeting shNT control plasmid and treated with vehicle control (95% ethanol). Phase contrast images, 20x.
(B) Quantification of the number of spheres/well in (A).
(C) Proliferation assay (19). HPET cells were transfected with 20 μg of shMDM2 plasmid and treated with 10−8 M DHT with/without 10−5 M OHF treated as indicated. Total cell numbers/well were determined using the Trypan Blue Viability assay.
(D) HPET cells were transfected with 20 μg of shMDM2 and expression of the stem cell factors were determined by RT-qPCR.
(E) HPET cells were transfected with 20 μg of shMDM2 and expression of prostate luminal epithelial cells factors were determined by RT-qPCR. Data are expressed as mean ± SEM; n = 4.
(F) Schematic representation of MDM2-mediated regulation of AR expression in modulating self-renewal and epithelial cell specification. Details are provided in the main text.
These observations primarily recapitulated those observed in HPET and HuSLCs expressing AR following treatment with MG132 or transfection with pSVARo. However, one difference was that not all of the stem cell factors decreased in response to DHT treatment (Figure 7D), suggesting that MDM2 may exert specificity in maintaining the steady-state expression of select stem/progenitor cell proteins. Whether MDM2-mediated degradation of AR involves p53 is unclear. HPET cells do not express p53 while HuSLCs express p53, suggesting that p53 activity is not essential for degrading AR (Supplementary Figure S6).
DISCUSSION
Mechanisms that prevent AR expression in normal prostate stem cells and prostate CSCs remain largely unknown. Our study provides the first evidence that in stem-like AR(−) CSCs isolated from PCa biopsies, MDM2 promotes the constant degradation of AR protein, thereby maintaining prostate CSC pluripotency and inhibiting epithelial cell lineage specification (summarized in Figure 7F). The AR(−) signature also facilitates CSC proliferation and expansion, while induction of AR via MDM2 down-regulation selectively induces a luminal epithelial cell phenotype and loss of cell growth.
Most studies on prostate CSCs and AR have been performed in side-fractions of CSC-like cells isolated from LNCaP, LNCaP derivative, and LAPC9 cell lines (35,36). Both AR mRNA and AR protein are down-regulated In LNCaP and LAPC9-derived PSA−/lo cells (6). In the CAstration-Resistant Nkx3.1-expressing cells (CARNs) mouse model, CARNs expressed AR; however genetic deletion of AR did not alter their luminal progenitor/stem cell properties (37). Only the rate of proliferation during prostate regeneration was reduced (37). Taken together, these observations imply that AR is not required for prostate CSC or normal prostate progenitor/stem cell function.
Our study supports the role of MDM2 in blocking AR protein expression and proposes that MDM2 exerts fundamentally different functions in AR(−) prostate CSCs as compared to non-CSC, AR(+) PCa cells which comprise the bulk of prostate tumor cells (summarized in Supplementary Table S4). In AR(−) prostate CSCs, MDM2 continuously degrades AR to maintain an AR(−) phenotype, self-renewal, and proliferative potential. MDM2 is also reported to promote stemness properties in other tissue-derived stem cells, e.g., in generating induced pluripotent stem cells from p53-deficint murine embryonic fibroblasts (MEFs) (38) and suppressing differentiation of human mesenchymal stem cells into osteoblasts; whereas MDM2 knockdown increases osteoblast differentiation (38). In a similar manner, MDM2 knockdown in HPET and HuSLCs induced terminal differentiation to a luminal epithelial phenotype. However in non-CSC AR(+) PCa cells, it is well-documented that MDM2 temporally modulates AR protein levels to attenuate AR-mediated transcription during normal cellular function and to regulate cell cycle progression while retaining basal levels of AR expression (7). Inhibition of MDM2 expression in LNCaP and androgen-resistant LNCaP (LNCaP-Res) cell lines down-regulates AR protein levels and decreases AR activity, however total AR expression is not lost during this process (39). Thus, the mechanism regulating MDM2-mediated knockdown of HPET and HuSLC AR protein to undetectable levels remains to be elucidated.
Therapeutic resistance remains a persistent challenge in the treatment of PCa. Consequently, targeting the AR and androgens is still central to the management of advanced PCa [reviewed in (40)]. The recent discovery that PCa cells synthesize steroids de novo has resulted in considerable interest in drugs that inhibit androgen synthesis (41). Regrettably, since CSCs do not appear to require AR, none of the second-generation ADT and antagonist drugs, e.g., abiraterone and enzalutamide, would theoretically eliminate CSCs from the PCa cell pool. Moreover, our study suggests that antiandrogen treatment alone paradoxically increases CSC self-renewal and cell proliferation. If indeed induction of AR expression causes CSC differentiation into AR(+) luminal cells, then this could potentially re-sensitize CSCs to ADT and eliminate them along with the bulk of responsive AR(+) PCa cells. Induction of AR could also promote terminal differentiation and eliminate CSCs through this mechanism.
The emergence of therapeutic resistance is also attributed to production of AR-Vs (11,12,42). AR-Vs are expressed in clinical PCa biopsy specimens and PCa cell lines (13,26) and their expression is upregulated in metastatic and treatment-resistant disease (11,43). One of the most commonly expressed variants is AR-V7. It is considered a valid therapeutic target in the treatment of CRPC; however the mechanisms by which AR-V7 drives CRPC progression remains to be elucidated (42). Our study observed that biopsy-derived CSCs only expressed AR-fl, but not AR-V, in response to blocking MDM2 activity. Further studies are required to determine a putative role of AR-Vs in prostate CSCs. In addition, HPET cells do not express p53 while HuSLCs express p53, yet AR is continuously degraded and self-renewal and proliferation are maintained in both CSC-like cell lines, suggesting that p53 activity is not essential for these processes. Thus, therapeutic approaches that target MDM2-p53 interactions would likely be ineffective in inhibiting prostate CSC growth. Other reported p53-independent MDM2 activities include the promotion of cancer progression through EMT in AR(−) DU-145 cells which express a mutant/non-functional p53 (44), and clonogenic survival in MCF7, SJSA, and Panc1 cell lines (38). This is in contrast to AR(+)p53(+) cells where targeting MDM2/p53 interactions inhibit tumor cell growth. The small molecule inhibitor MI-219 selectively disrupts MDM2/p53 interactions, thereby activating p53 signaling and inducing apoptosis in LNCaP cells in vitro and inhibiting LNCaP xenograft growth in vivo (45). Similarly, the MDM2 inhibitor Nutlin-3 activates p53 and inhibits the growth of SJSA-1 osteosarcoma xenografts by 90% (46). In CRPC, lncRNA (AR-repressed long noncoding RNA) is up-regulated and binds AR protein to impair AR-MDM2 interactions. Consequently, AR is not ubiquitinated and degraded, resulting in the upregulation of AR transcriptional activity and increased CRPC cell growth (47).
Collectively, these studies infer that selectively targeting MDM2 activity, and not MDM2-p53 or AR-p53 interactions, could potentially eliminate CSCs more effectively than second generation ADT or antagonist drugs. Our study suggests that MDM2 conserves the AR(−) CSC signature and that this may be a critical step towards stimulating CSC expansion during the emergence of therapeutic resistance. Furthermore, treatments that promote antiandrogenic activities may signal CSCs to initiate proliferation and expansion (48). Thus, selectively blocking MDM2 expression and/or MDM2-mediated activity in combination with AR/androgen-targeted treatments may offer a novel strategy for eliminating AR(−) CSCs as well as the bulk of AR(+) PCa cells to decrease tumor burden and metastasis, and/or inhibit the emergence of therapeutic resistant disease.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Findings provide a novel mechanistic aspect of prostate cancer cell stemness that advances our understanding of the diverse transcriptional activity that bypasses AR in contributing to therapeutic resistance, tumor progression and metastasis.
ACKNOWLEDGEMENTS
The authors thank Katherine A. Burns, PhD for her invaluable assistance in the preparation and proofreading of the manuscript.
Financial support : Funding for this work was provided by the National Institute of Diabetes & Digestive & Kidney Diseases (R01 DK60957, S.K.) and the United States Department of Defense (W81XWH-08-1-0662, S.K.).
Footnotes
Conflict of interest statement: The authors declare no potential conflicts of interest.
REFERENCES
- 1.Zhang K, Zhou S, Wang L, Wang J, Zou Q, Zhao W, et al. Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res 2007;67:4807–15 [DOI] [PubMed] [Google Scholar]
- 3.Hu WY, Hu DP, Xie L, Li Y, Majumdar S, Nonn L, et al. Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem Cell Res 2017;23:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aumuller G, Groos S, Renneberg H, Konrad L, Aumueller M. Embryological and Postnatal Development of the Prostate Foster C, Bostwick D, editors. Philadelphia, Pennsylvania: 19106: W.B. Saunders Company; 1998. [Google Scholar]
- 5.Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology 1991;128:2867–73 [DOI] [PubMed] [Google Scholar]
- 6.Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, et al. The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell 2012;10:556–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin HK, Wang L, Hu YC, Altuwaijri S, Chang C. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. Embo J 2002;21:4037–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burska UL, Harle VJ, Coffey K, Darby S, Ramsey H, O’Neill D, et al. Deubiquitinating enzyme Usp12 is a novel co-activator of the androgen receptor. J Biol Chem 2013;288:32641–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardozo CP, Michaud C, Ost MC, Fliss AE, Yang E, Patterson C, et al. C-terminal Hsp-interacting protein slows androgen receptor synthesis and reduces its rate of degradation. Archives of biochemistry and biophysics 2003;410:134–40 [DOI] [PubMed] [Google Scholar]
- 10.Li B, Lu W, Yang Q, Yu X, Matusik RJ, Chen Z. Skp2 regulates androgen receptor through ubiquitin-mediated degradation independent of Akt/mTOR pathways in prostate cancer. Prostate 2014;74:421–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonarakis ES, Armstrong AJ, Dehm SM, Luo J. Androgen receptor variant-driven prostate cancer: clinical implications and therapeutic targeting. Prostate Cancer Prostatic Dis 2016;19:231–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho Y, Dehm SM. Androgen Receptor Rearrangement and Splicing Variants in Resistance to Endocrine Therapies in Prostate Cancer. Endocrinology 2017;158:1533–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong D, Sethi S, Li Y, Chen W, Sakr WA, Heath E, et al. Androgen receptor splice variants contribute to prostate cancer aggressiveness through induction of EMT and expression of stem cell marker genes. Prostate 2015;75:161–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Xie N, Gleave ME, Rennie PS, Dong X. AR-v7 protein expression is regulated by protein kinase and phosphatase. Oncotarget 2015;6:33743–54 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Singh S, Chitkara D, Mehrazin R, Behrman SW, Wake RW, Mahato RI. Chemoresistance in prostate cancer cells is regulated by miRNAs and Hedgehog pathway. PLoS ONE 2012;7:e40021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathew G, Timm EA Jr., Sotomayor P, Godoy A, Montecinos VP, Smith GJ, et al. ABCG2-mediated DyeCycle Violet efflux defined side population in benign and malignant prostate. Cell Cycle 2009;8:1053–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res 2009;19:683–97 [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Lin K, Lu Y, Rycaj K, Zhong Y, Chao HP, et al. Developing a Novel Two-Dimensional Culture System to Enrich Human Prostate Luminal Progenitors that Can Function as a Cell of Origin for Prostate Cancer. Stem cells translational medicine 2017;6:748–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams K, Ghosh R, Vummidi Giridhar P, Gu G, Case T, SM B, et al. Inhibition of Stathmin1 Accelerates the Metastatic Process Cancer Res 2012;72:5407–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Gao N, Kasper S, Reid K, Nelson C, Matusik RJ. An androgen-dependent upstream enhancer is essential for high levels of probasin gene expression. Endocrinology 2004;145:134–48 [DOI] [PubMed] [Google Scholar]
- 21.Pitkanen-Arsiola T, Tillman JE, Gu G, Yuan J, Roberts RL, Wantroba M, et al. Androgen and anti-androgen treatment modulates androgen receptor activity and DJ-1 stability. Prostate 2006;66:1177–93 [DOI] [PubMed] [Google Scholar]
- 22.Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 1999;270:41–9 [DOI] [PubMed] [Google Scholar]
- 23.Mulholland DJ, Cheng H, Reid K, Rennie PS, Nelson CC. The androgen receptor can promote beta-catenin nuclear translocation independently of adenomatous polyposis coli. J Biol Chem 2002;277:17933–43 [DOI] [PubMed] [Google Scholar]
- 24.Tillman JE, Yuan J, Gu G, Fazli L, Ghosh R, Flynt AS, et al. DJ-1 binds androgen receptor directly and mediates its activity in hormonally treated prostate cancer cells. Cancer Res 2007;67:4630–7 [DOI] [PubMed] [Google Scholar]
- 25.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res 2009;69:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watson PA, Chen YF, Balbas MD, Wongvipat J, Socci ND, Viale A, et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc Natl Acad Sci U S A 2010;107:16759–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE 2011;6:e19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szutorisz H, Georgiou A, Tora L, Dillon N. The proteasome restricts permissive transcription at tissue-specific gene loci in embryonic stem cells. Cell 2006;127:1375–88 [DOI] [PubMed] [Google Scholar]
- 29.Sakurai M, Ayukawa K, Setsuie R, Nishikawa K, Hara Y, Ohashi H, et al. Ubiquitin C-terminal hydrolase L1 regulates the morphology of neural progenitor cells and modulates their differentiation. J Cell Sci 2006;119:162–71 [DOI] [PubMed] [Google Scholar]
- 30.Jian R, Cheng X, Jiang J, Deng S, Hu F, Zhang J. A cDNA-based random RNA interference library for functional genetic screens in embryonic stem cells. Stem Cells 2007;25:1904–12 [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Lee MJ. Emerging roles of the ubiquitin-proteasome system in the steroid receptor signaling. Archives of pharmacal research 2012;35:397–407 [DOI] [PubMed] [Google Scholar]
- 32.Szigyarto CA, Sibbons P, Williams G, Uhlen M, Metcalfe SM. The E3 ligase axotrophin/MARCH-7: protein expression profiling of human tissues reveals links to adult stem cells. J Histochem Cytochem 2010;58:301–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Wang W, Li C, Yu H, Yang A, Wang B, et al. WWP2 promotes degradation of transcription factor OCT4 in human embryonic stem cells. Cell Res 2009;19:561–73 [DOI] [PubMed] [Google Scholar]
- 34.Fang L, Zhang L, Wei W, Jin X, Wang P, Tong Y, et al. A methylation-phosphorylation switch determines Sox2 stability and function in ESC maintenance or differentiation. Mol Cell 2014;55:537–51 [DOI] [PubMed] [Google Scholar]
- 35.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946–51 [DOI] [PubMed] [Google Scholar]
- 36.Tang DG, Patrawala L, Calhoun T, Bhatia B, Choy G, Schneider-Broussard R, et al. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog 2007;46:1–14 [DOI] [PubMed] [Google Scholar]
- 37.Chua CW, Epsi NJ, Leung EY, Xuan S, Lei M, Li BI, et al. Differential requirements of androgen receptor in luminal progenitors during prostate regeneration and tumor initiation. eLife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wienken M, Dickmanns A, Nemajerova A, Kramer D, Najafova Z, Weiss M, et al. MDM2 Associates with Polycomb Repressor Complex 2 and Enhances Stemness-Promoting Chromatin Modifications Independent of p53. Mol Cell 2016;61:68–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mu Z, Hachem P, Hensley H, Stoyanova R, Kwon HW, Hanlon AL, et al. Antisense MDM2 enhances the response of androgen insensitive human prostate cancer cells to androgen deprivation in vitro and in vivo. Prostate 2008;68:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Graham L, Schweizer MT. Targeting persistent androgen receptor signaling in castration-resistant prostate cancer. Medical oncology 2016;33:44. [DOI] [PubMed] [Google Scholar]
- 41.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol 2009;27:3742–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uo T, Plymate SR, Sprenger CC. The potential of AR-V7 as a therapeutic target. Expert Opin Ther Targets 2018;22:201–16 [DOI] [PubMed] [Google Scholar]
- 43.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 2008;68:5469–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slabakova E, Kharaishvili G, Smejova M, Pernicova Z, Suchankova T, Remsik J, et al. Opposite regulation of MDM2 and MDMX expression in acquisition of mesenchymal phenotype in benign and cancer cells. Oncotarget 2015;6:36156–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A 2008;105:3933–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004;303:844–8 [DOI] [PubMed] [Google Scholar]
- 47.Zhang A, Zhao JC, Kim J, Fong KW, Yang YA, Chakravarti D, et al. LncRNA HOTAIR Enhances the Androgen-Receptor-Mediated Transcriptional Program and Drives Castration-Resistant Prostate Cancer. Cell reports 2015;13:209–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SO, Ma Z, Yeh CR, Luo J, Lin TH, Lai KP, et al. New therapy targeting differential androgen receptor signaling in prostate cancer stem/progenitor vs. non-stem/progenitor cells. Journal of molecular cell biology 2013;5:14–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology 2000;141:4698–710 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.