Abstract
Elevated expression of soluble vascular endothelial growth factor receptor-1 (sFlt-1) in preeclampsia plays a major role in the pathogenesis of this serious disorder of human pregnancy. Although reduced placental oxygenation is thought to be involved in the pathogenesis of preeclampsia, it is unclear how oxygen regulates placental sFlt-1 expression. The aims herein were to investigate sFlt-1 expression in in vivo and in vitro physiological and pathological models of human placental hypoxia and to understand the role of hypoxia inducible factor-1 (HIF-1) in regulating the expression of this molecule. sFlt-1 expression in placental villi was significantly increased under physiological low oxygen conditions in early first-trimester and in high-altitude placentae, as well as in pathological low oxygen conditions, such as preeclampsia. In high-altitude and in preeclamptic tissue, sFlt-1 localized within villi to perivascular regions, the syncytiotrophoblast layer, and syncytial knots. In first-trimester villous explants, low oxygen, but not hypoxiareoxygenation (HR), increased sFlt-1 expression. Moreover, exposure of villous explants to dimethyloxalyl-glycin, a pharmacological inhibitor of prolyl-hydroxylases, which mimics hypoxia by increasing HIF-1α stability, increased sFlt-1 expression. Conversely, HIF-1α knockdown using antisense oligonucleotides, decreased sFlt-1 expression. In conclusion, placental sFlt-1 expression is increased by both physiologically and pathologically low levels of oxygen. This oxygen-induced effect is mediated via the transcription factor HIF-1. Low oxygen levels, as opposed to intermittent oxygen tension (HR) changes, play an important role in regulating sFlt-1 expression in the developing human placenta and hence may contribute to the development of preeclampsia.
Keywords: hypoxia, hypoxia-reoxygenation, high altitude, preeclampsia
CONSIDERABLE EVIDENCE SUPPORTS a central role for local oxygen availability in human trophoblast cell differentiation and hence appropriate placental development and function (20). During early pregnancy, entry of maternal blood to the intervillous space of the placenta is limited by the endovascular tropho-blast, and thus oxygen levels around the interstitial and villous trophoblast are relatively low (~15 mmHg, equivalent to 2–3% O2) (17). This low-oxygen environment is essential for normal embryonic development (11). After 10–12 wk of gestation, maternal blood flow to the intervillous space increases and oxygen levels surrounding the placental villi reaches ~55 mmHg (~8% O2) (17). Uteroplacental blood flow rises exponentially in the second trimester of pregnancy and is associated with transformation of the proximal uterine artery Doppler waveform (37). Failure of this process to occur results in uteroplacental vascular insufficiency and chronic hypoxia, placing the pregnancy at risk of preterm delivery from preeclampsia (26) and/or intrauterine growth restriction (IUGR)(41).
The main cellular pathway by which oxygen regulates gene expression is the formation of the heteromeric protein complex known as hypoxia inducible factor (HIF). HIF comprises two distinct subunits: α and β. When oxygen tension is low, the labile α subunit forms a heterodimer with the constitutively expressed β subunit. The heterodimer is subsequently translocated inside the nucleus where it binds to short DNA motifs (known as HREs, or hypoxia responsive elements) in the promoter regions of a variety of genes, thereby activating their transcription. Under normoxia, HIF-1α is rapidly hydroxylated and hence targeted for proteosomal degradation (28). Among the many molecules whose expression is induced under low-oxygen conditions via the HIF pathway are vasculogenic factors, such as vascular endothelial growth factor (VEGF) (9) and its receptor VEGFR-1 (also known as Flt-1) (10). A splice variant of Flt-1, the soluble form of VEGFR-1 (sVEGFR-1), also known as sFlt-1 (19), comprises six extracellular IgG-like domains and a unique C terminus lacking the classical trans-membrane domain (19). sFlt-1 is expressed and secreted from several different tissues, including human endometrium (22), endothelial cells (12), and placental villous tissue (8).
The source of the pregnancy-associated increase in sFlt-1 production and secretion in humans is believed to be the placenta (3, 8). Studies using primary cultures of isolated human cytotrophoblast cells have recently demonstrated that low oxygen causes increased sFlt-1 expression (29).
Secreted sFlt-1 binds to VEGF and placental growth factor (PlGF) with high affinity, thereby decreasing their availability for binding the transmembrane receptors VEGFR-1 and VEGFR-2 (8, 34). As such, any increase in serum sFlt-1 will cause decrease in serum-free VEGF and PlGF. During normal pregnancy, sFlt-1 serum levels increase with advancing gestation but are markedly increased in both serum and placental tissue from preeclamptic pregnancies (21, 23, 43). Increased serum levels of sFlt-1 in preeclampsia have been argued as a potential direct cause of several manifestations of the disease (27, 40), and it has been postulated that in preeclampsia, the abnormal placentation following placental hypoxia may result in increased sFlt-1 levels (27), thus contributing to the pathogenesis of this disease.
Although it is now established that preeclampsia is associated with lowered placental oxygenation (35), the exact mechanisms by which oxygen increases sFlt-1 expression are presently unclear. The aims of this study were to examine placental sFlt-1 expression in models of placental hypoxia, including placental tissues obtained from various weeks of gestations across the first trimester (in vivo developmental hypoxia), from high-altitude (in vivo physiological chronic hypoxia) and preeclamptic pregnancies (in vivo pathological hypoxia). Additionally, using a well-established villous organ culture system, we have investigated the mechanisms by which reduced oxygenation (in vitro hypoxia) and hypoxia-reoxygenation (HR) regulate sFlt-1 expression. Finally, we have determined whether HIF, in particular HIF-1α, plays a direct role in regulating the expression of sFlt-1 in the human placenta.
MATERIALS AND METHODS
Tissue and blood collection.
Local Ethics Committee approval was obtained for the study from the participating institutions, and all women gave written informed consent. Tissue collection strictly adhered to the guidelines outlined in The Declaration of Helsinki. High-altitude (HA) placentae and blood samples were collected in Leadville, CO (HA, 3,179 m above sea level). HA placentae were obtained from healthy normotensive patients at term. Ten milliliters of blood were withdrawn from the mother’s antecubital vein at 36 wk or greater of gestation and at 3 mo postpartum. Blood was allowed to clot, and the serum was separated and stored in −80°C for later analysis. Sea level (SL) placental samples (also referred to as term control, or TC) were collected from term deliveries in Toronto, Ontario, Canada (~40 m). Control blood samples were collected in Denver, CO (1,600 m). Severe early-onset preeclampsia was diagnosed on the basis of the American College of Obstetrics and Gynecology criteria (1). Preeclamptic placentae (PE, n = 16) and preterm normotensive age-matched control placentae (preterm control, PTC, n = 12) were collected from deliveries at Mount Sinai Hospital in Toronto. Early-onset preeclampsia was defined when the patient was delivered before 34-wk gestation due to preeclampsia. All third-trimester specimens were obtained immediately after delivery from normal-looking cotyledons randomly collected. Areas with calcified, necrotic or visually ischemic tissue were omitted from sampling. Subjects suffering from diabetes, essential hypertension, or renal disease were excluded. Pregnancies affected by IUGR were also excluded. Preterm deliveries were due to multiple pregnancies (16%), preterm labor due to incompetent cervix (35%), premature preterm rupture of membrane (33%), and spontaneous preterm deliveries without identifiable cause (16%). All preterm and term control groups did not show clinical or pathological signs of preeclampsia, infections or other maternal or placental disease. Birth weight, gestational age, and laboratory values or clinical observations relevant to the health of the mother were abstracted from the clinical records. The clinical characteristics of the patients are shown in Table 1. First-trimester placental samples (5–12 wk of gestation, n = 55) were collected from elective first-trimester pregnancy terminations performed by dilatation and curettage. Gestational age was determined by the date of the last menstrual period and first-trimester ultrasound measurement of crown-rump-length.
Table 1.
Clinical parameters of participants
| Term Control | Preterm Control | High Altitude | Early Preeclampsia | |
|---|---|---|---|---|
| n | 15 | 12 | 16 | 18 |
| Maternal age, yr | 28.5 ± 3.7 | 30.5 ± 4.8 | 27.6 ± 7.1 | 28.9 ± 6.0 |
| Gestational age, wk | 39.2 ± 1.2 | 29.5 ± 4 | 39.8 ± 1.7 | 29.2 ± 2.9 |
| Gravidity/parity, mean | 2/1 | 2/0 | 2/0 | 2/1 |
| Blood pressure, mmHg | ||||
| Systolic | 113 ± 8 | 114 ± 4 | 113 ± 13 | 181 ± 10 |
| Diastolic | 73 ± 8 | 70 ± 7 | 74 ± 8 | 111 ± 6.0 |
| Proteinuria (plus protein) | Absent | Absent | Absent | 3.4 ± 1.5 |
| Edema (% of patients) | Absent | Absent | Absent | 82% |
| Birth weight, g | 3332 ± 346 | 1300 ± 730 | 3054 ± 276* | 1160 ± 352 |
| Mode of delivery (n) | ||||
| CS | 3 | 5 | 3 | 16 |
| VD | 12 | 7 | 13 | 2 |
Data are presented as means ± SD. CS, cesarian section; VD, vaginal delivery.
P < 0.05 high altitude vs. term control.
First-trimester human chorionic villous explant culture.
Chorionic villous explant culture was performed as previously described (5). Briefly, placental tissues (5–8 wk of gestation, 15 separate sets) were placed in ice-cold PBS and processed within 2 h of collection. Tissues were aseptically dissected to remove decidual tissue and fetal membranes. Small fragments of placental villi (25–45 mg wet weight) were teased apart, placed on Millicell-CM culture dish inserts (Millipore , Bedford, MA) precoated with 0.15 ml of undiluted Matrigel (Collaborative Biomedical Products, Bedford, MA), and transferred to a 24-well culture dish. Explants were cultured in serum-free DMEM/F12 (Gibco BRL, Grand Island, NY) supplemented with 100 &g/ml streptomycin, 100 U/ml penicillin, and incubated overnight at 37°C in 5% CO2 in air to allow attachment. Explants were maintained in standard condition (5% CO2 in 95% air) or in an atmosphere of 3% or 8% O2 (92% or 87% N2 and 5% CO2, respectively) for 72 h at 37°C. The morphological integrity and viability of villous explants and their extravillous trophoblast outgrowth and migration were monitored daily for the duration of the various experiments, as previously reported (5). In parallel experiments, HR was performed as previously described (15) by decreasing from 8% O2 (physiological oxygen tension at 10–12 wk gestation) to 2–3% O2 for 2–3 h, followed by exposure to 20% O2 standard conditions for 1 h.
Pharmacological stabilization and knockdown of HIF-1.
Explants kept in 20% O2 were treated with a 1.0 mM concentration of dimethyloxalyl-glycin (DMOG), an inhibitor of prolyl-hydroxylases activity mimicking hypoxia via stabilization of HIF-1α (16). Control cultures maintained in standard 20% O2 conditions were run in parallel in the presence of medium alone. HIF-1α knockdown studies were performed using a previously validated antisense approach (5). In brief, 10 μM of HIF-1α antisense and control sense oligos were added to the media of explants in 3% oxygen conditions.
After completion of experiments, conditioned media were collected and kept in −80°C for ELISA analysis of sFlt-1. Explants were collected and snap frozen in liquid nitrogen for gene analysis. Ex-plants in each experiment were also fixed in 4% paraformaldehyde for immunohistochemical analysis.
TUNEL assay.
An in situ cell death detection kit was purchased from Amersham Biosciences (Piscataway, NJ). Terminal deoxynucleotidyl transferase-dUTP-nick end labeling (TUNEL) assays were performed according to instructions provided by the manufacturer. Paraffin sections of villous explants and preeclamptic placental tissue were dewaxed in xylene, rehydrated in descending grades of ethanol, and finally soaked in PBS. Tissue sections were pretreated with proteinase K (10 μg/ml) in PBS for 10 min, washed, and then incubated in 3% hydrogen peroxide in methanol for 45 min. After washing in PBS, the slides were preincubated with 1 × One Phor All buffer in 0.1% Triton X-100 in water for 30 min and then incubated in TdT solution for 1.5 h at 37°C. Samples were washed in PBS, and avidin biotin complex (Vector Laboratories, Burlingame, CA) was applied for 1 h. Staining was detected with the diamino-benzidine chromogen after 10 min. Slides were counterstained with hematoxylin, dehydrated in ascending concentrations of ethanol, and fixed with xylene.
RNA isolation and quantitation using real-time RT-PCR (qPCR).
Total RNA was isolated from placental samples using a TRIzol-based approach, according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Total RNA was isolated from whole explants using RNeasy Mini Kit (Qiagen, Mississauga, Ontario, Canada), according to the manufacturer’s protocol. DNA contamination was enzymatically removed by DNase-I digestion before RNA reverse transcription. qPCR (sFlt-1) was performed on the MJ’s Opticon II Light Cycler system as previously described (36). TaqMan Universal MasterMix and specific Taqman primers and probe for sVEGFR-1 (sFlt-1, accession number: U01134) and 18S were used (Applied Biosystems, Foster City, CA) based on the manufacturer’s protocol (Applied Biosystems). Relative quantitation of data was performed using logarithmic curves. Expression level of sFlt-1 was normalized based on 18S expression using the 2ΔΔCt formula, as previously described (25, 36). Sequences for sFlt-1 primers and probe: forward: 5’-GGGAAGAAATCCTCCAGAAGAAAGA-3’; reverse: 5’-GAGATCCGAGAGAAAACAGCCTTT-3’; probe: 5’-CAGTGCTCACCTCTGATTG-3’. Total amplicon size is 79 base pairs.
Western blot analysis.
Western blot analysis for sFlt-1 and Flt-1 was performed using 50 μg of total placental protein lysates that were subjected to 6% (wt/vol) SDS-PAGE gel. After electrophoresis, proteins were transferred to polyvinylidene difluoride membranes. Nonspecific binding was blocked by incubation in 5% (wt/vol) BSA in Tris-buffered saline containing 0.1% (vol/vol) Tween-20 (TBST-20) for 60 min. Membranes were then incubated with either 1:200 diluted specific anti-soluble VEGFR-1 antibody (Zymed Laboratories, San Fransisco, CA) or 1:1,000 diluted specific monoclonal anti-Flt-1 antibody (abcam, ab9540, Cambridge, MA), in 5% (wt/vol) BSA in Tris-buffered saline-Tween (TBST) at 4°C. After overnight incubation, membranes were washed with TBST and incubated for 60 min at room temperature with 1:10,000 diluted horseradish peroxidase-conjugated anti-rabbit IgG (Santa Cruz, CA) in 5% (wt/vol) BSA in TBST. After washing with TBST, blots were exposed to chemiluminescent reagent (enhanced chemiluminescence; Amersham Pharmacia Biotech, Oakville, Ontario, Canada). All Western blots were checked for equal protein loading at all times using Ponceau staining.
Immunohistochemistry.
Paraffin sections were mounted on glass slides, dewaxed in xylene, and rehydrated in descending ethanol gradient. Antigen retrieval was performed by heating in sodium citrate solution (10 mmol). Endogenous peroxidase was quenched with 3% (vol/vol) hydrogen peroxide in PBS for 30 min. After blocking (5% normal goat serum for 1 h), the slides were incubated overnight with primary antibody (anti-human soluble VEGFR-1, 1:150 dilution). Slides were washed in 1×PBS and exposed to peroxidase-conjugated secondary antibody (1:300, goat anti-rabbit, Vector Laboratories) for 45 min at room temperature. Finally, avidin biotin complex (Vector Laboratories) was applied for 1 h, and staining was detected with the diamino-benzidine chromogen after 5 min. Slides were counterstained with hematoxylin. Primary antibody was omitted and replaced by blocking solution in the negative control conditions.
ELISA.
Serum samples from the same women who donated their placentae, as well as conditioned media from first-trimester villous explants, were collected. These were used to determine sFlt-1 expression using an ELISA performed in duplicate, according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). The minimal detectable concentration was 5 pg/ml of sFlt-1. Protein content in the conditioned media was normalized to the total protein concentration that was measured by Bradford protein assay. The intra-assay coefficient of variation for the HA and control serum samples was <9%, whereas the intra-assay coefficient of variation for the conditioned media samples obtained from the explant cultures was 5.7%.
Statistics.
Statistical analyses were performed using GraphPad Prism software. Data are represented as means ±SE of at least 3 separate experiments carried out in triplicate. For comparison of data between multiple groups, we used one-way ANOVA with post hoc Dunnett test. For comparison between two groups, we used the Mann-Whitney U test and paired or unpaired t- test when applicable. Significance was defined as P < 0.05. Results are expressed as the means ± SE.
RESULTS
Changes in sFlt-1 Expression During Placental Development.
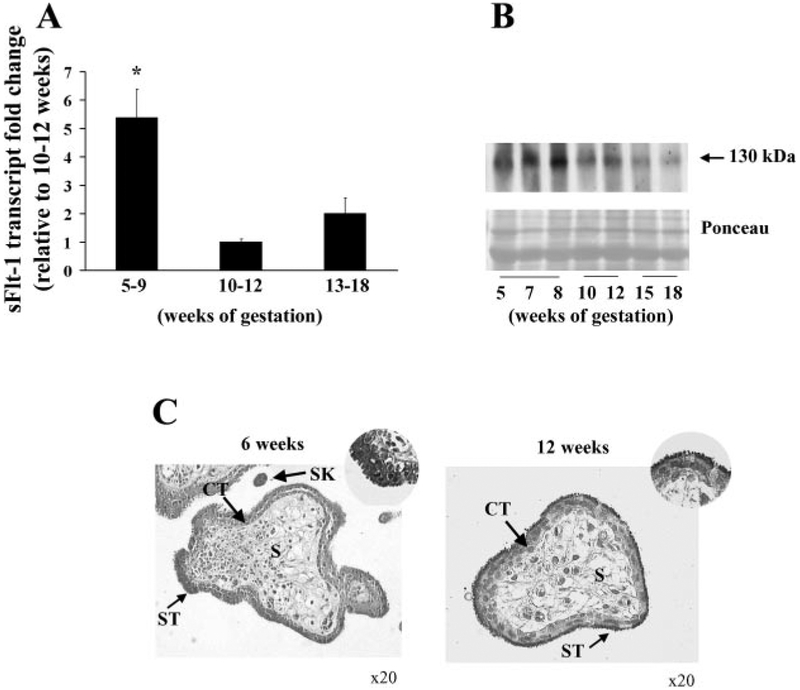
At the end of the first trimester of pregnancy (10–12th wk of gestation), when the intervillous space opens to maternal blood, the placenta experiences a surge in oxygenation (17). As such, we first examined whether sFlt-1 expression is affected by this physiological change in placental oxygenation in vivo. sFlt-1 transcript expression was significantly increased (5.3 ± 1.4 fold) in early first-trimester placental samples (5–9 wk of gestation) compared with late first-trimester (10–12 wk) samples (P < 0.05) (Fig. 1A). Although an increase in sFlt-1 transcript in samples from weeks 13 to 18 wk relative to weeks 10 to 12 wk (twofold) was noted, this increase was not statistically significant (Fig. 1A).
Fig. 1.
sFlt-1 expression during placental development. A: expression of sFlt-1 transcript in early first-trimester placental samples vs. later gestation as assessed by real-time PCR analysis (n = 11 for each gestational age tested); *P < 0.05, 5–9 wk vs. 10–12 wk. B: representative sFlt-1 immunoblot of first and early second-trimester samples. 5–9 wk, n = 5; 10–12 wk, n = 5; 13–18 wk, n = 7. C: immunolocalization of sFlt-1 in first-trimester tissue. Dark gray staining represents positive immunoreactivity. (CT, cytotrophoblasts; ST, syncytiotropho-blast, SK, syncytial knot; S, stroma). All values are represented as the means ± SE.
We next examined the protein expression of sFlt-1 during normal placental development. Western blot analysis of placental lysates from the same gestational age range showed a decrease in sFlt-1 protein level at 10–12 wk gestation (1.4 ± 0.18-fold increase at 5–9 wk relative to 10–12 wk) (Fig. 1B). Similar to the sFlt-1 transcript level, an increase in sFlt-1 protein was also noted at 13–18 wk of gestation. We next assessed the spatial localization of sFlt-1 in first-trimester samples using immunohistochemistry (IHC). At 6 wk of gestation, strong positive immunoreactivity for sFlt-1 was noted in the syncytiotrophoblast layer (ST) (Fig. 1C, left). After 10 wk of gestation, reduced immunoreactivity was observed in the ST and restricted mainly to the apical brush border (Fig. 1C, right). We also observed strong positive sFlt-1 immunostaining in syncytial knots in sections of first-trimester placental tissue. (Fig. 1C, left). No sFlt-1 staining was observed in stromal regions.
sFlt-1 expression is increased in high-altitude and preeclamptic placentae.
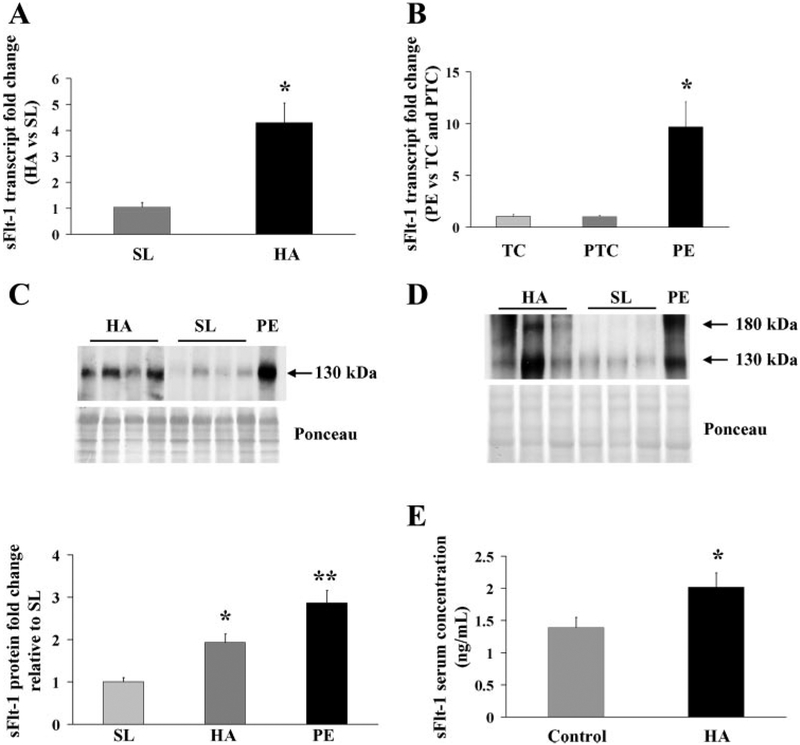
The effect of in vivo conditions of chronic placental hypoxia on sFlt-1 expression was next examined. HA placental tissues showed increased sFlt-1 transcript expression compared with SL samples (HA: 4.3 ± 0.7-fold increase vs. SL; P < 0.05) (Fig. 2A). Early onset severe preeclamptic placental samples, included as an internal positive control, also demonstrated a significant increased sFlt-1 expression compared with normotensive preterm age-matched control samples and term controls (PE: 9.7 ± 2.4-fold increase vs. PTC and TC controls; P < 0.05). sFlt-1 transcript level was similar in the PTC control and TC (Fig. 2B). The effect of mode of delivery on sFlt-1 expression in TC placentae was next examined by qPCR. No changes in sFlt-1 mRNA expression were observed in placentae tissue collected after cesarean section compared with spontaneous vaginal deliveries(1.05 ± 0.14-fold increase relative to CS). Western blot analyses performed on placental lysates from high altitude showed higher levels of both sFlt-1 and Flt-1 proteins compared with SL samples corroborating the transcript levels (Fig. 2, C and D, top). Densitometric analyses showed increased sFlt-1 expression in high-altitude samples relative to SL control tissues (Fig. 2C, bottom) (1.9 ± 0.2 vs. 1.0 ± 0.1 respectively, P < 0.01). Similar to the sFlt-1 protein expression in HA placental tissues, measurements of circulating sFlt-1 in serum of HA patients relative to lower altitudes (control), demonstrated a 45% greater concentration (HA: 2,018 ± 225 vs. control: 1,392 ± 159 pg/ml; P = 0.01) (Fig. 2D). sFlt-1 concentrations (Y) fell as the time past delivery (X) increased (best fit regression = y = 0.0033x2 –4.25x + 1,906 R2 = −0.92, P = 0.009, data not shown). In conjunction with the placental expression of sFlt-1 reported here, the elevated circulating concentrations in high-altitude pregnancy appear to be largely of placental origin. Values obtained 3–6 mo after delivery in the same women did not differ between low (272 ± 85 pg/ml) and high (305 ± 138 pg/ml) altitude (P = 0.42). sFlt-1 concentrations were not related to placental weight at either altitude, hence differences in placental mass do not account for the elevated values at high altitude.
Fig. 2.
sFlt-1 expression in high-altitude and preeclamptic placental samples. A: expression of sFlt-1 mRNA in high-altitude (HA) vs. sea level (SL) samples assessed by real-time PCR analysis. HA, n = 15, SL, n = 12. *P < 0.001, HA vs. SL. B: fold change in the transcript level of sFlt-1 in early severe preeclampsia (PE, n = 18) compared with age-matched controls (AMC, n = 12) assessed by real-time PCR. *P < 0.01, PE vs. term control (TC) and vs. preterm control (PTC), respectively. C, top: representative sFlt-1 Western blot analysis in placental tissues from high-altitude (HA, n = 14) and preeclamptic pregnancies (PE, n = 5), relative to SL controls (n = 11). Bottom: sFlt-1 protein densitometric analysis in HA, PE, and control SL. Data are normalized vs. sea-level samples. *P < 0.01, HA vs. SL; **P < 0.01, PE vs. HA and SL. D: representative Flt-1 Western blot analsis in placental tissues from HA (n = 6) and preeclamptic pregnancies (PE, n = 3), relative to SL controls (n = 5). E: relative levels of circulating sFlt-1 protein in serum of pregnant patients from HA and lower altitude (control) near term (n = 16). Values are mean ± SE. *P < 0.05, HA vs. control.
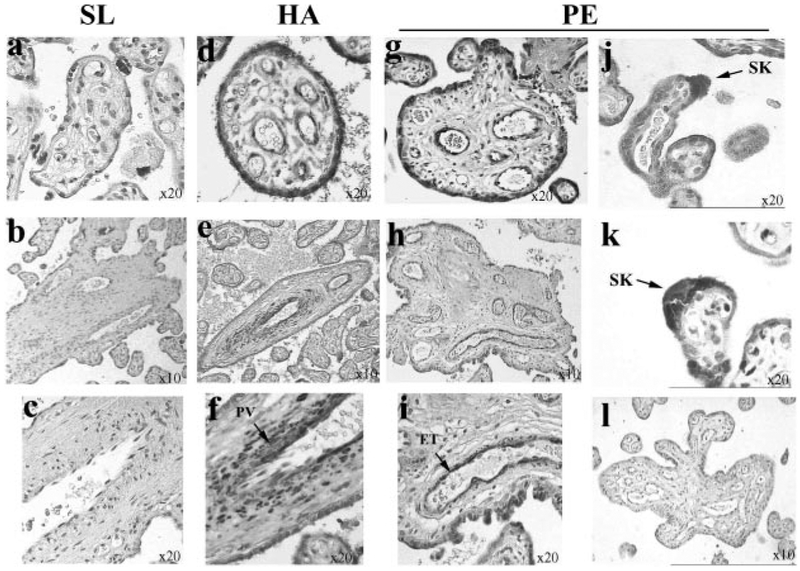
We next examined the spatial localization of sFlt-1 in HA, PE, and control (SL) placental tissues. (Fig. 3). IHC analysis showed strong positive immunoreactivity for sFlt-1 in ST layers and in vascular and perivascular regions of section from HA samples (Fig. 3, d, e, and f). Low/absent staining for sFlt-1 was noted in SL samples (Fig. 3, a–c). Increased ST staining, as well as vascular staining, was also observed in preeclamptic samples (Fig. 3, g–i). We also consistently observed strong positive immunoreactivity for sFlt-1 in syncytial knots mainly in PE samples (Fig. 3, j and k). No immunoreactivity was observed in control PE section where primary antibody was omitted (Fig. 3l).
Fig. 3.
Immunolocalization of sFlt-1 in representative HA, PE, and SL placental tissue. a–c: sea level controls, n = 10. d–f: high-altitude samples, n = 8. g–k: early preeclampsia samples, n = 6. l: negative control (no 1° antibody). Dark gray staining represents positive sFlt-1 immunostaining. ET, endothelium; PV, perivascular.
Hypoxia but not HR, increases expression of sFlt-1.
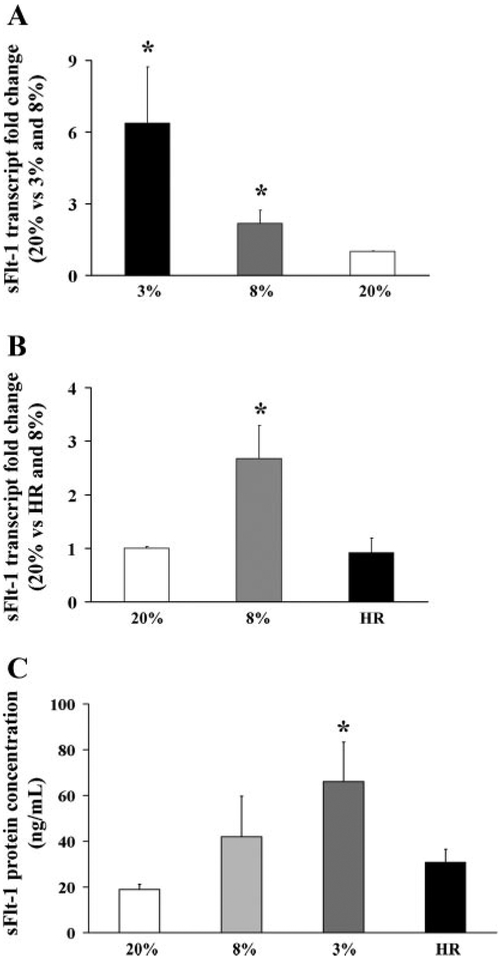
We next tested the effect of varying oxygenation on sFlt-1 expression in vitro using explant cultures. Exposure of villous explants to 3% oxygen resulted in increased sFlt-1 mRNA levels compared with explants cultured at 20% (6.3 ± 2.3 vs. 1.0 ± 0.02, P <0.05) (Fig. 4A). Exposure to 8% O2 resulted also in a signifi-cant increase in sFlt-1 expression (2.2 ± 0.5 vs. 1.0 ± 0.02). Next, we examined sFlt-1 protein expression and localization in explant tissue sections using IHC. Sections of explants exposed to 3% oxygen showed increased sFlt-1 staining in ST layer and proliferating extravillous trophoblast cells compared with sections from explants exposed to 20% oxygen (Fig. 5B, left and middle).
Fig. 4.
Effect of low oxygen and hypoxia-reoxygenation (HR) on sFlt-1 expression in first-trimester villous explants. A: expression of sFlt-1 mRNA in explants cultured at 3% and 8% vs. 20% O2 measured by qRT-PCR analysis, n = 7. *P < 0.05, 3% vs. 20% O2. B: real-time RT-PCR analysis of sFlt-1 mRNA in explants cultured in 20% and 8% O2 compared with HR conditions, n = 5. *P < 0.05, 8% vs. HR; *P < 0.05, 8% vs. 20% O2. C: sFlt-1 protein concentration measured by ELISA in conditioned media from first-trimester placental explants that were cultured in 20%, 8%, and 3% O2 compared with HR, n = 8. *P < 0.05, 3% vs. HR and 20%. Values are expressed as means ± SE of at least five separate experiments carried out in triplicate.
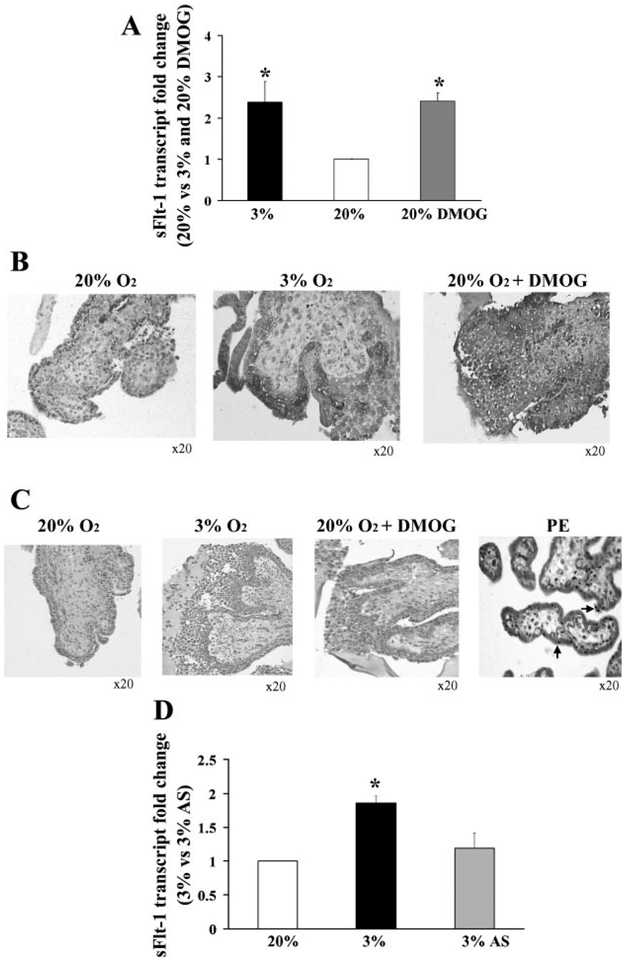
Fig. 5.
Effect of dimethyloxalyl-glycin (DMOG) and antisense oligonucleotides to hypoxia inducible factor (HIF-1α) on sFlt-1 expression in first-trimester placental explants. A: effect of DMOG treatment on sFlt-1 transcript in villous explants assessed by qRT-PCR, n = 3. *P < 0.05, 3% and 20% DMOG vs. 20% O2. B: effect of DMOG treatment on spatial localization of sFlt-1 protein in villous explants, n = 3. Dark gray staining represents positive immunoreactivity. C: TUNEL staining of villous explants and placental sample of early PE. Positive staining appears as black nuclear staining. D: effect of antisense oligonucleotides to HIF-1α (AS) on sFlt-1 mRNA expression in explants, n = 6. *P < 0.05, 3% O2 vs. 3% O2 +AS. Values are expressed as means ± SE of at least three separate experiments carried out in triplicate.
To determine whether intermittent change in oxygenation affects sFlt-1 expression, explants were next exposed to HR conditions. sFlt-1 transcript level was significantly higher in control explants exposed to 8% O2 than those treated by HR or 20% O2 standard conditions (8%, 2.5 ± 0.6-fold increase relative to HR; 8%, 2.6 ± 0.2-fold increase vs. 20%, P < 0.05) (Fig. 4B). Moreover, sFlt-1 transcript level was not changed following HR relative to standard 20% oxygen condition(0.9 ± 0.2 vs. 1 ± 0.03, respectively). Using ELISA, we confirmed that the expression of secreted sFlt-1 in conditioned media of explants exposed to 3% and to a lesser extent to 8% oxygen was increased relative to explants maintained in 20% O2. In contrast, HR exposure did not change sFlt-1 levels compared with 20% O2 controls (Fig. 4C).
sFlt-1 expression in low oxygen is mediated via HIF-1.
We show here that increased expression of sFlt-1 occurs where oxygen tension is lower both in in vivo and in vitro models; however, it is still unclear by what mechanism(s) hypoxia may regulate the expression of sFlt-1. Therefore, we investigated the impact of HIF-1 stabilization and knockdown on sFlt-1 expression. DMOG, an indirect stabilizer of the oxygen labile moiety of HIF-1 (16), was added to the media of explants cultured under 20% oxygen. The sFlt-1 mRNA expression of the DMOG-treated explants was significantly greater than that of explants kept at 20% O2 and was equivalent to control explants exposed to 3% O2 (2.4 ± 0.2 vs. 1.0 ± 0.03, respectively, P < 0.05), (Fig. 5A). As well, we observed that addition of DMOG to the 20% O2-treated explants resulted in increased sFlt-1 protein expression in all trophoblast layers, including EVT compared with explants exposed to 20% O2 in the absence of DMOG (Fig. 5B, right and left). DMOG-treated explants also exhibited the typical low oxygen-induced outgrowth compared with control explants maintained at 20% O2, and TUNEL assays indicated that the observed phenotype, due to either 3% O2 exposure or DMOG treatment, was not likely due to an increased incidence of apoptosis (Fig. 5C).
Finally, using a previously established antisense knockdown technique for HIF-1α (5), we demonstrated that antisense-treated explants under conditions of reduced oxygenation (3% O2) have significantly lower expression of sFlt-1 transcript relative to control cultures exposed to the same oxygenated environment in the presence of control sense or medium alone(1.19 ± 0.22 vs. 1.86 ± 0.1, respectively, P < 0.05) (Fig. 5D).
DISCUSSION
In the present study, we have demonstrated that, 1) reduced oxygenation in in vivo and in vitro placental hypoxia causes increased expression of sFlt-1, 2) low oxygenation as opposed to HR is the driving force for increased sFlt-1 production in human placental tissues, and 3) sFlt-1 expression under conditions of reduced oxygenation is mediated by HIF-1. Previous studies have reported that low oxygen conditions increase sFlt-1 expression in both primary cytotrophoblast cells, as well as in term placental villous explants (2, 29). Our expression profile of sFlt-1 during the first trimester indicates an inverse correlation of this soluble receptor with increasing physiological placental oxygenation occurring after the 10th wk of gestation. Hence, early on, when oxygen tension is relatively low, sFlt-1 transcript and protein are elevated. The fine-tuning of sFlt-1 expression during this critical developmental period highlights the importance of this soluble receptor in controlling the effects of its ligand VEGF, which is known to be highly expressed at this time (18). It is plausible that during early pregnancy, this soluble receptor, by antagonizing VEGF and PlGF function, may temporally restrict early development of the placental microvasculature, thereby diminishing the detrimental impact of early oxygenation (<10 wk), known to be associated with spontaneous miscarriage (11, 17). Beyond the critical early period of hypoxia and embryogenesis, subsequent to increased placental blood flow, a decline in sFlt-1 expression may allow vascular growth factors to increase placental vascularity in accordance with the needs of the developing fetus (18).
Placentae from high-altitude pregnancies exhibit significant morphological adaptation to chronic hypoxia, including increased vascularity of mature intermediate and terminal villi, resulting in reduced diffusional barrier (vasculosyncytial membrane) and increased density of terminal villi (42). Our findings suggest that even modestly reduced oxygen tension, estimated at ~20% reduction relative to sea level in our high-altitude placentae (42) correlates with increased expression of sFlt-1. It is quite possible that the excess sFlt-1 may function to restrict excessive peripheral vascular development under high-altitude conditions, an incomplete adaptation, as a greater incidence of chorangiomas is noteworthy in high-altitude placentae (4). The systemic effects of chronic hypoxia on sFlt-1 are also clinically significant. High-altitude residents are at two- to fourfold greater risk for the development of preeclampsia (30). Under reduced oxygenation, trophoblast cells may secrete more sFlt-1 than its ligands (29). Hence, increased sFlt-1 expression in normotensive high-altitude patients may explain the increased susceptibility to both preeclampsia and IUGR within this population.
Finally, we found that the primary sources of increased sFlt-1 expression in high-altitude placentae are the vascular and perivascular tissues and to a lesser extent the trophoblast cells. In contrast, in preeclamptic tissue, sFlt-1 is mainly localized to trophoblast layers. Although the endothelial expression of sFlt-1 has been described in other systems (12), our data provide the first evidence demonstrating a differential spatial vascular expression between normal (high-altitude preferential perivascular expression) and pathologic (preeclampsia preferential syncytiotrophoblast expression) human placentae. Recent evidence reports increased sFlt-1 expression in cord blood of newborns from preeclamptic pregnancies (39). Therefore, it is likely that increased circulating sFlt-1 levels found in physiological and pathological models of placental hypoxia may originate from placental trophoblast layers and/or perivascular tissue.
Mode of delivery and preterm birth are two factors that can differently affect gene expression in placental tissue. It has been shown that in vaginal deliveries accompanied by birth asphyxia there is increased expression of VEGF, Flt-1, and VEGFR-2 in placental tissue (38). Our results show that there is no difference in sFlt-1 expression between normal vaginal deliveries and cesarean section, indicating that sFlt-1 expression is not subject to changes in mode of delivery. Moreover, our findings demonstrating that sFlt-1 expression levels do not change between preterm and term control groups further indicate that gestational age at delivery is also not responsible for changes in sFlt-1.
Our in vitro model of placental hypoxia using first-trimester explants supports previous reports demonstrating increased sFlt-1 expression in hypoxic conditions in vitro both in primary isolated first-trimester trophoblast cells, as well as in term explants (2, 29). The stimulatory effect of lowered oxygen tension on sFlt-1 expression has been postulated to be mediated by HIF-1 (24). In silico analysis of the Flt-1 gene, which is the precursor of sFlt-1, HREs were revealed in its promoter region (10), which supports the contention that sFlt-1 may be regulated by HIF-1. Our experimental findings of increased sFlt-1 expression under DMOG-mediated HIF-1 stabilization or its decreased expression using HIF-1α knockdown under reduced oxygenation provides direct evidence that HIF-1α regulates sFlt-1 transcript expression. Previous studies indicate that HIF-1α expression is increased in preeclamptic (6, 31) and high-altitude placentae (7) and therefore may explain the increased sFlt-1 expression seen in these conditions.
Our in vitro studies demonstrate that sFlt-1 expression does not change after HR. Cycles of hypoxia followed by reoxygenation has been proposed as the underlying condition in preeclampsia that induces oxidative stress, thus causing placental damage (15). We specifically tested HR because both VEGF in rat myocardium (33) and TNF-α in placental explants (13) are elevated after an HR insult and associated with increased secretion of sFlt-1. Although HR may induce oxidative stress in the human placenta (14), our data here, the epidemiological data (42), and our recent data on aberrant global placental gene expression in the same in vitro and in vivo models tested here (35) suggest that chronically reduced oxygen may be the main trigger. Although we have shown that low oxygen tension contributes to aberrant global placental gene expression in early onset severe preeclampsia (35), we still note here that in preeclamptic placentae sFlt-1 expression was significantly more elevated than that of high-altitude placentae compared with controls, suggesting that other pathways may potentially be involved in regulating sFlt-1 expression in preeclampsia.
Preeclampsia is also characterized by excessive shedding/turnover of trophoblast microfragments and syncytial knots into maternal peripheral circulation, an event that has been hypothesized to contribute to generalized endothelial dysfunction (32). We find increased sFlt-1 protein expression in placental syncytial knots, particularly in preeclamptic placentae, suggesting that shed syncytial fragments may serve as a vehicle to carry excess sFlt-1 into the maternal circulation, hence enhancing the detrimental antiangiogenic function of sFlt-1 on the maternal peripheral vasculature.
In conclusion, increased sFlt-1 expression in the human placenta under low oxygen conditions in vivo and in vitro is mediated by HIF-1α. Chronically low oxygen tension, as opposed to HR, plays an important role in regulating the expression of this angiogenic antagonist in the human placenta. Increased sFlt-1 expression in high-altitude placentae could explain the greater population susceptibility to the development of preeclampsia in this environment.
ACKNOWLEDGMENTS
We thank Dr. Ljiljiana Petkovic for placental collection. We also thank Dr. Alan Bocking for his constant support and Dr. Martin Post for carefully reading the manuscript.
GRANTS
This work was supported by the Canadian Institutes of Health Research (CIHR) Grant (MT-1406) to I. C. Isabella Caniggia is recipient of an Ontario Women’s Health CIHR/IGH Mid-Career Award.
Footnotes
Publisher's Disclaimer: The American Journal of Physiology - Regulatory, Integrative and Comparative Physiology publishes original investigations that illuminate normal or abnormal regulation and integration of physiological mechanisms at all levels of biological organization, ranging from molecules to humans, including clinical investigations. It is published 12 times a year (monthly) by the American Physiological Society, 9650 Rockville Pike, Bethesda MD 20814–3991.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American College of Obstetrics and Gynecology. Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. Int J Gynaecol Obstet 77: 67–75, 2002. [PubMed] [Google Scholar]
- 2.Ahmad S and Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res 95: 884–891, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Banks RE, Forbes MA, Searles J, Pappin D, Canas B, Rahman D, Kaufmann S, Walters CE, Jackson A, Eves P, Linton G, Keen J, Walker JJ, and Selby PJ. Evidence for the existence of a novel preg nancy-associated soluble variant of the vascular endothelial growth factor receptor, Flt-1. Mol Hum Reprod 4: 377–386, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Benirschke K Recent trends in chorangiomas, especially those of multiple and recurrent chorangiomas. Pediatr Dev Pathol 2: 264–269, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, and Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGF-beta(3). J Clin Invest 105: 577–587, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caniggia I and Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and preeclamptic pregnancies–a review. Placenta 23 Suppl A: S47–S57, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Caniggia I, Wu Y, and Zamudio S. Overexpression of HIF-1 alpha in placentae from high altitude. Placenta 23: A.49–A.50, 2002. [Google Scholar]
- 8.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, and Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod 59: 1540–1548, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, and Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16: 4604–4613, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber HP, Condorelli F, Park J, and Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem 272: 23659–23667, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Hempstock J, Jauniaux E, Greenwold N, and Burton GJ. The contribution of placental oxidative stress to early pregnancy failure. Hum Pathol 34: 1265–1275, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Hornig C, Barleon B, Ahmad S, Vuorela P, Ahmed A, and Weich HA. Release and complex formation of soluble VEGFR-1 from endothelial cells and biological fluids. Lab Invest 80: 443–454, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Hung TH, Charnock-Jones DS, Skepper JN, and Burton GJ. Secretion of tumor necrosis factor-alpha from human placental tissues induced by hypoxia-reoxygenation causes endothelial cell activation in vitro: a potential mediator of the inflammatory response in preeclampsia. Am J Pathol 164: 1049–1061, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung TH, Skepper JN, and Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol 159: 1031–1043, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung TH, Skepper JN, Charnock-Jones DS, and Burton GJ. Hypoxiareoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 90: 1274–1281, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, and Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, and Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157: 2111–2122, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann P, Mayhew TM, and Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis II Changes during normal pregnancy. Placenta 25: 114–126, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Kendall RL and Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 90: 10705–10709, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kingdom JC and Kaufmann P. Oxygen and placental vascular development. Adv Exp Med Biol 474: 259–275, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, and Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab 88: 2348–2351, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Krussel JS, Casan EM, Raga F, Hirchenhain J, Wen Y, Huang HY, Bielfeld P, and Polan ML. Expression of mRNA for vascular endothelial growth factor transmembraneous receptors Flt1 and KDR, and the soluble receptor sflt in cycling human endometrium. Mol Hum Reprod 5: 452–458, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, and Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Gu B, Zhang Y, Lewis DF, and Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta 26: 210–217, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ and Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Lunell NO, Nylund LE, Lewander R, and Sarby B. Uteroplacental blood flow in pre-eclampsia measurements with indium-113m and a computer-linked gamma camera. Clin Exp Hypertens 1: 105–117, 1982. [DOI] [PubMed] [Google Scholar]
- 27.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Liber-mann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, and Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzen E and Ratcliffe PJ. HIF hydroxylation and cellular oxygen sensing. Biol Chem 385: 223–230, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, and Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145: 4838–4845, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, and Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180: 1161–1168, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Rajakumar A, Brandon HM, Daftary A, Ness R, and Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25: 763–769, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Sargent IL, Germain SJ, Sacks GP, Kumar S, and Redman CW. Trophoblast deportation and the maternal inflammatory response in preeclampsia. J Reprod Immunol 59: 153–160, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki H, Ray PS, Zhu L, Galang N, and Maulik N. Oxidative stress due to hypoxia/reoxygenation induces angiogenic factor VEGF in adult rat myocardium: possible role of NF-B. Toxicology 155: 27–35, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Sawano A, Takahashi T, Yamaguchi S, Aonuma M, and Shibuya M. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ 7: 213–221, 1996. [PubMed] [Google Scholar]
- 35.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, and Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab 90: 4299–4308, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soleymanlou N, Wu Y, Wang JX, Todros T, Ietta F, Jurisicova A, Post M, and Caniggia I. A novel Mtd splice isoform is responsible for trophoblast cell death in pre-eclampsia. Cell Death Differ 12: 441–452, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, and Brandes JM. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol 162: 121–125, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Trollmann R, Amann K, Schoof E, Beinder E, Wenzel D, Rascher W, and Dotsch J. Hypoxia activates the human placental vascular endothelial growth factor system in vitro and in vivo: up-regulation of vascular endothelial growth factor in clinically relevant hypoxic ischemia in birth asphyxia. Am J Obstet Gynecol 188: 517–523, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Tsao PN, Wei SC, Su YN, Chou HC, Chen CY, and Hsieh WS. Excess soluble fms-like tyrosine kinase 1 and low platelet counts in premature neonates of preeclamptic mothers. Pediatrics 116: 468–472, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, and Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab 88: 5555–5563, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Viero S, Chaddha V, Alkazaleh F, Simchen MJ, Malik A, Kelly E, Windrim R, and Kingdom JC. Prognostic value of placental ultrasound in pregnancies complicated by absent end-diastolic flow velocity in the umbilical arteries. Placenta 25: 735–741, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Zamudio S The placenta at high altitude. High Alt Med Biol 4: 171–191, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, and Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol 160: 1405–1423, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]