Abstract
The influence of hypoxia (lowered arterial blood and/or tissue PO2) on fetoplacental development and the role of hypoxia in preeclampsia are major research foci in perinatal biology. While animal and cell models are of utility, we do not know whether artificial hypoxic stimuli mimic the pathological conditions attributed to hypoxic stress in vivo; we cannot distinguish the effects of hypoxia from under- or overlying pathologies. High altitude (>2700 m) is the natural experiment we can use to distinguish pathology from adaptation in human pregnancy. The two best known impacts of high altitude on pregnancy outcome are reduced fetal growth and an increased incidence preeclampsia. This review focuses on the mechanisms by which altitude increases maternal risk for the development of preeclampsia. The review first considers the evidence that placental hypoxia is causally involved in the development of preeclampsia. It then focuses on how data from studies of pregnant women at high altitude support (or do not support) etiological models of preeclampsia. Considered are the theories that reduced uteroplacental blood flow, circulating factors of placental origin, placental oxidative stress and increased maternal vascular reactivity are etiological in preeclampsia. The data suggest that oxidative stress and endothelial dysfunction have pathophysiological origins that are independent of placental hypoxia. We conclude that altitude shifts the individual risk for the development of preeclampsia because of impacts on multiple physiological systems, no one of which can be specifically pointed to as causal.
Keywords: Hypoxemia, Oxidative Stress, Uteroplacental Blood Flow, Pregnancy, Growth Factors, Vascular Reactivity, Review
2. HIGH ALTITUDE AND PREECLAMPSIA
The ancient Greeks gave eclampsia (lightning) its name because the disease struck quickly and without warning. Pre-eclampsia, the prodromal state, was characterized by racing pulses, a symptom that would now be recognized as hypertension. After more than 2000 years, the cause of preeclampsia remains unknown.
Residence at high altitude (>2700 m) is the only external environmental factor that has been consistently linked with an increased incidence of preeclampsia (1–4). Despite the utility of experimental animal and cell culture models employing hypoxic stimuli, high altitude is the only in vivo human model with which to compare the results of in vitro and animal experimentation. To date we do not know whether artificial hypoxic stimuli mimic the pathological conditions attributed to hypoxic stress in vivo. This review evaluates how the data from high altitude support or do not support etiological theories of preeclampsia. We have used a variety of research designs, all with appropriate Institutional Review Board Approvals and participants’ informed consent, including cohort studies, birth-certificate analyses and prospective longitudinal physiological analyses. The data have consistently shown anywhere from a two- to a four-fold elevation in the incidence of preeclampsia at high altitude using both strict criteria (primiparas with documented proteinuria and hypertension that resolved following delivery) and less strict, but clinically relevant criteria (e.g. hypertension plus evidence of other organ system involvement, such as neurological symptoms, abnormal liver function or platelet consumption). Increased preeclampsia at high altitude is a global phenomenon, being observed in North and South America, the middle east and anecdotally among Chinese migrants to Tibet. It is not due to an altitude-associated increase in known maternal risk factors (e.g. obesity). The effect of altitude is independent of other risk factors, including socioeconomic status (5, 6). Since the most obvious effect of high altitude is lowered arterial oxygen tension (hypoxemia, lowered PO2), the increased incidence of preeclampsia and IUGR at high altitude supports that hypoxia contributes to the development of preeclampsia. Further support derives from the disease literature: women with congenital heart diseases associated with poor cardiac output or impaired lung transfer of oxygen to blood also have a markedly increased risk for preeclampsia (7).
A plethora of animal studies have investigated regulation of vascular tone and reactivity, uterine artery structure and growth and maternal physiology in pregnancy under hypoxic conditions (reviewed in 8–10). They converge in revealing how subtle changes in multiple physiological systems likely contribute to an increased risk for the development of preeclampsia at high altitude. The following section of this review examines some of the evidence that hypoxia is involved in the etiology of preeclampsia. We then examine 4 well-known etiological models of preeclampsia and consider to what extent data from high altitude are consistent with the etiological model.
2.1. Hypoxia and preeclampsia
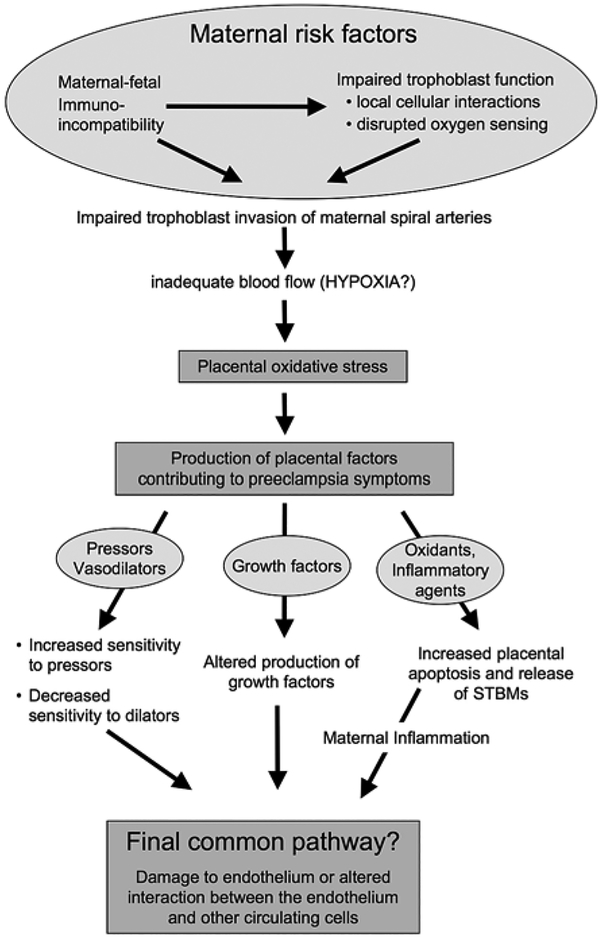
The model presented in Figure 1 is only one of many possibilities but will serve for this review as an organizational schema.
Figure 1.
Individual susceptibility is a prominent contributor to the development of preeclampsia; obesity, variation in thresholds for inflammatory response, vascular sensitivity, insulin sensitivity or other characteristics can contribute to increased susceptibility. At the top of the model we show that impaired trophoblast invasion is a common feature of the disease, and while as yet the cause of impaired invasion is unknown there is good evidence to support disrupted oxygen sensing and immunological interactions as playing a role (these issues are reviewed elsewhere in this volume). At high altitude trophoblast invasion is impaired and uterine blood flow is reduced. This review considers how these two observations may translate into specific features of preeclampsia, such as placental oxidative stress, altered production of growth factors and increased vascular responsiveness
The first question that must be answered if we are to consider high altitude a useful model for understanding the pathophysiology of preeclampsia is whether the evidence supports that preeclampsia is characterized by fetoplacental hypoxia. Hypoxia and/or ischemia-reperfusion injury are often invoked as a mechanism contributing to preeclampsia (11–17). Many studies since the early 1980s, pioneered by Stuart Campbell in the UK (uterine arteries) and Warwick Giles in Australia (umbilical arteries), have demonstrated that increased resistance to blood flow is present before the onset of symptoms in women who eventually develop preeclampsia and/or intrauterine growth restriction (18–20). Increased resistance implies reduced blood flow, though this correlation has not been directly tested. We found that reduced uterine artery blood flow is present at high altitude (21) and that reduced blood flow precedes the onset of symptoms in preeclampsia (22). The high-altitude fetus is thus subjected to the double insult of hypoxemia of the blood entering the placental intervillous space and decreased uteroplacental blood flow. This supports the idea that reduced oxygen delivery and/or PO2 contribute to the development of preeclampsia and IUGR at any altitude, and likely augments the risk at high altitude.
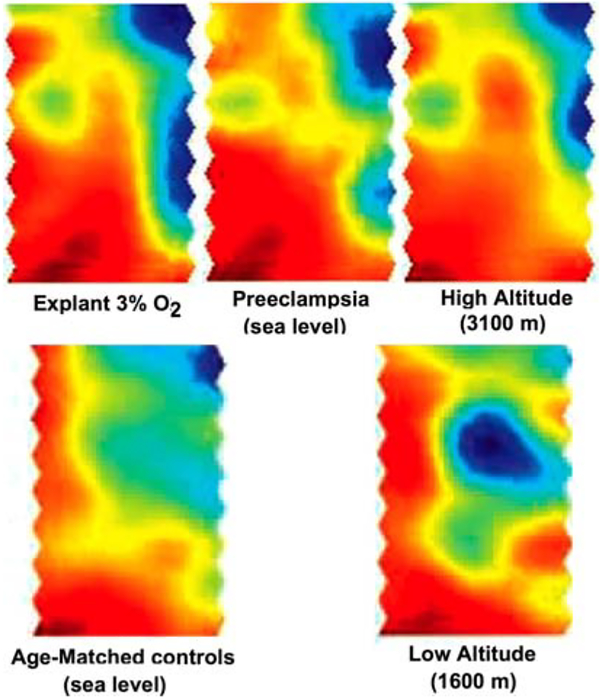
But does what is observed at high altitude mirror what is seen in preeclampsia? Both morphological and molecular evidence support that high altitude placentas resemble preeclampsia in some, but not all features. Fox, (23, 24) showed that hypoxia was associated with increased cytotrophoblast proliferation, a finding mirrored in high altitude placentas by an increased proportion of cytotrophoblast relative to other trophoblast cell populations (reviewed in 25). There is decreased remodeling of the uteroplacental arteries at the level of the basal plate in preeclampsia (26) and at high altitude, although not to the same extent as in preeclampsia. Increased vascularity (27) or a more tortuous and dense distribution of blood vessels at the level of the maternal myometrium is observed in preeclampsia, and in high altitude placentas (28). In contrast increased fibrin deposition and other evidence of syncytial damage is common in preeclampsia, but consistently decreased at high altitude (25). Placental expression of markers of hypoxia, such as erythropoietin receptor (29) and sFlt-1 (30) are increased at high altitude and/or in preeclampsia. Global profiles of gene expression were similar in placentas exposed to high altitude hypoxia, preeclampsia at sea level and term placental explants cultured under 3% oxygen conditions (31) (Figure 2). Confirmation of the changes in a number genes by Soleymanlou and colleagues (qPCR) indicated that the high altitude placenta retains an immature phenotype (consistent with lower oxygen tension) relative to the low altitude placenta. There is increased expression of Integrin alpha6, a marker of immature trophoblast phenotype, and increased markers of proliferation and hypoxia (e.g. VEGF). These molecular data are consistent with the morphological data (increased cytotrophoblast and vascular development). Correlation between the gene expression profiles demonstrated in Figure 2 was strong in preeclampsia versus high altitude (r=.5, p<.001), and greater still in preeclampsia versus term explants cultured under 3% oxygen (r=.6, p<.001) (31). There is thus good evidence to support the involvement of hypoxia in preeclampsia. However, hypoxia is neither necessary nor sufficient to cause preeclampsia. A doubling of the incidence at high altitude in the absence of differences in epidemiological risk factors indicates that hypoxia increases risk, but other factors must come into play in orderto tip one individual versus another over her particular tolerance into the preeclampsia syndrome. This is where the high altitude model is of special utility.
Figure 2.
Shown here are the results of microarray analysis of pooled placental samples from term explants cultured under 3% oxygen, preeclamptics, high altitude, age-matched controls for the preeclamptic patients and low altitude controls for the high altitude patients. This analysis is based on self-organizing maps. Of the 1700 genes represented on the microarray plate, areas of increased gene expression are shown in red and areas of decreased gene expression are shown in blue. The methodological details of the analysis, the results from a targeted analysis of the maximally different areas of these microarrays, the list of individual gene differences and the validation of differences in selected individual genes between altitude, explants, preeclampsia and controls are in reference 31. This figure is reprinted here to highlight the broad similarity in the pattern of gene expression between the in vitro and in vivo models of placental hypoxia (3% O2, preeclampsia and high altitude) and their divergence from the controls (AMC at sea level and low altitude). Reprinted by permission from 31.
3. ALTITUDE AND ETIOLOGICAL MODELS OF PREECLAMPSIA
3.1. Etiological model #1 - Reduced blood flow
The idea that placental ischemia or hypoxia ‘causes’ preeclampsia has a long and contentious history. Beker, in 1929, wrote that ‘an inadequate blood supply to the uterus may be the cause of toxaemia’ (32). Browne and Veall countered, in 1953, that ‘placental ischemia is the result, and not the cause of hypertension in toxaemia’ (33), a theory modified by Clemetson, who proposed that reduced or slowed cord blood flow causes fetal hypoxia (markedly lower umbilical artery PO2) and this in turn causes placental ‘hypoxia’ via the blood return through the umbilical arteries to the placental circulation (34). To date, the chicken and egg question of whether decrement in blood flow precedes the onset of symptoms remains contentious.
A majority of investigators believe impaired placental invasion of the maternal vasculature, possibly due to fetoplacental-maternal immuno-incompatibility (35), or to aberrant oxygen sensing (12, 36) is the primary initiating factor in the disease and ‘causes’ the reduced blood flow and resultant placental hypoxia (Figure 1). Table 1 summarizes the relevant publications on uteroplacental blood flow in normal and pathological human pregnancy. While many clinicians feel that uteroplacental blood flow cannot be reliably or accurately measured, data across 50 years and multiple techniques converge on an average blood flow of ~700 ml/min in late pregnancy. The ranges in Table 1 are noteworthy; normotensive women without complications have blood flows that could overlap with those noted in preeclampsia (Table 1), suggesting lowered blood flow alone is not causal in preeclampsia.
Table 1.
Reported values for uteroplacental blood flow in normal and abnormal near-term human pregnancy
| Group | Sample size | Technique | Range (ml/min) | Blood flow (ml/min) | Reduction versus normal | Reference |
|---|---|---|---|---|---|---|
| Normal | 8 | Isotopic | NR | 600 | 33 | |
| Chronic HTN | 3 | Isotopic | NR | −50% | 33 | |
| Chronic HTN+PE | 2 | Isotopic | NR | −70% | 33 | |
| Mild PE | 2 | Isotopic | NR | −60% | 33 | |
| Normal | 7 | Nitrous oxide | 610–925 | 750 | 107 | |
| Normal | 13 | Nitrous oxide | 175–840 | 492±188 | 108 | |
| Normal | 8 | Nitrous oxide | 255–480 | 500 | 109 | |
| Normal | 5 | Nitrous oxide | 308–651 | 465±136 | 110 | |
| Chronic HTN | 1 | Nitrous oxide | NR | −12% | 110 | |
| Normal | 50 | Isotopic | NR | 111 | ||
| Chrome HTN | 31 | Isotopic | NR | −20% | 111 | |
| Severe PE | 11 | Isotopic | NR | −29% | 111 | |
| Normal | 37 | Isotopic | NR | 116, 117 | ||
| Mild PE | 17 | Isotopic | NR | −35% | 116, 117 | |
| Severe PE | 8 | Isotopic | NR | −67% | 116, 117 | |
| PE+IUGR | 10 | Isotopic | NR | −54% | 116, 117 | |
| PE w/o IUGR | 15 | Isotopic | NR | −40% | 116, 117 | |
| IUGR w/o HTN | 19 | Isotopic | NR | −61% | 116, 117 | |
| Normal | 26 | Isotopic | NR | 1140 | 118 | |
| IUGR w/o HTN | 13 | Isotopic | NR | −57% | 118 | |
| Normal | 24 | Doppler | NR | 825 | 112 | |
| Normal | 18 | Doppler | NR | 921 | 113 | |
| Low altitude normal | 18 | Doppler | NR | 921 | 113, 21 | |
| High altitude normal | 23 | Doppler | NR | −33% | 21 | |
| Mild PE | 7 | Doppler | NR | −67% | 22 | |
| Normal | 18 | Doppler | NR | 830±284 | 114 |
NR = not reported; PE=preeclampsia; IUGR = Intrauterine growth restriction; HTN = hypertension
The underlying cause of reduced blood flow in preeclampsia is thought to be a relative failure of trophoblast to fully invade and remodel the maternal spiral arteries (37–43). Abnormal Doppler findings correlate with other placental morphological characteristics, such as reduced tertiary branching of the villous vascular tree, that would logically be associated with reduced blood flow or intermittent ischemia (13, 19, 40). We examined the decidual ends of uteroplacental arteries in placentas from high (3100 m) vs. low (1600 m) altitude. We found that while individually variable, remodeling was absent in 67% of all arteries examined in the high altitude placentas vs. 27% of the arteries examined in low altitude placentas (28). The latter contrasts with findings using the same technique at sea level, wherein 100% of arteries from normal placentas were remodeled (26). Other studies suggest that a gradient of remodeling is a much more likely scenario in normal human placentas (38). Thus there may be exquisite sensitivity of the trophoblast to oxygen tension such that even very small differences in tissue PO2 can effect the invasion process. To that end, a number of investigators are now testing their in vitro models across a range of oxygen concentrations, e.g. 1–8%, which would likely reflect the high and low extremes of blood circulating in the intervillous space near term (44–46).
The high altitude data are consistent with a variety of studies indicating that failed remodeling contributes to reduced uteroplacental blood flow. But there are nonetheless troubling inconsistencies in the data. The occurrence of placental ischemia (<50% reduction in blood flow) without hypertension or IUGR is noted in the literature (reviewed in Table 1). A small subset of women have impaired trophoblast invasion of the maternal spiral arteries, but develop neither preeclampsia nor IUGR, while other subsets of women have impaired invasion and develop IUGR, but not preeclampsia (37, 39, 42). Moreover having normal resistance indices does not necessarily mean that blood flow is normal. We found no evidence for increased vascular resistance in the uterine artery at high altitude, despite the relative lack of uteroplacental arterial remodeling, and despite lower uterine blood flow (47). Another study at even greater altitude (4300 m) found lower vascular resistance in the uterine artery (48). This means that resistance does not directly translate into volumetric flow: there can be a marked reduction in overall uteroplacental blood flow, which would reduce both oxygen and substrate delivery, without any obvious differences in resistance. In summary, with caveats regarding indirect measures of blood flow, the literature on preeclampsia and from high altitude are consistent in showing that reduced blood flow is present more often than in normal pregnancy. It seems reasonable to infer that reduced uteroplacental blood is not sufficient to produce the disease, but that reduced blood flow may increase the risk for development of preeclampsia in women who are otherwise susceptible.
3.2. Etiological model #2 - Circulating factors of placental origin
The idea that the hypoxic/ischemic or otherwise stressed placenta produces something toxic to the mother (toxaemia) is also a theory with a long history. Even the idea that oxidative mechanisms are involved can be traced back as far as 1949, when Thompson & Tickner proposed that ‘a mono-amine oxidase in the placenta is inactivated by placental ischemia; there is evidence that it can destroy vaso-constrictor amines and thus ischemia may cause vasoconstriction’ (49). Innumerable “factors” have since been suggested and tested (50, 51). Today’s equivalent of yesterday’s abnormal coagulation cascade is the angiogenic growth factors (reviewed in this volume), currently under scrutiny as a causal culprit (52). However it is worth noting that without exception, no circulating factor has ever been found where the ranges reported in preeclampsia do not overlap with those of normotensive women. Similarly, in those studies wherein serial samples were collected and retrospectively analyzed for the factor(s) of interest, sensitivity and specificity have not attained a consistency that would permit clinical use. Factors that do change prior to the onset of hypertension and other symptoms also overlap with the ranges observed in women who remain normotensive.
In this respect the data from high altitude are still being explored. We have shown greater maternal circulating concentrations and placental expression of the circulating anti-angiogenic growth factor sFlt-1 (30), favored as a potential cause of endothelial dysfunction (53). We have also shown greater expression of placental VEGF and circulating total VEGF (31, 54) at high altitude. However, additional data indicate that a significant portion of the sFlt-1 (and VEGF) measured in serum may actually be of platelet or other circulating cell origin. This means the circulating concentrations reported often reflect variation in sample collection and storage as opposed to reflecting the in vivo circulatory state (55–57). How local platelet or other circulating cells’ secretion of growth factors or cytokines may influence the development of preeclampsia is a difficult area of study, but one that merits greater attention (58–61). It is at least possible that our collective failure to find “the” circulating factor of placental origin that causes preeclampsia is first, that no single factor exists, and second, that virtually all the circulating factors reported to date as being “associated” with preeclampsia, whether prior to or during the course of the disease, are released (or not) to a variable degree after the blood has been collected. They therefore may reflect risk or susceptibility, but not the true “in vivo” circulating milieu. This would account for the lack of consistency between studies, and the substantial overlap that exists between what is observed in preeclampsia vs. normotensive pregnancy.
Nonetheless, one of the breakthroughs in theoretical models of preeclampsia was the idea that the plethora of symptoms, along with the inter-individual variability in symptoms, might be accounted for by a systemic dysfunction localized to a cell type as opposed to specific organ, i.e. disruption of the vascular endothelium (62). With respect to markers of endothelial cell activation or dysfunction, the circulating factors that have been studied thus far in high altitude pregnancy (other than sFlt-1 and total VEGF) are pro-versus anti-inflammatory cytokines, and endothelial cell adhesion molecules.
Increased Th1 (pro-inflammatory) cytokine activity in pregnancy is associated with preeclampsia (63–66). More proinflammatory cytokines are produced by lymphocytes from preeclamptic women than from women with normal pregnancies (67–69). This again emphasizes the idea that no one circulating factor is really important. Rather, local release of factors that interact with the endothelium, potentially escalating the cascade of preeclampsia symptoms, are more important. We found that maternal circulating concentrations of the pro-inflammatory cytokines IL-6, TNF-alpha, and IL-8 were all elevated late in normal pregnancy in women residing at high altitude, but did not differ even marginally in the non-pregnant state. The same subjects failed to increase their levels of anti-inflammatory (Th-2) IL-10 during pregnancy, causing a marked reduction in circulating concentrations relative to low altitude controls that was most pronounced in the third trimester when pregnancy complications develop (70). We suspect that the overall profile of cytokine production during pregnancy at high altitude is altered by sympathoadrenal activation secondary to the interaction of hypoxia and pregnancy (a general stress, see the section on vascular reactivity below). Alternatively, altered cytokine production or degradation may reflect underlying mechanisms that contribute both to the observed alterations in circulating concentrations, and to the development of preeclampsia, without one necessarily causing the other.
Elevation of pro-inflammatory cytokines such as IL-6 have been linked with an increase in circulating concentrations of endothelial cell adhesion molecules in preeclampsia (71). We investigated circulating concentrations of vascular cell adhesion molecule (VCAM-1), E-Selectin and platelet-endothelial cell adhesion molecule (PECAM). Not only were none of these circulating markers elevated relative to low altitude (71) but VCAM-1 was reduced at high compared with low altitude in normal pregnancies (72). The discordance between our findings at high altitude and in preeclampsia suggests that placental hypoxia is unlikely to be the cause of elevated circulating VCAM-1 concentrations in preeclampsia. Likewise, since prior data link an increase in pro-inflammatory cytokines with elevated VCAM-1 in preeclampsia, the high altitude data suggest that increased inflammation does not necessarily increase endothelial cell adhesion molecules under in vivo conditions of hypoxia. Insofar as these limited data permit us to infer, endothelial cell activation does not seem to be a generalized effect of maternal hypoxemia, or of mild placental hypoxia, and therefore may be uniquely associated with the pathophysiology of preeclampsia.
3.3. Etiological model #3 - Placental oxidative stress
In this theoretical model (Figure 1) there is an imbalance between the cellular generation of reactive oxygen species (ROS) and the capacity of anti-oxidants to prevent oxidative damage. This has been suggested as playing a pivotal role in preeclampsia (reviewed in 73). In this etiological model, placental oxidative stress is often considered as the event precipitating the increased placental apoptosis observed in preeclampsia (74–76). Increased apoptosis, in turn, is thought to increase the deportation of apoptotic syncytiotrophoblast fragments (STBMs) into the maternal circulation, and, for reasons that are not clear, such fragments are not cleared by the lungs as in normal pregnant women, and circulate in the mother. These fragments then increase inflammatory stress and endothelial cell damage (77). The oxidative stress model has both a maternal and a placental component. The maternal model suggests that reduced anti-oxidants (e.g. vitamins A, C, and E) in the mother increase her placental/endothelial susceptibility to oxidative stress, while the placental model argues that the placenta’s ability to buffer oxidative stress is either diminished or overwhelmed. The high altitude data can thus far address only the placental model. Antioxidant enzymes are markedly reduced in preeclamptic placentas (78) and oxidative stress is therefore increased (Table 2). Some studies show that there are compensatory mechanisms for oxidative stress in preeclamptic placentas (79) while others show specific defects that could limit the placenta’s ability to cope with normal levels of oxidative stress or which would contribute to unusually high burdens of oxidative stress (80, 81). Along with oxidative stress is increased nitrative stress (82), specifically of the syncytiotrophoblast and this too, is thought to contribute to increased apoptosis. Table 2 summarizes the results generated from the same laboratory with respect to preeclampsia and gestational age-matched controls (78) and normal pregnancies at high altitude versus low altitude (83). Clearly high-altitude placentas are similar to preeclamptic placentas in having a diminished anti-oxidant capacity, but unlike preeclampsia, they show no evidence of increased lipid peroxidation or protein carbonylation (Table 2). Lipid peroxidation is reduced by 47% in high-altitude relative to low-altitude placentas, and thus increased oxidative stress in preeclamptic placentas is unlikely to be due to chronic mild hypoxia. Moreover, we evaluated apoptosis in the high versus low altitude placentas (83), and there was no difference between altitudes; the values obtained were similar to other published data using similar techniques (84). Surprisingly, given the lack of lipid peroxidation and protein carbonylation in the high altitude placentas, we found that there were increased nitrotyrosine residues in the syncytiotrophoblast, a feature that has been consistently reported in preeclampsia (75). Thus the primary conclusion of these studies was that hypoxia does not necessarily increase oxidative stress, nor apoptosis. A second conclusion was that hypoxia may increase nitrative stress, likely via increased nitric oxide scavenging of oxygen radicals, but increased nitrative stress does not appear to contribute to apoptosis (83). Again, these data suggest that mechanisms other than lowered tissue PO2 contribute to the increased oxidative stress observed in preeclampsia.
Table 2.
Differences in concentrations of pro- versus anti-oxidant enzyme precursors, in enzyme activity levels and in measures of oxidative stress in low altitude vs. high altitude and preeclamptic vs. control placentas
| Markers of oxidative status | Preeclampsia (n=20) vs. age matched control (n=18) | High (n=18) vs low altitude (n=8) |
|---|---|---|
| Thioredoxin (ng/mg protein) | −15%1 | −34%2 |
| Thioredoxin Reductase Activity (U/g protein) | −48%1 | −46%2 |
| Ghitathione Peroxidase Activity (moles/min/mg protein) | −34%1 | −27%2 |
| Superoxide Dismutase Activity (U/mg protein) | −20%1 | −30% p=0.05 |
| Lipid Peroxidation (4-HNE/MDA uM/mg protein) | +291%1 | −47%2 |
| Protein Carbonyl (U/mg protein) | +18% | −34%2 |
Data calculated from 78, 83.
p<.05 preeclampsia versus gestational age matched control,
p<.05 low versus high altitude.
4HNE = 4-hydroxy-2(E)-nonenal, MDA = malondialdehyde
3.4. Etiological model #4 - Altered vascular sensitivity
Altered vascular responsiveness in preeclamptic women began to be investigated in the 1950s (85–87). The seminal publication in 1973 by Norman Gant, in which primigravid adolescents showed an increased vasopressor response to angiotensin II long before the onset of symptoms (88) further promoted the idea that an underlying, perhaps constitutional aberration in vascular sensitivity is present in women predisposed to develop preeclampsia. While compelling, this idea is hampered by the fact that the rate of repeat preeclampsia in subsequent pregnancies is only ~30%; the increased incidence of the disease in primiparous women argues against a constitutional predisposition of any great impact. Instead vascular sensitivity may vary from pregnancy to pregnancy, even within the same woman, due to the interaction of other pregnancy-related physiological changes. A substantial literature supports that human pregnancy is characterized by attenuated systemic vascular response to a number of pressor agents, as well as enhanced response to vasodilators, which contributes to the fall in systemic vascular resistance that, in turn, facilitates the normal increase in cardiac output and redistribution of blood flow to favor the uteroplacental circuit (88–92). But this varies considerably from individual to individual.
Enhanced pressor and systemic vascular resistance response to not only angiotensin II, but also catecholamines are noted in hypertensive pregnancy (87, 93, 94). Circulating norepinephrine and epinephrine levels correlate with elevated blood pressure, reduced plasma volume and elevated heart rate in preeclamptic, but not normotensive pregnant women (94). Directly measured sympathetic neural outflow (muscle sympathetic nerve activity) is greater in women who develop preeclampsia (95, 96). Moreover a preeclampsia like syndrome can be induced in animals by inducing sympathetic over-reactivity (97), and eclamptic seizures can occur in preeclamptic women given anticholinergics (98). These data imply that diminution of para-sympathetic activity potentiates an already hyper-reactive sympathetic vascular stimulation -one which may be constitutional or pregnancy-induced. This particular theory concerning the etiology of preeclampsia waxes and wanes in popularity. Nonetheless the evidence for SNS dysregulation and an exaggerated stress response in preeclampsia is too great to simply ignore the possibility (95, 99–103). Taken together, the data support that alpha-sympathetic activity in preeclamptic women is enhanced compared with normal pregnant women. The high altitude-data support that SNS activity may contribute to an increased risk for the disease. Urinary excretion of catecholamines was elevated in pregnancy at high altitude (70). Altitude-associated differences in both norepinephrine and epinephrine were most pronounced early in pregnancy (46% and 109% greater, respectively), although even non-pregnant values were 37% (norepinephrine) and 47% (epinephrine) greater among the high altitude women.
The idea that altered vascular reactivity is present in high altitude pregnancy has been vigorously pursued in a series of animal studies by several different laboratories (see the excellent review in 8). Taken together the data suggest that there is altered vascular reactivity in pregnancy at high altitude that favors increased vasoconstrictor over vasodilator responses. Increasingly sophisticated methods to explore the effects of shear stress, intraluminal pressure and other variables are being systematically applied to pregnancy- related vascular reactivity under conditions of normoxia and hypoxia (104–106). But the importance of altered vascular reactivity is central to each of the 3 hypotheses evaluated in sections 3.1–3.3 above, and may be the underlying correlate of the numerous alterations in maternal physiology present in high altitude pregnancy. These physiological studies are summarized in Table 3. The take-home message of the human data (reviewed in 10) is that maternal physiological adjustment to pregnancy is altered under conditions of chronic mild hypoxemia to a state that is intermediate between normal pregnancy and preeclampsia. The underlying cause of such systemic alterations can only be vascular.
Table 3.
Matemal physiological changes in normal, high altitude and preeclamptic pregnancy
| Changes | Sea Level Normal | High Altitude | Preeclampsia |
|---|---|---|---|
| Blood pressure | Declines to mid trimester, then rises | Gradual rise | Decline followed by sharp rise |
| Plasma volume | Increases | Increases, but lower baseline | No increase |
| Catecholamines | Decreased sensitivity | Higher concentrations, greater sensitivity | Increased sensitivity |
| Cytokines | Increased anti- vs. pro-inflammatory | Increased pro- vs. anti-inflammatory | Increased pro—vs. anti-inflammatory |
| Trophoblast Invasion | Virtually complete | Diminished | Further diminished |
| Uterine blood flow | Normal | ~30% lower | ~70% lower |
4. CONCLUSIONS AND PERSPECTIVES
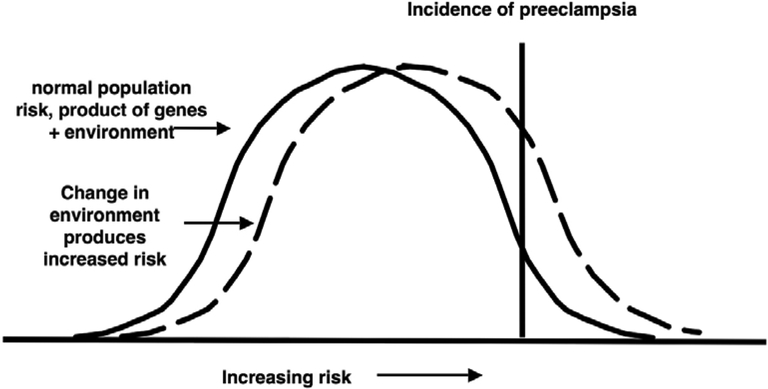
These 4 broad theories, reduced blood flow, circulating factors, placental oxidative stress and altered vascular function, are, of course, related and subject to numerous permutations in terms of the direction of causal arrows shown in Figure 1. The natural experiment of voluntary residence at high altitude clearly supports the idea that multiple systems are involved in preeclampsia. It also supports that while extremes of variation may be present in one individual’s measurements, there is virtually always overlap with the normal range when a large enough population is considered. The question that remains to be answered is if there is a single cause, or a final common pathway by which preeclampsia is induced, why would it be more common at high altitude? This rhetorical question implies the answer - there cannot be a single cause. Rather, the likely explanation for the link between maternal hypoxemia and an increased risk for preeclampsia lies in the impact of hypoxia on multiple physiological systems (Figure 3, Table 3). In this model it is not just one effect of hypoxia that ‘causes’ preeclampsia, rather it is the impact of hypoxia on several important adjustments to pregnancy that shifts the general population risk such that more women eventually develop the disease (Figure 3). In the model presented in Figure 3, we suggest that most physiological variables have a normal distribution, and that perturbation of the environment (e.g. by lowered oxygen pressure) can shift a greater proportion of individuals into a higher risk category for the development of a disease such as preeclampsia. None of the variables discussed above and listed in Tables 2 or 3, are sufficient to cause preeclampsia. Rather they are correlates of the disease, not markers of a single underlying cause, but far more likely to represent the range of variability present in human pregnancy. Altitude simply shifts the risk.
Figure 3.
Model for how chronic mild hypoxemia due to high-altitude residence, operating on multiple maternal physiological characteristics, may right-shift the population-wide risk of preeclampsia.
5. ACKNOWLEDGEMENTS
This work has been supported by funding from the American Heart Association Colorado Affiliate (CWGB 27) the American Heart Association (06–014200), and NIH grants HL 14985, HD 42737.
6. REFERENCES
- 1.Moore LG, Hershey DW, Jahnigen D & Bowes W Jr.: The incidence of pregnancy-induced hypertension is increased among Colorado residents at high altitude. Am J Obstet Gynecol 144, 423–9 (1982) [DOI] [PubMed] [Google Scholar]
- 2.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA & Moore LG: Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Ped Res 54, 20–5 (2003) [DOI] [PubMed] [Google Scholar]
- 3.Mahfouz AAR, El-Aid MM, Alakija W & Al-Erian RAG: Altitude and socio-biological determinants of pregnancy-associated hypertension. Int J Obstet Gynecol 44, 135–138 (1994) [DOI] [PubMed] [Google Scholar]
- 4.Palmer SK, Moore LG, Young D, Cregger B, Berman JC & Zamudio S: Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180, 1161–8 (1999) [DOI] [PubMed] [Google Scholar]
- 5.Jensen GM & Moore LG: The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health 87, 1003–1007 (1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giussani DA, Phillips PS, Anstee S & Barker DJP: Effects of altitude versus economic status on birth weight and body shape at birth. Ped Res 49, 490–494 (2000) [DOI] [PubMed] [Google Scholar]
- 7.Shime J, Mocarski EJ, Hastings D, Webb GD & McLaughlin PR: Congenital heart disease in pregnancy: short- and long-term implications. Am J Obstet Gynecol 156, 313–22 (1987) [DOI] [PubMed] [Google Scholar]
- 8.White MM & Zhang L: Effects of chronic hypoxia on maternal vasodilation and vascular reactivity in guinea pig and ovine pregnancy. High Alt Med Biol 4, 157–69 (2003) [DOI] [PubMed] [Google Scholar]
- 9.Moore LG: Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol 4, 141–56 (2003) [DOI] [PubMed] [Google Scholar]
- 10.Zamudio S: High altitude and hypertension in pregnancy. In: Ed: Lyall F. Cambridge University Press, Cambridge; (2006) [Google Scholar]
- 11.Genbacev O, Joslin R, Damsky CH, Polliotti BM & Fisher SJ: Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest 97, 540–50 (1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M & Lye SJ: Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest 103, 1641–50 (1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingdom JC & Kaufmann P: Oxygen and placental villous development: origins of fetal hypoxia. Placenta 18, 613–21; discussion 623–6 (1997) [DOI] [PubMed] [Google Scholar]
- 14.Caniggia I, Winter J, Lye SJ & Post M: Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta 21, S25–30 (2000) [DOI] [PubMed] [Google Scholar]
- 15.Jazayeri A, O’Brien WF, Tsibris JC & Spellacy WN: Are maternal diabetes and preeclampsia independent simulators of fetal erythropoietin production? Am J Perinatology 15, 577–80 (1998) [DOI] [PubMed] [Google Scholar]
- 16.Hung TH, Skepper JN & Burton GJ: In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol 159, 1031–43 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung TH, Skepper JN, Charnock-Jones DS & Burton GJ: Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res 90, 1274–81 (2002) [DOI] [PubMed] [Google Scholar]
- 18.Campbell S, Diaz-Recasens J, Griffin DR, Cohen-Overbeek TE, Pearce JM, Willson K & Teague MJ: New doppler technique for assessing uteroplacental blood flow. Lancet 1, 675–7 (1983) [DOI] [PubMed] [Google Scholar]
- 19.Giles WB, Trudinger BJ & Baird PJ: Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol 92, 31–38 (1985) [DOI] [PubMed] [Google Scholar]
- 20.Galan HL, Ferrazzi E & Hobbins JC: Intrauterine growth restriction (IUGR): biometric and Doppler assessment. Prenat Diagn, 22, 331–7(2002) [DOI] [PubMed] [Google Scholar]
- 21.Zamudio S, Palmer S, Droma T, Stamm E, Coffin C & Moore L: Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol 79, 7–14 (1995) [DOI] [PubMed] [Google Scholar]
- 22.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA & Moore LG: Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol 79, 15–22 (1995) [DOI] [PubMed] [Google Scholar]
- 23.Fox H: The villous cytotrophoblast as an index of placental ischemia. J Obstet Gynaecol Br Com 71, 885–93 (1964) [DOI] [PubMed] [Google Scholar]
- 24.Fox H: Effect of hypoxia on trophoblast in organ culture. Am J Obstet Gynecol 107, 1058–1064 (1970) [DOI] [PubMed] [Google Scholar]
- 25.Zamudio S: The placenta at high altitude. High Alt Med Biol 4, 171–91 (2003) [DOI] [PubMed] [Google Scholar]
- 26.Labarrere CA & Faulk WP: Antigenic identification of cells in spiral artery trophoblastic invasion: validation of histologic studies by triple-antibody immunocytochemistry. Am J Obstet Gynecol 171, 165–71 (1994) [DOI] [PubMed] [Google Scholar]
- 27.Starzyk KA, Salafia CM, Pezzullo JC, Lage JM, Parkash V, Vercruysse L, Hanssens M & Pijnenborg R: Quantitative differences in arterial morphometry define the placental bed in preeclampsia. Hum Pathol 28, 353–8 (1997) [DOI] [PubMed] [Google Scholar]
- 28.Tissot van Patot M, Grilli A, Chapman P, Broad E, Tyson W, Heller DS, Zwerdlinger L & Zamudio S: Remodelling of uteroplacental arteries is decreased in high altitude placentae. Placenta 24, 326–35 (2003) [DOI] [PubMed] [Google Scholar]
- 29.Zamudio S, Baumann MU & Illsley NP: Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta 27, 49–55 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, Zamudio S & Caniggia I: Increased Expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M & Caniggia I: Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrin Metab 90, 4299–308 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beker JC: The effects of pregnancy on blood circulation in their relation to so-called toxemia. Am J Obstet Gynecol 18: 368–374 (1929) [Google Scholar]
- 33.Browne J & Veall N: The maternal placental blood flow in normotensive and hypertensive women. J Obstet Gynaecol Br Emp 60, 141–147 (1953) [DOI] [PubMed] [Google Scholar]
- 34.Clemetson C & Churchman J: Oxygen and carbon dioxide content of umbilical artery and vein blood in toxaemic and normal pregnancy. J Obstet Gynaecol Br Com 60, 335–344 (1953) [DOI] [PubMed] [Google Scholar]
- 35.Jauniaux E, Poston L & Burton GJ: Placental-related diseases of pregnancy: involvement of oxidative stress and implications in human evolution. Hum Reprod Update, Advance access, 1–9 (2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajakumar A, Brandon HM, Daftary A, Ness R & Conrad KP: Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta 25, 763–9 (2004) [DOI] [PubMed] [Google Scholar]
- 37.Robertson WB, Khong TY, Brosens I, De Wolf F, Sheppard BL & Bonnar J: The placental bed biopsy: review from three European centers. Am J Obstet Gynecol 155, 401–12 (1986) [DOI] [PubMed] [Google Scholar]
- 38.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR & van Asshe A: A study of placental bed spiral arteries and trophoblast invasion in normal and severe preeclamptic pregnancies. Br J Obstet Gynaecol 101, 669–74 (1994) [DOI] [PubMed] [Google Scholar]
- 39.Pijnenborg R, Anthony J, Davey DA, Rees A, Tiltman A, Vercruysse L & van Assche A: Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol 98, 648–55 (1991) [DOI] [PubMed] [Google Scholar]
- 40.Kreczy A, Fusi L & Wigglesworth JS: Correlation between umbilical arterial flow and placental morphology. Int J Gynecol Pathol 14, 306–9 (1995) [DOI] [PubMed] [Google Scholar]
- 41.Macara L, Kingdom JC, Kaufmann P, Kohnen G, Hair J, More IA, Lyall F & Greer IA: Structural analysis of placental terminal villi from growth-restricted pregnancies with abnormal umbilical artery Doppler waveforms. Placenta 17, 37–48 (1996) [DOI] [PubMed] [Google Scholar]
- 42.Aardema MW, Oosterhof H, Timmer A, van Rooy I & Aarnoudse JG: Uterine artery doppler flow and uteroplacental vascular pathology in normal pregnancies and pregnancies complicated by pre-eclampsia and small for gestational age fetuses. Placenta 22, 405–411 (2001) [DOI] [PubMed] [Google Scholar]
- 43.Lin S, Shimizu I, Suehara N, Nakayama M & Aono T: Uterine artery Doppler velocimetry in relation to trophoblast migration in to the myometrium of the placental bed. Obstet Gynecol 85, 760–765 (1995) [DOI] [PubMed] [Google Scholar]
- 44.Soothill PW, Nicolaides KH, Rodeck CH & Campbell S: Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Therapy 1, 168–175 (1986) [DOI] [PubMed] [Google Scholar]
- 45.Rodesch F, simon P, Donner C & Jauniaux E: Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 80, 283–285 (1992) [PubMed] [Google Scholar]
- 46.Jauniaux E, Watson AL & Burton G: Evaluation of respiratory gases and acid-base gradients in fetal tissue and uteroplacental tissue between 7–16 weeks. Am J Obstet Gynecol 184, 998–1003 (2001) [DOI] [PubMed] [Google Scholar]
- 47.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C & Moore LG: Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol 79, 7–14 (1995) [DOI] [PubMed] [Google Scholar]
- 48.Krampl ER, Espinoza-Dorado J, Lees CC, Moscoso G, Bland JM & Campbell S: Maternal uterine artery Doppler studies at high altitude and sea level. Ultrasound Obstet Gynecol 18, 578–82 (2001) [DOI] [PubMed] [Google Scholar]
- 49.Thompson RHS & Tickner A: Observations on the mono-amine oxidase activity of placenta and uterus. Biochem J 45, 125–129 (1949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner CP: Preeclampsia-eclampsia syndrome and coagulation. Clinics in Perinatology 18, 713–26 (1991) [PubMed] [Google Scholar]
- 51.Taylor RN, de Groot CJ, Cho YK & Lim KH: Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol 16, 17–31 (1998) [DOI] [PubMed] [Google Scholar]
- 52.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP & Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. NEJM 350, 672–83 (2004) [DOI] [PubMed] [Google Scholar]
- 53.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP & Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111, 649–58 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zamudio S, Wheeler T, Anthony F & Moore LG: Vascular endothelial growth factor (VEGF), vascular resistance and villous angiogenesis at high altitude (3100 m). J Soc Gynecol Inv 9, 141A (2002) [Google Scholar]
- 55.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G & Markovic N: Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 26, 563–73 (2005) [DOI] [PubMed] [Google Scholar]
- 56.Dittadi R, Meo S, Fabris F, Gasparini G, Contri D, Medici M & Gion M: Validation of blood collection procedures for the determination of circulating vascular endothelial growth factor (VEGF) in different blood compartments. Int J Biol Markers 16, 87–96 (2001) [PubMed] [Google Scholar]
- 57.Jelkmann W: Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem 47, 617–23 (2001) [PubMed] [Google Scholar]
- 58.Faas MM, Schuiling GA, Linton EA, Sargent IL & Redman CW: Activation of peripheral leukocytes in rat pregnancy and experimental preeclampsia. Am J Obstet Gynecol 182, 351–7 (2000) [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Gu Y & Lucas MJ: Expression of thrombin receptors in endothelial cells and neutrophils from normal and preeclamptic pregnancies. J Clin Endocrinol Metab 87, 3728–34 (2002) [DOI] [PubMed] [Google Scholar]
- 60.Sheu JR, Hsiao G, Lin WY, Chen TF, Chien YY, Lin CH & Tzeng CR: Mechanisms involved in agonist-induced hyperaggregability of platelets from normal pregnancy. J Biomed Sci 9, 17–25 (2002) [DOI] [PubMed] [Google Scholar]
- 61.Clark P, Boswell F & Greer IA: The neutrophil and preeclampsia. Semin Reprod Endocrinology 16, 57–64 (1998) [DOI] [PubMed] [Google Scholar]
- 62.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA & McLaughlin MK: Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol 161, 1200–4 (1989) [DOI] [PubMed] [Google Scholar]
- 63.Saito S & Sakai M: Th1/Th2 balance in preeclampsia. J Reprod Immunol 59, 161–73 (2003) [DOI] [PubMed] [Google Scholar]
- 64.Omu AE, Al-Qattan F, Diejomaoh ME & Al-Yatama M: Differential levels of T helper cytokines in preeclampsia: pregnancy, labor and puerperium. Acta Obstet Gynecol Scand 78, 675–80 (1999) [PubMed] [Google Scholar]
- 65.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA & Socol ML: Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol 170, 1752–7; discussion 1757–9 (1994) [PubMed] [Google Scholar]
- 66.Greer IA, Lyall F, Perera T, Boswell F & Macara LM: Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol 84, 937–40 (1994) [PubMed] [Google Scholar]
- 67.Darmochwal-Kolarz D, Leszczynska-Gorzelak B, Rolinski J & J. 0leszczuk: T helper 1- and T helper 2-type cytokine imbalance in pregnant women with pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 86, 165–70 (1999) [DOI] [PubMed] [Google Scholar]
- 68.Saito S, Tsukaguchi N, Hasegawa T, Michimata T, Tsuda H & Narita N: Distribution of Th1, Th2, and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol 42, 240–5 (1999) [DOI] [PubMed] [Google Scholar]
- 69.Saito S, Umekage H, Sakamoto Y, Sakai M, Tanebe K, Sasaki Y & Morikawa H: Increased T-helper-1-type immunity and decreased T-helper-2-type immunity in patients with preeclampsia. Am J Reprod Immunol 41, 297–306 (1999) [DOI] [PubMed] [Google Scholar]
- 70.Coussons-Read ME, Mazzeo RS, Whitford MH, Schmitt M, Moore LG & Zamudio S: High altitude residence during pregnancy alters cytokine and catecholamine levels. Am J Reprod Immunol 48, 344–54 (2002) [DOI] [PubMed] [Google Scholar]
- 71.Lyall F, Greer IA, Boswell F, Macara LM, Walker JJ & Kingdom JC: The cell adhesion molecule, VCAM-1, is selectively elevated in serum in pre-eclampsia: does this indicate the mechanism of leucocyte activation? Br J Obstet Gynaecol 101, 485–7 (1994) [DOI] [PubMed] [Google Scholar]
- 72.Marks L, Zamudio S, Cousins F, Duffie E & Lyall F: Endothelial activation and cell adhesion molecule concentrations in pregnant women living at high altitude. J Soc Gynecol Invest In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hubel CA: Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 222, 222–35 (1999) [DOI] [PubMed] [Google Scholar]
- 74.Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H & Kaufmann P: Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblast into the maternal circulation. Placenta 24, 181–90 (2003) [DOI] [PubMed] [Google Scholar]
- 75.Myatt L & Cui X: Oxidative stress in the placenta. Histochem Cell Biol 122, 369–82 (2004) [DOI] [PubMed] [Google Scholar]
- 76.Burton GJ & Jauniaux E: Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Invest 11, 342–52 (2004) [DOI] [PubMed] [Google Scholar]
- 77.Redman CW & Sargent IL: Placental debris, oxidative stress and pre-eclampsia. Placenta 21, 597–602 (2000) [DOI] [PubMed] [Google Scholar]
- 78.Vanderlelie J, Venardos K, Clifton VL, Gude NM, Clarke FM & Perkins AV: Increased biological oxidation and reduced anti-oxidant enzyme activity in preeclamptic placentae. Placenta 26, 53–8 (2005) [DOI] [PubMed] [Google Scholar]
- 79.Shibata E, Ejima K, Nanri H, Toki N, Koyama C, Ikeda M & Kashimura M: Enhanced protein levels of protein thiol/disulphide oxidoreductases in placentae from pre-eclamptic subjects. Placenta 22, 566–72 (2001) [DOI] [PubMed] [Google Scholar]
- 80.Hass R & Sohn C: Increased oxidative stress in preeclamptic placenta is associated with altered proteasome activity and protein patterns. Placenta 24, 979–84 (2003) [DOI] [PubMed] [Google Scholar]
- 81.Wang Y & Walsh SW: Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta 19, 581–6 (1998) [DOI] [PubMed] [Google Scholar]
- 82.Myatt L, Kossenjans W, Sahay R, Eis A & Brockman D: Oxidative stress causes vascular dysfunction in the placenta. J Matern Fetal Med, 9, 79–82(2000) [DOI] [PubMed] [Google Scholar]
- 83.Zamudio S, Kovalenko O, Vanderlelie J, Illsley NP, Heller D, Belliappa S & Perkins AV: Chronic hypoxia in vivo reduces placental oxidative stress. Placenta Submitted [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Austgulen R, Chedwick L, Vogt Isaksen C, Vatten L & Craven C: Trophoblast apoptosis in human placenta at term as detected by expression of a cytokeratin 18 degradation product of caspase. Arch Pathol Lab Med 126, 1480–6 (2002) [DOI] [PubMed] [Google Scholar]
- 85.Gigee W, Raab W, Schroeder G & Wagner R: Vascular reactivity and electrolytes in normal and toxemic pregnancy; pathogenic considerations and a diagnostic pre-toxemia test. J Clin Endocrinol Metab 16, 1196–216 (1956) [DOI] [PubMed] [Google Scholar]
- 86.Chesley LC: Vascular reactivity in normal and toxemic pregnancy. Clin Obstet Gynecol 9, 871–81 (1966) [DOI] [PubMed] [Google Scholar]
- 87.Talledo OE, Chesley LC & Zuspan FP: Reninangiotensin system in normal and toxemic pregnancies. III. Differential sensitivity to angiotensin II and norepineprhine in toxemia of pregnancy. Am J Obstet Gynecol 100, 218–221 (1968) [Google Scholar]
- 88.Gant NF, Daley GL, Chand S, Whalley PJ & MacDonald PC: A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 52, 2682–9 (1973) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chesley L: Hypertensive Disorders of Pregnancy. Appleton-Century-Crofts, New York: (1978) [Google Scholar]
- 90.Nisell H, Hjemdahl P, Linde B & Lunell NO: Sympatho-adrenal and cardiovascular reactivity in pregnancy-induced hypertension. I. Responses to isometric exercise and a cold pressor test. Br J Obstet Gynaecol 92, 722–31 (1985) [DOI] [PubMed] [Google Scholar]
- 91.Nisell H, Hjemdahl P, Linde B & Lunell NO: Sympathoadrenal and cardiovascular reactivity in pregnancy-induced hypertension. II. Responses to tilting. Am J Obstet Gynecol 152, 554–60 (1985) [DOI] [PubMed] [Google Scholar]
- 92.Sladek SM, Magness RR & Conrad KP: Nitric oxide and pregnancy. Am J Physiol 272, R441–63 (1997) [DOI] [PubMed] [Google Scholar]
- 93.Raab W, Schroeder G, Wagner R & Gigee W: Vascular reactivity and electrolytes in normal and toxemic pregnancy. J Clin Endocrinol 16, 1196–1213 (1956) [DOI] [PubMed] [Google Scholar]
- 94.Nisell H, Hjemdahl P & Linde B: Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol 5, 479–93 (1985) [DOI] [PubMed] [Google Scholar]
- 95.Schobel HP, Fischer T, Heuszer K, Geiger H & Schmieder RE: Preeclampsia -- a state of sympathetic overactivity. NEJM 335, 1480–5 (1996) [DOI] [PubMed] [Google Scholar]
- 96.Greenwood JP, Scott EM, Walker JJ, Stoker JB & Mary DA: The magnitude of sympathetic hyperactivity in pregnancy-induced hypertension and preeclampsia. Am J Hypertens 16, 194–9 (2003) [DOI] [PubMed] [Google Scholar]
- 97.Kanayama N, Tsujimura R, She L, Maehara K & Terao T: Cold-induced stress stimulates the sympathetic nervous system, causing hypertension and proteinuria in rats. J Hypertens 15, 383–9 (1997) [DOI] [PubMed] [Google Scholar]
- 98.Kobayashi T, Sugimura M, Tokunaga N, Naruse H, Nishiguchi T, Kanayama N & Terao T: Anticholinergics induce eclamptic seizures. Semin Thromb Hemost 28, 511–4 (2002) [DOI] [PubMed] [Google Scholar]
- 99.Greenwood JP, Scott EM, Stoker JB, Walker JJ & Mary DA: Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation 104, 2200–4 (2001) [DOI] [PubMed] [Google Scholar]
- 100.Lewinsky RM & Riskin-Mashiah S: Autonomic imbalance in preeclampsia: evidence for increased sympathetic tone in response to the supine-pressor test. Obstet Gynecol 91, 935–9 (1998) [DOI] [PubMed] [Google Scholar]
- 101.Nisell H & Lunell NO: Sympatho-adrenal activity in different hypertensive disorders in pregnancy. A short review. Acta Obstet Gynecol Scand Suppl 118, 13–6 91984) [DOI] [PubMed] [Google Scholar]
- 102.Zuspan FP: Catecholamines. Their role in pregnancy and the development of pregnancy-induced hypertension. J Reprod Med 23, 143–50 (1979) [PubMed] [Google Scholar]
- 103.Zuspan FP: Pregnancy induced hypertension. 1. Role of sympathetic nervous system and adrenal gland. Acta Obstet Gynecol Scand 56, 283–6 (1977) [DOI] [PubMed] [Google Scholar]
- 104.Mateev S, Sillau AH, Mouser R, McCullough RE, White MM, Young DA & Moore LG: Chronic hypoxia opposes pregnancy-induced increase in uterine artery vasodilator response to flow. Am J Physiol Heart Circ Physiol 284, H820–9 (2003) [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Zheng J, Bird IM & Magness RR: Mechanisms of shear stress-induced endothelial nitric-oxide synthase phosphorylation and expression in ovine fetoplacental artery endothelial cells. Biol Reprod 70, 785–96 (2004) [DOI] [PubMed] [Google Scholar]
- 106.Li Y, Zheng J, Bird IM & Magness RR: Effects of pulsatile shear stress on nitric oxide production and endothelial cell nitric oxide synthase expression by ovine fetoplacental artery endothelial cells. Biol Reprod 69, 1053–9 (2003) [DOI] [PubMed] [Google Scholar]
- 107.Assali N, Rauramo L & Peltonen T: Measurement of uterine blood flow and uterine metabolism. Am J Obstet Gynecol 79, 86–98 (1953) [DOI] [PubMed] [Google Scholar]
- 108.Metcalfe J, Romney S, Ramsey L, Reid D & Burwell C: Estimation of uterine blood flow in normal human pregnancy at term. J Clin Inv 34, 1632–1638 (1955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Romney SL, Metcalfe J, Reid DE & Burwell CS: Blood flow of the gravid uterus. Ann N Y Acad Sci 75, 762–9 (1959) [DOI] [PubMed] [Google Scholar]
- 110.Blechner JN, Stenger VG & Prystowsky H: Uterine blood flow in women at term. Am J Obstet Gynecol 120, 633–40 (1974) [DOI] [PubMed] [Google Scholar]
- 111.Kaar K, Jouppila P, Kuikka J, Luotola H, Toivanen J & Rekonen A: Intervillous blood flow in normal and complicated late pregnancy measured by means of an intravenous 133Xe method. Acta Obstet Gynecol Scand 59, 7–10 (1980) [DOI] [PubMed] [Google Scholar]
- 112.Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I & Brandes JM: Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol 162, 121–125 (1990) [DOI] [PubMed] [Google Scholar]
- 113.Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E & Moore LG: Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol 80, 1000–6 (1992) [PubMed] [Google Scholar]
- 114.Konje JC, Howarth ES, Kaufmann P & Taylor DJ: Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. Br J Obstet Gynaecol 110, 301–5(2003) [PubMed] [Google Scholar]
- 115.Zamudio S, Palmer SK, Dahms TE, Berman JC, McCullough RG, McCullough RE & Moore LG: Blood volume expansion, preeclampsia, and infant birth weight at high altitude. J Appl Physiol 74, 1566–1573 (1993) [DOI] [PubMed] [Google Scholar]
- 116.Lunell NO, Nylund LE, Lewander R & Sarby B: Uteroplacental blood flow in pre-eclampsia. Measurements with indium- 113m and a computer-linked gamma camera. Clin Exp Hyperten, 1, 105–17 (1982) [DOI] [PubMed] [Google Scholar]
- 117.Lunell NO, Lewander R, Mamoun I, Nylund L, Sarby S & Thornstrom S: Uteroplacental blood flow in pregnancy induced hypertension. Scand J Clin Lab Invest Suppl, 169, 28–35 (1984) [DOI] [PubMed] [Google Scholar]
- 118.Nylund L, Lunell NO, Lewander R & Sarby B: Uteroplacental blood flow index in intrauterine growth retardation of fetal or maternal origin. Br J Obstet Gynaecol, 90, 16–20 (1983) [DOI] [PubMed] [Google Scholar]