Abstract
The diverse amino acid chemistries and secondary structures in peptides provide ‘minimalist’ mimics of motifs in proteins and offer many ideal properties for targeted delivery approaches. Several non-viral vectors (polymers and lipids) have been studied for their potential applications in gene delivery. However, non-specific uptake, lack of targeting, inability to escape endosomes, and inefficient nuclear delivery limit their application. Peptide-assisted trafficking of non-viral vectors can potentially overcome these biological barriers to improve gene delivery through targeted uptake using key cell-surface receptors (e.g., integrins, growth factor receptors, and G-protein coupled receptors); membrane disruption for endosomal escape; and nuclear importation. Furthermore, the capacity of peptides to regulate spatio-temporal control over gene delivery opens multi-faceted avenues for effective gene delivery in a variety of complex applications. Rigorous on-going in vitro and in vivo studies utilizing peptides for targeted and microenvironment-sensitive gene delivery could promote their widespread clinical usage.
Keywords: Cell penetrating peptide, DNA, gene delivery, non-viral vectors, peptide
Introduction
Gene therapy is a revolutionary approach in medicine that has been heralded for its potential capacity to address the root causes underlying various diseases and not merely the symptoms [1]. The foundation of gene therapy is the modulation of gene expression through delivery of exogenous genetic materials like DNA and RNA [2]. Gene therapy was originally envisioned for treatment of inherited disorders, but it has risen in prominence due to its broader potential in a wide variety of both hereditary and acquired conditions ranging from cardiovascular and monogenic diseases to cancers. Recent gene therapy approvals by the US FDA are remarkable milestones in gene delivery technology. For example, in fall of 2017, two chimeric antigen receptor (CAR) T cell immunotherapies (Kymriah [3] and Yescarta [4]) were approved by the US FDA for the treatment of advanced stage hematological malignancies [5], representing the first clinical approvals for gene therapy products in the US. The CAR-T technology involves reconstruction of CAR receptors on T cells to enable antigen recognition and destruction of malignant B cells [6]. Patient-derived T cells are genetically modified ex vivo and subsequently injected back into the bloodstream of the patients [7]. Meanwhile, in December, 2017, Luxturna became the first US FDA approved in vivo gene therapy for treatment of inherited retinal disease. Luxturna corrects mutations in the RPE65 gene by using an adeno-associated viral (AAV2) vector to carry the functional gene into the affected eye tissue [8]. These new approaches represent promising steps forward in the advancement of gene therapy products that are ultimately targeted to a wider array of tissues and administered using a broader range of methods.
The last two decades have seen continual increases in the number of clinical trials involving gene delivery technologies [9]. Among the gene carriers used in these trials, viral vectors such as adenoviruses, AAVs, lentiviruses, and retroviruses have been the dominant carriers used in nearly all gene therapy applications. However, only a few products (Kymriah, Yescarta, and Luxturna [US]; Gendicine and Oncorine [China]; Rexin G [Philippines]; Neovasculgen [Russia]; and Glybera [Europe]) are available in the worldwide market, attributable in part to the inherent shortcomings in viral vectors: complex and costly processes for production, limited DNA packaging ability, cytotoxicity, broad tropism, immunogenicity, and tumorigenicity [10]. These limitations have spurred development and advancement of a range of non-viral gene carriers fabricated using innovative synthesis schemes [11]. Two major categories of non-viral vectors include: cationic lipids [e.g., (N-[1-(2,3-dioleyloxy)propyl-]-N,N,N-trimethylammonium chloride (DOTMA); 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE); 1,2-Dioleyl-3-trimethylammonium propane (DOTAP)] and cationic polymers [e.g., linear, branched, and dendritic polyethyleneimine (PEI); poly(2-dimethylaminoethyl methacrylate) (PDMAEMA); poly(beta-amino esters) (PBAEs)] [12,13]. Spontaneous electrostatic interactions between these non-viral carriers and nucleic acids lead to the formation of lipoplexes and polyplexes, respectively, which offer useful properties for gene delivery [14]. Cationic lipids offer efficient binding to negatively charged cell surface constituents to stimulate uptake, and cationic lipids can further interact with the endosomal membrane to facilitate cytoplasmic delivery [15]. Alternatively, cationic polymers, including branched, linear, and dendritic structures, offer flexible polymer chemistries that facilitate design and formulation of nanoscale polyplexes with tailorable properties, with facile polymer modification for use in plethora of biomedical applications [16].
Peptides for gene delivery
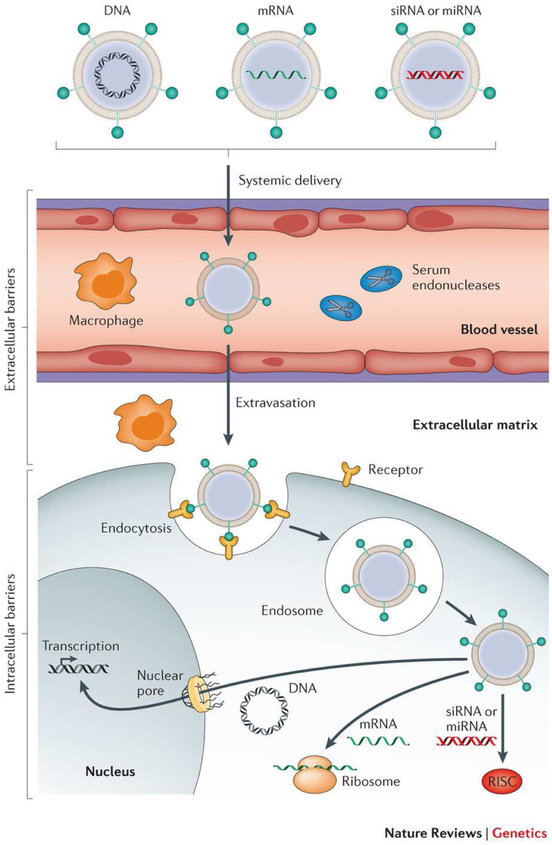
Non-viral vectors can encapsulate higher amounts of DNA and avoid detrimental immune responses associated with viral vectors. However, non-viral carriers exhibit gene expression levels that are typically several-fold lower than viral vectors. This inefficiency stems from the formidable extracellular and intracellular barriers precluding gene delivery using non-viral carriers (Figure 1). Recently, efforts have been focused on fine-tuning the functional components of non-viral vectors to enhance their biological efficacy.
Figure 1:
Barriers to successful in vivo delivery of nucleic acids using non-viral carriers. Various non-viral carriers can be used to deliver DNA, mRNA and short double-stranded RNA [e.g., small interfering RNA (siRNA) and microRNA (miRNA)]. If administered systemically, these carriers must help their cargoes avoid serum nuclease-mediated degradation, evade immune detection, and prevent nonspecific interactions with serum proteins. Moreover, for non-hepatic applications, these vectors need to avoid renal clearance, extravasate into desirable target tissues, and mediate both cell entry and appropriate subcellular trafficking. siRNA and miRNA ultimately must be loaded into the RNA-induced silencing complex (RISC), whereas mRNA must bind to the translational machinery. DNA must be further transported into the nucleus to exert its activity (Reproduced from reference [10], with permission from Springer Nature).
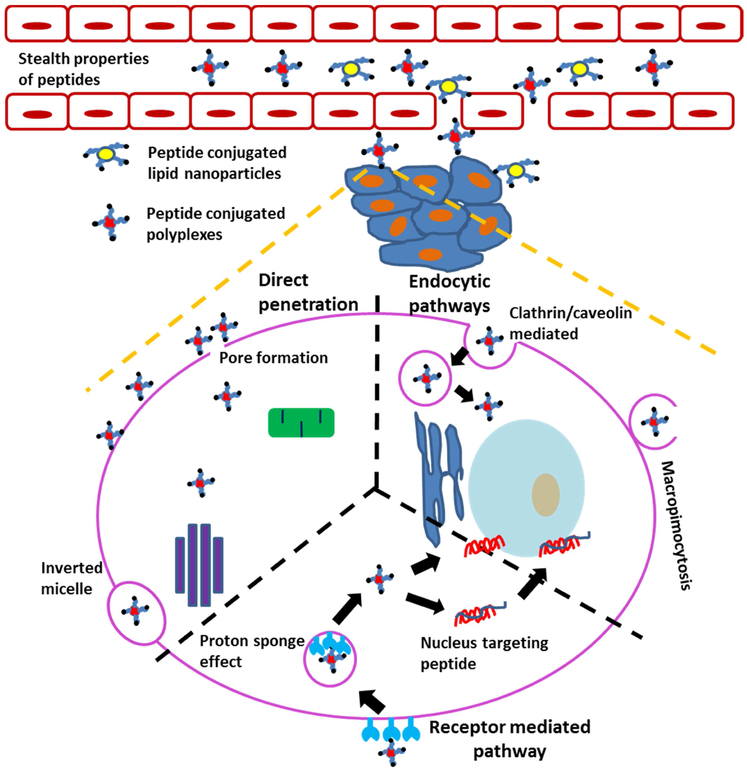
Peptides are short amino acid chains of approximately 50 amino acids or fewer that can be used as unique tools in drug/gene delivery. Peptides are biodegradable and biocompatible, and their amino acid building blocks can be easily manipulated to accelerate several functions of barrier penetration and targeting of gene cargoes [17]. Peptides facilitate access to a wide array of chemistries through incorporation of both naturally-occurring amino acid monomers as well as non-natural amino acids (>30), resulting in a broad chemical modification repertoire. Furthermore, peptides can incorporate a variety of useful structural and functional motifs, including protein fragments with similar secondary structure features as their parent domains, and high-affinity/high-specificity cell-binding motifs selected through phage display or other high-throughput technologies. Additional advantages of peptides as compared to proteins include ease of production (in bacterial or mammalian cells, or by using solid phase peptide synthesis), and stability during storage and handling under ambient conditions [18,19]. Given their unique properties and facile production, peptides have been widely explored for their capacity to help overcome the barriers for gene delivery (Figure 2), including cell penetration, endosomal escape, and organelle targeting [19]. Different pathways through which a peptide can function as a barrier penetrating peptide are presented in Figure 2.
Figure 2:
Schematic representation of pathways for peptide-mediated penetration of biological barriers to address key issues in non-viral gene carriers. Peptides can provide stealth properties to prevent opsonization while in circulation [20]. Furthermore, peptides can facilitate penetration of non-viral carriers across various cellular and sub-cellular barriers. Peptide-mediated transport mechanisms across the plasma membrane include uptake through endocytosis or macropinocytosis (right) [21]; active uptake through receptor-mediated endocytosis (bottom) [22]; and pore formation (left) [23]. Some peptides can mediate transcytosis through sequential endocytic uptake, subcellular transport, and exocytosis [24]. Within cells, peptides can facilitate endosomal escape [22] or nuclear targeting [25], leading to increased RNA delivery or gene transfection.
Cell penetrating peptides
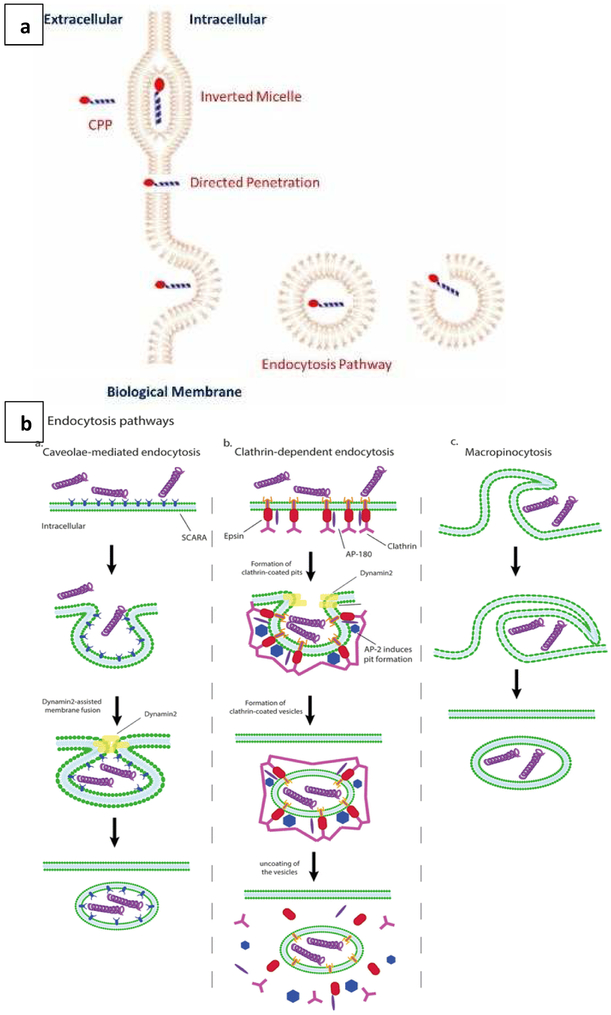
Cell penetrating peptides (CPPs) are positively charged sequences 10-30 amino acids in length that can complex with nucleic acids via electrostatic interactions, or alternatively, be conjugated to lipidic or polymeric components in gene carriers. CPPs are broadly categorized as: cationic peptides [comprising arginine and lysine residues; e.g., trans-activator of transcription (TAT) peptide, penetratin, and oligoarginines)]; amphipathic peptides (comprising both hydrophobic and hydrophilic segments; e.g., buforin 2, crotamine, and azurin p18), and hydrophobic peptides [e.g., peptide derived from vascular epithelial cadherin (pVEC) and Pep-7] [26]. CPPs can internalize into the cells through three main pathways as presented in Figure 3a. The first pathway is through direct penetration, wherein positively charged CPPs interact with phosphate groups on the lipid bilayer of the cell membrane leading to the formation of cavities that facilitate direct access into the cytoplasm. The second pathway is through induction of a transitory structure within the lipid bilayer that allows penetration and uptake (e.g., the inverted micelle structure formed by the penetratin dimer and negatively-charged phospholipids within the membrane). The third pathway is through endocytosis, an energy dependent pathway typically involving clathrin-mediated uptake, caveolar uptake, or macropinocytosis (detailed endocytic pathways are presented in Figure 3b) [23]. Both non-endocytic mechanisms involve CPP-mediated disturbances in the structural integrity of the cell membrane via non-covalent binding with membrane components, a process which allows the CPPs to translocate in an energy- and temperature-independent fashion. Meanwhile, CPP-triggered endocytosis is driven by CPP binding to membrane components, leading to energy- and temperature-dependent membrane invagination and uptake. For example, a study by Zhang et al. utilized HIV-1 TAT conjugated to PEI800 with 4’4-dithiodibutyric acid (DA) to develop CPP-labelled degradable gene carriers for efficient gene transfection (PEI-DA-TAT) [27]. These studies showed that the cationic HIV-1 TAT peptide interacted with the negatively charged cell membrane and thereby stimulated endocytosis of the peptide along with the gene carrier system. The resulting transfection efficiency of PEI-DA-TAT was increased by 8% as compared to PEI-DA and PEI25K. Another interesting study by Jiao et al. investigated the concentration dependence of arginine-rich CPPs on import pathways [28]. At low concentrations (< 2 μM), the uptake was mediated by direct translocation across the plasma membrane, whereas at higher concentrations (> 2 μM), glycosaminoglycan-dependent endocytosis was induced. Recent applications of CPPs for enhanced gene delivery applications are illustrated in Table 1.
Figure 3:
(a) Translocation mechanisms of CPPs into the cells through direct penetration, formation of inverted micelles, and endocytic mechanisms (Reproduced from reference [23], with permission from). (b) Endocytic pathways of CPPs through clathrin and caveolin mediated endocytosis, and macropinocytosis (Reproduced from reference [24], with permission from Springer Nature).
Table 1:
CPPs for gene delivery applications.
| Delivery carrier |
CPPs | Purpose | Delivery system |
Mechanism of uptake |
Ref |
|---|---|---|---|---|---|
| Peptide (CPP) | Arginine rich peptides containing unnatural amino acid 4-aminopiperidine-4-carboxylic acid (Api) or proline derivative with guanidine in the side chain (ProGu) | pDNA delivery | Peptide/pDNA complex | Endocytosis (pathway not specified) | [29] |
| Peptide (CPP) | Lysine with a guanidinylethyl (GEt) amine structure in the side chain [Lys(GEt)-peptide] | pDNA delivery | Lys(GEt)-peptide/pDNA complex | Endocytosis (pathway not specified) | [30] |
| Nanoparticle [magnetic nanoparticles (MNPs)] | PF14 (Stearyl-AGYLLGKLLOOL AAAALOOLL-NH2) | Splice correcting oligonucleotide (SCO) delivery | PF14-SCO-MNPs | Receptor-mediated endocytosis | [31] |
| Lipid [pH-sensitive cationic lipid (YSK05)] | Octaarginine (R8) | pDNA delivery | Lipid nanoparticles | Macropinocytosis | [32] |
| Peptide (CPP) | Human protein DMBT1 (deleted in malignant brain tumor 1)-derived CPP | siRNA delivery | DMBT1 peptide/siRNA complex | Direct membrane translocation and endocytosis | [33] |
Peptides for targeting cells and organelles
Peptides have generated considerable interest both as cellular targeting ligands able to initiate transport across specific cellular membranes through interactions with cell surface receptors, and as subcellular targeting ligands able to navigate barriers within the intracellular membrane network. The design of targeting peptides for specific receptors requires comprehensive information about the natural ligand’s binding site, secondary structure, and sequence to develop a biomimetic peptide. Alternatively, phage display can be used for screening a large library of peptides for desirable properties.
Peptides have been primarily explored against several major receptor classes including integrin receptors (e.g., αvβ3, αvβ5, and α5β1), growth factor receptors [e.g., epidermal growth factor receptor (EGFR), platelet derived growth factor receptor (PDGFR), and vascular endothelial growth factor receptor (VEGFR)], and G-protein coupled receptors (e.g., octreotide, exenatide, leuprolide, and abarelix). These receptor classes have generated enormous interest due to their broad relevance in cancers, cardiovascular conditions, tissue regeneration/repair, and other diseases (e.g., diabetes mellitus, obesity, infectious, and inflammatory diseases).
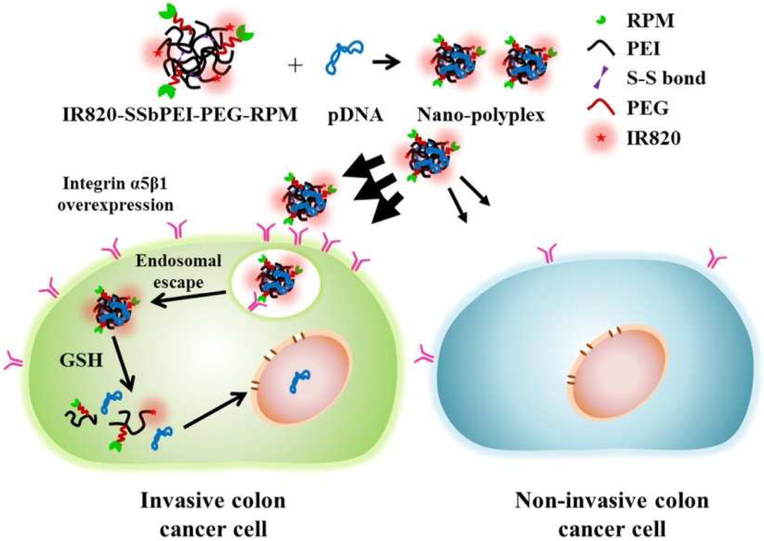
Integrins are heterodimeric cell surface receptors involved in cell adhesion, cell differentiation, cell proliferation, cell migration, and cell survival. Integrin signaling through receptors such as αvβ3, αvβ5, and α5β1 integrin is an important regulator of metastasis, and integrins also are involved in tumorigenesis through co-regulation of growth factor (e.g., EGFR) activity in cancers. The αvβ3 and αvβ5 integrins bind to the RGD domain in vitronectin. α5β1 integrin binds to the RGD domain in the 10th type III module (FNIII10) of fibronectin, as well as to the synergy site in the FNIII9 module, with the RGD motif forming, and the synergy site contacting only the head domain of the α subunit. Integrin-targeting approaches have especially benefitted from the design of integrin-targeting peptides that are able to mimic the presentation of native integrin ligands in extracellular matrix (ECM) and thereby garner increased receptor specificity. For example, the PR_b peptide (KSSPHSRNSGSGSGSGSGRGDSP) was designed to specifically target the α5β1 integrin via spatially-constrained presentation of the RGD and synergy motifs using an SG spacer sequence. This peptide was tested for its capacity to enhance the selectivity of liposome-mediated gene transfection in DLD1 colorectal cancer cells [21]. In these analyses, integrin binding by the PR_b peptide triggered a combination of macropinocytosis and caveolar endocytosis of liposomes. The transfection efficiency of the PR_b-modified liposomes in DLD1 cells was enhanced by approximately 8-fold and 5-fold when compared to PEI polyplexes and stealth liposomes, respectively, and targeting to the α5β1 integrin was confirmed by evaluation of the binding affinity of PR_b peptide to α5β1 integrin (76.3 ± 6.3 nM). In another example, a different peptide targeting the α5β1 integrin receptor (CPIEDRPMC; i.e., RPM) was used by Lee et al. for targeted delivery in a murine invasive colorectal cancer model [22]. RPM was conjugated to PEI-grafted PEG and incorporated into a polyplex system comprised of D-luciferin pDNA and PEI-grafted PEG (Figure 4) [22]. RPM stimulated α5β1-mediated endocytosis, resulting in a 2-fold increase in transfection efficiency in HT29 colorectal cancer cells and enhanced tumor-accumulation in HT29 xenografts in mice. The proton sponge effect of PEI assisted in the endosomal escape process for gene delivery. Altogether, integrin targeting peptides can facilitate specificity, integrin-mediated internalization, and efficient transfection for potential treatment of diseased cells.
Figure 4:
Schematic diagram of gene delivery using RPM-conjugated bioreducible gene carriers (SSbPEI-PEG-RPM) (Reproduced from reference [22], with permission from Elsevier). In this work, α5β1 integrin receptor-overexpressing invasive colon cancer cells (HT29) interacted with RPM peptides to enhance receptor-mediated endocytosis of the PEGylated polyplexes. Enhanced uptake was not observed in non-invasive colon cancer cells (HCT116). Following endosomal escape through the proton sponge effect of PEI, released pDNA was successfully transfected into the nucleus. IR820 was used for infrared imaging in transfected cells.
Growth factor receptors such as EGFR, PDGFR, and VEGFR are glycoproteins with an extracellular ligand-binding domain, a hydrophobic transmembrane region, and intracellular sequences containing regulatory and catalytic (kinase) domains that are overexpressed in many malignant gliomas. The versatility of peptide chemistries has proven especially useful in GFR targeting, with several examples demonstrating the benefits of phage display for selection of high affinity/high specificity GFR binders. For example, an EGFR-binding peptide (an 11-amino acid oligopeptide termed GE11) with high EGFR specificity and a KD of 22 nM was identified based upon phage display. GE11 was used for EGFR targeting within self-assembling peptide nanovesicles (SPVs) that were assembled from GE11-glycidyl hexadecyl dimethylammonium chloride (GE11-GHDC)/cholesterol/DOPE using thin-layer evaporation and reverse-phase evaporation methods [34]. The delivery of doxorubicin and the acetylcholinesterase gene using EGFR-targeted SPV resulted in excellent in vitro and in vivo drug/gene delivery with a significant growth-suppressing effect on liver cancer xenografts. Tumor weights in treated mice were 4-fold lower than tumor weights in animals treated with non-EGFR targeted SPV. Moreover, EGFR-specific nanovesicle targeting was clearly demonstrated based upon hyperspectral confocal fluorescence microscopy and in vivo tumor distribution in the xenograft mouse model, framing the benefits of peptides for enabling cell-targeted therapy.
G protein-coupled receptors (GPCRs) are the largest and most diverse group of membrane receptors and their overexpression is associated with promotion of cancer aggressiveness. A total of 118 peptide-binding GPCRs have been identified and hence peptides mimicking their natural agonists or antagonists can be used for targeting the orthosteric binding site of the receptor for cargo delivery. For example, a group of researchers designed a hybrid adeno-associated virus and phage (AAVP) vector displaying biologically active octreotide (Oct) for ligand-directed gene delivery of tumor necrosis factor (TNF) [35]. Oct can emulate the specific binding to somatostatin receptor 2 (SSTR2) in pancreatic neuroendocrine tumor (NET) cells to enhance uptake. A 5-fold increase in binding of Oct-AAVP-TNF to the NET cells was observed as compared to the untargeted control AAVP-TNF. The enhanced accumulation of Oct-AAVP-TNF in insulinomas of a human multiple endocrine neoplasia syndrome type 1 (MEN1) mouse model, and the subsequent TNF expression in tumors, provided clear evidence for improved targeted gene delivery by Oct. Taken together, the utilization of various natural ligand-mimetic peptides for GPCRs can enhance targeted cargo delivery of gene or small drug molecules to diseased cells for improvement in therapeutic efficacy.
Following endocytosis, many gene carriers become entrapped within endosomes, which can fuse with lysosomes or recycle their contents back to the cell surface. To avoid these fates, gene carriers must either escape endosomes or traffic within the cell through alternative trafficking strategies involving non-acidifying/non-recycling vesicles [36,37]. Several groups have taken advantage of the sophisticated chemical/physical properties of peptides to design peptide nanostructures with elegant membrane-disruptive capacity inside the acidic endosome. The mechanisms of action by such peptides include pore formation in the endosomal membrane [e.g., listeriolysin O, pneumococcal pneumolysin, virus inspired polymer for endosomal release (VIPER)] [38,39]; the proton sponge effect (e.g., histidine-rich peptides) [40]; or conformational changes in peptide secondary structure that assist membrane insertion and endosomal escape (e.g., hemagglutin derived peptide HA2, INF7, GALA) [41]. For example, a recent study by Asseline et al. demonstrated the cytosolic targeting ability of the histidine-rich peptide H5WYG (GLFHAIAHFIHGGWHGLIHGWYG) derived from the N-terminal segment of the HA-2 subunit of the influenza virus hemagglutinin following its conjugation to the 5’-end of the RNase H-incompetent antisense 2’-O-methyl-phosphodiester oligonucleotide (2’-Ome RNA705) [42]. (Note: the oligonucleotide targets an aberrant splicing of luciferase pre-mRNA in HeLa pLuc705 cells, such that successful delivery induces the increased expression of active luciferase.) The H5WYG peptide undergoes a conformational change between pH 7.0 and 6.0 after imidazole protonation, resulting in permeabilization of the cell membrane in a weakly acidic medium; accordingly, H5WYG is an excellent candidate for pH-mediated delivery of oligonucleotides to the cytosol from early endosomes. Confocal imaging demonstrated an approximately 5-fold higher level of cytosolic accumulation of H5WYG-2’-Ome-RNA705 as compared to 2’-Ome-RNA705; moreover, H5WYG-2’-Ome-RNA705 exhibited significant levels of nuclear accumulation due to the ability of cytosolic oligonucleotides to pass through the nuclear pores without the requirement of an active process. A 2660- and 400-fold increase in luminescence for H5WYG-2’-Ome-RNA705/lipofectamine treated HeLa cells was observed as compared to untreated control and 2’-Ome-RNA705/Lipofectamine treatments, respectively. This result is attributable to the successful entry, endosomal escape, and nuclear delivery of H5WYG-2’-Ome-RNA705 that directed the splicing machinery toward complete intron 2 removal by masking the cryptic splice site to induce luciferase expression.
Larger DNA cargoes typically require more active strategies to be trafficked into the nucleus, as most gene carriers are too large in size to passively diffuse through the nuclear pore membrane [43]. Peptides offer a potential solution to this issue through their ability to mimic the nuclear localization sequences (NLSs) found in proteins, viruses, and other native structures, particularly when NLS targeting is coupled to strategies that shuttle gene carriers into the perinuclear cytosol to access the nuclear uptake machinery. In one study, the simian virus 40 (SV40) large T-antigen derived NLS was conjugated to the LAHA4-L1 peptide, a pH-responsive (cationic amphipathic) peptide that promotes endosomal escape of nanocarriers. The coupled SV40 NLS-LAHA4-L1 peptide was assembled with luciferase DNA to form complexes that were ~130-165 nm in diameter, whereas LAHA4-L1/DNA complexes lacking the SV40 sequence formed ~227 nm complexes. DNA delivery efficiency was significantly improved in slow-dividing cells and dendritic cells, with up to a 10-fold increase in gene expression using NLS-linked LAH4-L1 as compared to LAHA4-L1 alone in slow-dividing epithelial cancer cells (Calu-3), macrophages (RAW264.7), dendritic cells (JAWSII), and thymidine-induced growth-arrested cells [25].
An alternative approach is the design of peptides that can shuttle gene carriers to the nucleus by using retrograde transport or other alternative trafficking patterns employed by some viruses and toxins. Ross, Munsell, and coworkers demonstrated the ability of histone tail motifs [histone 3 (H3)-derived peptides] to traffic DNA polyplexes into the nucleus by enhancing caveolar uptake and increasing polyplex transport through the Golgi/ER en route to the nucleus [36]. H3/PEI/DNA polyplexes trafficked through the Golgi/ER via a non-acidifying vesicular route that overlapped with H3K4 methyltransferase (H3K4MT)-linked pathways and also was regulated in part by Rab6 (a marker of retrograde transport from the Golgi to the ER). The H3/PEI/DNA polyplexes ultimately reached the nucleus through post-mitotic ER membrane redistribution in a process that was facilitated by the H3 NLS and importin-4 proteins [36,44]. Recently, the benefits of the H3-targeting approach were demonstrated in mesenchymal stem cell (MSC)-targeted gene delivery for bone repair applications. H3-targeted polyplexes induced a 4-fold enhancement in osteogenic bone morphogenetic protein-2 (BMP-2) expression by MSCs as compared to gene carriers lacking H3 [37]. Moreover, 100-fold more recombinant BMP-2 protein was needed to achieve similar levels of BMP-2-mediated chondrogenic gene and protein expression as H3-targeted BMP-2 gene transfer, demonstrating clear translational impacts.
Peptides for spatiotemporally-controlled gene delivery
Techniques that enable spatiotemporal control over which cells receive and express gene cargoes are of great interest to minimize off-target effects following gene transfer. The chemical versatility in peptides has been exploited for this purpose, often by designing peptides whose properties depend upon the application of an exogenous or endogenous stimulus. For example, an interesting photoswitchable CPP design offered a route to spatiotemporally control peptide activation and uptake in cancer cells [45]. The CPP was comprised of cell-penetrating oligoarginine (positively charged) linked to an inhibitory oligoglutamate (negatively charged) via an azobenzene (AB) moiety within the peptide backbone. The turn-like structure of the cis-form AB allowed efficient pairing of the oppositely charged peptide sequences, whereas the extended trans-form disrupted this pairing to release oligoarginine for cellular uptake. The light-responsive nature of AB allowed the structure and behavior of the peptide to be directly controlled by the application of light, with the cis-AB induced by application of certain wavelengths of light (λ ≈ 360 nm), and the trans-AB induced by distinct wavelengths (λ ≈ 440 nm). A 4- to 10-fold increase in cellular uptake of the trans-form vs. the cis-form occurred in cultured HeLa cells. This unique structural modification could potentially be further utilized to control uptake of gene carriers and other cargoes.
Studies by Urello et al. demonstrated an alternative approach to use ECM-inspired peptides to enable endogenous (proteolytic) control over the release/expression of gene cargoes from within collagen matrices. Their work employed collagen mimetic peptides (CMPs) to attach DNA polyplexes to collagen scaffolds such that the polyplexes were only released during matrix metalloproteinase (MMP)-mediated collagen remodeling and endocytic collagen uptake by fibroblasts [46-48]. CMPs are peptides comprised of GXY repeats such as the GPO triplet, and these peptides can strand invade into collagen to form a collagen triple helix incorporating one strand of the peptide. Urello and coworkers synthesized CMP-PEI conjugates, and they tuned CMP-PEI composition in DNA polyplexes to enable stable binding of CMP-polyplexes within the collagen gel matrix. Their approaches showed significant benefits for spatiotemporally-controlled platelet derived growth factor (PDGF) gene delivery in response to high levels of MMP expression, such as those present in the chronic wound bed. MMP activity mediated polyplex release through collagen degradation, and polyplex release could be modulated based upon the CMP conjugation ratio (0% to 50% CMP-PEI) to extend release from 20 days (for 0% CMP-PEI) to 35 days (for 50% CMP-PEI) [46]. In vitro wound closure with PDGF polyplexes was improved by 2-fold and 2.5-fold with 20% CMP-PEI polyplexes and mixed polyplexes (20% CMP-PEI and 50% CMP-PEI mixtures), respectively, as compared to non-CMP-modified polyplexes [47]. On-demand polyplex release and gene expression delivery was also were demonstrated in vivo, wherein gene expression could be tailored to peak at 6-20 days depending on the levels of CMP modification [48].
Spacing and arrangement of peptides on non-viral vehicles: Strategies and implications for gene delivery
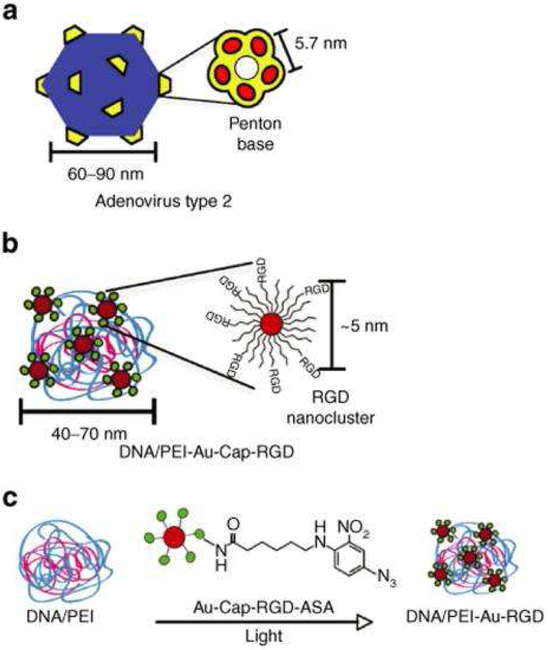
Nanocarrier modification with peptides not only requires careful peptide design, but also careful consideration of the number and arrangement of ligands required for cell binding, uptake, and/or trafficking. Ligand density is of critical importance, as it defines the binding avidity of the ligand and therefore strongly affects cellular responses. Furthermore, peptides can be arranged to mimic the specific presentation of clustered surface motifs in viruses and other naturally-occurring structures. For example, the effect of clustered RGD ligands on the transfection efficiency in DNA/PEI polyplexes was studied by covalent conjugation of RGD peptide-modified gold nanoparticles on the surfaces of polyplexes [49]. The clustering mimicked the physical structure of adenovirus type 2, which possesses five penton-base proteins located at each of the 12 vertices of the virus surface, with protruding RGD peptide sequences located 5.7 nm apart for viral cell entry (Figure 5). The presence of RGD nanoclusters on the DNA/PEI polyplex resulted in a 5.4-fold or 35-fold increase in gene transfer efficiency over unmodified polyplexes in HeLa cells with low- or high-integrin surface density, respectively.
Figure 5:
Schematic comparing adenovirus type 2 and DNA/PEI-Au-Cap-RGD-ASA polyplex (Au-Cap-RGD-ASA: azidosalicylic acid-RGD-modified gold nanoparticles). (a) Type 2 adenovirus with penton-base proteins. (b) DNA/PEI-Au-Cap-RGD-ASA polyplex with RGD nanoclusters. (c) Au-Cap-RGD-ASA nanoparticles are conjugated to preformed DNA/PEI polyplexes when exposed to the UV light (Reproduced with permission from reference [49]).
The spacer length for conjugated peptides is another important parameter that must be optimized to enable incorporation of bioactive peptide sequences without compromising the non-viral gene transfer activity. An interesting study by Stefanick et al. analyzed the effect of peptide linker length on cellular uptake of liposomes in breast cancer cells [50]. As compared with PEG2000 (~45 repeat units of ethylene glycol), the use of short linkers (EG12, with 12 repeats of ethylene glycol) for HER-2-targeting peptide conjugation significantly improved the uptake of liposomes by ~9-fold and ~ 100-fold, respectively, in breast cancer cells and multiple myeloma cells. These authors studied the effects of different linker lengths (EG6, EG12, EG18, EG24, EG30, EG36, EG45, and EG72) to determine that minimal uptake with the EG6 linker was either attributable to the inability of such a short linker to present the HER-2 peptide in a sufficiently extended fashion beyond the liposomal PEG coating, or to the insufficient linker length for allowing the peptide to reach the binding pocket on the receptor. Enhanced liposomal uptake was observed with EG12 and EG18 linkers, but gradually declined with EG24 and completely disappeared with EG45 and EG72. Shorter EG linkers (EG12, EG18) can adopt a more linear conformation than longer linkers (EG24, EG30, EG36, EG45, and EG72), which form globular mushroom-like structures with reduced capacity for peptide interactions.
Peptide-ligand combinations for synergistic enhancement in gene delivery
Gene delivery is affected by several intracellular barriers in sequence, including the cell membrane, endosomal membrane, and nuclear membrane. Accordingly, employing peptide-ligand combinations that can overcome different gene transfer barriers in series can provide synergistic improvements in gene transfection efficiency and reduced off-target effects. For example, use of a peptide for endosomal escape alone may not help in nuclear delivery of pDNA in nondividing cells because pDNA primarily enters the nucleus in conjuction with nuclear membrane breakdown during mitosis. This issue was addressed in a study exploring the synergistic effects of anchoring the fusogenic peptide GALA and the NLS maltotriose onto lipid nanocarriers [51]. The modification with fusogenic peptide GALA alone (for endosomal escape) enhanced transfection efficiency in liver of mice (following injection via tail vein) by 1 and 2 orders of magnitude for lipid nanocarriers prepared with DOTAP and DOTMA, respectively. Addition of maltotriose (for nuclear targeting) resulted in a further enhancement in transfection activity by 1 order of magnitude to reach the higher level obtained for a conventional lipoplex but with less hepatic toxicity. These results were attributable to the individual effects of GALA and maltotriose on endosomal escape and nuclear transfer to potentiate enhancement in transfection.
Future prospects
Recent gene therapy approvals by the FDA have encouraged a substantial investment in advanced gene delivery technologies. The chemical versatility and unique biochemical properties in peptides can be exploited to improve targeting specificity and enhance transfection in non-viral gene carriers. Furthermore, the stimuli-responsive properties in peptides offer new opportunities for spatiotemporal control in gene delivery and open avenues for multi-faceted, peptide-based systems in treatment of different diseases such as cancer, chronic wounds, and hereditary diseases. Appropriate chemical modifications of peptides that can sense changes in the disease microenvironment would be highly promising for effective treatment. Furthermore, peptides that are responsive to external stimuli such as light may be useful for switching the active/non-active forms of peptides to enable efficient uptake and transfection only in desired sites of interest.
Several PEI- and lipid-based nanoparticles for gene delivery have been developed for clinical trials, mostly focused on the treatment of different cancers [52]. However, peptide-mediated gene delivery is still in its infancy for successful application in the clinic. Rigorous in vitro and in vivo studies using peptide-mediated gene delivery are necessary to uncover the immunogenic properties, off-target effects, and efficacy of peptides in specific applications. The diverse amino acid chemistries and secondary structures will ultimately offer rich opportunities for targeted gene transfer in a variety of applications.
Highlights.
Peptides possess diverse amino acid chemistries and secondary structures suitable for targeted nucleic acid delivery.
Peptides can assist in nanocarrier trafficking by triggering cellular uptake, facilitating endosomal escape, and/or enabling nuclear targeting.
Peptides can promote spatio-temporal control over gene delivery.
Modified peptides responsive to external stimuli (e.g., light) can switch into active/non-active forms to assist uptake and transfection in target cells.
Acknowledgements
The authors thank the National Institute of Health (NIH) and National Science Foundation (NSF) for financial support through Grant No. NIH R01 AR067247, NIH R01 EB017766, NSF 1700980, and NSF 1605130. The statements and opinions herein are those of the authors and do not necessarily reflect the views of the NIH and NSF. R.K.T conducted literature searches, compiled references, wrote the paper, and designed original figures and tables. M.O.S. edited the paper and provided overall feedback and guidance during the writing process.
Abbreviations
- AAVP
adeno-associated virus and phage
- AB
azobenzene
- Api
Arginine rich peptides containing unnatural amino acid 4-aminopiperidine-4-carboxylic acid
- BMP-2
bone morphogenetic protein-2
- CAR
chimeric antigen receptor
- CMP
collagen mimetic peptide
- CPP
cell penetrating peptide
- DA
4’4-dithiodibutyric acid
- DMBT1
deleted in malignant brain tumor 1
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DOTAP
1,2-dioleyl-3-trimethylammonium propane
- DOTMA
N-[1-(2,3-dioleyloxy)propyl-]-N,N,N-trimethylammonium chloride
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- Get
Lysine with a guanidinylethyl
- GHDC
glycidyl hexadecyl dimethylammonium chloride
- GPCR
G protein-coupled receptor
- MEN1
multiple endocrine neoplasia syndrome type 1
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- NET
neuroendocrine tumor
- NLS
nuclear localization sequence
- Oct
octreotide
- PBAE
poly(beta-amino ester)
- PDGF
platelet derived growth factor
- PDGFR
platelet derived growth factor receptor
- PDMAEMA
poly(2-dimethylaminoethyl methacrylate)
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- ProGu
proline derivative with guanidine in the side chain
- pVEC
peptide derived from vascular epithelial cadherin
- RISC
RNA-induced silencing complex
- SCO
splice correcting oligonucleotide
- SSTR2
somatostatin receptor 2
- SV40
simian virus 40
- TAT
trans-activator of transcription
- TNF
tumor necrosis factor
- VEGFR
vascular endothelial growth factor receptor
- VIPER
virus inspired polymer for endosomal release.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest.
References:
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
• • of oustanding interest
- 1.Kay MA, Glorioso JC, Naldini L: Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med 2001, 7:33–40. [DOI] [PubMed] [Google Scholar]
- 2.Hill AB, Chen M, Chen CK, Pfeifer BA, Jones CH: Overcoming gene-delivery hurdles: Physiological considerations for nonviral vectors. Trends Biotechnol 2016, 34:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bach PB, Giralt SA, Saltz LB: FDA approval of tisagenlecleucel: Promise and complexities of a $475 000 cancer drug. JAMA 2017, 318:1861–1862. [DOI] [PubMed] [Google Scholar]
- 4.Ghobadi A: Chimeric antigen receptor T cell therapy for non-Hodgkin lymphoma. Curr Res Transl Med 2018, 66:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal GM, Pazdur R: Approvals in 2017: Gene therapies and site-agnostic indications. Nat Rev Clin Oncol 2018, 15:127–128. [DOI] [PubMed] [Google Scholar]
- 6.Smith AJ, Oertle J, Warren D, Prato D: Chimeric antigen receptor (CAR) T cell therapy for malignant cancers: Summary and perspective. Journal of Cellular Immunotherapy 2016, 2:59–68. [Google Scholar]
- 7.Levine BL, Miskin J, Wonnacott K, Keir C: Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev 2017, 4:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ameri H: Prospect of retinal gene therapy following commercialization of voretigene neparvovec-rzyl for retinal dystrophy mediated by RPE65 mutation. J Curr Ophthalmol 2018, 30:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J: Gene therapy clinical trials worldwide to 2012 – An update. J Gene Med 2013, 15:65–77. [DOI] [PubMed] [Google Scholar]
- 10.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG: Non-viral vectors for gene-based therapy. Nat Rev Genet 2014, 15:541. [DOI] [PubMed] [Google Scholar]; • Detailed overview on non-viral vectors for gene delivery.
- 11.Schlenk F, Grund S, Fischer D: Recent developments and perspectives on gene therapy using synthetic vectors. Ther Deliv 2013, 4:95–113. [DOI] [PubMed] [Google Scholar]
- 12.Ramamoorth M, Narvekar A: Non viral vectors in gene therapy- An overview. J Clin Diagn Res 2015, 9:GE01–GE06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majewski AP, Stahlschmidt U, Jérôme V, Freitag R, Müller AHE, Schmalz H: PDMAEMA-grafted core–shell–corona particles for nonviral gene delivery and magnetic cell separation. Biomacromolecules 2013, 14:3081–3090. [DOI] [PubMed] [Google Scholar]
- 14.Zu Rehman, Hoekstra D, Zuhorn IS: Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: Real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano 2013, 7:3767–3777. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Huang L: Lipid nanoparticles for gene delivery. Adv Genet 2014, 88:13–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mou Q, Ma Y, Jin X, Zhu X: Designing hyperbranched polymers for gene delivery. Mol Syst Des Eng 2016, 1:25–39. [Google Scholar]
- 17.Chuah JA, Matsugami A, Hayashi F, Numata K: Self-assembled peptide-based system for mitochondrial-targeted gene delivery: Functional and structural insights. Biomacromolecules 2016, 17:3547–3557. [DOI] [PubMed] [Google Scholar]
- 18.Zylberberg C, Gaskill K, Pasley S, Matosevic S: Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther 2017, 24:441–452. [DOI] [PubMed] [Google Scholar]
- 19.Komin A, Russell LM, Hristova KA, Searson PC: Peptide-based strategies for enhanced cell uptake, transcellular transport, and circulation: Mechanisms and challenges. Adv Drug Deliv Rev 2017, 110–111:52–64. [DOI] [PubMed] [Google Scholar]; • Detailed overview on peptide types, functions, and mechanisms for enhancing cellular uptake.
- 20.Ranalli A, Santi M, Capriotti L, Voliani V, Porciani D, Beltram F, Signore G: Peptide-based stealth nanoparticles for targeted and pH-triggered delivery. Bioconjug Chem 2017, 28:627–635. [DOI] [PubMed] [Google Scholar]
- 21.Adil MM, Erdman ZS, Kokkoli E: Transfection mechanisms of polyplexes, lipoplexes, and stealth liposomes in α5β1 integrin bearing DLD-1 colorectal cancer cells. Langmuir 2014, 30:3802–3810. [DOI] [PubMed] [Google Scholar]
- 22.Lee YM, Lee D, Kim J, Park H, Kim WJ: RPM peptide conjugated bioreducible polyethylenimine targeting invasive colon cancer. J Control Release 2015, 205:172–180. [DOI] [PubMed] [Google Scholar]
- 23.Jafari S, Maleki Dizaj S, Adibkia K: Cell-penetrating peptides and their analogues as novel nanocarriers for drug delivery. Bioimpacts 2015, 5:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gestin M, Dowaidar M, Langel Ü: Uptake mechanism of cell-penetrating peptides In Peptides and peptide-based biomaterials and their biomedical applications. Edited by Sunna A, Care A, Bergquist PL. Springer International Publishing; 2017:255–264. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Liang W, Qiu Y, Cespi M, Palmieri GF, Mason AJ, Lam JKW: Incorporation of a nuclear localization signal in pH responsive LAH4-L1 peptide enhances transfection and nuclear uptake of plasmid DNA. Mol Pharm 2016, 13:3141–3152. [DOI] [PubMed] [Google Scholar]
- 26.Hosseinkhani H, Abedini F, Ou KL, Domb AJ: Polymers in gene therapy technology. Polym Adv Technol 2015, 26:198–211. [Google Scholar]
- 27.Zhang B, Zhang H, Dai S, Bi J: Cell-penetrating peptide–labelled smart polymers for enhanced gene delivery. Eng Life Sci 2017, 17:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao CY, Delaroche D, Burlina F, Alves ID, Chassaing G, Sagan S: Translocation and endocytosis for cell-penetrating peptide internalization. J Biol Chem 2009, 284:33957–33965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato T, Yamashita H, Misawa T, Nishida K, Kurihara M, Tanaka M, Demizu Y, Oba M: Plasmid DNA delivery by arginine-rich cell-penetrating peptides containing unnatural amino acids. Bioorg Med Chem 2016, 24:2681–2687. [DOI] [PubMed] [Google Scholar]
- 30.Oba M, Kato T, Furukawa K, Tanaka M: A cell-penetrating peptide with a guanidinylethyl amine structure directed to gene delivery. Sci Rep 2016, 6:19913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowaidar M, Abdelhamid HN, Hällbrink M, Freimann K, Kurrikoff K, Zou X, Langel Ü: Magnetic nanoparticle assisted self-assembly of cell penetrating peptides-oligonucleotides complexes for gene delivery. Sci Rep 2017, 7:9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalil IA, Kimura S, Sato Y, Harashima H: Synergism between a cell penetrating peptide and a pH-sensitive cationic lipid in efficient gene delivery based on double-coated nanoparticles. J Control Release 2018, 275:107–116. [DOI] [PubMed] [Google Scholar]
- 33.Tuttolomondo M, Casella C, Hansen PL, Polo E, Herda LM, Dawson KA, Ditzel HJ, Mollenhauer J: Human DMBT1-derived cell-penetrating peptides for intracellular siRNA delivery. Mol Ther - Nucleic Acids 2017, 8:264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang X, Shi B, Wang K, Fan M, Jiao D, Ao J, Song N, Wang C, Gu J, Li Z: Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials 2016, 82:194–207. [DOI] [PubMed] [Google Scholar]
- 35.Smith TL, Yuan Z, Cardó-Vila M, Sanchez Claros C, Adem A, Cui MH, Branch CA, Gelovani JG, Libutti SK, Sidman RL, Pasqualini R, Arap W: AAVP displaying octreotide for ligand-directed therapeutic transgene delivery in neuroendocrine tumors of the pancreas. Proc Nat Acad Sci U S A 2016, 113:2466–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross NL, Munsell EV, Sabanayagam C, Sullivan MO: Histone-targeted polyplexes avoid endosomal escape and enter the nucleus during postmitotic redistribution of ER membranes. Mol Ther. Nucleic Acids 2015, 4:e226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munsell EV, Kurpad DS, Freeman TA, Sullivan MO: Histone-targeted gene transfer of bone morphogenetic protein-2 enhances mesenchymal stem cell chondrogenic differentiation. Acta Biomater 2018, 71:156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim NH, Provoda C, Lee KD: Design and characterization of novel recombinant listeriolysin O–protamine fusion proteins for enhanced gene delivery. Mol Pharm 2015, 12:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Y, Yumul RC, Pun SH: Virus-inspired polymer for efficient in vitro and in vivo gene delivery. Angew Chem Int Ed Engl 2016, 55:12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razzano V, Paolino M, Reale A, Giuliani G, Donati A, Giorgi G, Artusi R, Caselli G, Visintin M, Makovec F, Battiato S, Samperi F, Villafiortia-Monteleone F, Botta C, Cappelli A: Poly-histidine grafting leading to fishbone-like architectures. RSC Adv 2018, 8:8638–8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai W, Gao X: Functional peptides for siRNA delivery. Adv Drug Deliv Rev 2017, 110– 111:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asseline U, Gonçalves C, Pichon C, Midoux P: Improved nuclear delivery of antisense 2'-Ome RNA by conjugation with the histidine-rich peptide H5WYG. J Gene Med 2014, 16:157–165. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Li H, Liu Z, Yuan W: Lipopolyplex for therapeutic gene delivery and its application for the treatment of Parkinson’s disease. Front Aging Neurosci 2016, 8:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross NL, Sullivan MO: Importin-4 regulates gene delivery by enhancing nuclear retention and chromatin deposition by polyplexes. Mol Pharm 2015, 12:4488–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prestel A, Moller HM: Spatio-temporal control of cellular uptake achieved by photoswitchable cell-penetrating peptides. Chem Commun (Camb) 2016, 52:701–704. [DOI] [PubMed] [Google Scholar]; • Irradiation-based approach for activation/inactivation of peptides with the ability to modulate cellular uptake and subsequent delivery
- 46.Urello MA, Kiick KL, Sullivan MO: A CMP-based method for tunable, cell-mediated gene delivery from collagen scaffolds. J Mater Chem B 2014, 2:8174–8185. [DOI] [PubMed] [Google Scholar]; • • Peptide-based spatio-temporal control of gene delivery by harnessing ECM turnover.
- 47.Urello MA, Kiick KL, Sullivan MO: Integration of growth factor gene delivery with collagen-triggered wound repair cascades using collagen-mimetic peptides. Bioeng Transl Med 2016, 1:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urello MA, Kiick KL, Sullivan MO: ECM turnover-stimulated gene delivery through collagen-mimetic peptide-plasmid integration in collagen. Acta Biomater 2017, 62:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ng QKT, Sutton MK, Soonsawad P, Xing L, Cheng H, Segura T: Engineering clustered ligand binding into nonviral vectors: αvβ3 targeting as an example. Mol Ther 2009, 17:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]; • • Importance of peptide conformational arrangement (clustering) on efficient integrin targeting and gene transfer.
- 50.Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B: A systematic analysis of peptide linker length and liposomal polyethylene glycol coating on cellular uptake of peptide-targeted liposomes. ACS Nano 2013, 7:2935–2947. [DOI] [PubMed] [Google Scholar]; • • Role of peptide linkers in targeted cellular uptake.
- 51.Akita H, Masuda T, Nishio T, Niikura K, Ijiro K, Harashima H: Improving in vivo hepatic transfection activity by controlling intracellular trafficking: The function of GALA and maltotriose. Mol Pharm 2011, 8:1436–1442. [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Guo Z, Tian H, Chen X: Production and clinical development of nanoparticles for gene delivery. Mol Ther Methods Clin Dev 2016, 3:16023. [DOI] [PMC free article] [PubMed] [Google Scholar]