Abstract
Colorectal cancer has become a serious problem, especially in highly developed countries. As reported by the World Health Organization, the number of colon cancer cases in the world in 2012 amounted to 1.36 million. It is the second most common cancer in females (614,000 cases, 9.2% of the total) and the third in males (746,000 cases, 10.0% of the total) worldwide. It is believed that TGFβ pathway elements are involved in the pathogenesis of colorectal cancer. This study assessed one of these elements, the ACVR2A gene. Qualitative and quantitative analyses of the ACVR2A gene in 84 patients with colorectal cancer was performed. There was no statistically significant association between ACVR2A gene expression and age, gender, histological type, grading of tumor, vascular invasion, and presence of lymphocytes in tumor tissue. No association was observed between the ACVR2A gene expression level and the presence of metastases in regional lymph nodes and distant metastases. In this study, larger tumors (T3 and T4) were characterized by higher ACVR2A expression compared to smaller tumors (T1 and T2). This may indicate an association between ACVR2A expression and the severity of pathological changes in the tumor growth process.
Keywords: Activin A protein, ACVR2A gene expression, tumor suppressor, colorectal cancer, TGFß signaling pathway
Introduction
Risk factors for colorectal cancer can be divided into modifiable and non-modifiable types. The former one includes bad nutritional habits, physical inactivity, obesity, cigarette smoking, heavy alcohol consumption, and urban residence. The non-modifiable risk factors include age and hereditary factors. The likelihood of colorectal cancer diagnosis increases after the age of 40 and rises sharply after the age of 50. It is believed that about 5 to 10% of colorectal cancers are the result of genetic factors. Therefore, mutations in genes engaged in the DNA repair pathway like MLH1 and MSH2 genes should be mentioned as responsible for hereditary non-polyposis colorectal cancer (HNPCC). On the other hand, mutations in tumor suppressor genes are also associated with the risk of cancer, e.g. the APC gene is related in familial adenomatous polyposis – FAP (Haggar and Boushey, 2009).
In recent years, the TGFβ signaling pathway, which may act as a tumor suppressor or a promoter depending on the stage of the disease, has become the subject of many studies (Padua and Massagué, 2009; Yang et al., 2010; Heldin et al., 2012). The TGFβ signaling pathway begins with the connection of the ligand, e.g. TGFβs, bone morphogenetic proteins (BMPs), growth differentiation factors (GDFs), nodals, activins, and inhibins with the receptor. There are three types of receptors in the cell membrane. The TGFβ receptors type I and type II (defined as TβRI and TβRII) are directly involved in signal transduction (Alarmo et al., 2007; Yang et al., 2010; Olsen et al., 2015). Devoid of enzymatic activity, the TGFβ receptors type III (TβRIII) such as betaglycan regulate the availability of TβRI and TβRII for ligands (Padua and Massagué, 2009). Next to the binding of ligands to TβRII, TβRI is enlisted, transphosphorylated, and activated to phosphorylate the downstream mediators, receptor-regulated SMADs proteins. These transmit the signals to the cell nucleus where the gene transcription is modulated. In addition to the classical pathway, TGFβ signaling pathways independent of SMADs proteins can be distinguished. Also, PI3-kinase, p38 kinase, and small GTPase pathways such as RhoA, PKN, and Rock should be mentioned (Yang et al., 2010; Dean et al., 2017).
TGFβ as a tumor suppressor induces apoptosis or autophagy, inhibits cell cycle, as well as the expression of growth factor, cytokines and chemokines. Many mutations in genes encoding receptors and decreased expression of components of the TGFβ pathway are documented in carcinoma. Additionally, the lack or downregulation of TβRI, TβRII, or SMADs is often associated with a worse prognosis (Yang et al., 2010). TGFβ as a tumor promoter determines mesenchymal-epithelial transition (MET), increases the activity of proteases, decreases immune response, promotes angiogenesis, and modulates the cytoskeletal architecture and extracellular matrix (Padua and Massagué, 2009; Yang et al., 2010; Heldin et al., 2012). Aggressive and rapidly proliferating gliomas have a high TGFβ and SMADs activity. In animal models, an increase of metastasis to other organs was observed after enhancement of TGFβ signaling. The opposite effect, namely a reduction in the ability to metastasize, was observed after suppressed TGFβ transduction (Yang et al., 2010).
In this study, we focused on a gene encoding one of the proteins belonging to the TβRII receptors family (Jung et al., 2004). ACVR2A gene also called ACVR2, ACTRII, or ACTRIIA is located on the long arm of chromosome 2 (location 2q22.2-q23.3) and has the overall length of 83.3 kb (Fitzpatrick et al., 2009). The lead role of a protein encoded by this gene is the mediation of activin functions. The activin A receptor type 2A (ACVR2A) is constructed of 513 amino acids, and consists of an extracellular, a transmembrane and a cytoplasmic serine-threonine kinase domains. Except for the ability to transfer phosphate groups, the protein exhibits transferase and tyrosine kinase activity (Jung et al., 2004). Signal transduction begins with the connection of activin to the ACVR2 extracellular domain and the formation of heterodimer complex with ACVR1. Firstly, there is a phosphorylation of ACVR1 and intracellular effector proteins SMAD2 and SMAD3. Phosphorylated SMAD2 and SMAD3 form a complex with SMAD4, which is transported into the cell nucleus to regulate gene transcription (Hempen et al., 2003; Jung et al., 2004).
ACVR2A is believed to be a tumor suppressor that inhibits the growth and differentiation of cells. Its inactivation could lead to the development of colorectal cancer (Ballikaya et al., 2014). For this reason, in the study, the expression of the ACVR2A gene in patients with colorectal cancer was estimated. The level of ACVR2A expression for age and sex of respondents was assessed. The correlation with the level of ACVR2A expression and clinical stage was also determined according to the TNM classification and histological grade of the tumor (G).
Materials and Methods
Materials
Tissue specimen of colorectal carcinomas were obtained from the Department of Pathology at the Medical University of Lodz, Poland. All specimens were taken during the colon cancer surgery and diagnosed macroscopically by histopathological examination as a colorectal cancer tissue. Collected tissue samples were frozen in liquid nitrogen immediately after the surgery and then stored at -80 °C until processed for RNA isolation. All experiments were carried out with the license of the local ethics committee (No. RNN/8/08/KE) and patients informed consent.
RNA extraction
Total RNA was isolated from frozen sections of colorectal cancer tissue using the Total RNA Prep Plus Minicolumn Kit (A&A Biotechnology, Poland) according to the manufacturer’s protocol. The concentration of RNA in samples after isolation was measured using a spectrophotometer. Based on the obtained concentrations, the amount of RNA added to a reverse transcription reaction was determined and standardized in all samples. The isolated RNA has an A260/280 ratio of 1.8-2.0. Until analysis, RNA samples were stored at -80 °C.
Reverse transcription
Total RNA isolated from tissue specimens of colorectal cancer was reversely transcribed into complementary DNA (cDNA) in accordance with the High Capacity cDNA Reverse Transcription Kit protocol (Applied Biosystems, USA). The reverse transcription was performed under the following conditions: 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 min. The final concentration of RNA used to prepare the reaction mixture was 0.1 μg/μL for each sample. cDNA synthesized by RT reaction was stored at -20 °C until analysis. To check the presence of cDNA in each sample, the PCR amplification of ACTB gene was performed (sequences for ACTB primer set: forward 5’-GTGGGGCGCCCCAGGCACCA-3’; reverse 5’-CTCCTTAATGTCACGCACGATTTC-3’). ACTB gene encoding beta-actin, belonging to the housekeeping genes, was used as a reference. Only the samples that showed the presence of PCR product for ACTB gene (540 bp) were selected for further analysis.
PCR amplification
In the next step of the analysis, a qualitative assessment of ACVR2A gene expression was performed. In this analysis an ACVR2A gene fragment was amplified by polymerase chain reaction. The PCR reaction was carried out using ready-made solution containing Taq DNA polymerase, dNTPs, MgCl2 and reaction buffers (Biotool, Germany), according to the manufacturer’s protocol. The 20 μL reaction mixture for PCR consisted of 5 μL of 2x PCR Super Master Mix (Biotool, Germany), 0.7 μL of each primer at a concentration of 0.5 μM (sequences for the ACVR2A primer set were: forward 5’-AGGGTTCACTATCAGACTTTC-3’; reverse 5’-GTAAATATGCCAATCCTCTAGC-3’), 1 μL of cDNA template, and distilled water to a final reaction volume of 20 μL. In every experiment, a negative control sample (reaction mixture without cDNA template) was used. The PCR reaction conditions included the initial denaturation for 5 min at 95 °C, 34 cycles consisting of 3 steps (denaturation for 1 min at 95 °C, annealing for 1 min at 57 °C and elongation for 1 min at 72 °C) and final elongation for 7 min at 72 °C. PCR amplifications were carried out in MJ Mini Thermal Cycler (Bio-Rad, USA). The presence of 96 bp PCR products for the ACVR2A gene was assessed by electrophoresis in 2% agarose gels. For quantitative assessment, only samples that showed the mRNA expression of ACVR2A gene in qualitative analysis were included.
Real-time PCR
Real-time PCR was used for quantitative assessment of ACVR2A (investigated gene) and ACTB (reference gene) mRNA expression. Amplification reactions were performed using a MX3005P QPCR SYSTEM (Stratagene, USA) in accordance with the SYBR®Green JumpStart TaqReadyMix protocol (Sigma Aldrich, Germany). The reaction mixture for both genes consisted of 7.5 μL of JumpStart Taq ReadyMix (Sigma Aldrich, Germany), 0.7 μL of 10 μM solution of each primer, 1 μL of cDNA template, and distilled water to final reaction volume of 16 μL. The same set of primers of the qualitative analysis was used. All amplification reactions for the two genes were carried out in parallel, in separate tubes and in triplicate for each sample to ensure reproducibility of the reaction. In each experiment, a negative control sample also tested in triplicate was included. The real-time reaction conditions included the initial denaturation step at 95 °C for 10 min and 40 cycles consisting of 3 steps: denaturation at 95 °C for 30 s, annealing at 58 °C for 1 min, and elongation at 72 °C for 1 min. To assess the real-time reaction specificity, melting curve analysis was performed for all amplification products of ACVR2A and ACTB genes. The obtained Tm (melting temperature) for amplification products of ACVR2A was 79 °C and for ACTB it was 88 °C (Figure 1). The threshold was manually set at the same level for all analyzed samples to determine Ct values. The mean of the obtained Ct values for both genes was counted. The efficiency of the kinetic PCR reaction was estimated based on the analysis of the standard curves for both genes. The standard curves were obtained by five serial 10-fold dilutions of quantified PCR products. The efficiencies of PCR reactions were calculated from the slopes of the standard curves according to the equation E=10[-1/slope]-1. Because the values of efficiencies for both genes were similar (95% for ACVR2A, 93% for ACTB), the ΔΔCt method proposed by Livak and Schmittgen was used to calculate the relative level of ACVR2A expression. The mean Ct values for the ACTB and ACVR2A genes obtained for all tested samples were adopted as a calibrator.
Figure 1. mplification plot of qPCR reaction for samples with fluorescence threshold (A) and melting curve of amplification products (B). The melting temperature (Tm) for the reference gene ACTB was 88 °C and for the ACVR2A gene was 79 °C. The samples were made in triplicate.
Statistical analysis
The software STATISTICA12 (StatSoft, Inc., 2014) was used for statistical analyses. The collected quantitative data was checked for conformity with a normal distribution using the Shapiro-Wilk test. Due to the lack of conformity with normal distribution a comparative statistical analysis using the nonparametric U-Mann Whitney test was performed. A p < 0.05 was assumed as significant in all tests.
Results
Eighty-four cases of colorectal cancer were qualitatively analyzed to check the ACVR2A gene expression. ACVR2A gene expression was observed in all tested samples, which were used for further qualitative analysis using the real-time PCR. The statistical analysis included all 84 cases. In the studied group, the expression level of ACVR2A relative to ACTB was different and ranged from 0.0234 to 85.5424 with a median value 0.786. The detailed clinical description of all patients is shown in Table 1.
Table 1. Clinical characteristics of 84 patients with colorectal cancer. The p-values were calculated by Mann Whitney test.
| Variables | Patients (n = 84) | p-value |
|---|---|---|
| Sex: | ||
| male | 37 | 0.172 |
| female | 47 | |
| Age (years): | 63 (34-82) | 0.774 |
| Tumor localization: | ||
| cecum or colon | 53 | 0.051 |
| rectum | 27 | |
| Type of tumor: | ||
| tubular | 59 | 0.375 |
| mucinous | 10 | |
| Histological grading: | ||
| G1 or G2 | 59 | 0.692 |
| G3 | 24 | |
| TNM staging: | ||
| I or II | 46 | 0.261 |
| III or IV | 38 | |
| Size and depth of primary tumor invasion (T): | ||
| pT1 or pT2 | 22 | 0.04 |
| pT3 or pT4 | 62 | |
| Presence of metastases in the regional lymph nodes (N): | ||
| N0 | 48 | 0.439 |
| N1 or N2 | 31 | |
| Distant metastases (M): | ||
| presence of metastases | 14 | 0.782 |
| lack of metastases | 68 | |
| Lymphocytes in tumor tissue: | ||
| presence of lymphocytes | 37 | 0.179 |
| lack of lymphocytes | 47 | |
| Vessel invasion: | ||
| presence of vascular invasion | 53 | 0.219 |
| lack of vascular invasion | 31 |
The studied population consisted of 47 women and 37 men. Patients age was between 34 and 82 years, with the median age at the time of diagnosis of 63 years. The analyzed population was divided into two groups according to age. The first group included the 38 persons aged between 34 and 60 years old and the second group consisted of 46 people aged over 60 years. No statistical difference was observed between ACVR2A gene expression level and patients’ gender (p=0.172) or age (p=0.774).
In 53 of the cases, the tumors were located in the cecum or colon, the other 27 tumors were located in the rectum. Although a higher relative level of ACVR2A gene expression was observed in the group of patients with tumors located in the rectum, this difference was not statistically significant (p=0.051). All examined tumors were histologically classified as adenocarcinomas. The tubular type of tumor was predominant in the studied group (59 cases had a tubular type and 10 cases had a mucinous type). No correlation was found between tumor histological and the expression of the investigated gene (p=0.375).
Due to the histological grading, most tumors were classified as G1 or G2 grade (59 cases); the other 24 tumors had a G3 grade. In this study, cases were divided into 2 groups: cancers that were of low or intermediate grade (group 1) and of high grade (group 2). Comparison of ACVR2A expression level with histological grading between the groups did not show a statistical dependence (p=0.692).
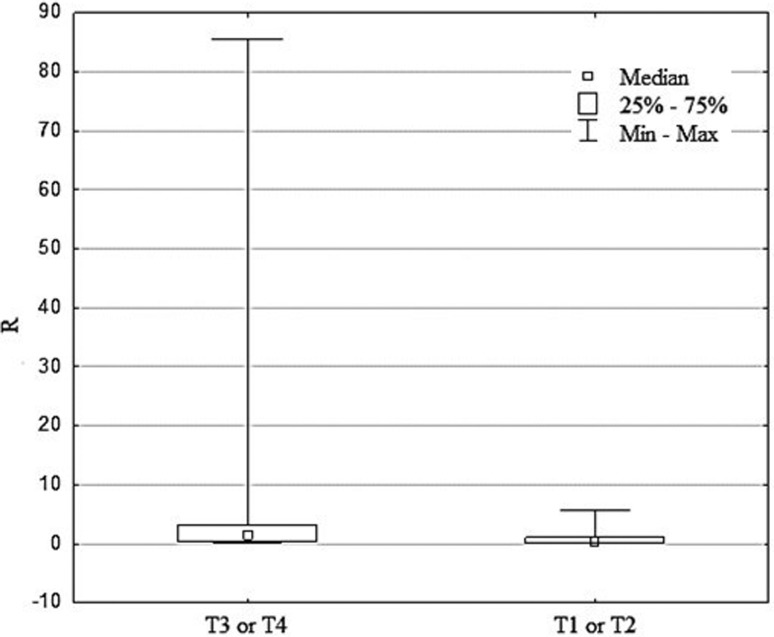
The ACVR2A gene expression level was compared with several clinicopathological parameters, such as the size and depth of primary tumor invasion (T), the presence of metastases in the regional lymph nodes (N), and the presence of distant metastases (M) according to the TNM classification. No dependence was observed when comparing ACVR2A gene expression with the lymph nodes and distant metastases (p=0.439, p=0.782 respectively for N and M). On the other hand, in cases with more advanced tumors (pT3 or pT4 wall depth penetration), the relative level of ACVR2A gene expression was higher than in cases with lower tumor invasion (pT1-pT2). This difference was statistically significant (p=0.04). In order to confirm that the expression level of the investigated gene was correlated with the stage of cancer, another evaluated parameter was pTNM staging. Based on this cancer staging method, the studied population was grouped into less advanced (46 cancers with stages I or II) and more advanced cases (38 cancers with stages III or IV). Comparison of ACVR2A gene expression level with pTNM staging between the groups did not show a significant dependence (p=0.261).
The presence of lymphocytes in tumor tissue is a positive prognostic indicator in advanced colon cancer. Therefore, in another analysis, the ACVR2A gene expression was compared with the presence of tumor-infiltrating lymphocytes. In 37 cases, lymphocytes were present in tumor tissue, while in 47 cases they were not. A lower level of ACVR2A expression was observed in patients with lymphocytic infiltration. We found that the ACVR2A expression level was not significantly related to the presence of lymphocytes (p=0.179).
The last analyzed parameter was the presence of vessel invasion, which is a poor prognosis factor in cancers. Vascular invasion was present in 53 cases and in this group of patients the relative level of ACVR2A gene expression was higher. Despite this, no association was observed between the level of ACVR2A expression and presence of vessel invasion (p=0.219).
Discussion
Numerous screening tests implemented in recent years have reduced mortality and incidence of colon cancer in the world. Nevertheless, the five-year survival remains low due to metastasis (Bauer et al., 2015). The process of metastasis is associated with reduced or transformed TGFβ susceptibility and increased expression or activation of the TGFβ ligands. Many studies were conducted to clarify the transition mechanism of the TGFβ signaling pathway from a tumor suppressor to promoter and as a result, two signaling SMAD-dependent and SMAD-independent pathways have been extracted. It is believed that both of them contribute to the development of cancer (Yang et al., 2010).
ACVR2A is a ligand of activin A protein, which is an important regulator of pregnancy. Activin A occurs physiologically, e.g., in the endometrium, placenta and vascular endothelium and regulates the remodeling of the uterine spiral arteries, which are reduced in preeclampsia. The elevated level of activin A in the serum of women with preeclampsia has been documented and for this reason is considered as a preeclampsia marker (Fitzpatrick et al., 2009). In addition to the ACVR2A mutations in preeclampsia, vascular dysfunction leading to hypertension, proteinuria, or edema are reported. Many sources also indicate the potential impact of ACVR2A gene mutations on multiple synostoses syndrome (Jung et al., 2004; Fitzpatrick et al., 2009; Van Dijk and Oudejans, 2011). Wang et al. (2014) demonstrated that the ACVR2A protein level correlates with the severity of sepsis. The contribution of ACVR2A has also been confirmed in the gastrulation, spermatocytogenesis and spermiogenesis processes ( AmiGO).
ACVR2A is involved in important signaling pathways, for example PEDF-induced signaling, TFG-β signaling pathway, or signaling pathways regulating the pluripotency of stem cells, which may be related to the initiation of the carcinogenesis process (PathCards). Among others things, the association of the ACVR2A gene mutation with the development of prostate and lung cancer has been confirmed (Rossi et al., 2005; Dean et al., 2017).
In this study, the level of mRNA were compared with the clinical stage of cancer (the TNM Classification of Malignant Tumors) and its components. There was no statistically significant association between ACVR2A gene expression and the presence of metastases in the regional lymph nodes (p=0.439) and distant metastases (p=0.782). A similar result was achieved by evaluating the level of ACVR2A gene expression with the TNM staging (p=0.261). One of the TNM classification components, namely the size and depth of primary tumor invasion, deserves more attention. After dividing the study cohort into two groups, it appeared that the more extensive tumor group (T3 or T4) had higher ACVR2A gene expression compared to the less advanced tumor group (T1 or T2). This difference was statistically significant (p=0.04) and shown in Figure 2.
Figure 2. Comparison of ACVR2A expression level (R) with size and depth of primary tumor invasion (T) according to TNM classification.
In an investigation, Jung et al. (2006) tested the effects of TGFBR2, BAX, and ACVR2 mutations according to tumor stage, grade, and size in MSI-H colon cancer. They found that poor histological grade and larger tumor volume were correlated with mutant ACVR2. In this experiment, it was observed that the majority of frameshift mutations were biallelic and lead to the loss of protein expression. Activin signaling is believed to be a growth suppressor and inhibitor of this signaling by mutated ACVR2A, and loss of protein expression may increase tumor growth (Deacu et al., 2004; Jung et al., 2006). In addition, the restoration of ACVR2A activity was seen to inhibit the growth of colon cancer cells (Deacu et al., 2004; Chung et al., 2008). This fact was explained by the increase of de novo synthesis of proteins as CALU, IBP2, LETM1, PRS8, SF3B3, and TNPO1 responsible for growth inhibition and induction of apoptosis (Ballikaya et al., 2014). In many studies, efforts have been made to restore the activation pathway via drugs or miRNAs. For instance, Fuchs et al. (2014) noted that miR-27 inhibits TGFβ signaling pathway genes, including ACVR2A (Fuchs et al., 2014). In a mouse model, acvr2a was also found to be regulated by miR-29b and miR-181a (Plank et al., 2015). Attempts have also been made to reduce the mutations in the ACVR2A gene by mesalazine. As reported by Campregher et al. (2010), 5-ASA reduces microsatellite instability and improves replication fidelity in repeat sequences of TGFBR2 and ACVR2A genes. Hence, taking into consideration the potential role of the TGFβ signaling pathway genes in metastases, the use of suitable drugs or miRNA may be helpful in the future to reduce the expression of these genes and avoid metastases in people at risk (Campregher et al., 2010; Fuchs et al., 2014).
The study by Jung et al. (2006) found in two cohorts of 172 and 503 patients a different incidence of ACVR2A mutations (4.5% vs 32.5% respectively), indicating a high variability of mutations in populations. In another study, the ACVR2A gene was seen mutated in 90.9% of MSI colorectal cancer cases (Pinheiro et al., 2015). In later a subsequent study by Jung et al. (2009), in which 51 cases of MSS colon cancers were examined, loss of ACVR2A expression was observed in only 14% of cases, and in an immunohistochemistry study conducted by Jung et al. (2004), the loss of ACVR2A expression was observed in 62% of MSI colorectal neoplasms and protein presence was observed in all MSS tumors. The presence of protein in all our samples might suggest that they originated from MSS colon cancers, but this must be determined at the protein level.
In addition, discrepancies may have resulted from a relatively small number of colorectal cases in our study (84 patients were surveyed) and minor differences within the study group in case of cancer invasion and metastasis. For a complete view of ACVR2A gene expression in different stages of cancer development it seems advisable to extend the study to include patients with colorectal cancer to compare ACVR2A expression in each of the T1, T2, T3, and T4 groups separately.
Our study apparently contradicts the results obtained by Jung et al. (2006), because more advanced tumors (according to the TNM classification) presented higher ACVR2A expression. Similarly, overexpression of the ACVR2A gene was found in multiple myeloma (Grcevic et al., 2010). In addition, ovarian cancer patients with higher ACVR2A expression had shorter disease-free survival compared to those with low expression (Dean et al., 2017).
Inconsistent results were found in other studies of primary tumor size and ACVR2A protein expression. It is possible that despite the high expression, the resulting post-translational protein is dysfunctional and deprived of activity. The phenotypic effect of these lesions may be a greater tumor size. Further research is needed to analyze the functionality of the resulting protein by proteomics techniques.
Acknowledgments
The present study was supported by the Statutory Funds of the Department of Pharmaceutical Biochemistry and Molecular Diagnostics, Medical University od Lodz no. 503/3-015-02/503-31-001; Funds of the Faculty of Pharmacy, Medical University of Lodz no. 502-03/3-015-02/502-34-073.
Footnotes
Associate Editor: Houtan Noushmehr
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicial to the impartiality of the reported research.
Author contributions
EB and AJ conceived and designed the study; DW and JP conducted the experiments; AW and RS analyzed the data; DW and AW wrote the manuscript. All authors read and approved the final version.
References
- Alarmo EL, Kuukasjärvi T, Karhu R, Kallioniemi A. A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat. 2007;103:239–246. doi: 10.1007/s10549-006-9362-1. [DOI] [PubMed] [Google Scholar]; Alarmo EL, Kuukasjärvi T, Karhu R and Kallioniemi A (2007) A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat 103:239-246. [DOI] [PubMed]
- Ballikaya S, Lee J, Warnken U, Schnölzer M, Gebert J, Kopitz J. De novo proteome analysis of genetically modified tumor cells by a metabolic labeling/azide-alkyne cycloaddition approach. Mol Cell Proteomics. 2014;13:3446–3456. doi: 10.1074/mcp.M113.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ballikaya S, Lee J, Warnken U, Schnölzer M, Gebert J and Kopitz J (2014) De novo proteome analysis of genetically modified tumor cells by a metabolic labeling/azide-alkyne cycloaddition approach. Mol Cell Proteomics 13:3446-3456. [DOI] [PMC free article] [PubMed]
- Bauer J, Ozden O, Akagi N, Carroll T, Principe DR, Staudacher JJ. Activin and TGFβ use diverging mitogenic signaling in advanced colon cancer. Mol Cancer. 2015;14:1–14. doi: 10.1186/s12943-015-0456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bauer J, Ozden O, Akagi N, Carroll T, Principe DR and Staudacher JJ (2015) Activin and TGFβ use diverging mitogenic signaling in advanced colon cancer. Mol Cancer 14:1-14. [DOI] [PMC free article] [PubMed]
- Campregher C, Honeder C, Chung H, Carethers JM, Gasche C. Mesalazine reduces mutations in Transforming Growth Factor β Receptor II and Activin Type II Receptor by improvement of replication fidelity in mononucleotide repeats. Clin Cancer Res. 2010;16:1950–1956. doi: 10.1158/1078-0432.CCR-09-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]; Campregher C, Honeder C, Chung H, Carethers JM and Gasche C (2010) Mesalazine reduces mutations in Transforming Growth Factor β Receptor II and Activin Type II Receptor by improvement of replication fidelity in mononucleotide repeats. Clin Cancer Res 16:1950-1956. [DOI] [PMC free article] [PubMed]
- Chung H, Young DJ, Lopez CG, Le TAT, Lee JK, Ream-Robinson D, Huang SC, Carethers JM. Mutation rates of TGFBR2 and ACVR2 coding microsatellites in human cells with defective DNA mismatch repair. PLoS One. 2008;3:e3463. doi: 10.1371/journal.pone.0003463. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chung H, Young DJ, Lopez CG, Le TAT, Lee JK, Ream-Robinson D, Huang SC and Carethers JM (2008) Mutation rates of TGFBR2 and ACVR2 coding microsatellites in human cells with defective DNA mismatch repair. PLoS One 3:e3463. [DOI] [PMC free article] [PubMed]
- Deacu E, Mori Y, Sato F, Yin J, Olaru A, Sterian A, Xu Y, Wang S, Schulmann K, Berki A, et al. Activin Type II Receptor restoration in ACVR2-deficient colon cancer cells induces Transforming Growth Factor-β response pathway genes. Cancer Res. 2004;64:7690–7696. doi: 10.1158/0008-5472.CAN-04-2082. [DOI] [PubMed] [Google Scholar]; Deacu E, Mori Y, Sato F, Yin J, Olaru A, Sterian A, Xu Y, Wang S, Schulmann K, Berki A et al. (2004) Activin Type II Receptor restoration in ACVR2-deficient colon cancer cells induces Transforming Growth Factor-β response pathway genes. Cancer Res 64:7690-7696. [DOI] [PubMed]
- Dean M, Davis DA, Burdette JE. Activin A stimulates migration of the fallopian tube epithelium, an origin of high-grade serous ovarian cancer, through non-canonical signaling. Cancer Lett. 2017;391:114–124. doi: 10.1016/j.canlet.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dean M, Davis DA and Burdette JE (2017) Activin A stimulates migration of the fallopian tube epithelium, an origin of high-grade serous ovarian cancer, through non-canonical signaling. Cancer Lett 391:114-124. [DOI] [PMC free article] [PubMed]
- Fitzpatrick E, Johnson MP, Dyer TD, Forrest S, Elliott K, Blangero J, Brennecke SP, Moses EK. Genetic association of the activin A receptor gene (ACVR2A) and pre-eclampsia. Mol Hum Reprod. 2009;15:195–204. doi: 10.1093/molehr/gap001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fitzpatrick E, Johnson MP, Dyer TD, Forrest S, Elliott K, Blangero J, Brennecke SP and Moses EK (2009) Genetic association of the activin A receptor gene (ACVR2A) and pre-eclampsia. Mol Hum Reprod 15:195-204. [DOI] [PMC free article] [PubMed]
- Fuchs H, Theuser M, Wruck W, Adjaye J. miR-27 negatively regulates pluripotency-associated genes in human embryonal carcinoma cells. PLoS One. 2014;9:e111637. doi: 10.1371/journal.pone.0111637. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fuchs H, Theuser M, Wruck W and Adjaye J (2014) miR-27 negatively regulates pluripotency-associated genes in human embryonal carcinoma cells. PLoS One 9:e111637. [DOI] [PMC free article] [PubMed]
- Grcevic D, Kuec R, Kovacic N, Lukic A, Lukic IK, Ivcevic S, Nemet D, Seiwerth RS, Ostojic SK, Croucher PI, et al. Bone morphogenetic proteins and receptors are over-expressed in bone-marrow cells of multiple myeloma patients and support myeloma cells by inducing ID genes. Leuk Res. 2010;34:742–751. doi: 10.1016/j.leukres.2009.10.016. [DOI] [PubMed] [Google Scholar]; Grcevic D, Kuec R, Kovacic N, Lukic A, Lukic IK, Ivcevic S, Nemet D, Seiwerth RS, Ostojic SK, Croucher PI et al. (2010) Bone morphogenetic proteins and receptors are over-expressed in bone-marrow cells of multiple myeloma patients and support myeloma cells by inducing ID genes. Leuk Res 34:742-751. [DOI] [PubMed]
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]; Haggar FA and Boushey RP (2009) Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 22:191-197. [DOI] [PMC free article] [PubMed]
- Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]; Heldin CH, Vanlandewijck M and Moustakas A (2012) Regulation of EMT by TGFβ in cancer. FEBS Lett 586:1959-1970. [DOI] [PubMed]
- Hempen P, Zhang L, Bansal RK, Iacobuzio-Donahue CA, Murphy KM, Maitra A, Vogelstein B, Whitehead RH, Markowitz SD, Willson JKV, et al. Evidence of selection for clones having genetic inactivation of the Activin A Type II Receptor (ACVR2) gene in gastrointestinal cancers. Cancer Res. 2003;63:994–999. [PubMed] [Google Scholar]; Hempen P, Zhang L, Bansal RK, Iacobuzio-Donahue CA, Murphy KM, Maitra A, Vogelstein B, Whitehead RH, Markowitz SD, Willson JKV et al. (2003) Evidence of selection for clones having genetic inactivation of the Activin A Type II Receptor (ACVR2) gene in gastrointestinal cancers. Cancer Res 63:994-999. [PubMed]
- Jung B, Doctolero RT, Tajima A, Nguyen AK, Keku T, Sandler RS, Carethers JM. Loss of Activin Receptor Type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology. 2004;126:654–659. doi: 10.1053/j.gastro.2004.01.008. [DOI] [PubMed] [Google Scholar]; Jung B, Doctolero RT, Tajima A, Nguyen AK, Keku T, Sandler RS and Carethers JM (2004) Loss of Activin Receptor Type 2 protein expression in microsatellite unstable colon cancers. Gastroenterology 126:654-659. [DOI] [PubMed]
- Jung B, Smith EJ, Doctolero RT, Gervaz P, Alonso JC, Miyai K, Keku T, Sandler RS, Carethers JM. Influence of target gene mutations on survival, stage and histology in sporadic microsatellite unstable colon cancers. Int J Cancer. 2006;118:2509–2513. doi: 10.1002/ijc.21710. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jung B, Smith EJ, Doctolero RT, Gervaz P, Alonso JC, Miyai K, Keku T, Sandler RS and Carethers JM (2006) Influence of target gene mutations on survival, stage and histology in sporadic microsatellite unstable colon cancers. Int J Cancer 118:2509-2513. [DOI] [PMC free article] [PubMed]
- Jung B, Gomez J, Chau E, Cabral J, Lee JK, Anselm A, Slowik P, Ream-Robinson D, Messer K, Sporn J, et al. Activin signaling in microsatellite stable colon cancers is disrupted by a combination of genetic and epigenetic mechanisms. PLoS One. 2009;4:e8308. doi: 10.1371/journal.pone.0008308. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jung B, Gomez J, Chau E, Cabral J, Lee JK, Anselm A, Slowik P, Ream-Robinson D, Messer K, Sporn J et al. (2009) Activin signaling in microsatellite stable colon cancers is disrupted by a combination of genetic and epigenetic mechanisms. PLoS One 4:e8308. [DOI] [PMC free article] [PubMed]
- Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I, Waage A, Sundan A, Holien T. Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal. 2015;13:1–7. doi: 10.1186/s12964-015-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I, Waage A, Sundan A and Holien T (2015) Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal 13:1-7. [DOI] [PMC free article] [PubMed]
- Padua D, Massagué J. Roles of TGFβ in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]; Padua D and Massagué J (2009) Roles of TGFβ in metastasis. Cell Res 19:89-102. [DOI] [PubMed]
- Pinheiro M, Pinto C, Peixoto A, Veiga I, Lopes P, Henrique R, Baldaia H, Carneiro F, Seruca R, Tomlinson I, et al. Target gene mutational pattern in Lynch syndrome colorectal carcinomas according to tumour location and germline mutation. Br J Cancer. 2015;113:686–692. doi: 10.1038/bjc.2015.281. [DOI] [PMC free article] [PubMed] [Google Scholar]; Pinheiro M, Pinto C, Peixoto A, Veiga I, Lopes P, Henrique R, Baldaia H, Carneiro F, Seruca R, Tomlinson I et al. (2015) Target gene mutational pattern in Lynch syndrome colorectal carcinomas according to tumour location and germline mutation. Br J Cancer 113:686-692. [DOI] [PMC free article] [PubMed]
- Plank MW, Maltby S, Tay HL, Stewart J, Eyers F, Hansbro PM, Foster PS. MicroRNA expression is altered in an ovalbumin-induced asthma model and targeting miR-155 with antagomirs reveals cellular specificity. PLoS One. 2015;10:e0144810. doi: 10.1371/journal.pone.0144810. [DOI] [PMC free article] [PubMed] [Google Scholar]; Plank MW, Maltby S, Tay HL, Stewart J, Eyers F, Hansbro PM and Foster PS (2015) MicroRNA expression is altered in an ovalbumin-induced asthma model and targeting miR-155 with antagomirs reveals cellular specificity. PLoS One 10:e0144810. [DOI] [PMC free article] [PubMed]
- Rossi MR, Ionov Y, Bakin AV, Cowell JK. Truncating mutations in the ACVR2 gene attenuates activin signaling in prostate cancer cells. Cancer Genet Cytogenet. 2005;163:123–129. doi: 10.1016/j.cancergencyto.2005.05.007. [DOI] [PubMed] [Google Scholar]; Rossi MR, Ionov Y, Bakin AV and Cowell JK (2005) Truncating mutations in the ACVR2 gene attenuates activin signaling in prostate cancer cells. Cancer Genet Cytogenet 163:123-129. [DOI] [PubMed]
- Van Dijk M, Oudejans CBM. STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J Pregnancy. 2011;2011:521826. doi: 10.1155/2011/521826. [DOI] [PMC free article] [PubMed] [Google Scholar]; Van Dijk M and Oudejans CBM (2011) STOX1: Key player in trophoblast dysfunction underlying early onset preeclampsia with growth retardation. J Pregnancy 2011:521826. [DOI] [PMC free article] [PubMed]
- Wang HJ, Wang BZ, Zhang PJ, Deng J, Zhao ZR, Zhang X, Xiao K, Feng D, Jia YH, Liu YN, et al. Identification of four novel serum protein biomarkers in sepsis patients encoded by target genes of sepsis-related miRNAs. Clin Sci. 2014;126:857–867. doi: 10.1042/CS20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang HJ, Wang BZ, Zhang PJ, Deng J, Zhao ZR, Zhang X, Xiao K, Feng D, Jia YH, Liu YN et al. (2014) Identification of four novel serum protein biomarkers in sepsis patients encoded by target genes of sepsis-related miRNAs. Clin Sci 126:857-867. [DOI] [PMC free article] [PubMed]
- Yang L, Pang Y, Moses HL. TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yang L, Pang Y and Moses HL (2010) TGF-β and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol 31:220-227. [DOI] [PMC free article] [PubMed]
Internet Resources
- AmiGo [February 20, 2018]. http://amigo.geneontology.org/amigo/landing.; AmiGo, http://amigo.geneontology.org/amigo/landing (February 20, 2018)
- Genecards Human Gene Database [October 25, 2017]. http://genecards.org/cgibin/carddisp.pl?gene=ACVR2A#aliases_descriptions.; Genecards Human Gene Database, http://genecards.org/cgibin/carddisp.pl?gene=ACVR2A#aliases_descriptions (October 25, 2017)
- PathCards Pathway Unification Database http://pathcards.genecards.org/ February 20, 2018.; PathCards Pathway Unification Database, http://pathcards.genecards.org/ (February 20, 2018)