Abstract
The Albert Einstein College of Medicine of Yeshiva University in New York, Rwanda Military Hospital and the University of Rwanda have established a research partnership, which has received funding from the US National Institutes of Health for investigation of HPV carcinogenesis, diagnosis and treatment. This includes two major studies, on cervical cancer screening and on HPV in men who have sex with men. The partnership aims to build research and laboratory capacity within Rwanda, including establishing a centre of excellence in HPV-related research. It has already led to development of further south-to-south collaborations. This paper presents preliminary results from a cervical cancer screening study of women living with HIV in Rwanda.
Background
The Albert Einstein College of Medicine of Yeshiva University in New York, Rwanda Military Hospital and the University of Rwanda have established a research partnership, which has received funding from the US National Institutes of Health for investigation of HPV carcinogenesis, diagnosis and treatment. This includes two major studies, on cervical cancer screening and on HPV in men who have sex with men. The partnership aims to build research and laboratory capacity within Rwanda, including establishing a centre of excellence in HPV-related research. It has already led to development of further south-to-south collaborations.
Study description
This presentation outlined preliminary findings of research aimed at comparing the clinical performance of different screening methods and biomarkers for triage of screen-positive women living with HIV infection in Rwanda. The full protocol [1] envisages recruiting a convenience sample of >5000 consenting women, aged 30–54 years and living with HIV, to complete an administered short risk-factor questionnaire and be screened for high-risk human papillomavirus (hrHPV) using the Xpert® HPV assay (Cepheid, Sunnyvale, CA, USA), unaided visual inspection after acetic acid (VIA) and aided VIA using the Enhanced Visual Assessment (EVA) system (Mobile ODT, Tel Aviv, Israel). Reasons for selecting the Xpert® HPV assay for evaluation include its fast (1 hour) turnaround, ease of use, scalability, capacity for cloud-based monitoring and already high penetrance in lower- and middle-income countries.
Women found to be screen-positive for hrHPV and/or unaided VIA undergo colposcopy, including the collection of two cervical specimens prior to a four-quadrant microbiopsy protocol. The colposcopy-collected specimens are tested by dual immunocytochemical staining for p16INK4a and Ki-67 (CINtec® PLUS Cytology, Ventana, Tucson, AZ, USA) and for E6 or E7 oncoprotein for eight hrHPV genotypes (HPV16, 18, 31, 33, 35, 45, 52 and 58) using the next-generation AV Avantage™ hrHPV E6/E7 test (Arbor Vita Corporation, Freemont, CA, USA). Women with a local pathology diagnosis of cervical intraepithelial neoplasia grade 2 (CIN2) or more severe (CIN2+) or pathology review diagnosis of CIN grade three or more severe (CIN3+) will receive treatment. In the full study, the clinical performance and cost-effectiveness (e.g. sensitivity, specificity and predictive values) of different screening strategies and algorithms will be evaluated.
Preliminary results and conclusions
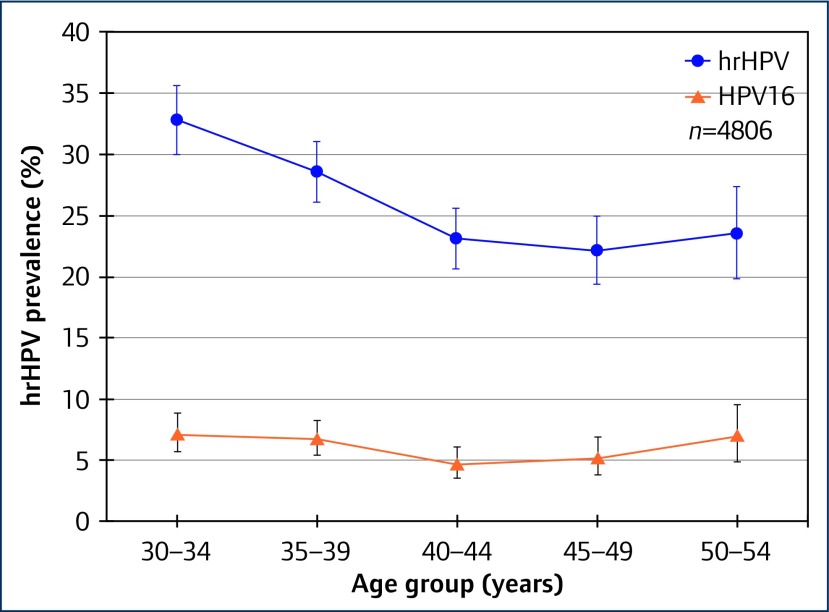
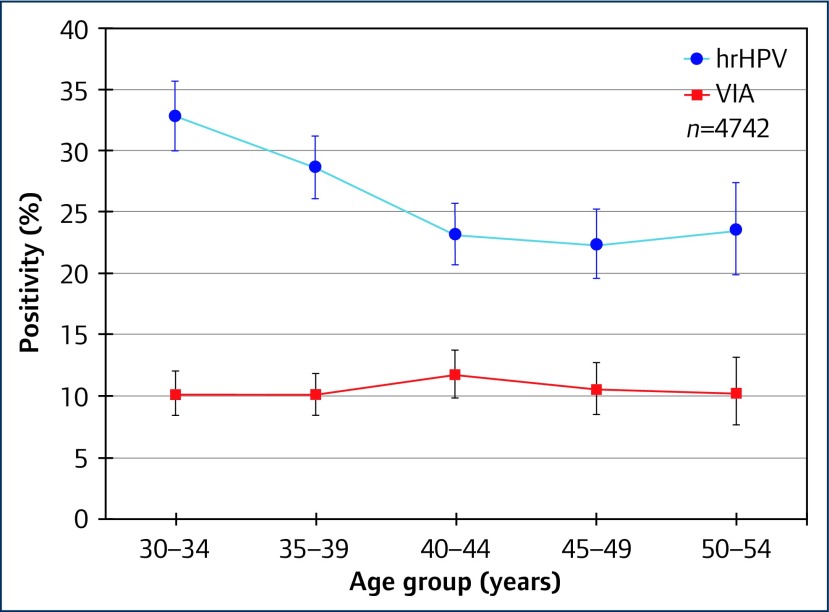
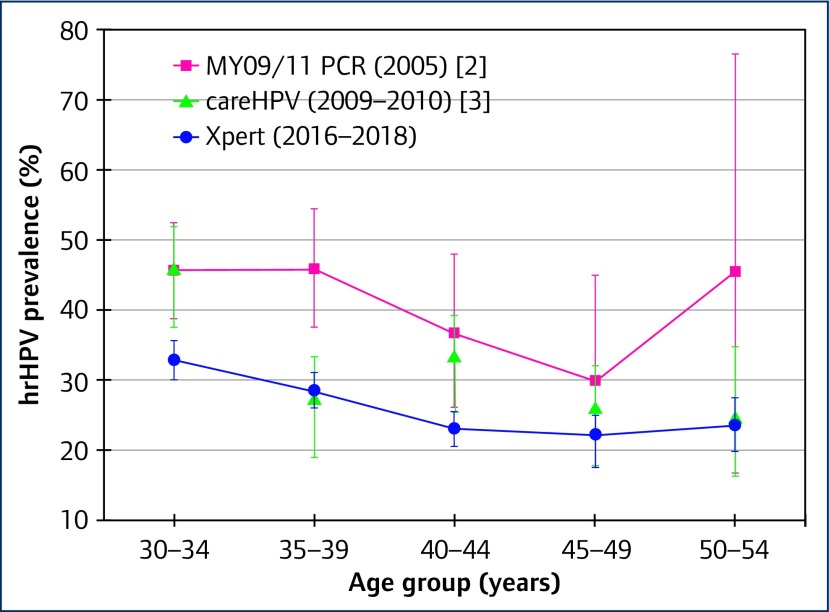
Among 4806 women living with HIV, we found an overall prevalence of hrHPV of 26.5%, with positivity for different HPV types across the five Xpert® assay channels as shown in Table 1. While overall prevalence of hrHPV declined with age (Ptrend<0.0001), this was not found for HPV16, nor for VIA positivity, as illustrated in Figures 1 and 2. Compared with earlier studies that enrolled women in 2005 [2] and 2010 [3], we found lower age-specific prevalence of hrHPV, as shown in Figure 3. This may perhaps be due to improvements in HIV management and care.
Table 1.
Prevalence of hrHPV among women aged 30–54 and living with HIV in Rwanda
| Prevalence, number (%) | |
|---|---|
| Total screened using Xpert® assay | 4806 (100) |
| hrHPV+ overall | 1273 (26.5) |
| Xpert® assay channels: | |
| HPV16+ | 292 (6.1) |
| HPV18/45+ | 262 (5.5) |
| HPV31/33/35/52/58+ | 675 (14.0) |
| HPV51/59+ | 174 (3.6) |
| HPV39/56/66/68+ | 293 (6.1) |
| Positive on one channel only | 950 (19.8) |
| Positive on two or more channels: | 323 (6.7) |
| Two channels | 240 (5.0) |
| Three channels | 66 (1.4) |
| Four channels | 17 (0.4) |
Figure 1.
Prevalence of hrHPV and HPV16 by age group among women living with HIV in Rwanda
Figure 2.
Prevalence of hrHPV and VIA positivity by age group among women living with HIV in Rwanda
Figure 3.
Prevalence of hrHPV among women living with HIV in Rwanda: comparison of studies across time
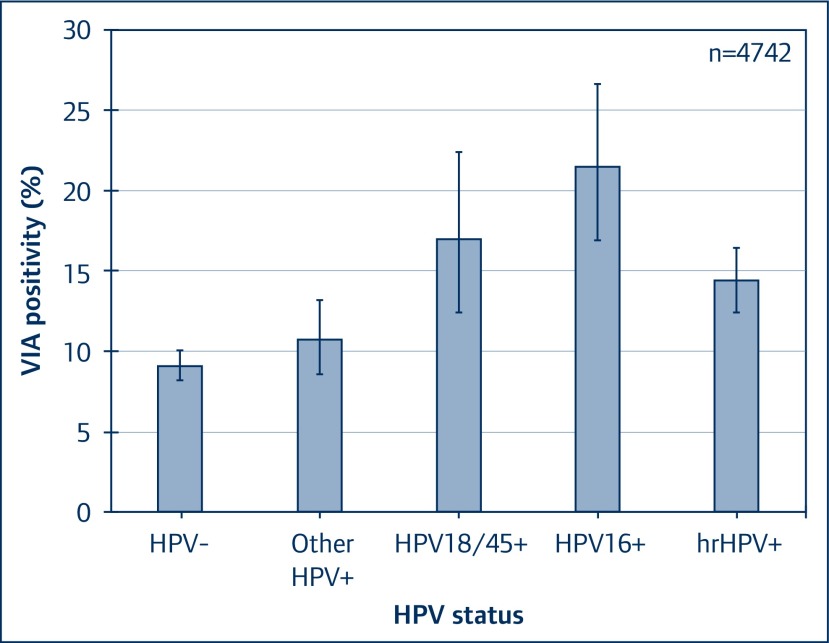
As expected, highest rates of VIA positivity were found in women testing positive for HPV16 infection, followed by HPV18/45, as illustrated in Figure 4.
Figure 4.
VIA positivity by hrHPV status among women living with HIV in Rwanda
The study is continuing and results will be compared with the clinical endpoint (CIN2+) when all pathology results become available.
Acknowledgements
This research is funded by NCI/NIH grant U54 CA 190163.
Rwanda Military Hospital, Kigali, Rwanda: JD Sinayobye, P Mugenzi, T Rurangwa, A Murangwa, TM Zawadi, A Munyaneza, JC Dusingize;
University of Rwanda: L Mutesa;
Albert Einstein College of Medicine, New York, NY, USA: T Hebert, A Adedimegi, K Anastos, PE Castle
The investigators especially thank the grant administration, the data manager, laboratory technicians, research nurses and all support staff.
Cepheid does not endorse the testing of alternate specimen types (specimen types that are not cleared/approved/registered by any regulatory body, per the package insert). If you choose to use the assay with alternate testing types, it is your laboratory’s responsibility to validate the assay for each alternate specimen type in accordance with federal, state, and local laws.
References
- 1. Murenzi G, Dusingize JC, Rurangwa T et al. Protocol for the study of cervical cancer screening technologies in HIV-infected women living in Rwanda. BMJ Open 2018; 8: e020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh DK, Anastos K, Hoover DR et al. Human papillomavirus infection and cervical cytology in HIV-infected and HIV-uninfected Rwandan women. J Infect Dis 2009; 199: 1851– 1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sinayobye Jd A, Sklar M, Hoover DR et al. Prevalence and risk factors for high-risk human papillomavirus (hrHPV) infection among HIV-infected and uninfected Rwandan women: implications for hrHPV-based screening in Rwanda. Infect Agent Cancer 2014; 9: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]