Abstract
Background
Preclinical studies in rodents have demonstrated inhibitory effects of glucagon-like peptide-1 (GLP-1) receptor stimulation on alcohol consumption. The effects of GLP-1 receptor stimulation on alcohol intake in primates have not been investigated.
Methods
We performed placebo-controlled studies on the effects of the GLP-1 receptor agonists exenatide and liraglutide on alcohol consumption in alcohol-preferring male African vervet monkeys. Monkeys selected for voluntary alcohol drinking were observed for at least ten days of baseline drinking and allocated to drug or vehicle (n=11–12 per group) balanced with respect to alcohol intake. Monkeys had access to alcohol 4h/day. In a first study, monkeys were treated with exenatide 0.04 mg/kg or vehicle once weekly for five weeks to obtain steady state plasma levels. In a second study, monkeys were treated daily with liraglutide (increasing dosing, 10 to 50 μg/kg/day) or vehicle over two weeks. In both studies, access to alcohol was suspended during drug up-titration. Then, alcohol was again made available 4h/day and treatment was continued for two weeks, during which alcohol intake was recorded. Observation of alcohol intake was continued for a week of drug washout.
Results
Liraglutide and to a lesser extent exenatide significantly reduced alcohol consumption without causing any signs of emesis and with no effect on water intake as compared to vehicle.
Conclusions
The present study demonstrates for the first time that GLP-1 receptor agonists can reduce voluntary alcohol drinking in non-human primates. The data substantiate the potential usefulness of GLP-1 receptor agonists in the treatment of alcohol use disorder.
Keywords: alcohol use disorder, non-human primate, pharmacotherapy, exendin-4, liraglutide, GLP-1
INTRODUCTION
Alcohol use disorder (AUD) is a major public health problem in large parts of the world including Europe and the United States, imposing a high cost on society and estimated to cause 6% of all deaths (WHO 2014; Laramee et al. 2013; CDC 2016). AUD is under-diagnosed and undertreated, and more than 2/3 of patients in abstinence-oriented treatment will relapse within the first year of achieving abstinence (Connor et al. 2016). Currently available pharmacotherapies such as disulfiram, naltrexone, acamprosate, nalmefene (in Europe) have low to moderate efficacy (Akbar et al. 2017; Del Re et al. 2013; Naglich et al. 2017; Yardley and Ray 2017). Thus, there remains a strong need for new molecular targets in the medical treatment of AUD.
Glucagon-like peptide-1 (GLP-1)-based therapy has been used in the treatment of type 2 diabetes since 2006. GLP-1 is an incretin hormone secreted from endocrine L-cells of the small intestine. GLP-1 has insulinotropic effects and inhibits glucagon release, which together lower blood glucose levels (Holst 2007). GLP-1 is also produced as a neurotransmitter in the brain and acts centrally to regulate nutrient intake, and GLP-1 receptors are expressed in brain areas involved with reward and addiction, such as the ventral tegmental area and nucleus accumbens in rodents, humans, and non-human primates (Alhadeff et al. 2012; Cork et al. 2015; Göke et al. 1995; Heppner et al. 2015; Merchenthaler et al. 1999). The GLP-1 system has therefore attracted interest as a potential target for treating addictions, and GLP-1 receptor agonists have shown promise in reducing alcohol intake in rodents (for review see Jerlhag 2018). Human gene variants of the GLP-1 receptor also show an association with the prevalence of AUD and with behavioral and neurological responses to alcohol in human laboratory studies (Suchankova et al. 2015).
Endogenous GLP-1 is degraded within minutes, but GLP-1 analogues with significantly longer half-lives have been developed as diabetes medications. Here, we tested two different clinically used peptide compounds: exenatide, in an extended-release formulation, and liraglutide. Pharmacokinetic properties of the two drugs in humans are summarized in Table 1. Evidence from rodent studies indicates that both exenatide and liraglutide penetrate the brain after systemic administration (Hunter and Holscher 2012; Kastin and Akerstrom 2003; Kim et al. 2010; Secher et al. 2014). In humans, exenatide (as the slow-release formulation Bydureon) requires several weeks to reach steady blood levels, and liraglutide requires careful up-titration to reduce side effects such as nausea and vomiting.
Table 1.
Comparison of the two tested treatments, human data (http://pro.medicin.dk/Medicin/Praeparater/4752 ; Jacobsen et al. 2016; Syed and McCormack 2015).
| Liraglutide (Victoza®) | Exenatide (Bydureon®) | |
|---|---|---|
| Dosing | 1.8 mg s.c. once daily | 2.0 mg s.c. once weekly |
| Tmax | 8–14 hours | Gradual increase in plasma levels over 6–7 weeks |
| Duration of action | 24 hours | Can be measured in plasma around 10 weeks after the last dose |
| T1/2 | 13 hours | See above |
| Bioavailability | 55% | 25% |
With the exception of an early observation of decreased alcohol drinking in diabetic patients treated with liraglutide (Kalra 2011), reports on the effects of GLP-1-based treatments on alcohol intake have so far been restricted to rodents, and most have focused on acute effects. A recent review of preclinical studies on the therapeutic effects of GLP-1 analogs in diabetes, which have been much more investigated than addiction-related effect, concluded that insights gained from rodent studies “need to be extended and validated in larger animal models, such as pigs and non-human primates (NHPs), before moving into clinical trials” (Renner et al. 2016). While the few reports examining GLP-1 receptor distribution in human and non-human primate brain indicate good concordance with rodent studies, some differences in expression patterns have been reported, including in areas relevant to reward and addiction (see Heppner et al. 2015; ten Kulve et al. 2015). Here, we tested the hypothesis that chronic administration of GLP-1 receptor agonists would decrease alcohol intake in alcohol-preferring vervet monkeys. Similar to humans, the feral Caribbean vervet monkey population comprises alcohol avoiders, occasional/moderate alcohol drinkers, and relatively heavy drinkers (about 17% of the population) that will consume alcohol to intoxication if available, for instance stolen from or offered by tourists (Ervin et al. 1990; Juarez et al. 1993). This offers a useful preclinical model of alcohol drinking that may more closely reflect some aspects of heavy or harmful drinking in humans, relative to typical rodent assays, as well as providing a more relevant pharmacokinetic profile for the tested drugs (Fiorentino et al. 2015; Gotfredsen et al. 2014).
MATERIALS AND METHODS
Subjects and housing
Thirty-two drug- and experimentally naïve young adult male (4.6–5.8 kg) vervet monkeys (Cercopithecus aethiops) were used (24 for the first study, supplemented with 8 for the second study). Subjects were screened for alcohol intake but did not participate in any studies prior to those reported here. The subjects were well-acclimated feral monkeys derived from an abundant, non-endangered local population (St Kitts and Nevis, the Caribbean) or colony-born at the Behavioural Science Foundation (BSF), Saint Kitts, and were socially housed in large outdoors wire cages as previously described (Palmour et al. 1998). To enable individual recording of drinking behaviors, subjects were singly housed for the duration of the experiments, in proximity to the group cages. Monkeys were maintained on chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI) and fresh local produce; water was available ad libitum throughout the experiment, which took place in July-September (exenatide; typical ambient temperature 27–29°C) and in January-March (liraglutide; 22–23°C). All experiments were conducted at the BSF. The project was reviewed and approved by the BSF Institutional Animal Care and Use Committee (BSF IACUC), operating under the auspices of the Canadian Council on Animal Care (Canadian Council on Animal Care Good Animal Practice registration A5028) and all of the procedures used in the study were covered by standard operating procedures approved by the BSF IACUC. The approval numbers are BSF 1505 for the exenatide study and BSF 1701 for the liraglutide study.
Screening for spontaneous alcohol preference
The feral population of monkeys were given a period of 6 weeks after entering the colony to adapt, after which they would be screened for alcohol preference. This was done by taking a group of 20–24, weighing each one, and housing them individually to be able to accurately measure alcohol and water consumption. Each animal was given one bottle containing water and one bottle containing an alcohol solution of 10% w/v. Monkeys were first allowed 24h/day access to alcohol for 5 days, then, access was reduced to 4h/day. Throughout this period, there was no food restriction and no behavioral stress except that of being singly caged. The restricted access allows an estimate of the extent to which the animal prefers alcohol to water and in both conditions, the gram alcohol/kg/day consumed indicating the maximum quantity an animal will consume when access is restricted (4h) or unrestricted (24h). This procedure was repeated at least twice.
For colony-bred monkeys, alcohol screening started at about three years of age and was repeated about every eight months for at least three times. Thereafter, only males continued to be tested, and the ones that were selected for the second study (liraglutide) were all tested four or five times before being selected for the study.
To select the 24 animals that participated in the first study (exenatide), 118 animals were initially assessed, and those that drank less than 1 g/kg of alcohol in the 4h period were excluded. The remaining 79 animals were tested a second time, and the most alcohol-consuming of those animals were tested further to ensure intake was maintained, and the 24 monkeys showing the highest alcohol intake were selected for study.
Because alcohol intake was not maintained at as high levels as anticipated in the exenatide study, for the subsequent liraglutide study, additional monkeys were screened and eight monkeys were replaced with new subjects (three in the liraglutide group and five in the vehicle group). The remaining monkeys used in the liraglutide study had previously been tested with exenatide (five in the liraglutide group and four in the vehicle group) or placebo (four in the liraglutide group, three in the vehicle group). Both the replacement of some of the more moderate alcohol consumers and escalation in alcohol intake over time, as previously observed (Ervin et al. 1990), resulted in higher baseline alcohol intake in the liraglutide study (Fig. S1 online).
Exenatide pilot study
Initially, a tolerability pilot experiment was performed in three monkeys each weighing around 5 kilograms. The monkeys were placed in single cages for seven weeks. The monkeys were dosed weekly with exenatide s.c. 0.08 mg/kg for 7 weeks. Blood was sampled just before each injection in order to estimate plasma levels, and possible production of antibodies against exenatide (Fineman et al. 2012). To this end, the monkeys were cage squeezed and anesthetized with ketamine s.c. and 2 ml of whole blood were drawn from the femoral vein. The animals were observed for possible side effects with special focus on vomiting and reduced food intake as well as irritation of the skin at the injection sites. The monkeys used in this pilot study were not screened for alcohol preference and were not used in the main studies.
Exenatide main study
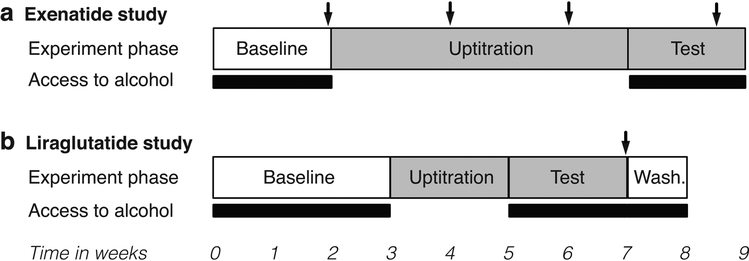
Monkeys had access to 10% (w/v) alcohol solution and water (two bottle-choice) 4h/day from 09:00 to 13:00, 5 days/week (Monday – Friday). Baseline drinking behavior was recorded for two weeks, at the end of which animals were assigned to drug or vehicle group balanced for alcohol intake. Then, access to alcohol was suspended for five weeks and exenatide or vehicle treatment was initiated (see Fig. 1). Exenatide was administered subcutaneously following cage squeezing once weekly at a dose of 0.04 mg/kg for seven weeks, five weeks of up-titration during which alcohol access was withheld, then after re-introduction of alcohol. Volumes of water and ethanol solution consumed were recorded by observers blinded to treatment condition.
Fig. 1. Diagram of the experimental designs.
Schematic representation of the experimental phases for exenatide (a) and liraglutide (b). For experiment phase, open boxes represent no treatment, grey boxes represent weekly (exenatide) or daily (liraglutide) administration of drug or vehicle. For access to alcohol, black bars represent daily 4h access, open bars represent no access. Arrows represent blood sampling.
Because exenatide can induce antibody formation in humans (Fineman et al. 2012), exenatide blood levels and exenatide-specific antibodies were monitored from blood samples collected before treatment start and after two and four weeks of treatment, and at study completion. At the last blood sampling, blood for measurement of blood alcohol concentrations was also collected.
Liraglutide pilot study
In a pilot study, three monkeys, weighing around 5 kilograms each, were cage squeezed daily and dosed with liraglutide s.c., 10 μg/kg for three days, 24 μg/kg for three days, 48 μg/kg for three days, and 72 μg/kg for three days. The animals were observed for possible side effects with special focus on vomiting, reduced food intake, or skin irritation at the injection sites. The initial dosing levels were selected based on previous studies with liraglutide in macaque monkeys (personal communication, Dr. Lotte Bjerre Knudsen, Novo Nordisk A/S). These monkeys were not screened for alcohol preference and were not used in the main experiments.
Liraglutide main study
Monkeys had access to alcohol from 09:00 to 13:00, 6 days/week (Monday – Saturday). Baseline drinking behavior was recorded for three weeks, at the end of which animals were assigned to liraglutide or vehicle group, balanced for alcohol intake. Then, access to alcohol was suspended and the monkeys were up-titrated over two weeks from an initial dose of 10 μg/kg/day to a target dose of 50 μg/kg/day (selected based on the pilot study), with a four days dose escalation interval (see Fig. 1). The up-titration was done individually dependent on the observation of side effects. Then, liraglutide treatment was continued for two weeks after the reintroduction of alcohol access. Finally, treatment was stopped and alcohol drinking was observed for one more week (washout period). In contrast to exenatide, the levels of anti-liraglutide antibodies in liraglutide-treated humans are low and do not impact safety or efficacy (Buse et al. 2011). Therefore, anti-liraglutide antibodies were not measured.
Blood Alcohol Analysis
Alcohol levels were determined at the Laboratory of Professor Kristian Linnet, Department of Forensic Medicines, University of Copenhagen, Denmark, using gas chromatography with a detection limit of 0.01 percent by volume (exenatide study), and at Laboratory of Neuropsychiatry using a commercial alcohol oxidase-based assay calibrated using 0.5 mg/ml alcohol standards (Analox Instruments Ltd, The Vale, London; liraglutide study, and repeat measure of the exenatide study).
Analysis of exenatide and anti-exenatide antibodies
Plasma levels of exenatide and production of anti-exenatide antibodies were determined at the Department of Biomedical Sciences, University of Copenhagen. The sensitivity of the exenatide assay was <1 pmol/L. Samples were diluted 10-fold in assay buffer. For estimation of antibodies against exenatide, plasma samples were incubated with 125-I-labelled exendin 9–39, which binds to the antiserum as full-length exenatide, and tracer antibody complexes separated from the mixture using plasma-coated charcoal as in the exenatide radioimmunoassay. Increases in tracer binding, above that observed in plasma from subjects never exposed to exenatide, is indicative of the presence of antibodies.
Drugs
Exenatide and liraglutide were purchased from the Rigshospitalet hospital pharmacy. Exenatide was purchased as the slow-release microsphere formulation Bydureon® weekly injection pens (AstraZeneca); the Bydureon vehicle solution was used as control. Liraglutide was purchased as Victoza® daily injection pens (Novo Nordisk); physiological saline was used as vehicle control. Alcohol was local rum purchased from St. Lucia (Denrose Strong Rum, 80% alcohol v/v) at St. Kitts and diluted to 20% in water, yielding an ethanol concentration of 10.15% (w/v).
Statistical analyses
Because baseline alcohol intake differed between studies, data were analyzed separately for each study. Alcohol intake and water intake were analyzed by mixed-model ANOVA with treatment group as a between-subjects factor and day as a repeated-measures factor. Intakes were analyzed separately for baseline, treatment, and washout periods. For exenatide, treatment was analyzed separately for the two treatment weeks due to the difference in blood levels before and after testing. Significant interactions were followed by multiple comparisons of treatment on each day (adjusted for false discovery rate). Blood alcohol levels were compared by two-tailed unpaired-sample t-test (drug vs. vehicle). All data are presented as means ± standard error of the mean (s.e.m.). Effects showing p<0.05 and which survived correction for false discovery rate (Benjamini-Hochberg procedure, false discovery rate limit set at 5%) were considered significant. Analyses were performed using Stata/SE for Mac. Post-hoc power analyses performed using G*power 3 (Faul et al. 2007) yielded a power of >0.95 for both treatments.
RESULTS
Exenatide pilot study
Plasma levels of exenatide were measured at high concentrations with peak levels at week five (4.08, 5.13, and 2.25 nM, see Table S1). Anti-exenatide antibodies were detected in two out of three monkeys. The antibody titer was low in monkey O8756 but in O8684 titer increased to high levels in the last two weeks. No emetic events, irritation of the skin at the injection sites, or other adverse effects were observed. No change in water or food intake was observed. Consequently, even though plasma levels of exenatide in the monkeys were substantially higher than in humans, we decided to use the same dose in the main exenatide experiment, i.e., 0.08 mg/kg s.c. once weekly.
Exenatide main study
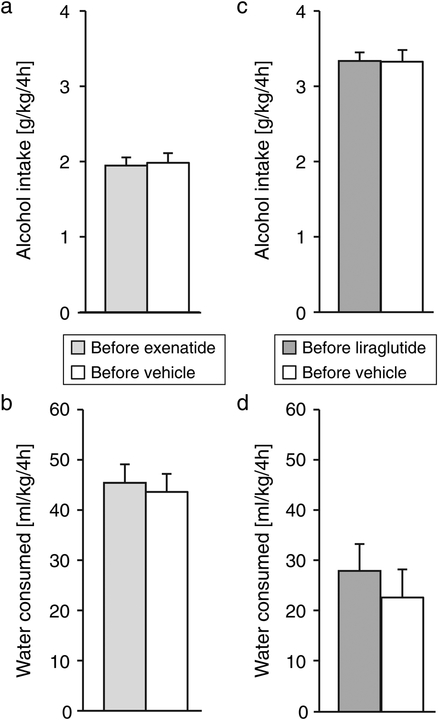
Alcohol intake and water intake did not differ between the vehicle and treatment group during the baseline period (p > 0.7; Fig. 2a). Both alcohol and water intake varied as a function of observation day ([F(9,189) = 23.6, p < 0.0001] and [F(9,189) = 5.71, p < 0.0001] respectively) but with no significant group by day interaction (Fig. S2a,b online).
Fig. 2. Intake of alcohol and water at baseline.
Daily alcohol and water intake averaged over the baseline assessment period for the exenatide study (a,b) and the liraglutide study (c,d). Ordinates: alcohol intake in g/kg/4h (top), water intake in ml/kg/4h (bottom). Data are group means, bars represent s.e.m. n=12, except for the exenatide group, n=11.
Exenatide plasma levels increased through the treatment period, with negligible plasma levels at week 2, rising to blood levels in the high therapeutic range in humans by week 4 (Fig. S3 online). One monkey was excluded from the study due to high antibody levels against exenatide.
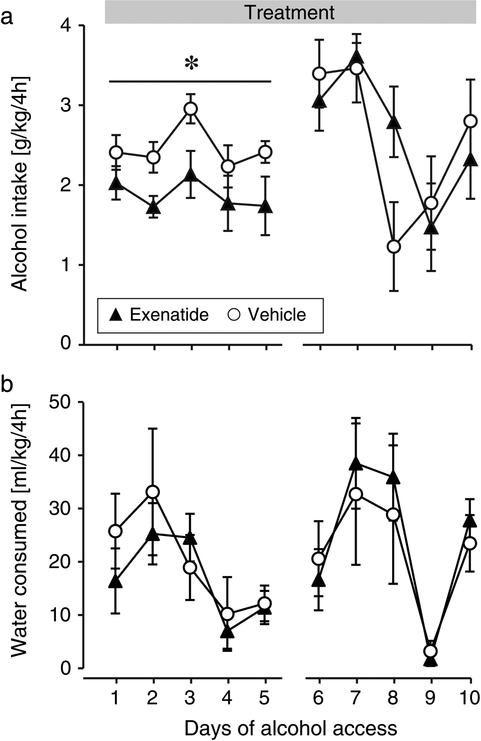
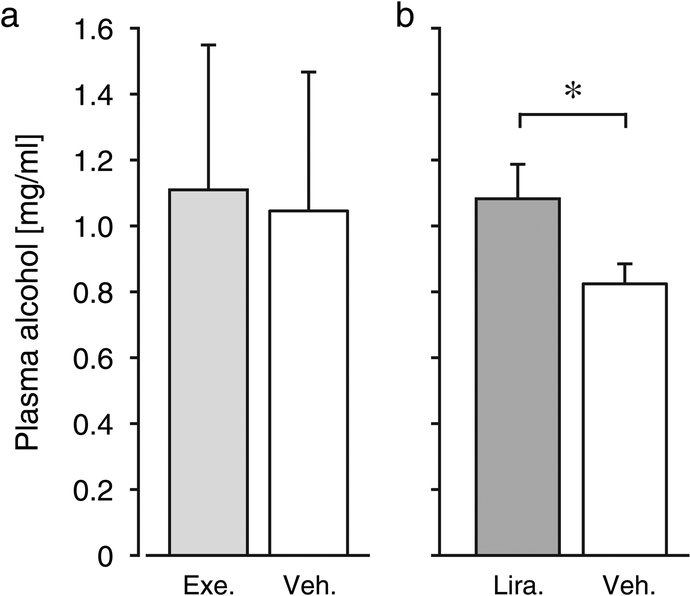
When alcohol was again made available, exenatide-treated animals drank significantly less alcohol relative to vehicle during the first week [F(1,21) = 6.80, p = 0.02], with no effect of day or treatment by day interaction (Fig. 3a). Water intake during the 4h daily observation periods was related to day only [F(4,84) = 5.87, p = 0.0003], with no effect of treatment or interaction (Fig. 3b). During the second week, there was no significant effect of treatment on alcohol intake or on water intake. Both alcohol and water intake varied by day during the second week ([F(4,84) = 6.83, p = 0.0001], [F(4,84) = 11.1, p < 0.0001]), with no significant treatment by day interaction. Alcohol intake appeared to vary more during this period, also in the vehicle group, relative to baseline or the first treatment period. Alcohol plasma concentrations in blood collected just after a drinking session (Wednesday of the second treatment week) did not differ between groups (Fig. 4a). Average alcohol intake did not correlate significantly with blood exenatide levels (data not shown). No emetic events, reduction in food intake, or irritation of the skin at the injection sites were observed at any time during the seven weeks of exenatide treatment.
Fig. 3. Effect of exenatide on intake of alcohol and water.
Daily alcohol (a) and water (b) intake during the exenatide treatment. Abscissa: days of alcohol access after initiation of the GLP-1 receptor agonist treatment. Ordinates and group sizes as in Fig. 2. *p < 0.05 exenatide vs. vehicle ANOVA main effect.
Fig. 4. Effect of exenatide and liraglutide on plasma alcohol levels.
Plasma alcohol levels from blood taken immediately after a 4h drinking session, taken the last liraglutide/vehicle treatment day, and Wednesday of the last exenatide/vehicle treatment week. *p < 0.05. Ordinates: plasma alcohol in mg/ml. Group sizes as in Fig. 2. Exe: exenatide, Veh: vehicle, Lira: liraglutide. *p < 0.05 liraglutide vs. vehicle.
Liraglutide pilot study
No emetic events or other signs of nausea or skin irritation were detected at any of the doses tested. Water and food intake were unchanged.
Liraglutide main study
Alcohol intake and water intake did not differ between the vehicle and treatment group during the baseline period (p > 0.5; Fig 2c,d). Both alcohol and water intake varied as a function of observation day ([F(11,242) = 4.79, p < 0.0001] and [F(11,242) = 7.36, p < 0.0001] respectively), with a significant group by day interaction for alcohol intake [F(11,242) = 3.93, p < 0.0001] and a trend for water intake (p = 0.06; Suppl. Fig 2c,d). However, there was no clear trend to this variation (Fig S2c online).
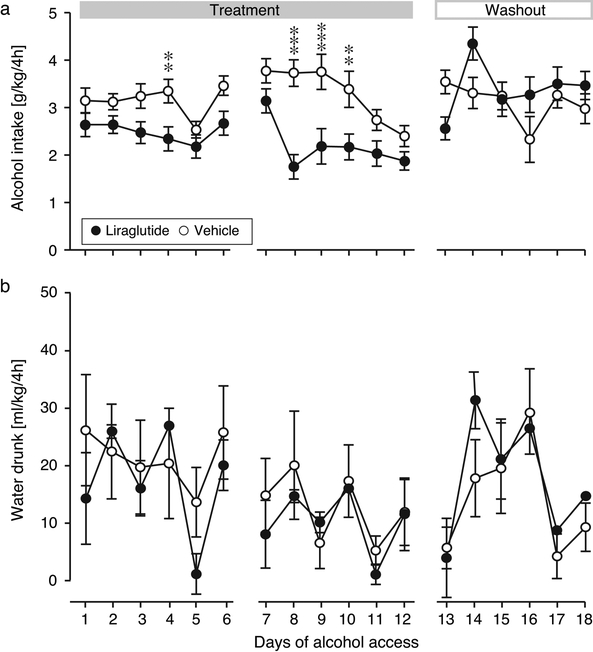
During the treatment period, liraglutide significantly decreased alcohol intake relative to the vehicle group [F(1,22) = 17.3, p = 0.0004] (Fig. 5a). Alcohol intake was also related to day [F(11,242) = 5.08, p < 0.0001], with a treatment by day interaction [F(11,242) = 2.44, p = 0.007]. Water intake was related to day only [F(11,242) = 2.80, p = 0.002], with no effect of treatment or interaction. Alcohol plasma concentrations in blood collected just after the last drinking session was significantly lower in the liraglutide group relative to vehicle (p < 0.05; Fig. 4b). During the washout period, the main effect of treatment on alcohol intake disappeared, but there was a significant effect of day [F(5,110) = 3.31, p = 0.008] and a treatment by day interaction [F(5,110) = 9.24, p < 0.0001]. The interaction reflects that alcohol intake remained reduced in the liraglutide group on the first day after the Sunday break (i.e., 2nd day drug-free), followed by an apparent rebound effect on the second day, after which intake returned to vehicle levels. No post-hoc comparisons during washout were significant after correcting for false discovery rate. Water intake remained related only to day [F(5,110) = 3.83, p = 0.003]. No emetic events, change in food intake, or irritation of the skin at the injection sites were observed.
Fig. 5. Effect of liraglutide on intake of alcohol and water.
Daily alcohol (a) and water (b) intake during the liraglutide treatment and washout. Abscissa, days of alcohol access after initiation of the GLP-1 receptor agonist treatment. Ordinates and group sizes as in Fig. 2. *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle.
DISCUSSION
The present study demonstrates that GLP-1 receptor agonist treatment can reduce voluntary alcohol drinking in non-human primates. Liraglutide, and to a lesser degree exenatide, reduced alcohol consumption without causing overt signs of nausea or other side effects, and with no effect on water intake, as compared to vehicle.
Since there were no records available regarding the use of the GLP-1 receptor agonist exenatide in vervet monkeys, we first performed a pilot experiment, testing exenatide in the once weekly formulation Bydureon®. In humans, Bydureon® is dosed 2 mg s.c. once weekly equivalent to 0.029 mg/kg for a person weighing 70 kg. Anticipating moderately faster metabolism in the monkeys, we decided to test a slightly higher dose of 0.04 mg/kg. Plasma levels of exenatide reached approximately ten to twenty times the steady-state levels reported in humans, which are about 0.05–0.2 nM (Fineman et al. 2011; Lonborg et al. 2012). Despite the high levels, exenatide was well tolerated with no signs of emesis, and the dose of 0.04 mg/kg was therefore used in the main exenatide experiment. As is sometimes observed in humans, some monkeys developed antibodies to exenatide. Liraglutide has previously been tested for tolerability and toxicity in macaque monkeys (macaca fascicularis; Gotfredsen et al. 2014; Nyborg et al. 2012) and was expected to be well tolerated in vervets, which we confirmed in the pilot study. In the main drug studies, both exenatide and liraglutide were well tolerated.
Chronic treatment with either GLP-1 agonist decreased alcohol intake relative to vehicle in the present investigation. These findings confirm and extend published studies in rats and mice. Both exenatide and liraglutide decreased alcohol intake in rats and mice when administered as acute treatment (Egecioglu et al. 2013; Shirazi et al. 2013; Sirohi et al. 2016; Vallöf et al. 2016). Liraglutide showed a more robust effect, but since we only tested a single dose of each drug, it is difficult to draw conclusions on the relative effectiveness of the two treatments. The higher baseline alcohol intake in the liraglutide study may have contributed to the apparent difference in efficacy. In rodents, at least some studies reported that GLP-1 receptor agonists reduced drinking only or more reliably in subjects with relatively higher intake of alcohol (Shirazi et al. 2013; Suchankova et al. 2015). It is also possible that the daily dosing used with liraglutide provided a more stable plasma level of the drug relative to the weekly exenatide dosing, which may not have produced as stable plasma levels in the vervet monkeys as is reported for humans with this formulation (Fineman et al. 2011).
Importantly, the present data indicate that GLP-1 receptor stimulation can produce sustained reduction in drinking without showing signs of tolerance after chronic treatment, and that such treatment is effective in subjects with a longer history of alcohol exposure. This is in agreement with the few rodent studies using repeated administration. The synthetic GLP-1 receptor agonist AC3174 only significantly reduced drinking after repeated administration, in mice having extended alcohol exposure (Suchankova et al. 2015). We recently showed that daily administration of exenatide for 16 days strongly reduced alcohol intake in mice, in a design similar to the present study (8 days of exenatide dosing during suspended alcohol access, continued treatment during resumed access, washout (Thomsen et al. 2017). One-week repeated administration of liraglutide similarly reduced drinking in rats (Vallöf et al. 2016). It is possible that stress related to the single housing of the animals during treatment and testing modulated effects of the treatment in the present investigation. Although our results in socially housed mice suggest this may not be an important factor (Thomsen et al. 2017), it would be valuable to be able to refine the present non-human primate paradigm to allow for the recording of individual drinking patterns under social housing conditions. Other limitations of the study include relatively short study duration, a lack of positive control e.g., naltrexone, and the inclusion of only male monkeys. In baboons, naltrexone reduced drinking to a degree comparable to our liraglutide effect (Holtyn et al. 2017). Finally, both this study and our previous mouse study (Thomsen et al. 2017) tested (sub)chronic GLP-1 receptor agonist administration initiated under conditions of suspended alcohol access (which may reflects aspects relevant to relapse in humans). For increased clinical relevance, it would be relevant to also test administration of the ligands in subjects that have continued access to alcohol during treatment.
Based on the present data, it cannot be excluded that GLP-1 receptor agonist treatments reduced alcohol intake by increasing alcohol’s potency, thus requiring less alcohol to produce a same level of intoxication, however that interpretation is unlikely for two main reasons. First, GLP-1 receptor agonists blunt alcohol-induced increases in striatal dopamine efflux, and decrease the rewarding effects of alcohol measured by conditioned place preference (Egecioglu et al. 2013; Shirazi et al. 2013; Vallöf et al. 2016). Second, we found that mice treated with exenatide waited significantly longer than vehicle-treated mice to take their first drink of alcohol after forced abstinence, consistent with decreased alcohol craving or decreased motivation to seek alcohol (Thomsen et al. 2017). GLP-1 receptor agonists also have a well-documented effect on food and fluid intake, raising the possibility that modulation of ingestive behaviors and calorie intake contributed to the observed decrease in alcohol drinking (Dickson et al. 2012; McKay et al. 2014; Tang-Christensen et al. 1996; Turton et al. 1996). However, the existing evidence indicates that GLP-1 receptor stimulation modulates the addictive/rewarding properties of alcohol in the brain, rather than modifying alcohol metabolism or reducing ingestive behaviors generally. The above-mentioned effects of GLP-1 analogs on dopamine release and reward support this interpretation (Egecioglu et al. 2013; Shirazi et al. 2013; Vallöf et al. 2016). Further, we showed that exenatide also decreased alcohol self-administered via the venous route in mice, bypassing any potential effects on oral ingestion (Sørensen et al. 2016). Studies using genetically engineered mice suggested that effects of exenatide on food intake are mediated partly in peripheral tissues while effects on alcohol intake are mediated in the brain (Sirohi et al. 2016). Finally, water intake was not reduced in the present study, in agreement with some rodent studies in which water intake was unchanged or increased (Egecioglu et al. 2013; Thomsen et al. 2017; Vallöf et al. 2016). Liraglutide (acute dosing) did not affect blood alcohol after an intraperitoneal injection of alcohol in mice (Vallöf et al. 2016), suggesting that modulation of alcohol metabolism is unlikely to explain the findings. It is, however, possible that GLP-1 receptor agonists decrease alcohol drinking through a qualitative change in the effects of alcohol, such as increased aversive properties, rather than, or in addition to, reducing the rewarding properties of alcohol.
In conclusion, we show for the first time that GLP-1 receptor agonists can reduce voluntary alcohol drinking in non-human primates. Both GLP-1 agonists investigated, exenatide and liraglutide, demonstrated efficacy without showing emesis or signs of nausea. The results are in concordance with effects of exenatide and liraglutide in rodents and strongly support initiation of clinical trials investigating the potential of GLP-1-based medications in patients suffering from AUD. Accordingly, we have initiated a randomized double-blinded placebo-controlled clinical trial investigating the effects of once-weekly exenatide on alcohol intake in patients with AUD (ClinicalTrials.gov identifier: NCT03232112; Antonsen et al. 2018)
Supplementary Material
ACKNOWLEDGEMENTS
We thank Professor Roberta Palmour, Department of Psychiatry, McGill University, Montreal, Canada and Behavioural Science Foundation, Saint Kitts, Eastern Caribbean for help with planning the experiment and interpreting the results. We thank Dr. Lotte Bjerre Knudsen, Novo Nordisk A/S for help in choosing the liraglutide dosing regimen. We also thank veterinarian Amy Beierschmitt, Behavioural Science Foundation, Saint Kitts, Eastern Caribbean for technical and animal care assistance.
Funding information and disclosures
Studies were funded by Psychiatric Center Copenhagen (AFJ). MT was supported by the Psychiatric Center Copenhagen research foundation and by grant R01AA025071 from the National Institutes of Health while preparing the manuscript. The sponsors played no role in study design, data interpretation, or decision to publish.
Footnotes
The authors declare no biomedical financial interests or potential conflicts of interest in relation to the work described.
REFERENCES
- (WHO) World Health Organization (2014) Global status report on alcohol and health 2014. WHO Library Cataloguing-in-Publication Data 2014; ed. [Google Scholar]
- Akbar M, Egli M, Cho YE, Song BJ, Noronha A (2017) Medications for alcohol use disorders: An overview. Pharmacol Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, Hayes MR (2012) GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsen KK, Kruse MK, Brunchmann AS, le Dous N, Jensen ME, Miskowiak KW, Fisher PM, Thomsen GK, Rindom H, Fahmy TP, Vollstädt-Klein S, Benveniste H, Volkow ND, Becker U, Ekstrøm C, Knudsen GM, Vilsbøll T, Fink-Jensen A. (2018) Does glucagon-like peptide-1 (GLP-1) receptor stimulation reduce alcohol intake in patients with alcohol dependence? Study protocol of a randomized, double-blinded, placebo-controlled clinical trial. BMJ Open 8:e019562. doi: 10.1136/bmjopen-2017-019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse JB, Garber A, Rosenstock J, Schmidt WE, Brett JH, Videbaek N, Holst J, Nauck M (2011) Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. J Clin Endocrinol Metab 96: 1695–702. [DOI] [PubMed] [Google Scholar]
- CDC Centers for Disease Control and Prevention (2016) Fact Sheets - Alcohol Use and Your Health. Available at: http://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm. Accessed September 25, 2017.
- Connor JP, Haber PS, Hall WD (2016) Alcohol use disorders. Lancet 387: 988–98. [DOI] [PubMed] [Google Scholar]
- Cork SC, Richards JE, Holt MK, Gribble FM, Reimann F, Trapp S (2015) Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol Metab 4: 718–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re AC, Maisel N, Blodgett J, Finney J (2013) The declining efficacy of naltrexone pharmacotherapy for alcohol use disorders over time: a multivariate meta-analysis. Alcohol Clin Exp Res 37: 1064–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012) The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E (2013) The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38: 1259–70. [DOI] [PubMed] [Google Scholar]
- Ervin FR, Palmour RM, Young SN, Guzman-Flores C, Juarez J (1990) Voluntary consumption of beverage alcohol by vervet monkeys: population screening, descriptive behavior and biochemical measures. Pharmacol Biochem Behav 36: 367–73. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–91. [DOI] [PubMed] [Google Scholar]
- Fineman M, Flanagan S, Taylor K, Aisporna M, Shen LZ, Mace KF, Walsh B, Diamant M, Cirincione B, Kothare P, Li WI, MacConell L (2011) Pharmacokinetics and pharmacodynamics of exenatide extended-release after single and multiple dosing. Clin Pharmacokinet 50: 65–74. [DOI] [PubMed] [Google Scholar]
- Fineman MS, Mace KF, Diamant M, Darsow T, Cirincione BB, Booker Porter TK, Kinninger LA, Trautmann ME (2012) Clinical relevance of anti-exenatide antibodies: safety, efficacy and cross-reactivity with long-term treatment. Diabetes Obes Metab 14: 546–54. [DOI] [PubMed] [Google Scholar]
- Fiorentino TV, Owston M, Abrahamian G, La Rosa S, Marando A, Perego C, Di Cairano ES, Finzi G, Capella C, Sessa F, Casiraghi F, Paez A, Adivi A, Davalli A, Fiorina P, Guardado Mendoza R, Comuzzie AG, Sharp M, DeFronzo RA, Halff G, Dick EJ, Folli F (2015) Chronic continuous exenatide infusion does not cause pancreatic inflammation and ductal hyperplasia in non-human primates. Am J Pathol 185: 139–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP (1995) Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–300. [DOI] [PubMed] [Google Scholar]
- Gotfredsen CF, Molck AM, Thorup I, Nyborg NC, Salanti Z, Knudsen LB, Larsen MO (2014) The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes 63: 2486–97. [DOI] [PubMed] [Google Scholar]
- Heppner KM, Kirigiti M, Secher A, Paulsen SJ, Buckingham R, Pyke C, Knudsen LB, Vrang N, Grove KL (2015) Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 156: 255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–39. [DOI] [PubMed] [Google Scholar]
- Holtyn AF, Kaminski BJ, Weerts EM (2017) Baclofen and naltrexone effects on alcohol self-administration: Comparison of treatment initiated during abstinence or ongoing alcohol access in baboons. Drug Alcohol Depend 179: 47–54. http://pro.medicin.dk/Medicin/Praeparater/4752wmdhpmdMPa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K, Holscher C (2012) Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci 13: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LV, Flint A, Olsen AK, Ingwersen SH (2016) Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics. Clin Pharmacokinet 55: 657–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E (2018) GLP-1 signaling and alcohol-mediated behaviors; preclinical and clinical evidence. Neuropharmacology. [DOI] [PubMed] [Google Scholar]
- Juarez J, Guzman-Flores C, Ervin FR, Palmour RM (1993) Voluntary alcohol consumption in vervet monkeys: individual, sex, and age differences. Pharmacol Biochem Behav 46: 985–8. [DOI] [PubMed] [Google Scholar]
- Kalra SK B Sharma A (2011) Change in Alcohol Consumption Following Liraglutide Initiation: A Real Life Experience. American Diabetes Association Annual Meeting 2011 Poster 1029. [Google Scholar]
- Kastin AJ, Akerstrom V (2003) Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27: 313–8. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Zhou J, Martin B, Carlson OD, Maudsley S, Greig NH, Mattson MP, Ladenheim EE, Wustner J, Turner A, Sadeghi H, Egan JM (2010) Transferrin fusion technology: a novel approach to prolonging biological half-life of insulinotropic peptides. J Pharmacol Exp Ther 334: 682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramee P, Kusel J, Leonard S, Aubin HJ, Francois C, Daeppen JB (2013) The economic burden of alcohol dependence in Europe. Alcohol Alcohol 48: 259–69. [DOI] [PubMed] [Google Scholar]
- Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Kober L, Treiman M, Holst JJ, Engstrom T (2012) Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J 33: 1491–9. [DOI] [PubMed] [Google Scholar]
- McKay NJ, Galante DL, Daniels D (2014) Endogenous glucagon-like peptide-1 reduces drinking behavior and is differentially engaged by water and food intakes in rats. J Neurosci 34: 16417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P (1999) Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–80. [DOI] [PubMed] [Google Scholar]
- Naglich AC, Lin A, Wakhlu S, Adinoff BH (2017) Systematic Review of Combined Pharmacotherapy for the Treatment of Alcohol Use Disorder in Patients Without Comorbid Conditions. CNS Drugs. [DOI] [PubMed] [Google Scholar]
- Nyborg NC, Molck AM, Madsen LW, Knudsen LB (2012) The human GLP-1 analog liraglutide and the pancreas: evidence for the absence of structural pancreatic changes in three species. Diabetes 61: 1243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmour RM, Ervin FR, Baker GB, Young SN (1998) An amino acid mixture deficient in phenylalanine and tyrosine reduces cerebrospinal fluid catecholamine metabolites and alcohol consumption in vervet monkeys. Psychopharmacology (Berl) 136: 1–7. [DOI] [PubMed] [Google Scholar]
- Renner S, Blutke A, Streckel E, Wanke R, Wolf E (2016) Incretin actions and consequences of incretin-based therapies: lessons from complementary animal models. J Pathol 238: 345–58. [DOI] [PubMed] [Google Scholar]
- Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, Schaffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L (2014) The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 124: 4473–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP (2013) Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One 8: e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Schurdak JD, Seeley RJ, Benoit SC, Davis JF (2016) Central & peripheral glucagon-like peptide-1 receptor signaling differentially regulate addictive behaviors. Physiol Behav 161: 140–4. [DOI] [PubMed] [Google Scholar]
- Sorensen G, Caine SB, Thomsen M (2016) Effects of the GLP-1 Agonist Exendin-4 on Intravenous Ethanol Self-Administration in Mice. Alcohol Clin Exp Res 40: 2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R, Jerlhag E, Engel JA, Hodgkinson CA, Egli M, Lopez MF, Becker HC, Goldman D, Heilig M, Ramchandani VA, Leggio L (2015) The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Translational psychiatry 5: e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YY, McCormack PL (2015) Exenatide Extended-Release: An Updated Review of Its Use in Type 2 Diabetes Mellitus. Drugs 75: 1141–52. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP (1996) Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol 271: R848–56. [DOI] [PubMed] [Google Scholar]
- ten Kulve JS, van Bloemendaal L, Balesar R, RG IJ, Swaab DF, Diamant M, la Fleur SE, Alkemade A (2016) Decreased Hypothalamic Glucagon-Like Peptide-1 Receptor Expression in Type 2 Diabetes Patients. J Clin Endocrinol Metab 101: 2122–9. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Dencker D, Wortwein G, Weikop P, Egecioglu E, Jerlhag E, Fink-Jensen A, Molander A (2017) The glucagon-like peptide 1 receptor agonist Exendin-4 decreases relapse-like drinking in socially housed mice. Pharmacol Biochem Behav 160: 14–20. [DOI] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR (1996) A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72. [DOI] [PubMed] [Google Scholar]
- Vallöf D, Maccioni P, Colombo G, Mandrapa M, Jornulf JW, Egecioglu E, Engel JA, Jerlhag E (2016) The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict Biol 21: 422–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley MM, Ray LA (2017) Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addict Biol 22: 581–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.