Abstract
Chronic pain is a debilitating condition that impacts tens of millions each year, resulting in lost wages for workers and exacting considerable costs in health care and rehabilitation. A thorough understanding of the neural mechanisms underlying pain and analgesia is critical to facilitate the development of therapeutic strategies and personalized medicine. Clinical and epidemiological studies report that women experience greater levels of pain than men and have higher rates of pain-related disorders. Studies in both rodents and humans report sex differences in the anatomical and physiologic properties of the descending antinociceptive circuit, mu opioid receptor (MOR) expression and binding, morphine metabolism, and immune system activation, all of which likely contribute to the observed sex differences in pain and opioid analgesia. Although more research is needed to elucidate the underlying mechanisms, these sex differences present potential therapeutic targets to optimize pain management strategies for both sexes.
Sex differences in pain and pain sensitivity
Pain is one of the most commonly reported health problems in the United States (Elzahaf et al., 2012; Kennedy et al., 2014). An NIH survey found that roughly 55% of American adults experienced acute pain within the previous three months, while more than 10% reported experiencing chronic pain, clinically defined as pain lasting longer than three months (Nahin, 2015). As both acute and chronic pain affect a significant portion of the population, the treatment of pain is of large-scale economic concern. Indeed, the United States annual cost of pain treatment is estimated at $600 billion (Medicine, 2011).
Epidemiological studies report that women suffer from chronic pain more frequently than men (LeResche, 2011; Greenspan et al., 2007). Women also experience higher rates of chronic pain conditions such as fibromyalgia, migraine, and osteoarthritis (Unruh, 1996; Fillingim et al., 2009; Mogil, 2012; Ruau et al., 2012; Kennedy et al., 2014). Although it is not clear if the increased incidence of chronic pain conditions observed in females results from greater susceptibility to such conditions or is due to women being more likely than men to report pain (for review see Berkley et al., 2006), women are more likely than men to use health care services for both painful and non-painful conditions (Bertakis et al., 2000; Prevention, 2014). Women are also more likely to experience significantly higher pain levels than men with the same diagnosis (Ruau et al., 2012).
Importantly, not all studies in humans report a sex difference in pain sensitivity (Fillingim et al., 2009). However, when results do suggest a sex difference, women are found to be more sensitive to pain than men and experience greater adverse effects of pain than men (Fillingim et al., 2009; Mogil, 2012).
Opioid Analgesia
Opioids are the most effective and common treatment for pain management (Trescot et al., 2008), with over 65 prescriptions written per 100 Americans in 2016 (CDC, 2017). Sex differences in the prescription of opioids have been reported, with women more likely than men to be prescribed opioids. Women are also prescribed higher doses than men (Campbell et al., 2010). Opioids (such as morphine and fentanyl) act by binding to neuronal mu-opioid receptors (MORs) to inhibit pain. However, opioids also induce respiratory depression, gut immotility, nausea, dysphoria, headache, and vomiting, with women more likely than men to experience many of these negative side effects (Myles et al., 1997; Cepeda et al., 2003; Fillingim et al., 2005; Comer et al., 2010). Long-term opiate use also results in the development of tolerance, leading to a reduction in opioid potency that is typically countered with dose escalation (Trescot et al., 2006; Trescot et al., 2008). The development of opioid tolerance and the resulting dose increase leads to a higher risk of addiction and overdose (Trescot et al., 2006). Clinically, men are two times more likely to have used opiates, including heroin, within the past month, year, and across the lifespan (NSDUH, 2016; see Riley et al. 2018 for review), and preclinical studies in rodents report that males experience longer and more severe withdrawal symptoms from opiates than females (Cicero et al., 2002; Diaz et al., 2005).
The existence of sex differences in opioid analgesia remains controversial. Although some studies report no sex difference in the analgesic efficacy of opioids in humans (Sarton et al., 2000; Fillingim et al., 2005; Bijur et al., 2008), other studies report that opioids exhibit decreased analgesic efficacy in women (Cepeda and Carr, 2003; Miller and Ernst, 2004). Importantly, these results are dependent on the setting (clinic versus laboratory), duration of pain (acute versus chronic) and type of pain (visceral, inflammatory or orofacial) for which opioids are prescribed. Preclinical studies using rodent models are more consistent, with the majority of studies reporting that morphine is more efficacious in modulating persistent pain in males than females. Indeed, using a variety of acute and chronic pain assays, researchers have shown that morphine’s median effective dose in female rodents is approximately twice the concentration of the dose needed for males to achieve comparable levels of pain relief (Kepler et al., 1989; Cicero et al., 2002; Ji et al., 2006; Loyd and Murphy, 2006; Loyd et al., 2008; Posillico et al., 2015). Researchers using other opioids have reported similar results (Barrett et al., 2002; Bai et al., 2015). Studies investigating the side-effects of opioids suggest that in rodents, females suffer from higher rates of opioid-induced hyperalgesia than males (Holtman and Wala, 2005; Juni et al., 2008). It is important to note that the origin of these sexually dimorphic properties may result from biological differences between sexes, environmental factors, or some interaction between the two. Researchers investigating the source of phenotypic differences between the sexes take into account multiple developmental elements that impact neurobiology and behavior such as genetic, hormonal, and epigenetic factors (for a thorough review, see Becker and Chartoff, 2018).
Mechanisms of analgesia: Endogenous
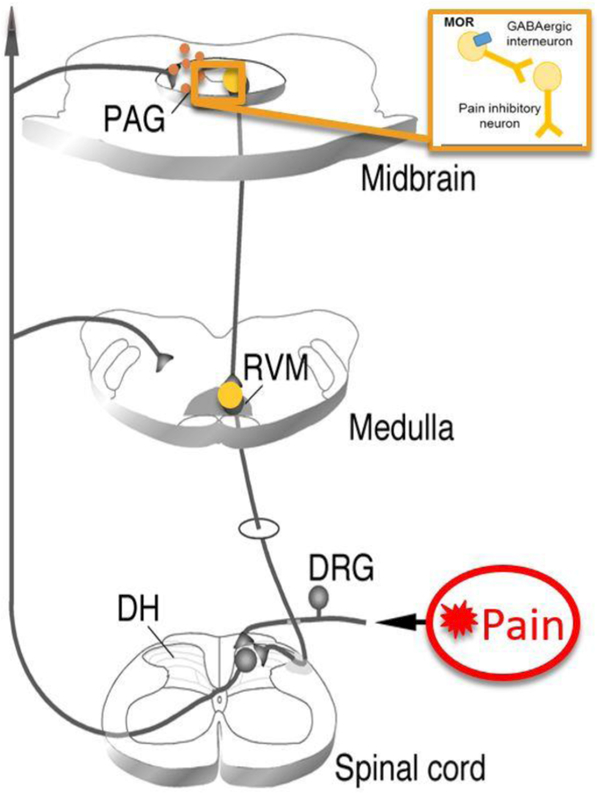
The CNS utilizes an endogenous descending neural pathway to reduce pain in a process called antinociception. Essential to this descending antinociceptive pathway is the midbrain periaqueductal gray (PAG) and its downstream targets, the rostral ventromedial medulla (RVM), and the spinal cord (Behbehani and Fields, 1979; Fields and Heinricher, 1985; see Figure 1). Indeed, electrical stimulation of the PAG induces a robust, opioid-dependent analgesia (Reynolds, 1969) that is attenuated by intra-PAG injection of the opioid receptor antagonist (−)-naloxone (Akil et al., 1976). Sex differences in pain sensitivity have been suggested to occur, in part, due to differences in endogenous opioid neurotransmission and in the circulation of steroid hormones (Micevych et al., 2003; LeResche et al., 2003; Smith et al., 2006; Pluchino et al. 2009). Specifically, studies in rodents indicate significant sex differences in the anatomical and physiological characteristics of the PAG-RVM-spinal cord circuit. Further, persistent inflammatory pain results in a greater activation of this descending circuit in males than in females. Similarly, systemic administration of morphine results in a significantly greater activation of this circuit in males than in females (Loyd and Murphy, 2006; Loyd et al., 2007).
Figure 1.
Noxious stimuli activate nociceptors located on primary afferents, which then relay nocispecific information to the dorsal horn of the spinal cord via the dorsal root ganglia (DRG). From here, nociceptive-specific information is relayed supraspinally to the PAG, thalamus and higher cortical regions. Both endogenous and exogenous opioids activate the PAG and its descending projections to the RVM and spinal cord to ultimately inhibit incoming pain signals (adapted from Guo et al., 2006).
Mechanisms of analgesia: Exogenous
Morphine and other exogenous opioids bind to MORs in the central nervous system to further modulate pain (Jensen and Yaksh, 1986; Loyd et al., 2008). MORs are G-protein coupled receptors whose activation inhibits neuronal activity (i.e. hyperpolarization) (Millan, 2002). The midbrain PAG, a critical structure in the descending modulation of pain, contains a high density of MOR+ neurons (Wang and Wessendorf, 2002; Commons et al., 2000), and we have previously reported that male rats show higher levels of PAG MOR expression and binding than females (Loyd et al., 2008). The binding of PAG MORs disinhibits tonically active GABAergic interneurons, resulting in the net activation of the PAG-RVM-spinal cord circuit and the inhibition of pain transmission (Al-Hasani and Bruchas, 2011; Lau and Vaughan, 2014). Intra-PAG administration of morphine or MOR selective agonists results in sex-dependent analgesia. For example, Krzanowska and Bodnar (Krzanowska and Bodnar, 1999) reported intra-PAG morphine ED50 values of 1.2 μg for male rats in comparison to 50 μg in estrus female rats. In a model of persistent inflammatory pain, we reported intra-PAG morphine ED50 values for males of 7.5 μg versus 15 μg for females (Loyd et al., 2008). Ablation of MOR+ neurons in the ventrolateral PAG reduces the analgesic efficacy of morphine in males but not in females, suggesting that PAG MOR expression is correlated with the degree of opioid analgesia in males (Loyd et al., 2008). Although sex differences in PAG MOR levels contribute to the dimorphic effects of morphine, several other factors have also been implicated, including neuroimmune signaling and opioid metabolism.
Opioids and Inflammation
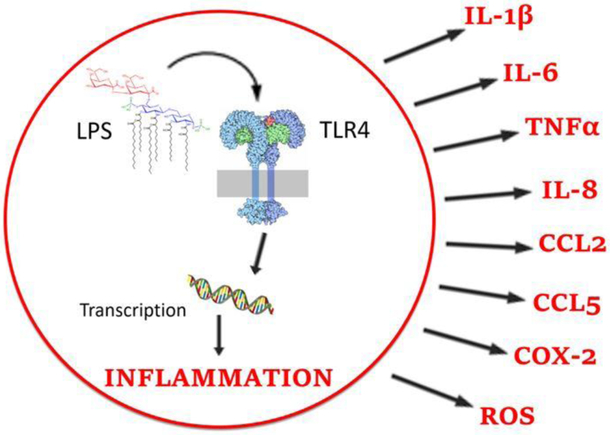
Neuroinflammation has been implicated in increased pain sensitivity and decreased opioid analgesia via the activity of glial cells, particularly microglia. Microglia survey the central nervous system for signals of cellular distress such as tissue injury and react to the presence of pathogens, including nitric oxide, substance P, and prostaglandins (Watkins et al., 2009). These molecules bind to receptors called toll-like receptors (TLRs), located primarily on the surface of microglia. Binding to microglial toll-like receptor 4 (TLR4) results in the transition of microglia from a resting state to an activated state, a process called reactive gliosis (Watkins and Maier, 2003; Hutchinson et al., 2008a; Watkins et al., 2009). In their activated state, microglia release pro-inflammatory molecules including cytokines (e.g. interleukins 1 and 6, (IL-1, IL-6), and tumor necrosis factor (TNF)), chemokines (e.g. IL-8, CCL2, and CCL5), cyclooxygenase-2 (COX-2), prostaglandins, and reactive oxygen species (ROS) (Bonizzi and Karin, 2004). Together these molecules down-regulate inhibitory GABAA receptors, up-regulate excitatory neuronal AMPA and NMDA receptors, and decrease glutamate transporter activity, effectively increasing neural excitability and opposing morphine action (Watkins et al., 2005; Yan et al., 2014; Eidson et al., 2016). Activated microglia are observed in virtually every known animal model of clinical pain, and the activation of microglia via complete Freund’s adjuvant (CFA) or the TLR4 agonist lipopolysaccharide (LPS), causes robust allodynia and hyperalgesia (Watkins et al., 1994; Sorge et al., 2011). Both the inhibition of microglial function and the blockade of pro-inflammatory molecule release prevent the development of hyperalgesia (Maier and Watkins, 1998; Ledeboer et al., 2005; Hutchinson et al., 2008).
Morphine and other opioids bind not only to neuronal MOR but also to microglial TLR4 and its co-receptor myeloid differentiation factor 2 (MD-2), which together with the recruitment of the adaptor protein MyD88, initiate a neuroinflammatory response that contributes to many of the negative side effects associated with opioid consumption, including hyperalgesia, respiratory depression, and tolerance (Hutchinson et al., 2007; Hutchinson et al., 2010; Eidson and Murphy, 2013; Thomas et al., 2015; see Figure 2). Recently, our lab has shown that morphine activates TLR4 within the midbrain PAG to induce cytokine release, resulting in the attenuation of morphine analgesia and the induction of tolerance (Eidson and Murphy, 2013; Eidson et al., 2016). Blockade of PAG TLR4 signaling, and the ensuing cytokine production, augments morphine-induced analgesia and attenuates the development of tolerance (Eidson and Murphy, 2013; Eidson et al., 2017; Doyle et al., 2017).
Figure 2.
TLR4 binding leads to the transcription and release of pro-inflammatory products.
Microglia and Sex Differences
Our recent studies suggest that sex differences in microglia phenotype within the PAG also contribute to the sexually dimorphic effects of morphine (Doyle et al., 2017; Doyle and Murphy, 2018). Specifically, we showed that although no sex differences in basal microglia expression (density) was observed within the PAG, the percentage of microglia showing an ‘activated’ phenotype at baseline was significantly higher in females than males. We further showed a significant relationship between morphine potency (i.e. ED50) and the percentage of activated microglia in the PAG of females, but not males.
As stated above, in addition to binding to neuronal MOR, most opioids, including morphine, bind to the the MD-2 co-receptor of TLR4 on microglia. Although the classical MOR binds only the (−)-stereoisomer of opioids, TLR4 binds opioids in a non-stereoselective manner, such that both the (−) and (+) isomers of opioid ligands modulate glial signaling. This unique feature has allowed researchers to investigate TLR4-mediated activity without affecting opioid receptor signaling (Wu et al. 2006). Our lab has recently reported that inhibition of PAG microglia with the selective TLR4 antagonist (+)-naloxone significantly potentiated morphine analgesia in females, but not males, abolishing the sex difference in opiate response. Indeed, morphine ED50 values for females co-administered (+)-naloxone decreased from 7.9 mg/kg to 3.16 mg/kg, a 2.5-fold reduction in ED50. These results suggest that PAG microglia are innately different in males and females in terms of their morphological state and implicate TLR4 in the attenuated response to morphine observed in females.
Morphine Metabolism
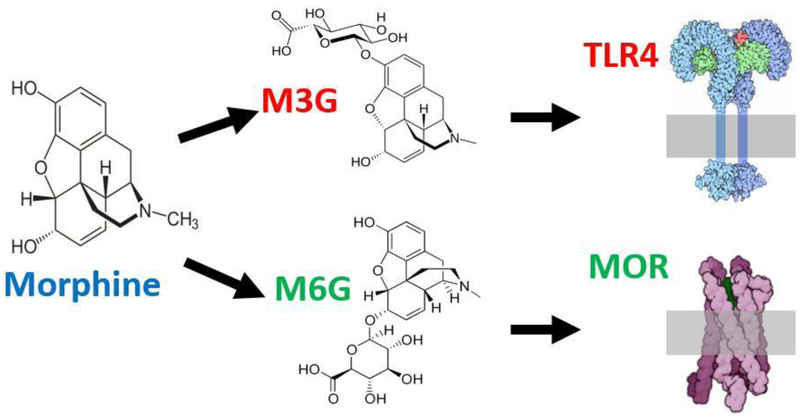
Morphine is metabolized by both the liver and the brain to produce two metabolites: morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) (Coffman et al., 1997; Togna et al., 2013). The metabolism of morphine results from the addition of a glucuronic acid component to the morphine substrate, a process called glucuronidation (Christrup, 1997). Multiple enzymes act to metabolize morphine, including 1A1, 1A6, 2B1, and importantly the family of uridine 5'-diphospho-glucuronosyltransferase (UGT) enzymes.
Morphine’s two primary metabolites have opposing effects on pain modulation. M3G binds to microglial TLR4 to produce hyperalgesia and allodynia (pain response from non-noxious stimuli) (Due et al., 2012), whereas M6G binds to MOR to produce potent pain inhibition (Wittwer and Kern, 2006; see Figure 3). Pharmacological studies show that administration of M3G correlates with nociception (Smith and Smith, 1995) and that M3G actively opposes opioid analgesia, as well as the analgesic effects of M6G (Ekblom et al., 1993; Roeckel et al., 2017;Doyle and Murphy, 2018).
Figure 3.
Morphine is metabolized via glucuronidation to produce M3G and M6G which bind to TLR4 and MOR, respectively (adapted from Doyle and Murphy 2018).
A number of studies point to a sex difference in the metabolism of morphine. For example, gene expression of UGT1 and UGT2 enzymes is significantly higher in females (Iwano et al., 2012), likely due to higher levels of estradiol. Studies on glucuronide concentrations in humans are inconclusive, as some report a sex difference (Murthy et al., 2002), while others do not (Sarton et al., 2000). However, animal studies consistently report that females have higher serum and plasma concentrations of M3G than males (South et al., 2001; South et al., 2009). Recent studies by our lab suggest that the increased production of M3G in females contributes to the attenuated response to morphine, implicating morphine metabolism as a vital factor in the sexually dimorphic effects of morphine (Doyle and Murphy, 2018).
Conclusions
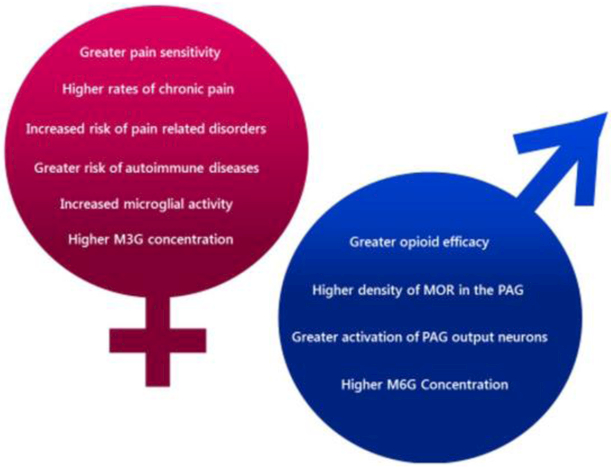
The neural pathways mediating pain and analgesia show sexual dimorphism at many points, notably in the PAG and its descending output, in the engagement of the immune system, and in the metabolism of morphine (see Figure 4). Although opioids are among the most efficacious and frequently prescribed medications for chronic pain, the sexually dimorphic mechanisms of analgesia are not yet fully elucidated. Future experiments on possible sex differences in the binding affinities of morphine, M6G, and M3G at MOR and MD-2, as well as dimorphisms in the internalization and downstream activity of MOR, will provide a more detailed model of the mechanisms underlying sex differences in opioid analgesia. Moreover, continued study of the origins of these sex differences (be they hormonal, autosomal, or epigenetic) will provide novel information on the developmental processes underlying the phenotypical differences between males and females.
Figure 4.
Summary of sex differences in pain and opioid analgesia.
To ensure effective management of chronic pain and its related conditions for both sexes, a research agenda focused on sex differences in opioid treatment is critical. The sex-specific differences presented in this review represent potential targets of future treatment strategies in the pursuit of developing effective analgesics for both sexes. Importantly, sex differences in the impact of opioids on mood, reward, and addiction are paramount to the development of personalized medicine and therefore integral components in the comprehensive study of opiates.
Highlights.
Research on pain and analgesia must include the role of sex
Discussion of several factors contributing to pain and analgesia
Sex differences in pain sensitivity and opioid efficacy
Sex-specific effects of morphine metabolism
Sexual dimorphism in inflammatory processes and immune signaling
Acknowledgements.
This work has been supported by NIH grants DA16272 and DA041529 awarded to AZM. Hillary Doyle was supported by a Honeycutt Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akil H, Mayer DJ, Liebeskind JC (1976) Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science 191:961–962. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, Bruchas MR (2011) Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115:1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Clark JD (2006) Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 104:570–587. [DOI] [PubMed] [Google Scholar]
- Bai L, Zhai C, Han K, Li Z, Qian J, Jing Y, Zhang W, Xu JT (2014) Toll-like receptor 4-mediated nuclear factor-kappaB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull 30:936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Zhang X, Li Y, Lu L, Li B, He X (2015) Sex differences in peripheral mu-opioid receptor mediated analgesia in rat orofacial persistent pain model. PLoS One 10:e0122924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Smith ES, Picker MJ (2002) Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. European journal of Pharmacology 452:163–173. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL (1979) Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Research 170:85–93. [DOI] [PubMed] [Google Scholar]
- **.Becker JB, Chartoff E (2018) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 0:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is an outstanding review highlighting the latest findings underlying hormonal, chromosomal, and epigenetic sex-dependent mechanisms underlying addiction.
- Berkley KJ, Zalcman SS, Simon VR (2006) Sex and gender differences in pain and inflammation: a rapidly maturing field. Am J Physiol Regul Integr Comp Physiol 291(2):R241–1 [DOI] [PubMed] [Google Scholar]
- Bertakis KD, Azari R, Helms LJ, Callahan EJ, Robbins JA (2000) Gender differences in the utilization of health care services. J Fam Pract 49:147–152. [PubMed] [Google Scholar]
- Bijur PE, Esses D, Birnbaum A, Chang AK, Schechter C, Gallagher EJ (2008) Response to morphine in male and female patients: analgesia and adverse events. The Clinical journal of pain 24:192–198. [DOI] [PubMed] [Google Scholar]
- Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, Pipy B, Bayard F, Arnal JF, Guery JC, Gourdy P (2010) 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. Journal of Immunology 185:1169–1176. [DOI] [PubMed] [Google Scholar]
- Campbell CI, Weisner C, Leresche L, Ray GT, Saunders K, Sullivan MD, Banta-Green CJ, Merrill JO, Silverberg MJ, Boudreau D, Satre DD, Von Korff M (2010) Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health 100:2541–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda MS, Carr DB (2003) Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesthesia and analgesia 97:1464–1468. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Farrar JT, Baumgarten M, Boston R, Carr DB, Strom BL (2003) Side effects of opioids during short-term administration: effect of age, gender, and race. Clinical pharmacology and therapeutics 74:102–112. [DOI] [PubMed] [Google Scholar]
- (CDC) Annual Surveillance Report OF Drug-related Rusks and Outcomes (2017) [Google Scholar]
- Christrup LL (1997) Morphine metabolites. Acta anaesthesiologica Scandinavica 41:116–122. [DOI] [PubMed] [Google Scholar]
- Cicero TJ, Nock B, O'Connor L, Meyer ER (2002) Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. The Journal of pharmacology and experimental therapeutics 300:695–701. [DOI] [PubMed] [Google Scholar]
- Coffman BL, Rios GR, King CD, Tephly TR (1997) Human UGT2B7 catalyzes morphine glucuronidation. Drug metabolism and disposition: the biological fate of chemicals 25:1–4. [PubMed] [Google Scholar]
- Comer SD, Cooper ZD, Kowalczyk WJ, Sullivan MA, Evans SM, Bisaga AM, Vosburg SK (2010) Evaluation of potential sex differences in the subjective and analgesic effects of morphine in normal, healthy volunteers. Psychopharmacology 208:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Aicher SA, Kow LM, Pfaff DW (2000) Preysnaptic and postsynaptic relation of mu-opioid receptors to gamma-aminobutyric acid-immunoreactive and medullary projecting periaqueductal gray neruons. J Comp Neurol 419(4):532–542. [DOI] [PubMed] [Google Scholar]
- Diaz SL, Kemmling AK, Rubio MC, Balerio GN (2005) Morphine withdrawal syndrome: involvement of the dopaminergic system in prepubertal male and female mice. Pharmacol. Biochem. Behav. 82:601–607 [DOI] [PubMed] [Google Scholar]
- **.Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ (2017) Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. The Journal of Neuroscience 37. 12: 3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first study to show that sex differences in microglia phenotype in the midbrain periaqueductal gray, a key brain region for the antinociceptive actions of opiates, contributes to the sexually dimorphic effects of morphine.
- **.Doyle HH, Murphy AZ (2018) Sex dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiology & Behavior 187:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first study to show sex-dependent effects of the morphine metabolites M3G and M6G, and demonstrate that blockade of glial signaling eliminates sex differences in morphine analgesia.
- Drew PD, Chavis JA (2000) Female sex steroids: effects upon microglial cell activation. Journal of neuroimmunology 111:77–85. [DOI] [PubMed] [Google Scholar]
- Due MR, Piekarz AD, Wilson N, Feldman P, Ripsch MS, Chavez S, Yin H, Khanna R, White FA (2012) Neuroexcitatory effects of morphine-3-glucuronide are dependent on Toll-like receptor 4 signaling. Journal of Neuroinflammation 9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Murphy AZ (2013) Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. The Journal of neuroscience: the official journal of the Society for Neuroscience 33:15952–15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **.Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ (2016) Toll-Like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Using selective TNF biologics, these authors demonstrate that blockade of glially-derived TNF within the midbrain periaqueductal gray complete eliminates the development of tolerance to morphine in male rats.
- Ekblom M, Gardmark M, Hammarlund-Udenaes M (1993) Pharmacokinetics and pharmacodynamics of morphine-3-glucuronide in rats and its influence on the antinociceptive effect of morphine. Biopharmaceutics & drug disposition 14:1–11. [DOI] [PubMed] [Google Scholar]
- Elzahaf RA, Tashani OA, Unsworth BA, Johnson MI (2012) The prevalence of chronic pain with an analysis of countries with a Human Development Index less than 0.9: a systematic review without meta-analysis. Current medical research and opinion 28:1221–1229. [DOI] [PubMed] [Google Scholar]
- Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S (2016) Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain, behavior, and immunity 52:18–26. [DOI] [PubMed] [Google Scholar]; This study administered LPS to healthy male and female volunteers and measured humoral and cognitive factors associated with sickness behavior. Despite launching a significantly higher neuroinflammatory response (IL-6, TNF, IL-10), females displayed comparable levels of mood, anxiety and non-specific sickness symptoms as males suggesting the existence of endogenous compensatory mechanisms.
- Fields HL, Heinricher MM (1985) Anatomy and physiology of a nociceptive modulatory system. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences 308:361–374. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd (2009) Sex, gender, and pain: a review of recent clinical and experimental findings. The journal of pain: official journal of the American Pain Society 10:447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R (2005) Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. The journal of pain: official journal of the American Pain Society 6:116–124. [DOI] [PubMed] [Google Scholar]
- Frenk SM, Porter KS, Paulozzi LJ (2015) Prescription opioid analgesic use among adults: United States, 1999–2012. NCHS Data Brief: 1–8. [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielse L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ (2007). Studying sex and gender differences in pain and analgesia: A consensus report. Pain, 132 Suppl 1, S26–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WI, Robbins MT, Wei F, Zou S, Dubner R, Ren K (2006) Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci 26:126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR Jr, Wala EP (2005) Characterization of morphine-induced hyperalgesia in male and female rats. Pain 114:62–70. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR (2007) Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. TheScientificWorldJournal 7:98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Brown K, Coats BD, Shridhar M, Sholar PW, Patel SJ, Crysdale NY, Harrison JA, Maier SF, Rice KC, Watkins LR (2008) Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4). The European journal of neuroscience 28:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR (2010) Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain, behavior, and immunity 24:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano S, Higashi E, Miyoshi T, Ando A, Miyamoto Y (2012) Focused DNA microarray analysis for sex-dependent gene expression of drug metabolizing enzymes, transporters and nuclear receptors in rat livers and kidneys. The Journal of toxicological sciences 37:863–869. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Yaksh TL (1986) Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Research 363:99–113. [DOI] [PubMed] [Google Scholar]
- Ji Y, Murphy AZ, Traub RJ (2006) Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. American journal of physiology Regulatory, integrative and comparative physiology 291: R307–314. [DOI] [PubMed] [Google Scholar]
- Juni A, Klein G, Kowalczyk B, Ragnauth A, Kest B (2008) Sex differences in hyperalgesia during morphine infusion: effect of gonadectomy and estrogen treatment. Neuropharmacology 54:1264–1270. [DOI] [PubMed] [Google Scholar]
- Karshikoff B, Lekander M, Soop A, Lindstedt F, Ingvar M, Kosek E, Olgart Hoglund C, Axelsson J (2015) Modality and sex differences in pain sensitivity during human endotoxemia. Brain, behavior, and immunity 46:35–43. [DOI] [PubMed] [Google Scholar]
- Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S (2014) Prevalence of persistent pain in the U.S. Adult population: new data from the 2010 national health interview survey. The journal of pain: official journal of the American Pain Society 15:979–984. [DOI] [PubMed] [Google Scholar]
- Kepler KL, Kest B, Kiefel JM, Cooper ML, Bodnar RJ (1989) Roles of gender, gonadectomy and estrous phase in the analgesic effects of intracerebroventricular morphine in rats. Pharmacology, biochemistry, and behavior 34:119–127. [DOI] [PubMed] [Google Scholar]
- Klein SL (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol 26:247–264. [DOI] [PubMed] [Google Scholar]
- Krzanowska EK, Bodner RJ (1999) Morphine antinociception elicited from the ventrolateral periaductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 821:224–230 [DOI] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ (2007) Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain 132 Suppl 1: S124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW (2014) Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol 29:159–164. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR (2005) Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115:71–83. [DOI] [PubMed] [Google Scholar]
- LeResche L, Mancl L, Sherman J, Gandara B, Dworkin S (2003). Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain 106(3):253–61 [DOI] [PubMed] [Google Scholar]
- LeResche L (2011). Defining Gender Disparities in Pain Management. Clinical Orthopaedics and Related Research, 469(7), 1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q (2012) Antagonists of toll like receptor 4 maybe a new strategy to counteract opioid-induced hyperalgesia and opioid tolerance. Medical Hypotheses 79:754–756. [DOI] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR (2012) Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology 37:1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Murphy AZ (2006) Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. The Journal of comparative neurology 496:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Morgan MM, Murphy AZ (2007) Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience 147:456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ (2008) Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. The Journal of neuroscience: the official journal of the Society for Neuroscience 28:14007–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manubay J, Davidson J, Vosburg S, Jones J, Comer S, Sullivan M (2015) Sex differences among opioid-abusing patients with chronic pain in a clinical trial. J Addict Med 9:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported that although the demographic profile of opioid abusing patients with chronic pain presenting for treatment in a clinical trial was similar between sexes, women reported a greater effect of pain on mood (increased depression) and social relations, while men reported more maladaptive behaviors, including alcohol and illicit drug abuse, dose escalation and arrest, and highlights the need for sex specific interventions.
- Marriott I, Huet-Hudson YM (2006) Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res 34:177–192. [DOI] [PubMed] [Google Scholar]
- Medicine Io (2011) Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Micevych PE, Rissman EF, Gustafsson JA, Sinchak K (2003) Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 71:802–810 [DOI] [PubMed] [Google Scholar]
- Millan MJ (1999) The induction of pain: an integrative review. Progress in Neurobiology 57:1–164. [DOI] [PubMed] [Google Scholar]
- Millan MJ (2002) Descending control of pain. Progress in neurobiology 66:355–474. [DOI] [PubMed] [Google Scholar]
- Miller PL, Ernst AA (2004) Sex differences in analgesia: a randomized trial of mu versus kappa opioid agonists. Southern medical journal 97:35–41. [DOI] [PubMed] [Google Scholar]
- Mogil JS (2012) Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature reviews Neuroscience 13:859–866. [DOI] [PubMed] [Google Scholar]
- Murthy BR, Pollack GM, Brouwer KL (2002) Contribution of morphine-6-glucuronide to antinociception following intravenous administration of morphine to healthy volunteers. Journal of clinical pharmacology 42:569–576. [DOI] [PubMed] [Google Scholar]
- Myles PS, Hunt JO, Moloney JT (1997) Postoperative 'minor' complications. Comparison between men and women. Anaesthesia 52:300–306. [DOI] [PubMed] [Google Scholar]
- *.Nahin RL (2015) Estimates of pain prevalence and severity in adults: United States, 2012. The journal of pain: official journal of the American Pain Society 16:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Survey study using a comprehensive rating scale to determine the percentage of the population experiencing various degrees of pain. Great and highly useful statistics.
- NSDUH (2016) National Survey on Drug Use and Health Rockville, Md: :Office of Applied Studies, Substance Abuse and Mental Health Services Administration, Dept. of Health & Human Services. [Google Scholar]
- Pluchino N, Ninni F, Casarosa E, Giannini A, Merlini S, Cubeddu A, Luisi M, Cela V, Genazzani A R (2009) Sex Differences in Brain and Plasma β-Endorphin Content following Testosterone, Dihydrotestosterone and Estradiol Administration to Gonadectomized Rats. Neuroendocrinology 89:411–423. [DOI] [PubMed] [Google Scholar]
- Posillico CK, Terasaki LS, Bilbo SD, Schwarz JM (2015) Examination of sex and minocycline treatment on acute morphine-induced analgesia and inflammatory gene expression along the pain pathway in Sprague-Dawley rats. Biol Sex Differ 6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention CfDCa (2014) Summary Health Statistics: National Health Interview Survey [Google Scholar]
- Reynolds DV (1969) Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164:444–445. [DOI] [PubMed] [Google Scholar]
- *.Roeckel L, Utard V, Reiss D, Mouheiche J, Maurin H, Robé A, Audouard E, Wood J N, Goumon Y, Simonin F, Gavériaux-Ruff C (2017). Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Scientific Reports. 7 10406 10.1038/s41598-017-11120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study reported an attenuation in opioid induced hyperalgesia in MOR KO, MOR flox or MOR-Nav1.8 sensory neuron conditional KO mice following M3G administration, adding to the controversy as to the role of microglia and neuronal MOR in mediating the negative side effects associated with opioid consumption.
- ***.Riley AL, Hempel BJ, Clasen MM (2018) Sex as a biological variable: Drug use and abuse. Physiol Behav. 187:79–96 [DOI] [PubMed] [Google Scholar]; *** This is an outstanding review that summarizes work from clinical and preclinical populations on sex differences in drug use and abuse, ranging from initiation to escalation/dysregulation and from drug cessation/abstinence to relapse.
- Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ (2012) Sex differences in reported pain across 11,000 patients captured in electronic medical records. The journal of pain: official journal of the American Pain Society 13:228–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A (2000) Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology 93:1245–1254; discussion 1246A. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD (2012) Sex, glia, and development: interactions in health and disease. Hormones and Behavior 62:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Smith MT (1995) Morphine-3-glucuronide: evidence to support its putative role in the development of tolerance to the antinociceptive effects of morphine in the rat. Pain 62:51–60. [DOI] [PubMed] [Google Scholar]
- Smith Y, Stohler C, Nichols T, Bueller J, Koeppe R, Zubieta J (2007) Pronociceptive and Antinociceptive Effects of Estradiol through Endogenous Opioid Neurotransmission in Women. J Neurosci. (21): 5777–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS (2011) Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. The Journal of neuroscience: the official journal of the Society for Neuroscience 31:15450–15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South SM, Edwards SR, Smith MT (2009) Antinociception versus serum concentration relationships following acute administration of intravenous morphine in male and female Sprague-Dawley rats: differences between the tail flick and hot plate nociceptive tests. Clinical and experimental pharmacology & physiology 36:20–28. [DOI] [PubMed] [Google Scholar]
- South SM, Wright AW, Lau M, Mather LE, Smith MT (2001) Sex-related differences in antinociception and tolerance development following chronic intravenous infusion of morphine in the rat: modulatory role of testosterone via morphine clearance. The Journal of pharmacology and experimental therapeutics 297:446–457. [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. Journal of Neuroinflammation 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ***.Thomas J, Mustafa S, Johnson J, Nicotra L, Hutchinson M (2015) The relationship between opioids and immune signalling in the spinal cord. Handb Exp Pharmacol 227:207–238. [DOI] [PubMed] [Google Scholar]; *** Excellent overview on opioid induced neuroinflammation in the spinal cord and the role of neural and glial mechanisms.
- Togna AR, Antonilli L, Dovizio M, Salemme A, De Carolis L, Togna GI, Patrignani P, Nencini P (2013) In vitro morphine metabolism by rat microglia. Neuropharmacology 75:391–398. [DOI] [PubMed] [Google Scholar]
- Trescot AM, Glaser SE, Hansen H, Benyamin R, Patel S, Manchikanti L (2008) Effectiveness of opioids in the treatment of chronic non-cancer pain. Pain physician 11: S181–200. [PubMed] [Google Scholar]
- Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, Abdi S, Jasper JF, Singh V, Jordan AE, Johnson BW, Cicala RS, Dunbar EE, Helm S 2nd, Varley KG, Suchdev PK, Swicegood JR, Calodney AK, Ogoke BA, Minore WS, Manchikanti L (2006) Opioid guidelines in the management of chronic non-cancer pain. Pain physician 9:1–39. [PubMed] [Google Scholar]
- Unruh AM (1996) Gender variations in clinical pain experience. Pain 65:123–167. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, Ingram SL, Connor MA, Christie MJ (1997) How opioids inhibit GABA-mediated neurotransmission. Nature 390:611–614. [DOI] [PubMed] [Google Scholar]
- Wang H, Wessendorf MW (2002) Mu- and delta-opioid receptor mRNAs are expressed in periaqueductal gray neruons projecting to the rostral ventromedial medulla. Neuroscience 109(3):619–34. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF (2003) Glia: a novel drug discovery target for clinical pain. Nature reviews Drug discovery 2:973–985. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF (2005) Glia: novel counter-regulators of opioid analgesia. Trends in Neurosciences 28:661–669. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF (2009) The "toll" of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends in pharmacological sciences 30:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF (1994) Characterization of cytokine-induced hyperalgesia. Brain Research 654:15–26. [DOI] [PubMed] [Google Scholar]
- Wittwer E, Kern SE (2006) Role of morphine's metabolites in analgesia: concepts and controversies. The AAPS Journal 8: E348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HE, Sun HS, Cheng CW, Terashvili M, Tseng LF (2006). Dextro-Naloxone or levonaloxone reverses the attenuation of morphine antinociception induced by lipopolysaccharide in the mouse spinal cord via a non-opioid mechanism". The European Journal of Neuroscience. 24 (9): 2575–2580 [DOI] [PubMed] [Google Scholar]
- Yan X, Yadav R, Gao M, Weng HR (2014) Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia 62:1093–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]