Abstract
Background:
Aortic syndromes (AS), including aortic dissection (AD), intramural hematoma (IMH) and penetrating aortic ulcer (PAU), carry significant acute and long-term morbidity and mortality. However, the contemporary incidence and outcomes of AS are unknown.
Methods and Results:
We utilized the Rochester Epidemiology Project record linkage system to identify all Olmsted County, Minnesota, residents with AS (1995–2015). Diagnostic imaging, medical records, and death certificates were reviewed to confirm the diagnosis and AS subtype. Age- and sex-adjusted incidence rates were estimated using annual county-level census data. Survival for patients with AS was compared to age- and sex-matched controls using Cox regression to adjust for comorbid conditions. We identified 133 patients with AS (77-AD, 21-IMH, and 35-PAU). Average age was 71.8 years (SD 14.1) and 57% were male. The age- and sex-adjusted incidence was 7.7 per 100,000 person-years, was higher for males than females (10.2 vs. 5.7 per 100,000 person-years), and increased with age. Among subtypes, the incidence of AD was highest (4.4 per 100,000 person-years), while the incidence of PAU and IMH were lower (2.1 and 1.2 per 100,000 person-years). Overall, the incidence of AS was stable over time (p trend=.33), although the incidence of PAU appeared to increase from 0.6 to 2.6 per 100,000 person-years (p=.008) with variability over the study interval. Patients with AS had more than twice the mortality rate at 5, 10, and 20 years when compared to population-based controls (5, 10 and 20-year mortality 39%, 57%, 91% versus 18%, 41%, and 66%; overall adjusted mortality HR=2.1, p<0.001). Survival was lower than expected up to 90 days after AS diagnosis and did not differ significantly by subtype or by 5-year strata of diagnosis.
Conclusions:
Overall, the incidence of AD and IMH has remained stable since 1995, despite the decline noted for other cardiovascular disease. AS confers increased early and long-term mortality that has not changed. These data highlight the need to improve long term care to impact the prognosis of this patient group.
Keywords: Aortic Dissection, Intramural Hematoma, Penetrating Aortic Ulcer, Epidemiology, Mortality/Survival
Introduction:
Aortic syndromes (AS) include aortic dissection (AD), intramural hematoma (IMH), and penetrating aortic ulcer (PAU) and represents an injury to the inner aortic layers resulting in emergent aortic pathology. These are highly morbid diagnoses that carry significant acute and long-term mortality risks. Acute dissections of the ascending aorta (Stanford A) carry a mortality that historically approaches 1% per hour.1 Although less morbid, acute dissections of the descending thoracic aorta (Stanford B) are associated with a 10–25% mortality at 30 days.2 Intramural hematoma mortality ranges from 10–50%, and 40% of the patients progress to overt dissection.2
Despite the known morbidity of these pathologies, the current epidemiology of them is unknown. Our knowledge of the epidemiology of these diseases in the United States is derived from data collected from 1980–1994, with a population based reported incidence of AD of 3.5 per 100,000 person years.3 However, our current understanding of the presentation, treatment patterns and outcomes are predominantly from registries at large referral centers,4 claims data,5, 6 or single center series that are more center based. Advances in the prevention and treatment of cardiovascular disease have resulted in a decline of acute myocardial infarction7 and abdominal aortic aneurysm.8, 9 However, it is unknown if there has been a concurrent decline in the incidence of these pathologies. Limited data from Sweden suggest that the incidence of AD has increased,10 yet no current data exists on the incidence of AS in the United States.
The purpose of this study was to measure the contemporary incidence of AS using a geographically defined population within southeast Minnesota and to assess their survival compared to the expected survival of community controls.
Methods:
Olmsted County is relatively isolated from other urban/suburban centers with only a few medical providers. These include Mayo Clinic and Olmsted Medical Center and their affiliated clinics. Due to the unique isolated nature of the region and few providers, billing data on all medical services are collated through the Rochester Epidemiology Project (REP).11, 12 This enables identification of incident diagnosis of medical conditions and permits review of treatments, evaluations, autopsy reports, and death certificates for decedents. We assembled a cohort of persons with AS among adults (≥18 years of age) residing in Olmsted County, Minnesota. The Mayo Clinic and Olmsted Medical Center Institutional Review Boards (IRB) approved this study. In addition, per Minnesota statutes, each patient identified with AS had provided authorization for the use of their medical record for research. No patients with AS were excluded due to lack or research authorization. The data cannot be made public since all residents were located in a specific Minnesota County. Any geographic subdivision smaller than a state cannot be de-identified according to the Health Insurance Portability and Accountability Act of 1996 definition of protected health information.
Identification of Incident Cohort and Controls
All unique patients with diagnosis code for AD, IMH or PAU using the International Classification of Disease (ICD), 9th revision diagnosis code (441.0–441.9), equivalent ICD-10 codes (I71.00-I71.03, I71.1-I71.6, I71.8, and I71.9, for October-December 2015) or Hospital Adaptation of the International Classification of Diseases, Second Edition (HICDA; a modification of the ICD-8)13 from REP providers were obtained from inpatient and outpatient encounters (1995–2015). All patient charts were reviewed including clinical data, imaging, autopsy reports, and death certificates to verify the diagnosis of AS. All patients were confirmed residents of Olmsted County at time of diagnosis to allow a true population based assessment of incidence. For diagnosis, patients were required to have imaging confirmation of AS (computed tomography (CT) with arterial contrast, magnetic resonance imaging (MRI), ultrasound, or conventional angiography), primary diagnosis of AS on their death certificate, or autopsy confirmation of AS. Categorization of AS was based on standard criteria; acute aortic dissection was defined as an intimal tear with the presence of a false lumen. Intramural hematoma was defined as crescent or circular thickening of the aortic wall without an intimal tear or dissection. Penetrating aortic ulcer was defined as a focal lesion of the aorta with erosion of the intima and the absence of intramural hematoma or dissection.14 Aortic dissection was classified using the DeBakey and Stanford Classification systems, and IMH classified with the Stanford system based on the involved portion of the aorta.14 In instances of uncertainty regarding the classification of AS based on imaging review, a vascular radiologist (T.M.) reviewed the imaging to render a final determination. If more than one aortic pathology was identified, primary categorization was based on the most severe pathology (AD>IMH>PAU). Additional pathologies were captured for further characterization. To ensure capture of all AS events, we also screened patients diagnosed with atherosclerosis of the aorta (ICD-9 440.0). A random 5% sample was reviewed, and only one PAU was identified (0.4%) of all screened patients; no further screening was performed. Patients were categorized according to the interval between onset of symptoms and diagnosis. Presentations were defined as acute (symptom onset within 14 days of diagnosis), subacute (symptom onset 15–90 days prior to diagnosis), chronic (symptom onset greater than 90 days prior to diagnosis) or unknown (if the exact date of symptom onset was never identified). For patients for whom onset of symptoms was not clearly documented, or whose pathology was asymptomatic (eg penetrating aortic ulcer), the date pathology was identified was considered the incident date.
We obtained two sets of population controls to assess the impact of AS on mortality. First, we utilized the expected survival of age- and sex-adjusted white residents of Minnesota (population controls), as the population in Olmsted County is predominantly white. Second, we randomly selected 3:1 Olmsted County residents as a control cohort (resident controls). Based on survival data for Olmsted County residents and those with AD, we calculated that a 3:1 matching ratio of controls to cases with an alpha of 0.05 and power of 0.8% would detect a minimum hazard ratio for death of 1.95. Resident controls were matched for birth year and sex to cases, and their charts were reviewed to confirm that no diagnosis of AS was present. As with AS cases, mortality and cause of death was ascertained in a similar manner.
The Charlson Comorbidities were obtained for AS cases and resident controls. Assignment of comorbidities used a refined algorithm that required two episodes of a diagnosis within the 5 years prior to the date of AS diagnosis as done previously within the REP.15 For resident controls, their matched AS case diagnosis date was used as the anchor date from which to base pre-existing comorbid conditions and define long term-events.
Assessment of Mortality
Mortality was assessed through two mechanisms. First, the REP data sources were queried for mortality status, and the death certificates were reviewed for cause. This included aortic-related (due to acute complications from AS or treatment of AS), cardiovascular-related (myocardial infarction, congestive heart failure, or stroke-related), or due to other reasons. Secondly, vital status and death date information were queried using an institutionally approved fee-based Internet research location service (Accurint, accurint.com) to ensure vital status was complete for all cases and resident controls. This service queries multiple databases for assessment of mortality and retrieval of death certificates. If death occurred outside Minnesota, death certificates were retrieved as permissible by the vital records statutes within the state in which the decedent passed away. Of all cases, three deaths occurred out of state, and the death certificates could not be obtained.
Statistical analysis
The incidence rates were estimated based on the number of new cases of AS in age, sex, and calendar year-specific stratum (numerator). The corresponding denominators were derived from annual census figures of Olmsted County, Minnesota, population (aged ≥18 years) from the 1990, 2000, and 2010 U.S. census, with linear interpolation for the inter-censal years, assuming the entire adult population was at risk. Incidence is reported as age- and sex-adjusted rates per 100,000 person-years, based on direct standardization against the 2010 U.S. white population, with 95% confidence intervals (CIs) estimated using the Poisson distribution.11 Multivariable Poisson regression modeling assessed the association of calendar year on incidence rate over the study period, adjusted for age and sex. Survival was evaluated as time to event and displayed as Kaplan Meier curves. The association of AS diagnosis on mortality was assessed by a stratified Cox proportional hazards modeling adjusting for age, sex, and Charlson comorbidity score to account for the case/control design. Patients with an autopsy diagnosis of AS were assigned a survival of zero days. Survival was also evaluated by AS subtype and 5 year epoch. Univariate associations of baseline characteristics with type of AS were made using ANOVA for continuous variables across the three groups and χ2 were used for categorical variables (with Fisher exact correction as needed for low event rates). Standardized mortality ratios were calculated using the observed versus expected risk of death based on age and sex of the study population with the risk of death among similar patients in Minnesota resident life tables.15 For statistical comparisons, a p value <0.05 was considered significant. Statistical analyses were performed with STATA (College Station, TX) and SAS (Cary, NC).
Results
Over the study interval, we identified 133 cases of AS (77 AD, 21 IMH, and 35 PAU). Overall, patients were predominantly white (86.5%) and male (57.1%). Mean age was 71.8 years (SD 14.1, range 28–93) and was lowest for AD and highest for PAU. Overall, 59.4% presented acutely, 3.0% subacute, 2.3% chronic, and 35.3% had an unknown event date prior to diagnosis and otherwise would constitute a chronic presentation (Table 1). Stanford A classification was most common for AD (58.4%) but Stanford B was more common for IMH (76.2%). Diagnosis was predominantly by CT scan for all subtypes; however, echocardiography was more frequent in patients with AD. Other details of presentation are presented in Table 1. Diagnosis was on autopsy for 6 patients (4.5%), all of which were after acute aortic dissection.
Table 1.
Baseline Characteristics of Incident Aortic Syndrome Cohort
| Total (N=133) |
Aortic Dissection (N=77) |
IMH (N=21) |
PAU (N=35) |
P |
|
|---|---|---|---|---|---|
|
Age (years, mean, SD) |
71.8 (14.1) | 68.9 (15.6) | 73.5 (11.5) | 77.1 (10.0) | 0.01 |
| Gender (male) | 76 (57.1%) | 46 (59.7%) | 11 (52.4%) | 19 (54.3%) | 0.77 |
| Ethnicity | 0.01 | ||||
| Hispanic/Latino | 2 (1.5%) | 2 (2.6%) | 0 (0.0%) | 0 (0.0%) | |
| Not Hispanic/Latino | 119 (89.5%) | 63 (81.8%) | 21 (100.0%) | 35 (100.0%) | |
| Unknown | 12 (9.0%) | 12 (15.6%) | 0 (0.0%) | 0 (0.0%) | |
| Race | 0.15 | ||||
| White | 115 (86.5%) | 64 (83.1%) | 18 (85.7%) | 33 (94.3%) | |
| Unknown | 11 (8.3%) | 10 (13.0%) | 1 (4.8%) | 0 (0.0%) | |
| African American | 3 (2.3%) | 1 (1.3%) | 1 (4.8%) | 1 (2.9%) | |
| Asian | 3 (2.3%) | 1 (1.3%) | 1 (4.8%) | 1 (2.9%) | |
| Hawaii/Pacific Island | 1 (0.8%) | 1 (1.3%) | 0 (0.0%) | 0 (0.0%) | |
| Acuity of diagnosis | <0.01 | ||||
| Acute | 79 (59.4%) | 52 (67.5%) | 17 (81.0%) | 10 (28.6%) | |
| Subacute | 4 (3.0%) | 2 (2.6%) | 1 (4.8%) | 1 (2.9%) | |
| Chronic | 3 (2.3%) | 2 (2.6%) | 0 (0.0%) | 1 (2.9%) | |
| Unknown | 47 (35.3%) | 21 (27.3%) | 3 (14.3%) | 23 (65.7%) | |
|
Other clinical features at presentation |
|||||
| Malperfusion | 6 (4.5%) | 5 (6.5%) | 1 (4.8%) | 0 (0.0%) | 0.36 |
| Rupture | 12 (9.0%) | 9 (11.7%) | 2 (9.5%) | 1 (2.9%) | 0.36 |
| Coronary Ischemia | 7 (5.3%) | 5 (6.5%) | 2 (9.5%) | 0 (0.0%) | 0.24 |
| Cardiac Tamponade | 10 (7.5%) | 10 (13.0%) | 0 (0.0%) | 0 (0.0%) | 0.02 |
| Aortic Valve Insufficiency |
14 (10.5%) | 14 (18.2%) | 0 (0.0%) | 0 (0.0%) | <0.01 |
|
Blood pressure at presentation |
|||||
| Systolic | 139.2 (33.3) | 136.8 (34.8) | 139.7 (35.3) | 143.6 (29.0) | 0.62 |
| Diastolic | 73.6 (16.8) | 74.6 (19.0) | 71.1 (17.2) | 73.1 (11.6) | 0.70 |
| Shock at presentation | 8 (6.3%) | 7 (9.9%) | 1 (4.8%) | 0 (0.0%) | 0.14 |
| BMI | 27.5 (6.8) | 27.4 (6.7) | 28.8 (7.1) | 26.9 (6.7) | 0.60 |
|
Medications at presentation |
|||||
| Aspirin | 58 (43.6%) | 28 (36.4%) | 12 (57.1%) | 18 (51.4%) | 0.13 |
| Beta blocker | 53 (39.8%) | 28 (36.4%) | 11 (52.4%) | 14 (40.0%) | 0.41 |
| ACEI/ARB | 43 (32.3%) | 21 (27.3%) | 9 (42.9%) | 13 (37.1%) | 0.31 |
| Statin | 42 (31.6%) | 21 (27.3%) | 6 (28.6%) | 15 (42.9%) | 0.25 |
|
Lab values at presentation |
|||||
| Hemoglobin | 12.7 (2.0) | 13.3 (1.9) | 12.1 (1.9) | 12.0 (1.9) | <0.01 |
| Creatinine | 1.3 (1.2) | 1.4 (1.6) | 1.1 (0.3) | 1.2 (0.4) | 0.44 |
| INR | 1.3 (0.6) | 1.3 (0.6) | 1.3 (0.6) | 1.3 (0.6) | 0.96 |
| Platelets | 214.6 (85.6) | 207.7 (69.3) | 220.3 (112.3) | 225.0 (98.2) | 0.61 |
| PTT | 37.0 (34.7) | 31.9 (16.3) | 50.9 (66.4) | 34.1 (14.5) | 0.25 |
| Risk factors | |||||
| Bicuspid Aortic Valve | 3 (2.3%) | 1 (1.3%) | 1 (4.8%) | 1 (2.9%) | 0.38 |
| Aortic Atherosclerosis | 111 (83.5%) | 57 (74.0%) | 20 (95.2%) | 34 (97.1%) | <0.01 |
| Prior Aortic Dilatation | 64 (51.6%) | 36 (50.7%) | 13 (68.4%) | 15 (44.1%) | 0.23 |
| Connective tissue disorder |
8 (6.0%) | 8 (10.4%) | 0 (0.0%) | 0 (0.0%) | 0.18 |
| Iatrogenic | 7 (5.3%) | 6 (7.8%) | 1 (4.8%) | 0 (0.0%) | 0.11 |
| Prior Aortic Surgery | 14 (10.5%) | 8 (10.4%) | 2 (9.5%) | 4 (11.4%) | 0.97 |
| Diagnostic modality | |||||
| ECHO | 15 (11.3%) | 13 (16.9%) | 2 (9.5%) | 0 (0.0%) | 0.01 |
| CT | 105 (78.9%) | 53 (68.8%) | 19 (90.5%) | 33 (94.3%) | <0.02 |
| MRI | 4 (3.0%) | 2 (2.6%) | 0 (0.0%) | 2 (5.7%) | 0.62 |
| Angiography | 3 (2.3%) | 3 (3.9%) | 0 (0.0%) | 0 (0.0%) | .32 |
| Stanford Class | |||||
| A | - | 45 (58.4%) | 5 (23.8%) | - | <.01 |
| B | - | 32 (41.6%) | 16 (76.2%) | - | |
| DeBakey Class | |||||
| I | - | 24 (31.2%) | - | - | |
| II | - | 21 (27.3%) | - | - | |
| IIIa | - | 8 (10.4%) | - | - | |
| IIIb | - | 24 (31.2%) | - | - | |
IMH – intramural hematoma, PAU – penetrating aortic ulcer.
The overall age- and sex-adjusted incidence of AS was 7.7 per 100,000 person-years (95% CI 6.4–9.0). There was no significant change in incidence over the study interval. We observed the lowest age- and sex-adjusted rate of 6.4 per 100,000 person-years from 2005–09 and a high of 9.8 in both the 2000–05 and 2010–15 (p=.33) time periods. The overall age- and sex-adjusted incidence of AD, IMH and PAU were 4.4 (95% CI 3.4–5.3), 1.2 (95% CI 0.7–1.8), and 2.1 (95% CI 1.4–2.7) per 100,000 person-years, respectively. The incidence of AD and IMH was stable. Although the incidence of PAU increased significantly from 0.6 to 2.6 per 100,000 person years (p=.008) this occurred in the setting of significant variability over the study interval (Table 2). AS was most common in males and those greater than 70 years of age (Table 3). Compared to county controls, those with AS had a higher prevalence of prior MI, peripheral vascular disease, chronic obstructive pulmonary disease, and chronic liver disease. Patients with AS had a higher Charlson comorbidity index compared to resident controls (2.6 vs. 1.7, p<0.001) at the time of diagnosis (Table 4).
Table 2.
Incidence of Aortic Syndrome and subtypes in Olmsted County Minnesota from 1995–2015
| Overall (1995–2015) | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2015 | P* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AS | Incidence | 95% CI | Incidence | 95% CI | Incidence | 95% CI | Incidence | 95% CI | Incidence | 95% CI | |
| Overall (age/sex adj) | 7.67 | 6.36–8.97 | 8.5 | 5.39–11.68 | 9.79 | 6.61–12.98 | 6.44 | 4.05– 8.835 | 9.78 | 6.71–12.87 | 0.33 |
| Women (age adj) | 5.69 | 4.20–7.19 | 6.78 | 3.18–10.38 | 6.65 | 3.24–10.06 | 4.94 | 2.13 7.75 | 7.26 | 3.69–10.84 | |
| Men (age adj) | 10.24 | 7.89–12.58 | 9.89 | 4.81–14.97 | 13.89 | 7.95–19.82 | 8.67 | 4.36–12.97 | 13.49 | 7.90–19.08 | |
| Aortic Dissection | |||||||||||
| Overall (age/sex adj) | 4.37 | 3.38– 5.35 | 4.36 | 3.93– 9.50 | 6.715 | 2.22– 6.35 | 4.29 | 1.83– 5.39 | 5.25 | 3.008– 7.50 | 0.21 |
| Women (age adj) | 3.07 | 1.98– 4.16 | 3.07 | 2.12– 8.51 | 5.31 | 2.38– 9.55 | 2.47 | 0.48– 4.50 | 3.72 | 1.13– 6.31 | |
| Men (age adj) | 5.86 | 4.14– 7.60 | 5.86 | 3.29–12.24 | 7.77 | 0.48– 4.45 | 5.97 | 1.92– 8.46 | 7.53 | 3.38–11.69 | |
| Intramural Hematoma | |||||||||||
| Overall (age/sex adj) | 1.24 | 0.70– 1.77 | 1.23 | 0.02 2.44 | 1.17 | 0.02– 2.31 | 1.17 | 0.14– 2.20 | 1.98 | 0.61– 3.36 | 0.66 |
| Women (age adj) | 0.99 | 0.61– 2.40 | 0.95 | 0.00– 2.27 | 1.06 | 0.00– 2.52 | 1.2 | 0.00– 2.79 | 1.24 | 0.00– 2.65 | |
| Men (age adj) | 1.50 | 0.38– 1.62 | 1.43 | 0.00– 3.42 | 1.26 | 0.00– 3.01 | 1.16 | 0.00– 2.56 | 2.85 | 0.33– 5.37 | |
| Penetrating Aortic Ulcer | |||||||||||
| Overall (age/sex adj) | 2.06 | 1.38– 2.75 | 0.59 | 0.00– 1.42 | 4.34 | 2.20– 6.48 | 1.65 | 0.43– 2.88 | 2.56 | 0.97– 4.14 | <0.01 |
| Women (age adj) | 1.62 | 0.82– 2.43 | 0.514 | 0.00– 1.52 | 3.13 | 0.78– 5.48 | 1.25 | 0.00– 2.67 | 2.29 | 0.27– 4.31 | |
| Men (age adj) | 2.88 | 1.57– 4.18 | 0.69 | 0.00– 2.05 | 6.66 | 2.26–11.05 | 2.3 | 0.03– 4.60 | 3.10 | 0.35– 5.86 | |
AS – Aortic syndrome, adj – adjusted. P values for overall trend from 1995–2015
Poisson model including age, sex, and calendar year of diagnosis, the p value is the significance of a calendar year change in incidence over the time interval.
Table 3.
Unadjusted Rates of Aortic Syndrome Across Age and Sex Strata in Olmsted County Minnesota from 1995–2015
| Incidence Rate (per 100,000 person-years) | |||
|---|---|---|---|
| Age Group | Male | Female | Total |
| 18–49 | 0.94 | 0.62 | 0.78 |
| 50–59 | 6.49 | 1.68 | 4.02 |
| 60–69 | 12.72 | 8.00 | 10.25 |
| 70–79 | 40.48 | 26.38 | 32.71 |
| 80+ | 62.96 | 32.82 | 43.14 |
| Total | 7.58 | 5.29 | 6.39 |
Table 4.
Charlson Comorbidities for Aortic Syndrome Cases and Controls in Olmsted County
| Charlson Scores | Case (N=133) |
Control (N=399) |
Total (N=532) |
P |
|---|---|---|---|---|
| Prior Myocardial Infarction | 19 (14.3%) | 13 (3.3%) | 32 (6.0%) | <0.01 |
| Congestive Heart Failure | 24 (18.0%) | 45 (11.3%) | 69 (13.0%) | 0.04 |
| Peripheral vascular disease | 54 (40.6%) | 56 (14.0%) | 110 (20.7%) | <0.01 |
| Cerebrovascular artery disease | 26 (19.5%) | 53 (13.3%) | 79 (14.8%) | 0.08 |
| Dementia | 9 (6.8%) | 23 (5.8%) | 32 (6.0%) | 0.67 |
| COPD | 31 (23.3%) | 58 (14.5%) | 89 (16.7%) | 0.02 |
| Gastric ulcer | 7 (5.3%) | 10 (2.5%) | 17 (3.2%) | 0.12 |
| Chronic Liver Disease | 11 (8.3%) | 15 (3.8%) | 26 (4.9%) | 0.04 |
| Diabetes Mellitus without complications |
23 (17.3%) | 75 (18.8%) | 98 (18.4%) | 0.69 |
| Rheumatic or Connective Tissue Disease |
7 (5.3%) | 20 (5.0%) | 27 (5.1%) | 0.91 |
| Diabetes Mellitus with end organ complications |
6 (4.5%) | 23 (5.8%) | 29 (5.5%) | 0.58 |
| Hemiplegia | 5 (3.8%) | 2 (0.5%) | 7 (1.3%) | <0.01 |
| Renal Insufficiency | 18 (13.5%) | 32 (8.0%) | 50 (9.4%) | 0.06 |
| Prior Malignancy | 26 (19.5%) | 80 (20.1%) | 106 (19.9%) | 0.90 |
| Moderate/Severe Liver Disease | 1 (0.8%) | 2 (0.5%) | 3 (0.6%) | 1 |
| Metastatic Solid Tumor | 5 (3.8%) | 8 (2.0%) | 13 (2.4%) | 0.33 |
| AIDS | 0 | 0 | 0 | 1 |
| Charlson comorbidity index | 2.6 (2.6) | 1.7 (2.1) | 1.9 (2.3) | <0.01 |
Over a median follow-up of 10.1 years, 73 deaths occurred in our AS cohort and 144 in Olmsted County controls. Mortality in cases was predominantly aortic-related (32%); cardiovascular-related death occurred in 29%, while 40% were due to other causes (8 cancer-related, 2 respiratory-related and 16 from other etiologies, 3 unknown). Overall survival was significantly lower for patients with AS compared to Olmsted County age-sex matched controls (resident controls), p<0.001, with median survivals of 7.3 and 14.7 years, respectively. Survival after diagnosis of AS was significantly lower than controls, both from Olmsted County resident controls and the expected survival of Minnesota residents (population controls, Figure 1). The 1-, 5-, 10- and 20-year survival for those with AS was 81%, 61%, 43%, and 9% compared to Olmsted County resident controls, 97%, 82%, 59%, and 34% (p<0.001) or expected survival of Minnesota white population controls (95%, 78%, 54%, and 27%, p<0.001). In a model including the case status along with age, sex, and Charlson comorbidity score, the adjusted hazard ratio (HR) for death for patients with AS compared to Olmsted County resident controls was 2.1 (95% CI 1.6–2.9, p<0.001). Given the high acute mortality after diagnosis, we also analyzed long-term survival excluding deaths within 14 days of diagnosis (followup started day 15, which removed 15 patients from the analysis), including the same additional variables in the model. AS was still associated with an increased age- and sex-adjusted risk for death when eliminating acute mortality compared to Olmsted County controls (HR 1.8, 95% CI 1.3–2.5, Figure 2). Survival was not different between AD, IMH, or PAU (p=.82, Figure 3), or between years of diagnosis (5-year increments, P=.92, Figure 4).
Figure 1.
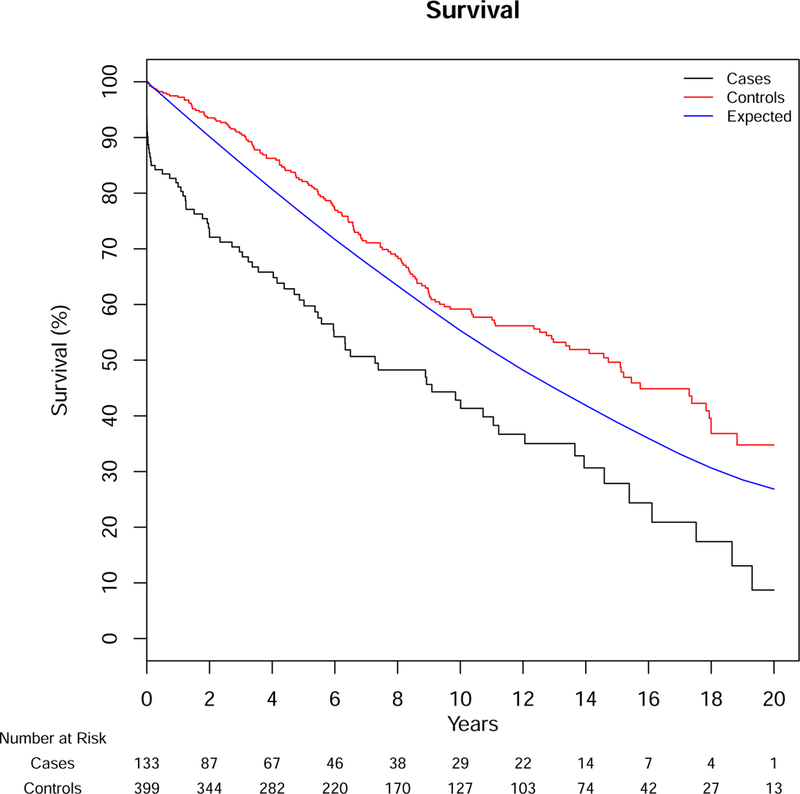
Survival for Patients with Aortic Dissection, Intramural Hematoma or Penetrating Ulcer Compared to Population Controls
Cases – Aortic Syndrome Patients, Controls – Matched Olmsted County Controls, Expected – Minnesota White Population. Log-rank for cases vs. controls p<0.001, cases vs. expected p<.001. SE<10%
Figure 2.
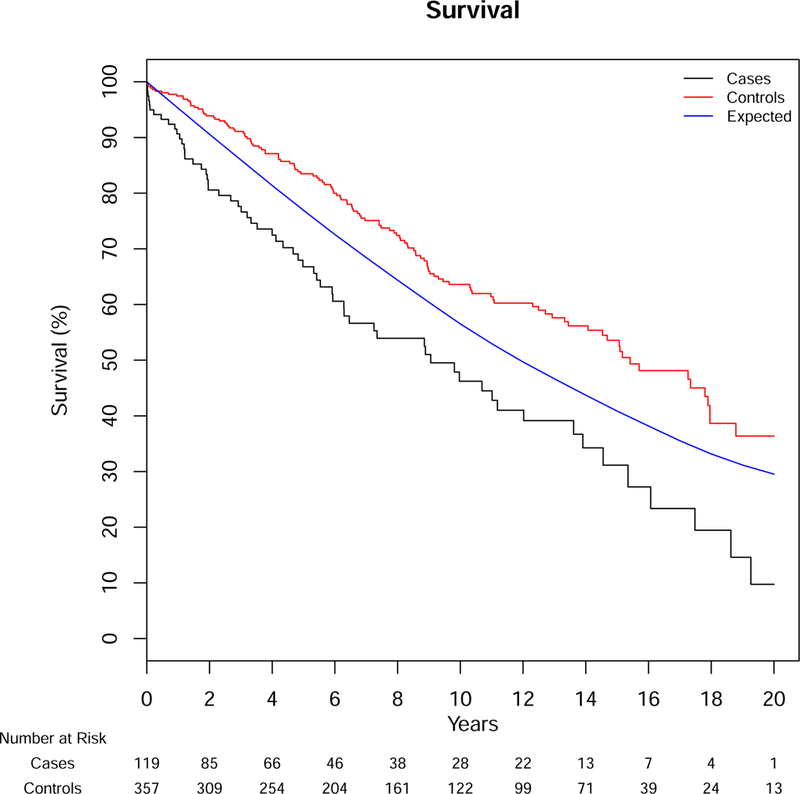
Survival for Patients with Aortic Dissection, Intramural Hematoma or Penetrating Ulcer Compared Population Controls Removing Deaths <14 days after Diagnosis
Cases – Aortic Syndrome Patients, Controls – Match Olmsted County Controls, Expected – Minnesota White Population. Log-rank for cases vs. controls p<0.001, cases vs. expected p<.001. SE<10%
Figure 3.
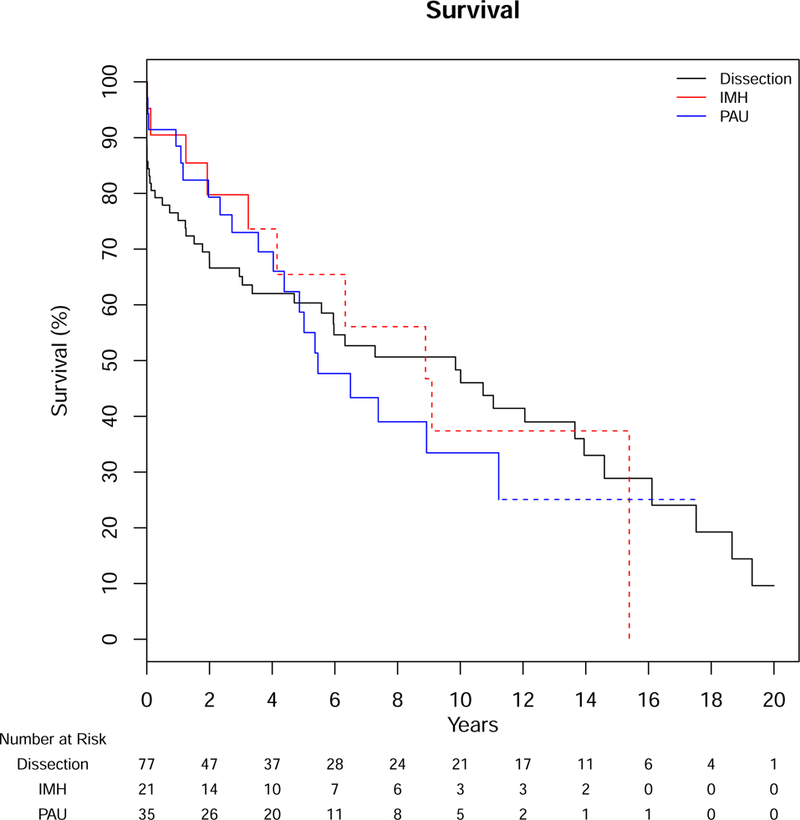
Survival After Diagnosis of Aortic Dissection, Intramural Hematoma and Penetrating Aortic Ulcer
IMH – intramural hematoma, PAU – penetrating aortic ulcer
Log-rank p=0.87, Dotted lines represent SE>10%
Figure 4.
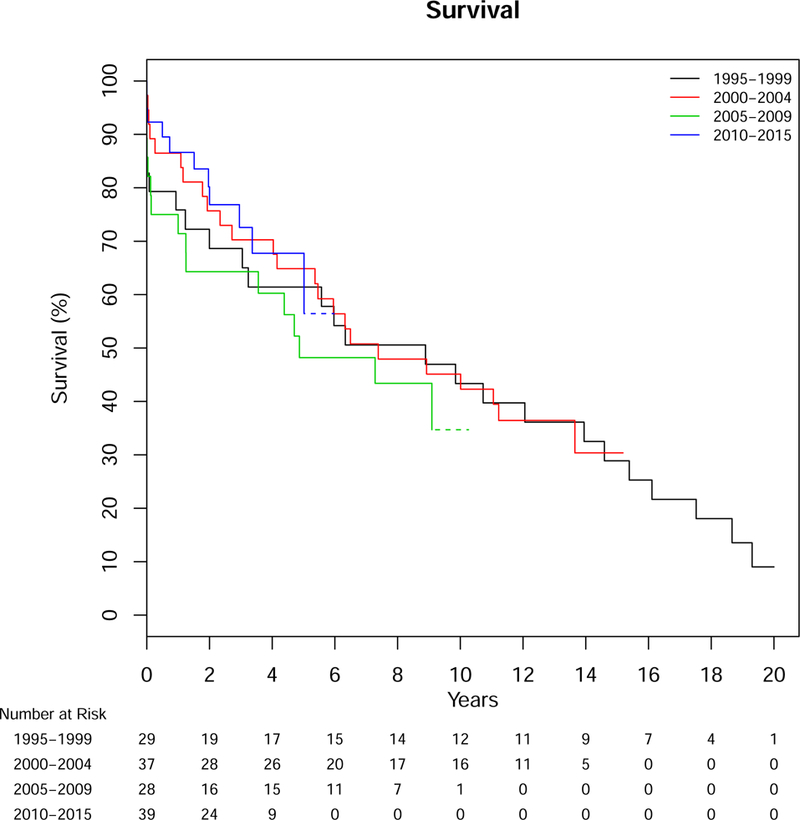
Survival After Diagnosis for Patients with Aortic Dissection, Intramural Hematoma or Penetrating Ulcer Over Time from 1995–2015
Log-rank p=.73. Dotted lines represent SE>10%
To assess mortality risk of AS after diagnosis at various time intervals, standardized mortality ratios (SMR) were calculated in patients with AS compared to similar age and sex residents of Minnesota. Patients with AS had increased risk of death at 0–14 days from diagnosis (SMR 66.8, 95% CI 37.4–110.18) and 15–90 days (4.4 95% CI 1.42–10.24,) and 1–2 years (2.36 95% CI 1.18–4.22, Figure 5).
Figure 5.
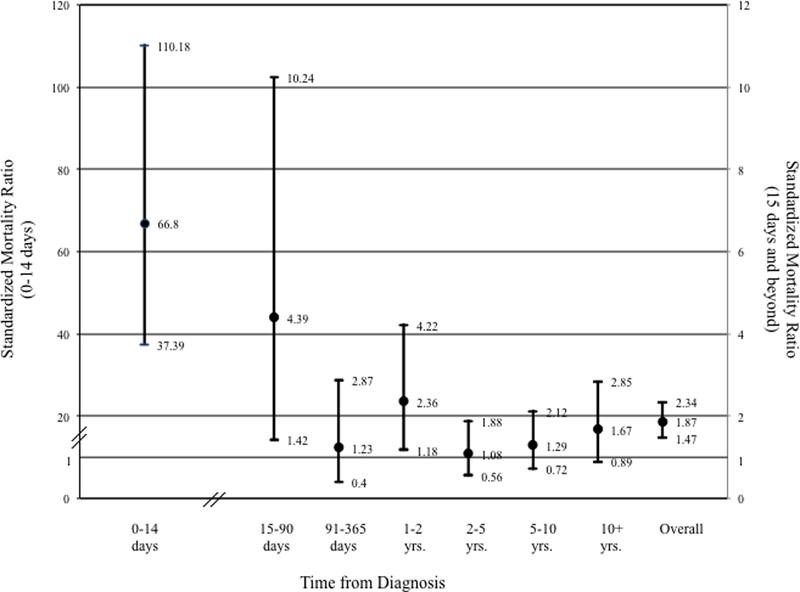
Standardized Mortality Rates after Diagnosis of Aortic Dissection, Intramural Hematoma or Penetrating Ulcer. Left sided Y-axis rates for 0–14 days after diagnosis. Right sided Y-axis values for 15 days and beyond.
Discussion
In this population-based assessment of AS, the incidence of AS has remained stable since 1995 at 7.7 per 100,000 person-years in Olmsted County, despite the known reduction in several cardiovascular diseases. Additionally, the incidence of AD and IMH has remained stable, and the incidence of PAU appears to have increased during that interval. The incidence of AS is higher for males (nearly two-fold) and those >70 years of age. Compared to local controls, patients with AS have higher rates of cardiac and vascular disease and chronic obstructive lung disease and carry a higher comorbidity burden than controls. Median survival after AS was 7.3 years and was lower than both population and resident control patients. Even with adjustment for acute mortality, long-term survival remained lower for patients with AS, and a dramatic increased risk of death extends to 90 days from diagnosis. Finally, there seems to be no obvious survival improvement after AS over time in our cohort. With these data, future work can focus on defining targets to improve the prognosis of these aortic pathologies.
The publication of the incidence of acute aortic dissection by Clouse et al. from 1980–1994 reported an age- and sex-adjusted incidence of 3.4 per 100,000 person-years (95% CI 2.4–4.6) based on the 1990 US white populations.3 In our study, which reported incidence from 1995–2015, we saw a similar incidence of acute dissection (4.4 per 100,000 person-years), suggesting little change in the incidence of this disease since 1980. Although Clouse et al. noticed an increasing trend in the incidence of AD (that did not reach significance), we did not see any notable increase in our population over the last 20 years. Only PAU had an increase in incidence, predominantly due to a sharp rise from 1995–1999 to the later years. However there was a peak from 2000–2004 and then subsequent decrease after this. Due to few events and this variability, it is difficult to definitely conclude PAU incidence is increasing. It is also unclear the cause for this apparent increase. One hypothesis may be increased axial imaging and the incidental identification of asymptomatic PAU. However, mortality for this pathology remained similar over time, symptomatic presentation was similar over time (40%), and the distribution of acute presentations was also similar over time (29%). Thus, our data do not support the hypothesis that we identified more asymptomatic disease. Symptomatic PAU has been included in the description of acute artic pathologies due to its potential fur symptoms and development if IMH. However, it is a distinct pathology from AD and IMH. As a chronic atherosclerotic process, the contributors to PAU are likely to be more centered on atherosclerotic risk factors while AD and IMH due to more structural and genetic factors. Further research on these patients is necessary to identify the etiology for this rise in incidence compared to AD and IMH.
The finding that overall AS incidence is stable contrasts sharply with what has been observed for other cardiovascular diseases. In particular, the incidence of coronary artery disease and abdominal aortic aneurysms are declining.9, 16 One of the key drivers for the decline in coronary disease and abdominal aortic aneurysm likely is the concomitant decline in smoking among adults.8 Interestingly, the association between tobacco use and AS is less established, and our finding may suggest it has a small impact on the development of AS. Within the same geographic region (Olmsted County, Minnesota) major changes in the epidemiology of MI have been reported. From 1987–2006, there has been a decline in the incidence of ST elevation MI, a decrease in the severity of infarction, and improvements in mortality after MI.7 Similar trends have been reported in other settings,17–19 and the discrepancy with the trends for AS was unanticipated. Lead time bias could be hypothesized, whereby more asymptomatic or incidental disease is identified. As discussed above, increased CT imaging that identifies more disease would explain our stable incidence, if this were true. Yet, we see similar mortality trends over the study interval. This would suggest that we continue to identify patients with similar acuity and prognosis and are likely not identifying a greater proportion of patients with less severe or morbid disease. With unchanged acuity or long-term hazards of AS over time, it is likely that the incidence has remained stable. Aortic dissection has several known non-modifiable risk factors, such as bicuspid aortic valve, connective tissue disease, and chromosomal abnormalities, and these would not be expected to alter with atherosclerotic disease trends. However, few patients had these risk factors identified in the medical record. Further understanding of modifiable and non-modifiable risk factors is needed to identify targets to reduce the incidence of AS. Further work on family trees and potential genetic triggers for aortic disease in this cohort is planned to try to determine the role genetics may play across all three pathologies.
Epidemiology data on thoracic aortic pathology is scarce in the literature. One of the only other recent epidemiological studies on aortic dissection and aneurysms came from the Swedish national health care registry.10 Our data differ from those in Sweden that suggest the incidence of thoracic aortic pathology (dissection and aneurysm) has increased from 1987–2002. Although their study interval differs slightly from ours, they demonstrated a rise in the incidence in men by 52% (10.7–16.3 per 100,000 person-years) and women by 28% (7.1–9.1 per 100,000 person-years). Comparison to our data is difficult since they did not report the specific incidence of aortic dissection; however, they report a similarly high rate of aortic-related deaths (39%). In addition, 31% of long-term deaths were due to cardiovascular disease. This is similar to our findings. Thus, aortic-related events are not only the most prevalent cause of acute mortality, but also for long-term death. The proclivity for AS to result in long-term aortic pathology is well known. Approximately 50% of patients will have aortic growth after acute Stanford B dissection, and over 25% will need intervention.20 Although lifetime blood pressure control has been the historic treatment paradigm after presentation, alternative treatment may be needed to improve these results. Thoracic endovascular aortic repair may improve long-term outcomes and could represent such an option.21 However, further work is needed to define what the optimal care for these patients should be beyond initial diagnosis and acute treatment.
Based on historical data, the increased mortality associated with AD was noted to be within 14 days and has defined the acute period.1 We confirmed that there is an excessive mortality risk in the acute (<14 day) time period for those with AS. However, we also noted an increased risk of death to 90 days, and this confirms that the “subacute” phase of these pathologies carries additional mortality risk. After this, mortality risk appears to be similar to the general population, with the exception of one and two years post diagnosis. This may be due to interventions performed or other cardiovascular events occurring at later times. Future work will look to define why this was observed, as we study fatal and non-fatal cardiovascular and aortic-related events for this cohort.
Our study has several limitations that should be acknowledged to aid in the interpretation of the results. The generalizability of our data is limited by the demographical characteristics of Olmsted County, notable a predominantly white population. However, prior data have shown that age, sex and ethnic characteristics of Olmsted County are similar to those of Minnesota and the upper Midwest. Additionally, mortality among Olmsted County is similar to the U.S. overall.22 We did note that the mortality rate of resident controls was better than the Minnesota white population, and this suggests residents in Olmsted County may be slightly healthier than state residents overall. Although we have tried to identify all possible cases, there may be patients with sudden death that did not undergo autopsy that would have been missed. Lastly, we have been inclusive in all dissection cases (including iatrogenic) to define the total incidence of the clinical pathology in our population beyond only spontaneous cases.
Our study is strengthened by the fact that this represents the most contemporary epidemiological assessment of AS in the U.S. There are limited resources to conduct such robust epidemiology research in the U.S. since our patient cohort is not subject to referral bias seen in registry or tertiary center reports, we can assess cause of death for nearly all patients, and all health care payers are included in the REP. With this, we have shown a stable incidence of AS since 1995, and these patients carry significantly higher rates of cardiac and peripheral vascular disease. Improvements in other cardiovascular trends have not translated to this patient group, and our findings strengthen the need to understand the modifiable and non-modifiable risk factors for AS to decrease its occurrence. Additionally, the overall survival after AS diagnosis is significantly reduced compared to population controls, and the mortality prognosis of AS appears unchanged. Improvements in post-diagnosis and long-term care are needed to improve the overall survival of this complex patient population. Further work will focus on these factors to improve outcomes and enhance guidelines for management of patients with AS.
Supplementary Material
What is known
Aortic dissection, intramural hematoma and penetrating ulcer are associated with significant aortic related mortality and morbidity
Historical estimates suggest an incidence of aortic dissection of 3.5 per 100,000 person-years, but this is from data collected prior to 1995
What the study adds
This study defines the contemporary incidence of aortic dissection, intramural hematoma and penetrating aortic ulcer using a population based approach
The incidence of aortic dissection and intramural hematoma have remained stable from 1996–2015 and the incidence of penetrating aortic ulcer may be increasing
Aortic dissection, intramural hematoma, and penetrating ulcer confer significant acute (within 14 day of diagnosis) and subacute (up to 90 days) mortality compared to population controls
Acknowledgments
Sources of Funding:
This study was supported by the American Heart Association (16SDG27250043). This study was also made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institutes of Health National Institute on Aging under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Data storage was performed with REDCap (UL1TR002377).
Footnotes
Disclosures:
None
References:
- 1.Hirst AE Jr., Johns Jr. and Kime SW Jr. Dissecting aneurysm of the aorta: a review of 505 cases. Medicine 1958;37:217–79. [DOI] [PubMed] [Google Scholar]
- 2.Nienaber CA and Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation 2003;108:628–35. [DOI] [PubMed] [Google Scholar]
- 3.Clouse WD, Hallett JW Jr., Schaff HV, Spittell PC, Rowland CM, Ilstrup DM and Melton LJ 3rd. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clinic proceedings 2004;79:176–80. [DOI] [PubMed] [Google Scholar]
- 4.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM and Eagle KA. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA : the journal of the American Medical Association 2000;283:897–903. [DOI] [PubMed] [Google Scholar]
- 5.Jones DW, Goodney PP, Nolan BW, Brooke BS, Fillinger MF, Powell RJ and Stone DH. National trends in utilization, mortality, and survival after repair of type B aortic dissection in the Medicare population. Journal of vascular surgery 2014;60:11–19 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mody PS, Wang Y, Geirsson A, Kim N, Desai MM, Gupta A, Dodson JA and Krumholz HM. Trends in aortic dissection hospitalizations, interventions, and outcomes among medicare beneficiaries in the United States, 2000–2011. Circulation Cardiovascular quality and outcomes 2014;7:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roger VL, Weston SA, Gerber Y, Killian JM, Dunlay SM, Jaffe AS, Bell MR, Kors J, Yawn BP and Jacobsen SJ. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation 2010;121:863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lederle FA. The rise and fall of abdominal aortic aneurysm. Circulation 2011;124:1097–9. [DOI] [PubMed] [Google Scholar]
- 9.Svensjo S, Bjorck M, Gurtelschmid M, Djavani Gidlund K, Hellberg A and Wanhainen A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011;124:1118–23. [DOI] [PubMed] [Google Scholar]
- 10.Olsson C, Thelin S, Stahle E, Ekbom A and Granath F. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation 2006;114:2611–8. [DOI] [PubMed] [Google Scholar]
- 11.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd and Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. American journal of epidemiology 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR and Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Commission on Professional and Hospital Activities. and National Center for Health Statistics (U.S.). H-ICDA; hospital adaptation of ICDA 2d ed. Ann Arbor,; 1973. [Google Scholar]
- 14.Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr., Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM, American College of Cardiology Foundation/American Heart Association Task Force on Practice G, American Association for Thoracic S, American College of R, American Stroke A, Society of Cardiovascular A, Society for Cardiovascular A, Interventions, Society of Interventional R, Society of Thoracic S and Society for Vascular M. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266–369. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM, Killian JM, Weston SA and Roger VL. Decade Long Trends in Atrial Fibrillation Incidence and Survival: A Community Study. The American journal of medicine 2015;128:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandiford P, Mosquera D and Bramley D. Trends in incidence and mortality from abdominal aortic aneurysm in New Zealand. Br J Surg 2011;98:645–51. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Jacobsen JB, Lash TL, Botker HE and Sorensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. Bmj 2012;344:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smolina K, Wright FL, Rayner M and Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. Bmj 2012;344:d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV and Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. The New England journal of medicine 2010;362:2155–65. [DOI] [PubMed] [Google Scholar]
- 20.Durham CA, Aranson NJ, Ergul EA, Wang LJ, Patel VI, Cambria RP and Conrad MF. Aneurysmal degeneration of the thoracoabdominal aorta after medical management of type B aortic dissections. Journal of vascular surgery 2015;62:900–6. [DOI] [PubMed] [Google Scholar]
- 21.Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, Glass A, Scheinert D, Czerny M, Kleinfeldt T, Zipfel B, Labrousse L, Fattori R, Ince H and trial I-X. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circulation Cardiovascular interventions 2013;6:407–16. [DOI] [PubMed] [Google Scholar]
- 22.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd and Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic proceedings 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


