This study by Fan et al1 in this issue of Acta Physiologica entitled “Orexin A increases sympathetic nerve activity through promoting expression of pro-inflammatory cytokines in Sprague-Dawley rats” sheds some light on the potential signalling pathways leading to cardiovascular activation with regard to increased sympathetic activity and neuro-inflammation and provides an interesting model for future work testing the neuro-inflammation-driven hypertension hypothesis.
Orexin A is an excitatory neuropeptide with high levels of expression during wakefulness. Low level of orexin A is associated with narcolepsy. This system is involved in a variety of cognitive, neuro-endocrine and cardiovascular processes.2 Orexin A binds to Gq protein-coupled receptors, orexin 1 receptor (OX1R) and orexin 2 receptor (OX2R) but has higher affinity to OX1R. Activation of OX1R results in increased intracellular calcium level and nerve discharge.3 The orexin A system is found in neurones within the dorsolateral hypothalamus, which projects to the paraventricular nucleus (PVN) and regulates sympathetic outflow. Therefore, research related to neural control of the cardiovascular system has identified it as a regulator of autonomic health. The study presented by Fan et al1 illustrates that PVN-rostro-ventrolateral medulla (RVLM)-projecting neurons express specifically OX1R and not OX2R. Microinjecting orexin A in the PVN increased splanchnic and renal sympathetic nerve activities. These effects were significantly suppressed when an OX1R antagonist was administered, further clarifying the contribution of OX1R over OX2R.
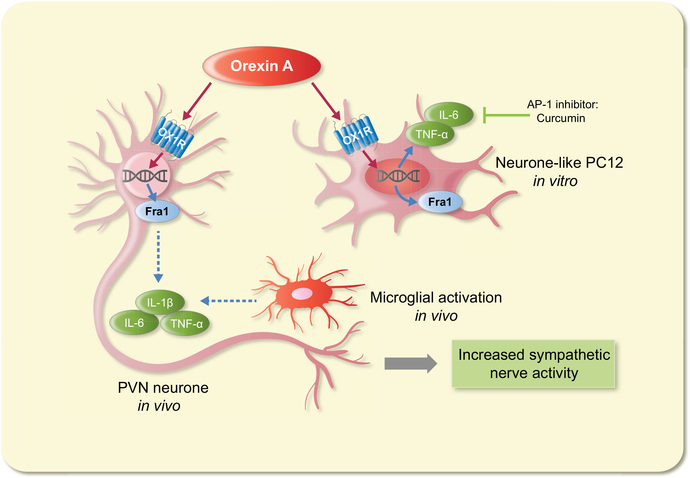
The authors have identified that, in cell cultures, neuron-like cells artificially expressing OX1R respond differently to orexin A than those that express OX2R. Exclusively, OX1R activation led to increased IL-6 and TNF-α, while OX2R does not impact pro-inflammatory cytokine levels. On the other hand, injecting orexin A into the PVN led to increased expression of IL-1β, in addition to IL-6 and TNF-α. Of importance, the neuron-like cells used in these cultures do not express IL-1β and the cultures also lacked glial cells to mimic the physiological environment of the brain. Increased sympathetic activity leads to microglial activation and increased levels of IL-1β, IL-6 and TNF-α in the PVN.4 Therefore, the present data indirectly confirm the critical role of glial cells in the production of IL-1β following orexin A stimulation within the PVN. Moreover, the effect of orexin A increases with longer exposure, peaking at 6 hours in vitro. Even without accounting for glial cell interactions contributing to inflammatory responses in the brain, the authors have provided convincing cell culture studies pointing to a significant contribution for the neurons alone.
The physiological relevance of this finding becomes more interesting, as orexin A levels follow a diurnal pattern with increased levels in the morning and a drop by nighttime. During early hours in the day, orexin A levels in human CSF are >700 pg/mL.5 An obvious extension of this work would be to clarify the levels of orexin A in hypertensive patients or animals. Hypertensive patients present their symptoms with elevated systolic blood pressure during wakefulness and an absence of the 10% dip during night-time, called the non-dipping response. As orexin expression levels are regulated according to the sleep-wake cycle, there is a potential correlation with the non-dipping response. However, a consensus about this correlation is still lacking. Interestingly, orexin deficiency in humans is associated with night-time BP dysregulation leading to increased cardiovascular risk. 6
Administering orexin A in the PVN increases excitability of magnocellular, parvocellular and spinally projecting neurons (SPN). However, the orexigenic projections that modulate sympathetic activity are mainly glutamatergic spinally projecting neurons.7 This phenotype goes along with the data by Fan et al1 that increased Fra-1 co-localization with OX1R-expressing neurons contributes to increased excitability of functional projections from the PVN towards the brainstem and spinal cord.
In Fan et al1 study, curcumin was used as an AP-1 blocker to prevent the increased expression of pro-inflammatory markers. Although it reduced the expression of IL-6 and TNF-α, it did not suppress Fra-1 expression. Fra-1 is used as a marker of chronic neuronal activation, and it is also a downstream molecule in the AP-1 signalling pathway. The data presented by Fan et al1 highlight that injecting orexin A in the PVN increases peripheral nerve activity within seconds. However, in cell cultures, the inflammatory response takes 6 hours to reach significant levels of cytokines mRNA expression. These observations leave room for further analysis, to verify the authors’ hypothesis that orexin A-induced inflammation leads to neuronal activation in the PVN and sympathetic nerve activity. The inflammatory factors do not appear to be the primary cause leading to increased nerve activity. Alternatively, it is conceivable that these inflammatory markers contribute to a feedforward mechanism with the resultant effect of increased neuronal excitability and sympathetic activity (Figure 1).
FIGURE 1.
Summary of the study presented by Fan et al.1 OX1R-mediated stimulation of PVN neurons leads to increased Fra-1 activation and peripheral sympathetic activity. In addition, orexin A-driven induction of pro-inflammatory cytokines in vitro can be blocked with an AP-1 inhibitor without affecting neuronal activity. These data provide an elegant model for further investigation of the neuro-inflammation-driven hypertension hypothesis
Elevated sympathetic nerve activity due to orexin A in the brain has been dealt with in cardiorespiratory, metabolic and cardiovascular studies. Clearly, the impact of orexin A is far reaching in the maintenance of cardiovascular health. However, several gaps in literature remain to be addressed, before the orexin system can be considered as a target for clinical trials in treating autonomic disorders. At present, the orexin system is a target for sleep disorders and dual orexin receptor antagonists have reached phase II trials.8
In conclusion, the study presented by Fan et al1 provides a greater understanding of the orexin system in regulating sympathetic output. In vitro studies convincingly complement the in vivo findings. They have identified the neuronal population within the PVN that exclusively expresses OX1R. The function of this receptor has been characterized considering neuronal activation, sympathetic output and inflammation. It also sheds light on areas that can be further explored such as the mechanism of orexin A-induced Fra-1 expression leading up to increased sympathetic output. The contribution of glial cells in mounting an inflammatory response to orexin A and other signalling mechanisms that might be involved in exciting the PVN and increasing peripheral sympathetic output are also of interest.
Footnotes
CONFLICT OF INTEREST
We have no conflict of interest to declare.
REFERENCES
- 1.Fan Y, Jiang E, Hahka T, Chen QH, Yan J, Shan Z. Orexin a increases sympathetic nerve activity through promoting expression of proinflammatory cytokines in Sprague Dawley rats. Acta Physiol (Oxf) 2017. Version of Record online: 23 October 2017. | 10.1111/apha.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Cid-Pellitero E, Garzon M. Hypocretin1/OrexinA-containing axons innervate locus coeruleus neurons that project to the Rat medial prefrontal cortex. Implication in the sleep-wakefulness cycle and cortical activation. Synapse. 2011;65:843–857. [DOI] [PubMed] [Google Scholar]
- 4.Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension. 2015;65:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalal MA, Schuld A, Haack M, et al. Normal plasma levels of orexin A (hypocretin-1) in narcoleptic patients. Neurology. 2001;56:1749–1751. [DOI] [PubMed] [Google Scholar]
- 6.Grimaldi D, Calandra-Buonaura G, Provini F, et al. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep. 2012;35:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dergacheva O, Yamanaka A, Schwartz AR, Polotsky VY, Mendelowitz D. Optogenetic identification of hypothalamic orexin neuron projections to paraventricular spinally projecting neurons. Am J Physiol Heart Circ Physiol. 2017;312:H808–H817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norman JL, Anderson SL. Novel class of medications, orexin receptor antagonists, in the treatment of insomnia - critical appraisal of suvorexant. Nat sci sleep. 2016;8:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]