Abstract
Soluble receptor of IL-6 (sIL-6R) and antagonist of the receptor complex, soluble glycoprotein 130 (sgp130) mediate opposite effects during inflammation. We measured the levels of these cytokines and their ratio in rat blood on the model of acute lung injury. The injury was modeled by the intratracheal administration of LPS. The levels of sgp130 and sIL-6R increased during the inflammatory process in the injured lungs. The sgp130/sIL-6R ratio increased or decreased depending on the intensity of the inflammatory process. sgp130/sIL-6R ratio might reflect the intensity of inflammation during lung injury.
Keywords: inflammation, soluble receptor of IL-6, sgp130, acute lung injury
Cytokines serve as transmitters in intracellular signaling interacting with specific receptor with very high affinity. Parameters of cytokine effects in organism depend on their concentration [1]. Cytokines in physiological concentrations participate in local inflammation, while in higher concentrations, they induce protective systemic inflammatory response and can even cause pathological changes in tissues in concentrations far surpassing the physiological levels.
IL-6, one of the most active cytokines taking part in the inflammatory processes, has pleiotropic effects in various tissues and organs [4]. Increased IL-6 level is associated with various pathological states [6] including lung injuries leading to acute respiratory distress syndrome [2,3]. Lung injury caused by LPS administration manifests itself in a cytokine-dependent damage to the endothelium, its high permeability with exudate accumulation and triggers systemic reactions, including septic shock [7]. LPS induces IL-6 production. Signal transduction from IL-6 can be realized via two pathways: classic, when IL-6 binds to cell surface receptor, and trans-signal, when IL-6 binds to soluble receptor (sIL-6R) with subsequent formation of the IL-6/sIL-6R complex [10]. The resultant complex widens the range of IL-6 targets as it transmits the signal in cells lacking membrane IL-6R.
Both signal transduction pathways require the presence of transmembrane glycoprotein 130, which is widely spread in cells and tissues of the human body and initiates signal transduction pathways. However, circulating soluble glycoprotein 130 (sgp130) can inactivate the IL-6/sIL-6R complex and disrupt trans-signal pathway [5]. Thus, both sIL-6R and sgp130 influence the development of IL-6-associated pathologies.
Here we evaluated the levels of the IL-6/sIL-6R/ sgp130 complex components in a rat model of acute lung injury.
MATERIALS AND METHODS
Experiments were performed on male Sprague-Dawley rats weighting 280–300 g. The animals were kept in a vivarium with complete ration and free access to water. The experiments were conducted in accordance with the standards of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (Strasbourg, 1986) Regulations on Good Laboratory Practice, order N. 199n of April 1, 2016, Russian Ministry of Health. Temperature and light regimen were similar for all rats. The animals were randomized into experimental (n=10) and control (n=10) groups. Acute lung injury was modeled by intratracheal administration of Salmonella enteri LPS (Sigma-Aldrich) [8,9]. Control animals received 0.9% NaCl.
The time of intratracheal administration of 5 mg/kg LPS to animals narcotized with Zoletil-xylazine (Virbac Sante Animale; 25 mg/kg intraperitoneally) was considered as the beginning of experiment [8,9].
The blood was taken from the caudal vein via a 24G catheter. Serum levels of IL-6 and sgp130 were measured before the start of the experiment (0 h) and 3, 6, 24 and 48 h after LPS injection by ELISA using Cusabio (IL-6), MyBioSource (sIL-6R), and Elabscience (sgp130) kits. Test sensitivity was 0.078 pg/ml, 0.05 ng/ml, and 0.1 ng/ml, respectively.
Statistical analysis was performed using SPSS Statistics 19.0 software. Parametric Student’s t test was used for between-group comparison of quantitative parameters. The data are presented as mean±standard deviation. The differences were considered significant at p<0.05.
RESULTS
Blood concentration of IL-6 in the control group practically did not change throughout the experiment and varied from 87±13 to 99±12 pg/ml (Table 1). Intratracheal administration of LPS induced a significant increase in blood IL-6 level. It reached the maximum (6496±530 pg/ml) 3 h after LPS injection. IL-6 concentration remained high in 6 and 24 h and significantly differed from the control (P< 0.01). The obtained results reflect the development of inflammatory processes after this type of LPS treatment. IL-6 concentration significantly decreased 48 h after LPS administration and reached the baseline value.
TABLE 1.
Effects of LPS on IL-6 Level (pg/ml) in Rat Blood on the Model of Acute Lung Injury (n=10; M±SD)
| Group | Time, h | ||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 24 | 48 | |
| Control | 97±12 | 94±11 | 87±13 | 99±12 | 96±12 |
| LPS, 5 mg/kg | 93±12 | 6496±530* | 5760±398* | 2396±362* | 102±16 |
Note.
p<0.01 in comparison with the initial level.
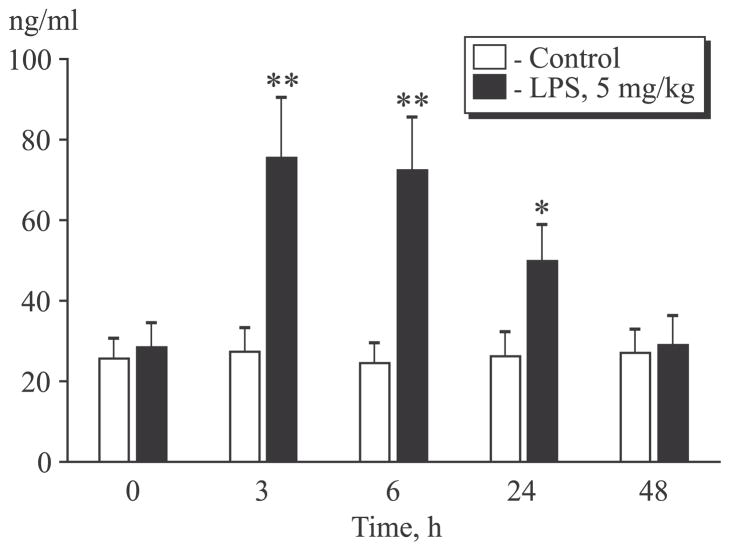
sIL-6R is an antagonist of IL-6-induced signal transduction [6]. LPS administration to animals was followed by a significant increase in sIL-6R level (Fig. 1). High level of sIL-6R was observed also in 3, 6, and 24 after LPS injection and decreased to the initial level within 48 h. As was mentioned before, IL-6/sIL-6R complex can potentiate the effects of IL-6 by providing signal transduction in cells lacking plasma membrane IL-6 receptor. Hence, increased sIL-6R level suggests a considerable contribution of trans-signal pathway to the inflammatory process in acute lung injury.
Fig. 1.
Changes in sIL-6R level in the dynamics of the inflammatory process. *p<0.05, **p<0.01 in comparison with the initial level.
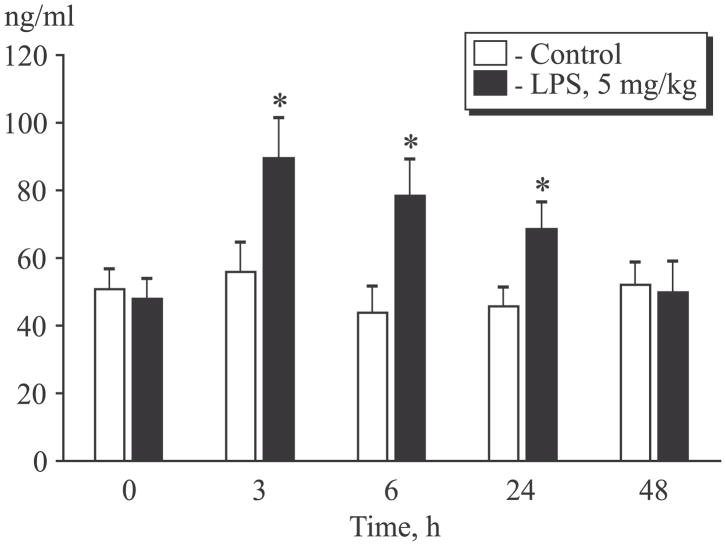
The level of sgp130 also significantly increased after LPS administration (Fig. 2). The highest concentration of sgp130 in the treatment group was observed in 3 h after LPS administration. In 24 h, when the inflammatory process was significantly less pronounced, sgp130 level remained increased in comparison with the control group. This parameter returned to the initial level in 48 after treatment and did not differ from that in the control group. sgp130 is an endogenous inhibitor of the trans-signal pathway mediated by IL-6/ sIL-6R complex [5]. Thus, an increase in sgp130 level can serve as a mechanism aimed at suppressing acute inflammation in lung injury.
Fig. 2.
Changes in sgp130 level in the dynamics of the inflammatory process. *p<0.05 compared to initial level.
As sIL-6R and sgp130 reveal differently directed effects on the development of inflammatory process, we calculated sgp130/sIL-6R ratio in the control and treatment groups (Table 2). sgp130/sIL-6R ratio in the control group practically did not change throughout the experiment. By contrast, in experimental group it decreased within the initial 6-h period after LPS injection, then increased after 24 h and only after 48 h reached the baseline level.
TABLE 2.
Ratio between the Levels of sgp130 and sIL-6R (n=10; arb. units; M±SD)
| Group | Time, h | ||||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 24 | 48 | |
| Control | 1.98±0.34 | 2.04±0.36 | 1.78±0.21 | 1.74±0.23 | 1.88±0.31 |
| LPS, 5 mg/kg | 1.75±0.23 | 1.19±0.17* | 1.08±0.13* | 1.4±0.25 | 1.82±0.27 |
Note.
p<0.05 in comparison with the initial level.
Thus, sgp10/sIL-6R ratio depends on the intensity of the inflammatory process. It can be hypothesized that the impaired balance between sIL-6R and sgp130 might affect progression of the inflammatory process. Long-term impairment can promote the development of chronic inflammation during lung injury. However, further investigations are needed to prove this hypothesis.
The experiments were supported by the National Institute of Health, USA (grant HL101902) and Russian Foundation of Basic Research (grant No. 16-04-00410).
References
- 1.Simbirtsev AS. Oytokines in the pathogenesis of infectious and noninfectious human diseases. Med Akad Zh. 2013;(3):18–41. Russian. [Google Scholar]
- 2.Gonzales JN, Lucas R, Verin AD. The acute respiratory distress syndrome: mechanisms and perspective therapeutic approaches. Austin J Vasc Med. 2015;2(1) pii: 1009. [PMC free article] [PubMed] [Google Scholar]
- 3.Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194(3):855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 5.Jostock T, Müllberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 6.Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 2002;1592(3):323–343. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, Yu X, Yu S, Kou J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int Immunopharmacol. 2015;29(2):937–946. doi: 10.1016/j.intimp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Li W, Pauluhn J, Trübel H, Wang C. Lipopolysaccharide-induced acute lung injury in rats: comparative assessment of intratracheal instillation and aerosol inhalation. Toxicology. 2013;304:158–166. doi: 10.1016/j.tox.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122(4):143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]