Abstract
Introduction:
Carbonyls, a class of compounds strongly linked to pulmonary disease in smokers, are probably the most reported non-nicotine toxicants found aerosols. Reported emissions vary from negligible quantities to those far exceeding combustible cigarettes. Observations of high emissions are commonly attributed to “dry puffing”, whereby the ECIG heating filament runs dry of liquid and reaches temperatures that induce thermal degradation of the ECIG vapor components at the filament’s metal surface. Using a pyrolysis flow reactor, in this study we examined the potential role of surface chemistry in the formation of carbonyl compounds in ECIGs, and whether the different commercially available filament materials could potentially impact their toxicant emissions through catalysis. This information could inform nascent efforts to regulate the design of ECIGs for public health ends.
Methods:
Nitrogen or air saturated with propylene glycol vapor was drawn through a temperature and residence time controlled tubular quartz pyrolysis flow reactor in which nichrome, Kanthal, or stainless steel ECIG heating filament wires were inserted. A control condition with no inserted wire was also included. Concentrations of carbonyl products at the reactor outlet were measured as a function of temperature, heating filament wire material, and carrier gas composition (N2 vs air). Carbonyls were sampled using DNPH cartridges and analyzed by HPLC.
Results:
ECIG heating filament wires were found to have a strong catalytic effect. Carbonyl formation initiated at temperatures lower than 250°C in the presence of the metallic wires, compared to 460°C without them. Carbonyl formation was found to be a function of the material of construction, and whether the wire was new or aged. New nichrome wires were the least reactive, but when aged they exhibited the highest reactivity. Carbonyls were formed via dehydration or oxidation reactions of PG.
Conclusions:
Carbonyl formation chemistry is catalyzed by commonly used ECIG heating filament materials, at temperatures that are well below those expected during “dry puffing”. The variability in the distribution and yield of carbonyl compounds across ECIG filament materials suggests that this heretofore unaccounted variable may partially explain the wide ranges reported in the literature to date. More importantly, it suggests that ECIG construction materials may be an important variable for regulations designed to protect public health.
Keywords: Electronic cigarette, atomizer material, coil wire, carbonyl, thermal degradation, catalysis, pyrolysis
Graphical abstract
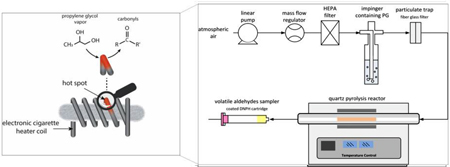
1. Introduction
Electronic cigarettes (ECIGs) have been marketed since at least 2003 [1]. Thereafter, the popularity of these new nicotine delivery systems continued to increase among active and former smokers, as well as among never smokers [2–4]. Particularly alarming is that the prevalence of ECIG use among teenagers in the US surpassed that of tobacco cigarettes for the first time in 2014 [5, 6]. These devices are usually advertised as safer alternatives to tobacco cigarettes and as possible smoking cessation tools [7–9]. However, despite emitting fewer categories of toxicants in comparison to tobacco cigarettes, it has been shown that carbonyl concentrations can at times exceed the levels that are measured in tobacco cigarettes [10, 11]. Carbonyls are mainly the products of either cracking or the oxidation reactions of solvents (propylene glycol (PG) and vegetable glycerin) and some flavorants and additives, when present in the ECIG liquid [12–16].
Carbonyls are the most studied toxicants in the liquids and aerosols of electronic cigarettes (ECIGs), yet a high variability is present in the levels and identities of the measured carbonyls, as detailed in Table S1 [12, 17–19]. Formaldehyde, one of the most abundant aldehydes in ECIGs, shows concentrations ranging from trace amounts to 50 μg/puff [10, 11, 13, 20]. This range has been attributed to the different characteristics of ECIG devices, liquids, and aerosol generation techniques. Several studies have attempted to correlate the carbonyl emissions with the liquids used, battery power outputs, ECIG design characteristics, user puffing behaviors, and the number, position, temperature, and age of the coils [13, 19–24]. A recent study showed that power per coil surface area is a better predictor of carbonyls emissions than power alone [25].
The power outputs commonly used in ECIGs provide coil temperatures sufficient to induce pyrolysis rather than combustion [26]. Occasional elevated temperatures and spikes of carbonyls have been associated with the “dry puff” phenomenon due to dry wicking [27]. While carbonyl formation during high temperature operation is well established [11], there is some evidence that carbonyls are formed in ECIGs at lower temperatures than expected from the extant knowledge on homogeneous carbonyl formation chemistry. Because the ECIG liquid is in direct contact with the metallic heating element, greater than expected carbonyl emissions might be linked to a catalytic interaction at the heater surface. If so, then use of different heating element materials may impact carbonyl emissions, and ultimately the toxicity of the inhaled aerosol. To our knowledge, no previous studies have taken a close look at the chemical reactions occurring at the PG ECIG metallic wires interface, hence favoring carbonyl production at lower temperatures.
In this study we examined the heterogeneous reaction of PG gas with several types of metallic wires used in ECIG heating elements. In an attempt to simulate the “dry puff” phenomenon, we used an experimental set up that consists of a tubular quartz flow reactor operating at various temperatures to measure carbonyl formation from the pyrolysis of PG. Emissions at the reactor outlet were sampled with 2, 4 dinitrophenylhydrazine (DNPH) cartridges. Carbonyl peak temperatures, and yields from PG pyrolysis in nitrogen and air, and in the presence of the widely used ECIG coil wire materials Kanthal, nichrome, and stainless steel, were also assessed.
2. Materials and Methods
2.1. Materials
High performance liquid chromatography (HPLC) grade acetonitrile and ethanol solvents, pure PG (CAS registry number 57 55 6), high purity silica adsorbent coated with DNPH H Series Cartridges H10, volume size 3 mL, standard DNPH derivatives (SUPELCO 47672 U) and Carbonyl DNPH Mix 1, 20 μg/mL in acetonitrile, ampule of 1 mL, were procured from Sigma Aldrich. Kanthal, nichrome, and stainless steel wire (24 gauge) were sourced from ECIG vendors in the USA. N2 gas was delivered from a 99.995% purity N2 tank. Gelman type A/E 47 mm Glass fiber filters were purchased from Pall Corporation.
2.2. Experimental setup
The setup is shown in Figure 1. A saturated flow of PG in HEPA filtered air or nitrogen was generated by bubbling the gas through pure PG in a midget impinger; the impinger and gas flows were at room temperature (22°C). The gas flow rate was regulated by a mass flow controller. A glass fiber filter was installed downstream of the impinger to remove fugitive liquid droplets potentially generated in the bubbler. The saturated gas stream then entered a 120 cm long horizontal heated quartz tube (0.65 cm diameter). A 45 cm section of the tube passed through a variable power electrically heated furnace. Five furnace power setpoints were used, resulting in gas temperatures of 80, 256, 360, 460, 565, and 670°C at the exit of the 45 cm heated zone, as measured using a K type thermocouple placed at the flow centerline. At each temperature setpoint, PG saturated gas was streamed for 20 min through the quartz tube in order to ensure complete saturation of the walls. Then, the effluent gas was collected on DNPH cartridges for 3 min. The temperature set was always run in the aforementioned order; this is due to the difficulty in cooling down the furnace from a higher to a lower temperature. The residence time of the gas inside the reactor (1.6 sec) was held constant by adjusting the flow rate for each temperature condition, using the ideal gas law (Table S2). Bundles consisting of thirty 45 cm long metal wires (24 gauge) of Kanthal, nichrome, or stainless steel were prepared and inserted into the heated section of the quartz tube. Each bundle occupied 18.6% of the total volume of the heated section of the quartz tube. Three aliquots of gases were taken at each temperature and for each type of wire, new metallic wires were used at the start of the experiment. The experiment with Nichrome was repeated after the Nichrome turned completely black. This is what is referred to as aged nichrome.
Figure 1.
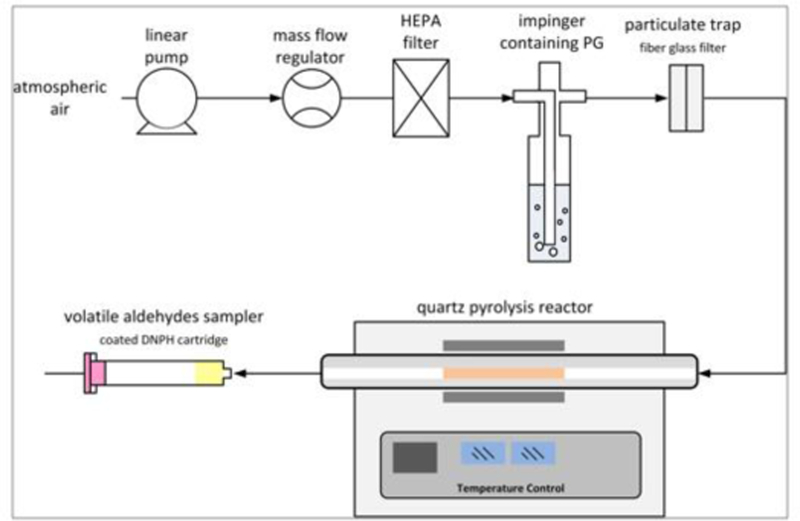
Illustration of the dry puff (left) and the schematic diagram of the propylene glycol (PG) pyrolysis reactor used in this study (right)
Carbonyl emissions were trapped at the outlet of the quartz tube on gas sampling DNPH coated H10 cartridges that were eluted with 5 ml of ethanol/acetonitrile (90/10 ratio) [28]. The eluent was delivered into amber vials. All samples were analyzed using HPLC (Agilent 1100) equipped with a photodiode array detector at k = 360 nm, and at a flow rate of 1 ml/min. The analytes were identified upon comparing their HPLC retention times with those of the standards. The HPLC method was adopted from the California Air Resources Board (CARB) method (SOP MLD 022), as well as the Environmental Protection Agency (EPA) method (TO 11A) (EPA, 1999), with some modifications in the time program of elution for better separation, as detailed in Rashidi et al. 2008 [29]. Gradient elution on a reverse phase C 18 column (25 cm, 4.6 mm, 5 lm) was performed. The solvents used were (A) water/ACN/ THF (6:3:1 v/v/v), (B) water/ACN (2:3 v/v), and (C) ACN. The elution profile varied linearly in time from pure A at t = 0 min to 25:75 A:B at t = 20 min and finally to pure C at t = 35 min. A background measurement for carbonyls was obtained by passing air through set up without heating the furnace.
Eight carbonyls (formaldehyde (FA), acetaldehyde (AA), acetone (Acet), acrolein (Acr), propionaldehyde (PA), butyraldehyde (BA), glyoxal (GA), and methylglyoxal (MGA)) were measured. The LOD and LOQ were respectively as follows (µg/mL): formaldehyde, 0·002 and 0·005; acetaldehyde, 0·005 and 0·016; acetone, 0·003 and 0·008; acrolein, 0·001 and 0·004; propionaldehyde, 0·002 and 0·006; crotonaldehyde, 0·004 and 0·013; methacrolein, 0·004 and 0·012; butyraldehyde, 0·004 and 0·013; benzaldehyde 0·011 and 0·038; and valeraldehyde, 0·003 and 0·010; glyoxal, 0.014 and 0.047; methyl glyoxal, 0.027 and 0.091.
3. Results
3.1. Thermal decomposition of PG in N2
Propionaldehyde (PA) and acetone (Acet) were formed at 413°C and 460°C, respectively. At 670°C, minimal amounts of formaldehyde (FA) and acetaldehyde (AA) were detected (Figure 2A) and of the total carbonyls PA, Acet, AA and FA constituted 57.8%, 28.8%, 6.4% and 3.2%, respectively.
Figure 2.
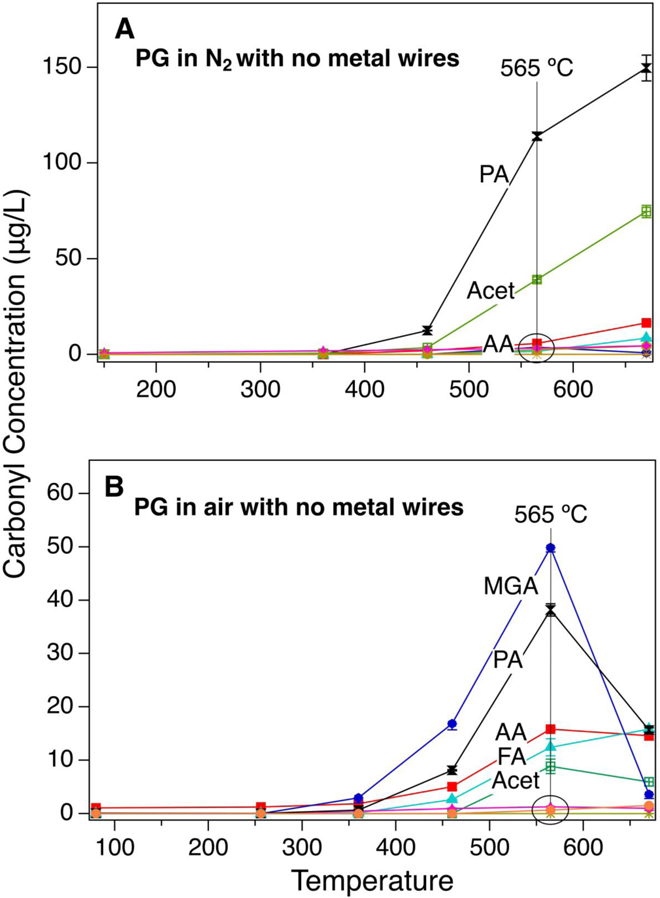
Homogenous heating of propylene glycol (PG) in nitrogen (A) and air (B)
3.2. Thermal decomposition of PG in air
The PG air gas and particle stream produced methylglyoxal (MGA), PA, Acet, FA, and AA. Formation began at 360°C and continued until 670°C, with a maximum at 565°C. At T > 565°C, the carbonyl concentrations begin to decrease, suggesting that they undergo further oxidation, possibly into acids or cracking into smaller molecules such as H2O, CO, and CO2 (Figure 2B). Of the total carbonyls that were measured at the peak (565°C), MGA, PA, AA, FA and Acet constituted 39.3%, 30.0%, 12.5%, 9.8% and 6.9%, respectively.
3.3. Thermal decomposition of PG in air in the presence of metallic wires
In the presence of Kanthal wires, MGA, FA, and AA were formed. The MGA peak appeared at temperatures close to 80°C and extended to 360°C with a maximum at 256°C. Two peaks were noted for FA and AA; one peak at 256°C °C and the second at 460°C (Figure 3A). Similar to Kanthal, the stainless steel wires led to the formation of MGA, FA, and AA at around 80°C (Figure 3B). Interestingly, butyraldehyde (BA) emission was also detected in this case at 360°C. In both cases, the presence of several maxima for FA and AA between 80 and 670°C alludes to the formation of these low carbonyls (C1 and C2) via several pathways. It was noted that the stainless steel wires changed to a copper like color by the end of the experiment.
Figure 3.
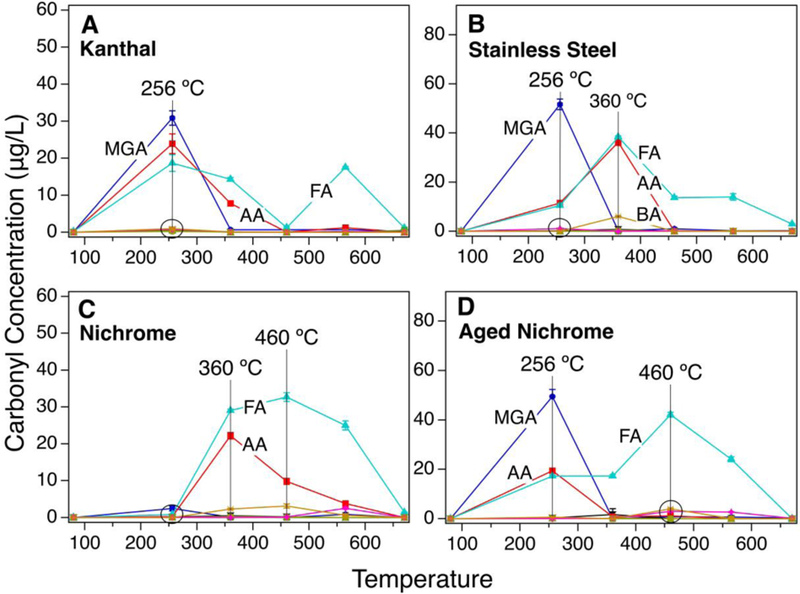
Heterogeneous heating of propylene glycol (PG) saturated gas in air in the presence of Kanthal (A), stainless steel (B), new nichrome (C), and aged nichrome wires (D)
The nichrome wires were less reactive. Only a small amount of MGA was observed at around 80°C, and FA and AA were detected between 256 and 670°C (Figure 3C). In addition to BA, glyoxal (GA) was seen for the first time in this case. Additionally, the nichrome wires turned black by the end of the experiment. Repeating the same experiment with the black nichrome wires showed a PG decomposition profile similar to Kanthal and stainless steel wires (Figure 3D). The MGA peak started at around 80°C with a maxima at 256°C, and the FA and AA peaks were broad, extending from 80 to 670°C. The levels of carbonyls produced with the aged (used for the second time) nichrome wire were also higher than those measured for the new nichrome wires.
4. Discussion
Scheme 1 summarizes the mechanisms of PG degradation under the different evaluated conditions. PG degradation in N2 is explained by a dehydration reaction mechanism that leads to PA and Acet through the formation of the intermediate propylene oxide, as recently suggested by DFT calculations [22, 30]. The minimal amounts of FA and AA that appeared at 670°C could be attributed to cracking at high temperatures. In air, PG degradation produced carbonyls via several reaction mechanisms, including dehydration, dehydrogenation, and glycol C C bond cleavage [31, 32]. The degradation of PG in air shows that the oxidation pathway is more prominent than the dehydration reaction. It is proposed that in the presence of metals and under oxygen rich conditions, PG adsorbed onto the metal surface produces 1,2 propylenedioxy at T < 80°C. With higher temperatures, propylenedioxy desorbs from the surface to form MGA via a double dehydrogenation reaction [33–35]. The same intermediate can decompose into FA and AA via C C bond cleavage, through the formation of adsorbed acetate and formate, respectively [22, 36].
Scheme 1.
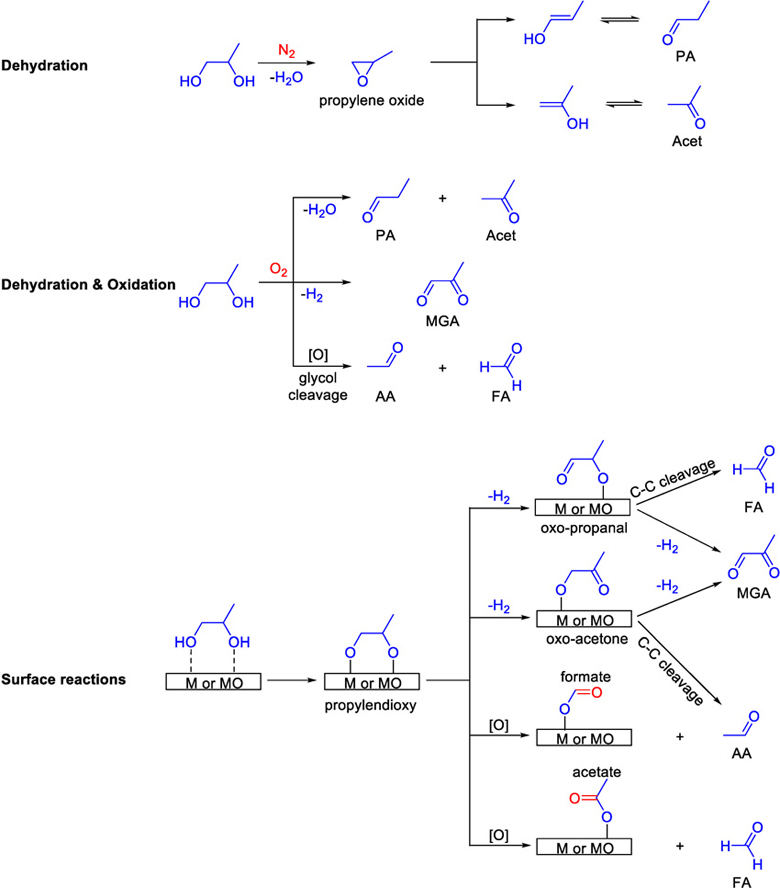
Propylene glycol (PG) saturated gas degradation pathways in the presence of N2, O2, and metallic coils
The reactions with the metallic wires suggest a catalytic effect on PG degradation that lowers the reaction’s activation energy and affects the yield and distribution of the produced carbonyls. Consequently, this suggests that ECIG coils can catalyze PG degradation, producing carbonyls at temperatures lower than what is expected for “dry puff” [19].
The metal wires also displayed different reactivities. Looking at the most toxic product, FA, and the total carbonyl concentration at the temperature thought to be similar to the power output of an ECIG (256°C), it was found that the yields differed with the type and age of the metal. The lowest carbonyl yields were measured in the presence of new nichrome wires when compared to Kanthal, stainless steel, and aged nichrome (Figure 4). The increase in the carbonyl yield with aged nichrome wires indicates that coil ageing plays an important factor that needs to be considered when comparing carbonyl yields in ECIGs [22]. The production of BA and GA by some metals can be explained by radical chain reactions in line with the previously reported studies about the detection of reactive oxygen species and radicals in ECIG aerosols [37, 38]. The disappearance of carbonyls at temperatures higher than 565°C is attributed to either extensive oxidation leading to acids or to cracking into smaller molecules like methane, carbon dioxide and water.
Figure 4.
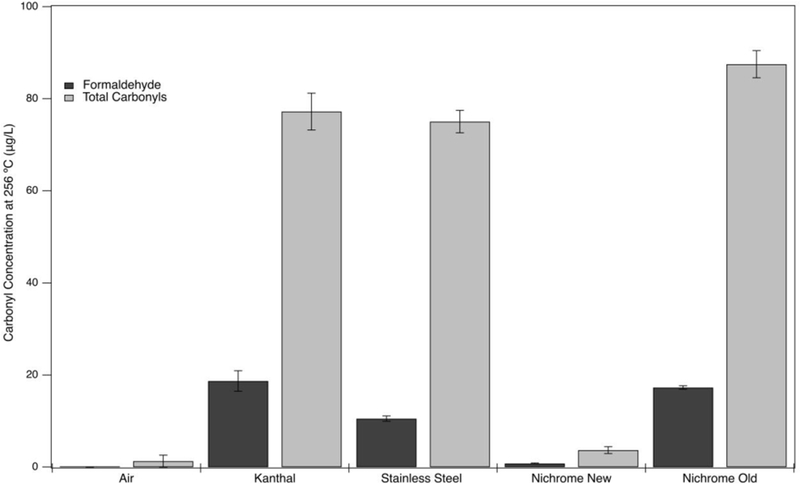
Formaldehyde and total carbonyls for PG pyrolysis at 256°C in air and in the presence of new Kanthal, stainless steel, nichrome, and aged nichrome wires
In brief, this study highlights the catalytic effect of metal wires on PG degradation especially in “dry puff” situations. We show, that under these conditions, carbonyl formation initiates at temperatures much lower than what is expected for “dry puff”. Both the type and age of the wires also contribute to the variability and yield of produced carbonyls. The results emphasize the importance of taking the construction materials of ECIGs into consideration as important variables for regulation.
Supplementary Material
Highlights.
ECIG heating filament wires were found to have a strong catalytic effect.
Carbonyl formation chemistry is catalyzed at temperatures that are well below those expected during “dry puffing”.
New nichrome wires were the least reactive, but when aged they exhibited the highest reactivity.
Results suggest that catalysis by ECIG filaments may partially explain the wide ranges of carbonyls.
Acknowledgments
Funding Sources
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (grant number P50DA036105) and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors have no conflict of interest to declare.
References
- [1].Kaisar MA, Prasad S, Liles T, Cucullo L, A decade of e-cigarettes: Limited research & unresolved safety concerns, Toxicology 365 (2016) 67–75, 10.1016/j.tox.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barrington-Trimis JL, Urman R, Leventhal AM, Gauderman WJ, Cruz TB, Gilreath TD, et al. , E cigarettes, Cigarettes, and the Prevalence of Adolescent Tobacco Use, Pediatrics 138 (2016) e20153983, 10.1542/peds.2015-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, Klein JD, Trends in Electronic Cigarette Use Among U.S. Adults: Use is Increasing in Both Smokers and Nonsmokers, Nicotine Tob. Res 17 (2015) 1195–1202, 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- [4].Coleman BN, Rostron B, Johnson SE, Ambrose BK, Pearson J, Stanton CA, et al. , Electronic cigarette use among US adults in the Population Assessment of Tobacco and Health (PATH) Study, 2013–2014, Tob. Control 26 (2017) e117–e126, 10.1136/tobaccocontrol-2016-053462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chapman R. California Department of Public Health, California Tobacco Control Program, State Health Officer’s Report on E Cigarettes: A Community Health Threat, Sacramento, CA: 2015 2015. Available from: http://tobaccofreeca.com/wp-content/uploads/2016/07/State-Health-e-cig-reportdigital.pdf [Google Scholar]
- [6].Dutra LM, Glantz SA, Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross sectional study, JAMA Pediatrics 168 (2014) 610–617, 10.1001/jamapediatrics.2013.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adriaens K, Van Gucht D, Declerck P, Baeyens F, Effectiveness of the Electronic Cigarette: An Eight Week Flemish Study with Six Month Follow up on Smoking Reduction, Craving and Experienced Benefits and Complaints, Int. J. Environ. Res. Public Health 11 (2014) 11220–11248, 10.3390/ijerph111111220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Foulds J, Veldheer S, Berg A, Electronic cigarettes (e cigs): views of aficionados and clinical/public health perspectives, Int. J. Clin. Pract 65 (2011) 1037–1042, 10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- [9].Etter JF, Electronic cigarettes: a survey of users, BMC Public Health 10 (2010) 231, 10.1186/1471-2458-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jensen RP , Luo W , Pankow JF , Strongin RM , Peyton DH Hidden Formaldehyde in E Cigarette Aerosols, N. Engl. J. Med 372 (2015) 392–394, 10.1056/NEJMc1413069. [DOI] [PubMed] [Google Scholar]
- [11].Talih S, Balhas Z, Salman R, Karaoghlanian N, Shihadeh A, “Direct Dripping”: A High Temperature, High Formaldehyde Emission Electronic Cigarette Use Method, Nicotine Tob. Res 18 (2016) 453–459, 10.1093/ntr/ntv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, Kunugita N, Carbonyl Compounds Generated from Electronic Cigarettes, Int. J. Environ. Res. Public Health 11 (2014) 11192–11200, 10.3390/ijerph111111192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, et al. , Carbonyl Compounds in Electronic Cigarette Vapors: Effects of Nicotine Solvent and Battery Output Voltage, Nicotine Tob. Res 16 (2014) 1319–1326, 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khlystov A, Samburova V, Flavoring Compounds Dominate Toxic Aldehyde Production during E Cigarette Vaping, Environ. Sci. Technol 50 (2016) 13080–13085, 10.1021/acs.est.6b05145. [DOI] [PubMed] [Google Scholar]
- [15].Kosmider L, Sobczak A, Prokopowicz A, Kurek J, Zaciera M, Knysak J, et al. , Cherry flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde, Thorax 71 (2016) 376–377, 10.1136/thoraxjnl-2015-207895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. , Flavoring Chemicals in E Cigarettes: Diacetyl, 2,3 Pentanedione, and Acetoin in a Sample of 51 Products, Including Fruit , Candy , and Cocktail Flavored E Cigarettes, Environ. Health Perspect 124 (2016) 733–739, 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].EL Hellani A, Salman R, El Hage R, Talih S, Malek N, Baalbaki R, et al. , Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design, Nicotine Tob. Res 20 (2016) 215–223, 10.1093/ntr/ntw280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jo SH, Kim KH, Development of a sampling method for carbonyl compounds released due to the use of electronic cigarettes and quantitation of their conversion from liquid to aerosol, J. Chromatogr. A 1429 (2016) 369–373, 10.1016/j.chroma.2015.12.061. [DOI] [PubMed] [Google Scholar]
- [19].Geiss O, Bianchi I, Barrero Moreno J, Correlation of volatile carbonyl yields emitted by e cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours, Int. J. Hyg. Environ. Health 219 (2016) 268–277, 10.1016/j.ijheh.2016.01.004. [DOI] [PubMed] [Google Scholar]
- [20].Gillman IG, Kistler KA, Stewart EW, Paolantonio AR, Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e cigarette aerosols, Regul. Toxicol. Pharmacol 75 (2016) 58–65, 10.1016/j.yrtph.2015.12.019. [DOI] [PubMed] [Google Scholar]
- [21].Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, et al. , Aldehyde Detection in Electronic Cigarette Aerosols, ACS Omega 2 (2017) 1207–1214, 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sleiman M, Logue JM, Montesinos VN, Russell ML, Litter MI, Gundel LA, et al. , Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals, Environ. Sci. Technol 50 (2016) 9644–9651, 10.1021/acs.est.6b01741. [DOI] [PubMed] [Google Scholar]
- [23].Hutzler C, Paschke M, Kruschinski S, Henkler F, Hahn J, Luch A, Chemical hazards present in liquids and vapors of electronic cigarettes, Arch. Toxicol 88 (2014) 1295–1308, 10.1007/s00204-014-1294-7. [DOI] [PubMed] [Google Scholar]
- [24].Jensen RP, Strongin RM, Peyton DH, Solvent Chemistry in the Electronic Cigarette Reaction Vessel, Sci. Rep 7 (2017) 42549, 10.1038/srep42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Talih S, Salman R, Karaoghlanian N, El Hellani A, Saliba N, Eissenberg T, et al. , “Juice Monsters”: Sub Ohm Vaping and Toxic Volatile Aldehyde Emissions, Chem. Res. Toxicol 30 (2017) 1791–1793, 10.1021/acs.chemrestox.7b00212. [DOI] [PubMed] [Google Scholar]
- [26].Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, et al. , A Device Independent Evaluation of Carbonyl Emissions from Heated Electronic Cigarette Solvents, PLOS ONE 12 (2017) e0169811, 10.1371/journal.pone.0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Farsalinos KE, Voudris V, Poulas K, E cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions, Addiction 110 (2015) 1352–1356, 10.1111/add.12942. [DOI] [PubMed] [Google Scholar]
- [28].Uchiyama S, Tomizawa T, Inaba Y, Kunugita N, Simultaneous determination of volatile organic compounds and carbonyls in mainstream cigarette smoke using a sorbent cartridge followed by two step elution, J. Chromatogr. A 1314 (2013) 31–37, 10.1016/j.chroma.2013.09.019. [DOI] [PubMed] [Google Scholar]
- [29].Al Rashidi M, Shihadeh A, Saliba NA, Volatile aldehydes in the mainstream smoke of the narghile waterpipe, Food Chem. Toxicol 46 (2008) 3546–3549, 10.1016/j.fct.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Laino T, Tuma C, Moor P, Martin E, Stolz S, Curioni A, Mechanisms of Propylene Glycol and Triacetin Pyrolysis, J. Phys. Chem. A 116 (2012) 4602–4609, 10.1021/jp300997d. [DOI] [PubMed] [Google Scholar]
- [31].Díaz E, Sad ME, Iglesia E, Homogeneous Oxidation Reactions of Propanediols at Low Temperatures, ChemSusChem 3 (2010) 1063–1070, 10.1002/cssc.201000142. [DOI] [PubMed] [Google Scholar]
- [32].Perlin AS, Glycol Cleavage Oxidation, Adv. Carbohydr. Chem. Biochem 60 (2006) 183–250, 10.1016/S0065-2318(06)60005-X. [DOI] [PubMed] [Google Scholar]
- [33].Salaev MA, Poleshchuk OK, Vodyankina OV, Propylene glycol oxidation over silver catalysts: A theoretical study, J. Mol. Catal. A: Chem 417 (2016) 36–42, 10.1016/j.molcata.2016.03.011. [DOI] [Google Scholar]
- [34].Ayre CR, Madix RJ, The adsorption and reaction of 1,2 propanediol on Ag(110) under oxygen lean conditions, Surf. Sci 303 (1994) 279–296, 10.1016/0039-6028(94)90776-5. [DOI] [Google Scholar]
- [35].Ayre CR, Madix RJ, C C and C H bond activation of 1,2 propanedioxy by atomic oxygen on Ag(110): Effects of CO adsorbed oxygen on reaction mechanism, Surf. Sci 303 (1994) 297–311, [Google Scholar]
- [36].Tuma C, Laino T, Martin E, Stolz S, Curioni A, Modeling the Impact of Solid Surfaces in Thermal Degradation Processes, ChemPhysChem 14 (2013) 88–91, 10.1002/cphc.201200921. [DOI] [PubMed] [Google Scholar]
- [37].Lerner CA, Sundar IK, Yao H, Gerloff J, Ossip DJ, McIntosh S, et al. , Vapors Produced by Electronic Cigarettes and E Juices with Flavorings Induce Toxicity, Oxidative Stress, and Inflammatory Response in Lung Epithelial Cells and in Mouse Lung, PLOS ONE 10 (2015) e0116732, 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goel R, Durand E, Trushin N, Prokopczyk B, Foulds J, Elias RJ, et al. , Highly Reactive Free Radicals in Electronic Cigarette Aerosols, Chem. Res. Toxicol 28 (2015) 1675–1677, 10.1021/acs.chemrestox.5b00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


