Abstract
Network neuroscience research is providing increasing specificity on the contribution of large-scale brain networks to creative cognition. Here, we summarize recent experimental work examining cognitive mechanisms of network interactions and correlational studies assessing network dynamics associated with individual creative abilities. Our review identifies three cognitive processes related to network interactions during creative performance: goal-directed memory retrieval, prepotent-response inhibition, and internally-focused attention. Correlational work using prediction modeling indicates that functional connectivity between networks—particularly the executive control and default networks—can reliably predict an individual’s creative thinking ability. We discuss potential directions for future network neuroscience, including assessing creative performance in specific domains and using brain stimulation to test causal hypotheses regarding network interactions and cognitive mechanisms of creative thought.
Keywords: creativity, default network, divergent thinking, executive control network, functional connectivity, network neuroscience
The cognitive neuroscience of creativity has made considerable progress by mapping brain networks involved in creative cognition. In a recent review of studies examining creative cognition and artistic performance, we reported a consistent pattern of functional network connectivity that was characterized by interactions between the Default Network (DN) and the Executive Control Network (ECN; [1]). The DN is a set of midline and posterior inferior parietal brain regions that support self-referential and spontaneous thought processes such as mind wandering, episodic and semantic memory retrieval, and mental simulation [2,3]. The ECN consists of lateral prefrontal and anterior inferior parietal regions that support cognitive control processes such as response inhibition, goal maintenance, and attention control [4]. Our previous review [1] proposed that, during creative task performance, the interaction of the DN and the ECN may reflect goal-directed, self-generated cognition, with DN involved in idea generation and ECN in guiding, constraining, and modifying DN processes to meet creative task goals (cf. [5–8]).
Despite signs of convergence in the literature, important questions remain: (a) What are the specific cognitive mechanisms that underlie network interactions during creative cognition? and (b) How might network dynamics relate to individual differences in creative thinking ability? The current review aims to update and extend the literature in light of several studies that have begun to address these questions. This research can be broadly categorized into experimental and correlational investigations, with experimental work largely focused on linking brain network interactions to specific cognitive mechanisms. Correlational work is further categorized into studies (a) using prediction methods to estimate individual creative ability from patterns of brain connectivity and (b) reporting correlations between various network properties and creative ability. We conclude the review by offering suggestions for future research to further isolate cognitive mechanisms and individual differences in the creative brain.
Cognitive Mechanisms and Brain Networks of Creative Cognition
Increasing behavioral and neuroimaging evidence suggests that creative cognition involves some aspects of cognitive control, including goal-directed memory retrieval: the ability to strategically search episodic and semantic memory for task-relevant information. A recent fMRI study [9] examined brain networks supporting episodic retrieval during divergent thinking. The study manipulated the kind of retrieval process engaged during creative cognition via an episodic specificity induction (ESI): brief training in recalling details of a recent event, which can prime or facilitate the involvement of episodic retrieval mechanisms in subsequent tasks, including creativity and imagination tasks (for review, see [10]). A behavioral study previously showed that ESI enhances divergent thinking performance on the AUT [11]. Consistent with this work, in the fMRI study [9], participants generated more novel and appropriate uses (i.e., flexibility measure on the AUT) following ESI compared to a control induction. Critically, functional connectivity analysis revealed stronger coupling between a cognitive control network and a core (default) network comprised of memory-related brain regions (hippocampus) after ESI than after a control induction. In this context, DN-ECN coupling appears to reflect goal-directed retrieval processes recruited to strategically search, select, and combine elements of past experience during divergent thinking.
Another cognitive control function linked to creative cognition is prepotent-response inhibition: the ability to suppress interference from dominant or salient response tendencies [12] such as obvious concepts or ideas that come to mind during divergent thinking [13]. In contrast to convergent thinking—which involves the discovery of the correct solution to a problem—divergent thinking measures people’s ability to generate several possible solutions to a problem or prompt, such as thinking of novel uses for common objects, as in the Alternate Uses Task (AUT)1. Behavioral work [12] has shown that divergent thinking ability is strongly correlated with performance on response inhibition tasks, suggesting that creative individuals may be better able to suppress interference from competing concepts during divergent thinking.
In a recent fMRI experiment of pre-potent response inhibition [14], we examined brain networks underlying semantic interference in the context of the classic verb generation task. During the initial phase, participants studied a list of noun and verb pairs; during the second phase, participants were presented with studied (“high-constraint”) and unstudied (“low-constraint”) nouns and asked to “think creatively” while searching for uncommon verbs to relate to each noun [15]. We found that the semantic distance between nouns and verbs, assessed computationally via latent semantic analysis, was greater in the low-constraint compared to the high-constraint condition, likely due to greater interference from the prepotent (studied) verb response disrupting remote conceptual combination in the high-constraint condition. Critically, functional connectivity analyses revealed stronger functional coupling of anterior DN and left ECN regions in the high- than the low-constraint condition. These findings highlight another mechanism of DN-ECN coupling: the activation of a prepotent, automatic response via the DN (cf. [16]) and its inhibition via the ECN.
Creative cognition has recently been hypothesized to invoke a state of internally-focused attention: the focusing of attention on self-generated thought processes and the shielding of internal processes from external interference [17]. A recent study [18] sought to dissociate neural circuits supporting external vs. internal attention and divergent vs. convergent thinking. The direction of attention was manipulated by controlling how stimuli were presented during divergent and convergent thinking tasks. In one condition, stimuli were visible for the duration of a trial, allowing participants to continuously view the stimulus (i.e., “external attention” condition); in another condition, stimuli were presented very briefly at the beginning of the trial and thus required internal maintenance (“internal attention” condition). Compared to external attention, divergent thinking requiring internal attention was related to increased activity of the right anterior inferior parietal lobule (IPL), corresponding to a posterior hub of the ECN. Functional connectivity analyses further revealed stronger coupling between the right IPL and visual cortex in the internal condition. Thus, posterior ECN regions may play a role in directing attentional resources during divergent thinking by attenuating sensory input and focusing attention to internally-directed cognitive processes.
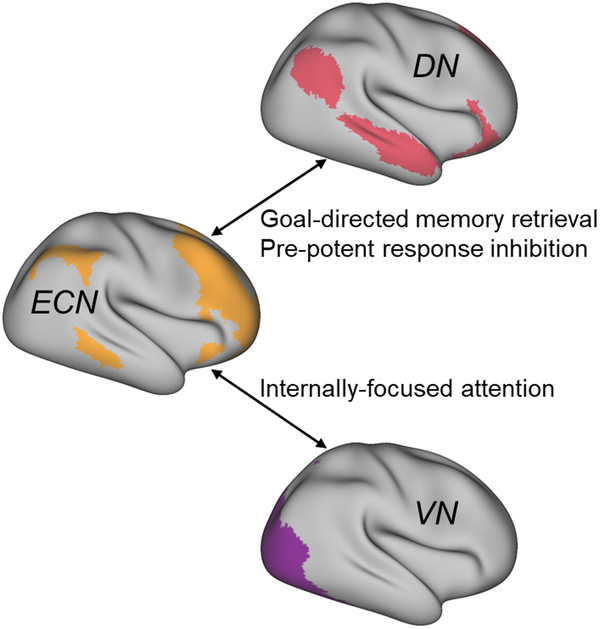
In sum, network neuroscience methods are beginning to provide insight into specific cognitive mechanisms related to network interactions during creative cognition. Figure 1 depicts the network interactions and corresponding cognitive mechanisms identified in the literature thus far. This work has demonstrated that DN-ECN coupling reflects both goal-directed episodic memory retrieval [9] and prepotent-response inhibition of semantic information [14]. Moreover, posterior ECN regions can interact with sensory cortices to attenuate external input and shield internal thought processes during idea generation [18]. Future research should continue to employ experimental paradigms to elucidate specific mechanisms underlying other modes of creative thought (e.g., figurative language production; [19]) and extend correlational findings using causal modeling to determine the direction of between-network information flow (cf. [20]).
Figure 1. Cognitive mechanisms of brain network interactions during creative cognition.
Notes. DN = default network; ECN = executive control network; VN = visual network.
Individual Differences in Brain Connectivity and Creative Ability
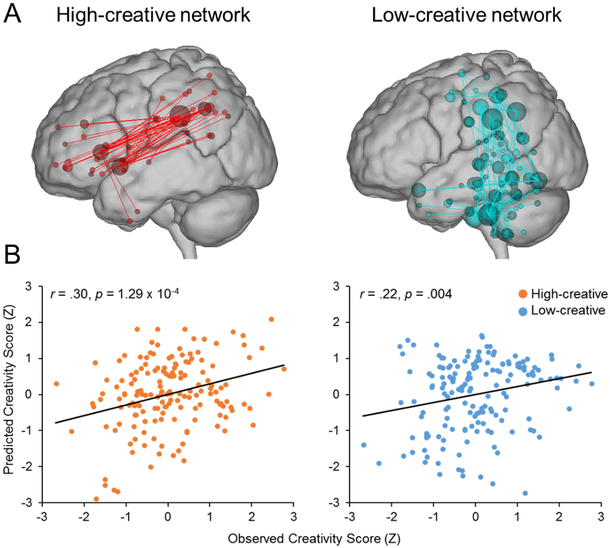
The past few years have seen a substantial increase in the number of studies examining how individual creative ability relates to variation in brain network connectivity. Table 1 lists the individual differences work conducted within the last two years (i.e., 2017–2018). New connectomic methods have been developed to characterize individual differences in personality and cognitive ability, such as connectome-based predictive modeling (CPM), which uses whole-brain connectivity patterns to predict individual traits and cognitive abilities [21–26]. CPM was recently used to identify functional connections correlated with high and low creative ability in a sample of 163 participants engaged in divergent thinking during fMRI [21]. A “high-creative” network consisted of default, salience, and executive network hubs; a “low-creative” network consisted of default, sensory, and cerebellar nodes (see Figure 2). Critically, the high-creative network generalized to predict divergent thinking ability in three independent samples of participants whose data were not used in model construction. Participants with stronger functional connections in this network thus tended to produce more original ideas.
Table 1.
Correlational studies of individual differences in creative ability and functional connectivity (2017-2018)
| Study | Sample Size | MRI Data | Creativity Task(s) | Network Analysis | Results |
|---|---|---|---|---|---|
| Beaty et al. (2018) | n = 163 | Task fMRI | AUT (originality) | Connectome-based predictive modeling | Network connectivity strength predicted verbal creativity in 4 datasets |
| Liu et al. (2018)* | n = 236 | Resting-state fMRI (and genetic data) | TTCT-V (composite) | Connectome-based predictive modeling | Network connectivity strength predicted verbal creativity in 2 datasets |
| Chen et al. (2018)* | n = 159 | Structural MRI (2-3 timepoints) | TTCT-V (composite) | Longitudinal VBM | ECN and FTN gray matter maturation predicted future verbal creativity |
| Zhu et al. (2017)* | n = 282 | Resting-state fMRI | TTCT-V (composite) and TTCT-F (composite) | ICA (mediation) | ECN mediated relation between DN and verbal, figural creativity |
| Sun et al. (2018)* | n = 574 | Resting-state fMRI | TTCT-V (composite) | Temporal variability of FC | DN between- and within-network FC variability correlated with verbal creativity |
| Bendetowicz et al. (2018) | n = 29 frontal patients, n = 54 controls | Structural MRI (lesion mapping) | CAT-V and FGAT | Voxel-based lesion-deficit mapping; disconnection-deficit mapping; network-based lesion deficit | MPFC (DMN) lesion disrupted remote concept generation; RLPFC (ECN) lesion disrupted remote concept combination but not generation |
| Gao et al. (2017)** | n = 22 HCG, n = 22 LCG | Resting-state fMRI | TTCT-F (composite) | Voxel-wise whole-brain FCS; seed-to-voxel; graph theory | HCG showed greater FCS across regions of multiple networks; network efficiency correlated with figural creativity score |
| Kenett et al. (2018) | n = 416 | DTI | TTCT-V (composite) | Network Control Theory | Network controllability of DLPFC (ECN) and other regions correlated with verbal creativity score |
| Li et al. (2017)** | n = 22 HCG, n = 22 LCG | Resting-state fMRI | TTCT-F (composite) | ICA; Dynamic FC | HCG showed more frequent transitions between brain states |
| Takeuchi et al. (2017) | n = 1277 | Resting-state fMRI | S-A creativity test | ReHO; seed-to-voxel; fALFF | Creativity score in females correlated with ReHo of MTG (DMN); RSFC between MPFC (DMN) and IFG (ECN); and fALFF in precuneus (DMN), MTG (DMN), and other regions |
Notes. = data from the Southwest University Longitudinal Imaging Multimodal (SLIM) Brain Data Repository (http://fcon_1000.projects.nitrc.org/indi/retro/southwestuni_qiu_index.html);
= data from the same subset of 180 undergraduates used to form the HCG and LCG. CAT-V = Combined Associates Task; DN = default network; DTI = diffusion tensor imaging; ECN = executive control network; fALFF = fractional amplitude of low frequency fluctuations; FC = functional connectivity; FCS = functional connectivity strength; FGAT = Free Generation of Remote Associates Task; fMRI = functional magnetic resonance imaging; FTN = fronto-temporal network; HCG = high-creative group; IFG = inferior frontal gyrus; ICA = independent components analysis; LCG = low-creative group; MPFC = medial prefrontal cortex; MTG = middle temporal gyrus; RLPFC = rostrolateral prefrontal cortex; TTCT-F = Torrance Test of Creative Thinking - Figural; TTCT-V = Torrance Test of Creative Thinking - Verbal; ReHo = regional homogeneity; SN = salience network; VBM = voxel-based morphometry.
Figure 2. Functional networks predictive of verbal divergent thinking ability identified via connectome-based predictive modeling.
Notes. Task-related fMRI data were acquired from participants (n = 163) engaged in an alternate uses divergent thinking task. (A) Functional networks were defined by extracting a latent factor of originality ratings, correlating these values with all possible connections (i.e., edges) in a whole-brain network (total possible edges = 35,778), and thresholding edges (p < .01) to retain the most significant edges, resulting in a “high-creative network” (224 edges) and a “low-creative” network (603 edges). (B) Scatterplots depicting correlations between observed creativity scores (x-axis) and model-predicted creativity score (y-axis) for the high- and low-creative networks. Adapted from [21].
Other work using similar prediction methods [27] has combined resting-state fMRI and genetic data to predict figural divergent thinking ability (i.e., visual-spatial; e.g., drawing). A model including both fMRI and genetic data showed better prediction of divergent thinking than models with separate fMRI and genetic data, and findings generalized to an independent sample of participants. Notably, although the “high-creative” network reported in this study showed some overlap with the high-creative network of the task-based CPM study noted above [21], the networks also showed considerable differences, likely due to variation in divergent thinking assessment (figural vs. verbal) and the type of imaging data (rest vs. task). Prediction modeling has also been used in longitudinal research to estimate future divergent thinking ability from structural brain networks [28]: executive network maturation, assessed via changes in grey-matter density, tracked improvements in divergent thinking ability three years later.
Several correlational studies have further investigated large-scale network characteristics associated with individual differences in creative thinking ability. Building on earlier seed-based studies reporting correlations between divergent thinking ability and resting-state functional connectivity (RSFC; e.g. [29–32]), a recent study [33] found that divergent thinking ability was related to increased RSFC between the left inferior frontal gyrus (IFG) of ECN and medial prefrontal cortex (MPFC) of the DN. This finding is consistent with earlier work [29] showing increased coupling between left IFG and MPFC in a high divergent thinking group. Several studies applied graph theoretical metrics such as global efficiency (i.e., the average shortest number of paths needed to traverse a given pair of brain regions) to assess information processing between network nodes. Other related work [34] found that a high divergent thinking group showed greater global efficiency within a resting-state network of executive and default nodes, similar to previous task-based research reporting a positive correlation between divergent thinking ability and global efficiency within a network of executive, salience, and default nodes [35]. The correspondence between resting-state and task-based networks was recently investigated in another study [36] that found executive-default coupling at rest predicted executive-default during divergent thinking, highlighting a link between network connectivity at rest and during task performance.
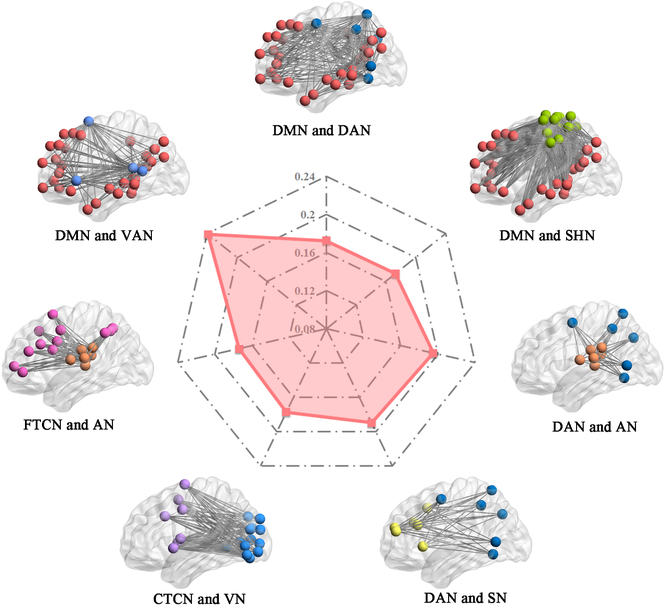
Dynamic connectivity research has complemented static connectivity findings by examining how network connectivity patterns shift over short time scales. One study [37] found that temporal variability of functional connectivity among executive (DLPFC) and default (precuneus and parahippocampal gyrus) network regions assessed at rest correlated with verbal creative thinking ability. The authors report several additional analyses examining within- and between-network variability and show that verbal creativity relates to between-network variability of other canonical networks beyond the DN and ECN (see Figure 3). Interestingly, of the 13 networks assessed in this study, only DN within-network variability correlated with creativity scores, highlighting a possible correspondence between neural variability within the DN and thought variability relevant to creative cognition.
Figure 3. Resting-state between-network variability correlated with figural divergent thinking.
Notes. Resting-state fMRI data were acquired from participants (n = 574) who completed a battery of verbal divergent thinking tasks outside the scanner. The radar plot in the middle displays correlations between pairs of functional networks whose resting-state signal variability significantly relates to verbal divergent thinking scores. AN = auditory network; CTCN = cingulo-opercular task control network; DAN = dorsal attention network; DMN = default mode network; FTCN = fronto-parietal task control network (executive control network); SHN = sensory/somatomotor hand network; SN = salience network; VAN = ventral attention network; VN = visual network.
Another recent study of connectivity dynamics [38] assessed network transitions in high and low divergent thinking groups and found that high divergent thinking ability was characterized by more frequent transitions between different brain connectivity “states” (i.e., recurring patterns of correlation between cortical networks), suggesting that flexible thinking may be marked by a more plastic brain. A related study exploring dynamic connectivity linked to Openness to Experience—a personality trait associated with creative thinking and default network functioning [39]—found that high Openness was related to increased time spent in a brain state characterized by positive correlations among the default, salience, executive, and dorsal attention networks [40]. Taken together with dynamic connectivity findings [37,38], it appears creative individuals benefit from an ability to dynamically shift between different patterns of brain connectivity.
Other studies have assessed variation in structural brain network connectivity in relation to creative thinking ability [41–44]. One such study [41] used network-based lesion-deficit mapping in a patient sample and found that MPFC lesions within the DN impaired remote concept generation, pointing to a role for the DN in spontaneous idea production; conversely, left rostrolateral prefrontal lesions within the ECN spared concept generation ability but impaired concept combination, consistent with role of ECN in higher-order control processes. Other recent work using network control theory analysis of white matter tracts has reported a correlation between divergent thinking ability and “modal controllability” in the right DLPFC of the ECN [42], suggesting that divergent thinking ability is characterized by an ability to “drive” the brain into difficult-to-reach cognitive states via the right DLPFC.
Notably, recent evidence suggests that correlations between creativity and structural brain connectivity vary as a function of sex. One study [43] found correlations between regional white matter volume and divergent thinking across diverse brain regions, but only in women. Other work [44] has reported decreased global network connectivity and clustering in women. Together, these findings highlight the importance of considering sex differences when assessing individual differences in creative thinking and brain network connectivity.
Summary and Future Directions
The cognitive neuroscience of creativity has benefited from recent innovations in network neuroscience methodology. This research is providing an increasingly sophisticated understanding of the complex mechanics of the creative brain, mapping neural dynamics to specific cognitive mechanisms and predicting individual creative abilities from patterns of brain connectivity. The literature has identified network dynamics supporting several cognitive processes relevant to creative thought (Figure 1), including goal-directed memory retrieval (executive-default; [9]), prepotent-response inhibition (executive-default; [14]), and internally-focused attention (executive-visual; [18]). Connectome prediction methods have been applied to estimate creative thinking ability from unique patterns of brain connectivity assessed both at rest [27] and during task performance [21], suggesting that variation in brain-network connectivity provides a reliable biomarker of creative thinking ability.
Future research should continue to map specific cognitive processes and individual differences supporting creative cognition. Network neuroscience methods provide a powerful approach, but activation studies continue to provide important insights into key cognitive mechanisms, including dissociating brain regions involved in generating “new” vs. “old” ideas [45], identifying neural correlates of remote conceptual combination [15] and expansion [46], and characterizing spontaneous cognitive processes related to DN activity and creative thought [47]. Moreover, research has thus far largely relied on correlational methods, so it is unclear whether connectivity patterns are causally related to creative performance. To address this issue, future research could employ new techniques in brain stimulation, such as transcranial alternating current stimulation, to causally manipulate interactions between large-scale brain networks. Although brain stimulation has already shown promise in identifying brain regions supporting creative thinking [48,49], an interesting next step would be to modulate interactions between these regions, particularly nodes within DN and ECN. Moreover, future individual differences research could examine whether connectivity patterns predictive of domain-general creative thinking (e.g., divergent thinking; [50]) extend to predict domain-specific creative performance [51,52], such as improvisation [53–58], poetry composition [59], visual creativity [5,60], or creative writing [61,62]. These are only a few potential directions for neuroscience research in what promises to be an exciting pursuit for the foreseeable future in mapping the creative brain.
Acknowledgements
Daniel L. Schacter was supported by National Institute of Mental Health grant MH060941 and National Institute on Aging grant AG008441.
Footnotes
AUT responses are commonly coded for fluency (i.e., total number of ideas), flexibility (i.e., total number of conceptual categories of ideas), and originality (i.e., creative quality of ideas).
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Beaty RE, Benedek M, Silvia PJ, Schacter DL: Creative cognition and brain network dynamics. Trends Cogn Sci 2016, 20:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckner RL, Andrews-Hanna JR, Schacter DL: The brain’s default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 2008, 1124:1–38. [DOI] [PubMed] [Google Scholar]
- 3.Raichle ME: The brain’s default mode network. Annu Rev Neurosci 2015, 38:433–447. [DOI] [PubMed] [Google Scholar]
- 4.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD: Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007, 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellamil M, Dobson C, Beeman M, Christoff K: Evaluative and generative modes of thought during the creative process. Neuroimage 2012, 59:1783–1794. [DOI] [PubMed] [Google Scholar]
- 6.Jung RE: The structure of creative cognition in the human brain. Front Hum Neurosci 2013, 7:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayseless N, Eran A, Shamay-Tsoory SG: Generating original ideas: The neural underpinning of originality. Neuroimage 2015, 116:232–239. [DOI] [PubMed] [Google Scholar]
- 8.Mok LW: The interplay between spontaneous and controlled processing in creative cognition. Front Hum Neurosci 2014, 8:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madore KP, Thakral PP, Beaty RE, Addis DR, Schacter DL: Neural mechanisms of episodic retrieval support divergent creative thinking. Cereb Cortex 2017, doi: 10.1093/cercor/bhx312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schacter DL, Madore KP: Remembering the past and imagining the future: Identifying and enhancing the contribution of episodic memory. Mem Stud 2016, 9:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madore KP, Addis DR, Schacter DL: Creativity and memory: Effects of an episodic specificity induction on divergent thinking. Psychol Sci 2015, 26:1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedek M, Jauk E, Sommer M, Arendasy M, Neubauer AC: Intelligence, creativity, and cognitive control : The common and differential involvment of executive functions in intelligence and creativity. Intelligence 2014, 46:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilhooly KJ, Fioratou E, Anthony SH, Wynn V: Divergent thinking: Strategies for generating alternative uses for familiar objects. Br J Psychol 2007, 98:611–625. [DOI] [PubMed] [Google Scholar]
- 14.Beaty RE, Christensen AP, Benedek M, Silvia PJ, Schacter DL: Creative constraints: Brain activity and network dynamics underlying semantic interference during idea production. Neuroimage 2017, 148:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green AE, Cohen MS, Raab HA, Yedibalian CG, Gray JR: Frontopolar activity and connectivity support dynamic conscious augmentation of creative state. Hum Brain Mapp 2015, 36:923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vatansever D, Menon DK, Stamatakis EA: Default mode contributions to automated information processing. Proc Natl Acad Sci 2017, 114:201710521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.*Benedek M: Internally directed attention in creative cognition In The Cambridge handbook of the neuroscience of creativity. Edited by Jung R, Vartanian O. Cambridge University Press; 2018. [Google Scholar]; This chapter summarizes brain behavioral and eye tracking research on internally directed attention and its relevance to creativity. Benedek characterizes creative thought as a mode of “perceptual decoupling” akin to mind-wandering that involves shielding internal processes from external stimulation. A series of neuroimaging studies show that internal attention during creative cognition involves posterior parietal regions including an fMRI study showing coupling between posterior parietal regions of the ECN and occipital cortices (see Benedek et al 2016 below).
- 18.Benedek M, Jauk E, Beaty RE, Fink A, Koschutnig K, Neubauer AC: Brain mechanisms associated with internally directed attention and self-generated thought. Sci Rep 2016, 6:22959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaty RE, Silvia PJ, Benedek M: Brain networks underlying novel metaphor production. Brain Cogn 2017, 111:163–170. [DOI] [PubMed] [Google Scholar]
- 20.**Vartanian O, Beatty EL, Smith I, Blackler K, Lam Q, Forbes S: One-way traffic: The inferior frontal gyrus controls brain activation in the middle temporal gyrus and inferior parietal lobule during divergent thinking. Neuropsychologia 2018, doi: 10.1016/j.neuropsychologia.2018.02.024. [DOI] [PubMed] [Google Scholar]; Although several studies have provided correlational evidence linking executive and default networks to divergent thinking, the causal relation between these networks has remained uncharacterized. Using dynamic causal modeling, Vartanian and colleagues provide the first causal evidence on this relationship, finding that the left inferior frontal gyrus exerts a top-down and unidirectional influence on activity within the middle temporal gyrus, thus extending correlational work by illustrating causal influences of executive brain regions in creative cognition.
- 21.Beaty RE, Kenett YN, Christensen AP, Rosenberg MD, Benedek M, Chen Q, Fink A, Qiu J, Kwapil TR, Kane MJ, et al. : Robust prediction of individual creative ability from brain functional connectivity. Proc Natl Acad Sci 2018, 115:1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Todd Constable R: Functional connectome fingerprinting: Identifying individuals based on patterns of brain connectivity. Nat Neurosci 2015, 18:1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu WT, Rosenberg MD, Scheinost D, Constable RT, Chun MM: Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc Cogn Affect Neurosci 2018, 13:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg MD, Finn ES, Scheinost D, Constable RT, Chun MM: Characterizing attention with predictive network models. Trends Cogn Sci 2017, 21:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg MD, Hsu WT, Scheinost D, Constable RT, Chun MM: Connectome-based models predict separable components of attention in novel individuals. J Cogn Neurosci 2018, 30:160–173. [DOI] [PubMed] [Google Scholar]
- 26.Yoo K, Rosenberg MD, Hsu WT, Zhang S, Li CSR, Scheinost D, Constable RT, Chun MM: Connectome-based predictive modeling of attention: Comparing different functional connectivity features and prediction methods across datasets. Neuroimage 2018, 167:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.*Liu Z, Zhang J, Xie X, Rolls ET, Sun J, Zhang K, Jiao Z, Chen Q, Zhang J, Qiu J, et al. : Neural and genetic determinants of creativity. Neuroimage 2018, 174:164–176. [DOI] [PubMed] [Google Scholar]; Using prediction modeling, Liu and colleagues combined resting-state fMRI and genetic data to estimate individual figural divergent thinking ability. The authors found that combining fMRI and genetic data provided a reliable and relatively accurate prediction of a person’s divergent thinking ability. They identify resting-state networks associated with high- and low-divergent thinking ability consisting of regions spanning the whole brain, with a preponderance of regions located within executive/default networks and default/sensory networks, respectively.
- 28.Chen Q, Beaty RE, Wei D, Yang J, Sun J, Liu W, Yang W, Zhang Q: Longitudinal alterations of frontoparietal and frontotemporal networks predict future creative cognitive ability. Cereb Cortex 2016, 28:103–115. [DOI] [PubMed] [Google Scholar]
- 29.Beaty RE, Benedek M, Wilkins RW, Jauk E, Fink A, Silvia PJ, Hodges DA, Koschutnig K, Neubauer AC: Creativity and the default network : A functional connectivity analysis of the creative brain at rest. Neuropsychologia 2014, 64:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Q-L, Xu T, Yang W-J, Li Y-D, Sun J-Z, Wang K-C, Beaty RE, Zhang Q-L, Zuo X-N, Qiu J: Individual differences in verbal creative thinking are reflected in the precuneus. Neuropsychologia 2015, 75:441–449. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi H, Taki Y, Hashizume H, Sassa Y, Nagase T, Nouchi R, Kawashima R: The association between resting functional connectivity and creativity. Cereb Cortex 2012, 22:2921–2929. [DOI] [PubMed] [Google Scholar]
- 32.Wei D, Yang J, Li W, Wang K, Zhang Q, Qiu J: Increased resting functional connectivity of the medial prefrontal cortex in creativity by means of cognitive stimulation. Cortex 2014, 51:92–102. [DOI] [PubMed] [Google Scholar]
- 33.**Takeuchi H, Taki Y, Nouchi R, Yokoyama R, Kotozaki Y, Nakagawa S, Sekiguchi A, Iizuka K, Yamamoto Y, Hanawa S, et al. : Regional homogeneity, resting-state functional connectivity and amplitude of low frequency fluctuation associated with creativity measured by divergent thinking in a sex-specific manner. Neuroimage 2017, 152:258–269. [DOI] [PubMed] [Google Scholar]; In a large sample of participants (n = 1277), Takeuchi et al. examine resting-state network connectivity related to divergent thinking ability using a range of analytic methods. The authors found that resting connectivity between executive (left inferior frontal gyrus) and default (medial prefrontal cortex) varied as a function of sex, with females showing a positive correlation and males showing a negative correlation.
- 34.Gao Z, Zhang D, Liang A, Liang B, Wang Z, Cai Y, Li J, Gao M, Liu X, Chang S, et al. : Exploring the associations between intrinsic brain connectivity and creative ability using functional connectivity strength and connectome analysis. Brain Connect 2017, 7:590–601. [DOI] [PubMed] [Google Scholar]
- 35.Beaty RE, Benedek M, Barry Kaufman S, Silvia PJ: Default and executive network coupling supports creative idea production. Sci Rep 2015, 5:10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.*Shi L, Sun J, Ren Z, Chen Q, Wei D, Yang W, Qiu J: Large-scale brain network connectivity underlying creativity in resting-state and task fMRI: Cooperation between default network and frontal-parietal network. Biol Psychol 2018, 135:102–111. [DOI] [PubMed] [Google Scholar]; This study is the first to examine both task-based and resting-state network connectivity related to divergent thinking in the same sample of participants. Results showed that divergent thinking is related to stronger default-executive (between-network) coupling and weaker executive-executive (within-network) coupling during divergent thinking, and the strength of default-executive coupling at rest correlated with originality ratings, providing evidence for a correspondence between resting and task networks in creative cognition.
- 37.**Sun J, Liu Z, Rolls ET, Chen Q, Yao Y, Yang W, Wei D, Zhang Q, Zhang J, Feng J, et al. : Verbal creativity correlates with the temporal variability of brain networks during the resting state. Cereb Cortex 2018, doi: 10.1093/cercor/bhy010. [DOI] [PubMed] [Google Scholar]; Sun et al. explore the question of whether cognitive flexibility, assessed via variability of functional connectivity within- and between-networks, relates to neural flexibility (divergent thinking ability) in a large sample of participants (n = 574) who completed resting-state scanning and a battery of verbal divergent thinking tasks outside the scanner. The authors find that verbal divergent thinking ability is characterized by within-network temporal variability (i.e., fluctuations in functional connectivity assessed over short time scales) of the default network and between-network variability of several networks, indicating that creative thinking ability is characterized by an ability to flexibly engage large-scale networks.
- 38.*Li J, Zhang D, Liang A, Liang B, Wang Z, Cai Y, Gao M, Gao Z, Chang S, Jiao B, et al. : High transition frequencies of dynamic functional connectivity states in the creative brain. Sci Rep 2017, 7:46072. [DOI] [PMC free article] [PubMed] [Google Scholar]; Several studies have examined static (or average) connectivity between regions and networks linked to creative cognition. This study is the first to assess dynamic shifts in connectivity associated with creativity and finds that high-divergent thinking ability is characterized by more frequent transitions between different connectivity “states” (or recurring patterns of network connectivity), providing a link between cognitive and neural flexibility.
- 39.Beaty RE, Kaufman SB, Benedek M, Jung RE, Kenett YN, Jauk E, Neubauer AC, Silvia PJ: Personality and complex brain networks : The role of openness to experience in default network efficiency. Hum Brain Mapp 2016, 779:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beaty RE, Chen Q, Christensen AP, Qiu J, Silvia PJ, Schacter DL: Brain networks of the imaginative mind: Dynamic functional connectivity of default and cognitive control networks relates to openness to experience. Hum Brain Mapp 2018, 39:811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.**Bendetowicz D, Urbanski M, Garcin B, Foulon C, Levy R, Bréchemier ML, Rosso C, De Schotten MT, Volle E: Two critical brain networks for generation and combination of remote associations. Brain 2018, 141:217–233. [DOI] [PubMed] [Google Scholar]; This paper presents a new assessment of creative concept generation (Free Generation of Associates Task) and combination (Combined Associates Task) based on the classic Remote Associates Test. Using lesion-based network mapping in a sample of frontal lesion patients and healthy controls, the authors dissociate brain regions and networks involved in concept generation and combination: lesions to the MPFC of the DN impaired generation but not combination and lesions to the RLPFC of the ECN impaired combination but spared generation.
- 42.Kenett YN, Medaglia JD, Beaty RE, Chen Q, Betzel RF, Thompson-Schill SL, Qiu J: Driving the brain towards creativity and intelligence: A network control theory analysis. Neuropsychologia 2018, doi: 10.1016/j.neuropsychologia.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi H, Taki Y, Nouchi R, Yokoyama R, Kotozaki Y, Nakagawa S, Sekiguchi A, Iizuka K, Yamamoto Y, Hanawa S, et al. : Creative females have larger white matter structures: Evidence from a large sample study. Hum Brain Mapp 2017, 38:414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryman SG, van den Heuvel MP, Yeo RA, Caprihan A, Carrasco J, Vakhtin AA, Flores RA, Wertz C, Jung RE: Sex differences in the relationship between white matter connectivity and creativity. Neuroimage 2014, 101:380–389. [DOI] [PubMed] [Google Scholar]
- 45.Benedek M, Schües T, Beaty RE, Jauk E, Koschutnig K, Fink A, Neubauer AC: To create or to recall original ideas: Brain processes associated with the imagination of novel object uses. Cortex 2018, 99:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham A, Rutter B, Bantin T, Hermann C: Creative conceptual expansion: A combined fMRI replication and extension study to examine individual differences in creativity. Neuropsychologia 2018, doi: 10.1016/j.neuropsychologia.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 47.*Marron TR, Lerner Y, Berant E, Kinreich S, Shapira-Lichter I, Hendler T, Faust M: Chain free association, creativity, and the default mode network. Neuropsychologia 2018, doi: 10.1016/j.neuropsychologia.2018.03.018. [DOI] [PubMed] [Google Scholar]; The default network is often considered a source of creative idea generation, but little evidence actually exists to support this claim. Marron and colleagues provide such evidence by examining brain regions associated with chain free association (FA), a relatively unconstrained task involving spontaneous production of contiguously related concepts. Behavioral indices of FA correlated with divergent thinking scores and brain activity during FA, including FA semantic distance and PCC activity during FA performance, pointing to a potential role of spontaneous episodic or semantic processes in idea production.
- 48.Luft CDB, Pereda E, Banissy MJ, Bhattacharya J: Best of both worlds: Promise of combining brain stimulation and brain connectome. Front Syst Neurosci 2014, 8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weinberger AB, Green AE, Chrysikou EG: Using transcranial direct current stimulation to enhance creative cognition: Interactions between task, polarity, andstimulation site. Front Hum Neurosci 2017, 11:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu W, Chen Q, Xia L, Beaty RE, Yang W, Tian F, Sun J, Cao G, Zhang Q, Chen X, et al. : Common and distinct brain networks underlying verbal and visual creativity. Hum Brain Mapp 2017, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boccia M: Where do bright ideas occur in our brain ? Meta-analytic evidence from neuroimaging studies of domain- specific creativity. Front Psychol 2015, 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi B, Cao X, Chen Q, Zhuang K, Qiu J: Different brain structures associated with artistic and scientific creativity: A voxel-based morphometry study. Sci Rep 2017, 7:42911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaty RE: The neuroscience of musical improvisation. Neurosci Biobehav Rev 2015, 51:108–117. [DOI] [PubMed] [Google Scholar]
- 54.Bashwiner DM, Wertz CJ, Flores RA, Jung RE: Musical creativity revealed in brain structure: Interplay between motor, default mode, and limbic networks. Sci Rep 2016, 6:20482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loui P: Rapid and flexible creativity in musical improvisation: Review and a model. Ann N Y Acad Sci 2018, doi: 10.1111/nyas.13628. [DOI] [PubMed] [Google Scholar]
- 56.Pinho AL, de Manzano O, Fransson P, Eriksson H, Ullen F: Connecting to create: Expertise in musical improvisation is associated with increased functional connectivity between premotor and prefrontal areas. J Neurosci 2014, 34:6156–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinho AL, Ullén F, Castelo-Branco M, Fransson P, De Manzano Ö: Addressing a paradox: Dual strategies for creative performance in introspective and extrospective networks. Cereb Cortex 2016, 26:3052–3063. [DOI] [PubMed] [Google Scholar]
- 58.Saggar M, Quintin EM, Bott NT, Kienitz E, Chien YH, Hong DWC, Liu N, Royalty A, Hawthorne G, Reiss AL: Changes in brain activation associated with spontaneous improvization and figural creativity after design-thinking-based training: A longitudinal fMRI study. Cereb Cortex 2017, 27:3542–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu S, Erkkinen MG, Healey ML, Xu Y, Swett KE, Chow HM, Braun AR: Brain activity and connectivity during poetry composition: Toward a multidimensional model of the creative process. Hum Brain Mapp 2015, 36:3351–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Pisapia N, Bacci F, Parrott D, Melcher D: Brain networks for visual creativity: A functional connectivity study of planning a visual artwork. Sci Rep 2016, 6:39185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lotze M, Erhard K, Neumann N, Eickhoff SB, Langner R: Neural correlates of verbal creativity: Differences in resting-state functional connectivity associated with expertise in creative writing. Front Hum Neurosci 2014, 8:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neumann N, Domin M, Erhard K, Lotze M: Voxel-based morphometry in creative writers: Gray-matter increase in a prefronto-thalamic-cerebellar network. Eur J Neurosci 2018, doi: 10.1111/ejn.13952. [DOI] [PubMed] [Google Scholar]