Abstract
p38γ is a member of p38 MAPK family which contains four isoforms p38α, p38β, p38γ and p38δ. p38γ MAPK has unique function and is less investigated. Recent studies revealed that p38γ MAPK may be involved in tumorigenesis and cancer aggressiveness. However, the underlying cellular/molecular mechanisms remain unclear. Epithelial-mesenchymal transition (EMT) is a process that epithelial cancer cells transform to facilitate the loss of epithelial features and gain of mesenchymal phenotype. EMT promotes cancer cell progression and metastasis, and is involved in the regulation of cancer stem cells (CSCs) which have self-renewal capacity and are resistant to chemotherapy and target therapy. We showed that p38γ MAPK positively increased EMT in breast cancer cells; over-expression of p38γ MAPK enhanced EMT while its down-regulation inhibited EMT. Meanwhile, p38γ MAPK augmented CSC population while knock down of p38γ MAPK decreased CSC ratio in breast cancer cells. MicroRNA-200b (miR-200b) was down-stream of p38γ MAPK and negatively regulated by p38γ MAPK; miR-200b mimics blocked p38γ MAPK-induced EMT while miR-200b inhibitors promoted EMT. p38γ MAPK regulated miR-200b through inhibiting GATA3. p38γ MAPK induced GATA3 ubiquitination, leading to its proteasome-dependent degradation. Suz12, a Polycomb group protein, was down-stream of miR-200b and involved in miR-200b regulation of EMT. Thus, our study established an important role of p38γ MAPK in EMT and identified a novel signaling pathway for p38γ MAPK–mediated tumor promotion.
Keywords: Cancer stem cells, p38γ MAPK, metastasis, microRNA
Introduction
p38 Mitogen Activated Protein Kinases (p38 MAPKs) play an important role in the cellular response to environmental stress. There are four p38 MAPK isoforms in mammalian cells encoded by different genes: p38α (MAPK14), p38β (MAPK 11), p38γ (MAPK 12), and p38δ (MAPK 13) [1]. The expression pattern of p38 MAPKs vary in different tissues. p38α is ubiquitously expressed in all cell types and tissues while p38β is highly expressed in the brain, thymus, and spleen; its expression is lower in the adrenals, lung, kidney, liver, pancreas, and heart, and it is not expressed in skeletal muscle [1]. p38γ is very abundant in skeletal muscle and its expression is lower in most other tissues is [1]. p38δ levels are high in the pancreas, intestine, adrenal gland, kidney, and heart [1]. All p38 MAPKs are Serine/Threonine kinases and activated by a wide variety of environmental and cellular stresses or inflammatory cytokines. p38α was the first p38 MAPK family member identified, therefore the most studied and bestcharacterized isoform; most of the literature on p38 MAPK refers to p38α.
Recently, p38γ MAPK has emerged as an oncogenic MAPK (MAPK) [2–6]. It was also found to be highly expressed in some human cancer cells [4, 5, 7, 8]. p38γ MAPK is shown to be involved in the regulation of cancer stem cells (CSCs), migration/invasion, tumorigenesis, and cell transformation [2–6, 9, 10]. However, the underlying mechanisms and cell signaling pathways are unclear.
Breast cancer is the most prevalent cancer in women worldwide and distant site metastasis is the main cause of death in breast cancer patients. Epithelial-mesenchymal transition (EMT) defined by the progressive loss of epithelial cell characteristics and the acquisition of mesenchymal features is a fundamental mechanism occurring during embryonic development and tissue differentiation. EMT also plays a crucial role for cancer progression, and contributes to the dissemination of cancer cells from solid tumors and the formation of detectable metastases [11]. EMT has also been implicated in therapy resistance, relapse, immune escape, and maintenance of cancer stem cell properties, such as self-renewal capacity [12, 13]. Understanding the complex events that lead to the EMT will therefore help to design new therapies against metastatic breast cancer. In this study, we investigated the novel role of p38γ MAPK in EMT and delineated a signaling pathway which may mediate the action of p38γ MAPK.
Materials and Methods
Materials
Anti-E-cadherin and Suz12 antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA). Protein A/G beads were obtained from Santa Cruz Biotechnology (San Diego, CA). Anti-Vimentin, p38α and p38γ antibodies were purchased from Santa Cruz Biotechnology (San Diego, CA). Anti-GAPDH antibody was obtained from Research Diagnostics, Inc. (Concord, MA). Anti-GATA3 antibody was obtained from Abcam Inc. (Cambridge, MA). Anti-E-cadherin monoclonal antibody was purchased from BD Transduction Laboratories (San Jose, CA). ALDEFLUOR kits and MammoCult™ Human Medium Kit were purchased from Stemcell Technologies (Vancouver, Canada). Ultra-low cluster plates were obtained from Corning Incorporated (Corning, NY). p38γ MAPK shRNA, control shRNA, GATA3 siRNA, and control siRNA were purchased from Santa Cruz Biotechnology (San Diego, CA). Wild type and mutated p38γ MAPK plasmids (p38WT and p38D179) were gifts from Dr. Oded Livnah (Hebrew University of Jerusalem, Jerusalem, Israel) [14]. Human GATA3 plasmid was obtained from Sino Biological Inc. (Beijing, China). miRNA mimic and inhibitors were purchased from Ambion (Thermo Fisher, Waltham, MA). Antibiotic-Antimycotic (AntiAnti) and cell culture mediums were obtained from Gibco (Thermo Fisher, Waltham, MA). Cyclohexamide was obtained from Biovision (Milpitas, CA). MG132 was purchased from EMD Millipore (Burlington, MA). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Cell culture and treatment
MCF7 cells were grown in DMEM medium containing 10% fetal bovine serum (FBS) and 1% Antibiotic-Antimycotic. MCF7 cells overexpressing ErbB2 (MCF7-ErbB2) were cultured in full DMEM medium with hydrocortisone (1 μg/ml) and insulin (10 μg/ml). BT474 cells were cultured in full RPMI medium with insulin. All cell lines were grown at 37°C with 5% CO2. For cyclohexamide treatment, culture medium was changed to serum free and treated with cyclohexamide (50 μg/ml) for indicated times. Cells were treated with chloroquine (100 μM) or MG132 (10 μM) for 6 hours followed by the collection of cell lysates.
Cell transfection and generation of stable cell lines
Transient transfection of mimics or inhibitors miR-200b and miR-34c, GATA3 siRNA (GATA3 si), control siRNA (con si) (San Cruz Biotech), GATA3 plasmid (GATA3 P), and control plasmid (con P) was performed using a Neon Transfection System (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, cells were electroporated and incubated with indicated miRNAs, siRNAs, and plasmids. Experiments were initiated forty-eight hours after the transfection.
For establishing stable transfectants, the plasmids of p38WT, p38D179, and control plasmids were transfected into MCF7 cells using a Neon Transfection machine (Life Technologies). Positive colonies were selected in standard cell culture media containing G418 (400 μg/ml). Short hairpin RNA (shRNA) of p38γ MAPK (p38γsh) or scrambled control shRNA (Santa Cruz Biotechnology) was transfected into MCF7, MCF7-ErbB2, and BT474 cells using a Neon Transfection machine (Life Technologies). Positive colonies were selected in standard cell culture media containing 4 μg/ml puromycin. Cell lysates were collected and analyzed by immunoblotting for the verification of the overexpression or silencing of p38γ MAPK.
ALDEFLUOR assay (Stem-like cell population assay)
The cancer stem-like cells (CSCs) were identified by measuring aldehyde dehydrogenase (ALDH) activity [12, 15]. The ALDEFLUOR assay (Stemcell Technologies) was performed according to the manufacturer’s protocol and the high ALDH enzymatic activity in cells were tested using a flow cytometer as described previously [4, 5]. Briefly, 106 cells were incubated in ALDEFLUOR assay buffer containing ALDH substrate (1 μMol/l per 1×106 cells) for 40 minutes at 37°C. Meanwhile, an aliquot of cells was treated under identical conditions with a specific ALDH inhibitor [50 mmol/l, diethylaminobenzaldehyde (DEAB)] as a negative control. CSCs (cells expressing high levels of ALDH) were identified by a FACSCalibur (Becton Dickinson) flow cytometer. The percentage of CSC population was analyzed and calculated using the WINMDI software.
Tumorsphere assay
Tumorsphere assay was performed as described previously [16, 17]. Briefly, cells were plated as a single cell suspension in ultra-low attachment 24-well plates (Corning) at 1000 cells/well. Cells were grown in serum-free MammoCult™ Human Medium (Stemcell Technologies) for 10 days. The number of tumorspheres (mammospheres) in each well that were 60 μm or larger in size were counted according to the manufacturer’s protocol (MammoCult™ Human Medium, Stemcell Technologies).
Immunoblotting and immunoprecipitation
Cells were lysed in modified RIPA buffer (150 mM NaCl, 50 mM Tris, 1% NP-40, 0.25% sodium deoxycholate) containing 1 mM sodium orthovanadate, 1 mM phenylmethanesulfonyl fluoride (PMSF), 5 μg/ml of aprotinin, and 2 μg/ml of leupeptin. The procedure for immunoblotting has been previously described [18]. Briefly, protein samples were clarified by centrifugation at 14,000 rpm for 10 min at 4°C and were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were transferred to nitrocellulose membranes. The membranes were probed with indicated primary antibodies, followed by the appropriate horseradish peroxidase-conjugated secondary antibodies, and developed by enhanced chemiluminescence. The intensity of specific proteins was quantified using Carestream Molecular Image Software.
For immunoprecipitation, equal amount of protein (500–800 μg) was incubated with anti-Ubiquitin antibody overnight at 4°C, followed by treatment with Protein A/G beads conjugated to agarose for 4 hours at 4°C. Immunoprecipitates were collected by centrifugation at 5,000 g for 5 min at 4°C. Samples were washed 5X with RIPA buffer, 1X with cold-TBS, and boiled in sample buffer (187.5 mM Tri-HCl, pH 6.8, 6% SDS, 30% glycerol, 150 mM DTT and 0.03% bromophenol blue). Proteins were resolved in SDS-PAGE and analyzed by immunoblotting.
Immunofluorescence microscopy
The procedure for immunofluorescent microscopy has been previously described [19, 20]. Briefly, cells were seeded on coverslips pre-coated with fibronectin (10 μg/ml). Cells were fixed with 3.7% paraformaldehyde for 10 min, washed 3 times in PBS, and permeabilized with 0.5% Triton X-100 for 5 min. Cells were blocked with 5% BSA, incubated with primary antibodies for 1 hour, and washed then treated with Alexa Fluorlabeled secondary antibodies. After rinsing with PBS, coverslips were mounted with Prolong Gold anti-fade reagent, and images were photographed using an Olympus 1X81 inverted fluorescent microscope with the same exposure time and amplifier offset.
RNA analysis and quantification
The expression of mRNA for E-cadherin, Vimentin, p38γ MAPK, and GAPDH was analyzed by quantitative RT-PCR. Briefly, total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. cDNAs were synthesized from 1 microgram RNA, respectively, using the Reverse Transcription System (New England Biolabs) according to the manufacturer’s instructions. cDNAs were amplified using DreamTaq Green PCR Master Mix (Themo Scientific) according to the manufacturer’s instructions. The following reverse (R) and forward (F) Primers were used: GAPDH F: 5’-CTCTCTGCTCCTCCTGTTCGAC-3’, GAPDH R: 5’TGAGCGATGTGGCTCGGCT-3’, E-cadherin F: 5’-GGGCACAGATGGTGTGATTA-3’ and E-cadherin R: 5’-GCGTGAGAGAAGAGAGTGTATG-3’, Vimentin F: 5’GATTCACTCCCTCTGGTTGATAC-3’, Vimentin R: 5’-CTTGTAGGAGTGTCGGTTGTT-3’, p38γMAPK F: 5’-CATCATCCACAGAGACCTGAAG-3’, p38γ MAPK R: 5’-GCTTGCATTGGTCAGGATAGA-3’. Each experiment was replicated at least 3 times.
The expression levels of miR-200b and miR-34c were analyzed by quantitative real-time PCR. Briefly, total RNA was extracted using TRIzol reagent (Thermo Fisher) according to the manufacturer’s instructions. cDNA was then synthesized using TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems) according to the manufacturer’s instructions. Real-time PCR was performed using the LightCycler SYBR Green I Master kit (Roche Applied Science, Mannheim, Germany) on Applied Biosystems StepOne Plus Real-Time PCR system (Life Technologies). The data were analyzed using 2^-ddCt method. The relative expression levels of miR-200b and miR-34c were normalized to SNORD68. Each experiment was replicated at least 3 times.
Statistics
Differences among treatment groups were analyzed using analysis of variance (ANOVA). Differences in which p was less than 0.05 were considered statistically significant. In cases where significant differences were detected, specific post-hoc comparisons between treatment groups were examined with Student-Newman-Keuls tests. The prevalence of metastasis between control and ethanol-treated groups was determined by the Fisher exact test.
Results
p38γ MAPK regulates the epithelial–mesenchymal transition (EMT) in breast cancer cells
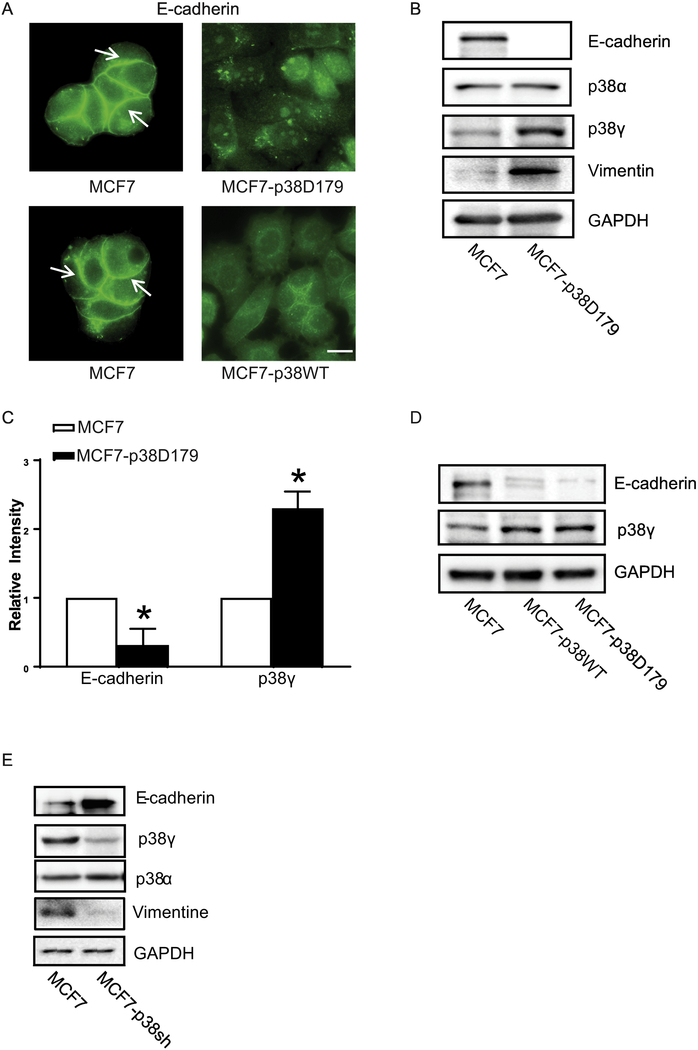
First, we investigated the role of p38γ MAPK in EMT and CSCs because MCF7 cells express low levels of p38γ MAPK and are less aggressive [5]. p38γ MAPK is activated through both overexpression and phosphorylation [3]. Asp to Ala mutation in the phosphorylation lip (D179) made p38γ MAPK constitutively active [14]. We stably transfected MCF7 cells with either wild type (MCF7-p38WT) or active p38γ MAPK (MCF7-p38D179) plasmids. Transfection of p38γD179 and p38γWT plasmids decreased the expression of E-cadherin at the adherens junctions (Fig. 1A). Immunoblotting data confirmed that overexpression of either wild type or p38γD179 decreased the levels of E-cadherin while increasing the levels of vimentin, suggesting the occurrence of EMT (Fig. 1B-C). Fig. 1C shows the quantitative results of the expression of E-cadherin and p38γ MAPK. Since p38γD179 or p38γWT plasmids had a similar effect (Fig. 1A and 1D), all subsequent experiments used p38γD179 plasmid only. To the contrast, knocking down of p38γ MAPK by p38γ shRNA decreased EMT in MCF7 cells, as indicated by an increase in E-cadherin and a decrease in vimentin (Fig. 1E). The expression of p38α MAPK was not altered by either over-expression or knock down of p38γ MAPK in MCF7 cells (Figs. 1B and 1E).
Figure 1.
Effect of p38γ MAPK on EMT in breast cancer cells. MCF7 cells were stably transfected with either WT p38γ MAPK (MCF7-p38WT) or the active form of p38γ MAPK (MCF7-p38D179) plasmids as described in the Materials and Methods. A: MCF7 and MCF7-p38D179 cells were cultured on coverslips. The expression of E-cadherin was detected by immunofluorescent staining as described in the Materials and Methods. Arrows indicate the E-cadherin on the cell borders. Bar = 20 μM. B and D: Cell lysates of MCF7, MCF7-p38WT or MCF7-p38D179 were collected and the expression of EMT markers (E-cadherin and Vimentin), and p38γ MAPK was analyzed by immunoblotting. GAPDH served as a loading control. C: The expression of E-cadherin and p38γ MAPK was quantified and normalized to the expression of GAPDH. The experiment was replicated at least three time. * denote significant difference from MCF7 cells, p < 0.05. E: MCF7 cells stably expressing control shRNA (MCF7) and p38γ MAPK shRNA (MCF7-p38sh) were established as described in the Materials and Methods. The expression of E-cadherin, Vimentin, p38γ and p38α MAPK was examined with immunoblotting.
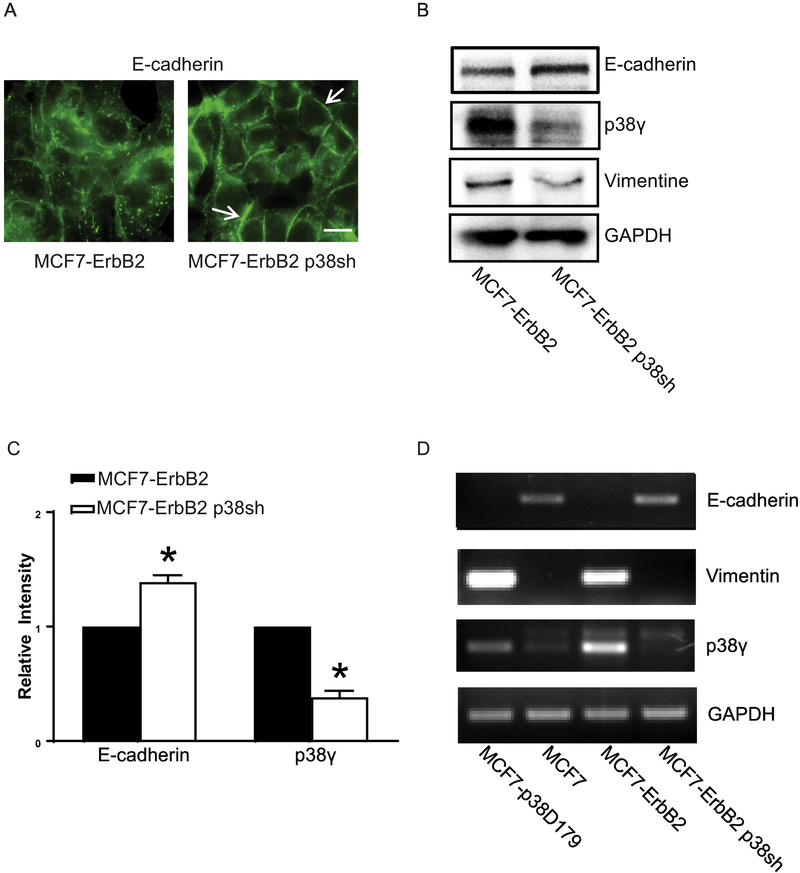
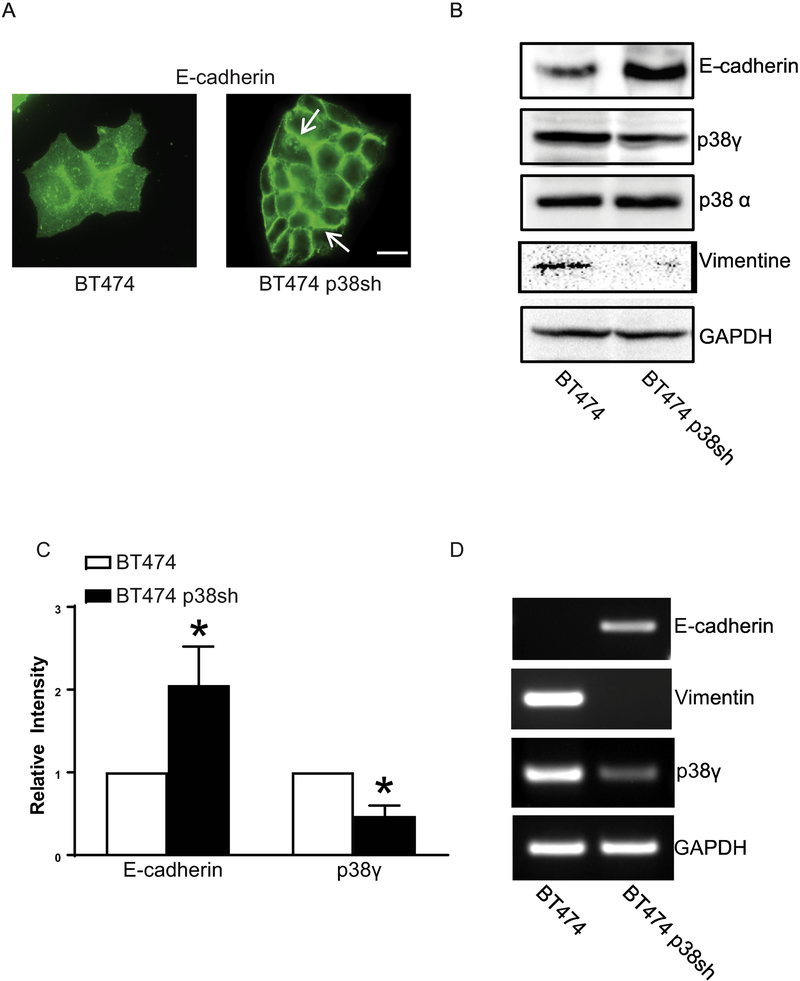
Breast cancer cells with high levels of ErbB2 express less or no E-cadherin and are more aggressive [11]. We sought to determine whether knocking down p38γ in these cells affected EMT. MCF7-ErbB2 cells are MCF7 cells overexpressing ErbB2 and BT474 cells are a breast cancer cell line naturally overexpressing ErbB2. We knocked down p38γ MAPK in these cells by stable transfection of p38γ shRNA. Down-regulation of p38γ MAPK caused an increase in E-cadherin and a decrease in vimentin (Figs 2 and 3), suggesting an inhibition of EMT. Breast cancer cells over-expressing ErbB2 lost Ecadherin at the intercellular junctions which are a feature of aggressiveness. In those breast cells when p38γ MAPK was knocked down, E-cadherin re-appeared at the intercellular junctions (Figs 2A and 3A). Immunoblotting data confirmed that knock down of p38γ MAPK increased the levels of E-cadherin (Fig. 2B and 2C; Fig. 3B and 3C). p38γ MAPK also regulated mRNA levels of E-cadherin or vimentin (Figs 2D and 3D). Thus, p38γ MAPK may positively regulate EMT through suppressing E-cadherin expression.
Figure 2.
Effect of p38γ MAPK on EMT in MCF7 cells over-expressing ErbB2. MCF7 cells over-expressing ErbB2 were stably transfected with control shRNA (MCF7-ErbB2) and p38γ MAPK shRNA (MCF7-ErbB2 p38sh) as described in the Methods. A: Cells were cultured on coverslips. The expression of E-cadherin was detected by immunofluorescent staining as described under the Materials and Methods. Arrows indicate the E-cadherin on the cell borders. Bar = 20 μM. B: Cell lysates of MCF7-ErbB2 and MCF7-ErbB2 p38sh cells were collected and the expression of EMT markers (Ecadherin and Vimentin), p38γ MAPK was analyzed by immunoblotting. GAPDH served as a loading control. C: The expression of E-cadherin and p38γ MAPK was quantified and normalized to the expression of GAPDH. The experiment was replicated at least three time. * denote significant difference from MCF7-ErbB2 cells, p < 0.05. D: The mRNA levels of E-cadherin, Vimentin, and p38γ MAPK in MCF7, MCF7-p38D179, MCF7-ErbB2 and MCF7-ErbB2 p38sh cells were examined by RT-PCR. The mRNA level of GAPDH was used as a loading control.
Figure 3.
Effect of p38γ MAPK on EMT in BT474 breast cancer cells. BT474 breast cancer cells were stably transfected with control shRNA (BT474) and p38γ shRNA (BT474 p38sh). A: The expression of E-cadherin was detected by immunofluorescent staining. Arrows indicate the E-cadherin on the cell borders. Bar = 20 μM. B: Cell lysates of BT474 and BT474 p38sh cells were collected and the expression of Ecadherin, Vimentin, p38γ, p38α MAPK, and GAPDH was analyzed by immunoblotting. C: The expression of E-cadherin and p38γ MAPK was quantified and normalized to the expression of GAPDH. The experiment was replicated at least three time. * denote significant difference from BT474 cells, p < 0.05. D: The mRNA levels of E-cadherin, Vimentin, and p38γ MAPK in BT474 and BT474 p38sh cells were analyzed by RT-PCR. The mRNA level of GAPDH was used as a loading control.
p38γ MAPK regulates the ratios of cancer stem cells (CSCs) in breast cancer cells
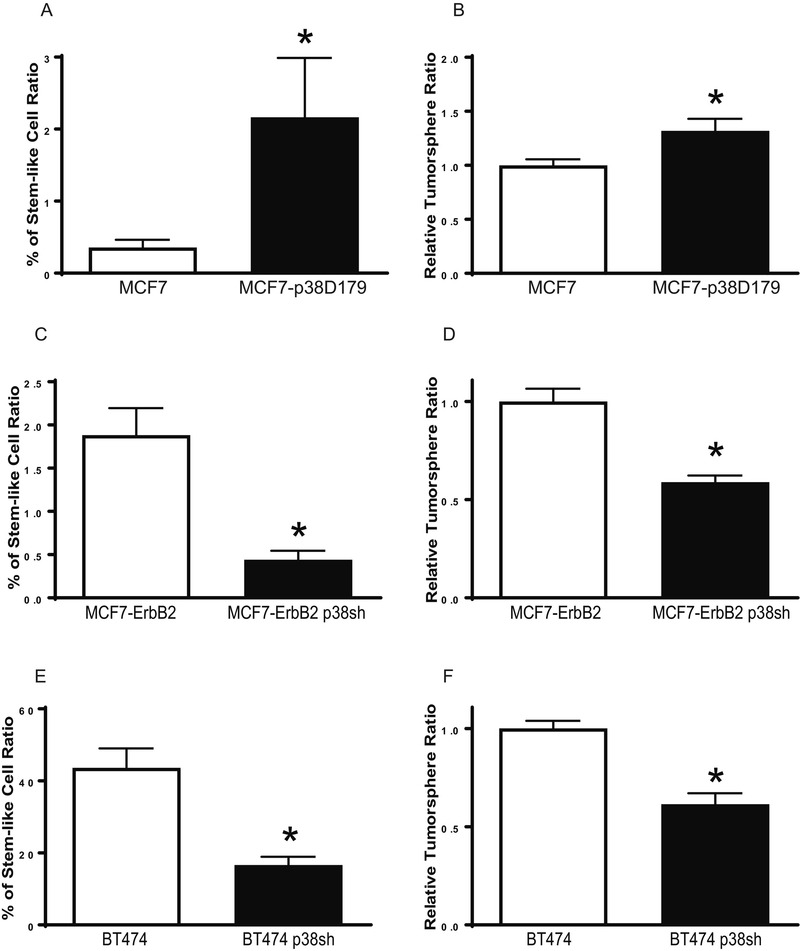
Since EMT in breast cancer is associated with CSC properties and self-renewal capabilities. We sought to determine whether p38γ MAPK regulated CSCs. We found that over-expression of p38γ MAPK increased CSC ratios and the formation of tumorspheres in MCF7 cells (Fig. 4A and 4B). In contrast, knocking down of p38γ MAPK decreased CSC population and tumorspheres in both MCF7-ErbB2 and BT474 cells (Figs. 4C-F). These data indicated that p38γ MAPK played a critical role in the regulation of CSCs.
Figure 4.
Effect of p38γ MAPK on CSCs and tumorsphere formation in breast cancer cells. A: MCF7 and MCF7-p38D179 cells were processed for ALDEFLUOR assay, followed by flow cytometry for the detection of CSCs. CSC population was calculated as percentage of total cells population. Each data point was mean ± SEM of three independent experiments. * p<0.05, denotes significant difference from the control groups. B: Cells (1,000 cells) were cultured on ultra-low attachment plates for assaying tumorsphere formation as described in the Materials and Methods. The number of tumorspheres was counted and calculated relative to MCF7 cells. Each data point was the mean ± SEM of three independent experiments. * p<0.05, denotes significant difference from MCF7 cells. C and D: MCF7-ErbB2 and MCF7-ErbB2 p38sh cells were analyzed for CSCs and tumorsphere formation as described above. * p < 0.05, denotes significant difference from MCF7- ErbB2 cells. E and F: BT474 and BT474 p38sh cells were analyzed for CSCs and tumorsphere formation as described above. * p < 0.05, denotes significant difference from BT474 cells.
miR-200b is down-stream of p38γ MAPK and regulates E-cadherin expression
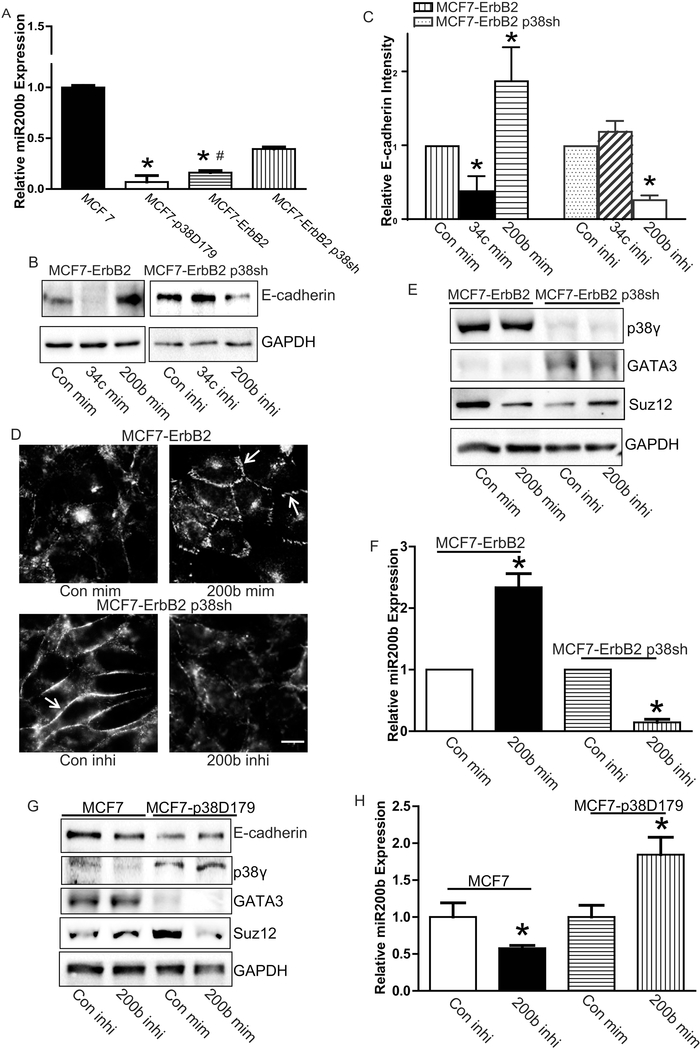
We showed that p38γ MAPK negatively regulated E-cadherin expression. Next, we sought to determine the underlying molecular mechanisms. Recently, miRNA 200 family has been implied in the regulation of EMT and associated with the suppression of cancers including breast cancers [21–27]. We have screened miRNA 200 family and found that the miR-200b levels were altered by p38γ MAPK in four breast cancer cell lines (MCF7, MCF-p38D179, MCF7-ErbB2, and MC F7-ErbB2 p38sh). We previously demonstrated that the p38γ MAPK level was low in MCF7, but high in MCF-ErbB2 cells [4, 5]. miR-200b levels in these cells inversely correlated with p38γ MAPK expression, i.e., breast cancer cells with higher expression levels of p38γ MAPK (MCF7-p38D179 and MCF7-ErbB2) had lower levels of miRNA 200b (Fig. 5A). On the other hand, breast cancer cells with lower levels of p38γ MAPK (MCF7 and MCF7-ErbB2 p38sh) expressed higher levels of miR-200b (Fig. 5A).
Figure 5.
p38γ MAPK and miR-200b in their regulation of E-cadherin. A: The expression of miR-200b in MCF7, and MCF7-p38D179, MCF7-ErbB2 and MCF7-ErbB2 p38sh cells was analyzed by real-time PCR as described in the Materials and Methods. Each data point was the mean ± SEM of three independent experiments. p< 0.05, * denotes significant difference from MCF7 cells. # denotes significant difference from MCF7-ErbB2 p38shCon Si. B: MCF7-ErbB2 and MCF7-ErbB2 p38sh cells were treated with either controls or mimics/inhibitors for miR-200b or miR-34c (con mim, con inhi,200b mim, 34c mim, 200b inhi or 34c inhi) as described in the Materials and Methods. After 48 hours, E-cadherin levels were analyzed by immunoblotting. C: The expression of E-cadherin was quantified and normalized to the expression of GAPDH. The experiment was replicated at least three time. * denote significant difference from con min or con inhi, p < 0.05. D: MCF7-ErbB2 and MCF7-ErbB2 p38sh cells were treated with miR-200b mimics or inhibitor for 48 hours, then the expression of E-cadherin was detected by immunofluorescent staining. Arrows indicate the E-cadherin on the cell borders. Bar = 20 μM. E: The protein levels of p38γ MAPK, GATA3, and Suz12 were determined by immunoblotting. F: The relative miR-200b levels were determined by real-time PCR. G: MCF7 or MCF7-p38D179 cells were treated with either miRNA-200b inhibitor (200b inhi) or mimics (200b mim) for 48 hours. The expression of E-cadherin, p38γ MAPK, GATA3, Suz12, and GAPDH was examined by immunoblotting. The experiments were replicated three times. H: The relative expression of miRNA-200b was determined by real-time PCR. Each data point was the mean ± SEM of three independent experiments *p < 0.05, denotes significant difference from respective controls.
To determine whether miR-200b can regulate E-cadherin expression, we treated the breast cancer cells with a high expression of miR-200b (MCF7 and MCF7-ErbB2 p38sh cells) with miR-200b inhibitor. In contrast, we transfected the breast cancer cells with a low expression of miR-200b (MCF7-p38D179 and MCF7-ErbB2 cells) with miR200b mimics. MCF7 and ErbB2 p38sh cells had the low p38γ MAPK levels but high expression of miR-200b and E-cadherin (Figs. 1, 2 and 5A). Treatment of miR-200b inhibitor decreased the expression of E-cadherin in these cells (Fig. 5B and 5C). Moreover, miR-200b inhibitor inhibited junctional E-cadherin expression (Fig. 5D, bottom panel). Reversely, miR-200b mimics increased E-cadherin expression in MCF7ErbB2 and MCF7-D179 cells (Fig. 5B and 5C) and enhanced the formation of junctional E-cadherin (Fig. 5D, top panel). However, neither miR-200b mimics nor inhibitors affected the p38γ MAPK levels (Fig. 5E and 5G), suggesting that p38γ MAPK was upstream of miR-200b. miR-34C was also reported to play a role in regulation of Ecadherin expression [28, 29]. miR-34C may inhibit E-cadherin expression through its regulation of Snail1 [29]. However, neither miR-34C mimics nor inhibitors reversed p38γ MAPK-regulated E-cadherin expression (Fig. 5B), suggesting that miR-34C was not involved in p38γ MAPK regulation of E-cadherin. Together, these results indicated that p38γ MAPK activation could suppress miR-200b which is a positive regulator of Ecadherin expression, resulting in enhanced EMT.
GATA3 mediates p38γ MAPK regulation of miR-200b
To understand the mechanisms of p38γ MAPK regulation of miR-200b, we examined the effect of p38γ MAPK on GATA3. GATA3 is a transcription factor that regulates the expression of miR-200s and miR-200b contains GATA3 binding sites in its promoter region [30]. Here, we showed that p38γ MAPK negatively regulated the expression of GATA3. Down-regulation of p38γ MAPK by shRNA increased the expression of GATA3 (Fig. 5E). In contrast, activation of p38γ MAPK inhibited the expression of GATA3 (Fig. 5G). However, neither miR-200b mimics nor inhibitors affected GATA3 levels (Figs. 5E and 5G), indicating that GATA3 is upstream of miR200b but downstream of p38γ MAPK. The effective manipulation of miR-200b levels by its inhibitor or mimics was confirmed (Fig. 5H).
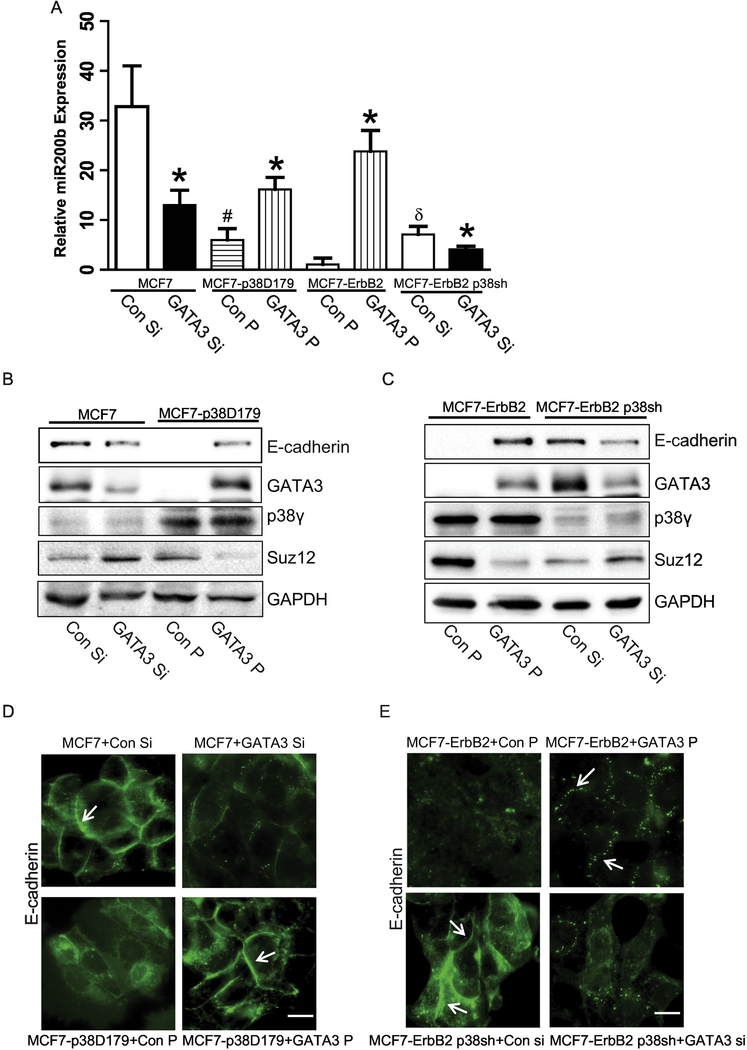
Next, we sought to confirm that GATA3 is a critical component of miR-200b/Ecadherin pathway. Indeed, As described above, breast cancer cells with low levels of p38γ MAPK (MCF7 and MCF7-ErbB2 p38sh cells) contained higher levels of GATA3, compared with cells with high levels of p38γ MAPK (MCF7-p38D179 and MCF7-ErbB2 cells)(Fig. 5E). Therefore, GATA3 siRNA was transfected in to the cells expressing higher levels of GATA3 (MCF7 and MCF7-ErbB2 p38sh cells); while GATA3 plasmids were transfected in to the cells with low expression of GATA3 (MCF7-p38D179 and MCF7-ErbB2 cells). Silencing of GATA3 suppressed miR-200b and E-cadherin in MCF7 and MCF7-ErbB2 p38sh cells (Figs. 6A-C). Furthermore, down-regulation of GATA3 inhibited the expression of E-cadherin in the intercellular junctions (Fig. 6D, top panel and Fig. 6E, bottom panel). To the contrary, overexpression of GATA3 reversed p38γ MAPK-induced down-regulation of miR-200b and E-cadherin (Figs. 6A-C). In addition, overexpression of GATA3 recovered p38γ MAPK-induced loss of E-cadherin in the adherens junctions (Fig 6D, bottom panel and Fig. 6E, top panel). Neither silence nor overexpression of GATA3 affected p38γ MAPK levels (Fig. 6B and 6C), confirming that GATA3 was downstream of p38γ MAPK. Taken together, these results demonstrated that p38γ MAPK/GATA3/miR-200b pathway played an important role in the regulation of EMT in breast cancer cells.
Figure 6.
Effect of GATA3 on the expression of E-cadherin and miRNA-200b in breast cancer cells. MCF7 or MCF7-ErbB2 p38sh cells were transiently transfected with GATA3 siRNA for 48 hours. MCF7-p38D179 or MCF7-ErbB2 cells were transfected with GATA3 plasmid for 48 hours. A: The relative levels of miR-200b were determined by real-time PCR. Each data point was the mean ± SEM of three independent experiments. p< 0.05, * denotes significant difference from match controls. # denotes significant difference from MCF7 Con Si. δ is significant difference from MCF7-ErbB2 Con P. B and C: The expression of E-cadherin, GATA3, p38γ MAPK, Suz12, and GAPDH in MCF7, MCF7-p38D179, MCF7-ErbB2, and MCF7-ErbB2 p38sh cells transfected with either GATA3 siRNA or GATA3 plasmid was examined by immunoblotting analysis. D and E: The expression of E-cadherin was examined by immunofluorescent staining. Arrows indicated E-cadherin on the cell borders. Arrows indicate the E-cadherin on the cell borders. Bar = 20 μM. The experiments were replicated three times.
The interaction between miR-200b and Suz12
Suz12 is a target gene for miR-200b [31]. Breast cancer cells expressing high levels of p38γ MAPK had more Suz12 expression. For example, MCF7-p38D179 and MCF7-ErbB2 had elevated Suz12 levels, compared to MCF7 or MCF7-ErbB2 p38sh cells (Figs. 5G). miR-200b mimics decreased Suz12 in MCF7-ErbB2 and MCF7p38D179 cells; whereas miR-200b inhibitors increased Suz12 expression in MCF7 and MCF7-ErbB2 p38sh cells (Fig. 5E and 5G). Furthermore, silencing GATA3 suppressed miR-200b and E-cadherin, but increased Suz12 in MCF7 and MCF7-ErbB2 p38sh cells (Fig 6B and 6C). To the contrary, overexpression of GATA3 decreased Suz12 levels in MCF7-ErbB2 and MCF7-p38D179 cells (Figs 6B and 6C). Therefore, these results confirmed the sequence of the signaling pathway for p38γ MAPK’s action: p38γ MAPK/GATA3/miR-200b/Suz12.
p38γ MAPK regulates GATA3 through the posttranslational process
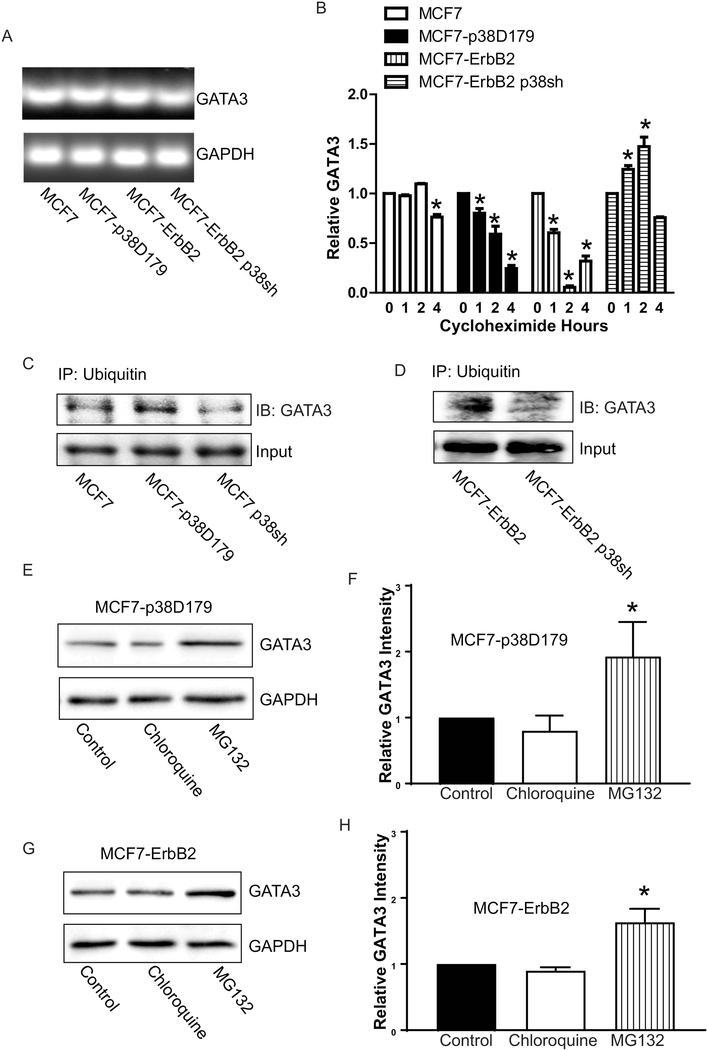
Since p38γ MAPK/GATA3 interaction is an important initial step for p38γ MAPK/GATA3/miR-200b/Suz12 pathway, we sought to determine how p38γ MAPK regulated GATA3. First, we examined the effect of p38γ MAPK on GATA3 mRNA levels. As shown in Fig. 7A, the manipulation of p38γ MAPK did not affect the expression of GATA3 mRNA. We then investigated the effect of p38γ MAPK on the stability of the GATA3 protein. As shown in Fig. 7B, high expression of p38γ MAPK increased the rate of GATA3 degradation, whereas knocking down stabilized GATA3. We next determined whether ubiquitination-dependent degradation was involved in p38γ MAPK regulation of GATA3 protein stability. Overexpression of active p38γ MAPK increased the levels of ubiquitinated GATA3, while silencing p38γ MAPK decreased GATA3 ubiquitination (Fig. 7C and 7D). To further examine p38γ MAPK-mediated GATA3 degradation, we treated the cells with either a proteasome inhibitor (MG132) or lysosome inhibitor (chloroquine). MG132 increased the levels of GATA3 in MCF7-p38D179 and MCF7-ErbB2 cells, suggesting that it blocked p38γ MAPK-mediated degradation of GATA3 (Fig. 7E-H). Chloroquine had no effect, suggesting that p38γ MAPK caused GATA3 degradation through ubiquitin-proteasome pathway rather than autophagy-lysosome pathway.
Figure 7.
Effect of p38γ MAPK GATA3 expression. A: mRNA levels of GATA3 in MCF7, MCF7-p38D179, MCF7-ErbB2 and MCF7-ErbB2 p38sh cells were determined by PCR. B: Changes in the protein levels of GATA3 over a course of time after cycloheximide (50 μg/ml) treatments were determined by immunoblotting. *denotes significant difference from 0 hours. C and D: The cell lysates from MCF7, MCF7-p38D179, MCF7 p38sh, MCF7-ErbB2, and MCF7-ErbB2 p38sh were immunoprecipitated with an antiubiquitin antibody, and then analyzed for GATA3 expression by immunoblotting. E and G: MCF7-p38D179 and MCF7-ErbB2 cells treated with either lysosome inhibitor (chloroquine, 100 μM)) or proteasome inhibitor (MG132, 10 μM) for 6 hours. The expression of GATA3 was then analyzed by immunoblotting. F and H: The expression of GATA3 was quantified and normalized to the expression of GAPDH. The experiment was replicated at least three time. * denote significant difference from respective controls, p < 0.05.
Discussion
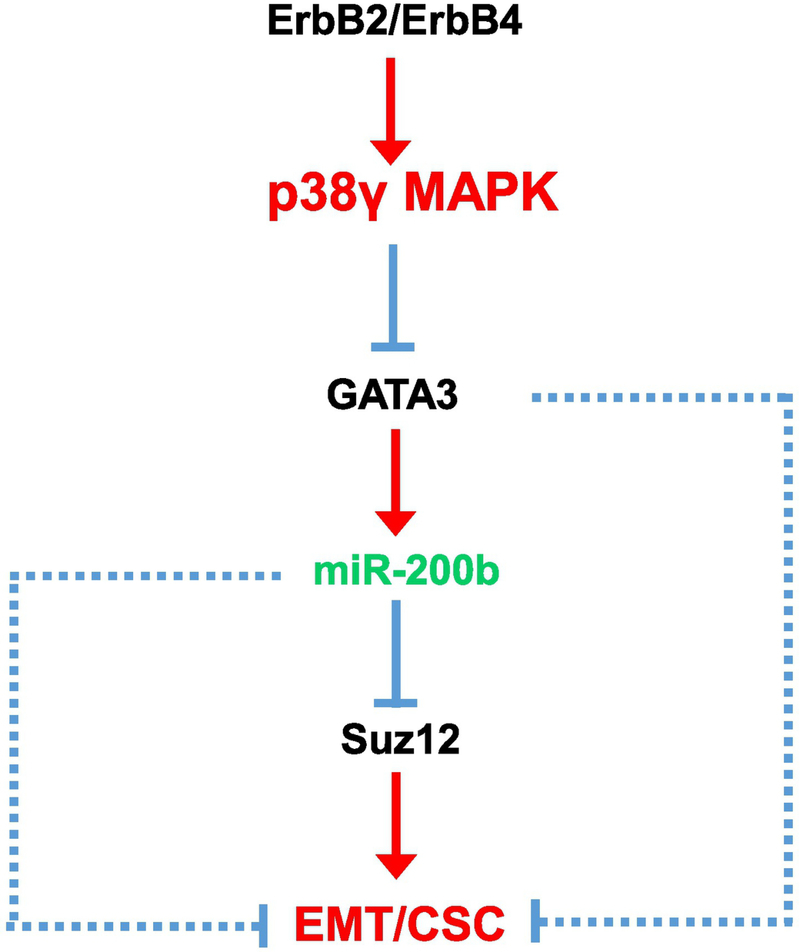
In this study, we showed that p38γ MAPK promoted EMT in breast cancer cells, that is over-expression or activation of p38γ MAPK enhanced EMT which was evident by decreased expression of E-cadherin and increased levels of vimentin. p38γ MAPK-enhanced EMT is accompanied by an increase in cancer stem cells (CSCs), which was consistent with the findings showing that induction of EMT is associated with the presence of cancer stem cells (CSCs) [13, 32]. We further delineated a down-stream signaling pathway that may mediate the action of p38γ MAPK (Fig. 8).
Figure 8.
Signaling pathway for p38γ MAPK-promoted EMT. p38γ MAPK is activated by its upstream receptor kinases, such as ErbB2 and ErbB4 [4, 5, 57]. The activation of p38γ MAPK destabilizes GATA3 by ubiquitin-proteasome-dependent degradation. Decreased levels of GATA3 causes the down-regulation of miR-200b, a negative regulator of Suz12. Suz12 is a promoter of EMT and CSC in breast cancer cells; therefore, down-regulation of miR-200b may promote EMT and CSC through the induction of Suz12. Alternatively, GATA3 may promote EMT and CSC through other miRNAs or unknown mechanisms (dotted lines)[48–50]. miR-200b could also promote EMT in breast cancer cells through mechanisms independent of Suz12 (dotted lines) [46].
miRNAs play essential roles in many biological processes and diseases, including cancers [33–37]. Dysregulation of miRNA expression has been associated with tumor development and metastasis [38, 39]. miRNAs are implicated in EMT in cancers including breast cancer [23, 40–42]. More specifically, the miRNA 200 family was shown to regulate EMT and progression of breast cancer [21–27]. We demonstrated that the activation of p38γ MAPK decreased the expression miR-200b which was a tumor suppressor for breast cancer cells (Fig. 5). However, modulation of miR-200b did not affect p38γ MAPK expression, indicating that miR200b was down-stream of p38γ MAPK. It appeared miR-200b mediated p38γ MAPK regulation of EMT. For example, miR-200b mimics inhibited p38γ MAPK-enhanced EMT while miR-200b inhibitor antagonized p38γ MAPK shRNA-inhibited EMT. Our finding was consistent with studies showing that miR200b was a suppressor of EMT in breast cancer cells [43–46]. Therefore, p38γ MAPK may promote EMT through inhibiting miR-200b.
GATA3, a transcription factor, plays an important role in mammary gland development and in breast cancer progression [47]. GATA3 expression is inversely associated with metastasis and poor prognosis, that is, low GATA3 levels correlate to aggressive phenotypes [48]. GATA3 was reported to regulate EMT and inhibit breast cancer development and metastasis [49]. GATA3 regulates the expression of various miRNAs including miR-200 family in cancer cells [48, 50, 51]. We showed that the activation of p38γ MAPK inhibited the expression of GATA3; whereas p38γ MAPK shRNA increased the expression of GATA3 (Fig. 5). Silencing of GATA3 suppressed miR-200b and E-cadherin in MCF7 and MCF7-ErbB2 p38sh cells (Figs. 6A-C), whereas overexpression of GATA3 reversed p38γ MAPK-induced down-regulation of miR-200b and E-cadherin (Figs. 6A-C). However, neither miR-200b mimics nor inhibitors affected GATA3 levels (Fig. 5). Together, these results indicate that GATA3 is upstream of miR200b but downstream of p38γ MAPK (Fig. 8). It is also possible that GATA3 may affect EMT by a mechanism other than targeting miR-200b. GATA3 was shown to inhibit breast cancer metastasis by attenuating EMT [49], and regulate prognosis and progression of breast cancer and other cancers by targeting other miRNAs [48, 50].
We further showed that p38γ MAPK regulation of GATA3 was likely mediated by a post-transcriptional mechanism through ubiquitin-proteasome pathway; that is, p38γ MAPK destabilized GATA3 by inducing its ubiquitination and proteasome-dependent degradation. Indeed, ubiquitin-proteasome pathway has been shown to play an important role in the regulation of GATA3 levels [52, 53]. More specifically, ubiquitin-proteasome pathway is critical in the maintenance of GATA3 in breast cancer cells [52].In future studies we may investigate the effect of p38γ MAPK on the interaction between GATA3 and Mdm2/FBXW7α (two potential ubiquitin ligases for GATA3 ubiquitination) to provide further insight into how p38γ MAPK promotes ubiquitination of GATA3.
Suz12 is a subunit of a polycomb repressor complex which has recently been implicated in tumorigenesis and cancer progression [54–56]. In particular, Suz12 is a positive regulator of EMT and CSC in breast cancer cells [31, 54]. Suz12 is a target gene for miR-200 and miR-200b inhibits Suz12 expression in various cancers including breast cancer stem cells [21, 31, 55, 56]. It has been proposed that miR-200b regulation of CSC and metastasis is mediated by Suz12 in breast cancer cells [21, 31]. We confirmed that Suz12 was down-stream of miR-200b and its expression was inhibited by miR-200b in breast cancer cells. Therefore, it is likely that miR-200b’s effect on EMT/CSC is mediated by the action of Suz12. Considering that miR-200b has various down-stream targets, however, it is also possible that miR-200b may promote EMT in breast cancer cells through mechanisms other than Suz12 [46].
Conclusions
In conclusion, our study established an important role of p38γ MAPK in EMT/CSCs. Overexpression of p38γ MAPK promoted EMT and CSCs. The increase in CSCs may results from the enhanced EMT. Mechanistically, we delineated a novel signaling pathway, namely, p38γ MAPK/GATA3/miR-200b/Suz12 that may mediate the EMT and aggressiveness of breast cancer. p38γ MAPK may regulate GATA3 through the posttranslational process.
Supplementary Material
Highlights.
p38γ MAPK increases EMT and cancer stem like cells in breast cancer cells.
The activation of p38γ MAPK causes ubiquitin-proteasome-dependent degradation of GATA3.
miR-200b is downstream of p38γ MAPK and GATA3, and regulates EMT.
Acknowledgements
We thank Dr. Oded Livnah (The Hebrew University of Jerusalem, Jerusalem, Israel) for providing plasmids for p38γ MAPK. This research is supported by grants from the National Institutes of Health (NIH) (AA017226 and AA015407). It is also supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development [Biomedical Laboratory Research and Development: Merit Review (BX001721)].
Abbreviation:
- EMT
Epithelial-mesenchymal transition
- CSC
cancer stem cell
- p38 MAPK
p38 Mitogen Activated Protein Kinases
- ALDH
aldehyde dehydrogenase
- miRNA
micro RNA
- shRNA
Short hairpin RNA
- RT-PCR
real-time polymerase chain reaction
Footnotes
Conflict of interest
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Escós A, Risco A, Alsina-Beauchamp D, Cuenda A, p38γ and p38δ mitogen activated protein kinases (MAPKs), new stars in the MAPK galaxy, Frontiers in cell and developmental biology, 4 (2016) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Meng F, Wu G, Is p38γ MAPK a metastasis-promoting gene or an oncogenic property-maintaining gene?, Cell Cycle, 12 (2013) 2329–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Qi X, Yin N, Ma S, Lepp A, Tang J, Jing W, Johnson B, Dwinell MB, Chitambar CR, Chen G, p38γ MAPK Is a Therapeutic Target for Triple‐Negative Breast Cancer by Stimulation of Cancer Stem‐Like Cell Expansion, Stem cells, 33 (2015) 2738–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xu M, Ren Z, Wang X, Comer A, Frank JA, Ke Z.-j., Huang Y, Zhang Z, Shi X, Wang S, ErbB2 and p38γ MAPK mediate alcohol-induced increase in breast cancer stem cells and metastasis, Molecular cancer, 15 (2016) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xu M, Wang S, Ren Z, Frank JA, Yang XH, Zhang Z, Ke ZJ, Shi X, Luo J, Chronic ethanol exposure enhances the aggressiveness of breast cancer: the role of p38gamma, Oncotarget, 7 (2016) 3489–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yin N, Qi X, Tsai S, Lu Y, Basir Z, Oshima K, Thomas J, Myers C, Stoner G, Chen G, p38γ MAPK is required for inflammation-associated colon tumorigenesis, Oncogene, 35 (2016) 1039. [DOI] [PubMed] [Google Scholar]
- [7].Meng F, Zhang H, Liu G, Kreike B, Chen W, Sethi S, Miller FR, Wu G, p38+¦ mitogen-activated protein kinase contributes to oncogenic properties maintenance and resistance to poly (ADP-ribose)-polymerase-1 inhibition in breast cancer, Neoplasia, 13 (2011) 472–IN425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yang K, Liu Y, Liu Z, Liu J, Liu X, Chen X, Li C, Zeng Y, p38γ overexpression in gliomas and its role in proliferation and apoptosis, Scientific reports, 3 (2013) 2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cerezo-Guisado MI, Reino P.d., Remy G, Kuma Y, Arthur JSC, GallegoOrtega D, Cuenda A, Evidence of p38γ and p38δ involvement in cell transformation processes, Carcinogenesis, 32 (2011) 1093–1099. [DOI] [PubMed] [Google Scholar]
- [10].Rosenthal DT, Iyer H, Escudero S, Bao L, Wu Z, Ventura AC, Kleer CG, Arruda EM, Garikipati K, Merajver SD, p38γ promotes breast cancer cell motility and metastasis through regulation of RhoC GTPase, cytoskeletal architecture, and a novel leading edge behavior, Cancer research, 71 (2011) 6338–6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu Y, Sarkissyan M, Vadgama JV, Epithelial-mesenchymal transition and breast cancer, Journal of clinical medicine, 5 (2016) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS, Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature, Cancer Res, 69 (2009) 1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kotiyal S, Bhattacharya S, Epithelial mesenchymal transition and vascular mimicry in breast cancer stem cells, Critical Reviews™ in Eukaryotic Gene Expression, 25 (2015). [DOI] [PubMed] [Google Scholar]
- [14].Avitzour M, Diskin R, Raboy B, Askari N, Engelberg D, Livnah O, Intrinsically active variants of all human p38 isoforms, The FEBS journal, 274 (2007) 963–975. [DOI] [PubMed] [Google Scholar]
- [15].Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G, ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome, Cell Stem Cell, 1 (2007) 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dontu G, Wicha MS, Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia, J. Mammary. Gland. Biol. Neoplasia, 10 (2005) 75–86. [DOI] [PubMed] [Google Scholar]
- [17].Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS, In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells, Genes Dev, 17 (2003) 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu M, Bower KA, Chen G, Shi X, Dong Z, Ke Z, Luo J, Ethanol enhances the interaction of breast cancer cells over-expressing ErbB2 with fibronectin, Alcohol Clin. Exp. Res, 34 (2010) 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA, Minnear FL, Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase, Am J Physiol Cell Physiol, 293 (2007) C1309–1318. [DOI] [PubMed] [Google Scholar]
- [20].Xu M, Bower KA, Wang S, Frank JA, Chen G, Ding M, Wang S, Shi X, Ke Z, Luo J, Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2, Mol. Cancer, 9 (2010) 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang H-F, Xu L-Y, Li E-M, A family of pleiotropically acting microRNAs in cancer progression, miR-200: potential cancer therapeutic targets, Current pharmaceutical design, 20 (2014) 1896–1903. [DOI] [PubMed] [Google Scholar]
- [22].Tryndyak VP, Beland FA, Pogribny IP, E‐cadherin transcriptional downregulation by epigenetic and microRNA ‐ 200 family alterations is related to mesenchymal and drug ‐ resistant phenotypes in human breast cancer cells, International journal of cancer, 126 (2010) 2575–2583. [DOI] [PubMed] [Google Scholar]
- [23].Guttilla IK, Adams BD, White BA, ERα, microRNAs, and the epithelial– mesenchymal transition in breast cancer, Trends in Endocrinology & Metabolism, 23 (2012) 73–82. [DOI] [PubMed] [Google Scholar]
- [24].Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ, The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1, Nature cell biology, 10 (2008) 593–601. [DOI] [PubMed] [Google Scholar]
- [25].Gregory PA, Bracken CP, Bert AG, Goodall GJ, MicroRNAs as regulators of epithelial-mesenchymal transition, Cell cycle, 7 (2008) 3112–3117. [DOI] [PubMed] [Google Scholar]
- [26].Davalos V, Moutinho C, Villanueva A, Boque R, Silva P, Carneiro F, Esteller M, Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis, Oncogene, 31 (2012) 2062–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ, A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition, Cancer research, 68 (2008) 78467854. [DOI] [PubMed] [Google Scholar]
- [28].Yu F, Jiao Y, Zhu Y, Wang Y, Zhu J, Cui X, Liu Y, He Y, Park E-Y, Zhang H, MicroRNA 34c gene down-regulation via DNA methylation promotes self-renewal and epithelial-mesenchymal transition in breast tumor-initiating cells, Journal of biological chemistry, 287 (2012) 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim NH, Kim HS, Li X-Y, Lee I, Choi H-S, Kang SE, Cha SY, Ryu JK, Yoon D, Fearon ER, A p53/miRNA-34 axis regulates Snail1-dependent cancer cell epithelial–mesenchymal transition, J Cell Biol, 195 (2011) 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang Y, Ahn Y-H, Gibbons DL, Zang Y, Lin W, Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA, The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200–dependent pathway in mice, The Journal of clinical investigation, 121 (2011) 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K, Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells, Molecular cell, 39 (2010) 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Findlay VJ, Wang C, Watson DK, Camp ER, Epithelial-to-mesenchymal transition and the cancer stem cell phenotype: insights from cancer biology with therapeutic implications for colorectal cancer, Cancer gene therapy, 21 (2014) 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sayed D, Abdellatif M, MicroRNAs in development and disease, Physiological reviews, 91 (2011) 827–887. [DOI] [PubMed] [Google Scholar]
- [34].Leung AK, Sharp PA, microRNAs: a safeguard against turmoil?, Cell, 130 (2007) 581–585. [DOI] [PubMed] [Google Scholar]
- [35].Kloosterman WP, Plasterk RH, The diverse functions of microRNAs in animal development and disease, Developmental cell, 11 (2006) 441–450. [DOI] [PubMed] [Google Scholar]
- [36].Croce CM, Calin GA, miRNAs, cancer, and stem cell division, Cell, 122 (2005) 67. [DOI] [PubMed] [Google Scholar]
- [37].Mendell JT, miRiad roles for the miR-17–92 cluster in development and disease, Cell, 133 (2008) 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang H, Li Y, Lai M, The microRNA network and tumor metastasis, Oncogene, 29 (2010) 937–948. [DOI] [PubMed] [Google Scholar]
- [39].Shenouda SK, Alahari SK, MicroRNA function in cancer: oncogene or a tumor suppressor?, Cancer and Metastasis Reviews, 28 (2009) 369. [DOI] [PubMed] [Google Scholar]
- [40].Riaz M, van Jaarsveld MT, Hollestelle A, Prager-van der Smissen WJ, Heine AA, Boersma AW, Liu J, Helmijr J, Ozturk B, Smid M, miRNA expression profiling of 51 human breast cancer cell lines reveals subtype and driver mutation-specific miRNAs, Breast Cancer Research, 15 (2013) R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hollestelle A, Elstrodt F, Timmermans M, Sieuwerts AM, Klijn JG, Foekens JA, den Bakker MA, Schutte M, Four human breast cancer cell lines with biallelic inactivating α-catenin gene mutations, Breast cancer research and treatment, 122 (2010) 125–133. [DOI] [PubMed] [Google Scholar]
- [42].O’Day E, Lal A, MicroRNAs and their target gene networks in breast cancer, Breast cancer research, 12 (2010) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ahmad A, Aboukameel A, Kong D, Wang Z, Sethi S, Chen W, Sarkar FH, Raz A, Phosphoglucose isomerase/autocrine motility factor mediates epithelialmesenchymal transition regulated by miR-200 in breast cancer cells, Cancer research, 71 (2011) 3400–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lu Z, Jiao D, Qiao J, Yang S, Yan M, Cui S, Liu Z, Restin suppressed epithelial-mesenchymal transition and tumor metastasis in breast cancer cells through upregulating mir-200a/b expression via association with p73, Molecular cancer, 14 (2015) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rhodes LV, Martin EC, Segar HC, Miller DF, Buechlein A, Rusch DB, Nephew KP, Burow ME, Collins-Burow BM, Dual regulation by microRNA-200b-3p and microRNA-200b-5p in the inhibition of epithelial-to-mesenchymal transition in triplenegative breast cancer, Oncotarget, 6 (2015) 16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang X, Hu Q, Hu L-X, Lin X-R, Liu J-Q, Lin X, Dinglin X-X, Zeng J-Y, Hu H, Luo M-L, miR-200b Regulates Epithelial-Mesenchymal Transition of Chemoresistant Breast Cancer Cells by Targeting FN1, Discovery medicine, 24 (2017) 75–85. [PubMed] [Google Scholar]
- [47].Chou J, Provot S, Werb Z, GATA3 in development and cancer differentiation: cells GATA have it!, Journal of cellular physiology, 222 (2010) 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chou J, Lin JH, Brenot A, J.-w. Kim, S. Provot, Z. Werb, GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression, Nature cell biology, 15 (2013) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yan W, Cao QJ, Arenas RB, Bentley B, Shao R, GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition, Journal of Biological Chemistry, 285 (2010) 14042–14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Jiang X, Chen Y, Du E, Yang K, Zhang Z, Qi S, Xu Y, GATA3-driven expression of miR-503 inhibits prostate cancer progression by repressing ZNF217 expression, Cellular signalling, 28 (2016) 1216–1224. [DOI] [PubMed] [Google Scholar]
- [51].Chen C, Xiang H, Peng Y.-l, Peng J, Jiang S.-w., Mature miR-183, negatively regulated by transcription factor GATA3, promotes 3T3-L1 adipogenesis through inhibition of the canonical Wnt/β-catenin signaling pathway by targeting LRP6, Cellular signalling, 26 (2014) 1155–1165. [DOI] [PubMed] [Google Scholar]
- [52].Song N, Cao C, Tang Y, Bi L, Jiang Y, Zhou Y, Song X, Liu L, Ge W, The Ubiquitin Ligase SCFFBXW7α Promotes ATA3 Degradation, Journal of cellular physiology, (2018). [DOI] [PubMed] [Google Scholar]
- [53].Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T, Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway, Journal of Biological Chemistry, 280 (2005) 29409–29419. [DOI] [PubMed] [Google Scholar]
- [54].Hu J, Su P, Jiao M, Bai X, Qi M, Liu H, Wu Z, Sun J, Zhou G, Han B, TRPS1 Suppresses Breast Cancer Epithelial-mesenchymal Transition Program as a Negative Regulator of SUZ12, Translational oncology, 11 (2018) 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Peng F, Jiang J, Yu Y, Tian R, Guo X, Li X, Shen M, Xu M, Zhu F, Shi C, Direct targeting of SUZ12/ROCK2 by miR-200b/c inhibits cholangiocarcinoma tumourigenesis and metastasis, British journal of cancer, 109 (2013) 3092–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].San K, Horita M, Ganapathy A, Chinnadurai G, Ezekiel UR, Deregulated expression of microRNA-200b/c and SUZ12, a Polycomb repressive complex 2 subunit, in chemoresistant colorectal cancer cells, Genes & cancer, 8 (2017) 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ramachandra CJ, Mehta A, Wong P, Shim W, ErbB4 activated p38γ MAPK isoform mediates early cardiogenesis through NKx2. 5 in human pluripotent stem cells, Stem Cells, 34 (2016) 288–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.