Abstract
Background
Brain tumours are uncommon, and have extremely poor outcomes. Patients and GPs may find it difficult to recognise early symptoms because they are often non-specific and more likely due to other conditions.
Aim
To explore patients’ experiences of symptom appraisal, help seeking, and routes to diagnosis.
Design and setting
Qualitative study set in the East and North West of England.
Method
In-depth interviews with adult patients recently diagnosed with a primary brain tumour and their family members were analysed thematically, using the Model of Pathways to Treatment as a conceptual framework.
Results
Interviews were carried out with 39 patients. Few participants (n = 7; 18%) presented as an emergency without having had a previous GP consultation; most had had one (n = 15; 38%), two (n = 9; 23%), or more (n = 8; 21%) GP consultations. Participants experienced multiple subtle ‘changes’ rather than ‘symptoms’, often noticed by others rather than the patient, which frequently led to loss of interest or less ability to engage with daily living activities. The most common changes were in cognition (speaking, writing, comprehension, memory, concentration, and multitasking), sleep, and other ‘head feelings’ such as dizziness. Not all patients experienced a seizure, and few seizures were experienced ‘out of the blue’. Quality of communication in GP consultations played a key role in patients’ subsequent symptom appraisal and the timing of their decision to re-consult.
Conclusion
Multiple subtle changes and frequent GP visits often precede brain tumour diagnosis, giving possible diagnostic opportunities for GPs. Refined community symptom awareness and GP guidance could enable more direct pathways to diagnosis, and potentially improve patient experiences and outcomes.
Keywords: central nervous system neoplasms, diagnosis, primary brain neoplasms, primary care, symptoms
INTRODUCTION
Primary malignant brain tumours are rarely diagnosed in primary care populations as the incidence is low: the age-adjusted incidence for the commonest type, glioma, is between 4.7 and 5.7 per 100 000 people.1,2 Outcomes remain poor despite improvements in treatment, so that, although brain tumours represent <2% of all cancers, they result in the most life-years lost of any cancer.3,4 Most patients with primary brain tumours have seen their GP before diagnosis, often several times,5 and >50% then present to, or are diagnosed by, accident and emergency services rather than by GPs or in specialist settings.6,7 Indeed, in the UK only 1% of patients are currently diagnosed via the ‘suspected cancer’ 2-week wait process, and via GP routine referrals,8 despite the publication of standardised National Institute for Health and Care Excellence guidelines in 2006,9 which were updated in 2018,10 and some intervening liberalisation of access to diagnostic imaging.11
More timely diagnosis could improve patient outcomes, yet patients and GPs may find it difficult to recognise the early symptoms of brain tumours. In primary care, these patients can present with symptoms that are far commoner manifestations of benign conditions, making the diagnostic process very challenging.12 Over the last decade, a number of studies have used routinely collected English primary care data to quantify the frequency of the commoner presenting symptoms and their predictive values.13–15 However, a systematic review found that, apart from new-onset epilepsy and headache, these symptoms have low positive predictive values for brain tumours: even headache has a positive predictive value of <1%.16 A recent analysis of 226 brain tumour cases from the national audit of cancer diagnosis in primary care showed that the commonest presentations were focal neurology (33%), ‘fits, faints, or falls’ (21%), and headache (21%).17
Little is known about how patients appraise possible symptoms of brain tumours and make decisions about when, why, or how they should seek help. However, qualitative research set among patients who were referred with possible symptoms of, or recently diagnosed with, other cancers has illuminated aspects of symptom appraisal processes.18–21 These include patients ‘normalising’ their symptoms if they consider them part of an expected ageing process, or if symptoms are vague, intermittent, or non-threatening. Therefore, this study aimed to use similar qualitative research methods among patients recently diagnosed with a brain tumour to develop a richer understanding of their experiences of symptoms preceding diagnosis, appraisal of these symptoms, help seeking, and routes to diagnosis to inform awareness and potentially drive service change.
How this fits in
National data suggest that people diagnosed with a brain tumour often see their GP several times before they are investigated or referred, frequently present as an emergency, and have poor outcomes. The findings from this study, in which people were interviewed soon after their brain tumour diagnosis, suggests that, although some patients present with headaches or major seizures, most experience subtle, intermittent, and multiple changes in their cognitive functioning, sleep, and other ‘head feelings’ for many months, suggesting possible missed diagnostic opportunities. As these interviews were undertaken with patients and their family members soon after diagnosis, potential recall and social desirability biases affecting their reported experiences should be minimised. Being aware of these subtle and intermittent changes or symptoms, and effective patient–GP communication with follow-up as safety netting, could alert GPs to more rapid investigation and referral, and possibly reduce development of the significant and major symptoms associated with brain tumours.
METHOD
Design
In-depth interviews were conducted face to face with adults who had been very recently diagnosed (within 4 weeks) with a primary brain tumour in the East and North West of England. The study was undertaken and reported in line with the standards for reporting qualitative research.22
Setting and recruitment
Potential participants were identified and recruited by neuro-oncology nurse specialists via weekly neurosurgery clinics at two regional hospitals: Cambridge University Hospitals NHS Foundation Trust and the Walton Centre NHS Foundation Trust in Liverpool. These hospitals together serve a population of approximately 6 million, and their multidisciplinary team meetings review more than 500 new cases of primary malignant brain tumours each year.
All adults aged ≥18 years who were newly diagnosed with a primary brain tumour at the two participating hospitals were eligible for inclusion unless the neuro-oncology nurse specialists felt that they were not suitable on clinical grounds (due to severe physical or mental health conditions). Patients were given or mailed an invitation letter with a patient information sheet. Purposive sampling strategies were used to recruit a range of participants by age, sex, and location to ensure a broad range of views and experiences, and were continued until data saturation was reached. Sampling decisions and illness were the main reasons for not selecting patients for interview.
Data collection
Semi-structured interviews were carried out with patients and their family members (usually a spouse or child) in their homes between September 2016 and June 2017, following their diagnosis and before undergoing brain surgery. All present gave their written informed consent to participate. An experienced researcher used an interview topic guide based on the aims of the research, the available research literature,13,16 and the authors’ collective expertise from interviewing patients recently diagnosed with other cancers.18–21 The guide was piloted, used, and revised during the study as new issues arose. It focused on when and how initial symptoms were noticed; the language used to describe symptoms and changes over time; the participant’s decision making and triggers to help seeking; and experiences of the diagnostic process from the patient’s perspective. Interviews lasted between 45 and 90 minutes, and were audiorecorded, professionally transcribed verbatim, checked, and anonymised. Reflexive field notes were made following the interviews.
Analysis
Transcripts were imported to NVivo (version 11) to support coding and data organisation. Inductive thematic analysis commenced soon after the beginning of data collection.23 Each participant-reported patient interval (time from first noticing a change to first presentation, including the appraisal and help-seeking intervals) and diagnostic interval (time from first presentation to diagnosis) was defined.24 The Model of Pathways to Treatment25,26 was then used as a conceptual model to underpin the remaining analysis. A coding frame based on this model was developed with the first few transcripts, applied to the subsequent transcripts, and refined iteratively, with a constant comparative method applied.27 When the first consultation did not result in referral, further iterative processes were also included in the analyses. Members of the core research team, from a range of clinical and non-clinical backgrounds, read all the transcripts, and met regularly to resolve coding issues, and further refine the coding framework. Codes were compared within and across interviews, and then grouped into key themes and sub-themes. These were agreed through a series of meetings with the core researchers, the two consumer members of the research team, and consensus meetings with the wider study team, which included neuro-oncology experts.
The analysis focused on two main areas. The symptoms that patients experienced before a brain tumour diagnosis and how these were reported and responded to in primary care are presented in this article. The psychological approaches underpinning the appraisal and help-seeking processes, often over several consultations, are reported elsewhere.28
Workshop: triangulation of early findings
Early findings of this study were shared at a workshop supported by the Brain Tumour Charity, which included GPs from London (n = 10), and patients (n = 7) and carers/family members (n = 9) affected by brain tumours drawn from across England. Four mixed and facilitated focus groups were undertaken for credibility checking. These lasted up to 1 hour each, were audiorecorded, transcribed verbatim, checked, anonymised, and analysed to search for concordant, discordant, or new data. With reference to this analysis, the findings were entirely supportive, and no new data were found.
RESULTS
A total of 39 patients were interviewed; their mean age was 53 years and 18 (46%) were female. The commonest diagnoses were glioblastomas, located in the frontal region (Table 1).
Table 1.
Characteristics of study participants diagnosed with brain tumours
| Variable |
Participants (n = 39) n (%) |
|---|---|
| Age at interview (mean age = 53 years, range = 22–74) | |
| 21–40 | 10 (26) |
| 41–60 | 15 (38) |
| ≥61 | 14 (36) |
|
| |
| Sex | |
| Male | 21 (54) |
| Female | 18 (46) |
|
| |
| Region of England | |
| Eastern | 30 (77) |
| North Western | 9 (23) |
|
| |
| Patient interval (first symptom to first presentation)a | |
| <7 days | 5 (13) |
| 1–4 weeks | 3 (8) |
| 1–6 months | 10 (26) |
| 7–12 months | 11 (28) |
| >12 months | 10 (26) |
|
| |
| Diagnostic interval (first presentation to diagnosis)a | |
| <7 days | 1 (3) |
| 1–4 weeks | 16 (41) |
| 1–6 months | 15 (38) |
| 7–12 months | 5 (13) |
| >12 months | 2 (5) |
|
| |
| Reported consultations with GPs in primary care | |
| 0 | 7 (18) |
| 1 | 15 (38) |
| 2 | 9 (23) |
| ≥3 | 8 (21) |
|
| |
| Reported consultations with emergency care | |
| Emergency only (no contact with GP) | 7 (18) |
| Emergency care with contact with GP | |
| • GP contact before emergency care | 14 (36) |
| • GP contact after emergency care | 1 (3) |
| • GP contact before and after emergency care | 5 (13) |
| No emergency care | 12 (31) |
|
| |
| Tumour type | |
| Diffuse astrocytoma | 5 (13) |
| Anaplastic astrocytoma | 4 (10) |
| Oligodendroglioma | 2 (5) |
| Anaplastic oligodendroglioma | 2 (5) |
| Glioblastoma | 22 (56) |
| Other astrocytic tumours | 2 (5) |
| Unknown | 2 (5) |
|
| |
| Tumour location | |
| Frontal (including frontoparietal × 2) | 20 (51) |
| Temporal | 10 (26) |
| Parietal (including parieno-occipital × 2) | 4 (10) |
| Occipital | 2 (5) |
| Other (thalamus × 1, tempero-insula × 1, N/A × 1) | 3 (8) |
|
| |
| WHO gradeb | |
| Low grade: II | 8 (21) |
| High grade: III/IV | 7/22 (18/56) |
| Ungraded | 2 (5) |
Participant account, not confirmed from clinical records.
Brain tumours are graded by the World Health Organization (WHO) from 1 −4, according to how they behave. Tumours graded 1 and 2 are slow growing, whereas tumours graded 3 and 4 are fast-growing, more aggressive tumours. N/A = not applicable.
Although headache and seizure (without preceding symptoms) are generally considered to be the most common presenting symptoms of brain tumours in primary and emergency care, headaches and seizures were only reported by half the participants (n = 21; 54%). More patients reported changes in cognition (n = 26; 67%) and sleep (n = 22; 56%). Furthermore, almost all participants (n = 38; 97%) had noticed multiple changes or symptoms before either routine or emergency presentations.
Four main themes were identified in participants’ narratives: people experience ‘changes’ rather than ‘symptoms’, which are often first noticed by others; multiple subtle changes precede brain tumour diagnosis; not all seizures are the same and few come ‘out of the blue’ (that is, without any prodrome); and quality of patient–GP communication. These themes are presented in detail below, with quotations contextualised with the participant’s ID number, sex, and age group.
People experience changes rather than symptoms, often first noticed by others
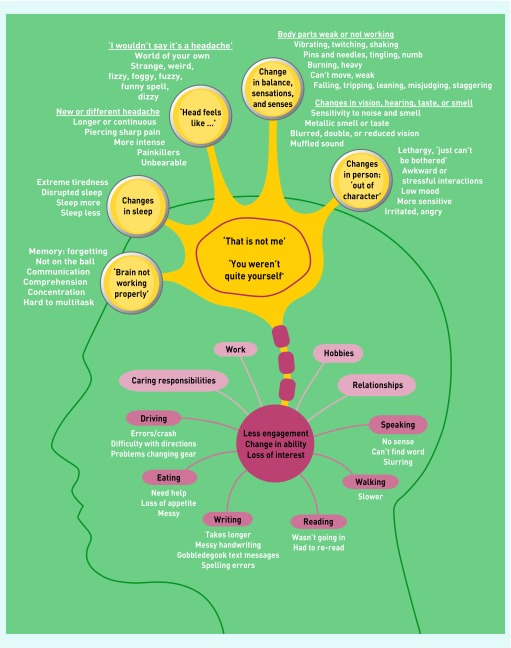
Participants frequently described ‘changes’ or ‘something not quite right’ rather than ‘symptoms’. Some changes related to their body, and others to the way they approached daily living activities, work, hobbies, or relationships (Figure 1).
Figure 1.
Changes or symptoms experienced by participants before their brain tumour diagnosis.
Some changes were very subtle and difficult to notice; sometimes the participant was unaware of a change until someone else pointed it out:
‘I felt OK, I didn’t feel as though there was anything wrong with me, but looking back now I can see what [my wife] was worried about.’
(Participant [P] 30, male [M], 71–80 years)
Sometimes friends or family had noticed a change, but did not say anything until after the participant had been diagnosed:
‘People … wouldn’t say anything at the time but now they say, “We noticed you haven’t been quite yourself for 6 months”, but they are little things that people wouldn’t say, “Go to the doctors and get sorted.”’
(P10, female [F], 61–70 years)
Multiple subtle changes precede brain cancer diagnosis
Figure 1 demonstrates the diverse range of changes experienced that made the participant think ‘that is not me’ or a person close to them report ‘you weren’t quite yourself’.
Experiencing these changes or symptoms often led to less engagement, less interest in or a change in ability to carry out work, hobbies, caring responsibilities, and daily living activities. ‘Seizure’ does not have a separate locus in Figure 1 as any of these changes could precede or be a part of a seizure. These changes are described in detail below. To summarise, changes were noted in cognitive function (‘brain not working properly’, changes in speaking and writing, changes in comprehension, changes in memory, and changes in concentration and ability to multitask); in sleep; in how the patient’s head feels (‘I wouldn’t say it’s a headache …’ and a new or different headache); in balance, sensations, and senses (body parts weak or not working, changes in vision, changes in hearing, and changes in taste or smell); and, in the person, being ‘out of character’.
Changes in cognitive function: ‘brain not working properly’
People described not being able to do things as they used to, or being less accurate or not ‘on the ball’:
‘I could feel myself getting a bit slower in work. I was struggling to do and think of all the things that I’d normally do … I just knew I wasn’t as quick and I couldn’t think ahead as much and it just wasn’t the same.’
(P37, F, 31–40 years)
Some people noticed very subtle changes, whereas others described having to put extensive thought into everyday tasks:
‘If I’m trying to do something, sometimes you have to stand there for about 5 to 10 minutes and figure out how I was going to do it.’
(P33, M, 51–60 years)
Cognitive changes often made people feel frustrated, irritated, confused, or anxious. Participants and their family members described four main types of changes in cognitive function as outlined below.
Changes in cognitive function: changes in speaking and writing
Many participants and their family members noticed that speech changed, often intermittently. Some experienced difficulty finding or saying words, whereas others noticed odd sentences with random words:
‘I’m trying to say something … I can think it, but it doesn’t really come out. I can’t find the right words, really.’
(P39, F, 21–30 years)
Many also mentioned problems with spelling, with text messages or emails becoming full of errors or lacking sense. Participants found texting became more difficult and took longer, leading some to replace text with emoticons or stop sending texts altogether:
‘I’m normally a pretty good speller, but I couldn’t remember how to spell words and writing up notes at work was a bit of a concern ‘cos I was forgetting how to spell.’
(P03, F, 31–40 years)
‘Total gobbledegook, because I pushed the wrong key, predictive text. So then I took the predictive text off, but then it was even worse. It could take me 10 minutes to write an email.’
(P32, M, 71–80 years)
Changes in cognitive function: changes in comprehension
Most participants noticed a change in their understanding or processing of information. Some people struggled to understand what was being said or how long it would take to complete tasks:
‘I’ve got this catch-up, it takes me a while to realise what they’ve said and quite often I’ll ask them to repeat it or sometimes it just takes longer to have got through.’
(P24, F, 41–50 years)
The greatest impact was on reading. Some patients reported having to frequently re-read text or stopped reading altogether:
‘Although I was having trouble reading, I was also having trouble understanding what the words were saying … I couldn’t be bothered to read because even if I could read it, I didn’t really understand what it was telling me anyway … The plot was disappearing every other page.’
(P32, M, 71–80 years)
Changes in cognitive function: changes in memory
Memory was affected in a range of situations, including people forgetting where they had left objects, what they were doing or thinking about in the moment, completing tasks as intended, or names or places:
‘Probably [for] the last 6 months, they all used to laugh at me and just say “Who’s got more dementia, you or your mum?”’ (P05, F, 41–50 years)
Family (Fa): ‘He kept forgetting things. He kept forgetting people’s names. And places. He couldn’t remember.’
Patient (P): ‘People I’ve known for 40 years, I couldn’t remember names. Really couldn’t and that’s what really upset me.’ (P33, M, 51–60 years)
Changes in cognitive function: changes in concentration and ability to multitask
Some noticed that they were struggling to concentrate or focus:
‘Whereas before that he could get to work with his eyes closed basically, he was actually questioning himself, “What have I got to do next? What am I here for?”, that sort of thing. He … kept saying, “I can’t concentrate.”’
(P19, M, 61–70 years)
Others found that they could only concentrate on one thing at a time, and therefore developed difficulty with multitasking:
‘I was trying to plait her hair and test her on the spellings and I had a funny turn and it was just too much, I just couldn’t cope with looking, listening, and doing.’
(P37, F, 31–40 years)
Changes in sleep
For some, sleep became disrupted and they often struggled to go to sleep, frequently waking up, not sleeping well, or waking earlier than usual. Others described sleeping more than normal, by going to bed earlier or waking up later. Some started having daytime naps because they felt an urgent need to close their eyes and rest, or because their night-time sleep was disrupted; these people described feelings of extreme tiredness:
‘I’ve literally pulled my car over, closed my eyes, and gone to sleep, which is unheard of [as] I’ve never found sleep that easy.’ (P03, F, 31–40 years)
Fa: ‘You always used to go to bed about half-past 10 didn’t you? But then that got to 8 o’clock didn’t it? … And then just before he was really poorly, come half-past 7 he would say, “I’m going up.”’ (P19, M, 61–70 years)
‘I mean, I was just, well, tired, exhausted … I was feeling absolutely totally shattered.’
(P15, M, 51–60 years)
‘Head feels like …’: ‘I wouldn’t say it’s a headache …’
Many participants felt strange sensations in their heads. Rather than calling them a headache or pain, they were described as feeling ‘muzzy’, ‘fuzzy’, ‘thick’, ‘fluttering’, or ‘coming in a wave’. Some spoke about temporary feelings of dizziness or light-headedness, described as ‘like being drunk’, and ‘giddy’. One patient had experienced such sensations every 2–3 weeks over the preceding year:
‘Not dizziness, not losing balance, not anything to do with your eyes, just literally the sensation of my head doing a little spin.’
(P12, F, 51–60 years)
‘I can only describe it like a sherbet bomb in my head … like that fizz bomb you have in the bath … but not painful, not uncomfortable, not anything … I would lay down and there would be a little bit of fizzing. No big deal, it wouldn’t last forever, but you know, just aware of what a strange sensation that is.’
(P27, F, 71–80 years)
‘I wouldn’t say it’s a headache, but you get like a little pain in the front of your head and it goes just like that. It’s almost like a wave.’
(P34, M, 41–50 years)
‘Head feels like …’: a new or different headache
Many participants spoke about having changing headaches such as new-onset headaches, a headache with increased intensity or duration, or one that felt different from ‘a normal headache’. Some described these headaches as constant or daily. Some spoke about experiencing painful headaches that were severe and unbearable: their pain was described as feeling like pressure, pulsations, piercing, drilling, and fireworks. Some noted they had started taking painkillers regularly:
Fa: ‘ ‘Before any of this started, [when] I’ve been getting up for work he’ll say, “God, I’ve got a terrific headache”, which … you never get headaches do you?’ (P30, M, F, 71–80 years)
‘Very piercing … they would shoot across the back of [my] head or across [my] forehead … I could stand there, I hadn’t got a headache, all of a sudden, it was like somebody had put a needle in [my] head.’
(P13, F, 31–40 years)
‘That was a pretty extreme headache because I don’t get headaches, but if I do, I really don’t normally need a paracetamol just to get through it.’
(P32, M, 71–80 years)
Changes in balance, sensations, and senses: body parts weak or not working
Participants spoke of weakness or numbness in parts of their body, or that body parts ‘just didn’t work’. Some experienced sensations such as pins and needles, tingling, twitching, or shaking in that part of the body, and, for some, this was later recognised as resulting from a seizure:
‘You know if you have a really dead arm, and then the feeling comes back, and it sort of prickles and it stings rather like stinging nettles, and feels very heavy.’
(P12, F, 51–60 years)
‘I always sat here with a roll-up … I did notice I was dropping it, it was annoying me that I was dropping it.’
(P27, F, 71–80 years)
‘I noticed that I was slurring my words and wasn’t able to say some syllables. When I was leaving a voicemail message on an answer machine, I had to repeat myself to get the thing out.’
(P21, M, 51–60 years)
When these sensations were noticed in upper limbs, some found that they dropped objects or felt clumsy or unable to hold everyday objects. Facial changes could result in dribbling, slurred speech, or difficulty talking. Weaker lower limbs were mainly manifested by slower walking or changes in a person’s ability to drive. Participants noticed changes in balance or becoming unstable. They spoke about leaning to one side, wobbling, staggering, tripping, stumbling, hovering, and sometimes falling:
Fa: ‘ ‘I noticed it because you were walking around as if you was about 90 years old, you sort of like hunched over and your legs were really slow and you were just wobbly the whole time.’
P: ‘Yes. I could hear my foot drag along the ground.’ (P16, M, 61–70 years)
‘I didn’t fall over, I was just a little bit unsteady. I can walk normally but not on a line, you know like when a policeman wants you to walk on a line for being tiddly? I couldn’t do that.’
(P11, M, 71–80 years)
Changes in balance, sensations, and senses: changes in vision
Some participants noticed that their eyesight or ability to focus deteriorated, experiencing blurred or double vision (sometimes intermittently), or were squinting or straining to see. For others, their field of vision was reduced, and a few participants spoke about experiencing a tic in their eye:
‘You know when you’re dreaming and it’s hazy or like you can’t see properly, it’s like that … like a blur … It’s more cloudy, like a mist comes over my eyes.’ (P06, 31–40 years)
P: ‘I was drifting to the left all the time.’
Fa: ‘ ‘He were driving right close to other cars that were parked or anything that’s this side … so I had to keep telling him to go back to the middle of the road.’ (P17, M, 61–70 years)
Changes in balance, sensations, and senses: changes in hearing
A few participants experienced sensitivity to noise:
‘If the kids make a noise upstairs I haven’t been able to handle it very well, so I’ve noticed a big increase in sensitivity to the light and the noise … everything was just heightened, the dog barking and things like that it would make me very irritable.’
(P03, F, 31–40 years)
Others felt their hearing was compromised, predominantly on one side, as if the sound was muffled or their ear was wrapped in cotton wool. Such changes meant that these people were straining to hear, asking people to repeat what they were saying or lip reading:
‘I found myself kind of turning to hear with my good ear and things like that … when my colleague speaks to me … she’s further into the room … I have to really try and lip read what she’s saying. I can still hear but just not very well.’
(P23, F, 21–30 years)
Changes in balance, sensations, and senses: changes in taste or smell
A few participants spoke about a metallic smell or taste that was particularly strong at times. This was sometimes subsequently attributed to a seizure:
‘I had had this funny smell … I don’t know if it’s a bit metal-y or a bit fuel-y, maybe workshop-y I suppose.’
(P07, F, 31–40 years)
Change in person: ‘out of character’
Participants and family members compared recent moods, attitudes, or behaviours with how the patient had previously been. Participants mostly knew they were ‘out of character’; some had been apologetic for their change, or articulated that they did not know why they were acting in a different manner. A number of personality or emotional changes were mentioned, including becoming more irritable, angry, anxious, overwhelmed, or sensitive than normal:
‘Not being as tolerant and as thoughtful and as considerate as I would probably expect myself to have been … I thought I was being totally irrational and not reasonable, and unfair and unkind.’
(P02, M, 51–60 years)
Other changes included lack of social inhibitions or change in social interactions:
Fa: ‘It was subtle changes in his personality where he was a little bit inappropriate. And I’ve never had to worry about that, you know, I mean he can be quite outrageous when he’s being funny, but it’s within the realms of absolutely fine. And then I started to worry about what he was going to say, who he was going to wind up, yeah, just how far he might go.’ (P20, M, 71–80 years)
Some were aware of a sense of lethargy that they had not had in the past, describing lack of motivation or lack of interest, and commonly using the phrase ‘just can’t be bothered’ about work and hobbies.
Not all seizures are the same or come ‘out of the blue’
Not all seizures were the same: they differed between participants and over time. Participants spoke of experiences that were ‘out of the norm’ and unwanted, yet still ‘understandable’ such as having déjà vu, panic attacks, sleepwalking, or intrusive daydreams.
Participants explained their symptoms as a ‘simple’ response to being tired, over-exercising, or not eating. Symptoms that were more intrusive led some participants and their family members to think they were experiencing a stroke.
People experiencing a seizure often did not seek help initially, and the timing of the decision to seek help was often driven by a seizure involving a collapse. However, not all patients collapsed, and some chose to visit their GP rather than the emergency department for seizure symptoms:
‘I would feel, “Ooh, that’s a bit strange”, I’m just overtired or there’s been a little reaction and it just didn’t seem to make sense … They were so spasmodic then that it was easy to put it down to just being over-tired and a bit overwrought at work, really.’
(P02, M, 51–60 years)
‘I thought it was like panic attacks … It started off with little twitches and obviously, you don’t really pay attention but now they’re getting like my arm will flare up, my leg will twitch up that way.’
(P06, F, 31–40 years)
‘I just thought they were intrusive daydreams or funny repetitive thoughts … it was that déjà vu sort of thing … I just thought this must be some weird baby brain.’
(P07, F, 31–40 years)
Quality of patient–GP communication
Patients spoke about the considerable consequences of subtle differences in discourse during GP consultations and these were reflected in the patients’, carers’, and GPs’ suggestions for how to improve GP consultations to reduce missed diagnostic opportunities (Box 1).
Box 1. Workshop participants’ views on how to improve GP consultations to reduce missed diagnostic opportunities.
| 1 | Ten-minute appointments or ‘one symptom per appointment’ are not sufficient to share subtle, intermittent changes or symptoms, and can lead to selective or limited disclosure. |
| 2 | Vague symptoms need thorough exploration by family doctors. Take a good history from family and friends if not forthcoming from the patient as patients may not notice all the symptoms themselves. |
| 3 | Improve how patients present their symptoms in the consultation (for example, encourage patients to bring written lists of symptoms, track multiple symptoms, and voice any concerns). |
| 4 | Aim at continuity of care so that GPs can have increased awareness of symptom changes over time. |
| 5 | Encourage follow-up appointments by making them before a patient leaves the surgery or giving a time limit for symptoms to resolve. |
| 6 | Empower patients to return if they think something is wrong or if they are unhappy with the plan. |
| 7 | Identify patients with repeated consultations with vague symptoms and have lower threshold for referral based on GP intuition. |
| 8 | When ordering investigations, most patients would rather be told that cancer is a differential diagnosis. |
Selective or limited disclosure
Patients discussed some of the changes or symptoms with their GP, but often failed to mention all the changes they had noticed because they forgot, or were reluctant to give more details, or found the consultation too short. Some noted that they were uncertain about which changes were important to discuss:
‘What did I tell him … I was feeling really lousy and what have you … at that point I didn’t actually say I’ve just crashed the car through … I didn’t.’ (P27, F, 71–80 years)
Fa: ‘You don’t know what information to offer the doctor because you don’t know what’s relevant and what isn’t. So for [patient] to go and not know, you needed someone to ask the leading questions … and then put a picture together for you. Rather than just sit there.’ (P26, F, 41–50 years)
Conversely, some patients were prompted by their GP to reveal more details, which, in turn, could lead to a decision to refer the patient for further investigations:
‘I said, “I’ve got a migraine” and that was it, that was pretty much all the information I was willing to give and then he said, “OK, well what else has been going on?” And then he made me go through the past 4 weeks like what you did and then got me to describe the migraine in detail and prompted as well.’
(P36, F, 21–30 years)
Alignment of views
Patients generally attended their GP with ideas about the cause of their symptoms. Sometimes patients felt disappointed when they were told nothing was ‘wrong’ yet they continued to experience symptoms, and spoke of believing they had been ‘fobbed off’ or ‘brushed off’. In some cases, the GP agreed with the patient’s views and subsequent investigations aligned with the patient’s suggestions.
However, GPs disagreed with the patient’s suggestions in many more instances. A brain tumour was not initially considered by the GP or the patient; instead, other diagnoses such as infection (viral, or ear, nose, and throat), hormonal changes (thyroid or menopause), eye problems, or mental health issues were considered more likely and investigated first. Once these diagnoses had been ruled out and the patient decided to re-visit their GP, a computed tomography scan was more likely to be arranged during subsequent consultations:
Fa: ‘You went to the GP about a month ago and said then that you’d been having these feelings of not feeling right and not being able to articulate your speech and thoughts … He did a preliminary dementia test, because [patient] was worried that he was getting Alzheimer’s.’ (P02, M, 51–60 years)
P: ‘I think I felt a bit fobbed off with it really … ‘cos [GP] would just look at you and if something wasn’t physical or if something wasn’t fitting in with what he thinks he just didn’t have anything to do with you.’
Fa: ‘You felt like as if you’ve been brushed off.’ (P31, M, 41–50 years)
GP response impacts the patient’s decision to re-consult
When GPs appraised symptoms and gave plausible explanations, patients spoke of feeling reassured; sometimes this gave patients less incentive to re-attend when symptoms continued. If patients felt their symptoms were dismissed or not given attention by the GP, this prompted them to downplay their symptoms and, again, their motivation to re-consult was low:
‘When I said, “Well, still got the headaches”, it didn’t [seem to] matter … So I never went back to my doctors, I just took it that they were saying that the headaches were OK.’
(P05, F, 41–50 years)
‘I was getting to the stage where I thought, well maybe it is just something that will disappear and, you know, they don’t seem too worried about it so I left it for a long time.’
(P23, F, 21–30 years)
Some patients spoke about the GP specifically asking the patient to return, with some booking the appointment for the patient. Others felt that there were missed opportunities to obtain a quicker diagnosis because they were not actively encouraged to re-attend if test results were negative, or symptoms persisted or developed. A few also spoke of long times between appointments, slow or forgotten referrals, or scan results not being reviewed:
‘Well, I just feel [my doctor] should have said, “look, if your eye test comes back clear, come back and we’ll have another look at you, and we’ll investigate it” … But you listen to what your doctor says and because they never said come back … I just feel they just sort of brush you off.’
(P05, F, 41–50 years)
‘You think that if you’d got something that is progressively carrying on over a long period of time that you would get to a stage where you think that you would investigate this a bit more.’
(P31, M, 41–50 years)
‘She said, “Don’t worry about it too much and come back and see me in a little bit,” which is fair enough … She made an appointment for me to come back I think.’
(P07, F, 31–40 years)
‘They didn’t send me to A&E, they sent me to a specific clinic where she’d obviously rung in front, because they were waiting, and a couple of days later, and she only works 2 days a week at the surgery, she actually phoned up [spouse] to check that everything went well. So I suspect when I walked in the door, she knew there was a problem.’
(P32, M, 71–80 years)
DISCUSSION
Summary
As far as the authors are aware, this is the first study to explore patient experiences along the pathway to primary brain tumour diagnosis. It provides a rich understanding of how people and their family and friends try to make sense of subtle, intermittent, and often multiple changes in their functioning and wellbeing before they are even identified as ‘symptoms’.
More than half of the study participants and their family members noticed a combination of physical and cognitive changes more than 6 months before seeking help, and many went on to have multiple encounters with GPs and other healthcare professionals before either referral and appropriate diagnostic imaging, or accelerating symptoms leading to emergency presentation.
Strengths and limitations
The study sample was diverse with respect to age, type, and stage of brain tumour at diagnosis, and was drawn from two regions of England characterised by diverse socioeconomic circumstances. Importantly, patients were interviewed within a few weeks of their diagnosis and before undergoing neurosurgery, facilitating recall of the subtle and intermittent ‘changes’ or symptoms they had experienced over the preceding months. They were also encouraged to have a family member present during the interview. Both approaches should minimise recall bias. The authors believe that data saturation was achieved as no new themes were identified in the later interviews. Furthermore, the workshop for GPs, other patients, and their family members provided an opportunity to triangulate the analysis and check the credibility of the study’s early findings, as well as confirming saturation.
The main limitation was that the participants were often unwell when they were interviewed, and sometimes apprehensive about their imminent major surgery. Although they were altruistic in wishing to contribute to the current research, their condition sometimes led to difficulties in communication, or to reliance on family members to ‘talk for them’. There may have been differences between the ‘public’ accounts given in the interviews, often in front of loved ones, and participants’ actual experiences and views. However, this work still provides important insights into the subtle and intermittent changes that can precede a brain tumour diagnosis and potential missed diagnostic opportunities.
Comparison with existing literature
These findings are consistent with previous work, which shows that non-specific symptoms often precede brain tumour diagnosis, making patient presentation and GP assessment for further investigation or referral problematic. This can result in multiple GP consultations, emergency presentations, and longer patient pathways.17,29
A recently reported epidemiological study, which analysed brain tumour cases from the national audit of cancer diagnosis in primary care, grouped neurological symptoms into six domains: headache, behavioural or cognitive change, focal neurology, ‘fits, faints, and falls’, non-specific neurological, and other or non-specific.17 However, the current study’s findings have identified a far wider range of subtle and often intermittent symptoms, more frequently referred to as ‘changes’ by the patients, and suggest that the GP case-note audit approach may not have identified people presenting with changes in sleep, and feelings in their head, not identified as a headache. There may also be a recording bias in primary care records studies because GPs tend to record a single symptom or the most significant symptoms, and there is neither the time nor the codes available to record more subtle or complex presentations, or the terms used by the patients to describe the changes.
Implications for research and practice
Clinicians continue to consider seizures and headache as the main presenting symptoms of brain tumours.30 However, the current study has shown that patients notice other changes and symptoms for many months before presentation. Studies using qualitative approaches have contributed to a deeper clinical understanding of the development of other serious conditions such as pancreatic cancer31 and meningococcal disease in children.32 Clearly, the current study’s qualitative findings, drawn from a small sample of patients soon after their brain tumour diagnosis, now need to be validated in a larger cohort. If generalisable, these findings could lead to tailored awareness campaigns for adult patients and educational approaches for GPs in a similar way to the HeadSmart campaign that was launched in the UK in 2011, following the recognition that the median total diagnostic interval was three times longer for children with brain tumours in the UK than in the US.33 The HeadSmart guidance on symptom awareness, assessment, investigation, and referral has been shown to enhance awareness among health professionals and the public, and appears to have led to a significant reduction in the UK’s total diagnostic interval.33,34
Non-specific early symptoms of brain tumours can contribute to diagnostic delays and possible disease progression. GPs seeing patients with these non-specific symptoms, such as mild cognitive changes or sleep changes, should be able to identify patients who warrant further investigation without an increase in unnecessary brain imaging, which may expose incidental abnormalities. GPs could benefit from a triage tool, particularly one that is low cost and accessible in primary care, such as the GP assessment of cognition (GPCOG) screening tool to elicit cognitive and functional changes indicative of dementia.35 A promising new approach is a serum-based spectroscopic tool that can detect disease-specific signatures;36 this has been shown to be a potentially cost-effective addition to the brain tumour diagnostic pathway,37 and is currently under development as a triage tool for primary care. Furthermore, recent advances in molecular biology have improved our understanding of glioma pathogenesis, with genomics now combined with histology in the revised 2016 World Health Organization classification of central nervous system tumours; this could contribute to biomarker-based early detection of brain tumours in the future.38
In summary, although subtle and intermittent changes or symptoms are almost universally experienced with age, and may occur with headache, GPs’ awareness of the changes preceding brain tumour diagnosis, and effective patient–GP communication with follow-up as safety netting, could alert them to more rapid investigation and referral, and possibly reduce development of the significant and major symptoms associated with brain tumours.
Acknowledgments
Thanks to all the patients and their family members who have so willingly shared their experiences at a very difficult time. Thanks also to those who contributed to recruitment at the Cambridge University Hospitals NHS Foundation Trust and the Walton Centre NHS Foundation Trust, Liverpool; to Sarah Curtis and Anna Wood for their contributions to data analysis; and to PPI representative Joyce Bell.
Funding
This study was supported by a grant from the Brain Tumour Charity (GN-000316 — BRACED: The BRAin Cancer Early Detection study). Fiona Walter and Willie Hamilton are directors of the multi-institutional CanTest Collaborative, which is funded by Cancer Research UK (CRUK) (C8640/A23385). Colin Watts is funded by the Brain Tumour Charity and CRUK.
Ethical approval
Ethical approval was granted by Cambridge South NRES Committee, East of England: 16/EE/0179.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a ‘state of the science’ review. Neuro Oncol. 2014;16(7):896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodbelt A, Greenberg D, Winters T, et al. Glioblastoma in England: 2007–2011. Eur J Cancer. 2015;51(4):533–542. doi: 10.1016/j.ejca.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Kirkby NF, Jefferies SJ, Jena R, Burnet NG. A mathematical model of the treatment and survival of patients with high-grade brain tumours. J Theor Biol. 2007;245(1):112–124. doi: 10.1016/j.jtbi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Burnet NG, Jefferies SJ, Benson RJ, et al. Years of life lost (YLL) from cancer is an important measure of population burden — and should be considered when allocating research funds. Br J Cancer. 2005;92(2):241–245. doi: 10.1038/sj.bjc.6602321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyratzopoulos G, Abel GA, McPhail S, et al. Measures of promptness of cancer diagnosis in primary care: secondary analysis of national audit data on patients with 18 common and rarer cancers. Br J Cancer. 2013;108(3):686–690. doi: 10.1038/bjc.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliss-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer: determining the patient journey using multiple routine data sets. Br J Cancer. 2012;107(8):1220–1226. doi: 10.1038/bjc.2012.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health England, National Cancer Registration and Analysis Service Brain routes to diagnosis 2015 update Routes to diagnosis 2015 update: brain tumours National Cancer Intelligence Network short report. http://www.ncin.org.uk/view?rid=3177 (accessed 4 Mar 2019).
- 8.Hamdan A, Mitchell P. The two-week wait guideline for suspected CNS tumours: a decade analysis. Br J Neurosurg. 2013;27(5):642–645. doi: 10.3109/02688697.2013.771725. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence Improving outcomes for people with brain and other central nervous system tumours CSG10. 2006 https://www.nice.org.uk/guidance/csg10 (accessed 27 Feb 2019). [Google Scholar]
- 10.Bates A, Gonzalez-Viana E, Cruickshank G, Roques T. Primary and metastatic brain tumours in adults: summary of NICE guidance. BMJ. 2018;362:k2924. doi: 10.1136/bmj.k2924. [DOI] [PubMed] [Google Scholar]
- 11.Simpson GC, Forbes K, Teasdale E, et al. Impact of GP direct-access computerised tomography for the investigation of chronic daily headache. Br J Gen Pract. 2010;60(581):897–901. doi: 10.3399/bjgp10X544069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penfold C, Joannides AJ, Bell J, Walter FM. Diagnosing adult primary brain tumours: can we do better? Br J Gen Pract. 2017;67(659):278–279. doi: 10.3399/bjgp17X691277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton W, Kernick D. Clinical features of primary brain tumours: a case-control study using electronic primary care records. Br J Gen Pract. 2007;57(542):695–699. [PMC free article] [PubMed] [Google Scholar]
- 14.Kernick DP, Ahmed F, Bahra A, et al. Imaging patients with suspected brain tumour: guidance for primary care. Br J Gen Pract. 2008;58(557):880–885. doi: 10.3399/bjgp08X376203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dommett RM, Redaniel MT, Stevens MC, et al. Features of cancer in teenagers and young adults in primary care: a population-based nested case-control study. Br J Cancer. 2013;108(11):2329–2333. doi: 10.1038/bjc.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Hansen M, Berendse S, Hamilton W. Symptomatic diagnosis of cancer of the brain and central nervous system in primary care: a systematic review. Fam Pract. 2015;32(6):618–623. doi: 10.1093/fampra/cmv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozawa M, Brennan PM, Zienius K, et al. Symptoms in primary care with time to diagnosis of brain tumours. Fam Pract. 2018;35(5):551–558. doi: 10.1093/fampra/cmx139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walter FM, Birt L, Cavers D, et al. ‘This isn’t what mine looked like’: a qualitative study of symptom appraisal and help seeking in people recently diagnosed with melanoma. BMJ Open. 2014;4(7):e005566. doi: 10.1136/bmjopen-2014-005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birt L, Hall N, Emery J, et al. Responding to symptoms suggestive of lung cancer: a qualitative interview study. BMJ Open Respir Res. 2014;1(1):e000067. doi: 10.1136/bmjresp-2014-000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall N, Birt L, Banks J, et al. Symptom appraisal and healthcare-seeking for symptoms suggestive of colorectal cancer: a qualitative study. BMJ Open. 2015;5(10):e008448. doi: 10.1136/bmjopen-2015-008448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mills K, Birt L, Emery JD, et al. Understanding symptom appraisal and help-seeking in people with symptoms suggestive of pancreatic cancer: a qualitative study. BMJ Open. 2017;7(9):e015682. doi: 10.1136/bmjopen-2016-015682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien BC, Harris IB, Beckman TJ, et al. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89(9):1245–1251. doi: 10.1097/ACM.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 23.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. [Google Scholar]
- 24.Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer. 2012;106(7):1262–1267. doi: 10.1038/bjc.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter F, Webster A, Scott S, Emery J. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy. 2012;17(2):110–118. doi: 10.1258/jhsrp.2011.010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott SE, Walter FM, Webster A, et al. The model of pathways to treatment: conceptualization and integration with existing theory. Br J Health Psychol. 2013;18(1):45–65. doi: 10.1111/j.2044-8287.2012.02077.x. [DOI] [PubMed] [Google Scholar]
- 27.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117. doi: 10.1186/1471-2288-13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott SE, Penfold C, Saji S, et al. ‘It was nothing that you would think was anything’: qualitative analysis of appraisal and help seeking for changes preceding brain cancer diagnosis. PLOS One. 2019 doi: 10.1371/journal.pone.0213599. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corner J, Hopkinson J, Fitzsimmons D, et al. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax. 2005;60(4):314–319. doi: 10.1136/thx.2004.029264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo M, Villani V, Taga A, et al. Headache as a presenting symptom of glioma: a cross-sectional study. Cephalalgia. 2018;38(4):730–735. doi: 10.1177/0333102417710020. [DOI] [PubMed] [Google Scholar]
- 31.Evans J, Chapple A, Salisbury H, et al. ‘It can’t be very important because it comes and goes’ — patients’ accounts of intermittent symptoms preceding a pancreatic cancer diagnosis: a qualitative study. BMJ Open. 2014;4(2):e004215. doi: 10.1136/bmjopen-2013-004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson MJ, Ninis N, Perera R, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367(9508):397–403. doi: 10.1016/S0140-6736(06)67932-4. [DOI] [PubMed] [Google Scholar]
- 33.Walker D, Hamilton W, Walter FM, Watts C. Strategies to accelerate diagnosis of primary brain tumors at the primary-secondary care interface in children and adults. CNS Oncol. 2013;2(5):447–462. doi: 10.2217/cns.13.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu TP, Shah A, Walker D, Coleman MP. Pattern of symptoms and signs of primary intracranial tumours in children and young adults: a record linkage study. Arch Dis Child. 2015;100(12):1115–1122. doi: 10.1136/archdischild-2014-307578. [DOI] [PubMed] [Google Scholar]
- 35.Brodaty H, Pond D, Kemp NM, et al. The GPCOG: a new screening test for dementia designed for general practice. J Am Geriatr Soc. 2002;50(3):530–534. doi: 10.1046/j.1532-5415.2002.50122.x. [DOI] [PubMed] [Google Scholar]
- 36.Hands JR, Clemens G, Stables R, et al. Brain tumour differentiation: rapid stratified serum diagnostics via attenuated total reflection Fourier-transform infrared spectroscopy. J Neurooncol. 2016;127(3):463–472. doi: 10.1007/s11060-016-2060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray E, Butler HJ, Board R, et al. Health economic evaluation of a serum-based blood test for brain tumour diagnosis: exploration of two clinical scenarios. BMJ Open. 2018;8(5):e017593. doi: 10.1136/bmjopen-2017-017593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446. doi: 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]